Wound Healing: Molecular Mechanisms, Antimicrobial Peptides, and Emerging Technologies in Regenerative Medicine
Abstract
1. Introduction
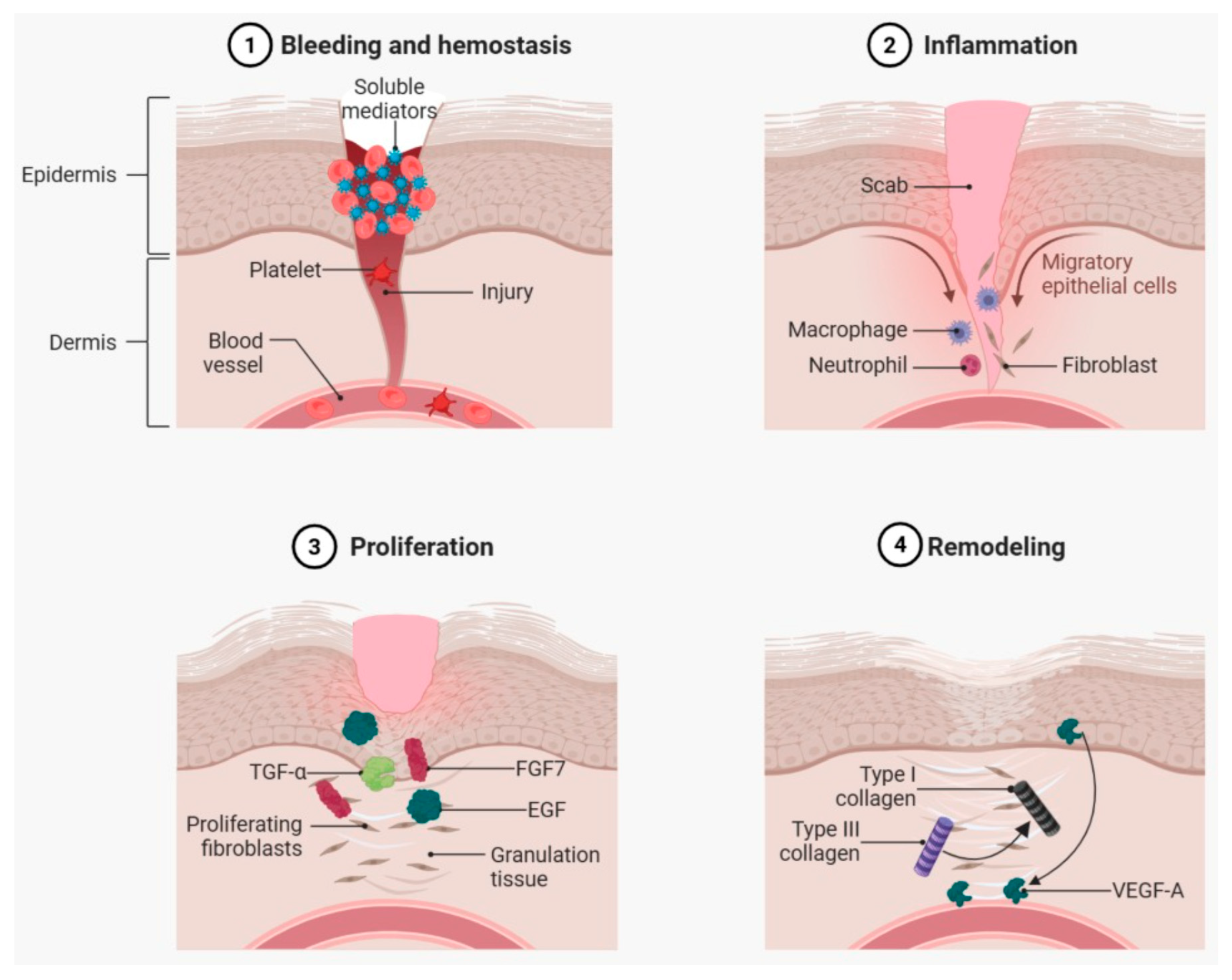
2. The Cellular and Molecular Landscape of Wound Healing
2.1. Clot Formation and Reepithelialization
2.2. Damage Signaling in Wounds
2.3. Role of Neutrophils and Macrophages in the Wound Healing Process
2.4. Angiogenesis in Wound Healing
2.5. Remodeling
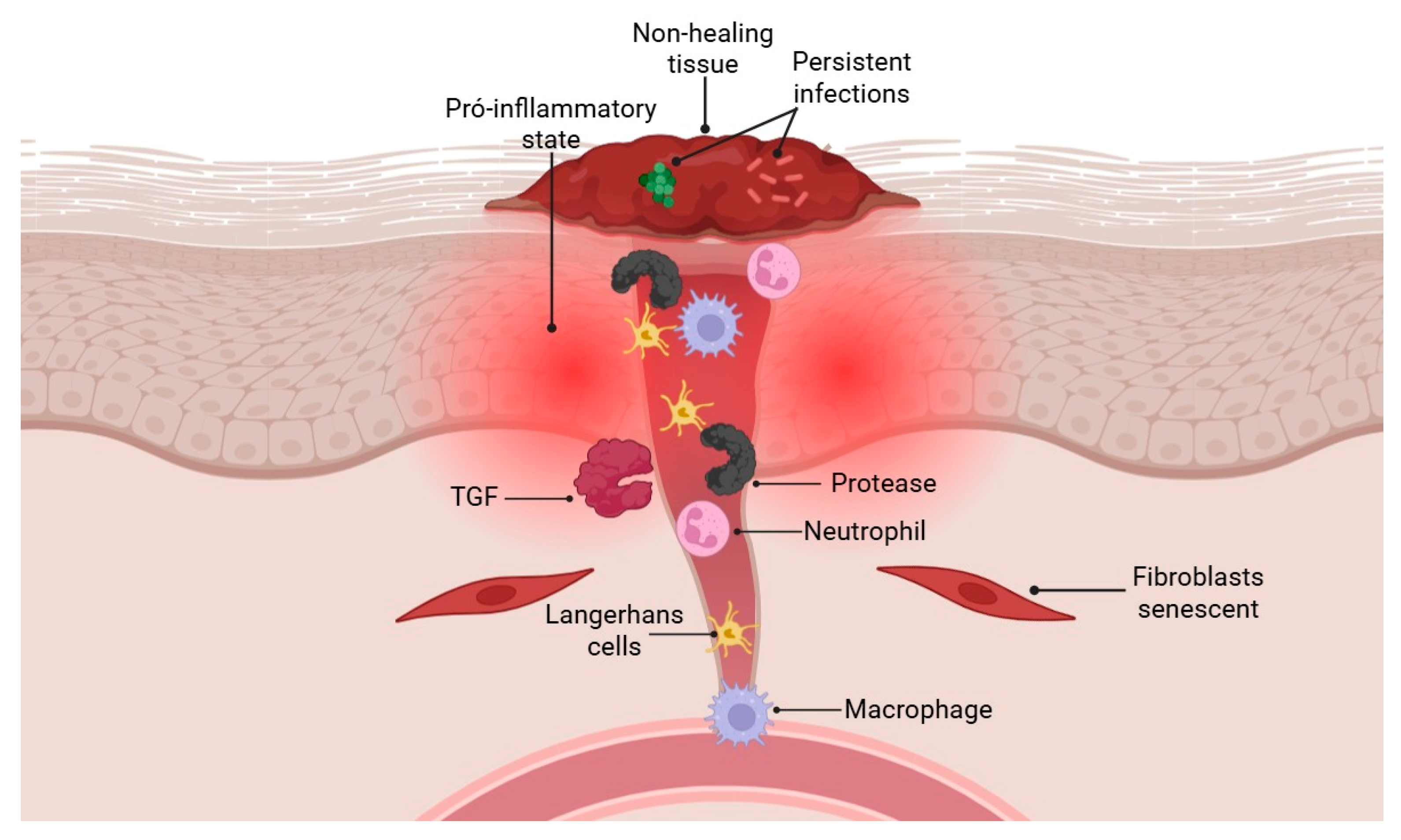
2.6. Failures in Tissue Repair
2.7. Fracture Healing
3. Antimicrobial Peptides in Wound Healing
3.1. Modulation of Innate Immunity and Direct Antimicrobial Action
3.2. Regulation of Cell Proliferation and Differentiation
3.3. Therapeutic Applications in Chronic Wounds
3.4. Limitations, Challenges and Strategies
4. Biofilms in Chronic Wounds
4.1. Mechanisms of Formation and Resistance of Bacterial Biofilms in Chronic Wounds
4.2. Impact of Biofilms on Chronic Inflammation and Delayed Healing
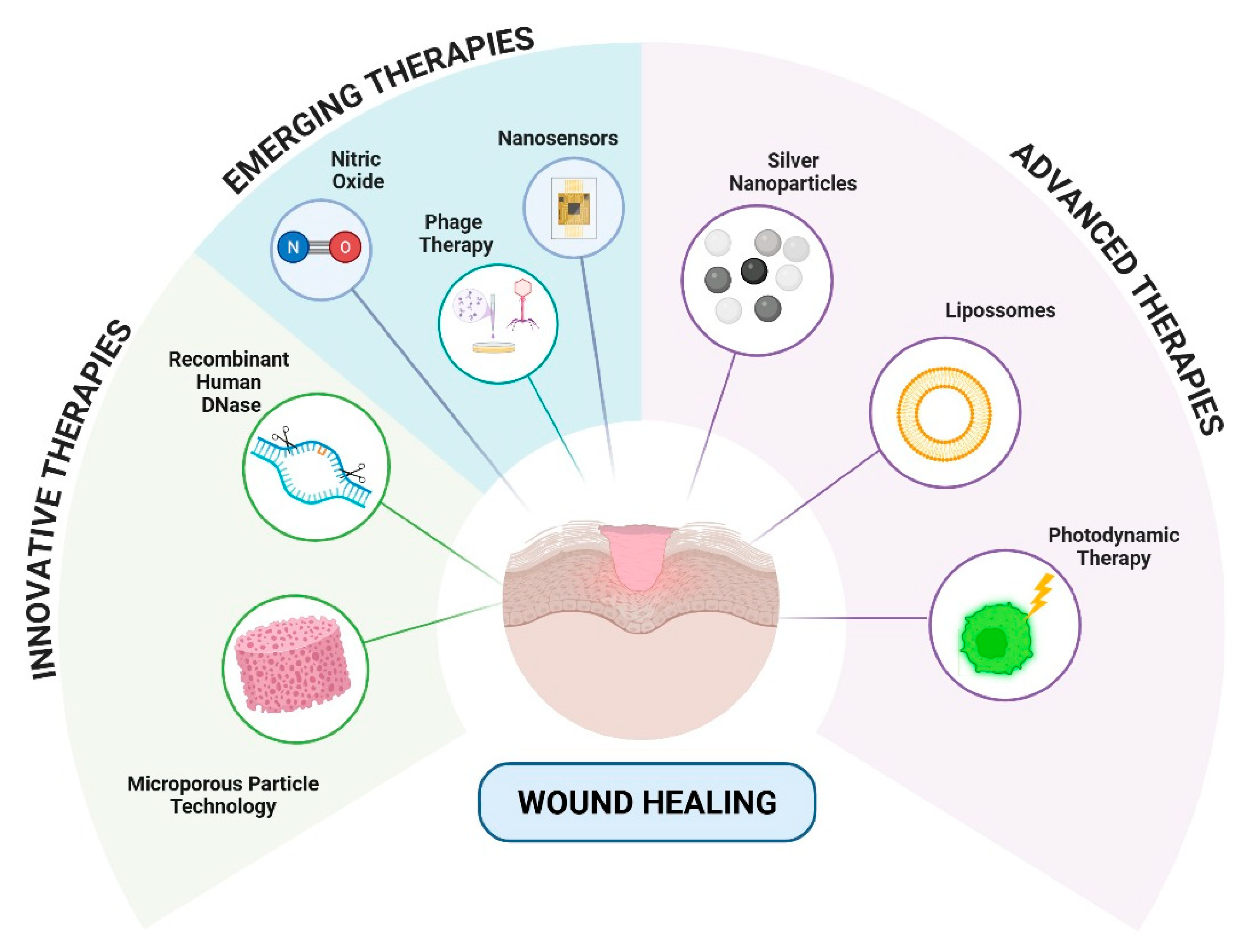
4.3. Advanced Therapeutic Approaches to Combating Biofilms Associated with Inflammation and Chronic Skin Wounds
4.3.1. Innovative Therapies
4.3.2. Advanced Therapies
4.3.3. Emerging Therapies
| Therapy | Classification | Mechanism of Action | References |
|---|---|---|---|
| Silver nanoparticles (AgNPs) | Advanced | Release of silver ions, oxidative damage to biofilm | [114] |
| Liposomal antibiotics | Advanced | Controlled release and biofilm targeting | [119] |
| Photodynamic Therapy (aPDT) | Advanced | Generation of reactive oxygen species via light and photosensitizer | [122] |
| Dornase alfa (DNase) | Innovative | Degradation of extracellular DNA from the biofilm matrix | [110] |
| Nitric oxide (NO) nanoparticles | Emerging | Biofilm dispersion and inflammatory modulation via NO | [123] |
| Bacteriophages | Emerging | Bacterial lysis and synergism with antibiotics | [126] |
| Photomodulated NO hydrogels | Emerging | Light-induced NO release | [124] |
| Microporous particles (MPPT) | Emerging | Passive removal of bacterial toxins and enzymes | [129] |
| Enzybiotic (phage endolysins) | Emerging | Specific enzymatic degradation of the bacterial wall | [128] |
| Dressings with smart sensors | Emerging | Biomarker monitoring and adaptive release | [130] |
| Silica nanoparticles with lectins | Emerging | Targeted delivery of antimicrobials to the biofilm matrix | [136] |
| Clay dressings with zwitterions | Emerging | Sustained release of antimicrobials with clay support | [135] |
5. Innovative Therapies and Technological Advances in Wound Healing
5.1. Biomaterials and Scaffolds for Tissue Regeneration
5.2. Gene Therapy and Cell-Based Treatments for Wound Regeneration
5.3. Three-Dimensional Printing and AI in Wound Management
5.4. Cutaneous Organoids in Wound Regeneration
6. Benefits of Using Peptides in Nanoparticles
| Type | Peptide/AMPc | Diameter (nm) | EE% | Concentration | Application | Outcome | References |
|---|---|---|---|---|---|---|---|
| Gold nanoparticles (AuNPs) | LL37 | 10 ± 1 | ND | 100 μg/mL | Therapy for diabetic wound healing | AuNPs@LL37 showed superior antibacterial action, synergistic effect with promotion of angiogenesis and accelerated healing of diabetic wounds, in addition to high biocompatibility in vitro and in vivo. | [199] |
| PLGA | Plectasin | 224 ± 3 to 215 ± 3 | 71–90 | 500 μL of peptide stock solution to PLGA solution (60 mg/mL) | Antimicrobial activity against Staphylococcus aureus | Plectasin-loaded nanoparticles demonstrated greater efficacy than free plectasin, without affecting the viability of eukaryotic cells at the concentrations tested. | [200] |
| Chitosan | Octominin | 372 ± 2 | 96.4 | Octoprohibitin (1 mg/mL) | Bacterial and fungal infections | Nanoencapsulated octominin demonstrated greater antimicrobial activity against C. albicans and A. baumannii compared to free Octominin. | [201] |
| PLGA | GIBIM-P5S9K (G17) and GAM019 (G19) | 1022 ± 3 and 1976 ± 4 | 41–67 | 10% PLGA (w/v) in ethyl acetate (EtAc) in a ratio of peptide solution to PLGA dispersion of 2:1 | Antimicrobial activity against Staphylococcus aureus (MRSA) and Escherichia coli | Nanoparticles loaded with G17 and G19 peptides showed slow release and bacteriostatic potential against E. coli O157:H7 and MRSA. | [197] |
| Chitosan | CAMA-CPP | 597 ± 1 | 75.2 | CS solution (0.50% w/v); Acetic acid (1%) and STPP (0.25%) solution. CAMA-CPP was added at a concentration of 0.10 mg/mL to the CS solution. | Antimicrobial activity against Salmonella enteritidis | ENC CAMA-CPP demonstrated pH-dependent sustained release, enzymatic and biological stability, in vitro safety, and antimicrobial and immunomodulatory efficacy against multidrug-resistant S. enteritidis. | [202] |
| Chitosan/alginate | Pexiganan (MSI-78) | ND | ND | PNPs 32 µg/mL | Helicobacter pylori infections | PNPs improved peptide stability in the stomach and demonstrated more effectiveness in eradicating H. pylori in the stomach of rats compared to pexiganan | [203] |
| Chitosan | Ultra short AMP (RBRBR) | 121 ± 1 | 51.3 | Chitosan 1.75% (v:v); acetic acid (1 mg/mL, 5 pH); TPP (1 mg/mL) and 500 µg of RBRBR. | Antimicrobial activity | RBRBR-CS-NPs demonstrated prolonged and selective action against several Gram-positive bacteria, including resistant strains of S. aureus | [204] |
| Solid Lipid Nanoparticle (SLN) | LL-37 and SERPINA A1 | 261 ± 4 | 83.3 | 8.48 μg for LL37 and 43.5 μg for A1 per mg of SLNs and 16.32 μg for LL37 and 62.47 μg for A1 per mg of SLNs | Wound healing and antimicrobial activity | LL37-A1-SLNs accelerated the wound healing process and improved antibacterial activity against S. aureus and E. coli compared with LL37 or A1 | [205] |
| Lipid-coated mesoporous silica nanoparticles | PA-targeting LL-37 peptide | 620 ± 10 | 79.3 | MSNs were loaded with Col by mixing 20 mL of MSN (10 mg/mL) with Col (20 mg, 17 μmol) | Antimicrobial activity against Pseudomonas aeruginosa | The Col@MSN@LL-(LL-37) nanocomposite demonstrated a 6.7-fold enhancement in antimicrobial activity relative to free Col | [206] |
| Chitosan | Antimicrobial Peptide Octoprohibitin | 246 ± 1 | 34.2 | 1 mg of Octominin-containing Octominin-CNPs | Antimicrobial activity against Acinetobacter baumannii | Octoprohibitin encapsulated in carbon nanoparticles (CNPs) exhibited potent antimicrobial activity against multidrug-resistant A. baumannii, effectively targeting both planktonic cells and established biofilms | [207] |
| PLGA | LL37 | 304.5 ± 10.0 | 70.2 | 20 mg of PLGA to 20 μg of LL37 (95.0% pure, Caslo ApS, DK) | Healing and antimicrobial activity against Escherichia coli | PLGA-LL37NP nanoparticles enhanced wound healing activity and significantly increased IL-6 and VEGFa at the mRNA level improving angiogenesis | [77] |
| Nesoporous polydopamine (MPDA) | RL-QN15 | 205 | 67.3 | 1 mg MPDA was dispersed in RL-QN15 | Therapies for the treatment of skin wounds | MPDA-RL-QN15 nanocomposites demonstrated up to 50-fold enhanced wound healing activity in animal models, with efficacy confirmed through histological analysis. | [208] |
