Ivermectin as an Alternative Anticancer Agent: A Review of Its Chemical Properties and Therapeutic Potential
Abstract
1. Introduction
Related Work
2. Results
2.1. Chemical Properties of IVM
2.2. IVM Effects Target Molecules and Signaling Pathways in Cancer Cells
3. Discussion
4. Methodology
4.1. Relevant Studies Selection
- Original experimental studies, excluding reviews, individual case reports, and bibliographic works.
- Research applying highly complex analytical techniques for molecular characterization and developing rigorous preclinical studies.
- Studies focused on the detailed analysis of the IVM’s chemical properties, as well as its potential anticancer effect.
- Research exploring the molecular mechanisms involved in oncological contexts.
- Studies using standardized methodologies to evaluate both the antitumor activity and cytotoxicity of IVM.
- Publications from 2014 to 2024, avoiding duplication or redundant records.
- Studies focused exclusively on the antiparasitic activity of IVM.
- Research conducted solely in silico without experimental validation.
- Studies that do not provide data on molecular mechanisms or chemical characterization.
- Research does not specifically evaluate anticancer effects.
4.1.1. Initial Search
4.1.2. Systematic Search
4.2. Scopus Data Collection
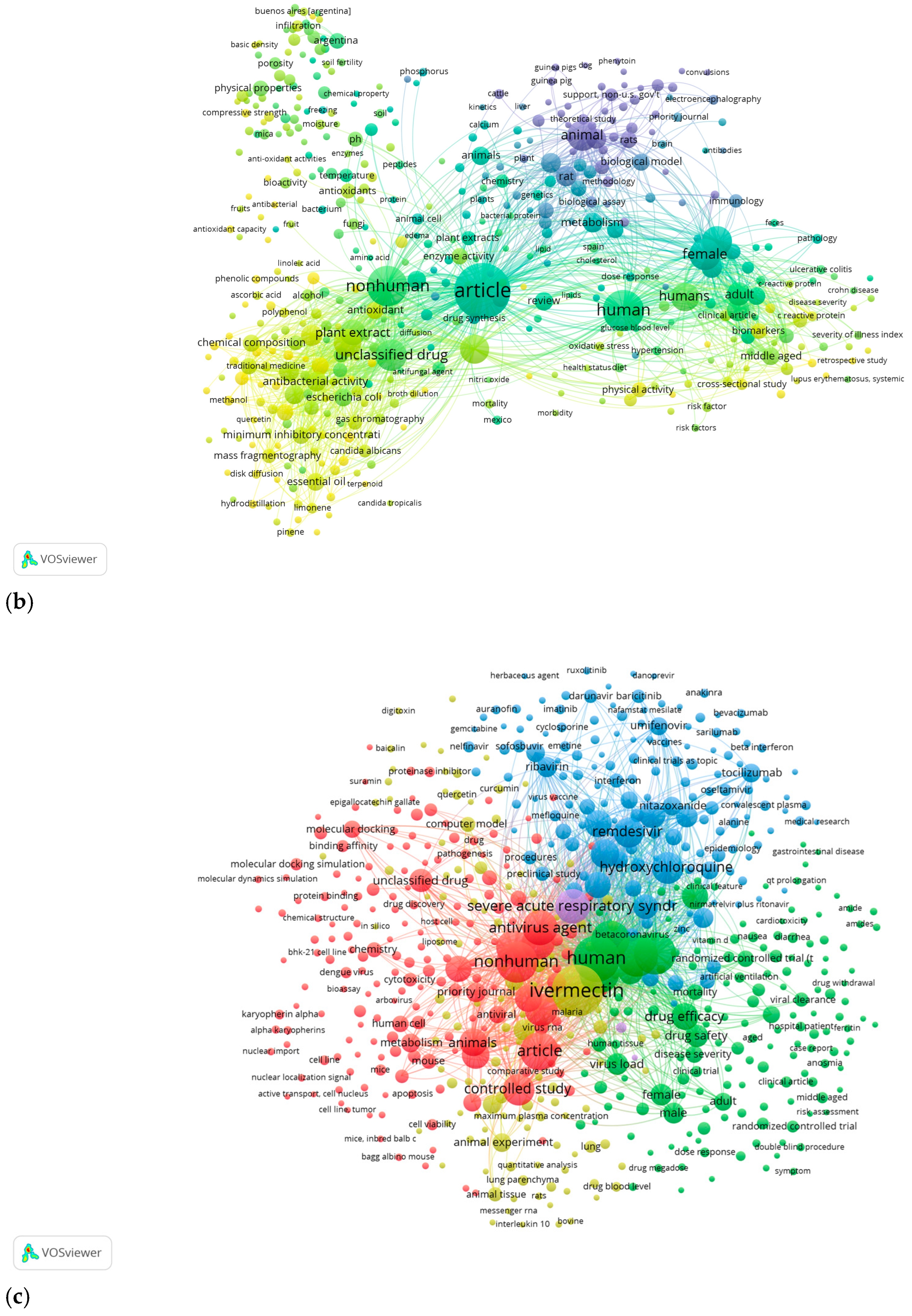
4.2.1. VOSViewer Bibliometric Map
4.2.2. Statistical Analysis with AI Tools
5. Conclusions
6. Limitations and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer [Fact Sheet]; WHO: Geneva, Switzerland, 2025; Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 3 February 2025).
