Chronic Administration of Calendula officinalis Ethanolic Extract Mitigates Anxiety-like Behavior and Cognitive Impairment Induced by Acute Scopolamine Exposure in Zebrafish
Abstract
1. Introduction
2. Results
2.1. Effects of CEE on Anxiety-like Behavior in Zebrafish
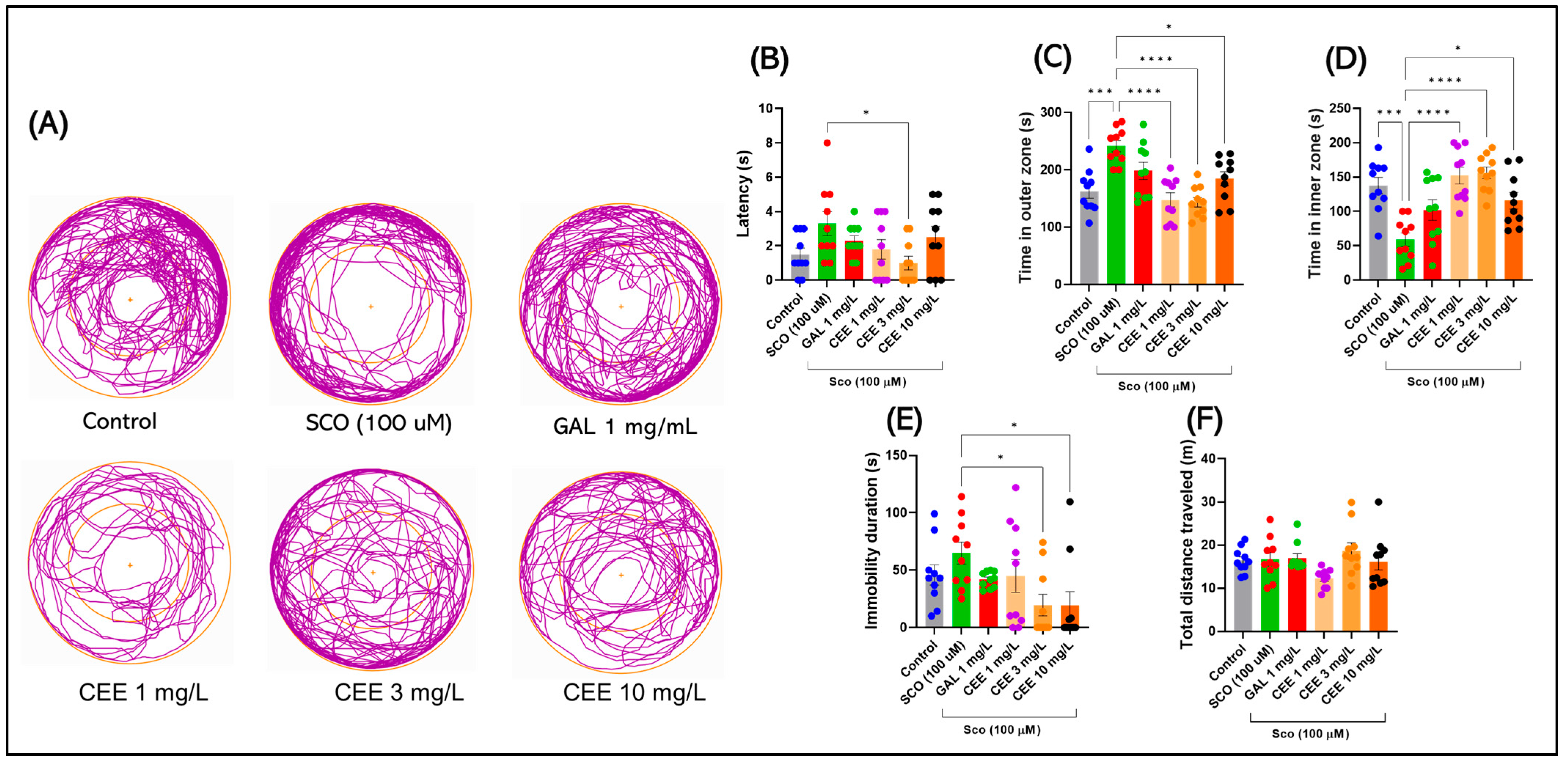
2.1.1. Novel Tank Diving Test (NTT)
2.1.2. Light/Dark Test (LDT)
2.1.3. Novel Approach Test (NAT)
2.2. Effects of CEE on Cognitive Performance
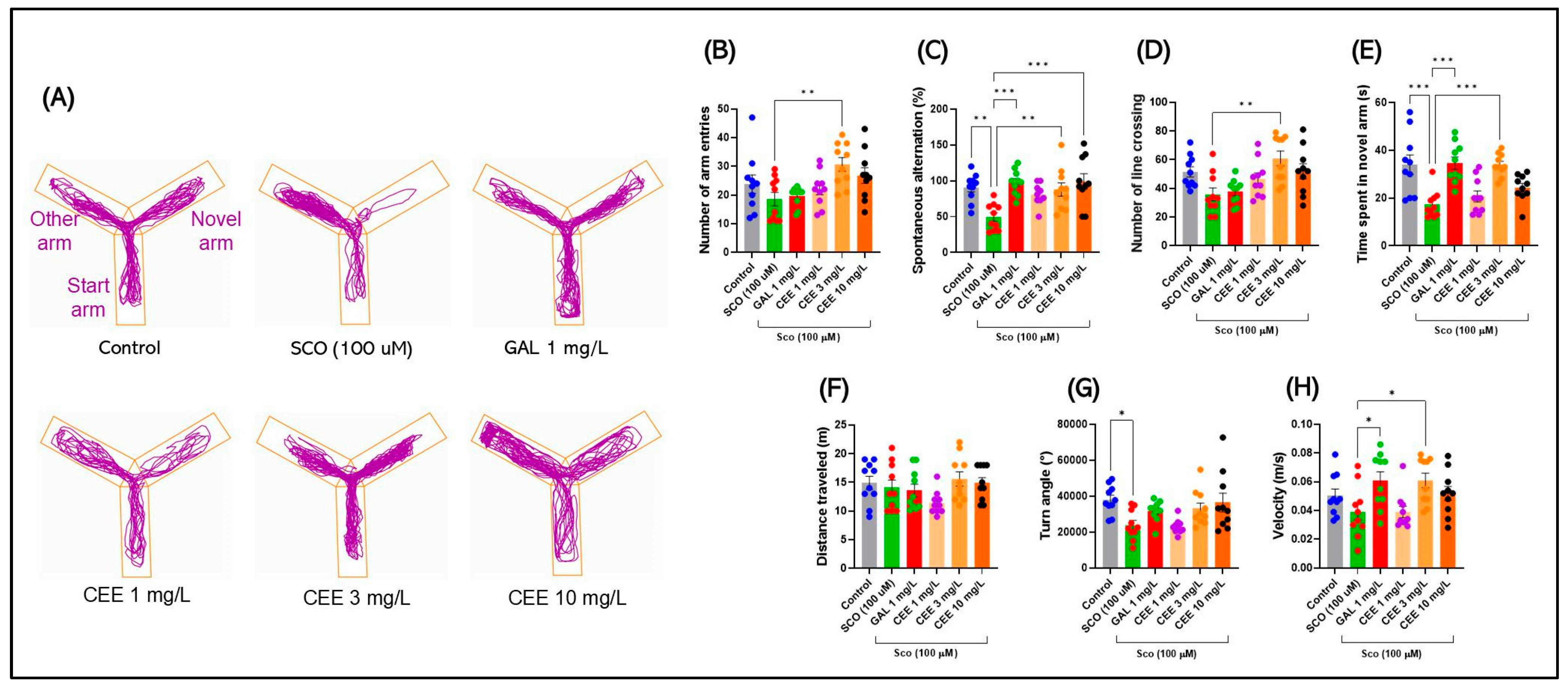
2.2.1. Y Maze
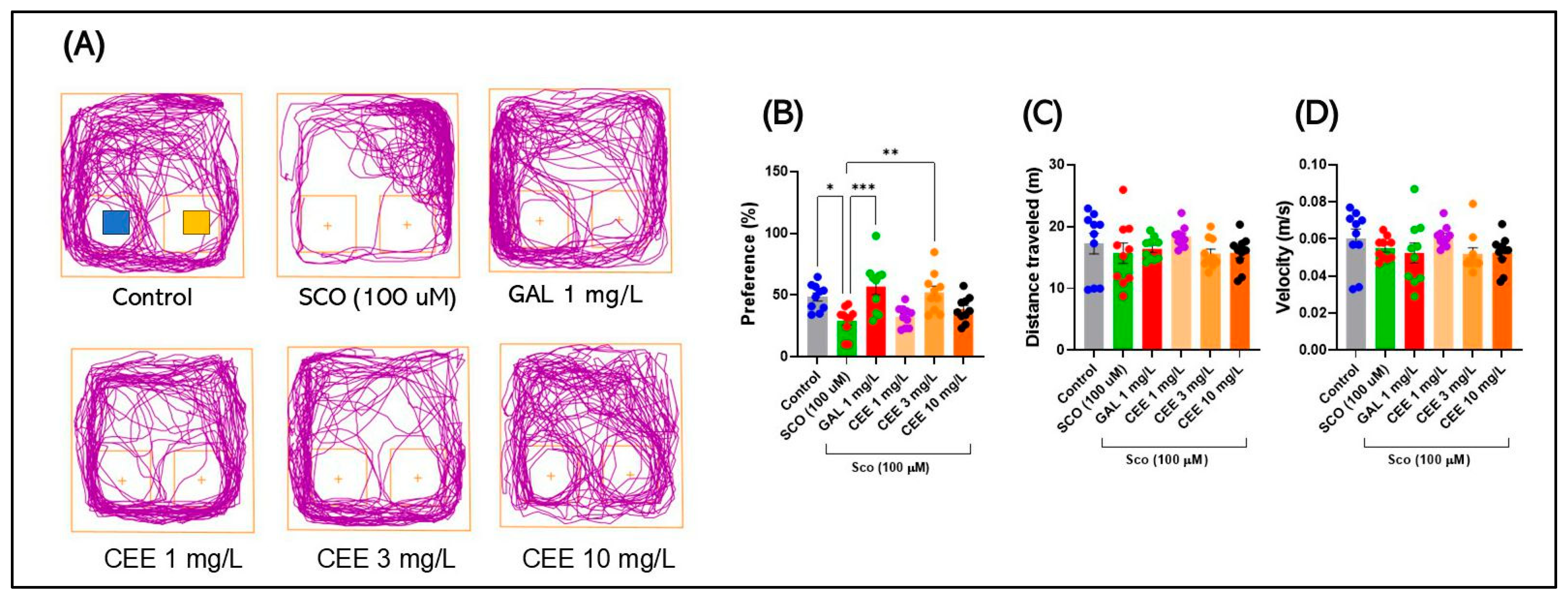
2.2.2. Novel Object Recognition (NOR)
2.3. Effects of CEE on Biochemical Parameters in Zebrafish
2.3.1. Acetylcholinesterase (AChE) Activity
2.3.2. Antioxidant Enzymes (SOD, CAT, GPx)
2.3.3. Glutathione (GSH) Levels
2.3.4. Oxidative Stress Markers (MDA, Protein Carbonyls)
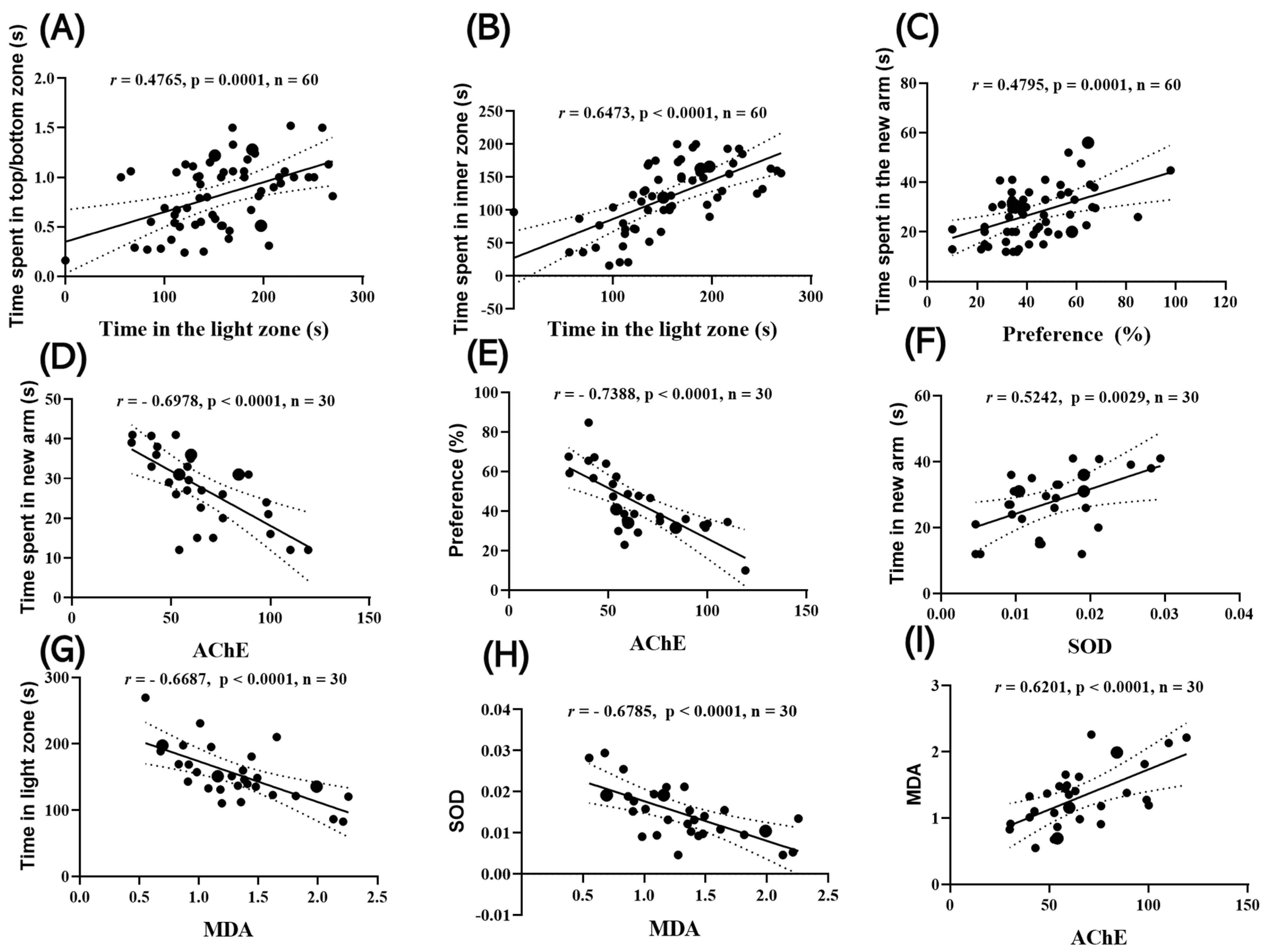
2.4. Correlations Between Behavioral Parameters and Biomarkers
3. Discussion
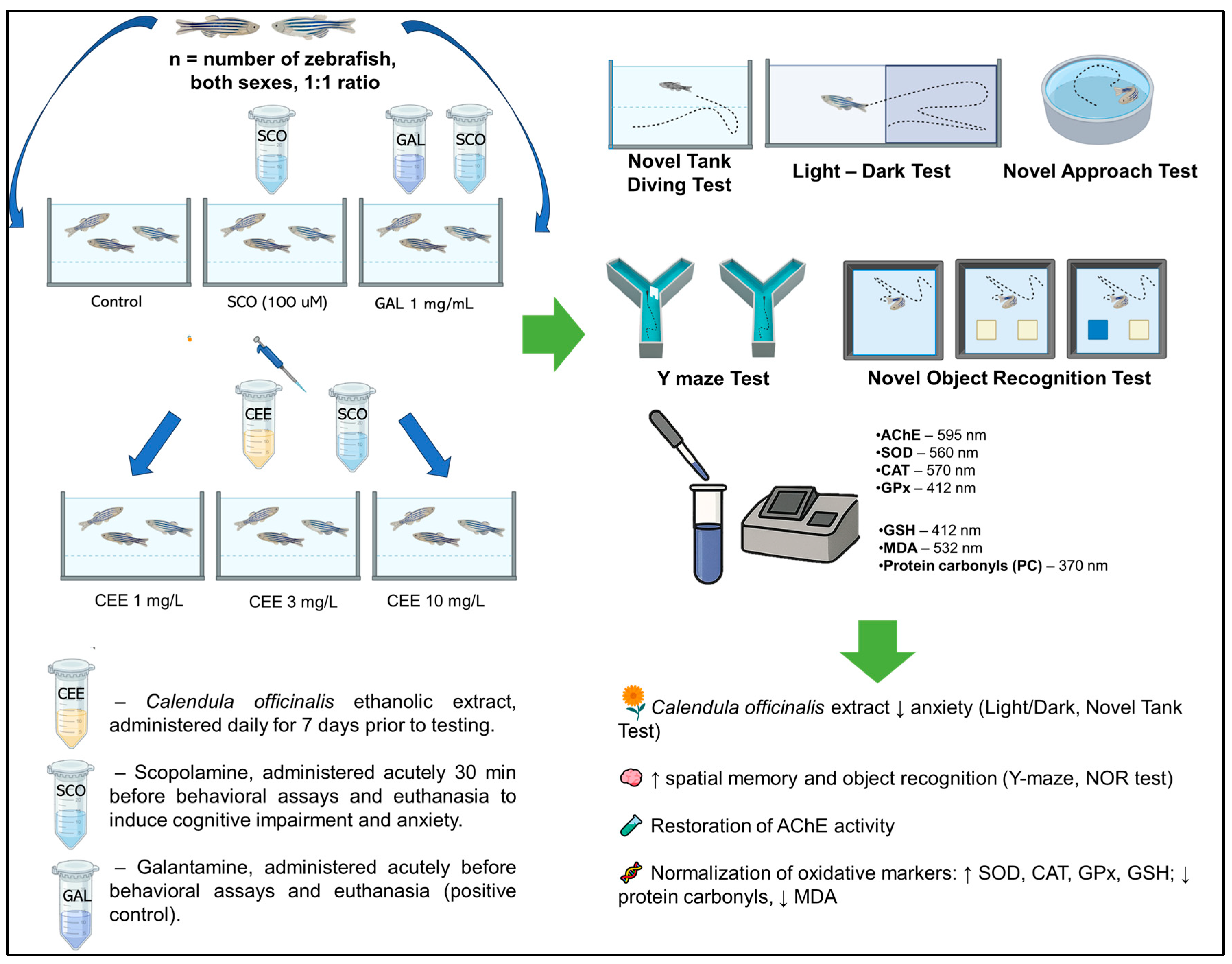
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Extracts
4.3. Animals
4.4. Treatment Administration
4.5. Behavioral Analysis
4.5.1. Novel Tank Diving Test (NTT)
4.5.2. Light/Dark Test (LDT)
4.5.3. Novel Approach Test (NAT)
4.5.4. Y-Maze Test
4.5.5. Novel Object Recognition (NOR)
4.6. Determination of Biochemical Parameters
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEE | Calendula officinalis ethanolic extract |
| SCO | Scopolamine |
| GAL | Galantamine |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| MCI | Mild cognitive impairment |
| BBB | Blood–brain barrier |
| NTT | Novel Tank Diving Test |
| LDT | Light/Dark Test |
| NAT | Novel Approach Test |
| NOR | Novel Object Recognition |
| BDNF | Brain-derived neurotrophic factor |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| GSH | Reduced glutathione |
| MDA | Malondialdehyde |
| ROS | Reactive oxygen species |
| FRAP | Ferric reducing antioxidant power |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical scavenging assay |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging assay |
| TBARS | Thiobarbituric acid reactive substances |
| DNPH | 2,4-dinitrophenylhydrazine |
| TXNIP | Thioredoxin-interacting protein |