| Hollow Silica Nanoparticles Loaded with RL-QN15 Peptide | RL-QN15 | 50 | ND | HPDA was mixed with CyRL-QN15 (1 nM) | Therapeutic strategy for clinical chronic skin wound healing. | The HPDAlCyRL-QN15/ZA hydrogel accelerated diabetic wound healing by promoting cell regeneration, angiogenesis, collagen deposition, and by reducing inflammation and oxidative stress. | [209] |
6.1. Types of Nanoparticles Used for Peptides
6.2. Clinical Applications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2018, 99, 665–706. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and Incidence of Chronic Wounds and Related Complications: A Protocol for a Systematic Review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Zamanifard, M.; Nasiri, M.; Yarahmadi, F.; Zonoori, S.; Razani, O.; Salajegheh, Z.; Imanipour, M.; Mohammadi, S.M.; Jomehzadeh, N.; Asadi, M. Healing of Diabetic Foot Ulcer with Topical and Oral Administrations of Herbal Products: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Int. Wound J. 2024, 21, e14760. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Adib, Y.; Bensussan, A.; Michel, L. Cutaneous Wound Healing: A Review about Innate Immune Response and Current Therapeutic Applications. Mediat. Inflamm. 2022, 2022, 5344085. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, Fracture and Bone Repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef]
- Schlundt, C.; Fischer, H.; Bucher, C.H.; Rendenbach, C.; Duda, G.N.; Schmidt-Bleek, K. The Multifaceted Roles of Macrophages in Bone Regeneration: A Story of Polarization, Activation and Time. Acta Biomater. 2021, 133, 46–57. [Google Scholar] [CrossRef]
- García-Domínguez, M. Pathological and Inflammatory Consequences of Aging. Biomolecules 2025, 15, 404. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Borchard, G.; Jordan, O. An Update on Antimicrobial Peptides (AMPs) and Their Delivery Strategies for Wound Infections. Pharmaceutics 2020, 12, 840. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chi, J.; Yan, Y.; Luo, R.; Feng, X.; Zheng, Y.; Xian, D.; Li, X.; Quan, G.; Liu, D.; et al. Membrane-Disruptive Peptides/Peptidomimetics-Based Therapeutics: Promising Systems to Combat Bacteria and Cancer in the Drug-Resistant Era. Acta Pharm. Sin. B 2021, 11, 2609–2644. [Google Scholar] [CrossRef]
- Asadi, N.; Pazoki-Toroudi, H.; Del Bakhshayesh, A.R.; Akbarzadeh, A.; Davaran, S.; Annabi, N. Multifunctional Hydrogels for Wound Healing: Special Focus on Biomacromolecular Based Hydrogels. Int. J. Biol. Macromol. 2021, 170, 728–750. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. A Review of Current Advancements for Wound Healing: Biomaterial Applications and Medical Devices. J. Biomed. Mater. Res. 2022, 110, 2542–2573. [Google Scholar] [CrossRef]
- Schulze, F.; Lang, A.; Schoon, J.; Wassilew, G.I.; Reichert, J. Scaffold Guided Bone Regeneration for the Treatment of Large Segmental Defects in Long Bones. Biomedicines 2023, 11, 325. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Seidi, F.; Youssefi Azarfam, M.; Khodadadi Yazdi, M.; Erfani, A.; Barani, M.; Chauhan, N.P.S.; Rabiee, N.; Kuang, T.; Kucinska-Lipka, J.; et al. Biopolymer-Based Composites for Tissue Engineering Applications: A Basis for Future Opportunities. Compos. Part B Eng. 2023, 258, 110701. [Google Scholar] [CrossRef]
- Garmany, A.; Terzic, A. Artificial Intelligence Powers Regenerative Medicine into Predictive Realm. Regen. Med. 2024, 19, 611–616. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Multimodal AI in Biomedicine: Pioneering the Future of Biomaterials, Diagnostics, and Personalized Healthcare. Nanomaterials 2025, 15, 895. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of Platelets, the Coagulation and Fibrinolytic Systems to Cutaneous Wound Healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, J.K. Back to Basics: The Coagulation Pathway. Blood Res. 2024, 59, 35. [Google Scholar] [CrossRef]
- Wilhelm, G.; Mertowska, P.; Mertowski, S.; Przysucha, A.; Strużyna, J.; Grywalska, E.; Torres, K. The Crossroads of the Coagulation System and the Immune System: Interactions and Connections. Int. J. Mol. Sci. 2023, 24, 12563. [Google Scholar] [CrossRef]
- Byun, K.-A.; Lee, J.H.; Lee, S.Y.; Oh, S.; Batsukh, S.; Cheon, G.; Lee, D.; Hong, J.H.; Son, K.H.; Byun, K. Piezo1 Activation Drives Enhanced Collagen Synthesis in Aged Animal Skin Induced by Poly L-Lactic Acid Fillers. Int. J. Mol. Sci. 2024, 25, 7232. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef]
- Smith, J.; Rai, V. Novel Factors Regulating Proliferation, Migration, and Differentiation of Fibroblasts, Keratinocytes, and Vascular Smooth Muscle Cells during Wound Healing. Biomedicines 2024, 12, 1939. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and Molecular Mechanisms of Skin Wound Healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Xin, Z.; Yu, D.; Yang, B.; Chen, L.; Hayouka, Z.; Chen, X.; Gong, Y.; Dai, H.; Wang, L.; Zhao, Y.; et al. Molecular Characterization, Expression and Immune Functions of Two C-Type Lectin from Venerupis Philippinarum. Fish Shellfish Immunol. 2020, 107, 260–268. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.; Bjorklund, M.; Xu, S. Mitochondrial Fragmentation and ROS Signaling in Wound Response and Repair. Cell Regen. 2022, 11, 38. [Google Scholar] [CrossRef]
- Moccia, F.; Fiorio Pla, A.; Lim, D.; Lodola, F.; Gerbino, A. Intracellular Ca2+ Signalling: Unexpected New Roles for the Usual Suspect. Front. Physiol. 2023, 14, 1210085. [Google Scholar] [CrossRef]
- Barnett, K.C.; Kagan, J.C. Lipids That Directly Regulate Innate Immune Signal Transduction. Innate Immun. 2020, 26, 4–14. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Song, Z.; Bhattacharya, S.; Clemens, R.A.; Dinauer, M.C. Molecular Regulation of Neutrophil Swarming in Health and Disease: Lessons from the Phagocyte Oxidase. iScience 2023, 26, 108034. [Google Scholar] [CrossRef]
- Zhou, Y.-Y.; Sun, B.-W. Recent Advances in Neutrophil Chemotaxis Abnormalities during Sepsis. Chin. J. Traumatol. 2022, 25, 317–324. [Google Scholar] [CrossRef]
- Brown, L.; Yipp, B.G. Neutrophil Swarming: Is a Good Offense the Best Defense? iScience 2023, 26, 107655. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Yang, J.; Man, J.; Wang, H.; Li, Y.; Liang, C.; Cao, J.; Chen, S.; Li, K.; et al. Rethinking Neutrophil Extracellular Traps. Int. Immunopharmacol. 2023, 124, 110834. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-Wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Guan, F.; Wang, R.; Yi, Z.; Luo, P.; Liu, W.; Xie, Y.; Liu, Z.; Xia, Z.; Zhang, H.; Cheng, Q. Tissue Macrophages: Origin, Heterogenity, Biological Functions, Diseases and Therapeutic Targets. Sig. Transduct. Target. Ther. 2025, 10, 93. [Google Scholar] [CrossRef]
- Yan, L.; Wang, J.; Cai, X.; Liou, Y.; Shen, H.; Hao, J.; Huang, C.; Luo, G.; He, W. Macrophage Plasticity: Signaling Pathways, Tissue Repair, and Regeneration. MedComm 2024, 5, e658. [Google Scholar] [CrossRef]
