1. Introduction
Neuropathic pain (NPP) is a chronic pain syndrome resulting from lesions or diseases affecting the somatosensory nervous system that impacts 6.9–10% of the general population [
1]. The defining characteristics of NPP include spontaneous pain, hyperalgesia (an exaggerated pain response to noxious stimuli), and allodynia (the perception of pain from normally non-painful stimuli) [
2]. As a major global public health challenge, NPP not only causes physical dysfunction but also contributes to comorbid psychological conditions, such as depression and anxiety, significantly impairing the quality of life and work capacity of patients [
3,
4]. Current clinical management commonly includes tricyclic antidepressants (TCAs), serotonin–norepinephrine reuptake inhibitors (SNRIs), gabapentinoids, opioids, topical agents, and botulinum toxin type A [
5,
6]. However, these pharmacotherapies are limited by dose-dependent adverse effects, such as somnolence, constipation, xerostomia, dizziness, weight gain, and localized reactions [
7].
The interpeduncular nucleus (IPN), located in the midbrain, plays a critical role in emotional control, pain modulation, reward processing, and learning/memory [
8,
9,
10]. The IPN is dominated by GABAergic neurons that receive glutamatergic input from the medial habenula (MHb) [
11,
12], and its activity is enhanced in rodent models of chronic constriction injury [
13] and sleep-deprivation-induced hyperalgesia [
14]. The IPN bidirectionally modulates pain perception and the affective state through reciprocal connections with the medial prefrontal cortex (mPFC), septum, hypothalamus, locus coeruleus, and nucleus incertus [
15,
16]. These neural networks facilitate the bidirectional transmission of reward signals and aversive information processing, potentially modulating both pain perception and spatial encoding.
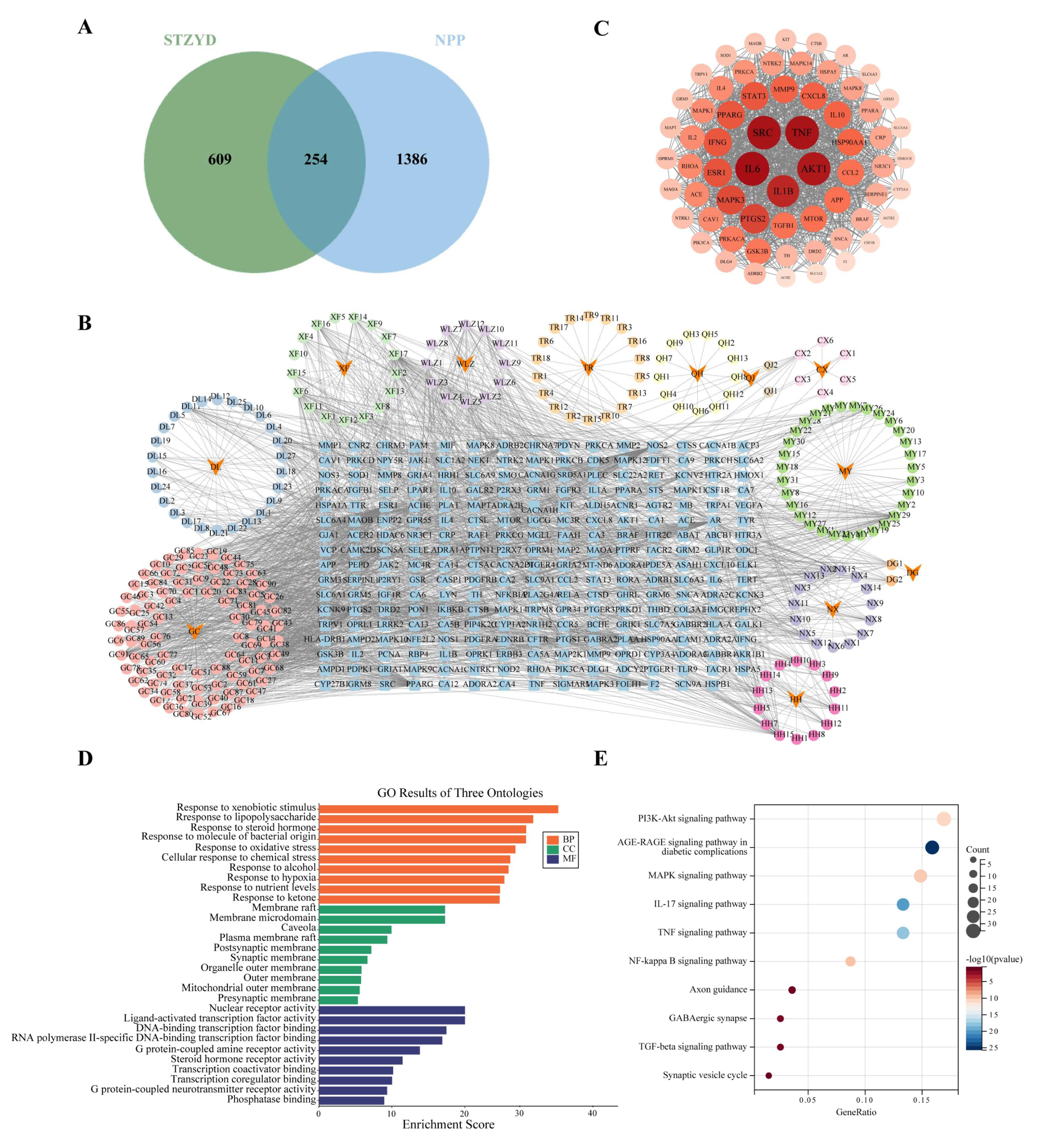
Shentong Zhuyu Decoction (STZYD), a classical formula documented in the Qing Dynasty (1636–1912 CE) text Yilin Gaicuo (Correcting Errors in Medical Classics) [
17,
18,
19], has been historically used to treat pain associated with “Bi” syndrome (painful obstruction syndrome). This polyherbal formulation comprises twelve botanical constituents. Modern pharmacological studies demonstrate that STZYD alleviates neuropathic pain through the modulation of the spinal cord MAPK pathway [
20]. Emerging evidence has identified specific bioactive components within STZYD, such as lumbrokinase (derived from
Pheretima aspergillum polypeptides),
Notopterygium incisum (Qianghuo), and paeoniflorin, as critical contributors to its analgesic efficacy [
18,
20]. Mechanistically, these compounds exhibit multitarget actions, such as the suppression of COX-2-mediated neuroinflammation, the regulation of TRPV1/Ca
2+ signaling, and the enhancement of GABAergic inhibitory neurotransmission. Currently, network pharmacology is being widely applied to investigate the mechanisms of traditional Chinese medicine (TCM) formulae and is regarded as an effective tool for elucidating the complex relationships between bioactive compounds, molecular targets, and therapeutic effects [
21,
22]. Previous studies have predicted the potential targets of STZYD in neuropathic pain models, including the spinal cords of rats, nucleus pulposus cells, and cancer-induced bone pain [
20]. However, the central nervous system (CNS) targets of STZYD have not yet been explored. In this study, we employed network pharmacology to construct a multilayered “drug–compound–target–disease” network, thereby providing a holistic and systematic perspective on the interactions between the formula and the human body.
3. Discussion