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and limitations in traditional anti-cancer therapies: A comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Tang, M.; Hu, X.; Wang, Y.; Yao, X.; Zhang, W.; Yu, C.; Cheng, F.; Li, J.; Fang, Q. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol. Res. 2021, 163, 105207. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, Y.J.; Song, X.H.; Sun, Y.Y.; Yang, W.Y.; Li, J.; Wu, Y.J. Ivermectin inhibits tumor metastasis by regulating the Wnt/β-catenin/integrin β1/FAK signaling pathway. Am. J. Cancer Res. 2022, 12, 4502. [Google Scholar]
- Sharmeen, S.; Skrtic, M.; Sukhai, M.A.; Hurren, R.; Gronda, M.; Wang, X.; Fonseca, S.B.; Sun, H.; Wood, T.E.; Ward, R.; et al. The antiparasitic agent ivermectin induces chloride-dependent membrane hyperpolarization and cell death in leukemia cells. Blood 2010, 116, 3593–3603. [Google Scholar] [CrossRef]
- Rabben, H.-L.; Andersen, G.T.; Ianevski, A.; Olsen, M.K.; Kainov, D.; Grønbech, J.E.; Wang, T.C.; Chen, D.; Zhao, C.-M. Computational drug repositioning and experimental validation of ivermectin in treatment of gastric cancer. Front. Pharmacol. 2021, 12, 625991. [Google Scholar] [CrossRef]
- Mohi-Ud-Din, R.; Chawla, A.; Sharma, P.; Mir, P.A.; Potoo, F.H.; Reiner, Ž.; Reiner, I.; Ateşşahin, D.A.; Sharifi-Rad, J.; Mir, R.H.; et al. Repurposing approved non-oncology drugs for cancer therapy: A comprehensive review of mechanisms, efficacy, and clinical prospects. Eur. J. Med. Res. 2023, 28, 345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Domínguez-Gómez, G.; Chavez-Blanco, A.; Medina-Franco, J.L.; Saldivar-Gonzalez, F.; Flores-Torrontegui, Y.; Juarez, M.; Díaz-Chávez, J.; González-Fierro, A.; Dueñas-González, A. Ivermectin as an inhibitor of cancer stem-like cells. Mol. Med. Rep. 2018, 17, 3397–3403. [Google Scholar] [CrossRef]
- Jiménez-Gaona, Y.; Vivanco-Galván, O.; Morales-Larreategui, G.; Cabrera-Bejarano, A.; Lakshminarayanan, V. Outcome of Ivermectin in Cancer Treatment: An Experience in Loja-Ecuador. Nurs. Rep. 2023, 13, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Kang, H.W.; Kim, S.Y.; Kim, M.J.; Jeong, J.W.; Hong, W.C.; Fang, S.; Kim, H.S.; Lee, Y.S.; Park, J.S. Ivermectin and gemcitabine combination treatment induces apoptosis of pancreatic cancer cells via mitochondrial dysfunction. Front. Pharmacol. 2022, 13, 934746. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Guan, X.; Chen, X.; Chen, J.; Zhang, X.; Lu, M. A Review of Ivermectin Use in Cancer Patients: Is It Time to Repurpose Ivermectin in Cancer Treatment? Acta Pol. Pharm. 2024, 81, 913–929. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, K.; Cheng, L.; Zhu, H.; Xu, T. Progress in understanding the molecular mechanisms underlying the antitumour effects of ivermectin. Drug Des. Dev. Ther. 2020, 14, 285–296. [Google Scholar] [CrossRef]
- Juarez, M.; Schcolnik-Cabrera, A.; Dueñas-Gonzalez, A. The multitargeted drug ivermectin:from an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer Res. 2018, 8, 317–331. [Google Scholar] [PubMed]
- Rujimongkon, K.; Namwat, N.; Kanchanapally, R.; Loilome, W.; Bhudhisawasdi, V. Ivermectin inhibits epithelial-to-mesenchymal transition via Wnt signaling in endocrine-resistant breast cancer cells. PLoS ONE 2025, 20, e0326742. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, H.; Ning, W.; Wu, X.; Xu, X.; Ma, Y.; Li, X.; Hu, J.; Wang, C.; Wang, J. Ivermectin has new application in inhibiting colorectal cancer cell growth. Front. Pharmacol. 2021, 12, 717529. [Google Scholar] [CrossRef]