- Brancewicz, J.; Wójcik, N.; Sarnowska, Z.; Robak, J.; Król, M. The Multifaceted Role of Macrophages in Biology and Diseases. Int. J. Mol. Sci. 2025, 26, 2107. [Google Scholar] [CrossRef]
- Chelmu Voda, C.; Stefanopol, I.A.; Gurau, G.; Hîncu, M.A.; Popa, G.V.; Mateescu, O.G.; Baroiu, L.; Mehedinti, M.C. Update on the Study of Angiogenesis in Surgical Wounds in Patients with Childhood Obesity. Biomedicines 2025, 13, 375. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Zou, B.; Yang, D.; Lu, Y.; Zhang, X.; Zhang, C.; Li, Y.; Zhu, C. Macrophage Regulation in Vascularization upon Regeneration and Repair of Tissue Injury and Engineered Organ Transplantation. Fundam. Res. 2025, 5, 697–714. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, X.; Wu, Z.; Qu, B.; Yuan, M.; Xing, Y.; Song, Y.; Wang, Z. Lymphatic Vessel: Origin, Heterogeneity, Biological Functions and Therapeutic Targets. Sig. Transduct. Target. Ther. 2024, 9, 9. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 81, pp. 241–330. ISBN 978-0-12-812151-1. [Google Scholar]
- Fan, J.; Xie, Y.; Liu, D.; Cui, R.; Zhang, W.; Shen, M.; Cao, L. Crosstalk Between H-Type Vascular Endothelial Cells and Macrophages: A Potential Regulator of Bone Homeostasis. J. Inflamm. Res. 2025, 18, 2743–2765. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, D.; Zhao, F. Updates on Recent Clinical Assessment of Commercial Chronic Wound Care Products. Adv. Healthc. Mater. 2023, 12, 2300556. [Google Scholar] [CrossRef]
- desJardins-Park, H.E.; Gurtner, G.C.; Wan, D.C.; Longaker, M.T. From Chronic Wounds to Scarring: The Growing Health Care Burden of Under- and Over-Healing Wounds. Adv. Wound Care 2022, 11, 496–510. [Google Scholar] [CrossRef]
- Zhang, M.; Gu, G.; Xu, Y.; Luan, X.; Liu, J.; He, P.; Wei, G. Injectable Self-Healing Antibacterial Hydrogels with Tailored Functions by Loading Peptide Nanofiber-Biomimetic Silver Nanoparticles. Macromol. Rapid Commun. 2024, 45, 2400173. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, R.; Xiong, Z.; Feng, Y.; Chen, L. Dysregulation of Autophagy during Photoaging Reduce Oxidative Stress and Inflammatory Damage Caused by UV. Front. Pharmacol. 2025, 16, 1562845. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, C. Cellular Senescence Is a Promising Target for Chronic Wounds: A Comprehensive Review. Burn. Trauma 2020, 8, tkaa021. [Google Scholar] [CrossRef]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Zander, R.; Khatun, A.; Kasmani, M.Y.; Chen, Y.; Cui, W. Delineating the Transcriptional Landscape and Clonal Diversity of Virus-Specific CD4+ T Cells during Chronic Viral Infection. eLife 2022, 11, e80079. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The Interplay of Fibroblasts, the Extracellular Matrix, and Inflammation in Scar Formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef]
- Nagy, T.; Fisi, V.; Frank, D.; Kátai, E.; Nagy, Z.; Miseta, A. Hyperglycemia-Induced Aberrant Cell Proliferation; A Metabolic Challenge Mediated by Protein O-GlcNAc Modification. Cells 2019, 8, 999. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Khosla, S. Fracture Healing in the Setting of Endocrine Diseases, Aging, and Cellular Senescence. Endocr. Rev. 2022, 43, 984–1002. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Aurora, R.; Silva, M.J. T Cells Heal Bone Fractures with Help from the Gut Microbiome. J. Clin. Investig. 2023, 133, e167311. [Google Scholar] [CrossRef]
- Ben Letaifa, R.; Klaylat, T.; Tarchala, M.; Gao, C.; Schneider, P.; Rosenzweig, D.H.; Martineau, P.A.; Gawri, R. Osteoimmunology: An Overview of the Interplay of the Immune System and the Bone Tissue in Fracture Healing. Surgeries 2024, 5, 402–414. [Google Scholar] [CrossRef]
- Weivoda, M.M.; Bradley, E.W. Macrophages and Bone Remodeling. J. Bone Miner. Res. 2023, 38, 359–369. [Google Scholar] [CrossRef]
- Frade, B.B.; Dias, R.B.; Gemini Piperni, S.; Bonfim, D.C. The Role of Macrophages in Fracture Healing: A Narrative Review of the Recent Updates and Therapeutic Perspectives. Stem Cell Investig. 2023, 10, 4. [Google Scholar] [CrossRef]
- Ono, T.; Okamoto, K.; Nakashima, T.; Nitta, T.; Hori, S.; Iwakura, Y.; Takayanagi, H. IL-17-Producing Γδ T Cells Enhance Bone Regeneration. Nat. Commun. 2016, 7, 10928. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, L.; Xiao, Z.; Li, M.; Wu, X.; Li, W.; Li, X.; Zhao, Q.; Wu, Y.; Zhang, H.; et al. Protective Role of γδ T Cells in Different Pathogen Infections and Its Potential Clinical Application. J. Immunol. Res. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound Microbiology and Associated Approaches to Wound Management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and Host-Defense Peptides as New Anti-Infective Therapeutic Strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E.W. Host Defense Peptides: Front-Line Immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef]
- Radic, M.; Muller, S. LL-37, a Multi-Faceted Amphipathic Peptide Involved in NETosis. Cells 2022, 11, 2463. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial Peptides: Pore Formers or Metabolic Inhibitors in Bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Hwang, I.; Lee, E.; Cho, H.-J.; Ryu, J.H.; Kim, T.-G.; Yu, J.-W. Antimicrobial Peptide LL-37 Drives Rosacea-like Skin Inflammation in an NLRP3-Dependent Manner. J. Investig. Dermatol. 2021, 141, 2885–2894. [Google Scholar] [CrossRef]
- Winn, N.C.; Volk, K.M.; Hasty, A.H. Regulation of Tissue Iron Homeostasis: The Macrophage “Ferrostat”. JCI Insight 2020, 5, e132964. [Google Scholar] [CrossRef]
- DeRosa, A.; Leftin, A. The Iron Curtain: Macrophages at the Interface of Systemic and Microenvironmental Iron Metabolism and Immune Response in Cancer. Front. Immunol. 2021, 12, 614294. [Google Scholar] [CrossRef]
- Lichtenberger, B.M.; Gerber, P.A.; Holcmann, M.; Buhren, B.A.; Amberg, N.; Smolle, V.; Schrumpf, H.; Boelke, E.; Ansari, P.; Mackenzie, C.; et al. Epidermal EGFR Controls Cutaneous Host Defense and Prevents Inflammation. Sci. Transl. Med. 2013, 5, 199ra111. [Google Scholar] [CrossRef]
- Scorza, C.A.; Marques, M.J.G.; da Silva, S.G.; da Graça Naffah-Mazzacoratti, M.; Scorza, F.A.; Cavalheiro, E.A. Status Epilepticus Does Not Induce Acute Brain Inflammatory Response in the Amazon Rodent Proechimys, an Animal Model Resistant to Epileptogenesis. Neurosci. Lett. 2018, 668, 169–173. [Google Scholar] [CrossRef]
- Gomes, A.; Teixeira, C.; Ferraz, R.; Prudêncio, C.; Gomes, P. Wound-Healing Peptides for Treatment of Chronic Diabetic Foot Ulcers and Other Infected Skin Injuries. Molecules 2017, 22, 1743. [Google Scholar] [CrossRef] [PubMed]
