Development of Clinical-Grade Durvalumab-680LT and Nivolumab-800CW for Multispectral Fluorescent Imaging of the PD-1/PD-L1 Axis of the Immune Checkpoint Pathway
Abstract
1. Introduction
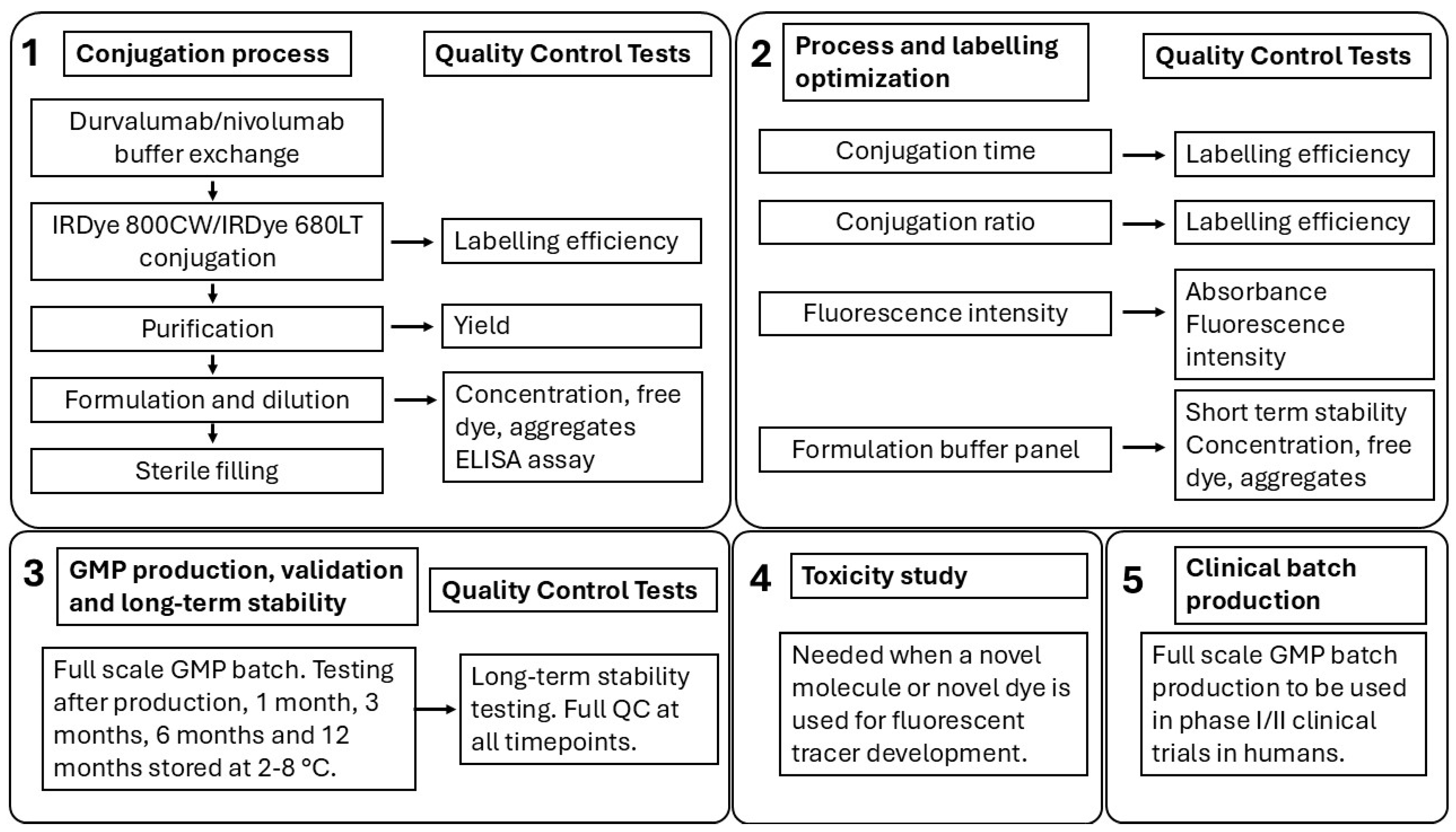
2. Results
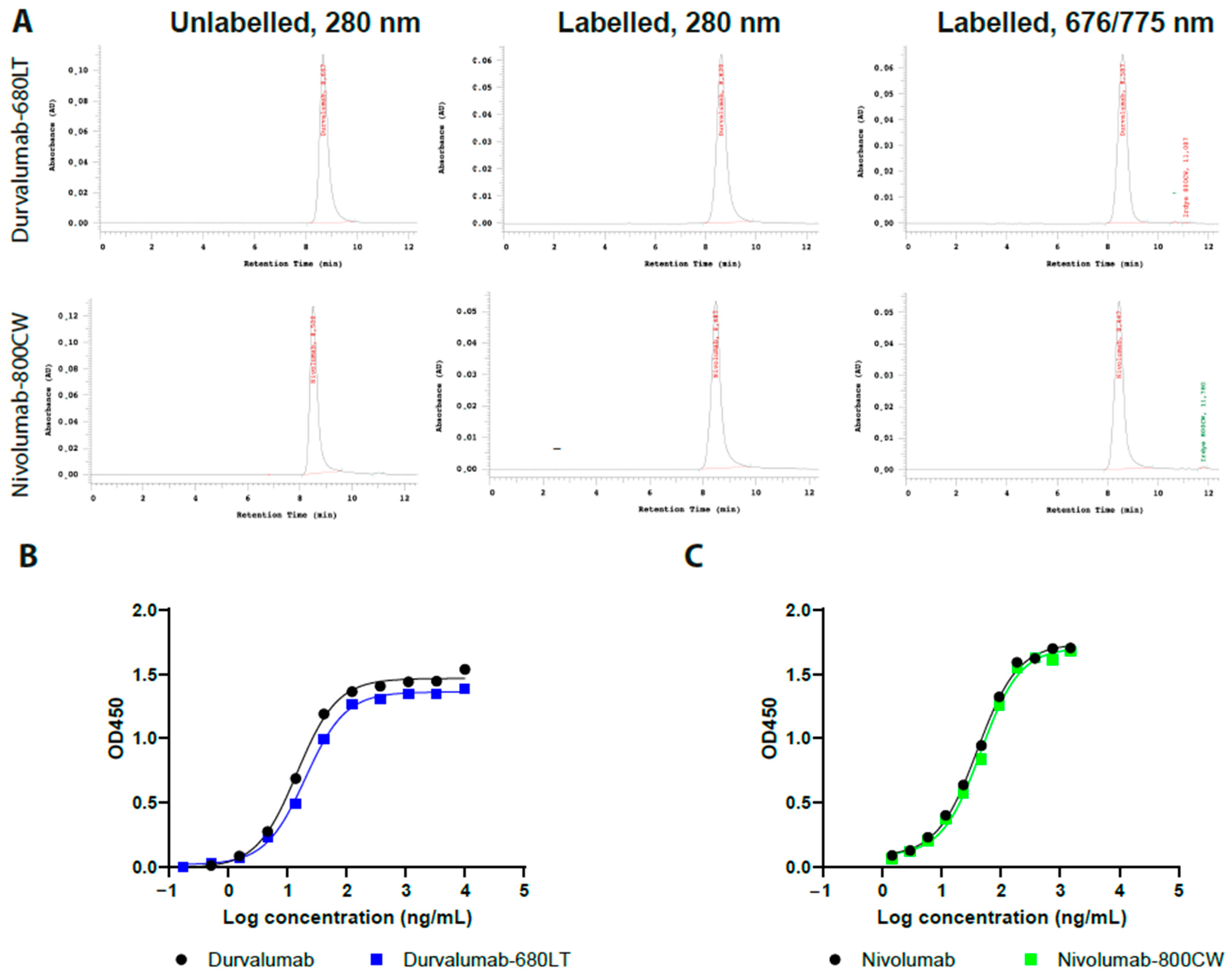
2.1. Conjugation, SE-HPLC Characterization, and ELISA
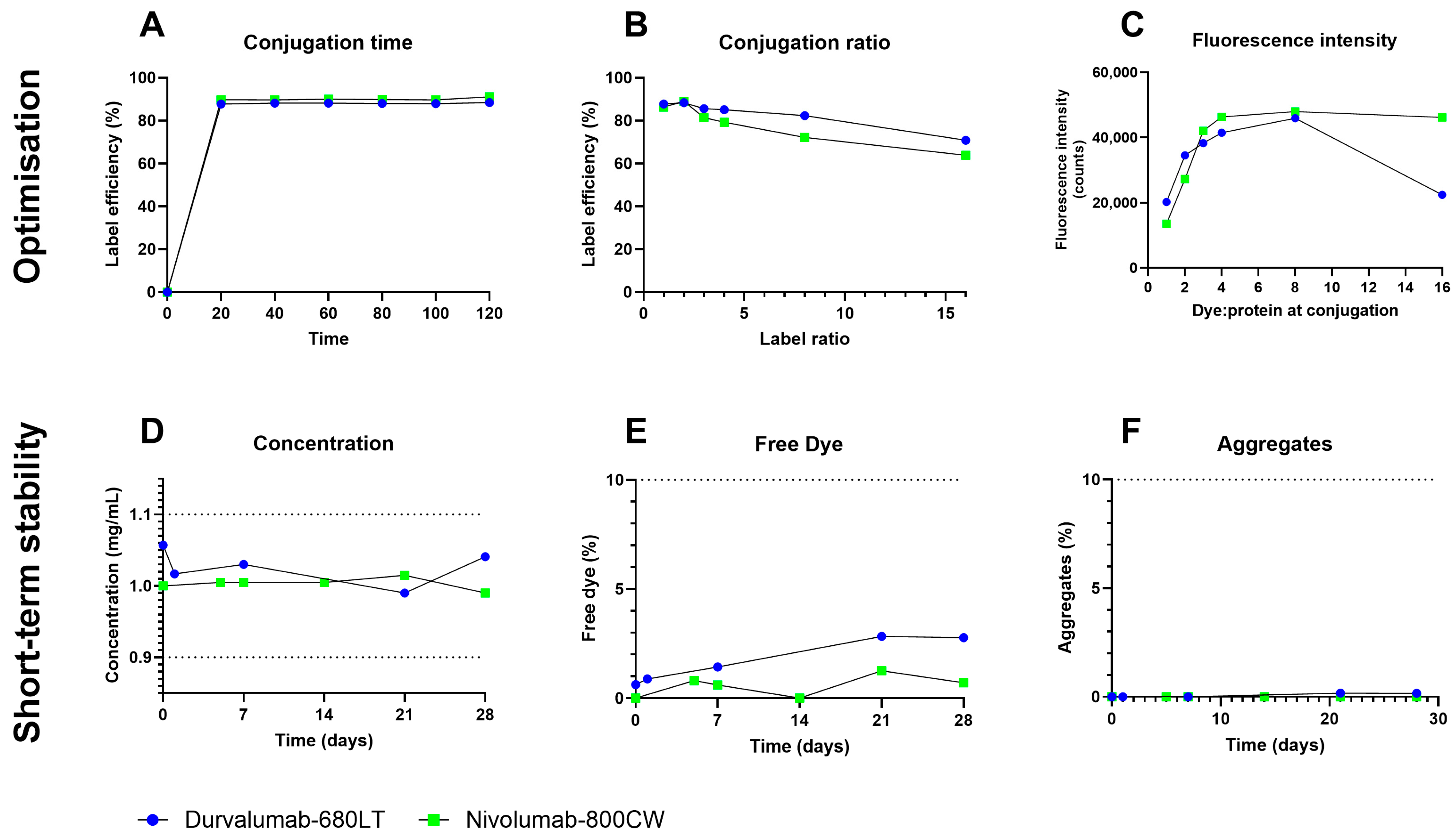
2.2. Process and Labelling Optimization
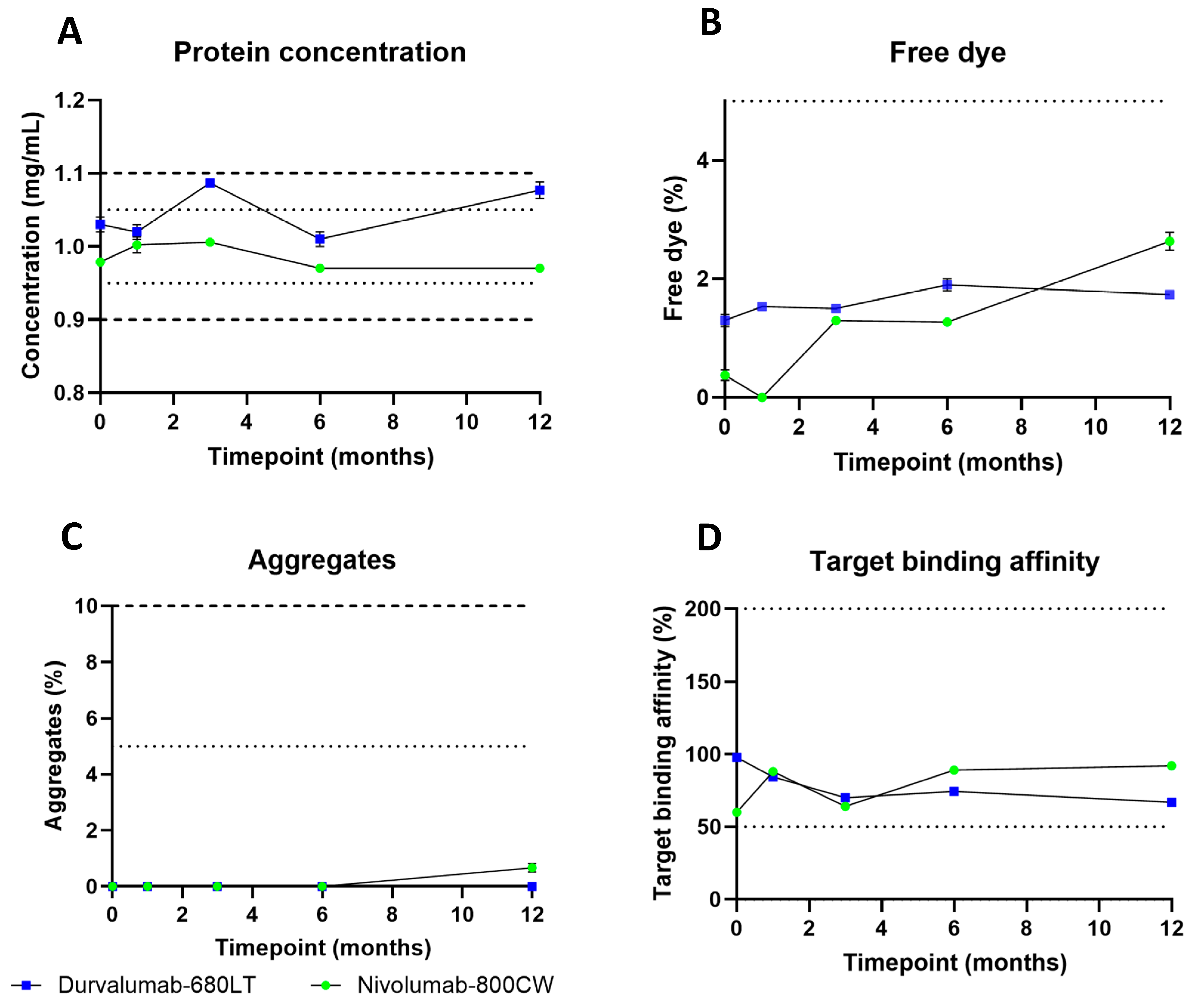
2.3. cGMP Production, Validation, and Long-Term Stability
2.4. Toxicity Study
3. Discussion
4. Materials and Methods
4.1. Labelling Procedure
4.2. Labelling Optimization
4.3. cGMP Production, Validation, and Long-Term Stability
4.4. Quality Control Testing
4.5. Toxicity Study
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cGMP | Current good manufacturing practices |
| ELISA | Indirect enzyme-linked immunosorbent assay |
| FMI | Fluorescent molecular imaging |
| HRP | Horseradish peroxidase |
| ICI | Immune checkpoint inhibitor |
| IHC | Immunohistochemistry |
| LE | Label efficiency |
| NSCLC | Non-small cell lung carcinoma |
| PD-1 | Programmed death receptor 1 |
| PD-L1 | Programmed death ligand 1 |
| PET | Positron emission tomography |
| SE-HPLC | Size-exclusion high-performance liquid chromatography |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
References
- Lin, E.M.; Gong, J.; Klempner, S.J.; Chao, J. Advances in Immuno-Oncology Biomarkers for Gastroesophageal Cancer: Programmed Death Ligand 1, Microsatellite Instability, and Beyond. World J. Gastroenterol. 2018, 24, 2686–2697. [Google Scholar] [CrossRef]
- Koemans, W.J.; Chalabi, M.; van Sandick, J.W.; van Dieren, J.M.; Kodach, L.L. Beyond the PD-L1 Horizon: In Search for a Good Biomarker to Predict Success of Immunotherapy in Gastric and Esophageal Adenocarcinoma. Cancer Lett. 2019, 442, 279–286. [Google Scholar] [CrossRef]
