Abstract
Background: Immune checkpoint inhibitors (ICIs) are effective against various advanced and metastatic cancers, but patient responses vary and can change over time, complicating treatment prediction. Therefore, better tools for patient stratification, response prediction, and response assessment are needed. This study presents the development and clinical translation of a fluorescently labelled ICI tracer pair used to perform multispectral fluorescent molecular imaging and simultaneously gain spatial and temporal insight in both programmed death ligand 1 (PD-L1) and programmed death receptor 1 (PD-1) expression. Methods: We conjugated the anti-PD-L1 antibody durvalumab to IRDye 680LT and the anti-PD-1 antibody nivolumab to IRDye 800CW. Tracers were developed and optimized for conjugation efficiency and purity to allow use in clinical trials. Stability was tested up to 12 months. An extended single-dose toxicity study in mice was performed for durvalumab-680LT and the unconjugated IRDye 680LT to demonstrate safety for first-in-human administration. Results: Durvalumab-680LT and nivolumab-800CW were successfully conjugated and purified. Conjugation optimization resulted in a robust production with labelling efficiencies of ≥88%. Long-term stability study of both tracers showed all parameters within end of shelf-life specifications for at least 12 months at 2–8 °C. No toxic effects were observed in doses up to 1000x the intended human dose for both IRDye 680LT and durvalumab-680LT, which are therefore considered safe for first-in-human use. Conclusions: We succeeded in the development and clinical translation of two novel fluorescent ICI tracers, durvalumab-680LT and nivolumab-800CW. Moreover, we demonstrated for the first time the safety of IRDye 680LT and durvalumab-680LT, enabling first-in-human use. Together, this makes durvalumab-680LT and nivolumab-800CW suitable for phase I/II clinical trials.
1. Introduction
Immune checkpoints play an essential part in the regulation of the immune system, protecting tissue from collateral damage during inflammation and preventing auto-immune reactions. One protein involved in immune checkpoint signalling is the programmed death receptor 1 (PD-1), which is expressed on activated T-cells. Binding of PD-1 to its ligand, programmed death receptor ligand 1 (PD-L1), results in the downregulation of the T-cell response and inhibition of the immune response. However, many cancer types exploit this response by upregulation of PD-L1 expression on tumour cells, avoiding T-cell mediated anti-tumour responses. This mechanism of immune evasion is targeted by treatment regimens that include immune checkpoint inhibitor antibodies (ICIs) targeting PD-1 or PD-L1, such as nivolumab and durvalumab [1,2,3,4].
Durvalumab and nivolumab have both been registered by the European Medicines Agency. After initial registration, the indications for these ICIs have been expanded, and recent studies have shown their efficacy in several forms of advanced and metastatic cancers, primarily non-small cell lung carcinoma (NSCLC) and urothelial carcinoma for durvalumab and NSCLC, renal cell carcinoma, Hodgkin lymphoma, urothelial carcinoma, and squamous cell carcinoma of the head and neck for nivolumab. Both ICIs have also been shown to provide a survival advantage over and generally fewer high-grade adverse events when compared to conventional chemotherapies in urothelial cell carcinoma and NSCLC [5,6,7]. However, response rates of these ICIs have been shown to vary between patients (10–70%, depending on type of disease) and can change over time, making it challenging to predict which patients will benefit from treatment [8,9,10,11]. Therefore, better tools for patient stratification, early response prediction, and response assessment are desirable to be able to select patients likely to benefit from ICI therapy. This may improve the effectiveness of cancer treatment, prevent unnecessary treatment, limit unwanted side-effects, and reduce medical costs [12].
Currently, patient selection for ICI therapy is performed mainly by the assessment of PD-L1 expression by immunohistochemistry (IHC) in biopsy specimens. However, accuracy is low due to several factors, including the size of the tissue specimens and heterogeneity of the tumour. This can lead to PD-L1 status misclassification in up to 52% of cases, which can be an issue for the administration of certain ICIs, as a positive IHC test is required for some indications like NSCLC [13,14,15]. Moreover, not all patients with high PD-L1-expressing tumours (on IHC) showed benefits from checkpoint inhibition therapy, while conversely, patients with negative PD-L1 expression showed response to anti PD-L1 treatment in several cases [16,17]. In contrast, small studies have shown that tumour uptake of 89Zr-atezolizumab, 89Zr-nivolumab, and 89Zr-pembrolizumab provided a more consistent positive correlation between tracer uptake and therapy response [18,19,20,21] when compared to IHC tissue staining. However, positron emission tomography (PET) imaging modalities have limitations, such as poor spatiotemporal resolutions and the inability for real-time imaging during endoscopy or surgery.
In contrast, fluorescent molecular imaging (FMI) can be used for real-time identification of tumours and tumour margins in oncologic surgery and endoscopy. This has previously been demonstrated in surgical interventions for, among others, breast cancer, peritoneal carcinomatosis, squamous cell head and neck carcinoma, and oesophageal adenocarcinoma [22,23,24,25]. Contrary to their radiolabelled counterparts, tissue samples of patients who have received fluorescent tracers can be stored after the procedure and can be analysed long after the procedure using ex vivo camera systems or fluorescence flatbed scanners, while fluorescent microscopy can be used to identify the cell types targeted by the tracer [26].
We hypothesize that FMI can also be performed using fluorescently labelled ICI antibodies, enabling real-time prediction and evaluation of ICI therapy response. This is a different approach to FMI than used in previous oncologic FMI studies with solid tumours and their markers, where tumour identification, margin assessment, and surgical resection were the main goals. We aim to achieve this novel approach to FMI by performing multispectral fluorescent imaging using two antibodies, one targeting PD-1 and the other targeting PD-L1, each conjugated to different near-infrared dyes with a distinct fluorescent spectrum; IRDye 800CW, the main dye used up until now, and the novel IRDye 680LT. This combination of dyes enables multispectral imaging due to their distinctive absorbance and emission spectra. IRDye 800CW has an absorption maximum at 774 nm and an emission maximum at 789 nm in PBS, whereas IRDye 680LT has an absorption maximum at 676 nm and an emission maximum at 693 nm in PBS. Multispectral imaging will be further enhanced by recent technological advancements in needle-based ultrasound-guided quantitative fluorescence molecular imaging, enabling fluorescence measurements in the whole body [27]. With this pair of tracers and this new technology, we aim to visualize both immune cells and tumour cells at both sides of the immune checkpoint pathway at the same time, getting more mechanistic insight into drug distribution of both therapeutic antibodies, the penetration of effector cells into the tumour microenvironment and interactions at a molecular immunological level within the tumour.
In this paper we describe the development process and clinical translation of such a tracer pair, consisting of durvalumab, conjugated to IRDye 680LT, and nivolumab, conjugated to IRDye 800CW. Moreover, we show the results of an extended single-dose toxicity study for both the unconjugated IRDye 680LT and the antibody–dye conjugate durvalumab-680LT, which have not seen in-human use previously.
