Design and Optimization of Spiro-Isatin-Thiazolidinone Hybrids with Promising Anticancer Activity
Abstract
1. Introduction
2. Results
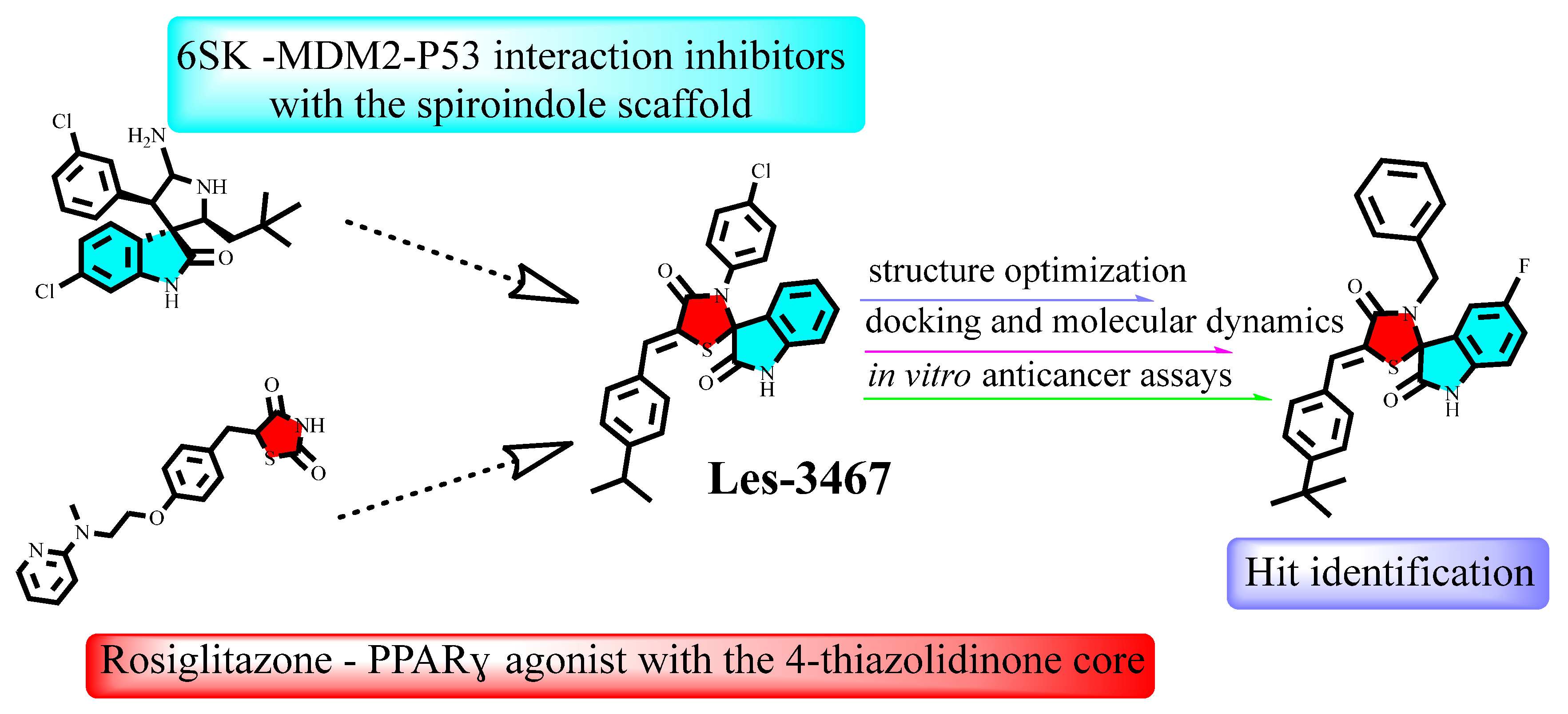
2.1. The Rationale of the Synthesis
- (a)
- Addition of a Halogen at the 5-position of the Isatin Ring: Adding a halogen (e.g., chlorine or fluorine) at the 5-position of the isatin ring is expected to improve the fit within the binding site. This modification can enhance binding through halogen bonding or hydrophobic interactions, contributing to better pocket occupancy and additional stabilization.
- (b)
- Replacement of the 4-Chlorophenyl Core with a Benzyl Substituent: Substituting the rigid 4-chlorophenyl core with a more flexible benzyl group could allow better suiting of the molecule to the binding pocket. This flexibility may lead to stronger hydrophobic interactions and better alignment with key residues, enhancing overall binding affinity.
- (c)
- Addition of a Substituent to the 5-Arylidene Portion of the Molecule: Incorporating a substituent (e.g., or polar group) on the 5-arylidene moiety could improve binding energy by introducing new interactions with residues in the binding site. This change could also stabilize the ligand’s position more effectively, ensuring a stronger and more specific fit.