NPP is one of the most intractable and debilitating forms of chronic pain, arising from disease or injury anywhere along the somatosensory pathway. From peripheral nociceptors to spinal dorsal horn neurons and from ascending tracts to cortical networks, maladaptive changes can initiate and perpetuate pain signaling at each level. In this context, central sensitization, driven by synaptic plasticity within the central nervous system (CNS), has emerged as the key mechanism by which transient peripheral insults are “memorized” as persistent pain. In this complex mechanism, network pharmacology provides a novel perspective. By integrating information at the molecular, cellular, and systems levels, network pharmacology facilitates the identification of key signaling pathways and therapeutic targets in NPP, thereby offering a multidimensional strategy for alleviating chronic pain. Accordingly, in this study, we first employed network pharmacology to predict the potential mechanisms by which STZYD exerts therapeutic effects on NPP, and we subsequently validated these predictions in animal experiments. Molecular, histological, and morphological analyses further demonstrated that NMDAR2B may serve as a critical membrane-associated target, suggesting that STZYD might modulate synaptic transmission in line with mechanisms involving NMDAR-mediated excitotoxicity and central sensitization. In addition, functional neuroscience experiments confirmed the role of GABAergic neurons within the IPN. Moreover, HPLC-Q-TOF-MS/MS analysis revealed that STZYD is capable of crossing the blood–brain barrier to reach the IPN and exhibits high affinity toward the NMDAR2B receptor.
Previous studies have demonstrated that STZYD exerts analgesic effects and modulates inflammation and immune responses primarily through the MAPK, PI3K/AKT, and NF-κB signaling pathways [
19,
20,
23]; however, these investigations focused on spinal cord or DRG-level targets. Specifically, while studies such as [
20,
24] have shown that STZYD or its active ingredients modulate MAPK/TRPV1 signaling at the spinal cord or DRG level to alleviate neuropathic pain, our work provides the first evidence for a circuit-specific mechanism centered on IPN GABAergic microcircuits, offering a central mechanism of action that is independent of spinal pathways. Building on this finding, we employed network pharmacology to predict, and subsequently validate, the involvement of NMDAR-2B, thereby shifting the focus from traditional spinal MAPK/TRPV1 pathways to central pain modulation. In the present study, network pharmacology analysis revealed multiple enriched biological processes and signaling pathways, including cytokine regulation, lipopolysaccharide response, membrane raft organization, MAPK signaling, and AGE-RAGE signaling. Although these results suggest diverse mechanisms of action, many of the enriched terms converge both functionally and spatially on calcium-dependent synaptic signaling. GO enrichment analysis indicated the significant involvement of membrane- and synapse-related components (e.g., membrane rafts, postsynaptic density), receptor activity, and cytokine regulatory processes, all of which are positioned upstream or downstream of NMDAR-mediated calcium influx. Likewise, KEGG pathway analysis highlighted calcium signaling and glutamatergic synapse pathways, both of which explicitly include NR2B-containing NMDARs and CaMKII as key nodes. Notably, CaMKII activation can interface with MAPK and PKA/PKC cascades, providing multiple parallel routes for CREB phosphorylation. Thus, the enrichment of MAPK signaling is better interpreted as a collateral pathway converging on CREB rather than an independent mechanism. Taken together, the NMDAR-2B/CaMKII/CREB axis occupies a central hub position by integrating upstream synaptic receptor activation and coordinating downstream transcriptional programs.
