Anti-Inflammatory Activity of 1,2-Benzothiazine 1,1-Dioxide Derivatives
Abstract
1. Introduction
2. In Vivo Studies
3. In Vitro Studies
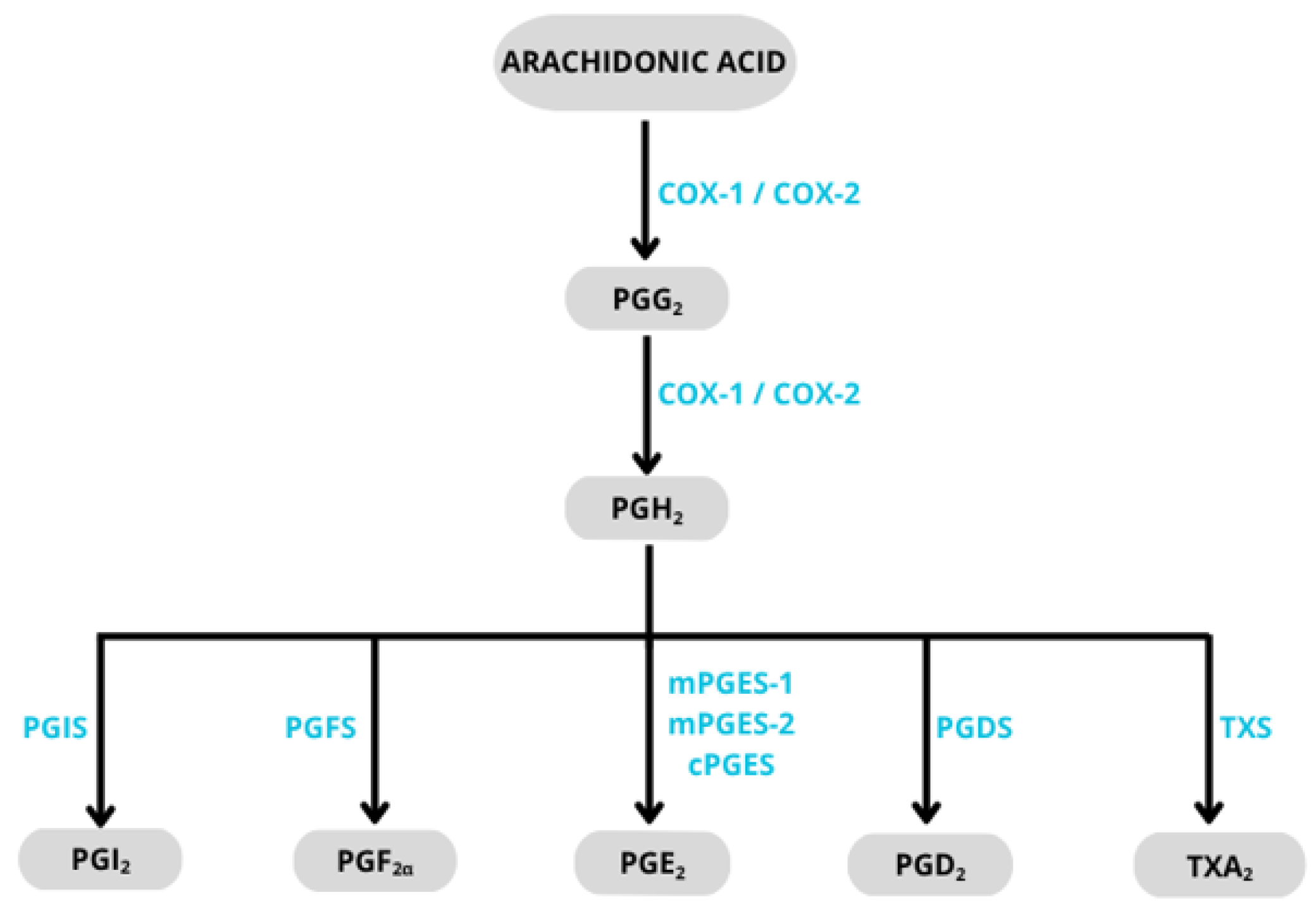
3.1. Inhibition of Cyclooxygenase (COX)
3.2. New Mechanisms of Anti-Inflammatory Action
3.2.1. Inhibition of Microsomal Prostaglandin E2 Synthase-1 (mPGES-1)
3.2.2. Inhibition of Pro-Inflammatory Cytokines
3.2.3. Inhibition of MAP Kinases
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB—blood–brain barrier | NAH—N-acylhydrazone |
| 11β-HSD1—11β-hydroxysteroid dehydrogenase type 1 | NHDF—normal human dermal fibroblasts |
| CBX—carbenoxolone | NO—nitric oxide |
| COX—cyclooxygenase | PCA—passive cutaneous anaphylaxis |
| ED50—effective dose for 50% of the population | PEC—peritoneal exudate cells (rat) |
| ERK—extracellular signal-regulated kinase | PGD2—prostaglandin D2 |
| ERK5—extracellular signal-regulated kinase 5 | PGE2—prostaglandin E2 |
| FHA—fast halo assay | PGF2α—prostaglandin F2 alpha |
| HO-1—heme oxygenase-1 | PGG2—prostaglandin G2 |
| ID50—inhibitory dose for 50% of the response | PGH2—prostaglandin H2 |
| IL-1β—interleukin 1 beta | PGI2—prostacyclin (prostaglandin I2) |
| IL-6—interleukin 6 | PMA—phorbol 12-myristate 13-acetate |
| IL-8—interleukin 8 | COPD—chronic obstructive pulmonary disease |
| JNK—c-Jun N-terminal kinase | RF—rheumatoid factor |
| LPS—lipopolysaccharide | ROS—reactive oxygen species |
| MAPK—mitogen-activated protein kinase | RA—rheumatoid arthritis |
| MCP-1—monocyte chemoattractant protein-1 | TBA—thiobarbituric acid |
| MDA—malondialdehyde | TLRs—Toll-like receptors |
| MCDA—multiple criteria decision analysis | TNF-α—tumor necrosis factor alpha |
| mPGES-1—microsomal prostaglandin E synthase-1 | TXA2—thromboxane A2 |
| MMP—matrix metalloproteinase |
References
- Sainsbury, M. Oxazines, Thiazines and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Ree, C.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 3, pp. 995–1038. [Google Scholar] [CrossRef]
- Weinreb, S.M.; Orr, R.K. 1,2-Thiazines and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Taylor, R.J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 8, pp. 513–566. [Google Scholar]
- Braun, J. Über Benzo-polymethylen-Verbindungen, X.: Oxydativer Abbau von Tetralin und substituierten Tetralinen zu Phthalonsäuren und Phthalsäuren. Chem. Ber. 1923, 56, 2332–2343. [Google Scholar] [CrossRef]
- Lombardino, J.G.; Wiseman, E.H.; McLamore, W.M. Synthesis and Antiinflammatory Activity of Some 3-Carboxamides of 2-Alkyl-4-hydroxy-2H-1,2-benzothiazine 1,1-Dioxide. J. Med. Chem. 1971, 14, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, D.; Cashin, C.H.; Kennedy, A.J.; Roberts, N.A. Pharmacological and biochemical activities of Tenoxicam (Ro 12-0068), a new nonsteroidal anti-inflammatory drug. Agents Actions 1984, 15, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.E. Derivatives of oxazinobenzothiazine-6,6-dioxide. U.S. Patent 4,563,452A, 7 January 1986. [Google Scholar]
- Marfat, A. Anti-Inflammatory 2-methyl-2H-1,2-benzo-(or -thieno-)thiazine-3-carboxamide 1,1-dioxide Derivatives, Compositions, and Method of Use Therefor. U.S. Patent 4,551,452A, 5 November 1985. [Google Scholar]
- Turck, D.; Busch, U.; Heinzel, G.; Narjes, H.; Nehmiz, G. Effect of Food on the Pharmacokinetics of Meloxicam after Oral Administration. Clin. Drug Investig. 1995, 9, 270–276. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Choudary, J.; Elsegood, M.R.J.; Siddiqui, H.L.; Khan, K.M. A facile synthesis of novel biologically active 4-hydroxy-N0-(benzylidene)-2H-benzo[e][1,2]thiazine-3-carbohydrazide 1,1-dioxides. Eur. J. Med. Chem. 2009, 44, 1311–1316. [Google Scholar] [CrossRef]
- Ahmad, M.; Siddiqui, H.; Zia-ur-Rehman, M.; Parvez, M. Anti-oxidant and anti-bacterial activities of novel N0-arylmethylidene-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3-c][1,2]benzothiazin-2(4H)-yl)acetohydrazides. Eur. J. Med. Chem. 2010, 45, 698–704. [Google Scholar] [CrossRef]
- Ahmad, M.; Rizvi, S.; Siddiqui, H.L.; Ahmad, S.; Parvez, M.; Suliman, R. Antioxidant and antimicrobial studies of novel N0-(substituted-2-chloroquinolin-3-yl)methylidene-4-hydroxy-2H-1,2-benzothiazine-3-carbo-hydrazides 1,1-dioxides. Med. Chem. Res. 2012, 21, 2340–2348. [Google Scholar] [CrossRef]
- Ahmad, N.; Zia-ur-Rehman, M.; Siddiqui, H.L.; Ullah, M.F.; Parvez, M. Microwave assisted synthesis and structureeactivity relationship of 4-hydroxy-N0-[1-phenylethylidene]-2H/2-methyl-1,2-benzothiazine-3-carbohydrazide 1,1-dioxides as anti-microbial agents. Eur. J. Med. Chem. 2011, 46, 2368–2377. [Google Scholar] [CrossRef]
- Sabatini, S.; Gosetto, F.; Serritella, S.; Manfroni, G.; Tabarrini, O.; Iraci, N.; Brincat, J.P.; Carosati, E.; Villarini, M.; Kaatz, G.W.; et al. Pyrazolo[4,3-c][1,2]benzothiazines 5,5-Dioxide: A Promising New Class of Staphylococcus aureus Nor A Efflux Pump Inhibitors. J. Med. Chem. 2012, 55, 3568–3572. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Choudary, J.; Ahmad, S.; Siddiqui, H.L. Synthesis of Potential Biologically Active 1,2 Benzothiazin-3-ylquinazolin-4(3H)-ones. Chem. Pharm. Bull. 2006, 54, 1175–1178. [Google Scholar] [CrossRef]