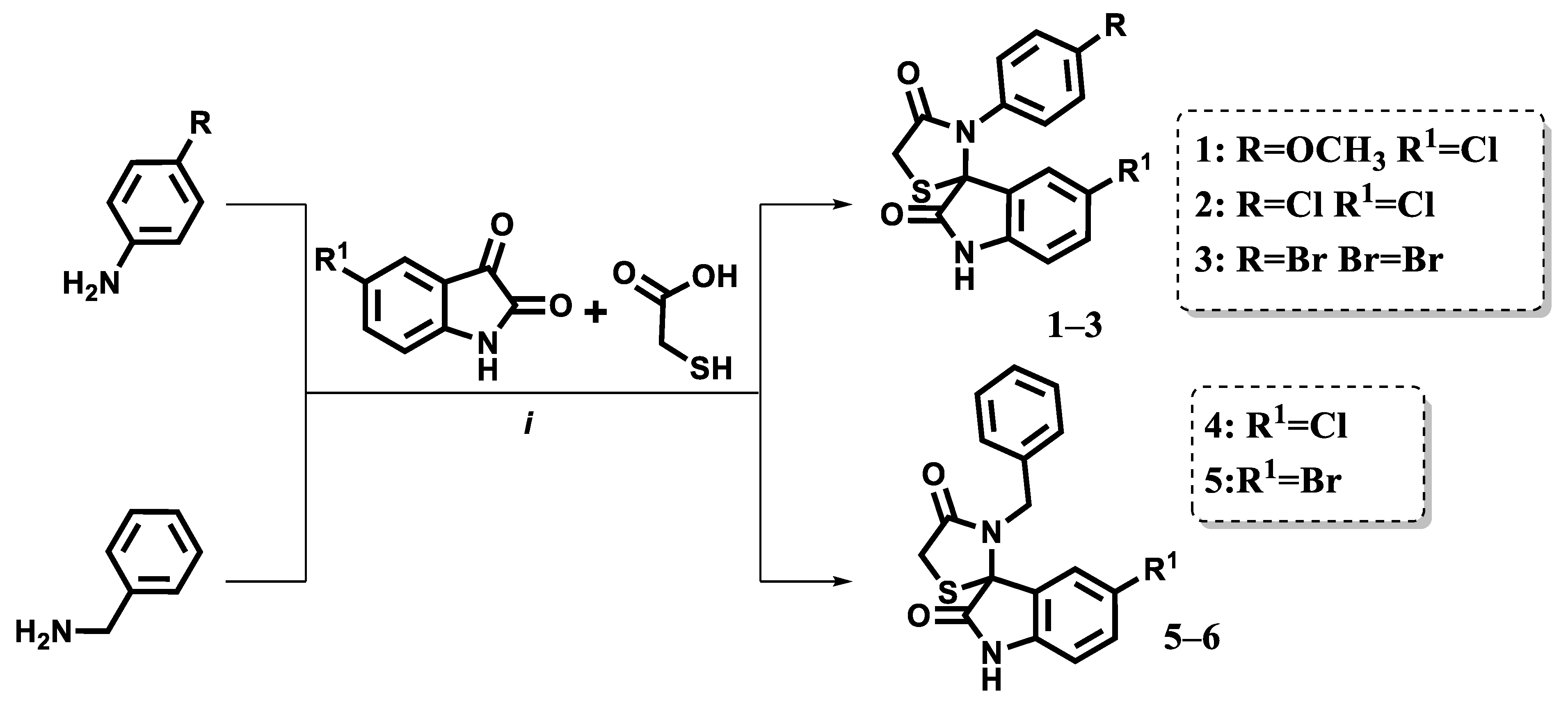
2.2. Synthesis
2.3. Anticancer Activity
2.4. In Silico Simulation
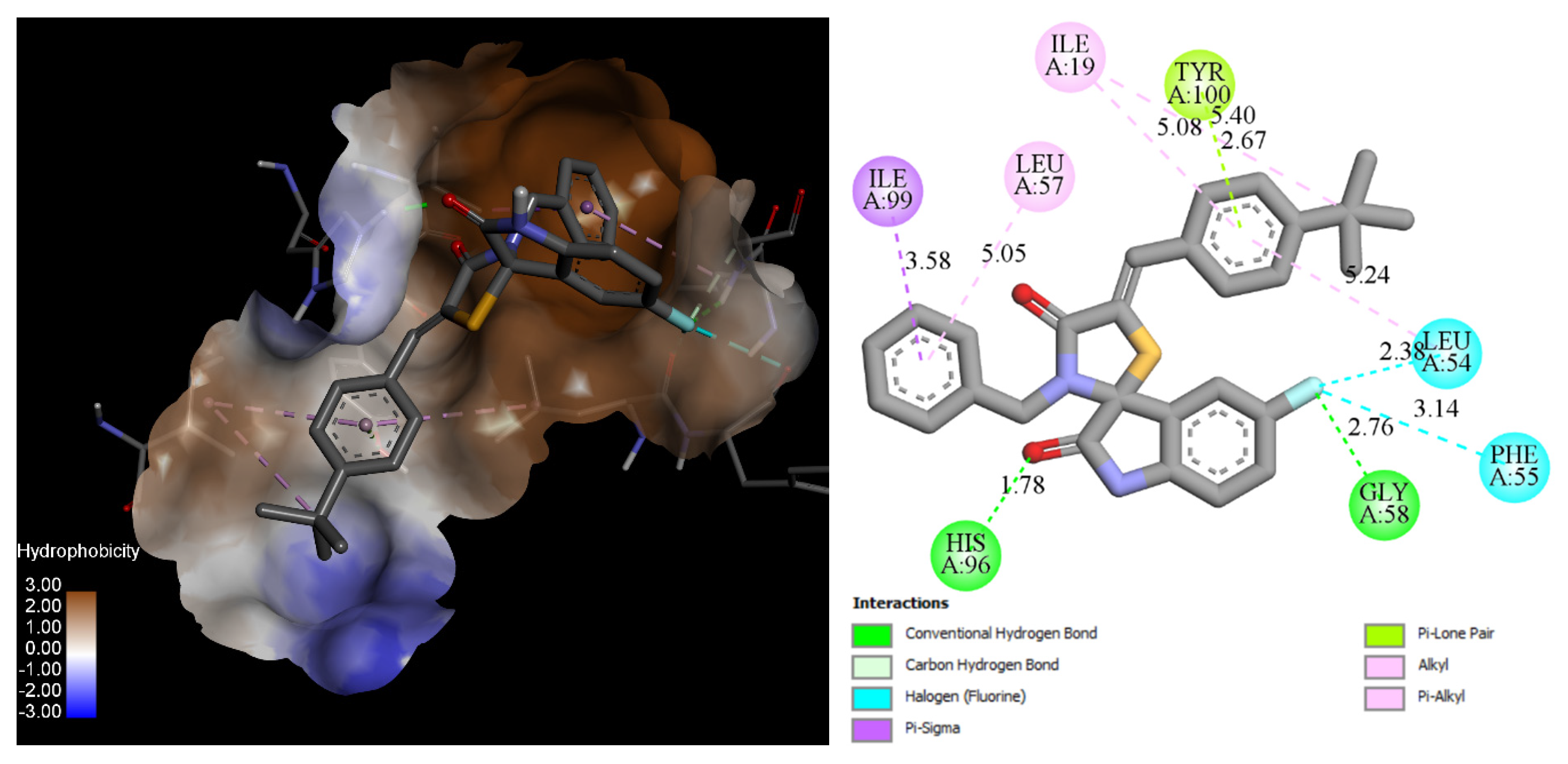
2.4.1. Docking
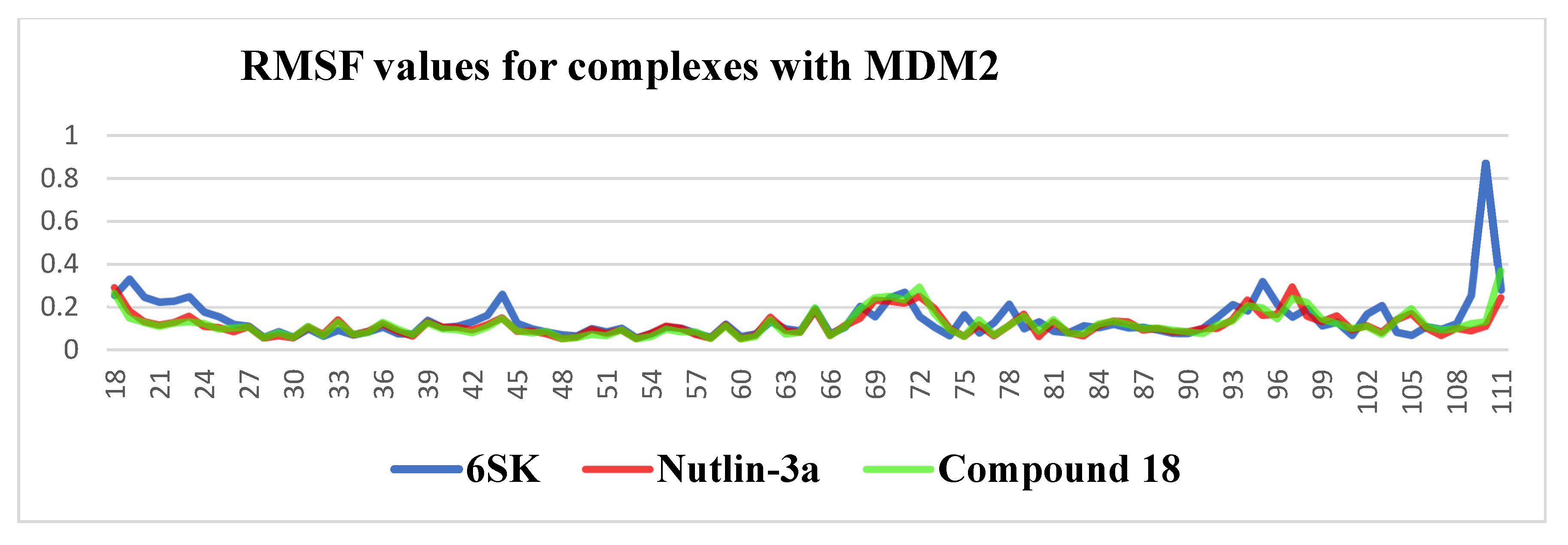
2.4.2. Ligand Stability in the MDM2 Binding Pocket: 100 ns MD Insights
- 6SK underwent a brief relaxation (~5 ns), after which its RMSD plateaued in the 0.02–0.12 nm range, implying a rigid, tightly anchored pose. The ligand remains locked in the hydrophobic cleft through a persistent π-stacking/van der Waals triad with Trp23, Leu54 and Leu57.
- Compound 18 showed a gradual rise in RMSD to ≈0.18 nm, indicating modest flexibility: its core contact pattern is retained while peripheral substituents periodically re-orient, suggesting scope for scaffold refinement without sacrificing affinity.
- Nutlin-3a displayed the largest excursions (0.18–0.28 nm) for most of the trajectory, reflecting partial displacement from the optimal binding pose and intermittent loss of key hydrogen bonds—behavior consistent with the higher dissociation constants reported for this chemotype.