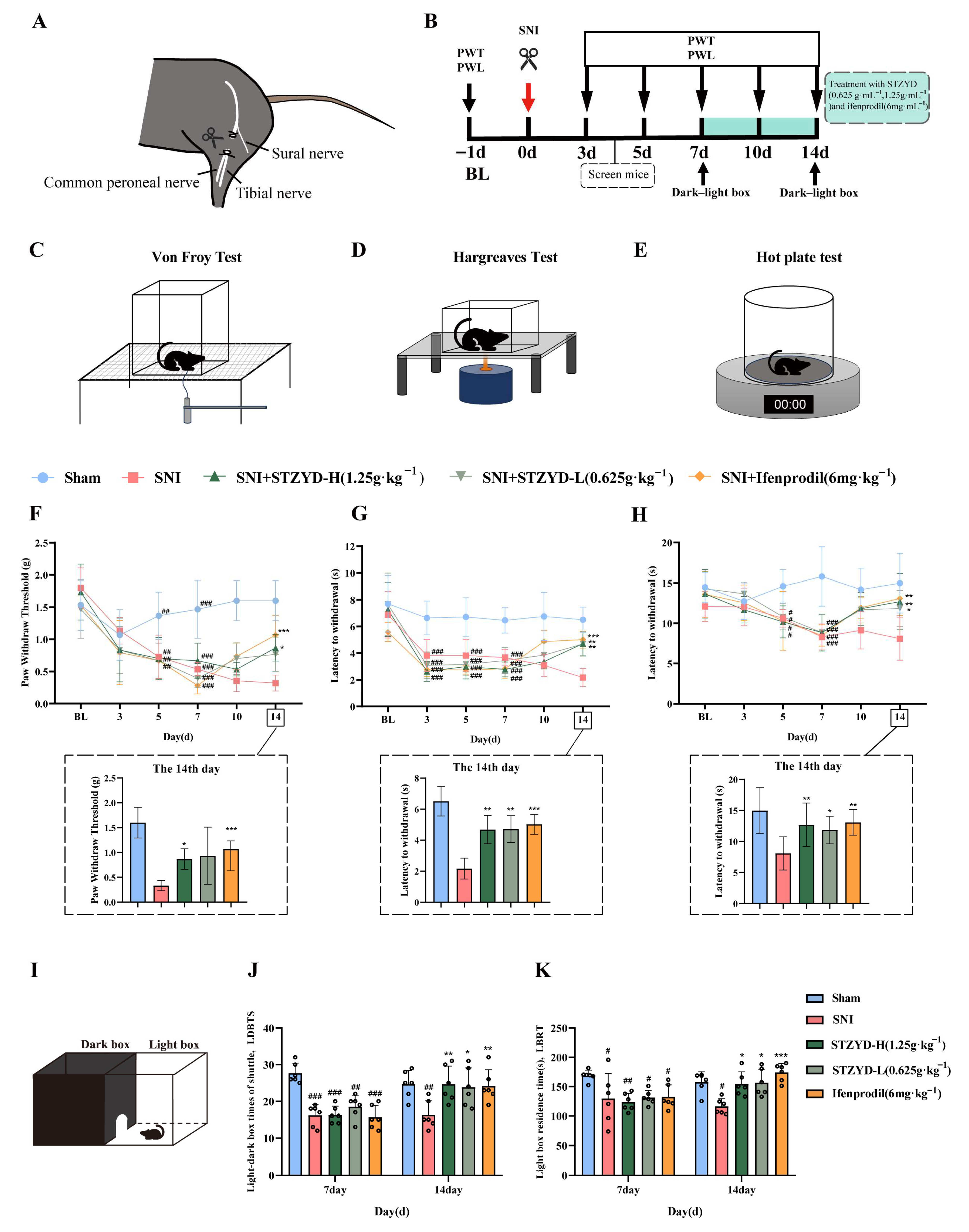
Behavioral studies demonstrated that STZYD treatment significantly increased both the PWTs and PWLs in SNI mice, consistent with previous findings [
20,
25,
26]. In the Von Frey test, low-dose STZYD did not yield a statistically significant effect. We posit that mechanical allodynia, compared with thermal pain, depends more on the sustained activation of spinal and supraspinal GluN2B-containing NMDA receptors. Partial target engagement at the low dose may suffice to attenuate thermal hypersensitivity but not to reverse mechanical allodynia, consistent with the significant increase in the mechanical withdrawal threshold observed only with the high dose. Moreover, thermal nociceptive pathways likely have a lower threshold for pharmacologic modulation and are thus more readily modulated by STZYD [
27]. Anxiety-like behaviors were assessed using the light–dark-box test. Similarly, STZYD treatment showed a tendency to reduce anxiety-like behaviors [
28], indicating its potential efficacy in alleviating both pain and anxiety. Given that STZYD attenuated both mechanical and thermal hypersensitivity in SNI mice, the increased time spent in, and transitions to, the light compartment in the light–dark box were likely secondary to analgesia rather than indicative of a primary anxiolytic effect. Demonstrating a pain-independent action will require testing STZYD in naïve, uninjured animals using validated anxiety paradigms (e.g., acute restraint or novelty-suppressed feeding). We will address this in future work by evaluating STZYD in assays specifically designed to isolate anxiety-related behaviors while minimizing confounding from analgesia.