- Brooke, E.; Davies, S.; Mulvaney, A.W.; Okada, M.; Pompeo, F.; Sim, E.; Vickers, R.J.; Westwood, I.M. Synthesis and In Vitro Evaluation of Novel Small Molecule Inhibitors of Bacterial Arylamine N-Acetyltransferases (NATs). Bioorg. Med. Chem. Lett. 2003, 13, 2527–2530. [Google Scholar] [CrossRef]
- Kim, S.; Ramu, R.; Kwon, S.W.; Lee, S.H.; Kim, C.H.; Kang, S.K.; Rhee, S.D.; Bae, M.A.; Ahn, S.H.; Ha, D.C.; et al. Discovery of cyclicsulfonamide derivatives as 11b-hydroxysteroid dehydrogenase 1 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, S.; Chu, S.Y.; Lee, J.H.; Narsaiah, B.; Kim, C.H.; Kang, S.K.; Kang, N.S.; Rhee, S.D.; Bae, M.A.; et al. Identification of Cyclicsulfonamide Derivatives with an Acetamide Group as 11b-Hydroxysteroid Dehydrogenase 1 Inhibitors. Chem. Pharm. Bull. 2011, 59, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Rhee, S.D.; Kang, N.S.; Jung, W.H.; Kim, H.Y.; Kim, J.H.; Kang, S.K.; Cheon, H.G.; Ahn, J.H.; Kim, K.Y. Anti-diabetic and anti-adipogenic effects of a novel selective 11b-hydroxysteroid-dehydrogenase type 1 inhibitor, 2-(3-benzoyl)-4-hydroxy-1,1-dioxo-2H-1,2-benzothiazine-2-yl-1-phenylethanone (KR-66344). Biochem. Pharmacol. 2011, 81, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Seo, H.; Kwak, H.J.; Kim, K.Y.; Ahn, J.H.; Bae, M.A.; Song, J.S. Pharmacokinetic characterization of 2-(3-benzoyl)-4-hydroxy-1,1-dioxo-2H-1,2-benzothiazine-2-yl-1-phenylethanone, a novel 11β-hydroxysteroid-dehydrogenase type 1 inhibitor in rats. Arch. Pharm. Res. 2016, 39, 492–498. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, S.; Yang, Y.; Hussain, S.; He, M.; Gui, D.; Ma, B.; Jing, C.; Qiao, Z.; Zhu, C.; et al. 1,2-Benzothiazine 1,1-dioxide carboxylate derivatives as novel potent inhibitors of aldose reductase. Bioorg. Med. Chem. 2011, 19, 7262–7269. [Google Scholar] [CrossRef]
- Hao, X.; Qin, X.; Hussain, S.; Parveen, S.; Zhang, W.; Fu, F.; Ma, B.; Zhu, C. Chiral resolution, determination of absolute configuration, and biological evaluation of (1,2-benzothiazin-4-yl)acetic acid enantiomers as aldose reductase inhibitors. J. Enzym. Inhib. Med. Chem. 2015, 30, 846–851. [Google Scholar] [CrossRef]
- Imani, A.; Soleymani, S.; Vahabpour, R.; Hajimahdi, Z.; Zarghi, A. Design, Synthesis, Docking Study and Biological Evaluation of 4-Hydroxy-2H-benzo[e][1,2]thiazine-3-carboxamide 1,1-dioxide Derivatives as Anti-HIV Agents. Iran. J. Pharm. Res. IJPR 2021, 20, 1–12. [Google Scholar]
- Ahmad, M.; Aslam, S.; Rizvi, S.U.F.; Muddassar, M.; Ashfaq, U.A.; Montero, C.; Ollinger, O.; Detorio, M.; Gardiner, J.M.; Schinazi, R.F. Molecular docking and antiviral activity of N-substituted benzyl/phenyl-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3-c][1,2]benzothiazin-2(4H)-yl)acetamides. Bioorg. Med. Chem. Lett. 2015, 25, 1348–1351. [Google Scholar] [CrossRef]
- Khalid, Z.; Aslam, S.; Ahmad, M.; Munawar, M.A.; Montero, C.; Detorio, M.; Parvez, M.; Schinazi, R.F. Anti-HIV activity of new pyrazolobenzothiazine 5,5-dioxide-based acetohydrazides. Med. Chem. Res. 2015, 24, 3671–3680. [Google Scholar] [CrossRef]
- Szczęśniak-Sięga, B.M.; Zaręba, N.; Czyżnikowska, Ż.; Janek, T.; Kepinska, M. Rational Design, Synthesis, Molecular Docking, and Biological Evaluations of New Phenylpiperazine Derivatives of 1,2-Benzothiazine as Potential Anticancer Agents. Molecules 2024, 29, 4282. [Google Scholar] [CrossRef]
- Neeraja, P.; Srinivas, S. Assembly of Benzothiazine and Triazole in a Single Molecular Entity: Synthesis of -Oxicam Derived Novel Molecules as Potential Antibacterial/Anti-cancer Agents. Mini Rev. Med. Chem. 2020, 20, 929–940. [Google Scholar] [CrossRef]
- Tamasi, G.; Serinelli, F.; Consumi, M.; Magnani, A.; Casolaro, M.; Cini, R. Release studies from smart hydrogels as carriers for piroxicam and copper(II)-oxicam complexes as anti-inflammatory and anti-cancer drugs. X-ray structures of new copper(II)-piroxicam and -isoxicam complex molecules. J. Inorg. Biochem. 2008, 102, 1862–1873. [Google Scholar] [CrossRef]
- Tamasi, G.; Casolaro, M.; Magnani, A.; Sega, A.; Chiasserini, L.; Messori, L.; Gabbiani, C.; Valiahdi, S.M.; Jakupec, M.A.; Keppler, B.K.; et al. New platinum-oxicam complexes as anti-cancer drugs. Synthesis, characterization, release studies from smart hydrogels, evaluation of reactivity with selected proteins and cytotoxic activity in vitro. J. Inorg. Biochem. 2010, 104, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Jannesari, Z.; Hadadzadeh, H.; Amirghofran, Z.; Simpson, J.; Khayamian, T.; Maleki, B. A mononuclear zinc(II) complex with piroxicam: Crystal structure, DNA- and BSA-binding studies; in vitro cell cytotoxicity and molecular modeling of oxicam complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Bluke, Z.; Paass, E.; Sladek, M.; Abel, U.; Kauss, V. Synthesis of 3,4-dihydro-2H-1,2-benzothiazine-3-carboxylic acid 1,1-dioxides and their evaluation as ligands for NMDA receptor glycine binding site. Enzym. Inhib. Med. Chem. 2016, 31, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Saddique, F.A.; Zaib, S.; Jalil, S.; Aslam, S.; Ahmad, M.; Sultan, S.; Naz, H.; Iqbal, M.; Iqbal, J. Synthesis, monoamine oxidase inhibition activity and molecular docking studies of novel 4-hydroxy-N′-[benzylidene or 1-phenylethylidene]-2-H/methyl/benzyl-1,2-benzothiazine-3-carbohydrazide 1,1-dioxides. Eur. J. Med. Chem. 2018, 143, 1373–1386. [Google Scholar] [CrossRef]