| NLRP3 | NOD-like receptor family pyrin domain-containing 3 inflammasome |
| PI3K/Akt | Phosphatidylinositol 3-kinase/protein kinase B pathway |
| ERK | Extracellular signal-regulated kinase |
| Hsp90α | Heat shock protein 90 alpha |
| AMPK | AMP-activated protein kinase |
| HIF-1α | Hypoxia-inducible factor 1 alpha |
| MMP-9 | Matrix metalloproteinase-9 |
| TNF-α | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
| BSA | Bovine serum albumin |
| SEM | Standard error of the mean |
| ns | Not significant |
References
- Xu, L.J.; Liu, A.L.; Du, G.H. Scopolamine. In Natural Small Molecule Drugs from Plants; Springer: Singapore, 2023; pp. 319–324. [Google Scholar] [CrossRef]
- Klinkenberg, I.; Blokland, A. The Validity of Scopolamine as a Pharmacological Model for Cognitive Impairment: A Review of Animal Behavioral Studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef]
- Blokland, A. Cholinergic Models of Memory Impairment in Animals and Man: Scopolamine vs. Biperiden. Behav. Pharmacol. 2022, 33, 231–237. [Google Scholar] [CrossRef]
- Miravalles, C.; Cannon, D.M.; Hallahan, B. The Effect of Scopolamine on Memory and Attention: A Systematic Review and Meta-Analysis. Eur. Psychiatry 2025, 68, e50. [Google Scholar] [CrossRef]
- Ebert, U.; Kirch, W. Scopolamine Model of Dementia: Electroencephalogram Findings and Cognitive Performance. Eur. J. Clin. Investig. 1998, 28, 944–949. [Google Scholar] [CrossRef]
- Saraf, M.K.; Anand, A.; Prabhakar, S. Scopolamine Induced Amnesia Is Reversed by Bacopa monniera through Participation of Kinase-CREB Pathway. Neurochem. Res. 2010, 35, 279–287. [Google Scholar] [CrossRef]
- Tang, K.S. The Cellular and Molecular Processes Associated with Scopolamine-Induced Memory Deficit: A Model of Alzheimer’s Biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, M.; Lee, J.S.; Oh, J.; Lim, J. Protocatechuic Acid from Euonymus alatus Mitigates Scopolamine-Induced Memory Impairment in Mice. Foods 2024, 13, 2664. [Google Scholar] [CrossRef]