- Li, N.; Zhan, X. Anti-parasite drug ivermectin can suppress ovarian cancer by regulating lncRNA-EIF4A3-mRNA axes. EPMA J. 2020, 11, 289–309. [Google Scholar] [CrossRef]
- Nunes, M.; Duarte, D.; Vale, N.; Ricardo, S. Pitavastatin and ivermectin enhance the efficacy of paclitaxel in chemoresistant high-grade serous carcinoma. Cancers 2022, 14, 4357. [Google Scholar] [CrossRef]
- Draganov, D.; Han, Z.; Rana, A.; Bennett, N.; Irvine, D.J.; Lee, P.P. Ivermectin converts cold tumors hot and synergizes with immune checkpoint blockade for treatment of breast cancer. Npj Breast Cancer 2021, 7, 22. [Google Scholar] [CrossRef]
- Khan, K.S.; Kunz, R.; Kleijnen, J.; Antes, G. Systematic reviews to support evidence-based medicine: How to review and apply findings of healthcare research. BMJ Evid.-Based Med. 2004, 9, 30. [Google Scholar] [CrossRef]
- Shubin, K.; Bērziņš, A.; Belyakov, S. Crystal Structures of New Ivermectin Pseudopolymorphs. Crystals 2021, 11, 172. [Google Scholar] [CrossRef]
- de Vos, L.; Gerber, M.; Liebenberg, W.; Wessels, J.C.; Lemmer, H.J. Co-Processed Crystalline Solids of Ivermectin with Span® 60 as Solubility Enhancers of Ivermectin in Natural Oils. AAPS PharmSciTech 2024, 25, 67. [Google Scholar] [PubMed]
- Vargas, F.; León, M.; Angulo, B.; Álvarez, Á.; González, J.; Maldonado, A. Evaluation of the Photostability of Ivermectin. Chem. Proc. 2024, 16, 78. [Google Scholar] [CrossRef]
- Velho, M.C.; Funk, N.L.; Deon, M.; Benvenutti, E.V.; Buchner, S.; Hinrichs, R.; Pilger, D.A.; Beck, R.C.R. Ivermectin-loaded mesoporous silica and polymeric nanocapsules: Impact on drug loading, in vitro solubility enhancement, and release performance. Pharmaceutics 2024, 16, 325. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Liu, K.; Liu, B.; Xu, W.; Gao, J.; Ding, L.; Tao, L. Ivermectin induces cell cycle arrest and apoptosis of HeLa cells via mitochondrial pathway. Cell Prolif. 2019, 52, e12543. [Google Scholar] [CrossRef]
- Liebig, M.; Alonso Fernandez, Á.; Blübaum-Grønau, E.; Boxall, A.; Brinke, M.; Carbonell, G.; Egeler, P.; Fenner, K.; Fernandez, C.; Fink, G.; et al. Environmental risk assessment of ivermectin: A case study. Integr. Environ. Assess. Manag. 2010, 6 (Suppl. 1), 567–587. [Google Scholar] [CrossRef]
- Xu, N.; Lu, M.; Wang, J.; Li, Y.; Yang, X.; Wei, X.; Si, J.; Han, J.; Yao, X.; Zhang, J.; et al. Ivermectin induces apoptosis of esophageal squamous cell carcinoma via mitochondrial pathway. BMC Cancer 2021, 21, 1307. [Google Scholar] [CrossRef] [PubMed]
- Jawad, M.J.; Richardson, A. Ivermectin Augments the Anti-Cancer Activity of Pitavastatin in Ovarian Cancer Cells. Diseases 2023, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, X.; Xu, R.; Ye, T. Drug repurposing of ivermectin abrogates neutrophil extracellular traps and prevents melanoma metastasis. Front. Oncol. 2022, 12, 989167. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Zhang, J.; Lu, X.; Zhou, D.; Deng, X.F.; Liu, Q.X.; Dai, J.G.; Zheng, H. Ivermectin induces nonprotective autophagy by downregulating PAK1 and apoptosis in lung adenocarcinoma cells. Cancer Chemother. Pharmacol. 2024, 93, 41–54. [Google Scholar] [CrossRef]
- Lv, S.; Wu, Z.; Luo, M.; Zhang, Y.; Zhang, J.; Pascal, L.E.; Wang, Z.; Wei, Q. Integrated analysis reveals FOXA1 and Ku70/Ku80 as targets of ivermectin in prostate cancer. Cell Death Dis. 2022, 13, 754. [Google Scholar] [CrossRef]