- Ashraf, A.; Ejaz, S.A.; Rahman, S.U.; Siddiqui, W.A.; Arshad, M.N.; Lecka, J.; Sévigny, J.; Zayed, M.E.M.; Asiri, A.M.; Iqbal, J.; et al. Hybrid compounds from chalcone and 1,2-benzothiazine pharmacophores as selective inhibitors of alkaline phosphatase isozymes. Eur. J. Med. Chem. 2018, 159, 282–291. [Google Scholar] [CrossRef]
- Wells, G.J.; Tao, M.; Josef, K.A.; Bihovsky, R. 1,2-Benzothiazine 1,1-dioxide P2-P3 peptide mimetic aldehyde calpain I inhibitors. J. Med. Chem. 2001, 44, 3488–3503. [Google Scholar] [CrossRef]
- Bihovsky, R.; Tao, M.; Mallamo, J.P.; Wells, G.J. 1,2-Benzothiazine 1,1-dioxide alpha-ketoamide analogues as potent calpain I inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 1035–1038. [Google Scholar] [CrossRef]
- Saeed, A.; Ehsan, S.; Zia-ur-Rehman, M.; Marshall, E.M.; Loesgen, S.; Saleem, A.; Giovannuzzi, S.; Supuran, C.T. Synthesis, characterization, antimicrobial, cytotoxic and carbonic anhydrase inhibition activities of multifunctional pyrazolo-1,2-benzothiazine acetamides. Beilstein J. Org. Chem. 2025, 21, 348–357. [Google Scholar] [CrossRef]
- Aslam, S.; Zaib, S.; Ahmad, M.; Gardiner, J.M.; Ahmad, A.; Hameed, A.; Furtmann, N.; Gütschow, M.; Bajorath, J.; Iqbal, J. Novel structural hybrids of pyrazolobenzothiazines with benzimidazoles as cholinesterase inhibitors. Eur. J. Med. Chem. 2014, 78, 106–117. [Google Scholar] [CrossRef]
- Fatima, M.; Siddiqui, W.A.; Choudhary, M.I.; Ashraf, A.; Niaz, S.; Raza, M.A.; Alam, S.M.; Ashfaq, M.; Tahir, M.N.; Dahlous, K.A. Synthesis of dimeric 1,2-benzothiazine 1,1-dioxide scaffolds: Molecular structures, Hirshfeld surface analysis, DFT and enzyme inhibition studies. RSC Adv. 2024, 14, 16935–16944. [Google Scholar] [CrossRef] [PubMed]
- Hina, S.; Zaib, S.; Uroos, M.; Zia-ur-Rehman, M.; Munir, R.; Riaz, H.; Syed, Q.; Abidi, S.H.I. N-Arylacetamide derivatives of methyl 1,2-benzothiazine-3-carboxylate as potential drug candidates for urease inhibition. R. Soc. Open Sci. 2023, 10, 230104. [Google Scholar] [CrossRef] [PubMed]
- Narsinghani, T.; Sharma, R. Lead Optimization on Conventional NSAIDs: An approach to reduce Gastrointestinal Toxicity. Chem. Biol. Drug Des. 2014, 84, 1–23. [Google Scholar] [PubMed]
- Yongzhong, W.; Smitha, A.; Meitzler, J.L.; Doroshow, J.H. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014, 345, 164–173. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Henshaw, F.R.; Dewsbury, L.S.; Lim, C.K.; Steiner, G.Z. The Effects of Cannabinoids on Pro- and Anti-Inflammatory Cytokines: A Systematic Review of In Vivo Studies. Cannabis Cannabinoid Res. 2021, 6, 177–195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikeda, T.; Kakegawa, H.; Miyataka, H.; Matsumoto, H.; Satoh, T. Anti-allergic and anti-inflammatory actions of 2′-(tetrazole-5-yl)-4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxanilide 1,1-dioxide. Bioorg. Med. Chem. Lett. 1992, 2, 709–714. [Google Scholar] [CrossRef]
- Szczęśniak-Sięga, B.M.; Mogilski, S.; Wiglusz, R.J.; Janczak, J.; Maniewska, J.; Malinka, W.; Filipek, B. Synthesis and pharmacological evaluation of novel arylpiperazine oxicams derivatives as potent analgesics without ulcerogenicity. Bioorg. Med. Chem. 2019, 27, 2075–2084. [Google Scholar] [CrossRef]
- Venkatachalam, G.; Arumugam, S.; Doble, M. Synthesis, Characterization, and Biological Activity of Aminated Zymosan. ACS Omega 2020, 5, 15973–15982. [Google Scholar] [CrossRef]
- Kwon, S.K.; Park, M.S. The synthesis of 1,2-benzothiazine-3-carboxamidylhydantoin derivatives and their antiinflammatory and analgesic activities. Arch. Pharm. Res. 1992, 15, 251–255. [Google Scholar] [CrossRef]
- Lee, E.; Kwon, S.K.; Kim, S.G. Synthesis and analgesic and anti-inflammatory activities of 1,2-benzothiazine derivatives. Arch. Pharm. Res. 1999, 22, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Sawhney, S. Synthesis and antiinflammatory activity of some 1,4-dihydro-3-methyl-1-(2-thiazolyl)pyrazolo[4,3-c][1,2]benzothiazine5,5-dioxides. Bull. Chem. Soc. Jpn. 1993, 66, 3843–3846. [Google Scholar] [CrossRef]
- Maso, M.D.; Perillo, I.A.; Schapira, C.B.; Gorzalczany, S.; Acevedo, M.C.; Sicardi, S.M. New 2-Substituted 4-Hydroxy-2H-1,2-benzothiazine-3-carboxamide 1,1-Dioxides: Spectral and Pharmacological Properties. J. Heterocycl. Chem. 1999, 36, 803–811. [Google Scholar] [CrossRef]
- Kacem, Y.; Kraiem, J.; Kerkeni, E.; Bouraoui, A.; Hassine, B.B. Synthesis and pharmacological profile of 6-methyl-3-isopropyl-2H-1,2-benzothiazin-4(3H)-one 1,1-dioxide derivatives: Nonsteroidal anti-inflammatory agents with reduced ulcerogenic effects in the rat. Eur. J. Pharm. Sci. 2002, 16, 221–228. [Google Scholar] [CrossRef]