- Myhrman, E.; Håkansson, J.; Lindgren, K.; Björn, C.; Sjöstrand, V.; Mahlapuu, M. The Novel Antimicrobial Peptide PXL150 in the Local Treatment of Skin and Soft Tissue Infections. Appl. Microbiol. Biotechnol. 2013, 97, 3085–3096. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardão, L.; Schmitt, F.; Soares, R.; Vilanova, M.; Domingues, L.; Gama, M. Wound Healing Activity of the Human Antimicrobial Peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Lessons Learned from Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, J.M.; Bandy, A. A Systematic Review of the Design and Applications of Antimicrobial Peptides in Wound Healing. Cureus 2024, 16, e58178. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Lim, S.H.; Sun, Y.; Thiruvallur Madanagopal, T.; Rosa, V.; Kang, L. Enhanced Skin Permeation of Anti-Wrinkle Peptides via Molecular Modification. Sci. Rep. 2018, 8, 1596. [Google Scholar] [CrossRef]
- Chan, L.Y.; Zhang, V.M.; Huang, Y.; Waters, N.C.; Bansal, P.S.; Craik, D.J.; Daly, N.L. Cyclization of the Antimicrobial Peptide Gomesin with Native Chemical Ligation: Influences on Stability and Bioactivity. ChemBioChem 2013, 14, 617–624. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, M. Antimicrobial Peptides: From Design to Clinical Application. Antibiotics 2022, 11, 349. [Google Scholar] [CrossRef]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial Contribution in Chronicity of Wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, D.; Hamilton, D.E.; Iloba, I.; Jensen, G.S. Secreted Metabolites from Pseudomonas, Staphylococcus, and Borrelia Biofilm: Modulation of Immunogenicity by a Nutraceutical Enzyme and Botanical Blend. Microorganisms 2024, 12, 991. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2022 Compendium of Estimates. Adv. Wound Care 2023, 12, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Fang, W.; Xu, S.; Luo, H.; Li, D.; Liu, Y.; Zeng, Z.; Tong, Y.; Zhao, L. Synergistic Quorum Sensing Inhibition and Mild-Temperature Photothermal Therapy of Integrated Nanoplatform for Implant-Associated Biofilm Infections. J. Colloid. Interface Sci. 2024, 663, 143–156. [Google Scholar] [CrossRef]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The Wound Microbiota: Microbial Mechanisms of Impaired Wound Healing and Infection. Nat. Rev. Microbiol. 2024, 22, 507–521. [Google Scholar] [CrossRef]
- Pugazhendhi, A.S.; Wei, F.; Hughes, M.; Coathup, M. Bacterial Adhesion, Virulence, and Biofilm Formation. In Musculoskeletal Infection; Coathup, M., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 19–64. ISBN 978-3-030-83250-6. [Google Scholar]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Fleming, D.; Niese, B.; Redman, W.; Vanderpool, E.; Gordon, V.; Rumbaugh, K.P. Contribution of Pseudomonas Aeruginosa Exopolysaccharides Pel and Psl to Wound Infections. Front. Cell. Infect. Microbiol. 2022, 12, 835754. [Google Scholar] [CrossRef] [PubMed]
- Omwenga, E.O.; Awuor, S.O. The Bacterial Biofilms: Formation, Impacts, and Possible Management Targets in the Healthcare System. Can. J. Infect. Dis. Med. Microbiol. 2024, 2024, 1542576. [Google Scholar] [CrossRef] [PubMed]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and Resistance of Microbial Biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, W.; Du, Q.; Zhang, H.; Han, D. Recent Advances in Bacterial Persistence Mechanisms. Int. J. Mol. Sci. 2023, 24, 14311. [Google Scholar] [CrossRef]
- Barman, S.; Kurnaz, L.B.; Leighton, R.; Hossain, M.W.; Decho, A.W.; Tang, C. Intrinsic Antimicrobial Resistance: Molecular Biomaterials to Combat Microbial Biofilms and Bacterial Persisters. Biomaterials 2024, 311, 122690. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Y.; Huang, B.; Bian, X.; Tang, B.Z. Aggregation-induced Emission Artificial Enzyme (AIEzyme) with DNase-like Activity: Imaging and Matrix Cleavage for Combating Bacterial Biofilm. Aggregate 2023, 4, e360. [Google Scholar] [CrossRef]
- Tan, Q.; Ai, Q.; He, Y.; Li, F.; Yu, J.P. Aeruginosa Biofilm Activates the NLRP3 Inflammasomes in Vitro. Microb. Pathog. 2022, 164, 105379. [Google Scholar] [CrossRef]
- Klopf, J.; Brostjan, C.; Eilenberg, W.; Neumayer, C. Neutrophil Extracellular Traps and Their Implications in Cardiovascular and Inflammatory Disease. Int. J. Mol. Sci. 2021, 22, 559. [Google Scholar] [CrossRef]
- Hook, J.S.; Patel, P.A.; O’Malley, A.; Xie, L.; Kavanaugh, J.S.; Horswill, A.R.; Moreland, J.G. Lipoproteins from Staphylococcus Aureus Drive Neutrophil Extracellular Trap Formation in a TLR2/1- and PAD-Dependent Manner. J. Immunol. 2021, 207, 966–973. [Google Scholar] [CrossRef]
- Speziale, P.; Pietrocola, G. Staphylococcus Aureus Induces Neutrophil Extracellular Traps (NETs) and Neutralizes Their Bactericidal Potential. Comput. Struct. Biotechnol. J. 2021, 19, 3451–3457. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Song, M.; Yan, G.; Wang, Q. The Biodegradability and in Vitro Cytological Study on the Composite of PLGA Combined With Magnesium Metal. Front. Bioeng. Biotechnol. 2022, 10, 859280. [Google Scholar] [CrossRef]
- Davis, S.C.; Avery, J.T.; Gil, J.; Solis, M.R.; Jozic, I.; Kimmerling, K.A.; Mowry, K.C. Protection with a Collagen Wound Matrix Containing Polyhexamethylene Biguanide Supports Innate Wound Healing in Biofilm-infected Porcine Wounds. Wound Repair Regen. 2025, 33, e70025. [Google Scholar] [CrossRef]
- Barreto, R.; Barrois, B.; Lambert, J.; Malhotra-Kumar, S.; Santos-Fernandes, V.; Monstrey, S. Addressing the Challenges in Antisepsis: Focus on Povidone Iodine. Int. J. Antimicrob. Agents 2020, 56, 106064. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Pradhan, D.; Halder, J.; Biswasroy, P.; Rai, V.K.; Dubey, D.; Kar, B.; Ghosh, G.; Rath, G. Metal Nanoparticles against Multi-Drug-Resistance Bacteria. J. Inorg. Biochem. 2022, 237, 111938. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, A.M.; Larrain, C.M.; Friedman, L.R.; Repkorwich, R.; Anidi, I.U.; Forrest, K.M.; Fennelly, K.P.; Carr, S.R. Novel Management of Pseudomonas Biofilm-like Structure in a Post-Pneumonectomy Empyema. Front. Cell. Infect. Microbiol. 2024, 14, 1458652. [Google Scholar] [CrossRef]
- Liu, D.; Yang, P.; Gao, M.; Yu, T.; Shi, Y.; Zhang, M.; Yao, M.; Liu, Y.; Zhang, X. NLRP3 Activation Induced by Neutrophil Extracellular Traps Sustains Inflammatory Response in the Diabetic Wound. Clin. Sci. 2019, 133, 565–582. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Yakoup, A.Y.; Kamel, A.G.; Elbermawy, Y.; Abdelsattar, A.S.; El-Shibiny, A. Characterization, Antibacterial, and Cytotoxic Activities of Silver Nanoparticles Using the Whole Biofilm Layer as a Macromolecule in Biosynthesis. Sci. Rep. 2024, 14, 364. [Google Scholar] [CrossRef]
- Jaligam, M.M.; Takahashi, C.; Heidt, B.; Shen, A.Q. Enhanced Antibacterial Efficacy: Rapid Analysis of Silver-Decorated Azithromycin-Infused Soluplus® Nanoparticles against E. Coli and S. Epidermidis Biofilms. Nanoscale 2024, 16, 17877–17885. [Google Scholar] [CrossRef]
- Lubis, F.A.; Malek, N.A.N.N.; Sani, N.S.; Jemon, K. Biogenic Synthesis of Silver Nanoparticles Using Persicaria Odorata Leaf Extract: Antibacterial, Cytocompatibility, and in Vitro Wound Healing Evaluation. Particuology 2022, 70, 10–19. [Google Scholar] [CrossRef]