2. Results
2.1. Conjugation, SE-HPLC Characterization, and ELISA
We successfully conjugated both nivolumab and durvalumab to both dyes. Fluorescence was confirmed at, respectively, 775 nm and 676 nm for the main protein peaks on the size-exclusion high-performance liquid chromatography (SE-HPLC) chromatograms for all tracers. SE-HPLC chromatograms at 280 nm were comparable to unconjugated antibody standard with regards to peak shape and retention time (Figure 1A, data only shown for durvalumab-680LT and nivolumab-800CW). Yield was ≥90% and label efficiency ≥77% for all tracers (Table 1). After purification of the labelled protein, aggregates were not detected and unconjugated dye was less than 0.50% for all tracers, except for nivolumab-680LT. As shown in Table 1, results for durvalumab-680LT and nivolumab-800CW were superior to their respective counterparts. Therefore, labelling optimization was performed only for durvalumab-680LT and nivolumab-800CW.
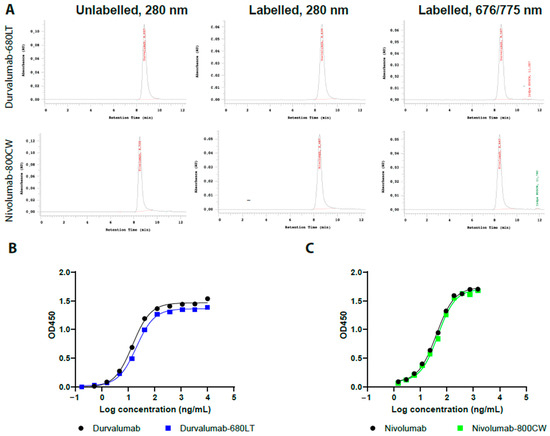
Figure 1.
(A) Representative size-exclusion high-performance liquid chromatography (SE-HPLC) chromatograms of durvalumab-680LT and nivolumab-800CW. First column represents both unconjugated antibodies at 280 nm. Here, the main peak of the antibody can be seen around the 9 min mark. Second column represents both antibodies conjugated to their respective dye at 280 nm. Here the main peak of the antibody–dye conjugate can be seen around the 9 min mark, whilst any aggregates would be visible before the main peak. Third column represents durvalumab-680LT at 676 nm and nivolumab-800CW at 775 nm, confirming successful conjugation of the dye to the protein. Here, the main peak of the antibody can be seen around the 9 min mark, whilst any aggregates would be visible before the main peak. Peaks for the free dye can be seen around the 12 min mark. Representative results of the indirect ELISA for (B) durvalumab-680LT and (C) nivolumab-800CW. (A–C) are single measurements, so no statistics were performed.

Table 1.
Initial labelling results for durvalumab-800CW, durvalumab-680LT, nivolumab-800CW, and nivolumab-680LT, comprised of three different labelling experiments per tracer. Mean ± standard deviation is shown where applicable.
An indirect enzyme-linked immunosorbent assay (ELISA) was set up for both of these tracers to determine the binding affinity of durvalumab-680LT and nivolumab-800CW to their respective target. Figure 1B–C shows initial results as sigmoid curves of both tracers with their respective standard, the unmodified antibodies.
2.2. Process and Labelling Optimization
After initial labelling experiments, optimization of the labelling process was performed for durvalumab-680LT and nivolumab-800CW to start translation for clinical use. Experiments were performed at each step of the conjugation process to create a robust production process resulting in a stable tracer.
Labelling efficiency of durvalumab-680LT without purification resulted in half of the labelling efficiency with purification (38% versus 84%, respectively). Durvalumab was therefore purified before conjugation, as the original buffer contains interfering components, primarily amino acids [28] (Table S1). No effect on the labelling efficiency was found with or without purification before the labelling, as the product buffer for nivolumab does not contain interfering components [29] (Table S1). However, nivolumab was purified before labelling to ensure optimal labelling conditions. For both durvalumab-680LT and nivolumab-800CW, peak label efficiency was achieved after 20 min, which stayed stable for the full labelling period of 120 min (Figure 2A). To ensure complete labelling and to account for analysis time, labelling time was set to 60–120 min.
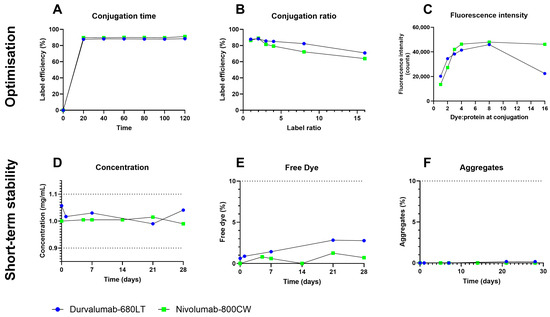
Figure 2.
Durvalumab-680LT and nivolumab-800CW conjugation optimization and small-scale stability. Optimization of the production process was achieved by investigating the optimum in conjugation time (A), the label efficiency at increasing amounts of dye per antibody (B), and the resulting fluorescent intensity of the signal after conjugation of higher amounts of dye (C). Optimized tracers were tested for preliminary stability by determining concentration of the tracer monomer (D) and presence of primary impurities-free IRDye (E) and antibody aggregates (F). Dotted lines in panels (D–F) indicate acceptance limits during stability study. All optimization experiments were only repeated once; therefore, no statistics were performed here.
The chromatography at higher label ratios yielded broader peaks as the label ratio increased. Label efficiency peaked at a molar dye to protein ratio of 2:1 for both tracers, after which it declined (Figure 2B). Free dye and aggregates also increased at labelling ratios higher than 2:1 (Tables S2 and S3). The fluorescence gained by higher label ratios also rapidly declined relative to the amount of dye conjugated. Fluorescence even began to diminish when labelled at a ratio of 16:1 (Figure 2C). Therefore, to ensure sufficient fluorescence in vivo, but also ensure long term stability, the molar dye to protein ratio of 2:1 was chosen for both tracers.
Using the described labelling process, a buffer panel and small-scale stability study were performed for both tracers. Stability results for 28 days for the optimal buffer for both tracers, a 50 mM sodium phosphate buffer of pH 7.0, are shown in Figure 2D–F. For both tracers, all parameters stayed within end of shelf-life specifications for at the duration of the stability study, with concentrations between 0.99 and 1.06 mg/mL for durvalumab-680LT and 0.99 to 1.02 mg/mL for nivolumab-800CW. Percentages of free dye stayed between 0.6 and 2.8% for durvalumab-680LT and 0 and 1.3% for nivolumab-800CW. No aggregate formation was observed during the stability study of nivolumab-800CW, while aggregates stayed between 0 and 0.2% for durvalumab-680LT. Results for the other tested buffers are shown in Figures S1 and S2.