- Dequeker, J.; Hawkey, C. Improvement in gastrointestinal tolerability of the selective cyclooxygenase (COX) 2 inhibitor, meloxicam, compared with piroxicam: Results of the safety and efficacy large-scale evaluation of COX-inhibiting therapies (SELECT) trial in osteoarthritis. Br. J. Rheumatol. 1998, 37, 946–951. [Google Scholar] [CrossRef]
- Kulkarni, S. Handbook of Experimental Pharmacology; Vallabh Prakashan: Delhi, India, 1993. [Google Scholar]
- Miranda, A.S.; Bispo Júnior, W.; Cupertino da Silva, Y.K.; Alexandre-Moreira, M.S.; Castro, R.; Sabino, J.R.; Lião, L.M.; Lima, L.M.; Barreiro, E.J. Design, synthesis, antinociceptive and anti-inflammatory activities of novel piroxicam analogues. Molecules 2012, 17, 14126–14145. [Google Scholar] [CrossRef]
- Park, S.B.; Park, J.S. Anti-inflammatory effect of a selective 11β-hydroxysteroid dehydrogenase type 1 inhibitor via the stimulation of heme oxygenase-1 in LPS-activated mice and J774.1 murine macrophages. J. Pharmacol. Sci. 2016, 131, 241–250. [Google Scholar] [CrossRef]
- Hatnapure, G.D.; Keche, A.P.; Rodge, A.H.; Birajdar, S.S.; Tale, R.H.; Kamble, V.M. Synthesis and biological evaluation of novel piperazine derivatives of flavone as potent anti-inflammatory and antimicrobial agent. Bioorg. Med. Chem. Lett. 2012, 22, 6385–6390. [Google Scholar] [CrossRef]
- Malinka, W.; Kaczmarz, M.; Filipek, B.; Sapa, J.; Głód, B.K. Preparation of novel derivatives of pyridothiazine-1,1-dioxide and their CNS and antioxidant properties. Farmaco 2002, 57, 737–746. [Google Scholar] [CrossRef]
- Dogruer, D.; Kupeli, E.; Yesilada, E.; Sahin, M.F. Synthesis of new 2-[1(2H)-phthalazinon-2-yl]acetamide and 3-[1(2H)-phthalazinon-2-yl]propanamide derivatives as antinociceptive and anti-inflammatory agents. Arch. Pharm. Med. Chem. 2004, 337, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Rouzer, C.A.; Marnett, L.J. Cyclooxygenases: Structural and functional insights. J. Lipid Res. 2009, 50, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase Isozymes: The Biology of Prostaglandin Synthesis and Inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef]
- Dhingra, A.; Chopra, B.; Dass, R.; Mittal, S.K. A review on COX and their inhibitors: Present and future. IPP 2014, 2, 470–485. [Google Scholar]
- Rouzer, C.A.; Marnett, L.J. Structural and chemical biology of the interaction of cyclooxygenase with substrates and nonsteroidal anti-inflammatory drugs. Chem. Rev. 2020, 120, 7592–7641. [Google Scholar] [CrossRef]
- Josephy, P.; Chiu, A.; Eling, T.E. Prostaglandin H synthase-dependent mutagenic activation of benzidine in a Salmonella typhimurium Ames tester strain possessing elevated N-acetyltransferase levels. Cancer Res. 1989, 49, 853–856. [Google Scholar]
- Goradel, N.H.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2018, 234, 5683–5699. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Adsule, S.; Li, Y.; Padhye, S. Back to the future: COX-2 inhibitors for chemoprevention and cancer therapy, Mini-Rev. Med. Chem. 2007, 6, 599–608. [Google Scholar]
- Chandrasekharan, N.V.; Dai, H.; Roos, K.L.T.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX- 3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc. Nat. Acad. Sci. USA 2002, 21, 13926–13931. [Google Scholar] [CrossRef]
- Hinz, B.; Cheremina, O.; Brune, K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008, 2, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Breyer, R.M.; Bagdassarian, C.K.; Myers, S.A.; Breyer, M.D. Prostanoid receptors: Subtypes and signaling. Ann. Rev. Pharm. Tox 2001, 41, 661–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Buchanan, F.; Wang, H.; Dey, S.K.; DuBois, R.N. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005, 65, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Krysan, K. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005, 65, 6275–6281. [Google Scholar] [CrossRef]
- Pan, M.R.; Hou, M.F.; Chang, H.-C.; Hung, W.-C. Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. J. Biol. Chem. 2008, 283, 11155–11163. [Google Scholar] [CrossRef]
- Szczęśniak-Sięga, B.; Gębczak, K.; Gębarowski, T.; Maniewska, J. Synthesis, COX-1/2 inhibition and antioxidant activities of new oxicam analogues designed as potential chemopreventive agents. Acta Biochim. Pol. 2018, 65, 199–207. [Google Scholar] [CrossRef]
- Lazer, E.S.; Miao, C.K.; Cywin, C.L.; Sorcek, R.; Wong, H.-C.; Meng, Z.; Potocki, I.; Hoermann, M.; Snow, R.J.; Tschantz, M.A.; et al. Effect of structural modification of enol-carboxamide-type nonsteroidal antiinflammatory drugs on COX-2/COX-1 selectivity. J. Med. Chem. 1997, 40, 980–989. [Google Scholar] [CrossRef]
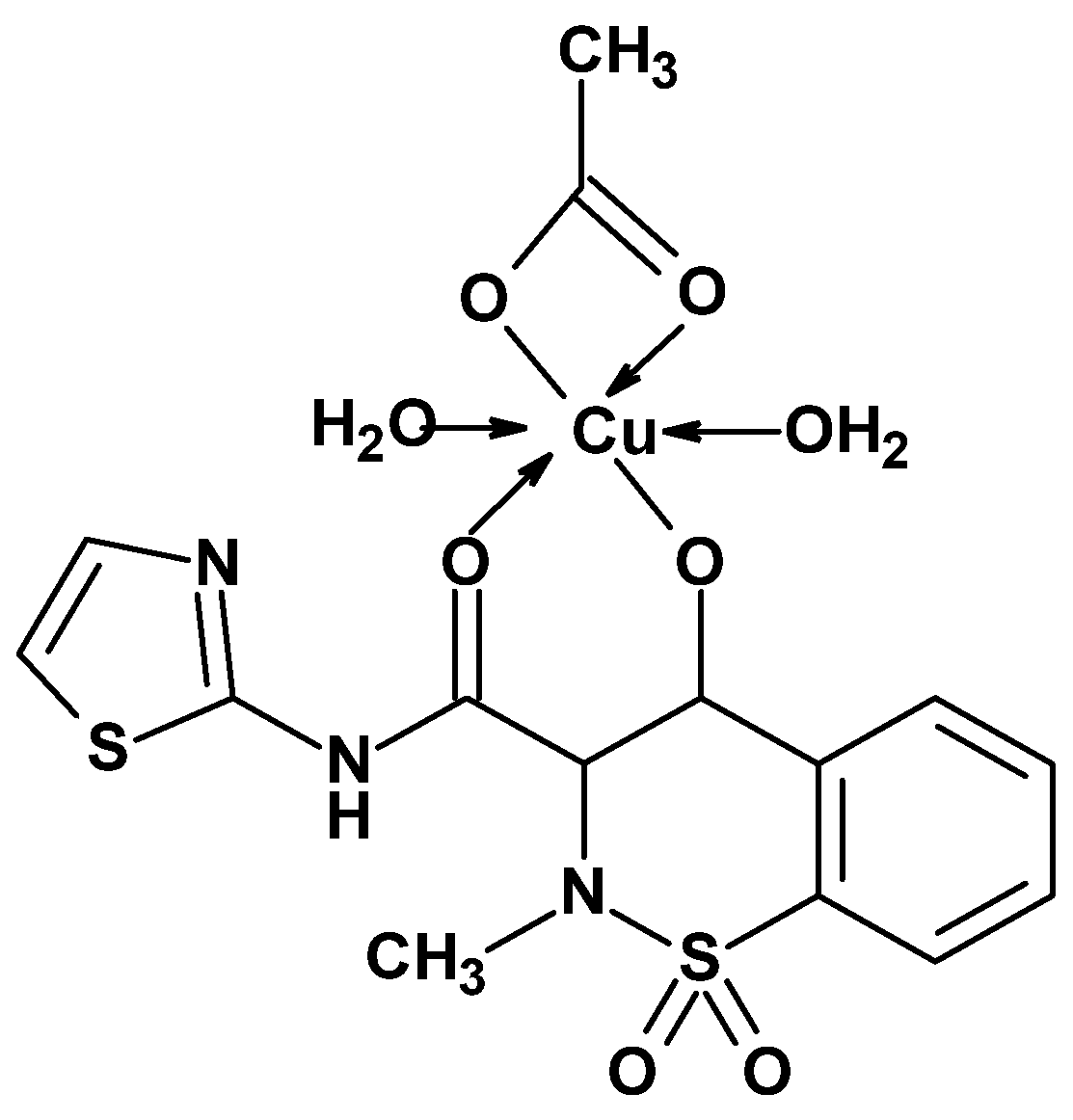
- Sherif, Y.E.-S.; El-Asmy, A.A.-H.; Lotfy, M. 4-Hydroxy-2-methyl-N-(2-thiazolyl)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxide (EX15) and Its Cu(II) Complex as New Oxicam COX-2 Selective Inhibitors. Croat. Chem. Acta 2012, 85, 19–26. [Google Scholar] [CrossRef]
- Sorenson, J.R.J. Copper chelates as possible active forms of the antiarthritic agents. J. Med. Chem. 1976, 19, 135–148. [Google Scholar] [CrossRef]