- Zhong, X.; Tong, C.; Liu, T.; Li, L.; Liu, X.; Yang, Y.; Liu, R.; Liu, B. Silver Nanoparticles Coated by Green Graphene Quantum Dots for Accelerating the Healing of MRSA-Infected Wounds. Biomater. Sci. 2020, 8, 6670–6682. [Google Scholar] [CrossRef]
- Baveloni, F.G.; Meneguin, A.B.; Sábio, R.M.; Camargo, B.A.F.D.; Trevisan, D.P.V.; Duarte, J.L.; Araújo, J.T.C.D.; Luiz, M.T.; Zanatta, M.B.T.; Silva, I.C.P.D.; et al. Antimicrobial Effect of Silver Nanoparticles as a Potential Healing Treatment for Wounds Contaminated with Staphylococcus Aureus in Wistar Rats. J. Drug Deliv. Sci. Technol. 2025, 103, 106445. [Google Scholar] [CrossRef]
- Saddik, M.S.; Al-Hakkani, M.F.; Abu-Dief, A.M.; Mohamed, M.S.; Al-Fattah, I.A.; Makki, M.; El-Mokhtar, M.A.; Sabet, M.A.; Amin, M.S.; Ahmed, H.A.; et al. Formulation and Evaluation of Azithromycin-Loaded Silver Nanoparticles for the Treatment of Infected Wounds. Int. J. Pharm. X 2024, 7, 100245. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Ali, A.A.; Ali, A.M.A.; Eissa, E.M.; Hassan, R.M.; Abo El-Ela, F.I.; Hassan, A.H. Potential Use of Tailored Citicoline Chitosan-Coated Liposomes for Effective Wound Healing in Diabetic Rat Model. Int. J. Nanomed. 2022, 17, 555–575. [Google Scholar] [CrossRef]
- Macartney, R.A.; Weaver, E.; Irwin, R.; Wylie, M.P.; Burke, G.A.; Lamprou, D.A. Co-Delivery of VEGF and Amoxicillin Using LP-Coated Co-Axial Electrospun Fibres for the Potential Treatment of Diabetic Wounds. Biomater. Adv. 2024, 158, 213765. [Google Scholar] [CrossRef]
- Duarte, A.C.; Fernández, L.; Jurado, A.; Campelo, A.B.; Shen, Y.; Rodríguez, A.; García, P. Synergistic Removal of Staphylococcus Aureus Biofilms by Using a Combination of Phage Kayvirus Rodi with the Exopolysaccharide Depolymerase Dpo7. Front. Microbiol. 2024, 15, 1438022. [Google Scholar] [CrossRef]
- Aguilera, L.F.; Araujo, L.O.; Facchinatto, W.M.; Lima, R.G.; Pontes, M.D.S.; Pulcherio, J.H.V.; Caires, C.S.A.; De Oliveira, K.T.; Oliveira, S.L.D.; Caires, A.R.L. Blue-Light Photoactivated Curcumin-Loaded Chitosan Nanoparticles Prepared by Nanoprecipitation and Ionic Gelation: A Promising Approach for Antimicrobial Photodynamic Inactivation. ACS Appl. Bio Mater. 2025, 8, 4055–4064. [Google Scholar] [CrossRef]
- Poh, W.H.; Rice, S.A. Recent Developments in Nitric Oxide Donors and Delivery for Antimicrobial and Anti-Biofilm Applications. Molecules 2022, 27, 674. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Q.; Qiang, H.; Wang, B.; Chen, J.; Xie, Y.; Peng, L.; Zhao, H.; Tian, J. Corrole-Based Photothermal Nanocomposite Hydrogel with Nitric Oxide Release for Diabetic Wound Healing. Acta Biomater. 2025, 192, 431–445. [Google Scholar] [CrossRef]
- Hasan, N.; Lee, J.; Kwak, D.; Kim, H.; Saparbayeva, A.; Ahn, H.-J.; Yoon, I.-S.; Kim, M.-S.; Jung, Y.; Yoo, J.-W. Diethylenetriamine/NONOate-Doped Alginate Hydrogel with Sustained Nitric Oxide Release and Minimal Toxicity to Accelerate Healing of MRSA-Infected Wounds. Carbohydr. Polym. 2021, 270, 118387. [Google Scholar] [CrossRef]
- Olawade, D.B.; Fapohunda, O.; Egbon, E.; Ebiesuwa, O.A.; Usman, S.O.; Faronbi, A.O.; Fidelis, S.C. Phage Therapy: A Targeted Approach to Overcoming Antibiotic Resistance. Microb. Pathog. 2024, 197, 107088. [Google Scholar] [CrossRef] [PubMed]
- Molina, W.P.C.; Riofrio, M.D.P.; Espinosa, P.M.J.; Jurado, M.A.S. Phage Therapy against Multiresistant Bacteria. Interam. J. Health Sci. 2024, 4, 91. [Google Scholar] [CrossRef]
- Murray, E.; Draper, L.A.; Ross, R.P.; Hill, C. The Advantages and Challenges of Using Endolysins in a Clinical Setting. Viruses 2021, 13, 680. [Google Scholar] [CrossRef]
- Liu, G.; Catacutan, D.B.; Rathod, K.; Swanson, K.; Jin, W.; Mohammed, J.C.; Chiappino-Pepe, A.; Syed, S.A.; Fragis, M.; Rachwalski, K.; et al. Deep Learning-Guided Discovery of an Antibiotic Targeting Acinetobacter Baumannii. Nat. Chem. Biol. 2023, 19, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Yang, F.; Jiang, Z.; Wu, K.; Hou, R.; Zhu, Y. Smart Wound Dressing for Advanced Wound Management: Real-Time Monitoring and on-Demand Treatment. Mater. Des. 2023, 229, 111917. [Google Scholar] [CrossRef]
- Mirani, B.; Hadisi, Z.; Pagan, E.; Dabiri, S.M.H.; Van Rijt, A.; Almutairi, L.; Noshadi, I.; Armstrong, D.G.; Akbari, M. Smart Dual-Sensor Wound Dressing for Monitoring Cutaneous Wounds. Adv. Healthc. Mater. 2023, 12, 2203233. [Google Scholar] [CrossRef]
- Vanaraj, R.; Arumugam, B.; Mayakrishnan, G.; Daniel, S.; Babu, C.M.; Kim, S.C. Chemical Engineering Innovations in Nanoparticle-Based Biosensors for Enhanced Detection of Biological Molecules. Chem. Eng. J. 2025, 507, 160081. [Google Scholar] [CrossRef]
- Hwang, D.; Dismuke, T.; Tikunov, A.; Rosen, E.P.; Kagel, J.R.; Ramsey, J.D.; Lim, C.; Zamboni, W.; Kabanov, A.V.; Gershon, T.R.; et al. Poly(2-Oxazoline) Nanoparticle Delivery Enhances the Therapeutic Potential of Vismodegib for Medulloblastoma by Improving CNS Pharmacokinetics and Reducing Systemic Toxicity. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102345. [Google Scholar] [CrossRef]
- Lin, P.; He, K.; Luo, J.; Wang, J.; Liu, Y.; Zhang, J.; Fan, H.; Huang, S.; Lan, W.; Wang, W.; et al. Multifunctional Nanofiber System with Photothermal-Controlled Drug Delivery and Motion Monitoring Capabilities as Intelligent Wound Dressing. Chem. Eng. J. 2025, 503, 158544. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, L.; Li, H.; Xiong, Z.; Fu, Z.; Zhang, Z.; Xie, W.; Cui, H.; Zhang, S.; Tang, Y.; et al. Construction of Tea Tree Oil/Salicylic Acid/Palygorskite Hybrids for Advanced Antibacterial and Anti-Inflammatory Performance. J. Mater. Chem. B 2023, 11, 4260–4273. [Google Scholar] [CrossRef]
- Nsayef Muslim, S.; Mohammed Ali, A.N.; Auda, I.G. Anti-biofilm and Anti-virulence Effects of Silica Oxide Nanoparticle–Conjugation of Lectin Purified from Pseudomonas Aeruginosa. IET Nanobiotechnology 2021, 15, 318–328. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Chapman, M.A. Regenerative Medicine: Charting a New Course in Wound Healing. Adv. Wound Care 2016, 5, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.-S. Enhancing Stem Cell-Based Therapeutic Potential by Combining Various Bioengineering Technologies. Front. Cell Dev. Biol. 2022, 10, 901661. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef]
- Braza, M.E.; Fahrenkopf, M.P. Split-Thickness Skin Grafts. In StatPearls; StatPearls Publishing: Petersburg, FL, USA, 2023. [Google Scholar]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin Tissue Engineering: Wound Healing Based on Stem-Cell-Based Therapeutic Strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, A.B.M. Vital Roles of Stem Cells and Biomaterials in Skin Tissue Engineering. World J. Stem Cells 2015, 7, 428. [Google Scholar] [CrossRef]
- Vyas, J.; Raytthatha, N.; Vyas, P.; Prajapati, B.G.; Uttayarat, P.; Singh, S.; Chittasupho, C. Biomaterial-Based Additive Manufactured Composite/Scaffolds for Tissue Engineering and Regenerative Medicine: A Comprehensive Review. Polymers 2025, 17, 1090. [Google Scholar] [CrossRef] [PubMed]