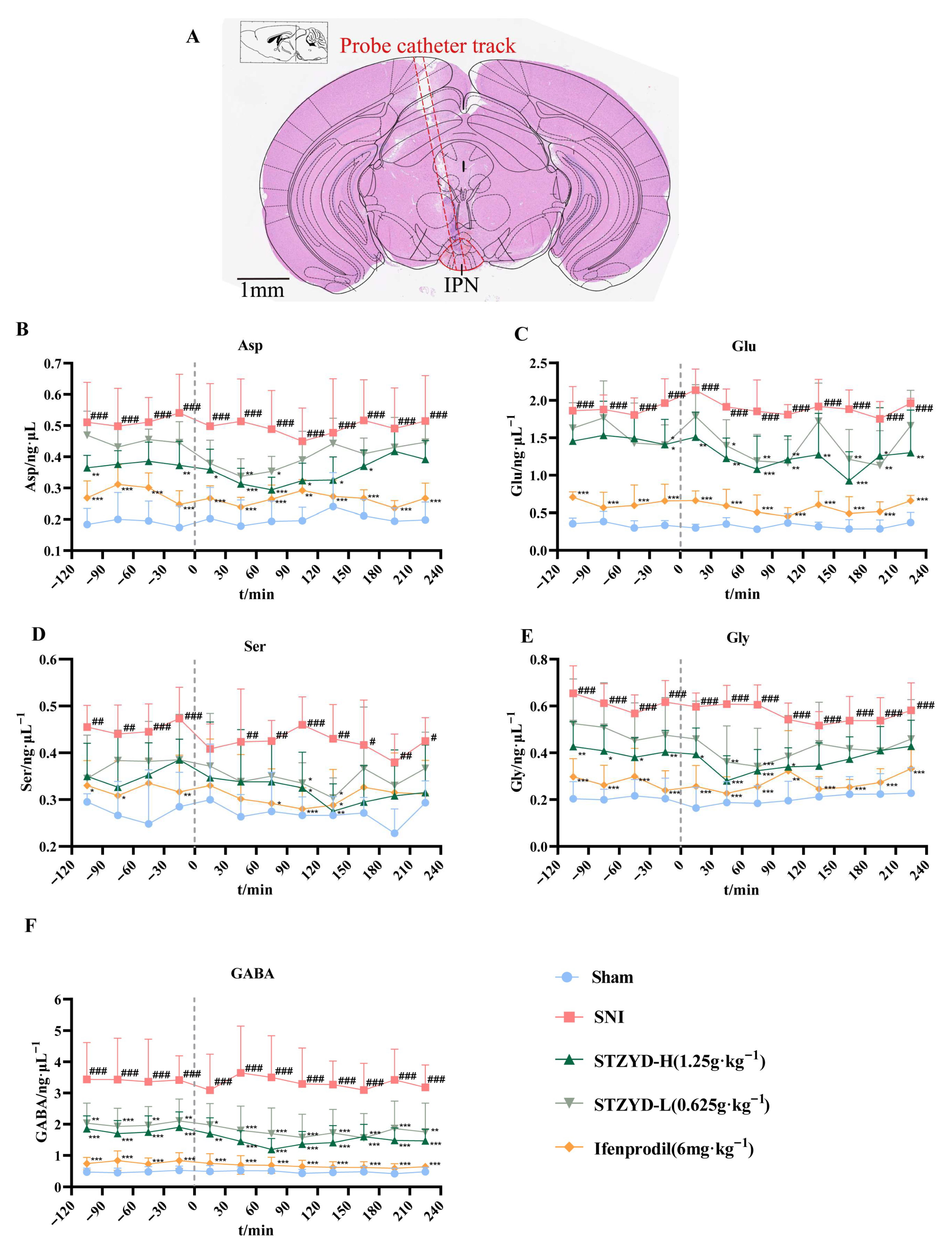
Building upon these findings, we employed intracerebral microdialysis to examine neurotransmitter alterations in the IPN region. The IPN receives glutamatergic projections from the upstream mHb and has been implicated in pain and depressive behaviors [
14,
29]. Our results revealed significant elevations in the Asp, Glu, D-Ser, and Gly levels within the IPNs of SNI mice. The observed changes in the Asp and Glu levels may be associated with excitotoxicity following nerve injury [
14,
29], which aligns with our experimental hypotheses. Glycine or D-serine binding further stabilizes the receptor’s active conformation, facilitating ion channel opening [
30]. Although studies have shown that prostaglandin E2 (PGE2) reduces glycine receptor responsiveness at the spinal level, particularly for receptors containing α3 subunits [
31], its role in the midbrain may differ substantially. The concurrent elevation of excitatory and inhibitory neurotransmitters in the IPN after SNI reflects a maladaptive compensatory loop rather than a physiological balance. Peripheral nerve injury drives excessive glutamatergic input from the MHb, which, in turn, overactivates IPN GABAergic neurons and provokes disproportionate inhibitory release [
11]. Concurrently, large amounts of GABA and Gly are released as compensatory inhibitory neurotransmitters, providing a negative feedback mechanism to counteract this hyperactivity; however, such compensation deviates from physiological homeostasis because its strength is directly coupled to excitatory input, thereby forming an abnormal cycle of “excess excitation → excessive inhibitory compensation” [
12]. Our immunofluorescence, fiber photometry, and chemogenetic data indicate that STZYD interrupts this cycle by suppressing aberrant GABAergic overactivation and re-establishing synaptic equilibrium.