- Güler, E.M.; Günaydın Akyıldız, A. Ivermectin induces oxidative stress and DNA damage in breast cancer cells. Bezmialem Sci. 2023, 11, 15–22. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, L.; Zuo, H.; Le, W.; Hu, J.; Zhang, T.; Li, M.; Yuan, Y. Ivermectin synergizes sorafenib in hepatocellular carcinoma via targeting multiple oncogenic pathways. Pharmacol. Res. Perspect. 2022, 10, e00954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, Y.; Chawla, J.; Parmar, M.S. Ivermectin in Cancer Treatment: Should Healthcare Providers Caution or Explore Its Therapeutic Potential? Curr. Oncol. Rep. 2025, 25, 1070–1079. [Google Scholar] [CrossRef]
- Halma, M.; Vottero, P. Ivermectin, a molecular Swiss army knife: A review of mechanisms, indications and safety concerns in drug repurposing. J. Indep. Med. 2025, 1, 5. [Google Scholar] [CrossRef]
- Kaduk, J.A.; Tanis, A.; Tovar, A.; Boaz, N.C.; Gindhart, A.M.; Blanton, T.N. Crystal structure of ivermectin hemihydrate ethanolate, (C48H74O14)(H2O)0.5(C2H5OH)0.82. Powder Diffr. 2021, 36, 247–256. [Google Scholar] [CrossRef]
- Cheng, X.; Qu, M.; Ren, J.; Gong, C.; Qi, L.; Shu, J.; Liu, B.; Sun, H. Solid–liquid equilibrium behavior and thermodynamic analysis of ivermectin using experiments and molecular simulations. J. Mol. Liq. 2024; advance online. [Google Scholar] [CrossRef]
- Juarez, M.; Schcolnik-Cabrera, A.; Dominguez-Gomez, G.; Chavez-Blanco, A.; Diaz-Chavez, J.; Duenas-Gonzalez, A. Antitumor effects of ivermectin at clinically feasible concentrations support its clinical development as a repositioned cancer drug. Cancer Chemother. Pharmacol. 2020, 85, 1153–1163. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, P.; Sun, Y.J.; Wu, Y.J. Ivermectin reverses the drug resistance in cancer cells through EGFR/ERK/Akt/NF-kappaB pathway. J. Exp. Clin. Cancer Res. 2019, 38, 265. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Chen, H.N.; Wang, K.; Yuan, K.; Lei, Y.; Li, K.; Lan, J.; Chen, Y.; Huang, Z.; Xie, N.; et al. Ivermectin Induces Cytostatic Autophagy by Blocking the PAK1/Akt Axis in Breast Cancer. Cancer Res. 2016, 76, 4457–4469. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V. Ivermectin: A Critical Review on Characteristics, Properties, and Analytical Methods. J. AOAC Int. 2023, 106, 534–557. [Google Scholar] [CrossRef] [PubMed]
- Goa, K.L.; McTavish, D.; Clissold, S.P. Ivermectin. A review of its antifilarial activity, pharmacokinetic properties and clinical efficacy in onchocerciasis. Drugs 1991, 42, 640–658. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Zhang, L.; Wang, Z.; Ding, H.; Yue, Z. Ivermectin Inhibits Bladder Cancer Cell Growth and Induces Oxidative Stress and DNA Damage. Anti-Cancer Agents Med. Chem.-Anti-Cancer Agents 2024, 24, 348–357. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Li, M.; Lin, Y.; Liu, Y.; Tang, S.; Dai, C. Ivermectin-Induced Apoptotic Cell Death in Human SH-SY5Y Cells Involves the Activation of Oxidative Stress and Mitochondrial Pathway and Akt/mTOR-Pathway-Mediated Autophagy. Antioxidants 2022, 11, 908. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhong, H.; Luo, X.; Yuan, J.; Gong, G.; Feng, Y.; Zhang, X.; Feng, X.; Jin, Y.; Li, J. Ivermectin induces oxidative stress and mitochondrial damage in Haemonchus contortus. Vet. Parasitol. 2025, 333, 110352. [Google Scholar] [CrossRef] [PubMed]