- Gervaso, F.; Sannino, A.; Peretti, G.M. The Biomaterialist’s Task: Scaffold Biomaterials and Fabrication Technologies. Joints 2013, 1, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. An Up-to-Date Review of Biomaterials Application in Wound Management. Polymers 2022, 14, 421. [Google Scholar] [CrossRef] [PubMed]
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063. [Google Scholar] [CrossRef] [PubMed]
- Downer, M.; Berry, C.E.; Parker, J.B.; Kameni, L.; Griffin, M. Current Biomaterials for Wound Healing. Bioengineering 2023, 10, 1378. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D Printing of PLA/n-HA Composite Scaffolds with Customized Mechanical Properties and Biological Functions for Bone Tissue Engineering. Compos. Part B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Hsu, F.-Y.; Hung, Y.-S.; Liou, H.-M.; Shen, C.-H. Electrospun Hyaluronate–Collagen Nanofibrous Matrix and the Effects of Varying the Concentration of Hyaluronate on the Characteristics of Foreskin Fibroblast Cells. Acta Biomater. 2010, 6, 2140–2147. [Google Scholar] [CrossRef]
- Chaudhari, A.; Vig, K.; Baganizi, D.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.; Pillai, S. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. IJMS 2016, 17, 1974. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, P.; Zhang, S.; Yang, G. Evaluation of Bacterial Nanocellulose-Based Uniform Wound Dressing for Large Area Skin Transplantation. Mater. Sci. Eng. C 2013, 33, 2995–3000. [Google Scholar] [CrossRef]
- Martin, C.A.; Radhakrishnan, S.; Nagarajan, S.; Muthukoori, S.; Dueñas, J.M.M.; Gómez Ribelles, J.L.; Lakshmi, B.S.; Nivethaa, E.A.K.; Gómez-Tejedor, J.A.; Reddy, M.S.; et al. An Innovative Bioresorbable Gelatin Based 3D Scaffold That Maintains the Stemness of Adipose Tissue Derived Stem Cells and the Plasticity of Differentiated Neurons. RSC Adv. 2019, 9, 14452–14464. [Google Scholar] [CrossRef]
- Pasini, C.; Re, F.; Trenta, F.; Russo, D.; Sartore, L. Gelatin-Based Scaffolds with Carrageenan and Chitosan for Soft Tissue Regeneration. Gels 2024, 10, 426. [Google Scholar] [CrossRef]
- Shan, Y.; Li, C.; Wu, Y.; Li, Q.; Liao, J. Hybrid Cellulose Nanocrystal/Alginate/Gelatin Scaffold with Improved Mechanical Properties and Guided Wound Healing. RSC Adv. 2019, 9, 22966–22979. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Jeschke, M.G. Combined Gene and Stem Cell Therapy for Cutaneous Wound Healing. Mol. Pharm. 2011, 8, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Branski, L.K.; Gauglitz, G.G.; Herndon, D.N.; Jeschke, M.G. A Review of Gene and Stem Cell Therapy in Cutaneous Wound Healing. Burns 2009, 35, 171–180. [Google Scholar] [CrossRef]
- Khavari, P.A.; Rollman, O.; Vahlquist, A. Cutaneous Gene Transfer for Skin and Systemic Diseases. J. Intern. Med. 2002, 252, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sprugel, K.H.; McPherson, J.M.; Clowes, A.W.; Ross, R. Effects of Growth Factors in Vivo. I. Cell Ingrowth into Porous Subcutaneous Chambers. Am. J. Pathol. 1987, 129, 601–613. [Google Scholar]
- Butt, M.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.; Hasan, M.; Khan, Y.; Hafeez, S.; Massoud, E.; Rahman, M.; et al. Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review. Genes 2022, 13, 1370. [Google Scholar] [CrossRef]
- Miller, A.D. Retrovirus Packaging Cells. Hum. Gene Ther. 1990, 1, 5–14. [Google Scholar] [CrossRef]
- Niidome, T.; Huang, L. Gene Therapy Progress and Prospects: Nonviral Vectors. Gene Ther. 2002, 9, 1647–1652. [Google Scholar] [CrossRef]
- Chesnoy, S.; Huang, L. Enhanced Cutaneous Gene Delivery Following Intradermal Injection of Naked DNA in a High Ionic Strength Solution. Mol. Ther. 2002, 5, 57–62. [Google Scholar] [CrossRef]
- Dinçer, S.; Türk, M.; Pişkin, E. Intelligent Polymers as Nonviral Vectors. Gene Ther. 2005, 12, S139–S145. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M. Gene Delivery Using Temperature-Responsive Polymeric Carriers. Drug Discov. Today 2002, 7, 426–432. [Google Scholar] [CrossRef]
- Nagasaki, T.; Atarashi, K.; Makino, K.; Noguchi, A.; Tamagaki, S. Synthesis of a Novel Water-Soluble Polyazobenzene Dendrimer and Photoregulation of Affinity Toward DNA. Mol. Cryst. Liq. Cryst. 2000, 345, 227–232. [Google Scholar] [CrossRef]
- Dean, J.; Hoch, C.; Wollenberg, B.; Navidzadeh, J.; Maheta, B.; Mandava, A.; Knoedler, S.; Sherwani, K.; Baecher, H.; Schmitz, A.; et al. Advancements in Bioengineered and Autologous Skin Grafting Techniques for Skin Reconstruction: A Comprehensive Review. Front. Bioeng. Biotechnol. 2025, 12, 1461328. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Q.; Tam, P.K.H. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. Int. J. Mol. Sci. 2022, 23, 10023. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chen, B.-T.; Liu, Y.; Wang, Y.; Xing, L.; Wang, H.; Zhou, T.-J.; Jiang, H.-L. Engineered Stem Cell-Based Strategy: A New Paradigm of next-Generation Stem Cell Product in Regenerative Medicine. J. Control. Release 2024, 365, 981–1003. [Google Scholar] [CrossRef]
- Al Abbar, A.; Ngai, S.C.; Nograles, N.; Alhaji, S.Y.; Abdullah, S. Induced Pluripotent Stem Cells: Reprogramming Platforms and Applications in Cell Replacement Therapy. BioResearch Open Access 2020, 9, 121–136. [Google Scholar] [CrossRef]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich Plasma and Regenerative Dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef]
- Cervelli, V.; Gentile, P.; De Angelis, B.; Calabrese, C.; Di Stefani, A.; Scioli, M.G.; Curcio, B.C.; Felici, M.; Orlandi, A. Application of Enhanced Stromal Vascular Fraction and Fat Grafting Mixed with PRP in Post-Traumatic Lower Extremity Ulcers. Stem Cell Res. 2011, 6, 103–111. [Google Scholar] [CrossRef]
- Moreira, T.D.; Martins, V.B.; Da Silva Júnior, A.H.; Sayer, C.; De Araújo, P.H.H.; Immich, A.P.S. New Insights into Biomaterials for Wound Dressings and Care: Challenges and Trends. Prog. Org. Coat. 2024, 187, 108118. [Google Scholar] [CrossRef]
- Yu, F.; Khan, A.U.R.; Li, Y.; Zhao, B.; Xie, X.; EL-Newehy, M.; EL-Hamshary, H.; Morsi, Y.; Li, J.; Pan, J.; et al. A Multifunctional Nanofiber Reinforced Photo-Crosslinking Hydrogel for Skin Wound Healing. Compos. Part B Eng. 2022, 247, 110294. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Echeverry-Rendón, M. 3D-Printed Biodegradable Polymer Scaffolds for Tissue Engineering: An Overview, Current Stage and Future Perspectives. Next Mater. 2025, 8, 100647. [Google Scholar] [CrossRef]
- Pedde, R.D.; Mirani, B.; Navaei, A.; Styan, T.; Wong, S.; Mehrali, M.; Thakur, A.; Mohtaram, N.K.; Bayati, A.; Dolatshahi-Pirouz, A.; et al. Emerging Biofabrication Strategies for Engineering Complex Tissue Constructs. Adv. Mater. 2017, 29, 1606061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fassi, F. Transforming Architectural Digitisation: Advancements in AI-Driven 3D Reality-Based Modelling. Heritage 2025, 8, 81. [Google Scholar] [CrossRef]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-Printed Chitosan-Based Scaffolds: An in Vitro Study of Human Skin Cell Growth and an in-Vivo Wound Healing Evaluation in Experimental Diabetes in Rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef]