- Cogdill, A.P.; Andrews, M.C.; Wargo, J.A. Hallmarks of Response to Immune Checkpoint Blockade. Br. J. Cancer 2017, 117, 1–7. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, Q.; Luo, X.S.; Yang, X.W.; Wang, H.D.; Guo, C.Y. Efficacy of PD-1/PD-L1 Inhibitors against Pretreated Advanced Cancer: A Systematic Review and Meta-Analysis. Oncotarget 2018, 9, 11846–11857. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Z.; Zheng, X.; Tao, H.; Zhang, S.; Ma, J.; Liu, Z.; Wang, J.; Qian, Y.; Cui, P.; et al. Response Efficacy of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 562315. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yuan, S.; Wang, C.; Zhu, H. Resistance to Immune Checkpoint Inhibitors in Gastric Cancer. Front. Pharmacol. 2023, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, J.; Zhang, Y.; Jiang, H.; Liu, D. Revisiting Immune Checkpoint Inhibitors: New Strategies to Enhance Efficacy and Reduce Toxicity. Front. Immunol. 2024, 15, 1490129. [Google Scholar] [CrossRef]
- Zdrenka, M.; Kowalewski, A.; Ahmadi, N.; Sadiqi, R.U.; Chmura, Ł.; Borowczak, J.; Maniewski, M.; Szylberg, Ł. Refining PD-1/PD-L1 Assessment for Biomarker-Guided Immunotherapy: A Review. Biomol. Biomed. 2024, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Hwang, D.M.; Albaqer, T.; Santiago, R.C.; Weiss, J.; Tanguay, J.; Cabanero, M.; Leung, Y.; Pal, P.; Khan, Z.; Lau, S.C.M.; et al. Prevalence and Heterogeneity of PD-L1 Expression by 22C3 Assay in Routine Population-Based and Reflexive Clinical Testing in Lung Cancer. J. Thorac. Oncol. 2021, 16, 1490–1500. [Google Scholar] [CrossRef]
- Memmott, R.M.; Wolfe, A.R.; Carbone, D.P.; Williams, T.M. Predictors of Response, Progression-Free Survival, and Overall Survival in Patients with Lung Cancer Treated with Immune Checkpoint Inhibitors. J. Thorac. Oncol. 2021, 16, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Daud, A.I.; Wolchok, J.D.; Robert, C.; Hwu, W.J.; Weber, J.S.; Ribas, A.; Hodi, F.S.; Joshua, A.M.; Kefford, R.; Hersey, P.; et al. Programmed Death-Ligand 1 Expression and Response to the Anti-Programmed Death 1 Antibody Pembrolizumab in Melanoma. J. Clin. Oncol. 2016, 34, 4102–4109. [Google Scholar] [CrossRef]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.A.M.S.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole Body PD-1 and PD-L1 Positron Emission Tomography in Patients with Non-Small-Cell Lung Cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef]