- Torres-Carrión, P.V.; González-Carrasco, I.; Cabezas-González, M.; Romero-Torres, Á. Methodology for systematic literature review applied to engineering and education. IEEE Rev. Iberoam. Tecnol. Del. Aprendiz. 2018, 13, 149–153. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Suryantoro, E.; Udin, U.; Qamari, I.N. A bibliometric analysis using VOSviewer: Leadership in infection prevention and control. Multidiscip. Sci. J. 2023, 5, 2023022. [Google Scholar] [CrossRef]





| Studying Type /Reference | Chemical Structure | Physicochemical Properties | Stability | Solubility | Influence on Biological Activity | Conclusion |
|---|---|---|---|---|---|---|
| Crystallography, thermal study [20] | Formula C48H74O14 (B1a) and C47H72O14 (B1b); macrocycle structure with disaccharides | Molecular weight ~875 Da; high log P; melting point ~157 °C; aqueous solubility ~1 µg/mL | Polymorphism and pseudopolymorphism affect stability depending on pH and temperature | Low in water, higher in organic solvents | Crystalline stability impacts bioavailability | Polymorphism affects solubility and stability of solid ivermectin |
| Co-crystals with Span® 60 (Sigma-Aldrich, MO, USA) [21] | Macrocycle B1a/B1b in solid co-crystalline matrix | Log P ~3.0–5.8; molecular weight 875 Da; solubility improves in oils (+169%) | Stable at room temperature and in thermal processes (DSC/TGA) | ↑solubility in oils due to co-crystals | Better penetration in lipid formulations | Co-crystallization improves solubility without altering the base structure |
| Experimental photostability [22] | Ivermectin B1a | Log P ~5.8; photolabile under UVA/UVC; susceptible to photodegradation | Degrades rapidly at extreme pH or under radiation | Low in water; stable in non-polar solvents | Photodegradation reduces biological efficacy | Requires light protection to maintain potency |
| Nanoencapsulation (silica and polymers) [23] | Lactone macrocycle B1a/B1b without alteration | BCS Class II; log P 5.83; solubility ~4 µg/mL | Stable in nano-containers | Aqueous solubility increases with nanoencapsulation | Higher bioavailability due to better release | Nanocarriers improve dissolution and activity |
| Crystallography [24] | Pseudopolymorphic variants (ethanol, GVL, MTBE) | — | Pseudopolymorphs are stable at different temperatures | Influenced by solvent in the crystal lattice | Crystalline forms with different release rates | Controlling solid form is key to standardizing activity |
| Experimental (environmental stability) [25] | Structure B1a/B1b | Optimal pH ~6.3; unstable under extremes (temperature, light) | Photolysis in a few hours; hydrolysis at extreme pH | No quantitative data; low in water | Reduces action time and environmental efficacy | Storage control is essential to maintain effectiveness |
| Type of Cancer/ Reference | Cell Lines Studied | Target Molecules/Pathways | Mechanism of Action | IVM Dose | Effects on Cancer Cells | Toxicity/IC50 in Normal Cells | Main Findings (Efficacy/Safety) |
|---|---|---|---|---|---|---|---|
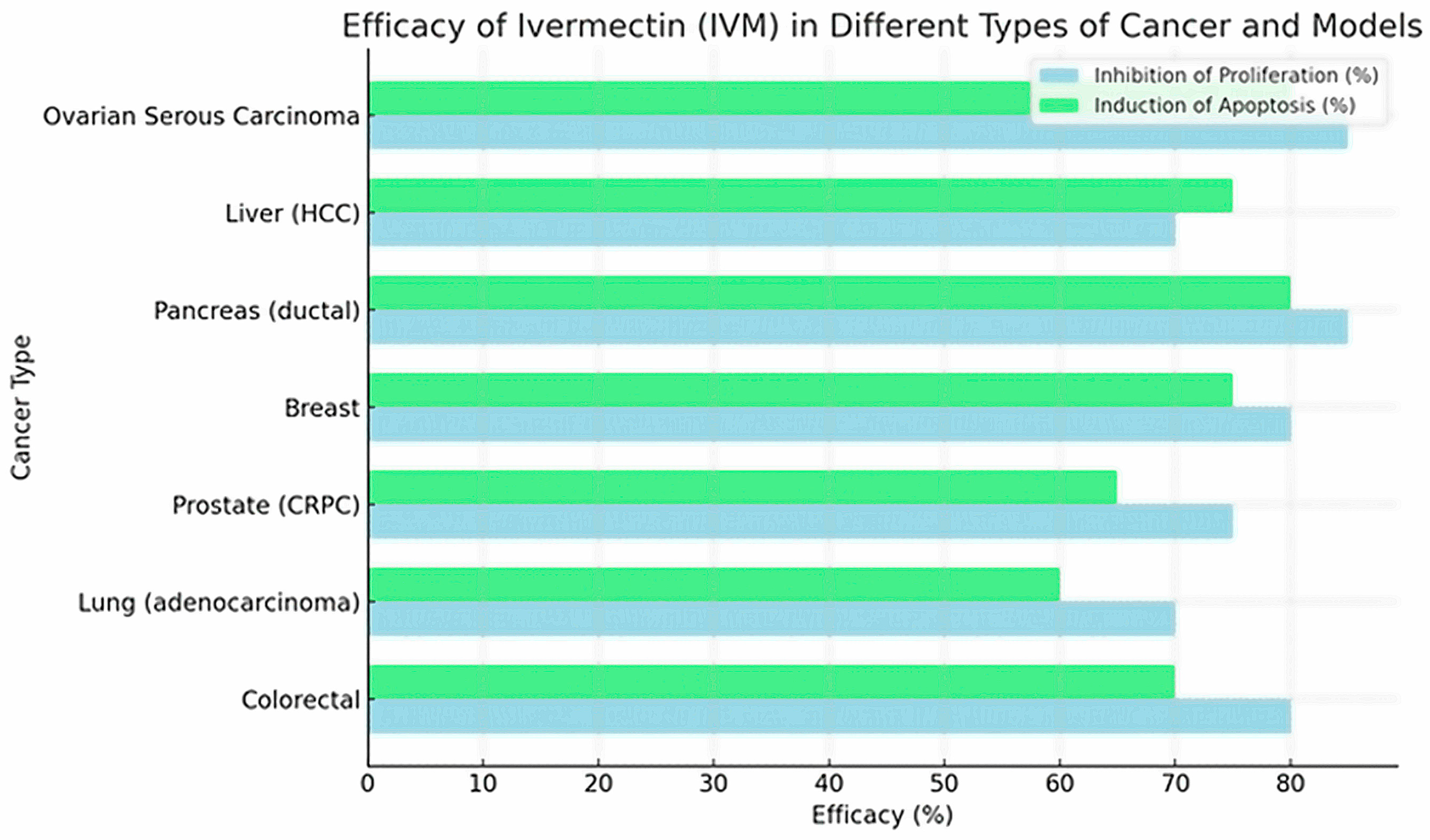
| Esophageal Squamous Cell Carcinoma [26] | KYSE-30, KYSE-70 (tumor); NE-3 (normal) | Mitochondrial pathway and NF-κB: ↑ROS, ↑Bax/↓Bcl-2, caspases | IVM induces mitochondrial dysfunction (↓ψm, ↓ATP) with ↑ROS, inhibits NF-κB (↓p-p65), increases Bax/Bcl-2 and activates caspases 9/3 → apoptosis | IC50 ≈6 μM (KYSE-30), =10 μM (KYSE-70) (estimated) | ↓Viability and proliferation, G1 arrest, ↑apoptosis (nuclear fragmentation, more Bax and caspases) | NE-3 normal cells: no toxicity up to ~15 μM; mild effect (~20% inhibition) at 20 μM | IVM (~10 μM) kills ESCC cells via mitochondrial apoptosis, without damaging normal cells at moderate doses |