2.3. cGMP Production, Validation, and Long-Term Stability
For both tracers, a validation batch at scale for clinical trial manufacturing was successfully produced in our current good manufacturing practice (cGMP) facility. The produced vials were all included into a long-term stability study at 2–8 °C and 15–25 °C. Table 2 provides the product specifications at release and at end of shelf life and their methods for testing. Figure 3 shows the results obtained after the first 12 months of the stability study for both tracers at 2–8 °C. Complete quality control (QC) data can be found in Tables S4 and S5. The protein concentration, the percentage of free dye and aggregates, and the target binding affinity complied with the release specifications and remained within the end of shelf-life specification for both products during the first 12 months of the stability study. Concentration after production was 1.03 ± 0.01 mg/mL for durvalumab-680LT and was 1.07 ± 0.01 mg/mL after 12 months of stability testing, with a high of 1.09 ± 0.006 mg/mL after 3 months and low of 1.01 ± 0.01 mg/mL after 6 months. Percentage of free dye was 1.3 ± 0.1% on release for durvalumab-680LT and was 1.7 ± 0.06% after 12 months of stability testing, with a high at 6 months of 1.9 ± 0.1%. No measurement was lower during the stability study than the first measurement of free dye. No aggregate formation was observed during the stability study of durvalumab-680LT. Target binding affinity stayed between 67 and 98% for durvalumab-680LT. Concentration after production was 0.98 ± 0.003 mg/mL for nivoumab-800CW and was 0.97 ± 0 mg/mL after 12 months of stability testing, with a high of 1.01 ± 0.006 mg/mL after 3 months and low of 0.97 ± 0.003 mg/mL after 6 months. Percentage of free dye was 0.4 ± 0.09% on release for nivolumab-800CW and was 2.6 ± 0.15% after 12 months of stability testing. All measurements stayed within this range for the free dye. Aggregate formation was only observed for nivolumab-800CW after 12 months of stability testing with 0.7 ± 0.15% of aggregates. Target binding affinity stayed between 60 and 92% for nivolumab-800CW. Consistently, all other QC tests stayed within the end of shelf-life specifications for both tracers.

Table 2.
Product specifications for durvalumab-680LT and nivolumab-800CW (both 1.0 mg/mL, 5.0 mL) at release and end of shelf life.
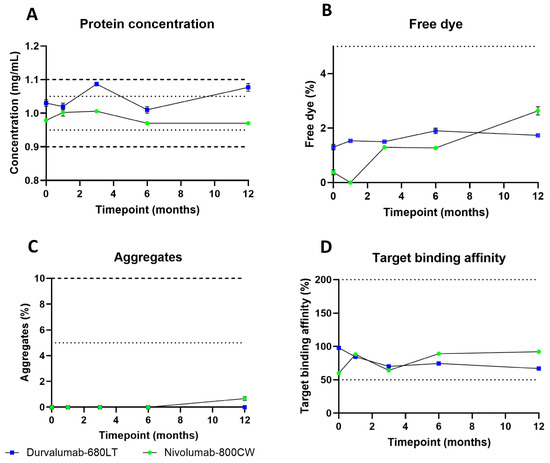
Figure 3.
Stability results of durvalumab-680LT and nivolumab-800CW at 2–8 °C. Release specifications are displayed with dotted lines and end of shelf-life specifications are displayed with dashed lines. (A) Protein concentration, (B) percentage of free dye, (C) percentage of aggregates, and (D) target binding affinity of both tracers. (A–C) are means ± standard deviation of three different measurements, and in (D) mean is reported of two measurements.
Stability data obtained during the first 12 months at 15–25 °C are presented in Figure S3, while complete QC data can be found in Tables S6 and S7. Both tracers also stayed within end of shelf-life specifications when stored at room temperature. Concentration after production was 1.03 ± 0.01 mg/mL for durvalumab-680LT and was 1.04 ± 0.006 mg/mL after 12 months of stability testing, with a high of 1.06 ± 0 mg/mL after 3 months and low of 1.01 ± 0.01 mg/mL after 6 months. Percentage of free dye was 1.3 ± 0.1% on release for durvalumab-680LT and was 1.9 ± 0.006% after 12 months of stability testing, with a high at 6 months of 2.1 ± 0.06%. No measurement was lower during the stability study than the first measurement of free dye. No aggregate formation was observed during the stability study of durvalumab-680LT. Target binding affinity stayed between 63 and 98% for durvalumab-680LT. Concentration after production was 0.98 ± 0.003 mg/mL for nivoumab-800CW and was 0.95 ± 0 mg/mL after 12 months of stability testing, with a high of 1.00 ± 0.006 mg/mL after 3 months and low of 0.97 ± 0.0006 mg/mL after 6 months. Percentage of free dye was 0.4 ± 0.09% on release for nivolumab-800CW and was 4.3 ± 0.06% after 12 months of stability testing. A lows was found after 1 month of 0.3 ± 0.1%. No measurement was lower during the stability study than the last measurement of free dye. Aggregate formation was only observed for nivolumab-800CW after 12 months of stability testing with 2.2 ± 0.06% of aggregates. Target binding affinity stayed between 60 and 89% for nivolumab-800CW. All other QC tests also stayed within the end of shelf-life specifications at 15–25 °C. This demonstrates the stability of both durvalumab-680LT and nivolumab-800CW for 12 months at both temperature ranges.
2.4. Toxicity Study
Single-dose intravenous administration of the tracer durvalumab-680LT to mice, at the doses of 9 mg/kg and 90 mg/kg, and IRDye 680LT, at the doses of 86 µg/kg and 860 µg/kg, did not result in mortalities. Body weight and food consumption were unaffected by the treatment at all doses tested for both dye and tracer (Tables S8 and S9). There were no changes related to the tracer or dye in the clinical pathology parameters, haematology, coagulation, clinical chemistry, urinalysis, terminal fasting body weights, organ weights, and organ weight to weight ratios (Tables S10 and S11). Grossly, bilateral blue discoloration of iliac lymph node and blue discoloration at injection site were observed at 90 mg/kg durvalumab-680LT in both sexes on day 2 and 15 without histopathologic correlation. The few incidences of inflammatory cell infiltration at the site of injection in control and treatment groups were considered as procedure-related and not related to test item administration.
3. Discussion
We successfully developed and produced durvalumab-680LT and nivolumab-800CW according to cGMP. To our knowledge this is the first time ICIs have been labelled with fluorescent dyes for first-in-human use in phase I/II clinical trials. Both tracers can now be combined in these trials, enabling multispectral fluorescent molecular imaging of the PD-1/PD-L1 axis. This is unique and of particular interest, as this is a novel approach to FMI, serving as a potential tool to overcome persistent challenges in ICI treatment. In this paper we describe the development and clinical translation of durvalumab-680LT and nivolumab-800CW.
Both tracers were developed using the “roadmap for development and clinical translation of 800CW optical tracers”, set up previously by our group [30]. Durvalumab was successfully conjugated to IRDye 680LT, whereas nivolumab was successfully conjugated to IRDye 800CW. Labelling optimization yielded parameters similar to previously developed tracers, including labelling time, label ratio, and used buffers, indicating that different antibodies display similar optimal labelling conditions and parameters [30,31,32]. Validation batches produced in our cGMP facility remained within all set specifications for 12 months at both 2–8 °C as 15–25 °C, indicating that the production process can be used to produce a stable tracer product fit for clinical production and subsequently for use in phase I/II clinical trials. Stability studies are ongoing and will collect data at 2–8 °C for up to 24 months to allow tracer use over a long shelf-life period.