- Kok, I.C.; Hooiveld, J.S.; van de Donk, P.P.; Giesen, D.; van der Veen, E.L.; Lub-de Hooge, M.N.; Brouwers, A.H.; Hiltermann, T.J.N.; van der Wekken, A.J.; Hijmering-Kappelle, L.B.M.; et al. 89Zr-Pembrolizumab Imaging as a Non-Invasive Approach to Assess Clinical Response to PD-1 Blockade in Cancer. Ann. Oncol. 2022, 33, 80–88. [Google Scholar] [CrossRef]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. 89Zr-Atezolizumab Imaging as a Non-Invasive Approach to Assess Clinical Response to PD-L1 Blockade in Cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, A.L.N.; Oprea-Lager, D.E.; Huisman, M.C.; Hoekstra, O.S.; Boellaard, R.; de Wit-Van der Veen, B.J.; Bahce, I.; Vugts, D.J.; van Dongen, G.A.M.S.; Thunnissen, E.; et al. Study of 89Zr-Pembrolizumab PET/CT in Patients with Advanced-Stage Non-Small Cell Lung Cancer. J. Nucl. Med. 2022, 63, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Nagengast, W.B.; Hartmans, E.; Garcia-Allende, P.B.; Peters, F.T.M.; Linssen, M.D.; Koch, M.; Koller, M.; Tjalma, J.J.J.; Karrenbeld, A.; Jorritsma-Smit, A.; et al. Near-Infrared Fluorescence Molecular Endoscopy Detects Dysplastic Oesophageal Lesions Using Topical and Systemic Tracer of Vascular Endothelial Growth Factor A. Gut 2019, 68, 7–10. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, S.J.; Voskuil, F.J.; Schmidt, I.; Karrenbeld, A.; Kats-Ugurlu, G.; Meersma, G.J.; Westerhof, J.; Witjes, M.J.H.; Van Dam, G.M.; Robinson, D.J.; et al. C-Met Targeted Fluorescence Molecular Endoscopy in Barrett’s Esophagus Patients and Identification of Outcome Parameters for Phase-I Studies. Issue 12 Theranostics 2020, 10, 5357–5367. [Google Scholar] [CrossRef]