| Colorectal Cancer [15] | SW480, SW1116 | Mitochondrial pathway and ROS: Bax/Bcl-2, caspases | ↑Total/mitochondrial ROS → mitochondrial damage → ↑Bax/↓Bcl-2, ↑caspase-3/7 → apoptosis; also, S phase arrest at low doses | 2.5–20 μM (e.g., 2.5–5 μM induced S phase arrest) | ↓Dose-dependent viability; ↑caspase-3/7, ↑cell apoptosis (markers: cleaved PARP) | Not evaluated in this study; focus on tumor cells | IVM suppresses CRC growth via ROS-mediated apoptosis (NAC reverses the effect) |
| Ovarian Cancer [27] | COV-318, OVCAR-5, CAOV-3, A2780, TOV-21G, SKOV-3 | Importin β (KPNB1)/PAK1 (indirect) | Alone: modulates KPNB1 → cycle arrest. In combination synergizes with pitavastatin, ↑apoptosis (caspase-3/7) | IVM alone: IC50 ~10–20 μM; in synergy tested ~20 μM fixed | IVM alone: moderate growth inhibition; combined: greater viability reduction (CI~0.6 in COV-318) and much ↑apoptosis (caspase-3/7 (2–4 fold) | IC50 ≫ safe plasma levels (e.g., 10–20 μM vs. ~0.05–0.3 μM possible in vivo) | IVM enhances pitavastatin in ovarian cancer, suggesting combination therapy; however, required in vitro concentrations are very high compared to safe human levels |
| Breast Cancer (Murine TNBC) [18] | 4T1 (mouse cell line) | ATP/P2X4/P2X7 axis, ICD mediators (ATP, HMGB1), MDSC/Tregs | IVM modulates ATP/P2X4/P2X7 channels → induces immunogenic cell death (releases ATP, HMGB1), selectively depletes MDSCs and Tregs → ↑CD8+ T infiltration and ↑Teff/Treg | 12 μM (used ex vivo in 4T1 cells for vaccine) | Alone: no noticeable antitumor effect. With anti-PD-1: synergy limits tumor growth (p = 0.03) and increases complete remissions. ↑immune response against rechallenge | Not reported (immunocompetent mouse model) | IVM acts as an immune modulator, “converts” cold tumors into hot ones; alone it doesn’t reduce tumors, but strongly boosts anti-PD-1 therapy |
| Melanoma (Metastasis) [28] | Neutrophils (mouse); B16F10 (murine) | Gasdermin D (GSDMD), NETs, MDSC | IVM binds to GSDMD (Kd ≈ 0.268 μM) and blocks its oligomerization → inhibits NET formation. Reduces infiltrated MDSCs and ↑CD8+ T in the lung | Kd ~0.268 μM (affinity to GSDMD); 5 μM did not affect B16F10 in 48h | No effect on primary tumor; ↓significant lung metastasis; ↓ctDNA of NETs in serum; ↑CD8+ in metastasis | B16F10: IC50 much higher than 5 μM; no viability reduction at 5 μM | IVM did not reduce the primary tumor, but stopped lung metastases in mice (via blocking NETs/GSDMD), suggesting potential anti-metastatic activity |
| Reference | Cancer Type | Model In Vitro/In Vivo | Treatment IVM Concentration | Main Findings (Efficacy) | Combination | Dose (I.V.) | Molecular Pathway/Target | Exposure Time | Toxicity/Selectivity |
|---|---|---|---|---|---|---|---|---|---|
| [15] | Colorectal | In vitro: SW480, SW1116 (human colorectal cancer). | IVM in increasing dilutions (0–30 µM). | Dose-dependent inhibition of viability and proliferation in SW480/SW1116; ↑apoptosis (Annexin-V+, ↑caspase-3/7 activity); ↑proportion of cells in S (S-phase arrest). Decreases Bcl-2; ↑Bax, ↑cleaved PARP. | -- | – | ↑ROS; mitochondrial apoptotic pathway (↑Bax, caspase-3); ↓Bcl-2; ↑cleaved PARP. | 6–36 h (depending on assay) | Not reported |