Several fluorescent tracers have been developed previously, including bevacizumab-800CW, cetuximab-800CW, panitimumab-800CW, and vedolizumab-800CW; however, these tracers were all based on a therapeutic antibody conjugated with IRDye 800CW [30,31,32,33,34,35]. To enable multispectral fluorescent imaging of the PD-1/PD-L1 axis, we aimed to use IRDye 680LT in addition to IRDye 800CW. IRDye 800CW safety has been previously demonstrated, both unconjugated and conjugated to bevacizumab [31,36]. Moreover, multiple clinical trials have been performed with antibodies conjugated to this dye [25,34,37,38,39]. Therefore, no additional safety testing was performed for nivolumab-800CW. In contrast, IRDye 680LT has never been used in humans. Therefore, an animal toxicity study in CD-1 mice was included in the development of durvalumab-680LT, assessing both the toxicity of IRDye 680LT and IRDye 680LT conjugated to durvalumab. Study results showed no observed toxic effects from the conjugated antibody or the dye, similar to the previously performed toxicity studies for 800CW-based tracers [31,36], supporting the safe use of IRDye 680LT in humans. This work thus opens up possibilities for conjugation of IRDye 680LT to other antibodies or molecules to image new targets of interest using IRDye 680LT or multispectral imaging.
Several optical tracers are already being used in a clinical trial setting, which have proven to be able to distinguish tumour tissue from normal tissue [25,34,37,38,39]. However, no fluorescent ICI tracers have been previously used in clinical trials, making durvalumab-680LT and nivolumab-800CW the first ones to be used. In contrast, the PET-tracers 89Zr-atezolizumab, 89Zr-nivolumab, and 89Zr-pembrolizumab have been used before in clinical studies to assess ICI therapy response. For these tracers, correlations were found between tracer uptake in the tumour pretreatment and both response to therapy and (progression-free) survival [18,19,20]. In addition, a one-armed antibody targeting CD8 has also been labelled with 89Zr to characterize the dynamics of CD8+ T cells in the context of ICI treatment [40]. As CD8+ T-cell proliferation at baseline in the tumour area is associated with increased ICI therapy response and tumour reduction, these radiolabelled tracers are a great addition for patient stratification already. Moreover, fluorescent imaging can be a great complementary technique, especially when using multispectral imaging [41,42,43]. Whereas PET imaging can cover the whole body and has a high penetration depth, fluorescence imaging provides high-resolution and real-time visualization of the tumour in vivo during surgery or endoscopy, limiting the impact of the lower penetration depth of fluorescence imaging. Moreover, since fluorescent tracers do not have a radioactive half-life, ex vivo analysis can be performed to get a more mechanistic insight into drug distribution of the antibodies, yielding insight into the heterogeneity of the tumour on a cellular level, the penetration of effector cells into the tumour microenvironment and interactions at a molecular immunological level within the tumour [44,45]. Multispectral imaging can further enhance these insights and could give additional information on interactions between targets. Together with novel techniques like needle-based ultrasound guided quantitative fluorescence molecular imaging, these results can be linked to the immune composition of the tumour microenvironment giving additional insights into tumour heterogeneity and can thereby potentially help to better understand why certain patients respond to ICI therapy and others do not. This would potentially improve patient stratification, as well as enable treatment response assessment and response prediction.
Durvalumab-680LT is currently being used in a first-in-human study (NCT05450484) at the University Medical Center Groningen to assess the tumour heterogeneity of PD-L1 expression in oesophageal cancer before and after neoadjuvant chemoradiotherapy. Nivolumab-800CW is currently being prepared for use in a clinical trial setting, where it will be combined with durvalumab-680LT, enabling multispectral imaging of the immune checkpoint pathway in oesophageal cancer. Moreover, durvalumab-680LT and nivolumab-800CW will be combined for multispectral imaging in locally advanced rectal cancer (NCT06304597). Depending on the results of these first clinical studies, this multispectral imaging approach using durvalumab-680LT and nivolumab-800CW can be investigated in several forms of advanced and metastatic cancers.
4. Materials and Methods
4.1. Labelling Procedure
For the development of durvalumab-680LT we used the registered drug product Imfinzi® (AstraZeneca, Cambridge, UK) and for the development of nivolumab-800CW we used the registered drug product Opdivo® (Bristol-Myers Squibb, New York, NY, USA). As an initial test, both nivolumab and durvalumab were labelled with both IRDye 800CW and IRDye 680LT (both LI-COR biosciences Lincoln, NE, USA), yielding durvalumab-800CW, durvalumab-680LT, nivolumab-800CW, and nivolumab-680LT. Tracer production procedures were derived from the development procedures of bevacizumab-800CW, cetuximab-800CW, and trastuzumab-800CW [30,31]. Briefly, durvalumab or nivolumab were buffer exchanged to labelling buffer, a 50 mM sodium phosphate buffer pH 8.5 (Apotheek A15, Gorinchem, The Netherlands), using PD-10 desalting columns (Cytiva lifesciences, Chicago, IL, USA), which were pre-equilibrated with labelling buffer. Afterwards, IRDye 800CW or IRDye 680LT, both dissolved in DMSO (Merck, Darmstadt, Germany), were added to the protein solution in a molar dye to protein ratio of 2:1. The solution was mixed gently and left to incubate protected from light for 1–2 h at room temperature. After incubation, the solution was purified from any unconjugated dye by buffer exchange on PD-10 desalting columns using the optimal formulation buffer: a 50 mM sodium phosphate buffer at pH 7.0 (Apotheek A15, Gorinchem, The Netherlands). Afterwards, the tracer solution was diluted with the same phosphate buffer to a concentration of 1.0 mg/mL.
The tracers were analysed several times during the production process using SE-HPLC to assess the critical process parameters and results of conjugation. At the end of the conjugation reaction, the percentage of dye conjugated to the antibody relative to the total dye added, the label efficiency, was determined. After purification, a second analysis was performed to determine the tracer concentration, which was used to dilute the tracer to the target concentration of 1.0 mg/mL. Afterwards a final test was performed to determine concentration and the percentage of free unconjugated dye and protein aggregates, as well as tracer identity and integrity and yield of the tracer.
4.2. Labelling Optimization
Based on the initial labelling results, optimization of the labelling process was performed for durvalumab-680LT and nivolumab-800CW.
First, the influence of amino acids present in the original formulation of the antibodies on the labelling process was investigated. Labelling with a buffer exchange into a 50 mM sodium phosphate buffer pH 8.5, using PD-10 desalting columns pre-equilibrated with the same buffer, was compared to labelling without buffer exchange.
Second, the incubation time of the antibody–dye solution was assessed. Standard labelling protocol was followed. The beginning of the labelling process was the addition of the dye to the antibody solutions. Samples were taken at regular intervals of 20 min up to 120 min. LE was measured at all time points.