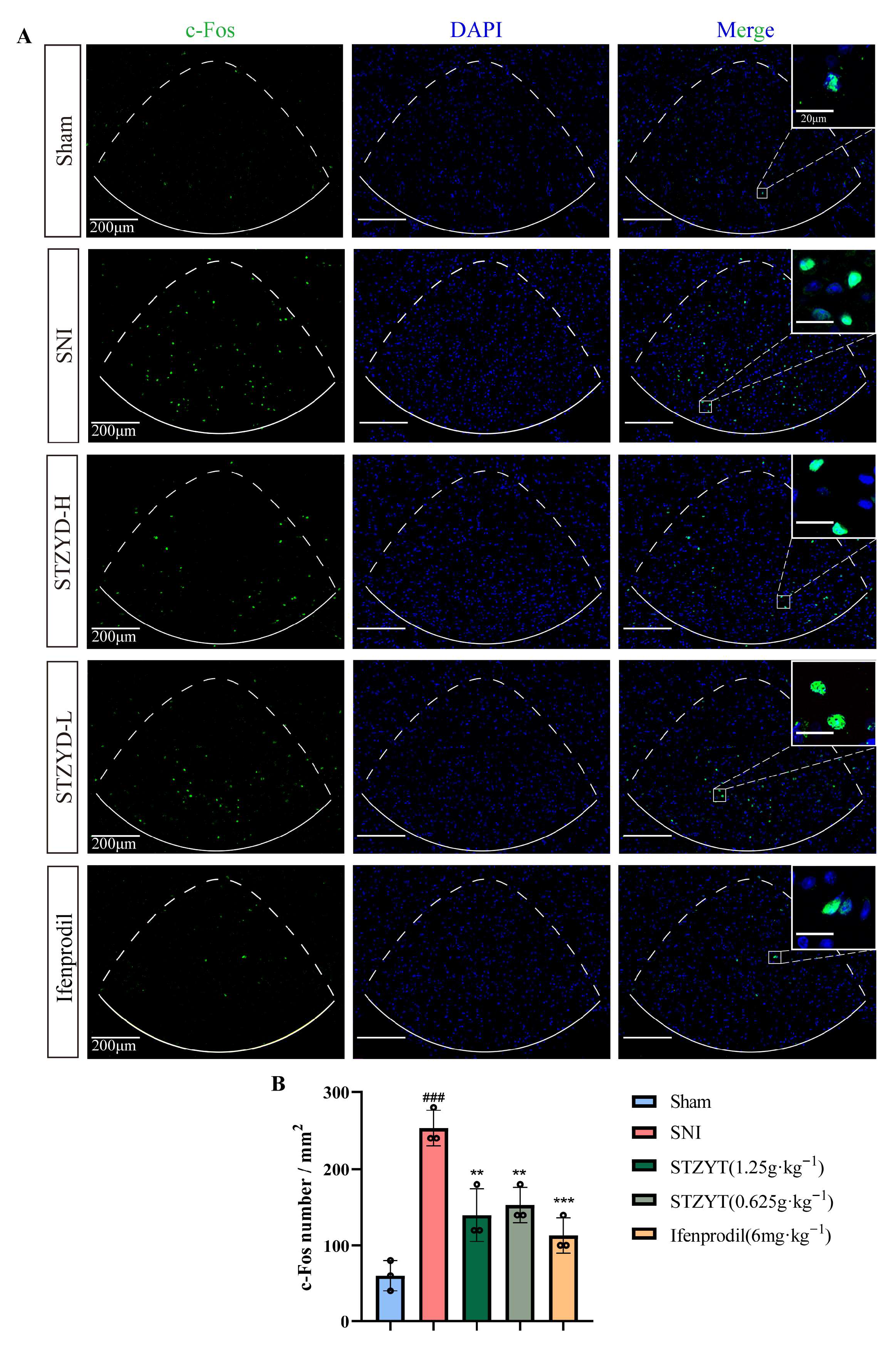
Studies have shown that c-Fos expression can promote the transcription of multiple pain-related genes, including c-Jun and dynorphin, whose upregulation contributes to the development and maintenance of neuropathic pain [
32]. As a marker of neuronal activity, we detected elevated c-Fos protein levels in the IPNs of SNI mice, indicating IPN activation during pain processing. Emerging evidence [
12,
33,
34,
35] has demonstrated that anxiety-like behaviors induced by withdrawal increase the c-Fos expression in the IPN, enhancing the GABAergic neuronal activity and spontaneous firing frequency, but not glutamatergic neurons. Similarly, acute restraint stress increases the c-Fos expression in the IPN [
36]. Given that anxiety and stress often accompany pain responses, it is plausible that the IPN plays a comparable role in pain modulation, particularly by regulating emotional responses that influence pain perception.