- Fu, C.W.; Tong, S.K.; Liu, M.X.; Liao, B.K.; Chou, M.Y. Scopolamine Affects Fear Learning and Social Recognition in Adult Zebrafish. Neuroscience 2025, 568, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.J.; Morrill, A.; Lucas, K.; Gallup, J.; Harris, M.; Healey, M.; Pitman, T.; Schalomon, M.; Digweed, S.; Tresguerres, M. Establishing Zebrafish as a Model to Study the Anxiolytic Effects of Scopolamine. Sci. Rep. 2017, 7, 15081. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Despande, B. Antioxidant Activity in the Leaves and Petals of Calendula officinalis Linn. Artic. Asian Pac. J. Health Sci. 2022, 9, 130–132. [Google Scholar] [CrossRef]
- Jan, N.; Iqbal Andrabi, K.; John, R. Calendula officinalis-An Important Medicinal Plant with Potential Biological Properties. Proc. Indian. Natl. Sci. Acad. 2017, 83, 769–787. [Google Scholar] [CrossRef]
- Dey, H.; Tarannum, M.; Kumar, P.; Agarwal, V.; Parvez, E.; Ali, N.; Shafieq, S.; Sharma, V. Calendula officinalis—A Crucial and Promising Antifungal Agent with Cytotoxic Biological Attributes. Sch. Acad. J. Pharm. 2024, 13, 30–36. [Google Scholar] [CrossRef]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals 2023, 16, 611. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef]
- Zarrelli, A.; Kowalski, R.; Baj, T.; Vella, F.M.; Pignone, D.; Laratta, B. The Mediterranean Species Calendula officinalis and Foeniculum Vulgare as Valuable Source of Bioactive Compounds. Molecules 2024, 29, 3594. [Google Scholar] [CrossRef]
- Alruhaimi, R.S.; Kamel, E.M.; Alnasser, S.M.; Alzoghaibi, M.A.; Lamsabhi, A.M.; Mahmoud, A.M. Mechanistic Insights into Carbonic Anhydrase IX Inhibition by Coumarins from Calendula officinalis: In Vitro and in Silico Approaches. RSC Adv. 2024, 14, 33602–33618. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.N.; Yeong, K.Y. Scopolamine, a Toxin-Induced Experimental Model, Used for Research in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2020, 19, 85–93. [Google Scholar] [CrossRef]
- Popovici, L.F.; Brinza, I.; Gatea, F.; Badea, G.I.; Vamanu, E.; Oancea, S.; Hritcu, L. Enhancement of Cognitive Benefits and Anti-Anxiety Effects of Phytolacca Americana Fruits in a Zebrafish (Danio rerio) Model of Scopolamine-Induced Memory Impairment. Antioxidants 2025, 14, 97. [Google Scholar] [CrossRef]