Third, the optimal molar label ratio between the antibody and the dye was assessed. Both tracers were labelled with ratios of 1:1, 2:1, 4:1, 8:1, and 16:1 (dye to protein) following standard labelling protocol. LE, protein concentration, and the percentage of aggregates and free dye were measured. Moreover, the fluorescence and absorbance for all label ratios was measured using a BioTek Synergy H4 plate reader (Winooski, VT, USA). Two concentrations of the tracer, 0.5 mg/mL and 0.1 mg/mL were added to a 96-well plate for all label ratios. Absorption spectra were measured in a range of 230 to 850 nm, whilst fluorescence intensity was measured with λex of 700 nm and λem of 789 nm for the IRDye 800CW and with λex of 632 nm and λem of 676 nm for the IRDye 680LT. Data were corrected for blank (unconjugated) antibody intensity.
Fourth, eight buffers for formulation of the final products were assessed by formulating both tracers in all different buffers after labelling following standard protocol. The composition of the different buffers, as well as the original buffers of both durvalumab and nivolumab, can be found in Table S1. A buffer panel and a small-scale stability study (28 days) were set up for both tracers for all buffers. The formulation buffers tested were modified sodium phosphate buffers, partially based on the original formulation buffer of the antibodies.
4.3. cGMP Production, Validation, and Long-Term Stability
Using the optimized labelling conditions, the production process was translated to our cGMP facility to produce a full-scale clinical batch of both tracers. Before the first clinical production, a validation batch was produced, validating the production process and finalizing the product specifications before production of the first clinical batch. The produced vials were all used for a long-term stability study at two different temperatures according to the ICH Q1A guideline: 2–8 °C (refrigerated) for 24 months and 15–25 °C (room temperature) for 12 months. Stability time points and performed tests can be found in Table S12.
cGMP production of durvalumab-680LT and nivolumab-800CW consisted of conjugation, purification, and sterile filtration. Briefly, durvalumab or nivolumab was buffer exchanged to labelling buffer, a 50 mM sodium phosphate buffer pH 8.5 using PD-10 desalting columns, which were pre-equilibrated with labelling buffer. Afterwards, cGMP-grade IRDye 800CW (nivolumab) or cGMP-grade IRDye 680LT (durvalumab), both dissolved in DMSO, was added to the protein solution in a molar dye to a protein ratio of 2:1. The solution was mixed gently and left to incubate protected from light for 1–2 h at room temperature. After incubation, the solution was purified from any unconjugated dye by buffer exchange on PD-10 desalting columns using the optimal formulation buffer: a 50 mM sodium phosphate buffer at pH 7.0 (Apotheek A15, Gorinchem, The Netherlands). Afterwards, the tracer solution was diluted with the same phosphate buffer to a concentration of 1.0 mg/mL. Subsequently, the solution was filtered over a sterile 0.2 µm filter (Sartopore® 2 gamma filter capsule), which was connected to a Flexboy® bag (3 L, Sartorius Stedim, Göttingen, Germany). Next, aseptic filling over a sterile 0.2 µm filter (Millex GP, Merck KGaA, Darmstadt, Germany) into 10R vials (APG Pharma, Uithoorn, The Netherlands) was performed, after which vials were closed using a 20 mm bromobutyl rubber stopper (Datwyler, Altdorf, Switzerland) and 20 mm aluminium closure (Datwyler, Altdorf, Switzerland). Closures were crimped using a compressed air-powered semi-automatic crimping tool.
A schematic overview of the whole development and optimization process, including the long-term stability testing and all relevant QC tests, is displayed in Figure 4.
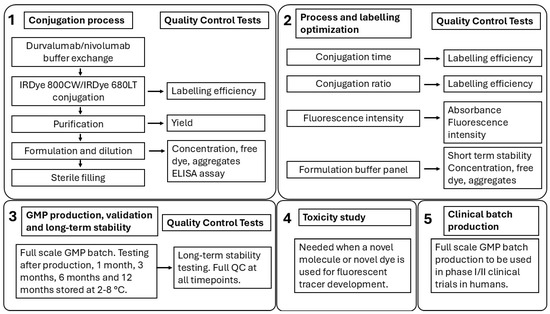
Figure 4.
A schematic overview of the development and optimization process of fluorescent tracer development with all relevant QC tests.
4.4. Quality Control Testing
Product specifications of durvalumab-680LT and nivolumab-800CW at release and at end of shelf life are presented in Table 2. In addition to pharmacopeial specifications, specific product specifications are based on development and optimization data. All specifications are identical for both tracers, except for the tests that involve the absorption spectrum of the specific dye.
The tracers were analysed using SE-HPLC as described previously [30]. During analysis, the absorption spectrum was measured from 200 to 900 nm. Using this absorption spectrum, HPLC analysis for the IRDye 800CW and IRDye 680LT was performed. The absorbance spectra of peaks from conjugated antibody and unconjugated dye were analysed to determine the absorption peak of the dye before and after conjugation. For protein measurements, peaks were analysed at 280 nm. A wavelength of 780 nm was used for the analysis of IRDye 800CW conjugated to antibodies and 775 nm was used for the analysis of unconjugated IRDye 800CW. For IRDye 680LT, 679 nm was used for dye conjugated to an antibody and 676 nm was used to determine the unconjugated dye.
Target affinity was assessed by an in-house developed indirect ELISA. ELISA set-up and design was performed as described in previous protocols [30]. Individual assay development and optimization was performed for both nivolumab-800CW and durvalumab-680LT. Briefly, a 96-well plate was coated overnight with recombinant human PD-L1 (Sino Biologicals Europe GmbH, Eschborn, Germany) (0.5 µg/mL) at 2–8 °C for durvalumab-680LT or recombinant human PD-1 (Sino Biologicals Europe GmbH, Eschborn, Germany) (0.5 µg/mL) at 2–8 °C for nivolumab-800CW. The next day, the plate was blocked with 1% bovine serum albumin (VWR international BV, Amsterdam, The Netherlands). Dilution series of both the commercially available durvalumab and durvalumab-680LT (10 µg/mL to 10 ng/mL) or of both the commercially available nivolumab and nivolumab-800CW (1.5 µg/mL to 1.5 ng/mL) were made and incubated on the plate in duplicate. Bound antibody was detected using an anti-human IgG secondary antibody labelled with horseradish peroxidase (HRP) (Jackson Immunoresearch laboratories, West Grove, PA, USA), specific against the Fab part of human IgG. Signal was generated by addition of 3,3′,5,5′-tetramethylbenzidine (TMB, Thermo Fisher, Waltham, MA, USA) and the reaction was stopped with 2 M sulfuric acid. TMB absorption was read at 450 nm. A four-parameter logistic regression was performed on the data to fit a sigmoidal curve. From the sigmoidal fit, the EC50 value was calculated. Target affinity was calculated by comparing the EC50 of the tracer and the reference commercially available antibody, yielding the relative binding affinity of the tracer.