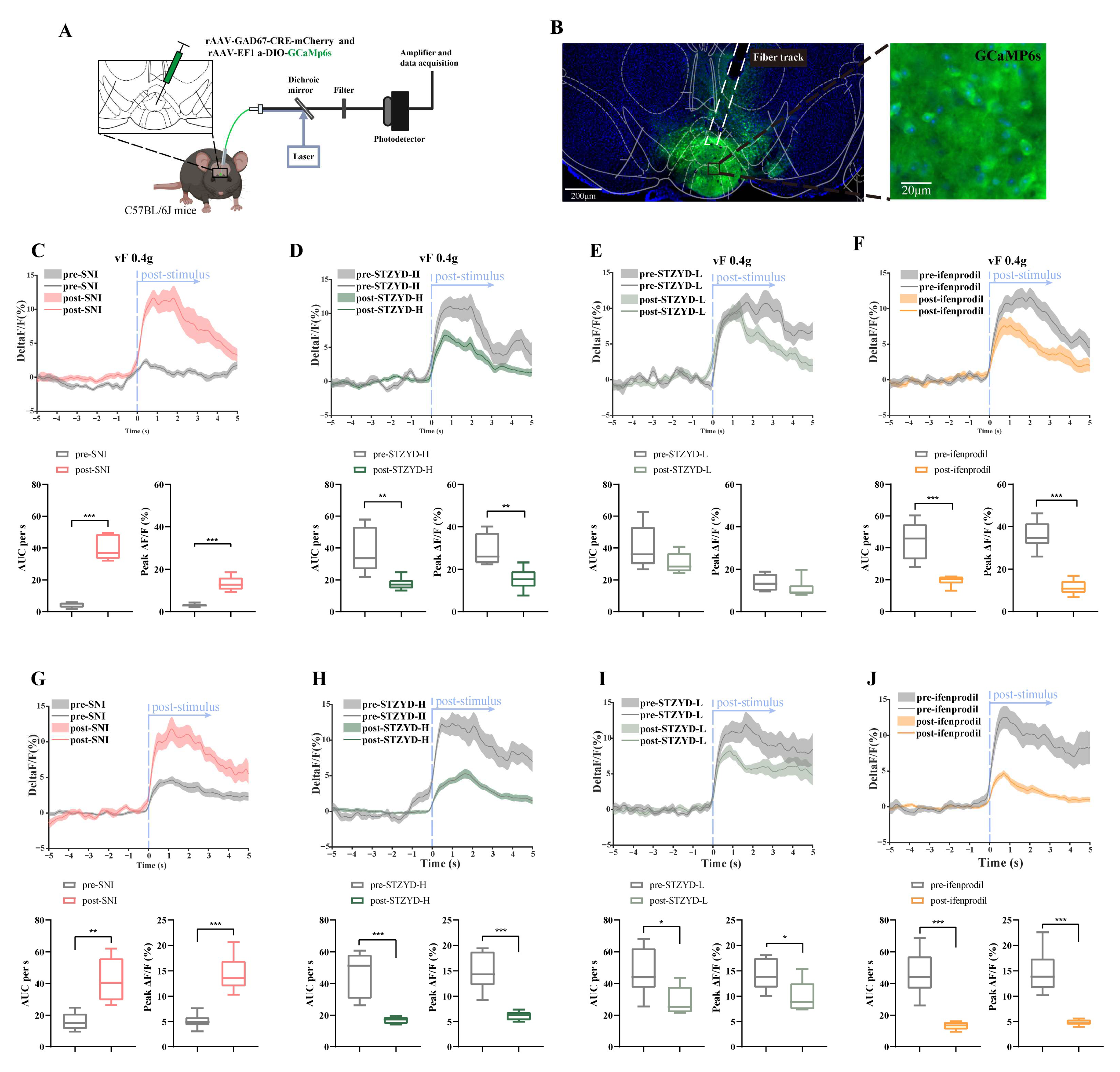
To further investigate GABAergic neurons, we recorded robust GCaMP signal fluctuations in the IPN of freely moving SNI mice upon subthreshold mechanical stimulation (0.4 g). Notably, the high-dose STZYD treatment effectively attenuated this GCaMP signal increase, consistent with previous reports of its analgesic effects in neuropathic pain models [
20]. In this study, we achieved enhanced analgesia using ifenprodil, an NMDA receptor antagonist that selectively inhibits receptors containing the NR2B subunit. Ifenprodil is widely used in the study of NMDA receptor subtypes and has demonstrated potent analgesic effects in both acute and chronic pain models with minimal side effects [
37,
38,
39,
40]. These findings suggest that STZYD may exert its analgesic effects by modulating GABAergic neurons via the NMDAR-2B receptor.