- Szczęśniak-Sięga, B.M.; Wiatrak, B.; Czyżnikowska, Ż.; Janczak, J.; Wiglusz, R.J.; Maniewska, J. Synthesis and biological evaluation as well as in silico studies of arylpiperazine-1,2-benzothiazine derivatives as novel anti-inflammatory agents. Bioorg. Chem. 2021, 106, 104476. [Google Scholar] [CrossRef]
- Szczęśniak-Sięga, B.M.; Maniewska, J.; Wiatrak, B.; Janek, T.; Nowotarska, P.; Czyżnikowska, Ż. Anti-inflammatory properties of novel 1,2-benzothiazine derivatives and their interaction with phospholipid model membranes. Membranes 2024, 14, 274. [Google Scholar] [CrossRef]
- Maniewska, J.; Wiatrak, B.; Czyżnikowska, Ż.; Szczęśniak-Sięga, B.M. Synthesis of new tricyclic 1,2-thiazine derivatives with anti-inflammatory activity. Int. J. Mol. Sci. 2021, 22, 7818. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.M.I.M.; Zarghi, A. Selective COX-2 inhibitors as anticancer agents: A patent review (2014–2018). Expert Opin. Ther. Pat. 2019, 29, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Wiatrak, B.; Krzyżak, E.; Szczęśniak-Sięga, B.M.; Szandruk-Bender, M.; Szeląg, A.; Nowak, B. Effect of tricyclic 1,2-thiazine derivatives in neuroinflammation induced by preincubation with lipopolysaccharide or coculturing with microglia-like cells. Pharm. Rep. 2022, 74, 890–908. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Sabzwari, S.R.A.; Vargowa, V. Cardiovascular risk of nonsteroidal anti-inflammatory drugs: An under-recognized public health issue. Cureus 2017, 9, 1144. [Google Scholar] [CrossRef]
- Ikeda-Matsuo, Y. The role of mPGES-1 in inflammatory brain diseases. Biol. Pharm. Bull. 2017, 40, 557–563. [Google Scholar] [CrossRef]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef]
- Mattila, S.; Tuominen, H.; Koivukangas, J.; Stenbäck, F. The terminal prostaglandin synthases mPGES-1, mPGES-2, and cPGES are all overexpressed in human gliomas. Neuropathology 2009, 29, 156–165. [Google Scholar] [CrossRef]
- Seo, M.-J.; Oh, D.-K. Prostaglandin synthases: Molecular characterization and involvement in prostaglandin biosynthesis. Prog. Lipid Res. 2017, 66, 50–68. [Google Scholar] [CrossRef]
- Hara, S. Prostaglandin terminal synthases as novel therapeutic targets. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 703–721. [Google Scholar] [CrossRef]
- Huang, Q.; Lai, T.; Wang, Q.; Luo, L. mPGES-1 inhibitor discovery based on computer-aided screening: Pharmacophore models, molecular docking, ADMET, and MD simulations. Molecules 2023, 28, 6059. [Google Scholar] [CrossRef]
- Wang, J.; Limburg, D.; Carter, J.; Mbalaviele, G.; Gierse, J.; Vazquez, M. Selective inducible microsomal prostaglandin E2 synthase-1 (mPGES-1) inhibitors derived from an oxicam template. Bioorg. Med. Chem. Lett. 2010, 20, 1604–1609. [Google Scholar] [CrossRef]
- Mbalaviele, G.; Pauley, A.M.; Shaffer, A.F.; Zweifel, B.S.; Mathialagan, S.; Mnich, S.J.; Nemirovskiy, O.V.; Carter, J.; Gierse, J.K.; Wang, J.L.; et al. Distinction of microsomal prostaglandin E synthase-1 (mPGES-1) inhibition from cyclooxygenase-2 inhibition in cells using a novel, selective mPGES-1 inhibitor. Biochem. Pharmacol. 2010, 79, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dhalla, N.S. The role of pro-inflammatory cytokines in the pathogenesis of cardiovascular disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation and pain. Int. Anesth. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Gannaparu, M.R.; Vasamsetti, S.B.; Punna, N.; Royya, N.K.; Pamulaparthy, S.R.; Nanubolu, J.B.; Kotamraju, S.; Banda, N. Synthesis of novel 1,2-benzothiazine 1,1-dioxide-3-ethanone oxime N-aryl acetamide ether derivatives as potent anti-inflammatory agents and inhibitors of monocyte-to-macrophage transformation. Eur. J. Med. Chem. 2014, 75, 143–150. [Google Scholar] [CrossRef]
- Gilowska, I. CXCL8 (interleukin 8)—The key inflammatory mediator in chronic obstructive pulmonary disease? Postep. Hig. Med. Dosw. 2014, 68, 842–850. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Gannarapu, M.R.; Vasamsetti, S.B.; Punna, N.; Kotamraju, S.; Banda, N. Synthesis of novel 1-substituted triazole linked 1,2-benzothiazine 1,1-dioxido propenone derivatives as potent anti-inflammatory agents and inhibitors of monocyte-to-macrophage differentiation. MedChemComm. 2015, 6, 1418–1424. [Google Scholar] [CrossRef]
- Bryk, D.; Olejarz, W.; Zapolska-Downar, D. Kinazy aktywowane mitogenami i ich znaczenie w patogenezie miażdżycy. Postep. Hig. Med. Dosw. 2014, 68, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A comprehensive review on MAPK: A promising therapeutic target in cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Saklatvala, J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr. Opin. Pharmacol. 2004, 4, 372–377. [Google Scholar] [CrossRef]
- Feng, Y.J.; Li, Y.Y. The role of p38 mitogen-activated protein kinase in the pathogenesis of inflammatory bowel disease. J. Dig. Dis. 2011, 12, 327–332. [Google Scholar] [CrossRef]
- Patterson, H.; Nibbs, R.; McInnes, I.; Siebert, S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin. Exp. Immunol. 2014, 176, 1–10. [Google Scholar] [CrossRef]
- Phan, T.; Zhang, X.H.; Rosen, S.; Melstrom, L.G. P38 kinase in gastrointestinal cancers. Cancer Gene Ther. 2023, 30, 1181–1189. [Google Scholar] [CrossRef]