2.4.3. ADMET Profile
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General Information
4.1.2. Synthesis and Characterization of Spiro[3H-indol-3,2′-thiazolidin]-2,4′(1H)-diones (Compounds 1–5)
Characterization of Compounds 1–5
- 5-Chloro-3′-(4-methoxyphenyl)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (1)
- 5-Chloro-3′-(4-chlorophenyl)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (2)
- 5-Bromo-3′-(4-bromophenyl)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (3)
- 3′-Benzyl-5-fluorospiro[indoline-3,2′-thiazolidine]-2,4′-dione (4)
- 3′-Benzyl-5-chlorospiro[indoline-3,2′-thiazolidine]-2,4′-dione (5)
4.1.3. Synthesis and Characterization of 5-Arylidene Derivatives of Spiro[3H-indol-3,2′-thiazolidin]-2,4′(1H)-diones (Compounds 6–19)
Characterization of Compounds 6–19
- (Z)-5-Chloro-3′-(4-methoxyphenyl)-5′-((E)-3-phenylallylidene)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (6)
- (Z)-5-Chloro-5′-(4-chlorobenzylidene)-3′-(4-methoxyphenyl)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (7)
- (Z)-5-Chloro-3′-(4-methoxyphenyl)-5′-(4-morpholinobenzylidene)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (8)
- (Z)-5-Chloro-3′-(4-chlorophenyl)-5′-(4-ethoxybenzylidene)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (9)
- (Z)-5-Chloro-3′-(4-chlorophenyl)-5′-(4-morpholinobenzylidene)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (10)
- (Z)-5-Chloro-5′-(4-chlorobenzylidene)-3′-(4-chlorophenyl)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (11)
- (Z)-5-Chloro-3′-(4-methoxyphenyl)-5′-(4-(pyridin-2-yl)benzylidene)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (12)
- (Z)-5-Chloro-3′-(4-chlorophenyl)-5′-(4-(pyridin-2-yl)benzylidene)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (13)
- (Z)-5-Bromo-3′-(4-bromophenyl)-5′-(4-ethoxybenzylidene)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (14)
- (Z)-5′-(4-(Tert-butyl)benzylidene)-5-chloro-3′-(4-chlorophenyl)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (15)
- (Z)-5-Bromo-3′-(4-bromophenyl)-5′-(4-(tert-butyl)benzylidene)spiro[indoline-3,2′-thiazolidine]-2,4′-dione (16)
- (Z)-3′-Benzyl-5′-(4-(tert-butyl)benzylidene)-5-chlorospiro[indoline-3,2′-thiazolidine]-2,4′-dione (17)
- (Z)-3′-Benzyl-5′-(4-(tert-butyl)benzylidene)-5-fluorospiro[indoline-3,2′-thiazolidine]-2,4′-dione (18)
- (Z)-3′-Benzyl-5′-(4-ethoxybenzylidene)-5-fluorospiro[indoline-3,2′-thiazolidine]-2,4′-dione (19)
4.2. Cell Lines and Culture Conditions
4.3. The MTT Assay
4.4. Molecular Docking Protocol
4.5. Molecular Dynamics
4.6. ADMET Profiles
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMET | Absorption, Distribution, Metabolism, Excretion, and Toxicity |
| HBA | Hydrogen Bond Acceptor |
| HBD | Hydrogen Bond Donor |
| IC50 | Half-Maximal Inhibitory Concentration |
| LD50 | Median Lethal Dose |
| MD | Molecular Dynamics |
| MDM2 | Mouse Double Minute 2 homolog |
| NMR | Nuclear Magnetic Resonance |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| RMSD | Root-Mean-Square Deviation |
| RMSF | Root-Mean-Square Fluctuation |
References
- López-Gómez, M.; Malmierca, E.; de Górgolas, M.; Casado, E. Cancer in Developing Countries: The next Most Preventable Pandemic. The Global Problem of Cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Horgan, D.; Mia, R.; Erhabor, T.; Hamdi, Y.; Dandara, C.; Lal, J.; Domgue, J.; Ewumi, O.; Nyawira, T.; Meyer, S.; et al. Fighting Cancer around the World: A Framework for Action. Healthcare 2022, 10, 2125. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/News-Room/Fact-Sheets/Detail/Cancer (accessed on 5 May 2025).
- Savage, S.R.; Yi, X.; Lei, J.T.; Wen, B.; Zhao, H.; Liao, Y.; Jaehnig, E.J.; Somes, L.K.; Shafer, P.W.; Lee, T.D.; et al. Pan-Cancer Proteogenomics Expands the Landscape of Therapeutic Targets. Cell 2024, 187, 4389–4407.e15. [Google Scholar] [CrossRef]