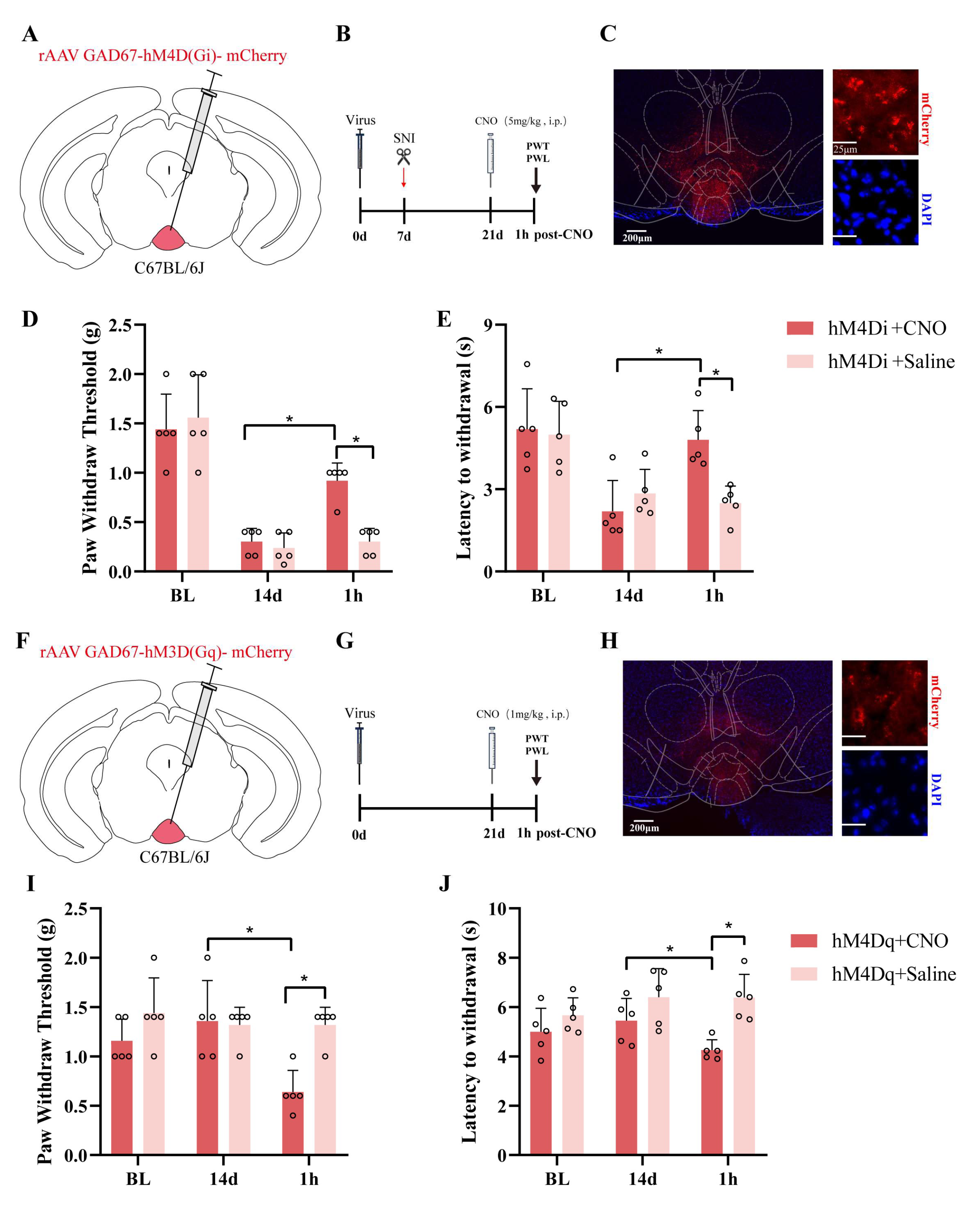
Furthermore, we successfully utilized chemogenetic technology to achieve the targeted manipulation of GABAergic neurons in the IPN, systematically exploring the functional role of this brain region in pain modulation. By selectively expressing the inhibitory receptor hM4D(Gi) and the excitatory receptor hM3D(Gq), in combination with CNO administration, we revealed the bidirectional regulation of nociceptive behavior mediated by IPN GABAergic neurons in both chronic neuropathic pain and pain induction models. In the SNI model, activation of the inhibitory DREADD receptor hM4D(Gi) significantly increased the PWT and PWL, suggesting that the inhibition of GABAergic neurons in the IPN can alleviate pain-related behaviors. These findings support the inhibitory role of the IPN in chronic pain modulation and are consistent with previous studies showing the involvement of the IPN in emotional and reward circuit regulation. Additionally, our use of a GAD67 promoter-driven viral vector strategy ensured high expression specificity, which enhanced the precise dissection of the GABAergic neuronal function in this brain region. Interestingly, activation of the excitatory DREADD receptor hM3D(Gq) in uninjured mice resulted in significant reductions in both the PWT and PWL, indicating that the excessive activation of GABAergic neurons alone can induce pain-like responses, which suggests that under normal conditions, IPN GABAergic neurons may play a potential pronociceptive role, and that their overactivity could amplify pain signals under pathological conditions. These findings complement the analgesic effects observed in the hM4D(Gi) group, supporting the plasticity and bidirectional regulatory function of the IPN in pain perception. Although the chemogenetic data demonstrate bidirectional pain control via IPN GABAergic neurons, the initial n = 5 per group limits the statistical power; an independent replication (total n ≈ 10–11) and larger follow-up studies are required to confirm the reproducibility.