- Escher, G.B.; Borges, L.D.C.C.; Santos, J.S.; Cruz, T.M.; Marques, M.B.; Do Carmo, M.A.V.; Azevedo, L.; Furtado, M.M.; Sant’ana, A.S.; Wen, M.; et al. From the Field to the Pot: Phytochemical and Functional Analyses of Calendula officinalis L. Flower for Incorporation in an Organic Yogurt. Antioxidants 2019, 8, 559. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, V.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Vojvodić, S.; Čonić, B.S.; Torović, L. Safety Assessment of Herbal Food Supplements: Ethanol and Residual Solvents Associated Risk. J. Food Compos. Anal. 2023, 122, 105483. [Google Scholar] [CrossRef]
- Popovici, L.F.; Oancea, S. Calendula officinalis—Overview On Applications And Pharmaceutical Uses. Curr. Trends Nat. Sci. 2024, 13, 268–279. [Google Scholar] [CrossRef]
- Basagni, F.; Ortega, J.A.; Bertozzi, S.M.; Armirotti, A.; Summa, M.; Bertorelli, R.; Bartolini, M.; Mellor, I.R.; Bedeschi, M.; Bottegoni, G.; et al. Galantamine-Memantine Hybrids for Alzheimer’s Disease: The Influence of Linker Rigidity in Biological Activity and Pharmacokinetic Properties. Eur. J. Med. Chem. 2023, 261, 115803. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an Emerging Model for Studying Complex Brain Disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A.V. Zebrafish Models for Translational Neuroscience Research: From Tank to Bedside. Trends Neurosci. 2014, 37, 264. [Google Scholar] [CrossRef]
- Cognato, G.d.P.; Bortolotto, J.W.; Blazina, A.R.; Christoff, R.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Y-Maze Memory Task in Zebrafish (Danio rerio): The Role of Glutamatergic and Cholinergic Systems on the Acquisition and Consolidation Periods. Neurobiol. Learn. Mem. 2012, 98, 321–328. [Google Scholar] [CrossRef]
- Cachat, J.M.; Canavello, P.R.; Elkhayat, S.I.; Bartels, B.K.; Hart, P.C.; Elegante, M.F.; Beeson, E.C.; Laffoon, A.L.; Haymore, W.A.M.; Tien, D.H.; et al. Video-Aided Analysis of Zebrafish Locomotion and Anxiety-Related Behavioral Responses. Neuromethods 2011, 51, 1–14. [Google Scholar]
- Singsai, K.; Saksit, N.; Chaikhumwang, P. Brain Acetylcholinesterase Activity and the Protective Effect of Gac Fruit on Scopolamine-Induced Memory Impairment in Adult Zebrafish. IBRO Neurosci. Rep. 2024, 16, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. The Frequency of U-Shaped Dose Responses in the Toxicological Literature. Toxicol. Sci. 2001, 62, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Bondy, S.C. The Hormesis Concept: Strengths and Shortcomings. Biomolecules 2023, 13, 1512. [Google Scholar] [CrossRef]