- Xu, W.; Molino, B.Z.; Cheng, F.; Molino, P.J.; Yue, Z.; Su, D.; Wang, X.; Willför, S.; Xu, C.; Wallace, G.G. On Low-Concentration Inks Formulated by Nanocellulose Assisted with Gelatin Methacrylate (GelMA) for 3D Printing toward Wound Healing Application. ACS Appl. Mater. Interfaces 2019, 11, 8838–8848. [Google Scholar] [CrossRef]
- Reifs Jiménez, D.; Casanova-Lozano, L.; Grau-Carrión, S.; Reig-Bolaño, R. Artificial Intelligence Methods for Diagnostic and Decision-Making Assistance in Chronic Wounds: A Systematic Review. J. Med. Syst. 2025, 49, 29. [Google Scholar] [CrossRef]
- Chino, D.Y.T.; Scabora, L.C.; Cazzolato, M.T.; Jorge, A.E.S.; Traina, C., Jr.; Traina, A.J.M. Segmenting Skin Ulcers and Measuring the Wound Area Using Deep Convolutional Networks. Comput. Methods Programs Biomed. 2020, 191, 105376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Currie, E.H.; Kavoussi, L.; Rabbany, S.Y. Laser Scanner for 3D Reconstruction of a Wound’s Edge and Topology. Int. J. CARS 2021, 16, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Filko, D.; Marijanović, D.; Nyarko, E.K. Automatic Robot-Driven 3D Reconstruction System for Chronic Wounds. Sensors 2021, 21, 8308. [Google Scholar] [CrossRef]
- Shankhwar, N.; Verma, A.K.; Noumani, A.; Singh, T.; Rao, K.S.; Vivekanand; Sharma, N.R.; Singh, S. Integrating Advanced Synthesis Techniques and AI-Driven Approaches with Nanofiber Technology: A State-of-the-Art Approach to Wound Care Management. Next Nanotechnol. 2025, 8, 100147. [Google Scholar] [CrossRef]
- Ramachandram, D.; Ramirez-GarciaLuna, J.L.; Fraser, R.D.J.; Martínez-Jiménez, M.A.; Arriaga-Caballero, J.E.; Allport, J. Fully Automated Wound Tissue Segmentation Using Deep Learning on Mobile Devices: Cohort Study. JMIR Mhealth Uhealth 2022, 10, e36977. [Google Scholar] [CrossRef]
- Singh, R.; Srinivas, S.P.; Kumawat, M.; Daima, H.K. Ligand-Based Surface Engineering of Nanomaterials: Trends, Challenges, and Biomedical Perspectives. OpenNano 2024, 15, 100194. [Google Scholar] [CrossRef]
- Cho, S.K.; Mattke, S.; Gordon, H.; Sheridan, M.; Ennis, W. Development of a Model to Predict Healing of Chronic Wounds Within 12 Weeks. Adv. Wound Care 2020, 9, 516–524. [Google Scholar] [CrossRef]
- Han, X.; Cai, C.; Deng, W.; Shi, Y.; Li, L.; Wang, C.; Zhang, J.; Rong, M.; Liu, J.; Fang, B.; et al. Landscape of Human Organoids: Ideal Model in Clinics and Research. Innov. 2024, 5, 100620. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, F.; Lang, H.; Ren, H.; Zhang, Q.; Huang, X.; He, C.; Xu, C.; Tan, C.; Ma, J.; et al. Organoids in Skin Wound Healing. Burn. Trauma 2025, 13, tkae077. [Google Scholar] [CrossRef]
- Kwak, S.; Song, C.L.; Lee, J.; Kim, S.; Nam, S.; Park, Y.-J.; Lee, J. Development of Pluripotent Stem Cell-Derived Epidermal Organoids That Generate Effective Extracellular Vesicles in Skin Regeneration. Biomaterials 2024, 307, 122522. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhou, D.; Wang, F.; Chen, X.; Li, M.; Su, J. Organoids for Tissue Repair and Regeneration. Mater. Today Bio 2025, 33, 102013. [Google Scholar] [CrossRef]
- Hsiung, N.; Ju, Y.; Yang, K.; Yang, P.; Zeng, W.; Zhao, H.; Zou, P.; Ye, J.; Yi, K.; Wang, X. Organoid-Based Tissue Engineering for Advanced Tissue Repair and Reconstruction. Mater. Today Bio 2025, 33, 102093. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liu, R.; Cen, X.; Dong, W.; Chen, Q.; Lin, J.; Wang, X.; Ling, Y.; Mao, R.; Sun, H.; et al. Preclinical Study of Engineering MSCs Promoting Diabetic Wound Healing and Other Inflammatory Diseases through M2 Polarization. Stem Cell Res. Ther. 2025, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-Chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef]
- Morais, A.S.; Mendes, M.; Cordeiro, M.A.; Sousa, J.J.; Pais, A.C.; Mihăilă, S.M.; Vitorino, C. Organ-on-a-Chip: Ubi Sumus? Fundamentals and Design Aspects. Pharmaceutics 2024, 16, 615. [Google Scholar] [CrossRef]
- Yao, B.; Xie, J.; Liu, N.; Hu, T.; Song, W.; Huang, S.; Fu, X. Direct Reprogramming of Epidermal Cells toward Sweat Gland-like Cells by Defined Factors. Cell Death Dis. 2019, 10, 272. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Xie, L.; Hassanin, A.A.; Zuo, E.; Lu, Y. The Potential of CRISPR/Cas9 Gene Editing as a Treatment Strategy for Inherited Diseases. Front. Cell Dev. Biol. 2021, 9, 699597. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sequeda, N.; Ruiz, J.; Ortiz, C.; Urquiza, M.; Torres, R. Potent and Specific Antibacterial Activity against Escherichia Coli O157:H7 and Methicillin Resistant Staphylococcus Aureus (MRSA) of G17 and G19 Peptides Encapsulated into Poly-Lactic-Co-Glycolic Acid (PLGA) Nanoparticles. Antibiotics 2020, 9, 384. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, D.; Skibba, M.; Brasier, A.R. The IRE1α–XBP1s Arm of the Unfolded Protein Response Activates N-Glycosylation to Remodel the Subepithelial Basement Membrane in Paramyxovirus Infection. Int. J. Mol. Sci. 2022, 23, 9000. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Her, C.-H.; Comune, M.; Moia, C.; Lopes, A.; Porporato, P.E.; Vanacker, J.; Lam, M.C.; Steinstraesser, L.; Sonveaux, P.; et al. PLGA Nanoparticles Loaded with Host Defense Peptide LL37 Promote Wound Healing. J. Control. Release 2014, 194, 138–147. [Google Scholar] [CrossRef]
- Water, J.J.; Smart, S.; Franzyk, H.; Foged, C.; Nielsen, H.M. Nanoparticle-Mediated Delivery of the Antimicrobial Peptide Plectasin against Staphylococcus Aureus in Infected Epithelial Cells. Eur. J. Pharm. Biopharm. 2015, 92, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Jayathilaka, E.H.T.T.; Nikapitiya, C.; De Zoysa, M.; Whang, I. Antimicrobial Peptide Octominin-Encapsulated Chitosan Nanoparticles Enhanced Antifungal and Antibacterial Activities. Int. J. Mol. Sci. 2022, 23, 15882. [Google Scholar] [CrossRef] [PubMed]
- Gourkhede, D.P.; Dani Nishanth, M.A.; Ram, V.P.; Abishad, P.; Yasur, J.; Pollumahanti, N.; Vergis, J.; Singh Malik, S.V.; Barbuddhe, S.B.; Rawool, D.B. Antimicrobial Efficacy of Chitosan Encapsulated Cecropin- A (1–7)- Melittin-Cell-Penetrating Peptide against Multi-Drug-Resistant Salmonella Enteritidis. J. Drug Deliv. Sci. Technol. 2022, 78, 103981. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Jiang, A.-M.; Ma, Z.-Y.; Li, X.-B.; Xiong, Y.-Y.; Dou, J.-F.; Wang, J.-F. The Synthetic Antimicrobial Peptide Pexiganan and Its Nanoparticles (PNPs) Exhibit the Anti-Helicobacter Pylori Activity in Vitro and in Vivo. Molecules 2015, 20, 3972–3985. [Google Scholar] [CrossRef]
- Almaaytah, A.; Mohammed, G.; Abualhaijaa, A.; Al-Balas, Q. Development of Novel Ultrashort Antimicrobial Peptide Nanoparticles with Potent Antimicrobial and Antibiofilm Activities against Multidrug-Resistant Bacteria. Drug Des. Dev. Ther. 2017, 11, 3159–3170. [Google Scholar] [CrossRef]