The general appearance, turbidity, visible particles, and colour of both tracers were assessed visually. Bacterial endotoxins, bioburden, residual solvents (DMSO), osmolality, pH, and sub-visible particles were tested according to their respective compendial Ph. Eur. monographs [46].
For both tracers, ELISA and HPLC methods were validated according to ICH Q2 guidelines. All other tests were validated according to their respective Ph. Eur. monographs [46].
4.5. Toxicity Study
An extended single-dose toxicity study in CD-1 mice was conducted according to the ICH M3 R2 guidelines. Moreover, in accordance with FDA guideline exploratory IND studies, a low dose (100× intended clinical study dose, based on microdosing of 30 nmol) and a high dose (1000× intended clinical study dose) of both the tracer durvalumab-680LT and the dye IRDye 680LT were administered. This corresponds with a single intravenous bolus injection of durvalumab-680LT (9 or 90 mg/kg body weight) or IRDye 680LT (86 or 860 µg/kg body weight). The study was conducted by Eurofins Advinus Ltd. (Bengaluru, India). The study protocol was reviewed and approved by the Institutional Animal Ethics Committee (IAEC), which is equivalent to Institutional Animal Care and Use Committee (IACUC). All procedures were in compliance with the guidelines issued by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
This toxicity study was set up to assess the toxic potential of both durvalumab-680LT and unconjugated IRDye 680LT when administered as a single intravenous dose to CD-1 mice and to study the reversibility of possible effects. No cross-reactivity between durvalumab and mouse PD-L1 was expected [47]. The mice, CRL:CD-1 (ICR), were obtained from HyLasco Biotechnology (India) and breeders were originally obtained from Charles River (Sulzfeld, Germany). At the commencement of treatment, on average, males weighed 33.26 g and females 26.82 g. Weight variation of mice did not exceed ±20% of the mean body weight in each sex and group. Females were nulliparous and non-pregnant.
Mice were assigned to groups, as shown in Table 3. Randomization of the mice was performed using a computer-generated algorithm according to body weight. The mice received a single intravenous bolus injection of durvalumab-680LT (9 or 90 mg/kg body weight), IRDye 680LT (86 or 860 µg/kg body weight), or vehicle (saline) via the tail vein. Ten mice of each sex were sacrificed two days after injection; the remaining mice were observed during a two-week follow-up and were sacrificed on day 15. At the time of administration, the animals were approximately 6 weeks old. The mice were observed at least twice daily for morbidity and mortality. Moreover, changes in skin, fur, eyes, mucous membranes, excretion, secretion and autonomic activity, body weight, and food consumption were measured at weekly intervals after administration. After sacrificing of the mice, organ and tissue weight were determined, as well as changes in haematology, clinical chemistry, and histopathology. The ProvantisTM software (Instem, Stone, Sheffordshire, UK) was used to collect all of this data.

Table 3.
Design of the toxicity study for durvalumab-680LT and IRDye 680LT.
4.6. Statistical Analysis
Data captured with ProvantisTM were analysed using built-in statistical analysis. A decision tree was used in analysis. First, test variance homogeneity was tested by Levene’s method. When variances were heterogenous, suitable transformation was performed automatically by the software. Second, further one-way analysis of variance (ANOVA) was performed. If ANOVA was significant, Dunnett’s vehicle control versus treatment group mean comparison was performed. Thirdly, for two groups, the comparison of mean between treatment and control group was performed using student’s t-test. For SE-HPLC analysis, mean values and standard deviations were calculated unless stated otherwise. For other QC tests, means were calculated where applicable.
5. Conclusions
In conclusion, in this study we developed the fluorescent tracers durvalumab-680LT, targeting PD-L1, and nivolumab-800CW, targeting PD-1, which are suitable for human use and can be applied in phase I/II clinical trials. Moreover, we have shown that both IRDye 680LT and durvalumab-680LT showed no toxic effects and are therefore safe for in-human use. Together, these ICI tracers enable a multispectral imaging approach on the PD-1/PD-L1 axis, giving insight into heterogeneity of the tumour and interactions between targets. Ultimately, this may be a major step in improving ICI patient stratification, enabling better response prediction or response assessment and ultimately improving ICI treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph18101501/s1: Table S1: Composition of different buffers for the buffer panel of durvalumab-680LT and nivolumab-800CW; Table S2: Label ratio results for durvalumab-680LT; Table S3: Label ratio results for nivolumab-800CW; Table S4: Stability results of durvalumab-680LT at storage for 12 months at 2–8 °C; Table S5: Stability results of nivolumab-800CW at storage for 12 months at 2–8 °C; Table S6: Stability results of durvalumab-680LT at storage for 12 months at 15–25 °C; Table S7: Stability results of nivolumab-800CW at storage for 12 months at 15–25 °C; Table S8: Summary of toxicity results of male mice: clinical signs, mean body weight, and food consumption; Table S9: Summary of toxicity results of female mice: clinical signs, mean body weight, and food consumption; Table S10: Clinical parameters of male mice; Table S11: Clinical parameters of female mice; Table S12: Stability testing, timepoints, tests, and both release and shelf-life specifications; Figure S1: Stability results of the buffer panel of durvalumab-680LT at 15–25 °C; Figure S2: Stability results of the buffer panel of nivolumab-800CW at 15–25 °C; Figure S3: Stability results of both durvalumab-680LT and nivolumab-800CW at 15–25 °C.