- Pfohl, U.; Pflaume, A.; Regenbrecht, M.; Finkler, S.; Graf Adelmann, Q.; Reinhard, C.; Regenbrecht, C.; Wedeken, L. Precision Oncology Beyond Genomics: The Future Is Here—It Is Just Not Evenly Distributed. Cells 2021, 10, 928. [Google Scholar] [CrossRef]
- Shin, S.; Moon, J.; Oum, C.; Kim, S.; Cho, S.I.; Lim, Y.; Ock, C.-Y.; Shin, S. Discontinuation Risk from Adverse Events: Immunotherapy Alone vs. Combined with Chemotherapy: A Systematic Review and Network Meta-Analysis. BMC Cancer 2024, 24, 152. [Google Scholar] [CrossRef]
- Middha, P.; Thummalapalli, R.; Quandt, Z.; Balaratnam, K.; Cardenas, E.; Falcon, C.J.; Margaret Lung Group, P.; Gubens, M.A.; Huntsman, S.; Khan, K.; et al. Germline Prediction of Immune Checkpoint Inhibitor Discontinuation for Immune-Related Adverse Events. J. Immunother. Cancer 2025, 13, e011273. [Google Scholar] [CrossRef]
- Hoog, C.J.P.O.H.; Mehra, N.; Maliepaard, M.; Bol, K.; Gelderblom, H.; Sonke, G.S.; de Langen, A.J.; van de Donk, N.W.C.J.; Janssen, J.J.W.M.; Minnema, M.C.; et al. Dose Selection of Novel Anticancer Drugs: Exposing the Gap between Selected and Required Doses. Lancet Oncol. 2024, 25, e340–e351. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, H.; Wang, C.; Qin, R.; Yang, Q.; Hou, Y.; Zhang, M.; Zhang, C.; Wang, N.; Feng, Y. Chemotherapy Cardiotoxicity Research in Cancer Patients: A Bibliometric and Visual Analysis (1994–2024). Front. Oncol. 2025, 15, 1502361. [Google Scholar] [CrossRef]
- Chen, C.; Garcia, Z.; Chen, D.; Liu, H.; Trelstad, P. Cost and Supply Considerations for Antibody Therapeutics. MAbs 2025, 17, 2451789. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, K.; Shu, P. Cost-Effectiveness Analysis of Pembrolizumab plus Chemotherapy as First-Line Treatment for Advanced Biliary Tract Cancer: Perspectives from US and Chinese Payers. BMJ Open 2025, 15, e094047. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.; Song, C.; Chen, Y.; Long, H.; Yang, L. Small-Molecule Anti-Cancer Drugs from 2016 to 2020: Synthesis and Clinical Application. Nat. Prod. Commun. 2021, 16, 1934578X211040326. [Google Scholar] [CrossRef]
- Bashi, A.C.; Coker, E.A.; Bulusu, K.C.; Jaaks, P.; Crafter, C.; Lightfoot, H.; Milo, M.; McCarten, K.; Jenkins, D.F.; van der Meer, D.; et al. Large-Scale Pan-Cancer Cell Line Screening Identifies Actionable and Effective Drug Combinations. Cancer Discov. 2024, 14, 846–865. [Google Scholar] [CrossRef]
- Southey, M.W.Y.; Brunavs, M. Introduction to Small Molecule Drug Discovery and Preclinical Development. Front. Drug Discov. 2023, 3, 1314077. [Google Scholar] [CrossRef]
- Barrios, C.; de Lima Lopes, G.; Yusof, M.M.; Rubagumya, F.; Rutkowski, P.; Sengar, M. Barriers in Access to Oncology Drugs—A Global Crisis. Nat. Rev. Clin. Oncol. 2023, 20, 7–15. [Google Scholar] [CrossRef]
- Subhasree, N.; Jiangjiang, Q.; Kalkunte, S.; Minghai, W.; Ruiwen, Z. The MDM2-P53 Pathway Revisited. J. Biomed. Res. 2013, 27, 254. [Google Scholar] [CrossRef]
- Koo, N.; Sharma, A.K.; Narayan, S. Therapeutics Targeting P53-MDM2 Interaction to Induce Cancer Cell Death. Int. J. Mol. Sci. 2022, 23, 5005. [Google Scholar] [CrossRef]
- Moll, U.M.; Petrenko, O. The MDM2-P53 Interaction. Mol. Cancer Res. 2003, 1, 1001–1008. [Google Scholar]
- Efeyan, A.; Serrano, M. P53: Guardian of the Genome and Policeman of the Oncogenes. Cell Cycle 2007, 6, 1006–1010. [Google Scholar] [CrossRef]
- Teodoro, J.G.; Evans, S.K.; Green, M.R. Inhibition of Tumor Angiogenesis by P53: A New Role for the Guardian of the Genome. J. Mol. Med. 2007, 85, 1175–1186. [Google Scholar] [CrossRef]
- Duffy, M.J.; Synnott, N.C.; McGowan, P.M.; Crown, J.; O’Connor, D.; Gallagher, W.M. P53 as a Target for the Treatment of Cancer. Cancer Treat. Rev. 2014, 40, 1153–1160. [Google Scholar] [CrossRef]
- Carson, D.A.; Lois, A. Cancer Progression and P53. Lancet 1995, 346, 1009–1011. [Google Scholar] [CrossRef]