- Gui, J.; Zahedi, F.; Ortiz, A.; Cho, C.; Katlinski, K.V.; Alicea-Torres, K.; Li, J.; Todd, L.; Zhang, H.; Beiting, D.P.; et al. Activation of p38α stress-activated protein kinase drives the formation of the pre-metastatic niche in the lungs. Nat. Cancer 2020, 1, 603–619. [Google Scholar] [CrossRef]
- Lin, H.; Dixon, S.G.; Wei Hu, W.; Hamlett, E.D.; Jin, J.; Ergul, A.; Wang, G.Y. p38 MAPK is a major regulator of amyloid beta-induced IL-6 expression in human microglia. Mol. Neurobiol. 2022, 59, 5284–5298. [Google Scholar] [CrossRef]
- Wang, G.; Pan, J.; Chen, S.-D. Kinases and kinase signaling pathways: Potential therapeutic targets in Parkinson’s disease. Prog. Neurobiol. 2012, 98, 207–221. [Google Scholar] [CrossRef]
- Sabatini, S.; Manfroni, G.; Barreca, M.L.; Bauer, S.M.; Gargaro, M.; Cannalire, R.; Astolfi, A.; Brea, J.; Vacca, C.; Pirro, M.; et al. The pyrazolobenzothiazine core as a new chemotype of p38 alpha mitogen-activated protein kinase inhibitors. Chem. Biol. Drug Des. 2015, 86, 531–545. [Google Scholar] [CrossRef]
- Cuenda, A.; Rouse, J.; Doza, Y.N.; Meier, R.; Cohen, P.; Gallagher, T.F.; Young, P.R.; Lee, J.C. SB203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995, 364, 229–233. [Google Scholar]
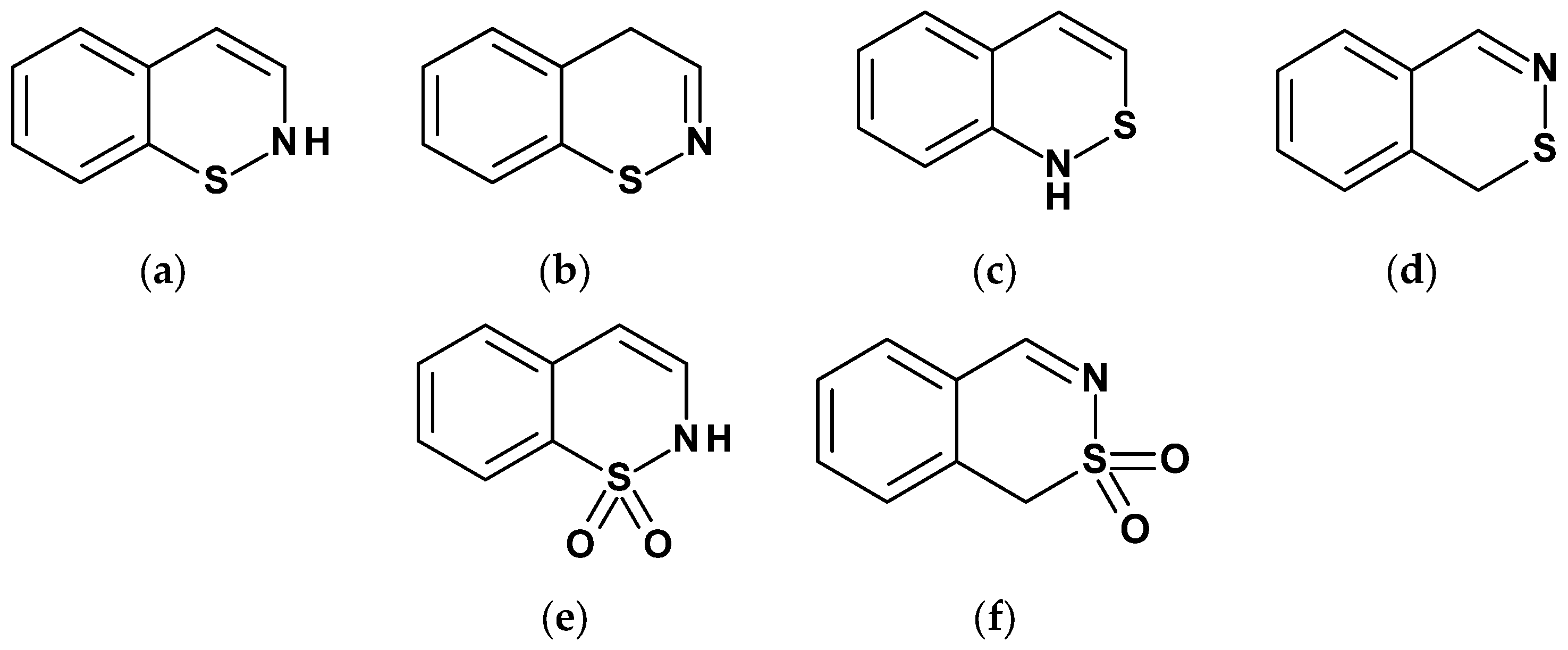

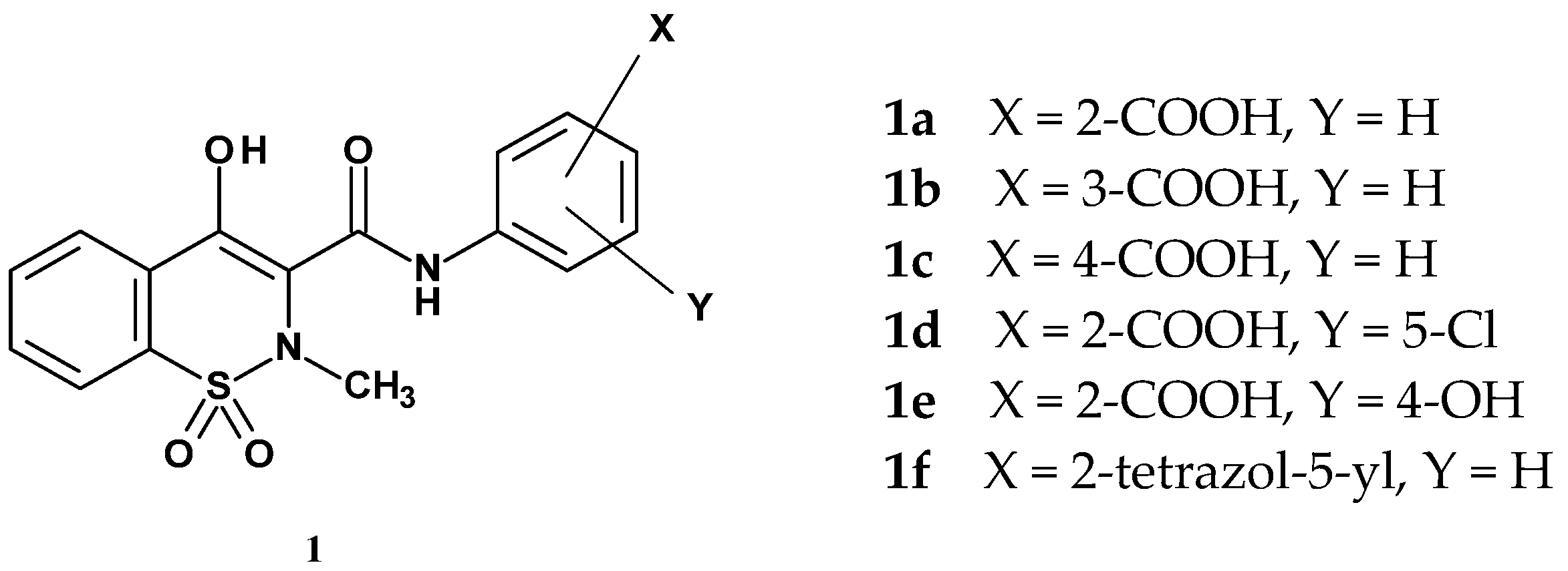

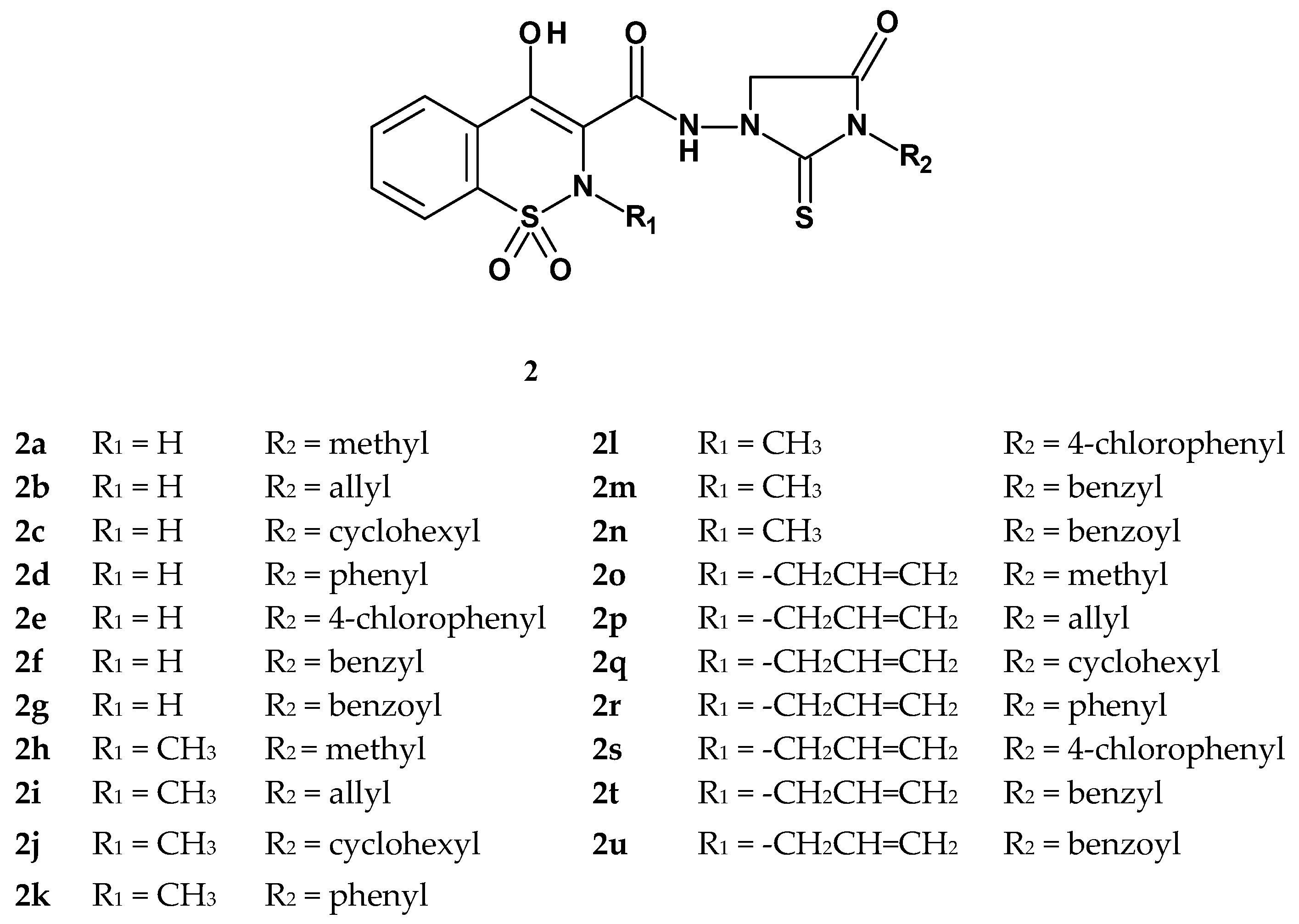

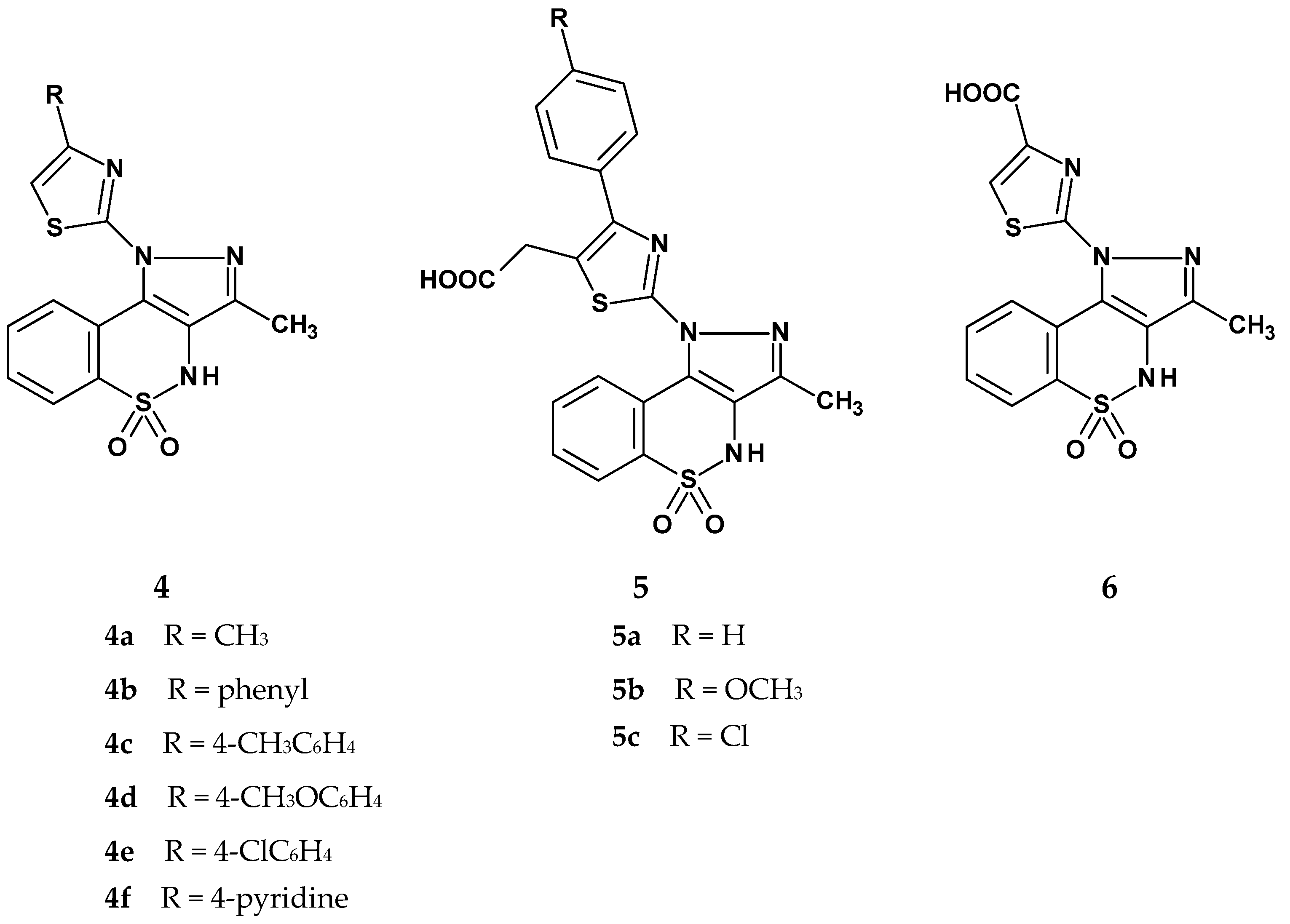
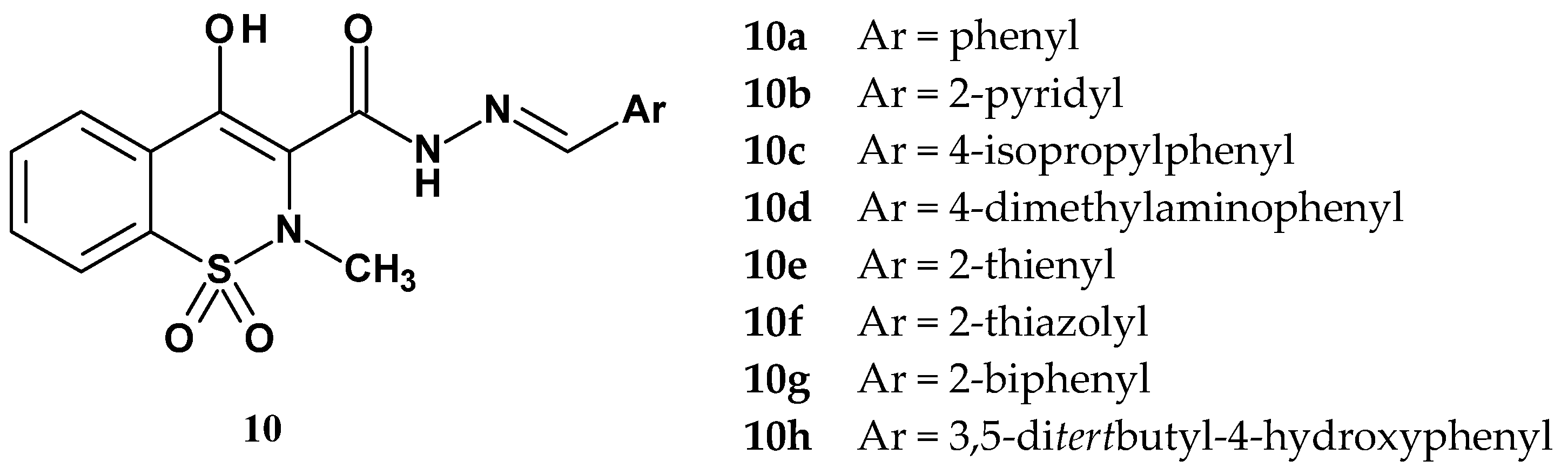







| Compound | Inhibition (%) |
|---|---|
| 1a | 47.7 |
| 1b | 25.2 |
| 1c | 21.7 |
| 1d | 72.3 |
| 1e | 94.2 |
| 1f | 102.7 |
| tranilast | 42.2 |
| piroxicam | 92.5 |
| Compound | Antiinflammatory a | Analgesic b | Compound | Antiinflammatory a | Analgesic b |
|---|---|---|---|---|---|
| 2a | IN | IN | 2m | MO | MK |
| 2b | IN | SL | 2n | MO | IN |
| 2c | IN | SL | 2o | MO | SL |
| 2d | IN | MO | 2p | IN | IN |
| 2e | IN | SL | 2q | IN | SL |
| 2f | IN | SL | 2r | IN | MO |
| 2g | IN | MO | 2s | MO | MO |
| 2h | MK | MK | 2t | IN | SL |
| 2i | MK | MK | 2u | IN | IN |
| 2j | MK | MK | indomethacin | MK | MO |
| 2k | MK | MK | aspirin | - | MO |
| 2l | MK | MO |
| Treatment | Carrageenan Test (Increase Percent of Paw Volume) | Randall–Selitto Assay ED50 (mg/kg) | ||||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 30 min | 60 min | |
| 3a | 64.1 ± 9.5 | 67.0 ± 7.8 | 82.8 ± 6.8 | 92.8 ± 10.6 | - | - |
| 3b | 71.6 ± 2.4 | 77.5 ± 1.1 | 80.1 ± 1.9 | 87.3 ± 2.4 | 79.9 | 215.9 |
| 3c | 69.0 ± 5.8 | 70.4 ± 5.8 | 73.0 ± 5.9 | 82.0 ± 5.8 | 34.7 | 944.5 |
| control | 56.9 ± 6.1 | 57.8 ± 6.1 | 75.3 ± 10.6 | 79.2 ± 7.5 | - | - |
| piroxicam | 39.2 ± 4.0 | 40.1 ± 4.9 | 39.0 ± 3.7 | 48.1 ± 6.6 | 5.2 | 37.5 |
| ibuprofen | - | - | - | - | 120.1 | 166.6 |
| Compound | Inhibition (%) | |||
|---|---|---|---|---|
| p.o. | i.p. | |||
| 2 h | 3.5 h | 2 h | 3.5 h | |
| 4a | 19 | - | 34 | 35 |
| 4b | 17 | 5 | 61 | 47 |
| 4c | - | - | 30 | 37 |
| 4d | - | 5 | 61 | 40 |
| 4e | 7 | 22 | 65 | 64 |
| 4f | 36 | 22 | 53 | 34 |
| 5a | 32 | 19 | 66 | 46 |
| 5b | 41 | 16 | 53 | 40 |
| 5c | 34 | 22 | 38 | 29 |
| 6 | 30 | 12 | 53 | 59 |
| ibuprofen | 62 | 65 | 70 | 74 |
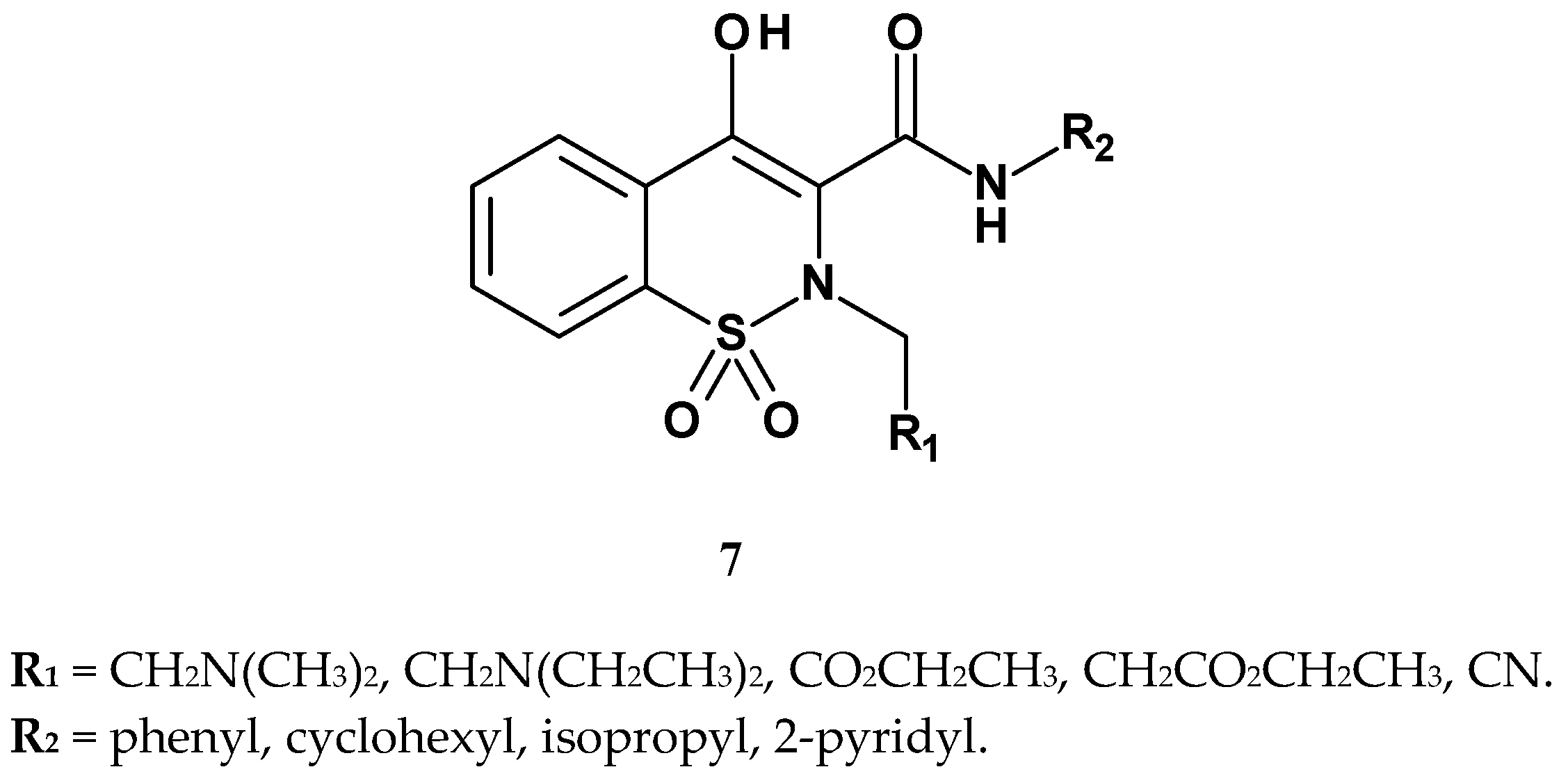
| Compound | R1 | R2 | Writhing Assay | Carrageenan Assay |
|---|---|---|---|---|
| ID50 | Inhibition (%) | |||
| 7b | CH2N(CH2CH3)2 | phenyl | 20.20 | 76.47 |
| 7c | CO2CH2CH3 | phenyl | 6.07 | 81.51 |
| 7d | CH2CO2CH2CH3 | phenyl | 7.24 | 82.36 |
| 7e | CN | phenyl | 17.50 | 77.31 |
| 7j | CH2N(CH2CH3)2 | isopropyl | >50 | 54.54 |
| 7k | CH2N(CH3)2 | isopropyl | >50 | 53.78 |
| piroxicam | H | pyridyl | 0.76 | 85.71 |
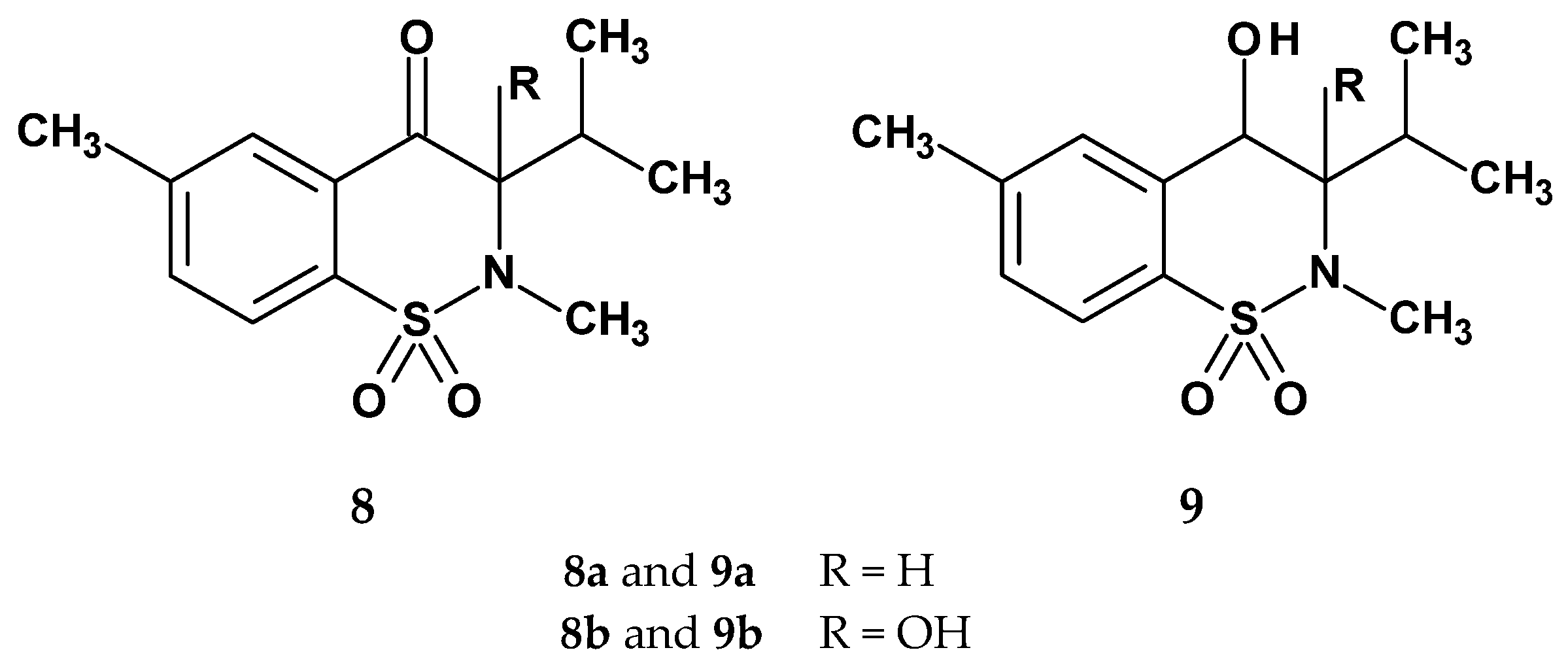
| Compound | Inhibition (%) after 3 h | ED50 |
|---|---|---|
| 8a | 27.8 | 29.8 |
| 8b | 47.0 | 9.2 |
| 9a | 32.4 | 23.4 |
| 9b | 54.5 | 4.7 |
| control | - | - |
| piroxicam | 67.3 | 4.4 |
| Compound | Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| The Writhing Test | The Formalin Test Phase 2 | The Zymosan Test | Carrageenan Test | COX-1 | COX-2 | |
| 10a | 76.1 | 11.1 | 43.7 | 57.4 | - | - |
| 10b | 58.6 | 38.4 | 73.9 | 22.5 | N/A | N/A |
| 10c | 75.1 | 9.1 | 56.6 | 62.2 | - | - |
| 10d | 57.9 | 16.8 | 72.1 | 42.6 | - | - |
| 10e | 90.4 | 32.5 | 37.1 | 74.2 | - | - |
| 10f | 62.7 | 60.1 | 78.7 | 73.2 | N/A | N/A |
| 10g | 84.0 | 54.2 | 81.3 | 77.5 | - | - |
| 10h | 82.7 | 27.5 | 82.6 | 81.8 | - | - |
| piroxicam | 95.4 | 53.9 | 57.4 | 25.8 | - | - |
| Compound | ED50 (mg/kg) Early Phase | ED50 (mg/kg) Late Phase | Compound | ED50 (mg/kg) Early Phase | ED50 (mg/kg) Late Phase |
|---|---|---|---|---|---|
| 11a | 11.59 | 6.18 | 12a | 13.95 | 8.85 |
| 11b | 27.02 | 7.14 | 12b | - | 7.93 |
| 11c | - | 9.03 | 12c | 22.78 | 7.22 |
| 11d | - | 4.01 | 12d | 12.83 | 6.24 |
| piroxicam | - | 18.85 | 12e | 11.92 | 8.25 |
| Compound | R1 | R2 | Ar | COX-1 (%) | COX-2 (%) |
|---|---|---|---|---|---|
| 13a | H | CH3 | 2-thiazole | 51 | 35 |
| 13b | H | CH3 | 5-ethyl-2-thiazole | 30 | 1 |
| 13c | H | CH3 | 5-phenyl-2-thiazole | 97 | 19 |
| 13d | H | CH3 | 5-methyl-2-thiadiazole | 2 | 23 |
| 13e | H | CH3 | 4-methyl-2-oxazole | −7 | −7 |