Author Contributions
Conceptualization, W.B.N. and M.N.L.-d.H.; methodology, D.P.A., B.G., B.G.J.D., W.B.N., and M.N.L.-d.H.; validation, H.K.H., W.T.R.H., and M.D.L.; formal analysis, H.K.H., W.T.R.H., and M.D.L.; investigation, H.K.H., W.T.R.H., and M.D.L.; resources, D.P.A., B.G., B.G.J.D., and M.N.L.-d.H.; writing—original draft preparation, H.K.H., W.T.R.H., and M.D.L.; writing—review and editing, D.P.A., B.G., B.G.J.D., W.B.N., and M.N.L.-d.H.; visualization, H.K.H., W.T.R.H., and M.D.L.; supervision, W.B.N. and M.N.L.-d.H.; project administration, H.K.H., W.T.R.H., and M.D.L.; funding acquisition, W.B.N. and M.N.L.-d.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Innovative Medicines Initiative 2 Joint Undertaking, grant number 831514 (Immune-Image). This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA. The APC was funded by Innovative Medicines Initiative 2 Joint Undertaking (Immune-Image).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Ethics Committee (IAEC) (G21275, May 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors would like to acknowledge P. Koopmans and H.H. Westra for their help in the development and productions of both tracers.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| cGMP | Current good manufacturing practices |
| ELISA | Indirect enzyme-linked immunosorbent assay |
| FMI | Fluorescent molecular imaging |
| HRP | Horseradish peroxidase |
| ICI | Immune checkpoint inhibitor |
| IHC | Immunohistochemistry |
| LE | Label efficiency |
| NSCLC | Non-small cell lung carcinoma |
| PD-1 | Programmed death receptor 1 |
| PD-L1 | Programmed death ligand 1 |
| PET | Positron emission tomography |
| SE-HPLC | Size-exclusion high-performance liquid chromatography |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
References
- Lin, E.M.; Gong, J.; Klempner, S.J.; Chao, J. Advances in Immuno-Oncology Biomarkers for Gastroesophageal Cancer: Programmed Death Ligand 1, Microsatellite Instability, and Beyond. World J. Gastroenterol. 2018, 24, 2686–2697. [Google Scholar] [CrossRef]
- Koemans, W.J.; Chalabi, M.; van Sandick, J.W.; van Dieren, J.M.; Kodach, L.L. Beyond the PD-L1 Horizon: In Search for a Good Biomarker to Predict Success of Immunotherapy in Gastric and Esophageal Adenocarcinoma. Cancer Lett. 2019, 442, 279–286. [Google Scholar] [CrossRef]
- Cogdill, A.P.; Andrews, M.C.; Wargo, J.A. Hallmarks of Response to Immune Checkpoint Blockade. Br. J. Cancer 2017, 117, 1–7. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, Q.; Luo, X.S.; Yang, X.W.; Wang, H.D.; Guo, C.Y. Efficacy of PD-1/PD-L1 Inhibitors against Pretreated Advanced Cancer: A Systematic Review and Meta-Analysis. Oncotarget 2018, 9, 11846–11857. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Z.; Zheng, X.; Tao, H.; Zhang, S.; Ma, J.; Liu, Z.; Wang, J.; Qian, Y.; Cui, P.; et al. Response Efficacy of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 562315. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yuan, S.; Wang, C.; Zhu, H. Resistance to Immune Checkpoint Inhibitors in Gastric Cancer. Front. Pharmacol. 2023, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, J.; Zhang, Y.; Jiang, H.; Liu, D. Revisiting Immune Checkpoint Inhibitors: New Strategies to Enhance Efficacy and Reduce Toxicity. Front. Immunol. 2024, 15, 1490129. [Google Scholar] [CrossRef]
- Zdrenka, M.; Kowalewski, A.; Ahmadi, N.; Sadiqi, R.U.; Chmura, Ł.; Borowczak, J.; Maniewski, M.; Szylberg, Ł. Refining PD-1/PD-L1 Assessment for Biomarker-Guided Immunotherapy: A Review. Biomol. Biomed. 2024, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Hwang, D.M.; Albaqer, T.; Santiago, R.C.; Weiss, J.; Tanguay, J.; Cabanero, M.; Leung, Y.; Pal, P.; Khan, Z.; Lau, S.C.M.; et al. Prevalence and Heterogeneity of PD-L1 Expression by 22C3 Assay in Routine Population-Based and Reflexive Clinical Testing in Lung Cancer. J. Thorac. Oncol. 2021, 16, 1490–1500. [Google Scholar] [CrossRef]
- Memmott, R.M.; Wolfe, A.R.; Carbone, D.P.; Williams, T.M. Predictors of Response, Progression-Free Survival, and Overall Survival in Patients with Lung Cancer Treated with Immune Checkpoint Inhibitors. J. Thorac. Oncol. 2021, 16, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Daud, A.I.; Wolchok, J.D.; Robert, C.; Hwu, W.J.; Weber, J.S.; Ribas, A.; Hodi, F.S.; Joshua, A.M.; Kefford, R.; Hersey, P.; et al. Programmed Death-Ligand 1 Expression and Response to the Anti-Programmed Death 1 Antibody Pembrolizumab in Melanoma. J. Clin. Oncol. 2016, 34, 4102–4109. [Google Scholar] [CrossRef]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.A.M.S.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole Body PD-1 and PD-L1 Positron Emission Tomography in Patients with Non-Small-Cell Lung Cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef]
- Kok, I.C.; Hooiveld, J.S.; van de Donk, P.P.; Giesen, D.; van der Veen, E.L.; Lub-de Hooge, M.N.; Brouwers, A.H.; Hiltermann, T.J.N.; van der Wekken, A.J.; Hijmering-Kappelle, L.B.M.; et al. 89Zr-Pembrolizumab Imaging as a Non-Invasive Approach to Assess Clinical Response to PD-1 Blockade in Cancer. Ann. Oncol. 2022, 33, 80–88. [Google Scholar] [CrossRef]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. 89Zr-Atezolizumab Imaging as a Non-Invasive Approach to Assess Clinical Response to PD-L1 Blockade in Cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, A.L.N.; Oprea-Lager, D.E.; Huisman, M.C.; Hoekstra, O.S.; Boellaard, R.; de Wit-Van der Veen, B.J.; Bahce, I.; Vugts, D.J.; van Dongen, G.A.M.S.; Thunnissen, E.; et al. Study of 89Zr-Pembrolizumab PET/CT in Patients with Advanced-Stage Non-Small Cell Lung Cancer. J. Nucl. Med. 2022, 63, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Nagengast, W.B.; Hartmans, E.; Garcia-Allende, P.B.; Peters, F.T.M.; Linssen, M.D.; Koch, M.; Koller, M.; Tjalma, J.J.J.; Karrenbeld, A.; Jorritsma-Smit, A.; et al. Near-Infrared Fluorescence Molecular Endoscopy Detects Dysplastic Oesophageal Lesions Using Topical and Systemic Tracer of Vascular Endothelial Growth Factor A. Gut 2019, 68, 7–10. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, S.J.; Voskuil, F.J.; Schmidt, I.; Karrenbeld, A.; Kats-Ugurlu, G.; Meersma, G.J.; Westerhof, J.; Witjes, M.J.H.; Van Dam, G.M.; Robinson, D.J.; et al. C-Met Targeted Fluorescence Molecular Endoscopy in Barrett’s Esophagus Patients and Identification of Outcome Parameters for Phase-I Studies. Issue 12 Theranostics 2020, 10, 5357–5367. [Google Scholar] [CrossRef]
- van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative Tumor-Specific Fluorescence Imaging in Ovarian Cancer by Folate Receptor-α Targeting: First in-Human Results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Voskuil, F.J.; de Jongh, S.J.; Hooghiemstra, W.T.R.; Linssen, M.D.; Steinkamp, P.J.; de Visscher, S.A.H.J.; Schepman, K.P.; Elias, S.G.; Meersma, G.J.; Jonker, P.K.C.; et al. Fluorescence-Guided Imaging for Resection Margin Evaluation in Head and Neck Cancer Patients Using Cetuximab-800CW: A Quantitative Dose-Escalation Study. Theranostics 2020, 10, 3994–4005. [Google Scholar] [CrossRef]
- Gabriëls, R.Y.; van der Waaij, A.M.; Linssen, M.D.; Dobosz, M.; Volkmer, P.; Jalal, S.; Robinson, D.; Hermoso, M.A.; Lub-De Hooge, M.N.; Festen, E.A.M.; et al. Fluorescently Labelled Vedolizumab to Visualise Drug Distribution and Mucosal Target Cells in Inflammatory Bowel Disease. Gut 2024, 73, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.; Zhao, X.; van der Waaij, A.M.; Meersma, G.J.; Dijkstra, F.A.; Haveman, J.W.; van Etten, B.; Robinson, D.J.; Kats-Ugurlu, G.; Nagengast, W.B. Ultrasound-Guided Quantitative Fluorescence Molecular Endoscopy for Monitoring Response in Patients with Esophageal Cancer Following Neoadjuvant Chemoradiotherapy. Clin. Cancer Res. 2024, 30, 3211–3219. [Google Scholar] [CrossRef]
- AstraZeneca Summary of Product Characteristics—Durvalumab. Available online: https://www.ema.europa.eu/en/documents/product-information/imfinzi-epar-product-information_en.pdf (accessed on 23 May 2025).