- Osakabe, N.; Fushimi, T.; Fujii, Y. Hormetic Response to B-Type Procyanidin Ingestion Involves Stress-Related Neuromodulation via the Gut-Brain Axis: Preclinical and Clinical Observations. Front. Nutr. 2022, 9, 969823. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Hayes, A.W.; Pressman, P.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. Quercetin Induces Its Chemoprotective Effects via Hormesis. Food Chem. Toxicol. 2024, 184, 114419. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Pereyra, A.; Patriarca, A.; Mazzobre, M.; Polak, T.; Abram, V.; Buera, M.; Poklar Ulrih, N. (PDF) Phenolic Compounds in Extracts from Eucalyptus Globulus Leaves and Calendula officinalis Flowers. JNPR 2016, 2, 53–57. [Google Scholar]
- Thawkar, B.S.; Kaur, G. Betanin Mitigates Scopolamine-Induced Cognitive Impairment by Restoring Cholinergic Function, Boosting Brain Antioxidative Status, and Increasing BDNF Level in the Zebrafish Model. Fish. Physiol. Biochem. 2023, 49, 335–349. [Google Scholar] [CrossRef]
- Patel, C.; Patel, P.; Sarkar, D.; Bulsara, J.; Soni, A.; Isapure, K.; Acharya, S. Neuroprotective Effect of Lutein in Scopolamine-Induced Alzheimer’s Disease in Mice and Zebrafish. Rev. Bras. De Farmacogn. 2021, 31, 762–771. [Google Scholar] [CrossRef]
- Taha, D.E.; Kabel, A.M.; Yassin, A.I.; Abdin, A.A. The Potential Mitigating Effect of Febuxostat Alone or with Donepezil on Scopolamine-Induced Alzheimer’s Disease in Rats: The Role of TXNIP/NLRP3 Inflammasome Signaling Pathway. Eur. J. Pharmacol. 2025, 1002, 177875. [Google Scholar] [CrossRef]
- Rastinpour, Z.; Fakhri, S.; Abbaszadeh, F.; Ranjbari, M.; Kiani, A.; Namiq Amin, M.; Echeverría, J. Neuroprotective Effects of Astaxanthin in a Scopolamine-Induced Rat Model of Alzheimer’s Disease through Antioxidant/Anti-Inflammatory Pathways and Opioid/Benzodiazepine Receptors: Attenuation of Nrf2, NF-ΚB, and Interconnected Pathways. Front. Pharmacol. 2025, 16, 1589751. [Google Scholar] [CrossRef]