- van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative Tumor-Specific Fluorescence Imaging in Ovarian Cancer by Folate Receptor-α Targeting: First in-Human Results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Voskuil, F.J.; de Jongh, S.J.; Hooghiemstra, W.T.R.; Linssen, M.D.; Steinkamp, P.J.; de Visscher, S.A.H.J.; Schepman, K.P.; Elias, S.G.; Meersma, G.J.; Jonker, P.K.C.; et al. Fluorescence-Guided Imaging for Resection Margin Evaluation in Head and Neck Cancer Patients Using Cetuximab-800CW: A Quantitative Dose-Escalation Study. Theranostics 2020, 10, 3994–4005. [Google Scholar] [CrossRef]
- Gabriëls, R.Y.; van der Waaij, A.M.; Linssen, M.D.; Dobosz, M.; Volkmer, P.; Jalal, S.; Robinson, D.; Hermoso, M.A.; Lub-De Hooge, M.N.; Festen, E.A.M.; et al. Fluorescently Labelled Vedolizumab to Visualise Drug Distribution and Mucosal Target Cells in Inflammatory Bowel Disease. Gut 2024, 73, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.; Zhao, X.; van der Waaij, A.M.; Meersma, G.J.; Dijkstra, F.A.; Haveman, J.W.; van Etten, B.; Robinson, D.J.; Kats-Ugurlu, G.; Nagengast, W.B. Ultrasound-Guided Quantitative Fluorescence Molecular Endoscopy for Monitoring Response in Patients with Esophageal Cancer Following Neoadjuvant Chemoradiotherapy. Clin. Cancer Res. 2024, 30, 3211–3219. [Google Scholar] [CrossRef]
- AstraZeneca Summary of Product Characteristics—Durvalumab. Available online: https://www.ema.europa.eu/en/documents/product-information/imfinzi-epar-product-information_en.pdf (accessed on 23 May 2025).
- Bristol-Myers Squibb Summary of Product Characteristics—Nivolumab. Available online: https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf (accessed on 23 May 2025).
- Linssen, M.D.; Ter Weele, E.J.; Allersma, D.P.; Lub-de Hooge, M.N.; Van Dam, G.M.; Jorritsma-Smit, A.; Nagengast, W.B. Roadmap for the Development and Clinical Translation of Optical Tracers Cetuximab-800CW and Trastuzumab-800CW. J. Nucl. Med. 2019, 60, 418–423. [Google Scholar] [CrossRef]
- Ter Weele, E.J.; Terwisscha Van Scheltinga, A.G.T.; Linssen, M.D.; Nagengast, W.B.; Lindner, I.; Jorritsma-Smit, A.; De Vries, E.G.E.; Kosterink, J.G.W.; Lub-De Hooge, M.N. Development, Preclinical Safety, Formulation, and Stability of Clinical Grade Bevacizumab-800CW, a New near Infrared Fluorescent Imaging Agent for First in Human Use. Eur. J. Pharm. Biopharm. 2016, 104, 226–234. [Google Scholar] [CrossRef]