- Marvalim, C.; Datta, A.; Lee, S.C. Role of P53 in Breast Cancer Progression: An Insight into P53 Targeted Therapy. Theranostics 2023, 13, 1421–1442. [Google Scholar] [CrossRef]
- Gao, C.; Xiao, G.; Piersigilli, A.; Gou, J.; Ogunwobi, O.; Bargonetti, J. Context-Dependent Roles of MDMX (MDM4) and MDM2 in Breast Cancer Proliferation and Circulating Tumor Cells. Breast Cancer Res. 2019, 21, 5. [Google Scholar] [CrossRef]
- Takahashi, S.; Fujiwara, Y.; Nakano, K.; Shimizu, T.; Tomomatsu, J.; Koyama, T.; Ogura, M.; Tachibana, M.; Kakurai, Y.; Yamashita, T.; et al. Safety and Pharmacokinetics of Milademetan, a MDM2 Inhibitor, in Japanese Patients with Solid Tumors: A Phase I Study. Cancer Sci. 2021, 112, 2361–2370. [Google Scholar] [CrossRef]
- Schoeffski, P.; Lorusso, P.; Yamamoto, N.; Lugowska, I.; Moreno Garcia, V.; Lauer, U.; Hu, C.; Jayadeva, G.; Lahmar, M.; Gounder, M. 673P A Phase I Dose-Escalation and Expansion Study Evaluating the Safety and Efficacy of the MDM2–P53 Antagonist Brigimadlin (BI 907828) in Patients (Pts) with Solid Tumours. Ann. Oncol. 2023, 34, S472–S473. [Google Scholar] [CrossRef]
- Khanna, V.; Eslami, G.; Reyes, R.; Diep, R.; Fernandez-Pol, S.; Stehr, H.; Suarez, C.J.; Pinto, H.; Ford, J.M.; Zhang, T.Y.; et al. MDM2 Inhibition Is Associated with the Emergence of TP53-Altered Clonal Hematopoiesis. npj Precis. Oncol. 2025, 9, 34. [Google Scholar] [CrossRef]
- Xie, X.; Xiong, S.-S.; Li, X.; Huang, H.; Wu, F.-B.; Shen, P.-F.; Peng, C.; He, G.; Han, B. Design and Organocatalytic Synthesis of Spirooxindole–Cyclopentene–Isoxazole Hybrids as Novel MDM2–P53 Inhibitors. Org. Chem. Front. 2021, 8, 1836–1843. [Google Scholar] [CrossRef]
- Giofrè, S.V.; Cirmi, S.; Mancuso, R.; Nicolò, F.; Lanza, G.; Legnani, L.; Campisi, A.; Chiacchio, M.A.; Navarra, M.; Gabriele, B.; et al. Synthesis of Spiro[Isoindole-1,5′-Isoxazolidin]-3(2 H)-Ones as Potential Inhibitors of the MDM2-P53 Interaction. Beilstein J. Org. Chem. 2016, 12, 2793–2807. [Google Scholar] [CrossRef]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.M.M.; Ghabbour, H.A. Design and Synthesis of New Substituted Spirooxindoles as Potential Inhibitors of the MDM2–P53 Interaction. Bioorg. Chem. 2019, 86, 598–608. [Google Scholar] [CrossRef]
- Kaminskyy, D. A Facile Synthesis and Anticancer Activity Evaluation of Spiro[Thiazolidinone-Isatin] Conjugates. Sci. Pharm. 2011, 79, 763–777. [Google Scholar] [CrossRef]
- Vintonyak, V.V.; Warburg, K.; Over, B.; Hübel, K.; Rauh, D.; Waldmann, H. Identification and Further Development of Thiazolidinones Spiro-Fused to Indolin-2-Ones as Potent and Selective Inhibitors of Mycobacterium Tuberculosis Protein Tyrosine Phosphatase B. Tetrahedron 2011, 67, 6713–6729. [Google Scholar] [CrossRef]
- Alkorbi, F.; Alshareef, S.A.; Abdelaziz, M.A.; Omer, N.; Jame, R.; Alatawi, I.S.; Ali, A.M.; Omran, O.A.; Bakr, R.B. Multicomponent Reaction for Synthesis, Molecular Docking, and Anti-Inflammatory Evaluation of Novel Indole-Thiazole Hybrid Derivatives. Mol. Divers. 2024, 29, 3945–3956. [Google Scholar] [CrossRef]
- Kosińska, K.; Skóra, B.; Holota, S.; Shepeta, Y.; Tabęcka-Łonczyńska, A.; Lesyk, R.; Szychowski, K.A. Role of 4-Thiazolidinone–Pyrazoline/Indoline Hybrids Les-4369 and Les-3467 in BJ and A549 Cell Lines. Cells 2024, 13, 1007. [Google Scholar] [CrossRef]
- Dang, Y.F.; Jiang, X.N.; Gong, F.L.; Guo, X.L. New Insights into Molecular Mechanisms of Rosiglitazone in Monotherapy or Combination Therapy against Cancers. Chem. Biol. Interact. 2018, 296, 162–170. [Google Scholar] [CrossRef]
- Ballav, S.; Biswas, B.; Sahu, V.K.; Ranjan, A.; Basu, S. PPAR-γ Partial Agonists in Disease-Fate Decision with Special Reference to Cancer. Cells 2022, 11, 3215. [Google Scholar] [CrossRef]
- Jang, J.Y.; Bae, H.; Lee, Y.J.; Choi, Y.I.; Kim, H.-J.; Park, S.B.; Suh, S.W.; Kim, S.W.; Han, B.W. Structural Basis for the Enhanced Anti-Diabetic Efficacy of Lobeglitazone on PPARγ. Sci. Rep. 2018, 8, 31. [Google Scholar] [CrossRef]