- Gholami, M.; Sarahroodi, S.; Abbasi-Maleki, S. Assessment of Gentisic Acid’s Protective Effects on Scopolamine-Induced Alzheimer’s Disease in Male Mice. Phytomedicine Plus 2025, 5, 100872. [Google Scholar] [CrossRef]
- Wang, M.; Ye, H.; Jiang, P.; Liu, J.; Wang, B.; Zhang, S.; Sik, A.; Li, N.; Liu, K.; Jin, M. The Alleviative Effect of Calendula officinalis L. Extract against Parkinson’s Disease-like Pathology in Zebrafish via the Involvement of Autophagy Activation. Front. Neurosci. 2023, 17, 1153889. [Google Scholar] [CrossRef]
- Zournatzis, I.; Liakos, V.; Papadopoulos, S.; Wogiatzi, E. Calendula officinalis—A Comprehensive Review. Pharmacol. Res.—Nat. Prod. 2025, 6, 100140. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Finiuk, N.; Stoika, R.; Lin, H.; Wang, X.; Jin, M. Active Compounds from Calendula officinalis Flowers Act via PI3K and ERK Signaling Pathways to Offer Neuroprotective Effects against Parkinson’s Disease. Food Sci. Nutr. 2024, 12, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Jasoria, Y.; Agrawal, M.; Kumar, S.; Chaudhary, H.; Sahu, K.K.; Singhal, M.; Arora, S.; Chandolia, P.; Saha, S.; Singh, K.; et al. In-Vivo Evaluation of Neuroprotective Effect of Chinese Plant Calendula officinalis Linn. Flower Extract against Aluminium Chloride-Induced Alzheimer’s in Wistar Rats. Pharmacol. Res.—Mod. Chin. Med. 2024, 12, 100458. [Google Scholar] [CrossRef]
- Salama, A.; EL-Kassaby, M.I.; Refaat, A.; Mohasib, R.M.M. GC–MS and Molecular Docking Analyses of Phytochemicals from Calendula officinalis L. Hexane Extract and Evaluation of Its Antioxidant and Wound Healing Properties in Rats. Egypt. J. Chem. 2024, 67, 1037–1058. [Google Scholar] [CrossRef]
- Zhao, L.; Lan, T.; Jiang, G.; Yan, B. Protective Effect of the Gold Nanoparticles Green Synthesized by Calendula officinalis L. Extract on Cerebral Ischemia Stroke-Reperfusion Injury in Rats: A Preclinical Trial Study. Inorg. Chem. Commun. 2022, 141, 109486. [Google Scholar] [CrossRef]
- Cueto-Escobedo, J.; German-Ponciano, L.J.; Guillén-Ruiz, G.; Soria-Fregozo, C.; Herrera-Huerta, E.V. Zebrafish as a Useful Tool in the Research of Natural Products With Potential Anxiolytic Effects. Front. Behav. Neurosci. 2022, 15, 795285. [Google Scholar] [CrossRef]