- Linssen, M.D.; Hooghiemstra, W.T.R.; Jorritsma-Smit, A.; Allersma, D.P.; Dijkstra, G.; Nagengast, W.B. Development and Characterisation of Antibody-Based Optical Imaging Probes for Inflammatory Bowel Disease. Pharmaceuticals 2021, 14, 92. [Google Scholar] [CrossRef]
- Rosenthal, E.L.; Warram, J.M.; De Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M.; Strong, T.V.; Schmalbach, C.E.; Morlandt, A.B.; Agarwal, G.; et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin. Cancer Res. 2015, 21, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Rosenthal, E.L.; Martin, B.A.; Lu, G.; Nishio, N.; Colevas, D.; Chirita, S.U.; Fakurnejad, S.; Birkeland, A.; van Keulen, S.; et al. The Clinical Application of Fluorescence-Guided Surgery in Head and Neck Cancer. J. Nucl. Med. 2019, 60, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Patel, N.L.; Wei, L.; Riffle, L.A.; Kalen, J.D.; Hill, G.C.; Jacobs, P.M.; Zinn, K.R.; Rosenthal, E. Synthesis and Biological Evaluation of Panitumumab-IRDye800 Conjugate as a Fluorescence Imaging Probe for EGFR-Expressing Cancers. Medchemcomm 2014, 5, 1337. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.V.; Draney, D.; Sevick-Muraca, E.M.; Olive, D.M. Single-Dose Intravenous Toxicity Study of IRDye 800CW in Sprague-Dawley Rats. Mol. Imaging Biol. 2010, 12, 583–594. [Google Scholar] [CrossRef]
- Harlaar, N.J.; Koller, M.; de Jongh, S.J.; van Leeuwen, B.L.; Hemmer, P.H.; Kruijff, S.; van Ginkel, R.J.; Been, L.B.; de Jong, J.S.; Kats-Ugurlu, G.; et al. Molecular Fluorescence-Guided Surgery of Peritoneal Carcinomatosis of Colorectal Origin: A Single-Centre Feasibility Study. Lancet Gastroenterol. Hepatol. 2016, 1, 283–290. [Google Scholar] [CrossRef]
- de Jongh, S.J.; Tjalma, J.J.J.; Koller, M.; Linssen, M.D.; Vonk, J.; Dobosz, M.; Jorritsma-Smit, A.; Kleibeuker, J.H.; Hospers, G.A.P.; Havenga, K.; et al. Back-Table Fluorescence-Guided Imaging for Circumferential Resection Margin Evaluation in Locally Advanced Rectal Cancer Patients Using Bevacizumab-800CW. J. Nucl. Med. 2020, 61, 655–661. [Google Scholar] [CrossRef]