- Popowicz, G.M.; Dömling, A.; Holak, T.A. The Structure-Based Design of Mdm2/Mdmx–P53 Inhibitors Gets Serious. Angew. Chem. Int. Ed. 2011, 50, 2680–2688. [Google Scholar] [CrossRef]
- SD, N.; Kumar, D.U.; Ghate, S.D.; Dixit, S.R.; Awasthi, A.; Revanasiddappa, B.C. Benzothiazole Derivatives as P53-MDM2 Inhibitors: In-Silico Design, ADMET Predictions, Molecular Docking, MM-GBSA Assay, MD Simulations Studies. J. Biomol. Struct. Dyn. 2025, 43, 2993–3004. [Google Scholar] [CrossRef]
- Finiuk, N.; Kryshchyshyn-Dylevych, A.; Holota, S.; Klyuchivska, O.; Kozytskiy, A.; Karpenko, O.; Manko, N.; Ivasechko, I.; Stoika, R.; Lesyk, R. Novel Hybrid Pyrrolidinedione-Thiazolidinones as Potential Anticancer Agents: Synthesis and Biological Evaluation. Eur. J. Med. Chem. 2022, 238, 114422. [Google Scholar] [CrossRef]
- Gollner, A.; Rudolph, D.; Arnhof, H.; Bauer, M.; Blake, S.M.; Boehmelt, G.; Cockroft, X.-L.; Dahmann, G.; Ettmayer, P.; Gerstberger, T.; et al. Discovery of Novel Spiro[3 H-Indole-3,2′-Pyrrolidin]-2(1 H)-One Compounds as Chemically Stable and Orally Active Inhibitors of the MDM2–P53 Interaction. J. Med. Chem. 2016, 59, 10147–10162. [Google Scholar] [CrossRef]
- Halgren, T.A. MMFF VII. Characterization of MMFF94, MMFF94s, and Other Widely Available Force Fields for Conformational Energies and for Intermolecular-Interaction Energies and Geometries. J. Comput. Chem. 1999, 20, 730–748. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Yusuf, D.; Davis, A.M.; Kleywegt, G.J.; Schmitt, S. An Alternative Method for the Evaluation of Docking Performance: RSR vs. RMSD. J. Chem. Inf. Model. 2008, 48, 1411–1422. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Drwal, M.N.; Banerjee, P.; Dunkel, M.; Wettig, M.R.; Preissner, R. ProTox: A Web Server for the in Silico Prediction of Rodent Oral Toxicity. Nucleic Acids Res. 2014, 42, W53–W58. [Google Scholar] [CrossRef]





| Compound | MDM2 (PDB 5LAV) | PPARγ (PDB 5YCP) |
|---|---|---|
| Les-3390 | −9.342 | −7.765 |
| Les-3467 | −8.801 | −8.189 |
| 6SK | −10.151 | - |
| BRL (Rosiglitazone) | - | −8.939 |
| Comp. | HCT116 | MCF-7 | MDA-MB-231 | 4T1 | KB3-1 | K562 | U373 | HaCaT (Pseudo-Normal) | NIH3T3 (Pseudo-Normal) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | >100 | >100 | 85.31 ± 1.15 | 89.39 ± 1.93 | 62.3 ± 0.51 | 71.84 ± 7.79 | 76.70 ± 0.54 | 96.73 ± 1.02 | 77.56 ± 1.82 |
| 2 | >100 | 84.93 ± 6.51 | 62.69 ± 0.22 | 73.03 ± 7.02 | 51.77 ± 0.26 | 9.96 ± 1.17 | 77.91 ± 0.56 | 82.78 ± 1.94 | 98.25 ± 0.64 |
| 3 | 88.93 ± 6.33 | 64.02 ± 4.77 | 56.49 ± 1.93 | 43.81 ± 1.76 | 46.71 ± 0.54 | 7.99 ± 0.12 | 49.44 ± 1.33 | 38.43 ± 0.77 | 82.93 ± 2.05 |
| 4 | >100 | >100 | 99.21 ± 0.41 | 91.81 ± 1.55 | 60.21 ± 0.72 | 88.75 ± 4.06 | 98.60 ± 1.92 | >100 | >100 |
| 5 | 92.79 ± 1.09 | 42.60 ± 1.24 | 68.79 ± 0.62 | 95.27 ± 1.07 | 52.09 ± 0.67 | 95.51 ± 2.64 | 76.21 ± 0.80 | >100 | >100 |
| 6 | >100 | 83.28 ± 6.03 | 55.85 ± 5.67 | 67.98 ± 2.60 | 45.23 ± 0.36 | >100 | 69.51 ± 0.90 | 85.34 ± 1.27 | >100 |
| 7 | >100 | 52.18 ± 2.15 | 8.55 ± 0.06 | 28.96 ± 0.92 | 17.16 ± 0.21 | 27.66 ± 3.02 | 72.34 ± 0.80 | 8.63 ± 0.57 | 79.74 ± 0.93 |
| 8 | >100 | >100 | >100 | >100 | 75.93 ± 4.21 | >100 | >100 | >100 | >100 |
| 9 | >100 | 74.46 ± 4.73 | 7.89 ± 0.33 | 7.84 ± 0.49 | 22.3 ± 1.17 | 67.11 ± 0.70 | 98.22 ± 0.87 | >100 | >100 |
| 10 | >100 | >100 | >100 | >100 | 16.92 ± 0.86 | >100 | >100 | >100 | >100 |
| 11 | >100 | 6.00 ± 0.28 | 6.37 ± 0.11 | 5.66 ± 0.27 | 6.95 ± 0.14 | 74.35 ± 0.66 | 66.91 ± 0.83 | 8.67 ± 0.49 | >100 |
| 12 | >100 | >100 | >100 | >100 | 0.97 ± 0.11 | >100 | >100 | >100 | >100 |
| 13 | 41.38 ± 0.62 | >100 | >100 | >100 | 0.99 ± 0.08 | 5.55 ± 0.17 | >100 | >100 | >100 |
| 14 | >100 | >100 | 60.24 ± 0.62 | >100 | 19.55 ± 0.23 | >100 | 89.52 ± 0.81 | >100 | >100 |