| [29] | Lung (adenocarcinoma) | In vitro: LUAD lines (e.g., A549 or H1975; non-specific); In vivo: LUAD xenografts in nude mice. | IVM (exact µM not detailed in summary; usually 5–20 µM) in culture; in mice 10 mg/kg i.p., 3×/week. | Marked inhibition of colony formation and proliferation of LUAD cells; significant induction of apoptosis and autophagy (non-cytoprotective). In vivo, suppresses lung adenocarcinoma tumor growth. | -- | In vivo: 10 mg/kg 3×/week (mouse) | ↓PAK1 (kinase linked to proliferation); ↑autophagy (non-cytoprotective); ↑apoptosis. | 48–72 h (in vitro); 3 weeks (in vivo) | Not reported (no specific adverse effects reported) |
| [30] | Prostate (CRPC) | In vitro: LNCaP, C4-2, 22Rv1 (AR+ prostate); In vivo: 22Rv1 xenograft in castrated mice. | IVM 4–12 µM in culture (48 h); In vivo: 10 mg/kg i.p., 3×/week. | G0/G1 arrest, apoptosis, and DNA damage in CRPC cells. ↓AR (full-length and variants) and PSA; ↓E2F1 and AR signaling by FOXA1 blockade; ↑γH2AX (DSB). In vivo reduces tumor volume 22Rv1 (↓Ki67, PSA). | Enzalutamide trial—synergy (IVM IC50 ↓ with enzalutamide) | In vivo: 10 mg/kg 3×/week (mouse) | Direct target: FOXA1 and Ku70/Ku80 (DSB repair); affects AR/E2F1 signaling; ↑apoptotic cascade (PARP). | 48 h (in vitro); 3–4 weeks (mice) | Preferential in AR+ cells (IC50 2–3 × lower in AR+ vs. AR−); no significant systemic toxicity reported. |
| [31] | Breast | In vitro: MCF-7 (ER+), MDA-MB-231 (triple-negative); Normal cells 184A1. | IVM 2.5–30 µM in culture (24 h). | Inhibits viability of MCF-7 (IC5024 µM) and MDA-MB-231 (IC5034 µM) much more than normal cells 184A1 (IC50~68 µM). ↑Dose-dependent apoptosis (AO/EB) in MCF-7/MDA-231. ↑ROS, ↑DNA damage (comet assay), MMP-Δψm in cancer cells. | -- | – | Oxidative stress and mitochondrial apoptosis pathway (↑ROS, ↓glutathione); DNA damage (comet assay). | 24 h | Normal IC50 >> tumor (better selectivity in cancer); low cytotoxicity in normal cells (collagen). |
| [10] | Pancreas (ductal) | In vitro: MiaPaCa-2, PANC-1 (pancreatic cells); ex vivo: patient organoids; In vivo: xenografts in mice. | IVM (± Gemcitabine 5 µM): IVM 2.5–10 µM (48–72 h); with gemcitabine 5 µM. | IVM + gemcitabine synergy: ↑proliferative inhibition (↓CI50). The combination induced G1 arrest (↓cyclins D1, ↓mTOR/STAT3) and ↑mitochondrial apoptosis (↑ROS, ↓mitochondrial Δψ). Decreases OCR and inhibits mitophagy. In vivo, IVM + gemcitabine suppresses tumorigenesis more than gemcitabine alone. | Gemcitabine (5 µM)—clear synergy | In vivo: (not specified) | ↓mTOR/STAT3; G1 arrest (↓cyclins D1/CDK4); ↑ROS/↓mitochondrial Δψ; ↓mitophagy. | 48–72 h (in vitro); 3 weeks (xenografts) | No notable adverse effects reported; IVM + gemcitabine better inhibition vs. gemcitabine alone (synergism). |
| [32] | Liver (hepatocellular carcinoma, HCC) | In vitro: HuH6, HepG2, SNU-182 (hepatocellular cancer); In vivo: HCC xenografts in mice. | IVM 5–20 µM in culture; In vivo: (dose details not specified) | IVM inhibited dose-dependent proliferation of HCC lines and ↑apoptosis. Inhibits migration, colonies, and CSC function. Suppresses oncogenic signaling mTOR/STAT3 and EMT and “stemness” markers. In mice, IVM reduced tumor formation and growth without apparent toxicity; also showed synergy with sorafenib. | Sorafenib—marked synergy (CI < 1) | – | ↓mTOR/STAT3; ↓EMT pathway (↓E-cad, ↓N-cad); ↓stem markers (Nanog, c-Myc). | 48–72 h (in vitro); 4–6 weeks (xenografts) | No systemic toxic effects in mice; IVM synergistic with sorafenib; global inhibition of essential oncogenic pathways. |