- Nishio, N.; van den Berg, N.S.; van Keulen, S.; Martin, B.A.; Fakurnejad, S.; Zhou, Q.; Lu, G.; Chirita, S.U.; Kaplan, M.J.; Divi, V.; et al. Optimal Dosing Strategy for Fluorescence-Guided Surgery with Panitumumab-IRDye800CW in Head and Neck Cancer. Mol. Imaging Biol. 2019, 22, 156–164. [Google Scholar] [CrossRef]
- Kist de Ruijter, L.; van de Donk, P.P.; Hooiveld-Noeken, J.S.; Giesen, D.; Elias, S.G.; Lub-de Hooge, M.N.; Oosting, S.F.; Jalving, M.; Timens, W.; Brouwers, A.H.; et al. Whole-Body CD8+ T Cell Visualization before and during Cancer Immunotherapy: A Phase 1/2 Trial. Nat. Med. 2022, 28, 2601–2610. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Ma, S.; Liu, Q.; Huang, L.; Chen, X.; Lou, K.; Wang, W. Recent Developments in Multimodality Fluorescence Imaging Probes. Acta Pharm. Sin. B 2018, 8, 320–338. [Google Scholar] [CrossRef]
- Zhou, L.; El-Deiry, W.S. Multispectral Fluorescence Imaging. J. Nucl. Med. 2009, 50, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Nguyen, Q.T. Molecular Imaging for Cancer Diagnosis and Surgery. Adv. Drug Deliv. Rev. 2014, 66, 90. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhao, H.; Hu, L.; Shao, X.; Lu, Z.; Wang, Y.; Ling, P.; Li, Y.; Zeng, K.; Chen, Q. Enhanced Optical Imaging and Fluorescent Labeling for Visualizing Drug Molecules within Living Organisms. Acta Pharm. Sin. B 2024, 14, 2428–2446. [Google Scholar] [CrossRef]
- EDQM Council of Europe. European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2023. [Google Scholar]
- Schofield, D.J.; Percival-Alwyn, J.; Rytelewski, M.; Hood, J.; Rothstein, R.; Wetzel, L.; McGlinchey, K.; Adjei, G.; Watkins, A.; Machiesky, L.A.; et al. Activity of Murine Surrogate Antibodies for Durvalumab and Tremelimumab Lacking Effector Function and the Ability to Deplete Regulatory T Cells in Mouse Models of Cancer. MAbs 2021, 13. [Google Scholar] [CrossRef] [PubMed]
| Tracer | Label Efficiency | Protein Yield | Concentration Sufficient | Protein Aggregates | Unconjugated Dye |
|---|---|---|---|---|---|
| Durvalumab-800CW | 85.2 ± 1.1% | 90.1 ± 2.3% | Yes | N.D. | 0.38 ± 0.57% |