| 13f | H | CH3 | 5-methyl-3-isoxazole | 10 | 9 |
| 13g | H | CH3 | phenyl | 26 | −18 |
| 13h | 6-OCH3 | CH3 | 5-methyl-2-thiazole | −36 | 40 |
| 13i | 6-CH3 | CH3 | 5-methyl-2-thiazole | 24 | 50 |
| 13j | 6-Cl | CH3 | 5-methyl-2-thiazole | 62 | 76 |
| 13k | 6-F | CH3 | 5-methyl-2-thiazole | 56 | 72 |
| 13l | 7-OCH3 | CH3 | 5-methyl-2-thiazole | 50 | 70 |
| meloxicam | H | CH3 | 5-methyl-2-thiazole | 39 | 77 |
| piroxicam | H | CH3 | 2-pyridine | 35 | 62 |
| Compound | R1 | R2 | Ar | COX-1 (%) | COX-2 (%) |
|---|---|---|---|---|---|
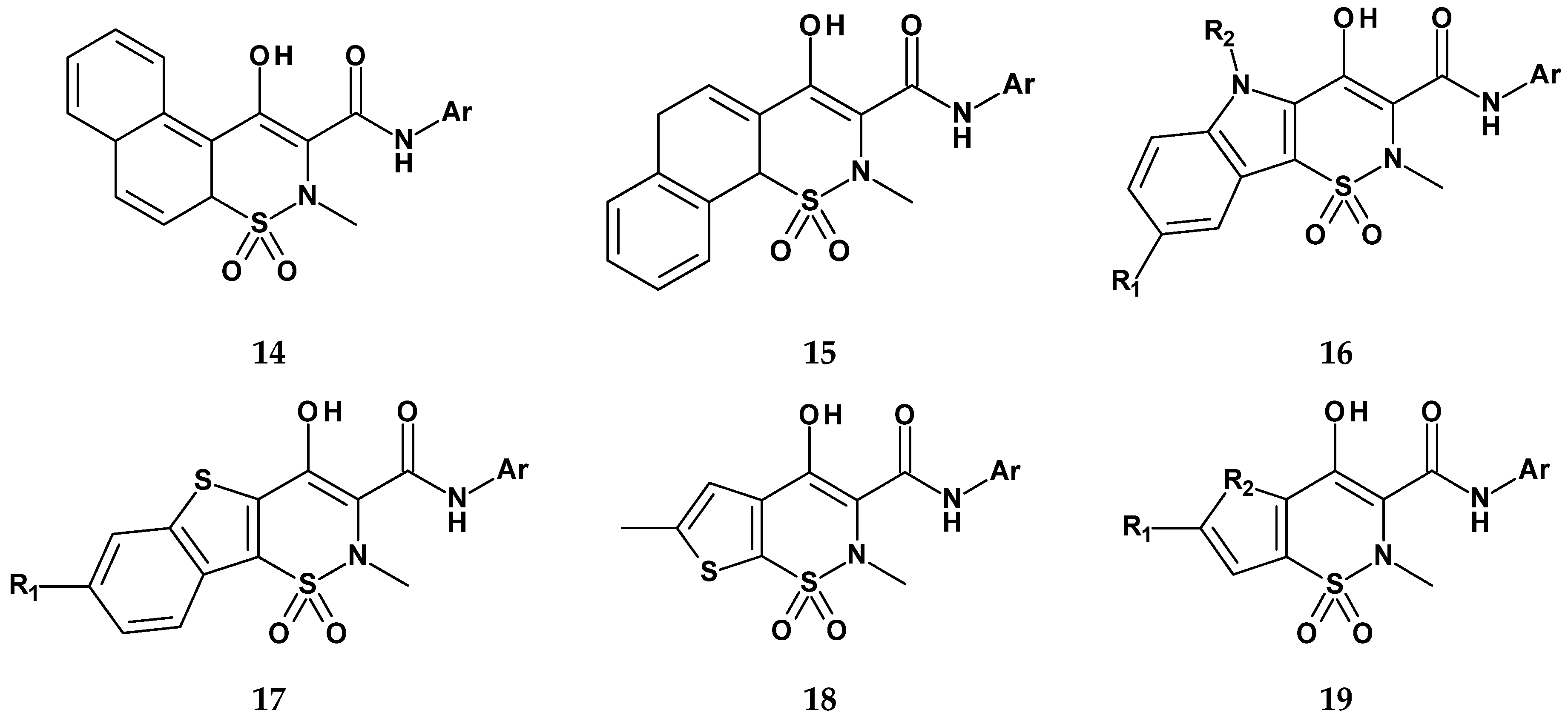
| 14 | - | - | 2-thiazole | 69 | 1 |
| 15 | - | - | 2-thiazole | 100 | 43 |
| 16 | H | CH2CH3 | 2-thiazole | 86 | 1 |
| 17 | Cl | - | 2-thiazole | 100 | 37 |
| 18 | - | - | 2-thiazole | −24 | −7 |
| 19 | H | S | 5-methyl-2-thiazole | 41 | 77 |
| meloxicam | - | - | - | 39 | 77 |
| Compound | R1 | R2 | Ar | COX-1 (%) | COX-2 (%) |
|---|---|---|---|---|---|
| 20a | 6-Cl | methyl | 2,4-dichlorophenyl | 100 | 100 |
| 20b | 6-Cl | methyl | 2-chlorophenyl | 100 | 100 |
| 20c | 6-Cl | methyl | 2,4-dibromophenyl | 99 | 97 |
| 20d | 6-Cl | benzyl | 2,4-dichlorophenyl | 100 | 92 |
| 20e | 6-Cl | ethyl | 2,4-dichlorophenyl | 99 | 100 |
| 20f | 6-Cl | cyclopropyl | 2,4-dichlorophenyl | 100 | 100 |
| 21 | - | - | 4-chlorophenyl | 100 | 93 |
| 22 | - | - | phenyl | 72 | 13 |
| 23 | Cl | S | 4-dichlorophenyl | 91 | 74 |
| 24 | - | - | 4-chlorophenyl | 100 | 96 |
| meloxicam | - | - | - | 39 | 77 |
| Group | Dose (mg/200 g) | Pain Tolerance n = 6 | Proximal Joint Mobilization Tolerance n = 6 | MDA (nmol/mL) n = 6 | RF (IU/mL) n = 6 |
|---|---|---|---|---|---|
| non-arthritic non-treated | solvent (SCMC) | 1.0 ± 0.00 | 1.48 ± 1.18 | 2.74 ± 2.1 | 178 ± 2.1 |
| arthritic non-treated | solvent (SCMC) | 3.9 ± 0.10 | 3.15 ± 0.55 | 4.0 ± 0.39 | 528 ± 71.0 |
| Cu(Ac)2·2H2O | 0.22 | 2.5 ± 0.09 | 2.58 ± 0.15 | 2.8 ± 0.33 | 385 ± 21.0 |
| piroxicam | 0.36 | 2.1 ± 0.12 | 2.07 ± 0.28 | 2.8 ± 0.2 | 325 ± 10.0 |
| sudoxicam | 0.36 | 2.8 ± 0.14 | 2.58 ± 0.6 | 1.5 ± 0.42 | 258 ± 31.0 |
| Cu complex | 0.96 | 1.7 ± 0.09 | 1.67 ± 0.11 | 1.8 ± 0.42 | 190 ± 9.0 |
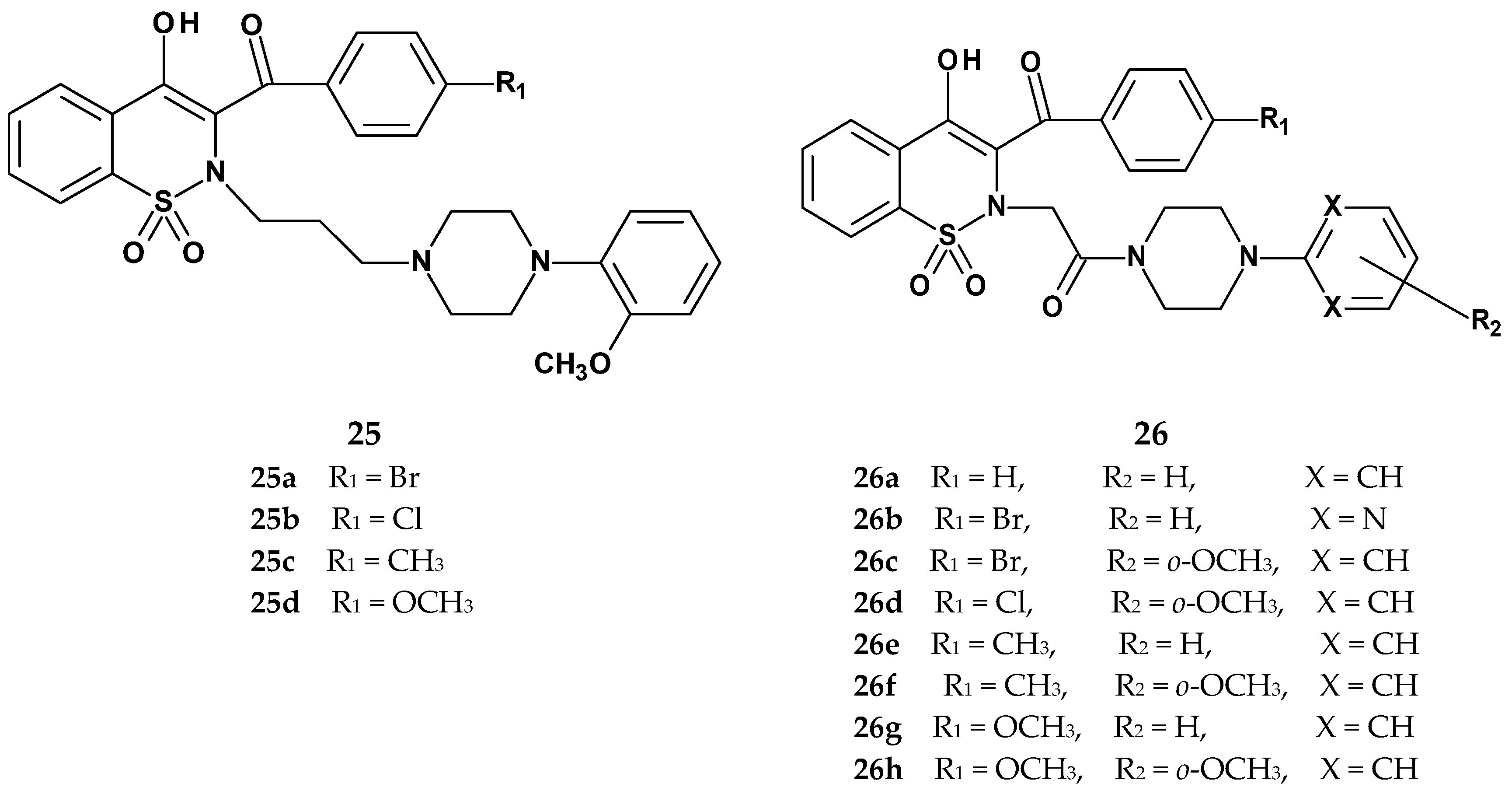
| Compound | Cyclooxygenase Inhibition Assay IC50 (µM) | COX Selectivity Ratio IC50(COX-2)/IC50(COX-1) | |
|---|---|---|---|
| COX-1 | COX-2 | ||
| 25a | 380.5 | N/A | - |
| 25b | 158.4 | N/A | - |
| 25c | 192.4 | N/A | - |
| 25d | 103.5 | N/A | - |
| 26a | 87.6 | 125.2 | 1.43 |
| 26b | 146.4 | 115.8 | 0.79 |
| 26c | 101.8 | N/A | - |
| 26d | 204.0 | 205.6 | 1.01 |
| 26e | 82.9 | 94.0 | 1.13 |
| 26f | 70.4 | 158.6 | 2.25 |
| 26g | 64.1 | 116.7 | 1.82 |
| 26h | 79.1 | N/A | - |
| meloxicam | 83.7 | 59.2 | 0.71 |
| Compound | Cyclooxygenase Inhibition Assay | Selectivity Ratio (COX-2/COX-1) | MTT Assay IC50 (µM) | |
|---|---|---|---|---|
| COX-1 | COX-2 | |||
| IC50 (µM) | IC50 (µM) | |||
| 27a | 241.64 ± 4.2 | 13.19 ± 2.1 | 0.05 | 228.40 ± 28.1 |
| 27b | 130.96 ± 7.5 | 62.63 ± 1.7 | 0.48 | 200.10 ± 20.9 |
| 27c | 128.73 ± 9.1 | 18.20 ± 4.3 | 0.14 | 203.28 ± 22.9 |
| 27d | 124.81 ± 6.7 | 17.80 ± 2.1 | 0.14 | 232.51 ± 11.8 |
| 28a | 126.54 ± 3.6 | 26.34 ± 3.4 | 0.21 | 163.46 ± 15.7 |
| 28b | 116.89 ± 4.3 | 25.55 ± 2.0 | 0.22 | 190.60 ± 12.5 |
| 28c | 121.51 ± 3.4 | 59.22 ± 3.7 | 0.49 | 168.50 ± 30.4 |
| 29 | 95.88 ± 5.7 | 12.46 ± 1.9 | 0.13 | 179.74 ± 13.6 |
| meloxicam | 267.71 ± 8.1 | 112.67 ± 3.3 | 0.42 | 174.23 ± 20.3 |
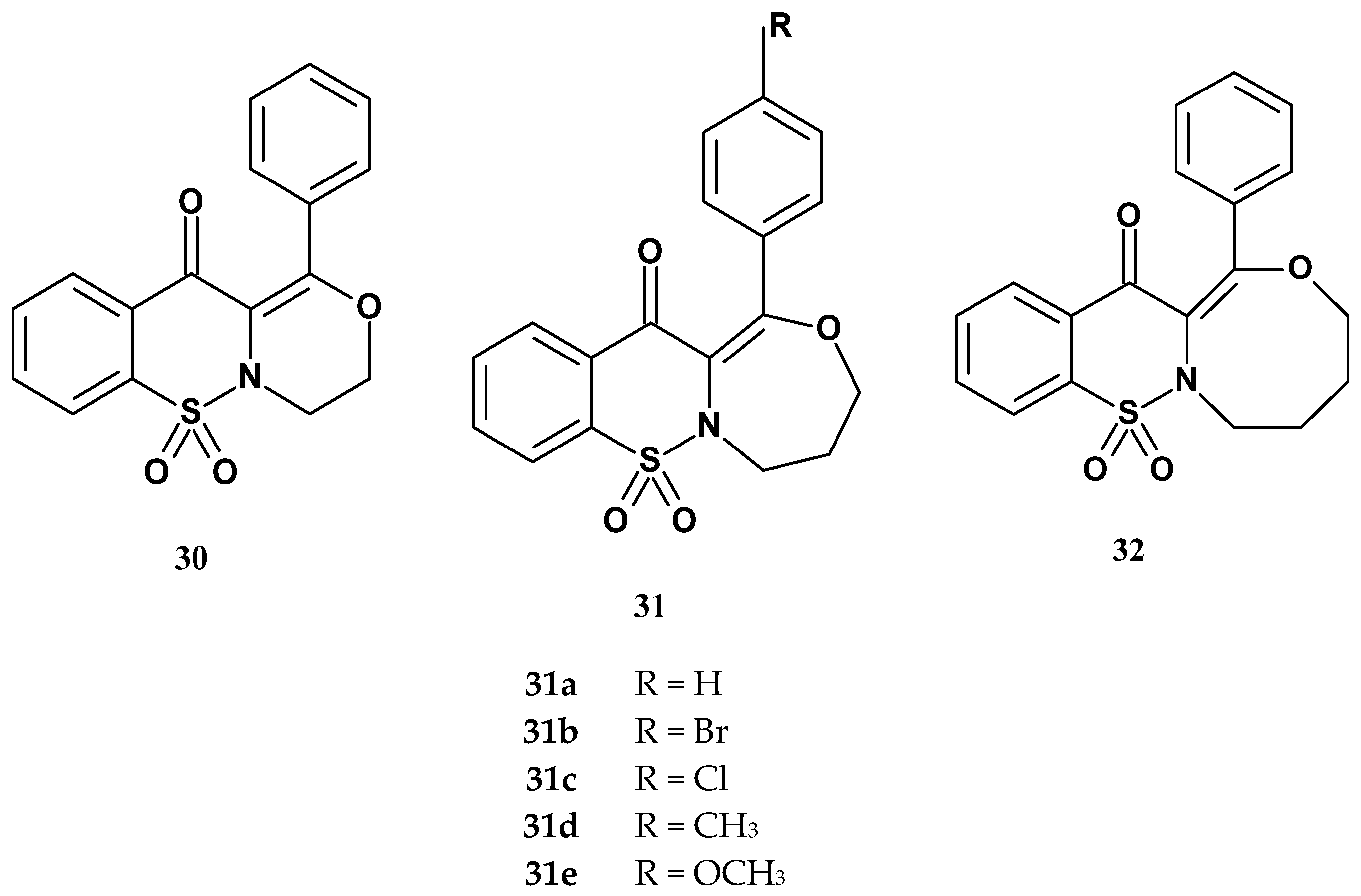
| Compound | IC50 (µM) | Ratio: COX-2/COX-1 | |
|---|---|---|---|
| COX-1 | COX-2 | ||
| Xa | 91.2 (0.18) | 54.6 (0.06) | 0.60 |
| Xb | 89.0 (0.16) | 55.9 (0.08) | 0.63 |
| Xc | 89.3 (0.09) | 55.3 (0.10) | 0.62 |
| 30 | 66.1 (0.08) | 56.9 (0.13) | 0.86 |
| 31a | 105.5 (0.15) | 89.9 (0.14) | 0.85 |
| 31b | 86.9 (0.15) | 95.1(0.10) | 1.09 |
| 31c | 89.8 (0.14) | 94.1 (0.12) | 1.05 |
| 31d | 125.7 (0.07) | 92.9 (0.09) | 0.74 |
| 31e | 115.3 (0.11) | 56.9 (0.06) | 0.49 |
| 32 | 86.1 (0.08) | 54.0 (0.09) | 0.63 |
| meloxicam | 83.7 (0.10) | 59.2 (0.12) | 0.71 |
 | |||
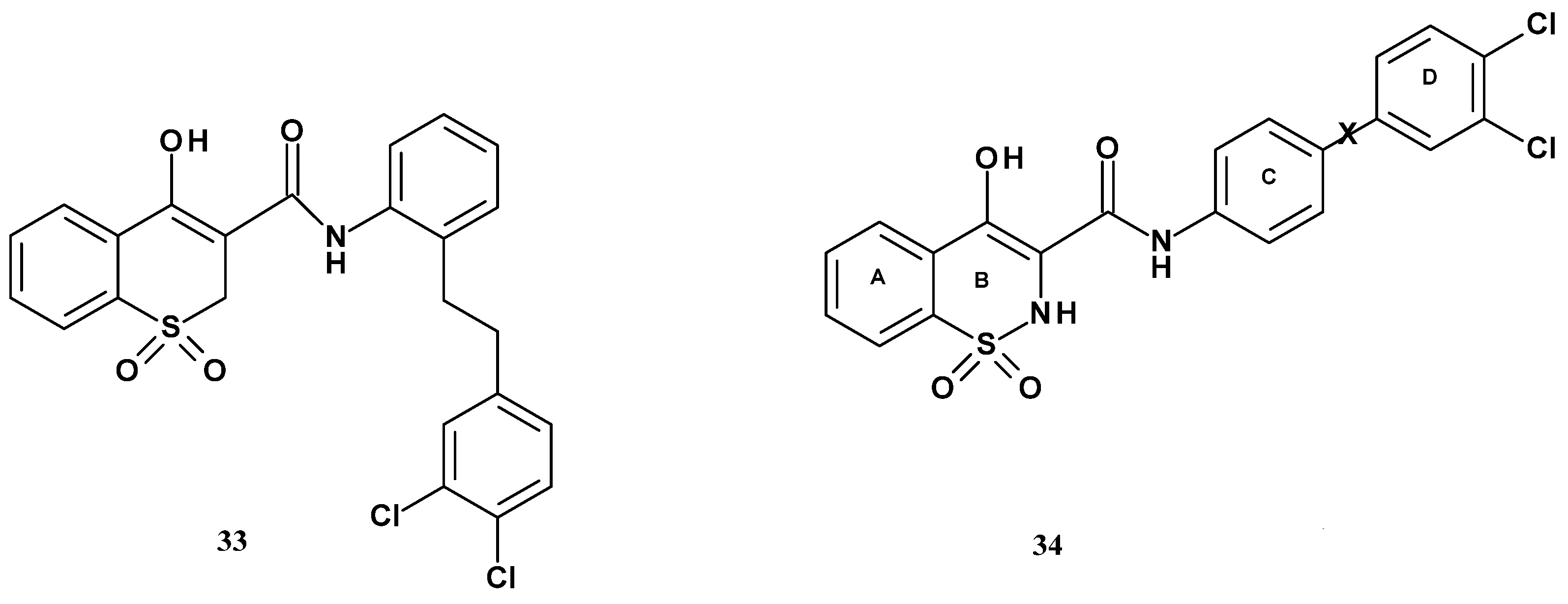
| Compound | R | mPGES-1 IC50 (µM) | mPGES-1/COX-2 Selectivity |
| 35a | 4-(4-chlorobiphenyl) | 0.29 | 23 |
| 35b | 4-(4-chlorophenoxy)phenyl | 0.53 | 10 |
| 35c | N-(4-chlorophenylo)-4-aniline | 0.15 | >23 |
| 35d | 4-(4-chlorobenzoyl)-3-chlorophenyl | 0.11 | 84 |
| 35e | 3-(4-chlorobenzoyl)phenyl | 3.91 | 44 |
 | ||||
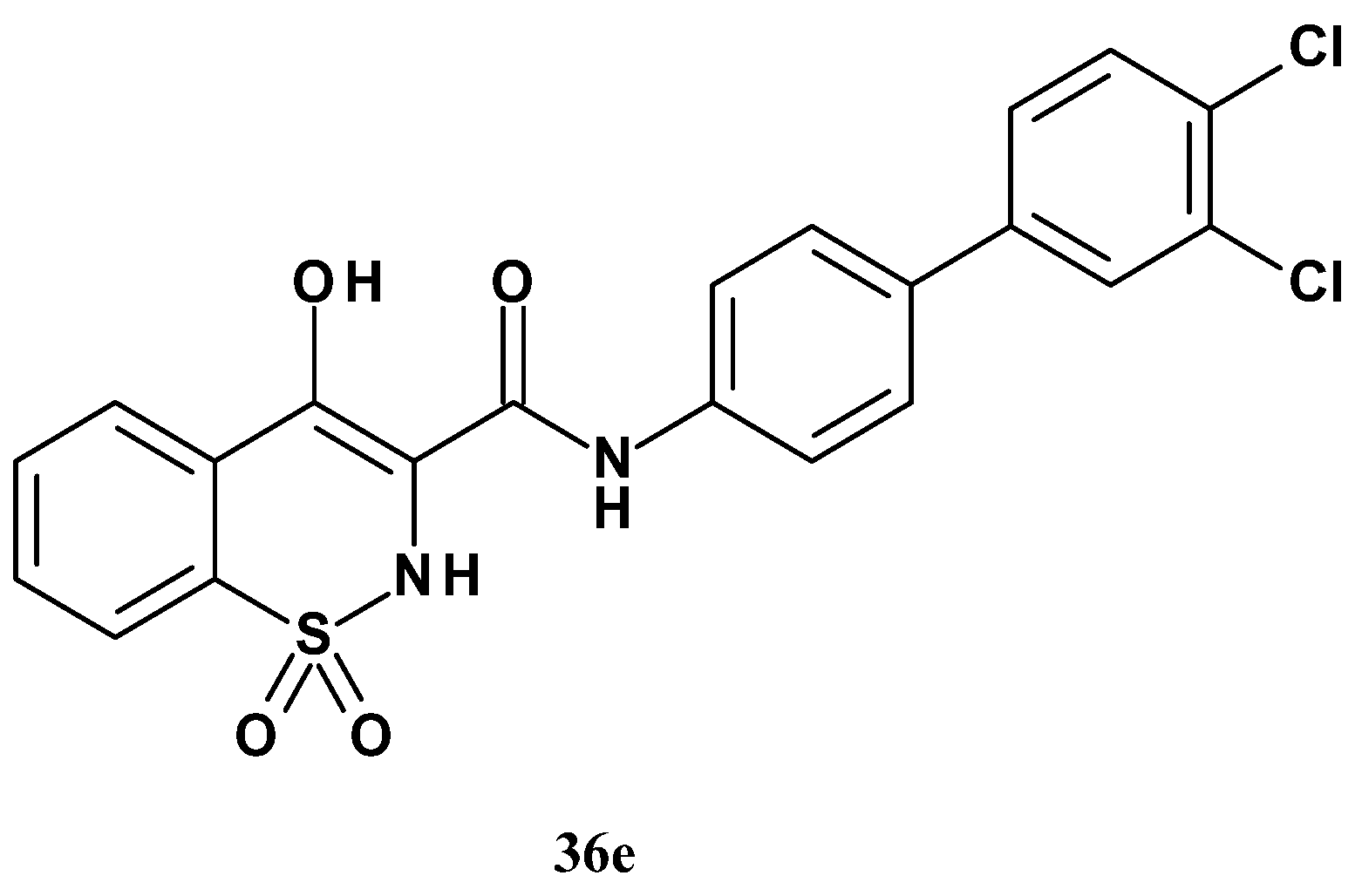
| Compound | R1 | R2 | mPGES-1 IC50 (µM) | mPGES-1/COX-2 Selectivity |
| 36a | H | 3-Cl | 0.86 | 22 |
| 36b | H | 4-CH3 | <0.07 | >45 |
| 36c | H | 4-CN | 3.96 | - |
| 36d | H | 4-H | 3.13 | 14 |
| 36e | 3-Cl | 4-Cl | 0.016 | >238 |
| 36f | 2-Cl | 3-Cl | 0.038 | >48 |
| 36g | 2-Cl | 4-Cl | 0.043 | >98 |
| 36h | 3-OCH3 | 4-OCH3 | 32.2 | >77 |
| 36i | 3-CH3 | 4-CH3 | <0.026 | 14 |
| 36j | 2-CH3 | 3-CH3 | 1.00 | - |
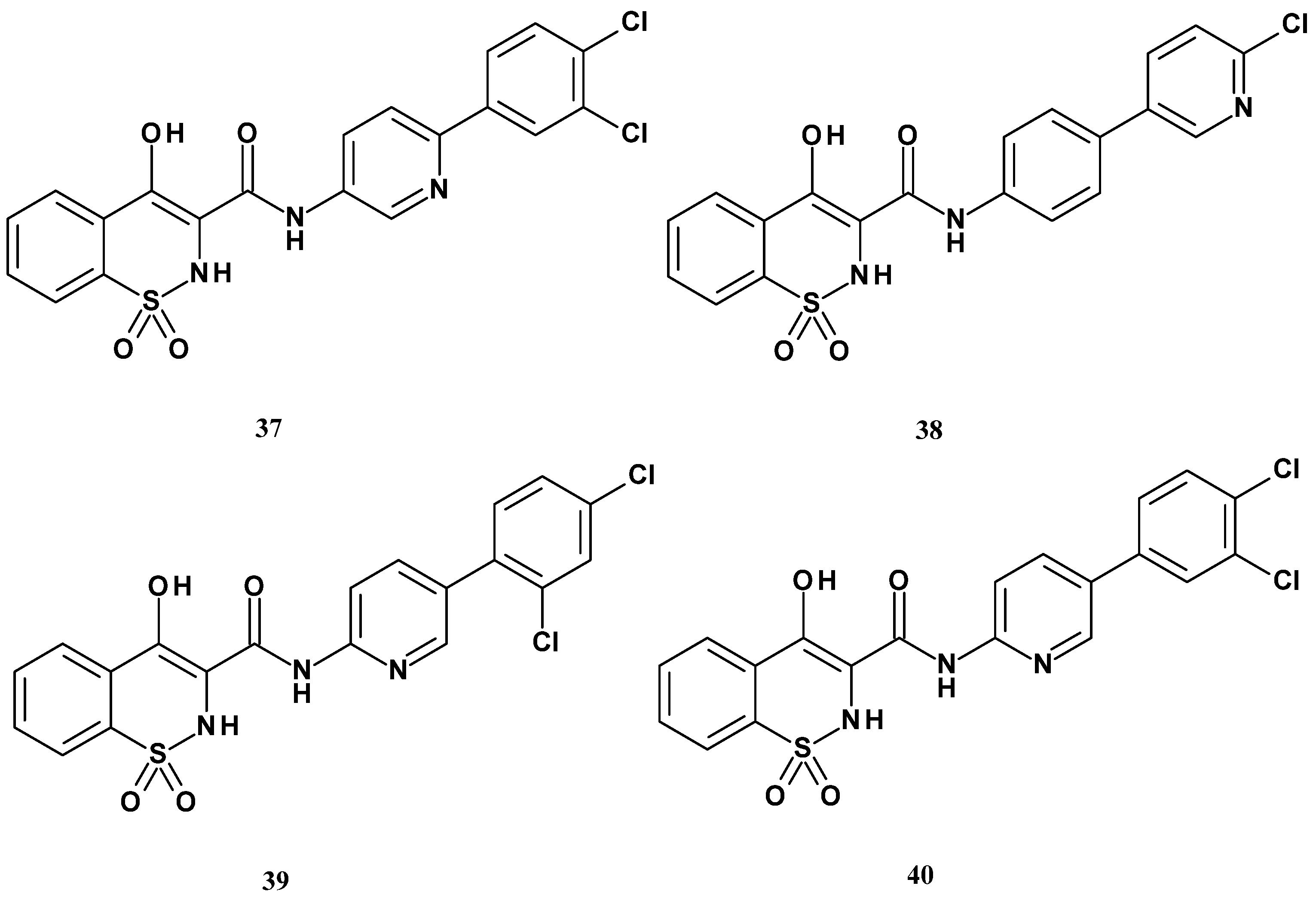
| Compound | mPGES-1 IC50 (µM) |
|---|---|
| 37 | 0.16 |
| 38 | 12.7 |
| 39 | 11.7 |
| 40 | 65.6 |
 | |||||
| Compound | R1 | R2 | R3 | mPGES-1 IC50 (µM) | mPGES-1/COX-2 Selectivity |
| 41a | H | H | OCH3 | 0.69 | >77 |
| 41b | H | H | OH | 0.098 | >69 |