- Fumakia, M.; Ho, E.A. Nanoparticles Encapsulated with LL37 and Serpin A1 Promotes Wound Healing and Synergistically Enhances Antibacterial Activity. Mol. Pharm. 2016, 13, 2318–2331. [Google Scholar] [CrossRef]
- Rathnayake, K.; Patel, U.; Pham, C.; McAlpin, A.; Budisalich, T.; Jayawardena, S.N. Targeted Delivery of Antibiotic Therapy to Inhibit Pseudomonas Aeruginosa Using Lipid-Coated Mesoporous Silica Core–Shell Nanoassembly. ACS Appl. Bio Mater. 2020, 3, 6708–6721. [Google Scholar] [CrossRef]
- Jayathilaka, E.H.T.T.; Han, J.; De Zoysa, M.; Whang, I. Antimicrobial Peptide Octoprohibitin-Encapsulated Chitosan Nanoparticles Enhanced Antibacterial Activity against Acinetobacter baumannii. Pharmaceutics 2024, 16, 1245. [Google Scholar] [CrossRef]
- Qin, P.; Meng, Y.; Yang, Y.; Gou, X.; Liu, N.; Yin, S.; Hu, Y.; Sun, H.; Fu, Z.; Wang, Y.; et al. Mesoporous Polydopamine Nanoparticles Carrying Peptide RL-QN15 Show Potential for Skin Wound Therapy. J. Nanobiotechnol 2021, 19, 309. [Google Scholar] [CrossRef]
- Qin, P.; Tang, J.; Sun, D.; Yang, Y.; Liu, N.; Li, Y.; Fu, Z.; Wang, Y.; Li, C.; Li, X.; et al. Zn2+ Cross-Linked Alginate Carrying Hollow Silica Nanoparticles Loaded with RL-QN15 Peptides Provides Promising Treatment for Chronic Skin Wounds. ACS Appl. Mater. Interfaces 2022, 14, 29491–29505. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial Biohybrid Nanofibers for Wound Dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of Bioactive Peptides Derived from Food Proteins across the Intestinal Epithelial Membrane: A Review. Trends Food Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
- Sun, X.; Acquah, C.; Aluko, R.E.; Udenigwe, C.C. Considering Food Matrix and Gastrointestinal Effects in Enhancing Bioactive Peptide Absorption and Bioavailability. J. Funct. Foods 2020, 64, 103680. [Google Scholar] [CrossRef]
- Ruden, S.; Hilpert, K.; Berditsch, M.; Wadhwani, P.; Ulrich, A.S. Synergistic Interaction between Silver Nanoparticles and Membrane-Permeabilizing Antimicrobial Peptides. Antimicrob. Agents Chemother. 2009, 53, 3538–3540. [Google Scholar] [CrossRef] [PubMed]
- Nizam, A.A.K.; Masri, S.; Fadilah, N.I.M.; Maarof, M.; Fauzi, M.B. Current Insight of Peptide-Based Hydrogels for Chronic Wound Healing Applications: A Concise Review. Pharmaceuticals 2025, 18, 58. [Google Scholar] [CrossRef]
- Ilieş, B.D.; Yildiz, I.; Abbas, M. Peptide-conjugated Nanoparticle Platforms for Targeted Delivery, Imaging, and Biosensing Applications. ChemBioChem 2024, 25, e202300867. [Google Scholar] [CrossRef] [PubMed]
- Bartczak, D.; Kanaras, A.G. Preparation of Peptide-Functionalized Gold Nanoparticles Using One Pot EDC/Sulfo-NHS Coupling. Langmuir 2011, 27, 10119–10123. [Google Scholar] [CrossRef]
- Casciaro, B.; d’Angelo, I.; Zhang, X.; Loffredo, M.R.; Conte, G.; Cappiello, F.; Quaglia, F.; Di, Y.-P.P.; Ungaro, F.; Mangoni, M.L. Poly(Lactide-co-Glycolide) Nanoparticles for Prolonged Therapeutic Efficacy of Esculentin-1a-Derived Antimicrobial Peptides against Pseudomonas Aeruginosa Lung Infection: In Vitro and in Vivo Studies. Biomacromolecules 2019, 20, 1876–1888. [Google Scholar] [CrossRef]
- Kogan Bocian, M.; Olmedo, I.; Hosta, L.; Guerrero, A.; Cruz, L.; Albericio, F. Peptides and Metallic Nanoparticles for Biomedical Applications. Nanomedicine 2007, 2, 287–306. [Google Scholar] [CrossRef]
- Wang, S.; Yan, C.; Zhang, X.; Shi, D.; Chi, L.; Luo, G.; Deng, J. Antimicrobial Peptide Modification Enhances the Gene Delivery and Bactericidal Efficiency of Gold Nanoparticles for Accelerating Diabetic Wound Healing. Biomater. Sci. 2018, 6, 2757–2772. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Sun, H.; Wu, Y.; Li, C.; Wang, Y.; Liu, Y.; Li, Y.; Nie, J.; Sun, D.; Zhang, Y.; et al. A Cyclic Heptapeptide-Based Hydrogel Boosts the Healing of Chronic Skin Wounds in Diabetic Mice and Patients. NPG Asia Mater. 2022, 14, 99. [Google Scholar] [CrossRef]
- Grönberg, A.; Mahlapuu, M.; Ståhle, M.; Whately-Smith, C.; Rollman, O. Treatment with LL-37 Is Safe and Effective in Enhancing Healing of Hard-to-heal Venous Leg Ulcers: A Randomized, Placebo-controlled Clinical Trial. Wound Repair Regen. 2014, 22, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Miranda, E.; Bramono, K.; Yunir, E.; Reksodiputro, M.H.; Suwarsa, O.; Rengganis, I.; Harahap, A.R.; Subekti, D.; Suwarto, S.; Hayun, H.; et al. Efficacy of LL-37 Cream in Enhancing Healing of Diabetic Foot Ulcer: A Randomized Double-Blind Controlled Trial. Arch. Dermatol. Res. 2023, 315, 2623–2633. [Google Scholar] [CrossRef]
- Shi, R.; Qiao, J.; Sun, Q.; Hou, B.; Li, B.; Zheng, J.; Zhang, Z.; Peng, Z.; Zhou, J.; Shen, B. Self-assembly of PEG–PPS Polymers and LL-37 Peptide Nanomicelles Improves the Oxidative Microenvironment and Promotes Angiogenesis to Facilitate Chronic Wound Healing. Bioeng. Transl. Med. 2024, 9, e10619. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, W.; Kong, L.; Fu, J.; Zhang, Q.; Liu, H. PLGA@IL-8 Nanoparticles-Loaded Acellular Dermal Matrix as a Delivery System for Exogenous MSCs in Diabetic Wound Healing. Int. J. Biol. Macromol. 2023, 224, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chu, R.; Li, H.; Hua, T.; Chen, H.; Li, R.; Zhou, D.; Cao, S.; Ye, S.; Li, H. A Novel Wound Dressing Based on a Gold Nanoparticle Self-Assembled Hydrogel to Promote Wound Healing. Mater. Adv. 2023, 4, 2918–2925. [Google Scholar] [CrossRef]
- Papaioannou, A.; Liakopoulou, A.; Papoulis, D.; Gianni, E.; Gkolfi, P.; Zygouri, E.; Letsiou, S.; Hatziantoniou, S. Effect of Peptides on the Synthesis, Properties and Wound Healing Capacity of Silver Nanoparticles. Pharmaceutics 2023, 15, 2471. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boleti, A.P.d.A.; Jacobowski, A.C.; Frihling, B.E.F.; Cruz, M.V.; Santos, K.F.D.P.; Migliolo, L.; de Andrade, L.R.M.; Macedo, M.L.R. Wound Healing: Molecular Mechanisms, Antimicrobial Peptides, and Emerging Technologies in Regenerative Medicine. Pharmaceuticals 2025, 18, 1525. https://doi.org/10.3390/ph18101525
Boleti APdA, Jacobowski AC, Frihling BEF, Cruz MV, Santos KFDP, Migliolo L, de Andrade LRM, Macedo MLR. Wound Healing: Molecular Mechanisms, Antimicrobial Peptides, and Emerging Technologies in Regenerative Medicine. Pharmaceuticals. 2025; 18(10):1525. https://doi.org/10.3390/ph18101525
Chicago/Turabian StyleBoleti, Ana Paula de Araújo, Ana Cristina Jacobowski, Breno Emanuel Farias Frihling, Maurício Vicente Cruz, Kristiane Fanti Del Pino Santos, Ludovico Migliolo, Lucas Rannier Melo de Andrade, and Maria Ligia Rodrigues Macedo. 2025. "Wound Healing: Molecular Mechanisms, Antimicrobial Peptides, and Emerging Technologies in Regenerative Medicine" Pharmaceuticals 18, no. 10: 1525. https://doi.org/10.3390/ph18101525
APA StyleBoleti, A. P. d. A., Jacobowski, A. C., Frihling, B. E. F., Cruz, M. V., Santos, K. F. D. P., Migliolo, L., de Andrade, L. R. M., & Macedo, M. L. R. (2025). Wound Healing: Molecular Mechanisms, Antimicrobial Peptides, and Emerging Technologies in Regenerative Medicine. Pharmaceuticals, 18(10), 1525. https://doi.org/10.3390/ph18101525