| Durvalumab-680LT | 88.4 ± 2.0% | 98.6 ± 2.5% | Yes | N.D. | 0.48 ± 0.39% |
| Nivolumab-800CW | 89.9 ± 0.5% | 98.2 ± 2.7% | Yes | N.D. | 0.50 ± 0.20% |
| Nivolumab-680LT | 77.8 ± 3.0% | 91.9 ± 1.7% | Yes | N.D. | 2.0 ± 2.5% |
| Test | Method | Release Specification | End of Shelf-Life Specification |
|---|---|---|---|
| Protein monomer concentration | SE-HPLC | 0.95–1.05 mg/mL | 0.90–1.10 mg/mL |
| Protein aggregates | SE-HPLC | ≤5.0% | ≤10% |
| Unconjugated IRDye 800CW/680LT | SE-HPLC | ≤5.0% | ≤10% |
| Protein monomer identity | SE-HPLC | Retention time comparable to reference standard | Retention time comparable to reference standard |
| Protein monomer integrity | SE-HPLC | Peak shape comparable to reference standard; no shoulders, minimal tailing | Peak shape comparable to reference standard; no shoulders, minimal tailing |
| UV-VIS absorption peaks | SE-HPLC | Nivolumab-800CW: peaks at 280 ± 3 nm 775 ± 3 nm Durvalumab-680LT: peaks at 280 ± 3 nm and 679 ± 3 nm | Nivolumab-800CW: peaks at 280 ± 3 nm 775 ± 3 nm Durvalumab-680LT: peaks at 280 ± 3 nm and 679 ± 3 nm |
| Target binding affinity | Indirect ELISA | 50–200% | 50–200% |
| Appearance (turbidity) | Visual inspection | Clear to slightly opalescent solution | Clear to slightly opalescent solution |
| Appearance (colour) | Visual comparison to colour standards | Colour tone: comparable to reference | Colour tone: comparable to reference |
| Container closure and label | Visual inspection | Closure intact, label legible and intact | Closure intact, label legible and intact |
| Extractable volume | Ph. Eur. 2.9.17 | ≥5.0 mL | ≥5.0 mL |
| pH | Ph. Eur. 2.2.3 | 6.9–7.1 | 6.9–7.1 |
| Osmolality | Ph. Eur. 2.2.35 | 270–310 mOsmol/kg | 270–310 mOsmol/kg |
| Residual solvents (DMSO) | Ph. Eur. 2.4.24 Ph. Eur. 5.4 | ≤50.0 mg/L | ≤50.0 mg/L |
| Bacterial endotoxins | Ph. Eur. 2.6.14 | ≤5.0 EU/mL | ≤5.0 EU/mL |
| Sterility | Ph. Eur. 2.6.1 | Sterile | Sterile |
| Visible Particles | Ph. Eur. 2.9.20 | Practically free of visible particles | Practically free of visible particles |