| [17] | High-grade serous carcinoma (ovarian cancer) | In vitro: Chemoresistant high-grade serous ovarian cancer cell lines: OVCAR8 and OVCAR8 PTX^RP (resistant to carboplatin/paclitaxel) and a non-tumoral line HOSE6.3 (normal ovarian epithelium). | Paclitaxel alone and in combination with repositioned drugs (pitavastatin, metformin, ivermectin, itraconazole, alendronate). Cells were exposed to Ivermectin in the range 0.39–50 μM (48 h), and pitavastatin in 0.04–5 μM (48 h). The combinations of Paclitaxel with each drug were administered simultaneously in a fixed ratio (0.25–4× IC50 of each drug). | Combination of Paclitaxel + Ivermectin or Paclitaxel + Pitavastatin produced maximum cytotoxicity and strong synergy in both chemoresistant lines, surpassing the effect of each drug alone. | -- | Ivermectin was combined with Paclitaxel in fixed ratios (0.25–4× IC50). The combination Paclitaxel + Ivermectin (and similarly Paclitaxel + Pitavastatin) showed the highest synergy and antitumor effect. (Combinations with metformin, itraconazole, etc., were also tested with lower relative synergy) | Pitavastatin: 0.04–5 μM (48 h) Ivermectin: 0.39–50 μM (48 h) Paclitaxel: initial dose unknown, combined at 0.25–4× IC50 with each drug | No specific molecular targets were investigated in this experiment; it was assumed each drug acts via independent mechanisms (mutually exclusive model in synergy analysis). | 48 h (continuous exposure) |
| Topic | Keywords Boolean Search |
|---|---|
| IVM in cancer treatments | (ivermectin AND this AND application AND in AND cancer AND treatment). |
| Chemical Properties of Ivermectin | (((Ivermectin Physicochemical Properties *) AND (stability *) AND (solubility *)) OR (chemical family)) OR ((physical properties *) OR (chemical properties)) OR ((biological activity *) |
| Oncological Properties of Ivermectin | ((cancer * OR signaling in cancer cells AND morphologic AND feature *) AND (receptors AND enzymes AND metabolic pathways *))) |
| Analysis Unit | |
|---|---|
| Co-authorship | Authors |
| Organizations | |
| Countries | |
| Co-occurrence | Keywords |
| Author Keywords | |
| Index Keywords | |
| Citation | Documents |
| Sources | |
| Authors | |
| Organizations | |
| Countries | |
| Bibliographic Coupling | Documents |
| Sources | |
| Authors | |
| Organizations | |
| Countries | |
| Co-citation | Cited references |
| Cited sources | |
| Cited authors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robalino, K.N.; Vivanco-Galván, O.; Romero-Benavides, J.C.; Jiménez-Gaona, Y. Ivermectin as an Alternative Anticancer Agent: A Review of Its Chemical Properties and Therapeutic Potential. Pharmaceuticals 2025, 18, 1459. https://doi.org/10.3390/ph18101459
Robalino KN, Vivanco-Galván O, Romero-Benavides JC, Jiménez-Gaona Y. Ivermectin as an Alternative Anticancer Agent: A Review of Its Chemical Properties and Therapeutic Potential. Pharmaceuticals. 2025; 18(10):1459. https://doi.org/10.3390/ph18101459
Chicago/Turabian StyleRobalino, Kimberly Naula, Oscar Vivanco-Galván, Juan Carlos Romero-Benavides, and Yuliana Jiménez-Gaona. 2025. "Ivermectin as an Alternative Anticancer Agent: A Review of Its Chemical Properties and Therapeutic Potential" Pharmaceuticals 18, no. 10: 1459. https://doi.org/10.3390/ph18101459
APA StyleRobalino, K. N., Vivanco-Galván, O., Romero-Benavides, J. C., & Jiménez-Gaona, Y. (2025). Ivermectin as an Alternative Anticancer Agent: A Review of Its Chemical Properties and Therapeutic Potential. Pharmaceuticals, 18(10), 1459. https://doi.org/10.3390/ph18101459