| 41c | H | OCH3 | H | 3.05 | - |
| 41d | H | OH | H | 0.18 | - |
| 41e | CH3 | H | H | 0.28 | - |
| 41f * | H | H | H | 0.19 | >149 |
| 42 | - | - | - | 5.76 | >3 |
| 43 | - | - | - | 29.6 | - |
| Compound | Secretion Inhibition Efficacy IC50 (µM) | Cell Viability (%) | ||
|---|---|---|---|---|
| TNF-α | IL-8 | MCP-1 | ||
| 44a | NA | NA | NA | 93.05 ± 1.09 |
| 44b | NA | NA | NA | 91.95 ± 2.09 |
| 44c | NA | NA | NA | 96.25 ± 1.22 |
| 44d | NA | NA | NA | 94.23 ± 3.25 |
| 44e | NA | 16.7 ± 1.9 | 5.7 ± 1.1 | 93.00 ± 1.26 |
| 44f | NA | NA | NA | 97.50 ± 1.29 |
| 44g | NA | NA | NA | 92.34 ± 2.01 |
| 44h | NA | NA | NA | 90.25 ± 2.87 |
| 45a | NA | NA | NA | 94.12 ± 1.69 |
| 45b | NA | NA | 9.4 ± 0.7 | 95.50 ± 3.11 |
| 45c | NA | NA | NA | 92.45 ± 1.65 |
| 45d | NA | NA | NA | 97.50 ± 1.73 |
| 45e | 12.1 ± 1.1 | NA | 4.0 ± 1.0 | 93.00 ± 1.41 |
| 45f | NA | NA | NA | 95.12 ± 2.51 |
| 45g | NA | NA | NA | 92.15 ± 1.52 |
| 45h | 6.0 ± 0.7 | NA | 2.5 ± 0.9 | 91.25 ± 1.26 |
| piroxicam | NA | NT | NA | - |
| pioglitazone | NT | NT | 18.6 ± 2.1 | - |
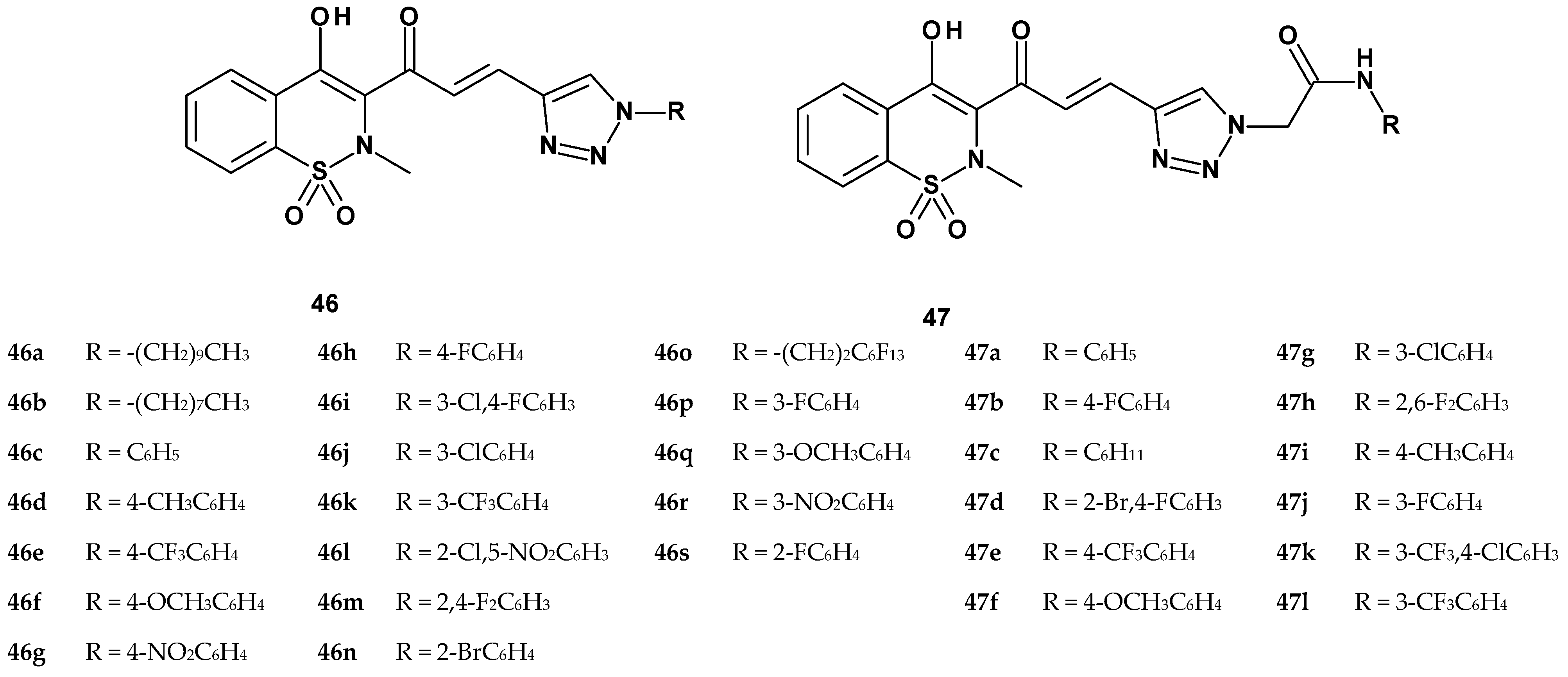
| Compound | Inhibition (%) | Cell Viability | ||
|---|---|---|---|---|
| TNF-α Secretion | IL-1β Secretion | MCP-1 Secretion | ||
| 46b | 2.9 ± 1.2 | 64.7 ± 5.4 | 40.9 ± 6.5 | 92.32 ± 1.20 |
| 46c | 24.0 ± 3.4 | 56.9 ± 7.8 | 31.9 ± 3.1 | 94.20 ± 3.36 |
| 46d | NA | 50.2 ± 6.5 | 45.2 ± 5.3 | 94.32 ± 1.08 |
| 46e | NA | 17.7 ± 2.4 | 12.7 ± 2.9 | 96.62 ± 2.42 |
| 46f | NA | 53.9 ± 4.1 | 58.9 ± 6.8 | 94.40 ± 1.92 |
| 46g | NA | 36.5 ± 3.7 | 31.5 ± 2.6 | 92.98 ± 2.08 |
| 46h | NA | 43.9 ± 3.5 | 58.9 ± 5.6 | 95.32 ± 1.54 |
| 46i | NA | 16.3 ± 6.4 | NA | 94.26 ± 2.60 |
| 46j | NA | 37.3 ± 8.6 | 5.4 ± 1.2 | 96.03 ± 1.52 |
| 46k | NA | 23.9 ± 4.5 | NA | 93.80 ± 3.24 |
| 46l | 52.5 ± 6.3 | 61.8 ± 2.8 | 67.1 ± 4.5 | 94.26 ± 2.06 |
| 46m | 11.2 ± 6.5 | 47.7 ± 3.8 | 29.6 ± 3.2 | 96.20 ± 2.30 |
| 46n | 13.7 ± 2.9 | 45.9 ± 4.3 | 16.2 ± 3.9 | 94.40 ± 1.54 |
| 46p | 48.3 ± 3.4 | NA | NA | 93.05 ± 2.16 |
| 46q | NA | 42.6 ± 2.9 | NA | 94.15 ± 1.29 |
| 46r | NA | 25.6 ± 4.7 | 30.7 ± 5.3 | 92.60 ± 2.20 |
| 46s | NA | 40.2 ± 4.5 | NA | 96.06 ± 1.15 |
| 47a | 33.3 ± 4.5 | 49.3 ± 3.2 | 76.7 ± 6.2 | 96.23 ± 1.02 |
| 47b | 73.4 ± 6.5 | 42.4 ± 4.5 | 41.4 ± 4.5 | 92.5 ± 2.16 |
| 47c | 39.7 ± 2.8 | 47.7 ± 5.3 | 56.1 ± 5.5 | 95.18 ± 1.19 |
| 47d | 59.8 ± 3.8 | 63.8 ± 4.5 | 65.4 ± 3.2 | 93.56 ± 2.02 |
| 47e | 94.2 ± 4.5 | 91.1 ± 3.2 | 81.1 ± 6.8 | 95.08 ± 1.19 |
| 47f | 62.9 ± 5.6 | 49.8 ± 5.8 | 64.8 ± 8.2 | 94.45 ± 2.23 |
| 47g | 91.1 ± 4.5 | 80.4 ± 6.2 | 84.0 ± 4.4 | 98.23 ± 1.45 |
| 47h | 31.3 ± 3.2 | 46.3 ± 4.6 | 41.3 ± 2.8 | 95.62 ± 2.08 |
| 47i | 84.7 ± 6.2 | 74.9 ± 7.2 | 87.2 ± 5.3 | 97.03 ± 1.25 |
| 47j | 70.7 ± 5.6 | 69.5 ± 6.5 | 48.2 ± 4.8 | 96.45 ± 2.22 |
| 47k | 93.2 ± 2.8 | 93.6 ± 4.8 | 88.4 ± 3.6 | 94.28 ± 2.04 |
| 47l | 87.6 ± 4.8 | 83.6 ± 5.6 | 85.1 ± 5.3 | 96.20 ± 3.03 |
| celecoxib | 37.4 ± 8.3 | 31.1 ± 3.2 | 20.1 ± 3.2 | - |
| piroxicam | 25.8 ± 4.5 | 21.8 ± 6.2 | 11.4 ± 2.3 | - |
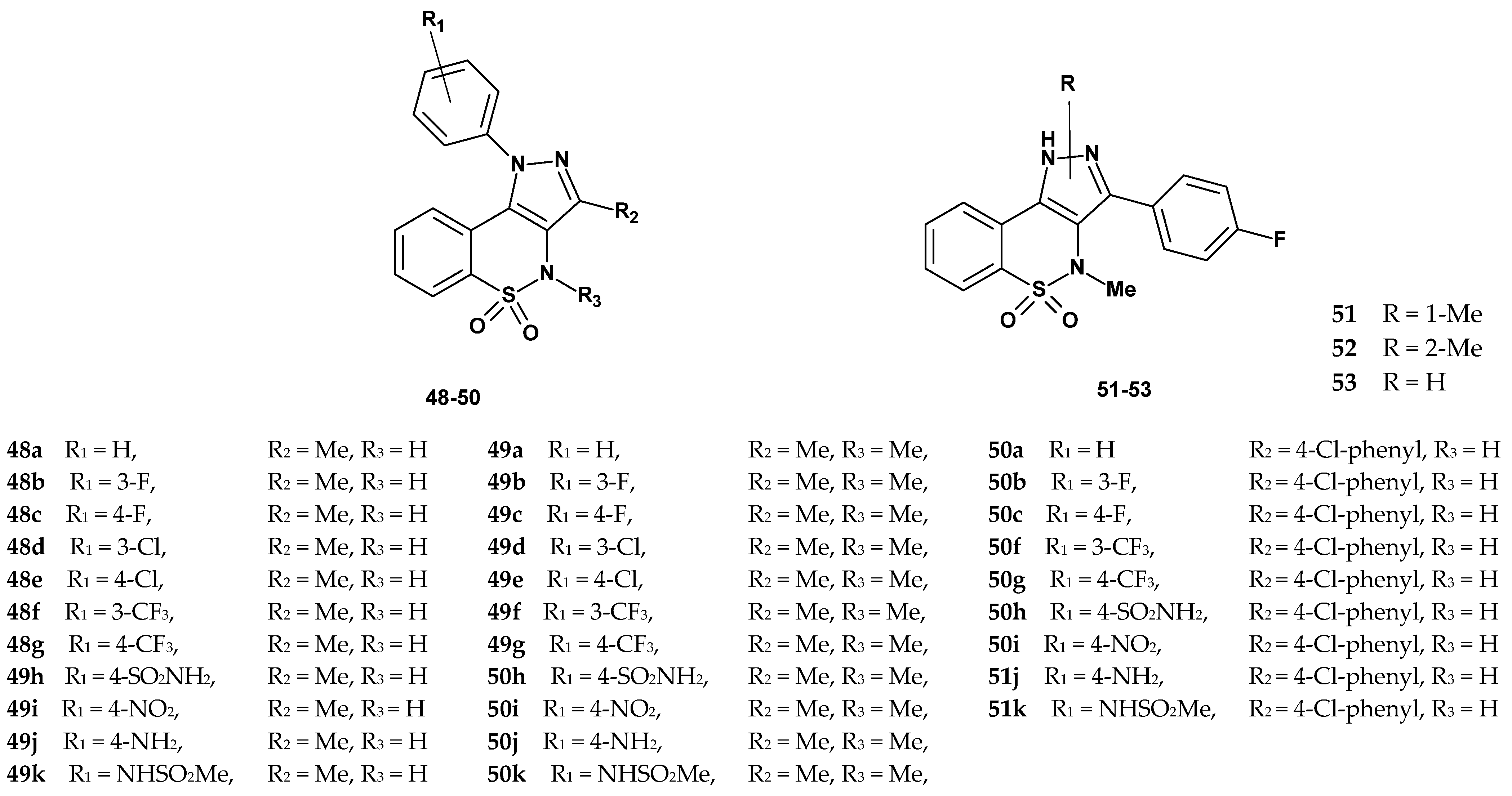
| Compound | Inhibition (%) p38α | IC50 (µM) | Compound | Inhibition (%) p38α | IC50 (µM) | ||
|---|---|---|---|---|---|---|---|
| p38α | TNF-α | p38α | TNF-α | ||||
| 48a | 26.4 | NC | 92.3 ± 5.6 | 49g | 26.6 | NC | 25 ± 2.1 |
| 48b | 52.0 | NC | >100 | 49h | 15.0 | NC | >100 |
| 48c | 46.8 | NC | >100 | 49i | 28.2 | NC | >100 |
| 48d | 91.0 | 0.936 ± 0.296 | 18 ± 3.5 | 49j | 25.5 | NC | >100 |
| 48e | 24.5 | NC | >100 | 49k | 33.9 | NC | >100 |
| 48f | 74.7 | 0.967 ± 0.137 | >100 | 50a | 65.8 | 7.727 ± 1.196 | >100 |
| 48g | 41.3 | NC | >100 | 50b | 79.5 | 2.863 ± 0.169 | 52 ± 2.5 |
| 48h | 60.1 | NC | >100 | 50c | 87.0 | 1.949 ± 0.314 | 43 ± 2.8 |
| 48i | 67.8 | 2.784 ± 0.694 | >100 | 50f | 75.3 | 3.332 ± 0.168 | >100 |
| 48j | 30.0 | NC | >100 | 50g | 51.5 | NC | 82.7 ± 5.2 |
| 48k | 85.1 | 0.593 ± 0.099 | 20 ± 2.6 | 50h | 78.8 | 2.749 ± 0.294 | >100 |
| 49a | 14.4 | NC | >100 | 50i | 76.5 | 3.529 ± 0.119 | 51 ± 3.2 |
| 49b | 19.5 | NC | >100 | 50j | 50.0 | NC | >100 |
| 49c | 36.4 | NC | >100 | 50k | 81.7 | 1.124 ± 0.357 | 32.4 ± 2.8 |
| 49d | 27.7 | NC | 31 ± 1.8 | 51 | 96 | 0.431 ± 0.053 | >100 |
| 49e | 35.6 | NC | >100 | 52 | 82 | 2.424 ± 0.154 | 12 ± 1.8 |
| 49f | 16.8 | NC | >100 | 53 | 95 | 0.457 ± 0.054 | 0.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczęśniak-Sięga, B.M.; Topolska, I. Anti-Inflammatory Activity of 1,2-Benzothiazine 1,1-Dioxide Derivatives. Pharmaceuticals 2025, 18, 1484. https://doi.org/10.3390/ph18101484
Szczęśniak-Sięga BM, Topolska I. Anti-Inflammatory Activity of 1,2-Benzothiazine 1,1-Dioxide Derivatives. Pharmaceuticals. 2025; 18(10):1484. https://doi.org/10.3390/ph18101484
Chicago/Turabian StyleSzczęśniak-Sięga, Berenika M., and Izabela Topolska. 2025. "Anti-Inflammatory Activity of 1,2-Benzothiazine 1,1-Dioxide Derivatives" Pharmaceuticals 18, no. 10: 1484. https://doi.org/10.3390/ph18101484
APA StyleSzczęśniak-Sięga, B. M., & Topolska, I. (2025). Anti-Inflammatory Activity of 1,2-Benzothiazine 1,1-Dioxide Derivatives. Pharmaceuticals, 18(10), 1484. https://doi.org/10.3390/ph18101484