- Bristol-Myers Squibb Summary of Product Characteristics—Nivolumab. Available online: https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf (accessed on 23 May 2025).
- Linssen, M.D.; Ter Weele, E.J.; Allersma, D.P.; Lub-de Hooge, M.N.; Van Dam, G.M.; Jorritsma-Smit, A.; Nagengast, W.B. Roadmap for the Development and Clinical Translation of Optical Tracers Cetuximab-800CW and Trastuzumab-800CW. J. Nucl. Med. 2019, 60, 418–423. [Google Scholar] [CrossRef]
- Ter Weele, E.J.; Terwisscha Van Scheltinga, A.G.T.; Linssen, M.D.; Nagengast, W.B.; Lindner, I.; Jorritsma-Smit, A.; De Vries, E.G.E.; Kosterink, J.G.W.; Lub-De Hooge, M.N. Development, Preclinical Safety, Formulation, and Stability of Clinical Grade Bevacizumab-800CW, a New near Infrared Fluorescent Imaging Agent for First in Human Use. Eur. J. Pharm. Biopharm. 2016, 104, 226–234. [Google Scholar] [CrossRef]
- Linssen, M.D.; Hooghiemstra, W.T.R.; Jorritsma-Smit, A.; Allersma, D.P.; Dijkstra, G.; Nagengast, W.B. Development and Characterisation of Antibody-Based Optical Imaging Probes for Inflammatory Bowel Disease. Pharmaceuticals 2021, 14, 92. [Google Scholar] [CrossRef]
- Rosenthal, E.L.; Warram, J.M.; De Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M.; Strong, T.V.; Schmalbach, C.E.; Morlandt, A.B.; Agarwal, G.; et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin. Cancer Res. 2015, 21, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Rosenthal, E.L.; Martin, B.A.; Lu, G.; Nishio, N.; Colevas, D.; Chirita, S.U.; Fakurnejad, S.; Birkeland, A.; van Keulen, S.; et al. The Clinical Application of Fluorescence-Guided Surgery in Head and Neck Cancer. J. Nucl. Med. 2019, 60, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Patel, N.L.; Wei, L.; Riffle, L.A.; Kalen, J.D.; Hill, G.C.; Jacobs, P.M.; Zinn, K.R.; Rosenthal, E. Synthesis and Biological Evaluation of Panitumumab-IRDye800 Conjugate as a Fluorescence Imaging Probe for EGFR-Expressing Cancers. Medchemcomm 2014, 5, 1337. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.V.; Draney, D.; Sevick-Muraca, E.M.; Olive, D.M. Single-Dose Intravenous Toxicity Study of IRDye 800CW in Sprague-Dawley Rats. Mol. Imaging Biol. 2010, 12, 583–594. [Google Scholar] [CrossRef]
- Harlaar, N.J.; Koller, M.; de Jongh, S.J.; van Leeuwen, B.L.; Hemmer, P.H.; Kruijff, S.; van Ginkel, R.J.; Been, L.B.; de Jong, J.S.; Kats-Ugurlu, G.; et al. Molecular Fluorescence-Guided Surgery of Peritoneal Carcinomatosis of Colorectal Origin: A Single-Centre Feasibility Study. Lancet Gastroenterol. Hepatol. 2016, 1, 283–290. [Google Scholar] [CrossRef]
- de Jongh, S.J.; Tjalma, J.J.J.; Koller, M.; Linssen, M.D.; Vonk, J.; Dobosz, M.; Jorritsma-Smit, A.; Kleibeuker, J.H.; Hospers, G.A.P.; Havenga, K.; et al. Back-Table Fluorescence-Guided Imaging for Circumferential Resection Margin Evaluation in Locally Advanced Rectal Cancer Patients Using Bevacizumab-800CW. J. Nucl. Med. 2020, 61, 655–661. [Google Scholar] [CrossRef]
- Nishio, N.; van den Berg, N.S.; van Keulen, S.; Martin, B.A.; Fakurnejad, S.; Zhou, Q.; Lu, G.; Chirita, S.U.; Kaplan, M.J.; Divi, V.; et al. Optimal Dosing Strategy for Fluorescence-Guided Surgery with Panitumumab-IRDye800CW in Head and Neck Cancer. Mol. Imaging Biol. 2019, 22, 156–164. [Google Scholar] [CrossRef]
- Kist de Ruijter, L.; van de Donk, P.P.; Hooiveld-Noeken, J.S.; Giesen, D.; Elias, S.G.; Lub-de Hooge, M.N.; Oosting, S.F.; Jalving, M.; Timens, W.; Brouwers, A.H.; et al. Whole-Body CD8+ T Cell Visualization before and during Cancer Immunotherapy: A Phase 1/2 Trial. Nat. Med. 2022, 28, 2601–2610. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Ma, S.; Liu, Q.; Huang, L.; Chen, X.; Lou, K.; Wang, W. Recent Developments in Multimodality Fluorescence Imaging Probes. Acta Pharm. Sin. B 2018, 8, 320–338. [Google Scholar] [CrossRef]
- Zhou, L.; El-Deiry, W.S. Multispectral Fluorescence Imaging. J. Nucl. Med. 2009, 50, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Nguyen, Q.T. Molecular Imaging for Cancer Diagnosis and Surgery. Adv. Drug Deliv. Rev. 2014, 66, 90. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhao, H.; Hu, L.; Shao, X.; Lu, Z.; Wang, Y.; Ling, P.; Li, Y.; Zeng, K.; Chen, Q. Enhanced Optical Imaging and Fluorescent Labeling for Visualizing Drug Molecules within Living Organisms. Acta Pharm. Sin. B 2024, 14, 2428–2446. [Google Scholar] [CrossRef]
- EDQM Council of Europe. European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2023. [Google Scholar]
- Schofield, D.J.; Percival-Alwyn, J.; Rytelewski, M.; Hood, J.; Rothstein, R.; Wetzel, L.; McGlinchey, K.; Adjei, G.; Watkins, A.; Machiesky, L.A.; et al. Activity of Murine Surrogate Antibodies for Durvalumab and Tremelimumab Lacking Effector Function and the Ability to Deplete Regulatory T Cells in Mouse Models of Cancer. MAbs 2021, 13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).