| 15 | >100 | 77.42 ± 2.90 | 30.72 ± 0.45 | 46.60 ± 2.98 | 9.77 ± 0.78 | >100 | 79.33 ± 1.31 | >100 | 90.32 ± 0.68 |
| 16 | >100 | >100 | 53.27 ± 0.51 | 69.05 ± 5.11 | 29.47 ± 0.33 | >100 | >100 | >100 | >100 |
| 17 | 8.24 ± 0.26 | 6.64 ± 0.39 | 13.52 ± 2.21 | 65.98 ± 4.48 | 5.85 ± 0.42 | 67.86 ± 0.81 | 32.26 ± 0.25 | 10.56 ± 0.21 | 91.00 ± 1.34 |
| 18 | 8.37 ± 0.51 | 6.99 ± 0.16 | 6.67 ± 0.15 | 46.74 ± 1.19 | 7.92 ± 0.65 | 37.37 ± 0.49 | 29.81 ± 0.35 | >100 | 98.39 ± 0.66 |
| 19 | 62.68 ± 0.76 | 63.90 ± 0.59 | 59.31 ± 2.27 | 60.51 ± 1.84 | 34.98 ± 0.48 | >100 | 54.89 ± 0.33 | 96.79 ± 0.78 | 94.35 ± 0.87 |
| Dox | 0.90 ± 0.11 | 0.62 ± 0.12 | 0.60 ± 0.08 | 0.73 ± 0.10 | 0.53 ± 0.08 | 0.95 ± 0.11 | 0.35 ± 0.09 | 3.1 ± 0.18 | 0.63 ± 0.12 |
| Compound | MDM2 (PDB 5LAV) | PPARγ (PDB 5YCP) |
|---|---|---|
| 1 | −7.263 | −6.273 |
| 2 | −7.781 | −6.538 |
| 3 | −7.202 | −7.399 |
| 4 | −8.934 | −7.212 |
| 5 | −8.522 | −6.234 |
| 6 | −8.480 | −5.868 |
| 7 | −9.528 | −5.861 |
| 8 | −8.621 | −4.434 |
| 9 | −8.243 | −5.952 |
| 10 | −8.408 | −4.789 |
| 11 | −8.694 | −5.755 |
| 12 | −8.638 | −4.520 |
| 13 | −8.427 | −4.449 |
| 14 | −7.280 | −4.745 |
| 15 | −8.141 | −4.411 |
| 16 | −7.964 | −1.728 |
| 17 | −9.720 | −7.861 |
| 18 | −10.160 | −8.513 |
| 19 | −9.423 | −7.978 |
| NUT (Nutlin-3a) | −8.632 | - |
| 8LX (Lobeglitazone) | - | −9.755 |
| Residue (Interaction) | Compound 18 Time of Interaction (%) | 6SK Time of Interaction (%) | Nutlin-3a Time of Interaction (%) |
|---|---|---|---|
| His96 (H-bond, hyd) | 75 | 55 | 27 |
| Leu54 (H-bond, halogen/hydrophobic) | 65 | 85 | 45 |
| Phe55 (halogen/hydrophobic) | 63 | 30 | 10 |
| Leu57 (hydrophobic) | 43 | 80 | 43 |
| Gly58 (H-bond hydrophobic) | 58 | 30 | 11 |
| Tyr100 (hydrophobic, halogen) | 44 | 75 | 15 |
| Compound | Lipinski’s Rules of Five | Toxicity Profile | |||||
|---|---|---|---|---|---|---|---|
| HBA | HBD | M | MLogP | Violation | TClass | LD50 | |
| 1 | 3 | 1 | 360.81 | 2.17 | 0 | IV | 693 |
| 2 | 2 | 1 | 365.23 | 3.00 | 0 | IV | 1098 |
| 3 | 2 | 1 | 454.14 | 3.24 | 0 | IV | 1313 |
| 4 | 3 | 1 | 328.36 | 2.62 | 0 | IV | 1313 |
| 5 | 2 | 1 | 344.82 | 2.73 | 0 | IV | 1098 |
| 6 | 3 | 1 | 474.96 | 3.75 | 0 | IV | 1600 |
| 7 | 3 | 1 | 483.37 | 3.89 | 0 | IV | 1098 |
| 8 | 4 | 1 | 534.03 | 2.87 | 1 | IV | 1600 |
| 9 | 3 | 1 | 497.39 | 4.09 | 0 | IV | 1600 |
| 10 | 3 | 1 | 538.44 | 3.67 | 1 | IV | 1600 |
| 11 | 2 | 1 | 487.79 | 4.72 | 0 | IV | 693 |
| 12 | 4 | 1 | 526.01 | 3.39 | 1 | IV | 1600 |
| 13 | 3 | 1 | 530.42 | 4.29 | 1 | IV | 693 |
| 14 | 3 | 1 | 586.30 | 4.29 | 1 | IV | 1000 |
| 15 | 2 | 1 | 509.45 | 5.04 | 2 | IV | 693 |
| 16 | 2 | 1 | 598.35 | 5.24 | 2 | IV | 1600 |
| 17 | 2 | 1 | 489.02 | 4.78 | 1 | IV | 1098 |
| 18 | 3 | 1 | 472.57 | 4.68 | 0 | IV | 1024 |
| 19 | 4 | 1 | 460.52 | 3.72 | 0 | IV | 1313 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khylyuk, D.; Holota, S.; Finiuk, N.; Stoika, R.; Rumynska, T.; Lesyk, R. Design and Optimization of Spiro-Isatin-Thiazolidinone Hybrids with Promising Anticancer Activity. Pharmaceuticals 2025, 18, 1502. https://doi.org/10.3390/ph18101502
Khylyuk D, Holota S, Finiuk N, Stoika R, Rumynska T, Lesyk R. Design and Optimization of Spiro-Isatin-Thiazolidinone Hybrids with Promising Anticancer Activity. Pharmaceuticals. 2025; 18(10):1502. https://doi.org/10.3390/ph18101502
Chicago/Turabian StyleKhylyuk, Dmytro, Serhii Holota, Natalia Finiuk, Rostyslav Stoika, Tetyana Rumynska, and Roman Lesyk. 2025. "Design and Optimization of Spiro-Isatin-Thiazolidinone Hybrids with Promising Anticancer Activity" Pharmaceuticals 18, no. 10: 1502. https://doi.org/10.3390/ph18101502
APA StyleKhylyuk, D., Holota, S., Finiuk, N., Stoika, R., Rumynska, T., & Lesyk, R. (2025). Design and Optimization of Spiro-Isatin-Thiazolidinone Hybrids with Promising Anticancer Activity. Pharmaceuticals, 18(10), 1502. https://doi.org/10.3390/ph18101502