| Sub-visible Particles | Ph. Eur. 2.9.19 | Particles ≥10 μm: ≤6000/vial Particles ≥25 μm: ≤600/vial | Particles ≥10 μm: ≤6000/vial Particles ≥25 μm: ≤600/vial |
| Group | Treatment | Dose | No. of Mice * | Sex |
|---|---|---|---|---|
| G1 | Vehicle control (saline) | N.A. | 16 | M |
| 16 | F | |||
| G2 | Durvalumab-680LT | 9 mg/kg body weight | 10 | M |
| 10 | F | |||
| G3 | 90 mg/kg body weight | 16 | M | |
| 16 | F | |||
| G4 | IRDye 680LT | 86 µg/kg body weight | 10 | M |
| 10 | F | |||
| G5 | 860 µg/kg body weight | 16 | M | |
| 16 | F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huizinga, H.K.; Hooghiemstra, W.T.R.; Linssen, M.D.; Allersma, D.P.; Gareb, B.; Dekkers, B.G.J.; Nagengast, W.B.; Lub-de Hooge, M.N. Development of Clinical-Grade Durvalumab-680LT and Nivolumab-800CW for Multispectral Fluorescent Imaging of the PD-1/PD-L1 Axis of the Immune Checkpoint Pathway. Pharmaceuticals 2025, 18, 1501. https://doi.org/10.3390/ph18101501
Huizinga HK, Hooghiemstra WTR, Linssen MD, Allersma DP, Gareb B, Dekkers BGJ, Nagengast WB, Lub-de Hooge MN. Development of Clinical-Grade Durvalumab-680LT and Nivolumab-800CW for Multispectral Fluorescent Imaging of the PD-1/PD-L1 Axis of the Immune Checkpoint Pathway. Pharmaceuticals. 2025; 18(10):1501. https://doi.org/10.3390/ph18101501
Chicago/Turabian StyleHuizinga, Henrik K., Wouter T. R. Hooghiemstra, Matthijs D. Linssen, Derk P. Allersma, Bahez Gareb, Bart G. J. Dekkers, Wouter B. Nagengast, and Marjolijn N. Lub-de Hooge. 2025. "Development of Clinical-Grade Durvalumab-680LT and Nivolumab-800CW for Multispectral Fluorescent Imaging of the PD-1/PD-L1 Axis of the Immune Checkpoint Pathway" Pharmaceuticals 18, no. 10: 1501. https://doi.org/10.3390/ph18101501
APA StyleHuizinga, H. K., Hooghiemstra, W. T. R., Linssen, M. D., Allersma, D. P., Gareb, B., Dekkers, B. G. J., Nagengast, W. B., & Lub-de Hooge, M. N. (2025). Development of Clinical-Grade Durvalumab-680LT and Nivolumab-800CW for Multispectral Fluorescent Imaging of the PD-1/PD-L1 Axis of the Immune Checkpoint Pathway. Pharmaceuticals, 18(10), 1501. https://doi.org/10.3390/ph18101501