- Sachett, A.; Gallas-Lopes, M.; Benvenutti, R.; M M Conterato, G.; P Herrmann, A.; Piato, A. How to Prepare Zebrafish Brain Tissue Samples for Biochemical Assays V1; Protocols.io: Berkeley, CA, USA, 2020. [Google Scholar] [CrossRef]
- Clayman, C.L.; Connaughton, V.P. Neurochemical and Behavioral Consequences of Ethanol and/or Caffeine Exposure: Effects in Zebrafish and Rodents. Curr. Neuropharmacol. 2022, 20, 560. [Google Scholar] [CrossRef]
- Popovici, L.F.; Oancea, S. Comparative Study On Phenolics, Flavonoids, Tannins And Antioxidant Properties Of Calendula officinalis Extracts Obtained By Different Extraction Techniques. Int. Multidiscip. Sci. GeoConference Surv. Geol. Min. Ecol. Manag. SGEM 2024, 24, 141–148. [Google Scholar] [CrossRef]
- Brinza, I.; Boiangiu, R.S.; Mihasan, M.; Gorgan, D.L.; Stache, A.B.; Abd-Alkhalek, A.; El-Nashar, H.; Ayoub, I.; Mostafa, N.; Eldahshan, O.; et al. Rhoifolin, Baicalein 5,6-Dimethyl Ether and Agathisflavone Prevent Amnesia Induced in Scopolamine Zebrafish (Danio rerio) Model by Increasing the MRNA Expression of Bdnf, Npy, Egr-1, Nfr2α, and Creb1 Genes. Eur. J. Pharmacol. 2024, 984, 177013. [Google Scholar] [CrossRef]
- Magno, L.D.P.; Fontes, A.; Gonçalves, B.M.N.; Gouveia, A. Pharmacological Study of the Light/Dark Preference Test in Zebrafish (Danio rerio): Waterborne Administration. Pharmacol. Biochem. Behav. 2015, 135, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, F.V.; Fontana, B.D.; Ziani, P.R.; Müller, T.E.; Mezzomo, N.J.; Rosemberg, D.B. Exploring Object Discrimination in Zebrafish: Behavioral Performance and Scopolamine-Induced Cognitive Deficits at Different Retention Intervals. Zebrafish 2019, 16, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Mullins, M.C. Dissection of Organs from the Adult Zebrafish. JoVE (J. Vis. Exp.) 2010, 37, e1717. [Google Scholar] [CrossRef]
- Brinza, I.; Guliev, C.; Oresanya, I.O.; Gok, H.N.; Orhan, I.E.; Hritcu, L. Solanum macrocarpon L. Ethanolic Leaf Extract Exhibits Neuroprotective and Anxiolytic Effects in Scopolamine-Induced Amnesic Zebrafish Model. Pharmaceuticals 2025, 18, 706. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kremen, W.S.; Beck, A.; Elman, J.A.; Gustavson, D.E.; Reynolds, C.A.; Tu, X.M.; Sanderson-Cimino, M.E.; Panizzon, M.S.; Vuoksimaa, E.; Toomey, R.; et al. Influence of Young Adult Cognitive Ability and Additional Education on Later-Life Cognition. Proc. Natl. Acad. Sci. USA 2019, 116, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Hawkins, R.E.; Brian, M.; Carrell, R.W. The Estimation of Red Cell Superoxide Dismutase Activity. J. Lab. Clin. Med. 1975, 85, 337–341. [Google Scholar]
- Sinha, A.K. Colorimetric Assay of Catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Tokumura, A. Glutathione Peroxidase Activity in Tissues of Vitamin E-Deficient Mice. J. Nutr. Sci. Vitaminol. 1976, 22, 405–407. [Google Scholar] [CrossRef]
- Salbitani, G.; Vona, V.; Bottone, C.; Petriccione, M.; Carfagna, S. Sulfur Deprivation Results in Oxidative Perturbation in Chlorella sorokiniana (211/8k). Plant Cell Physiol. 2015, 56, 897–905. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-Related Changes in Oxidized Proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovici, L.-F.; Brinza, I.; Oancea, S.; Hritcu, L. Chronic Administration of Calendula officinalis Ethanolic Extract Mitigates Anxiety-like Behavior and Cognitive Impairment Induced by Acute Scopolamine Exposure in Zebrafish. Pharmaceuticals 2025, 18, 1483. https://doi.org/10.3390/ph18101483
Popovici L-F, Brinza I, Oancea S, Hritcu L. Chronic Administration of Calendula officinalis Ethanolic Extract Mitigates Anxiety-like Behavior and Cognitive Impairment Induced by Acute Scopolamine Exposure in Zebrafish. Pharmaceuticals. 2025; 18(10):1483. https://doi.org/10.3390/ph18101483
Chicago/Turabian StylePopovici, Lucia-Florina, Ion Brinza, Simona Oancea, and Lucian Hritcu. 2025. "Chronic Administration of Calendula officinalis Ethanolic Extract Mitigates Anxiety-like Behavior and Cognitive Impairment Induced by Acute Scopolamine Exposure in Zebrafish" Pharmaceuticals 18, no. 10: 1483. https://doi.org/10.3390/ph18101483
APA StylePopovici, L.-F., Brinza, I., Oancea, S., & Hritcu, L. (2025). Chronic Administration of Calendula officinalis Ethanolic Extract Mitigates Anxiety-like Behavior and Cognitive Impairment Induced by Acute Scopolamine Exposure in Zebrafish. Pharmaceuticals, 18(10), 1483. https://doi.org/10.3390/ph18101483