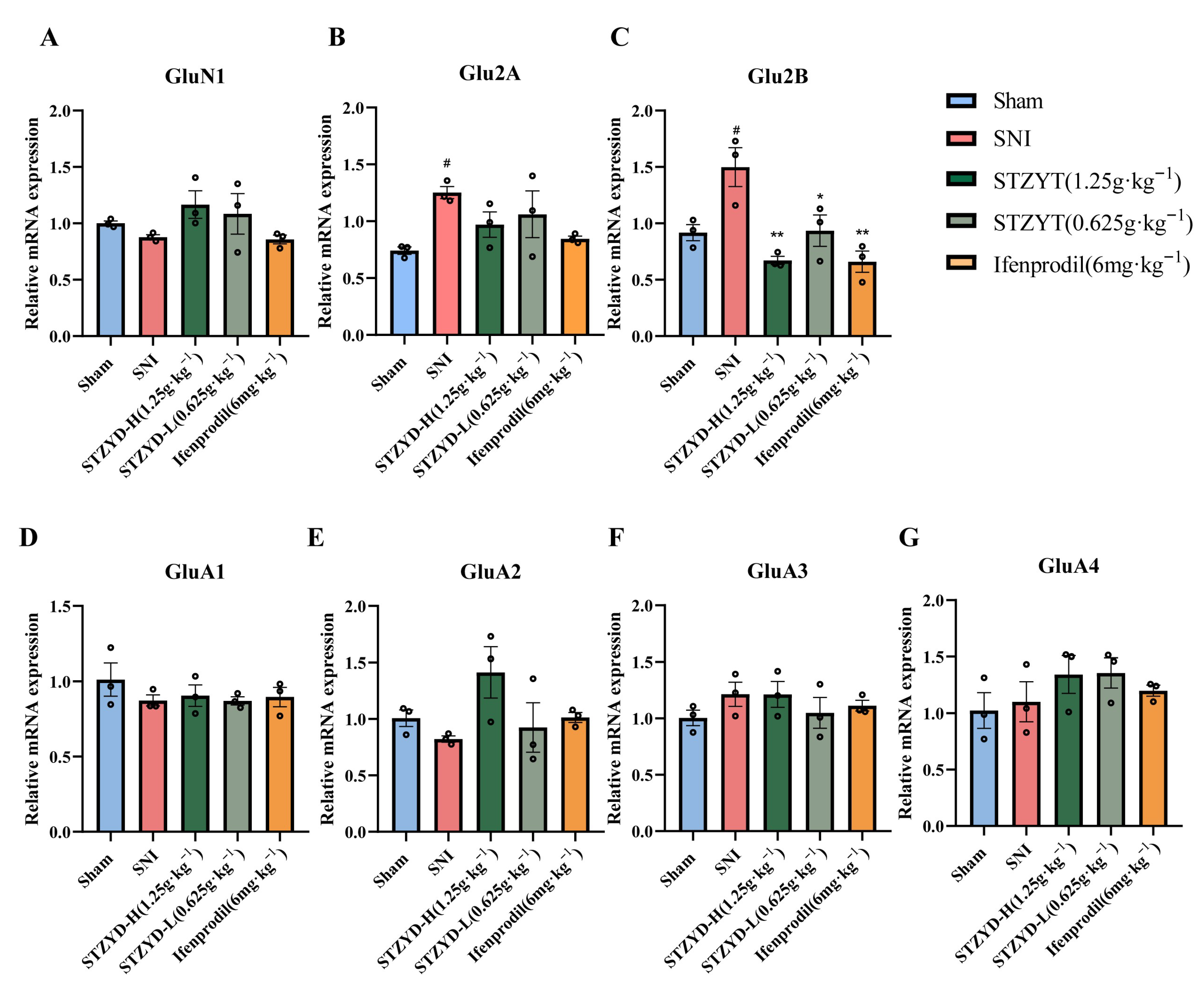
As a “molecular switch” of central sensitization, the NMDAR integrates peripheral nociceptive signals, central plasticity, and emotional regulation through a “subtype-spatiotemporal-specific” mechanism. Research shows that NMDAR upregulation and hyperactivation occur across diverse pain models, including spinal cord injury and inflammatory pain [
41,
42,
43,
44,
45,
46,
47,
48,
49,
50]. Notably, the NR2A and NR2B subunits are implicated in nociceptive transmission within key pain-processing regions: the caudal subnucleus of the spinal trigeminal nucleus, dorsal root ganglia (DRG), spinal dorsal horn, prefrontal cortex, and amygdala [
44,
45,
46,
47,
48,
49,
50]. The GRIN2B (encoding GluN2B) rs1806201 variant has been associated with susceptibility to chronic postsurgical pain, suggesting that NMDAR subtype expression levels may serve as biomarkers for pain classification and therapeutic response prediction [
51]. Our study further demonstrated that the mRNA and protein levels of the NMDA2B subunit, but not of other subtypes, were significantly elevated in the IPNs of the SNI mice. Notably, both the high-dose STZTD treatment and ifenprodil administration effectively downregulated this enhanced NMDA2B expression. These findings suggest that the excessive release of amino acid neurotransmitters (e.g., glutamate) may overactivate excitatory receptors, such as the NMDAR, leading to pathological calcium influx in neurons. This cascade exacerbates neural damage and perpetuates maladaptive pain signaling [
52]. Rather than relying on a single “key molecule”, the in vivo efficacy of STZYD likely stems from additive or synergistic GluN2B occupancy by licoricesaponin K2 and senkyunolide F, coupled with allosteric modulation via lower-affinity ligands such as azelaic acid and L-arginine. This multicomponent, multi-site binding mode may explain why the high-dose decoction, achieving the delivery of a combined compound mixture at supra-threshold brain levels, attains the complete suppression of the GluN2B/CaMKII/CREB axis, an effect rarely achieved with individual NMDAR blockers.
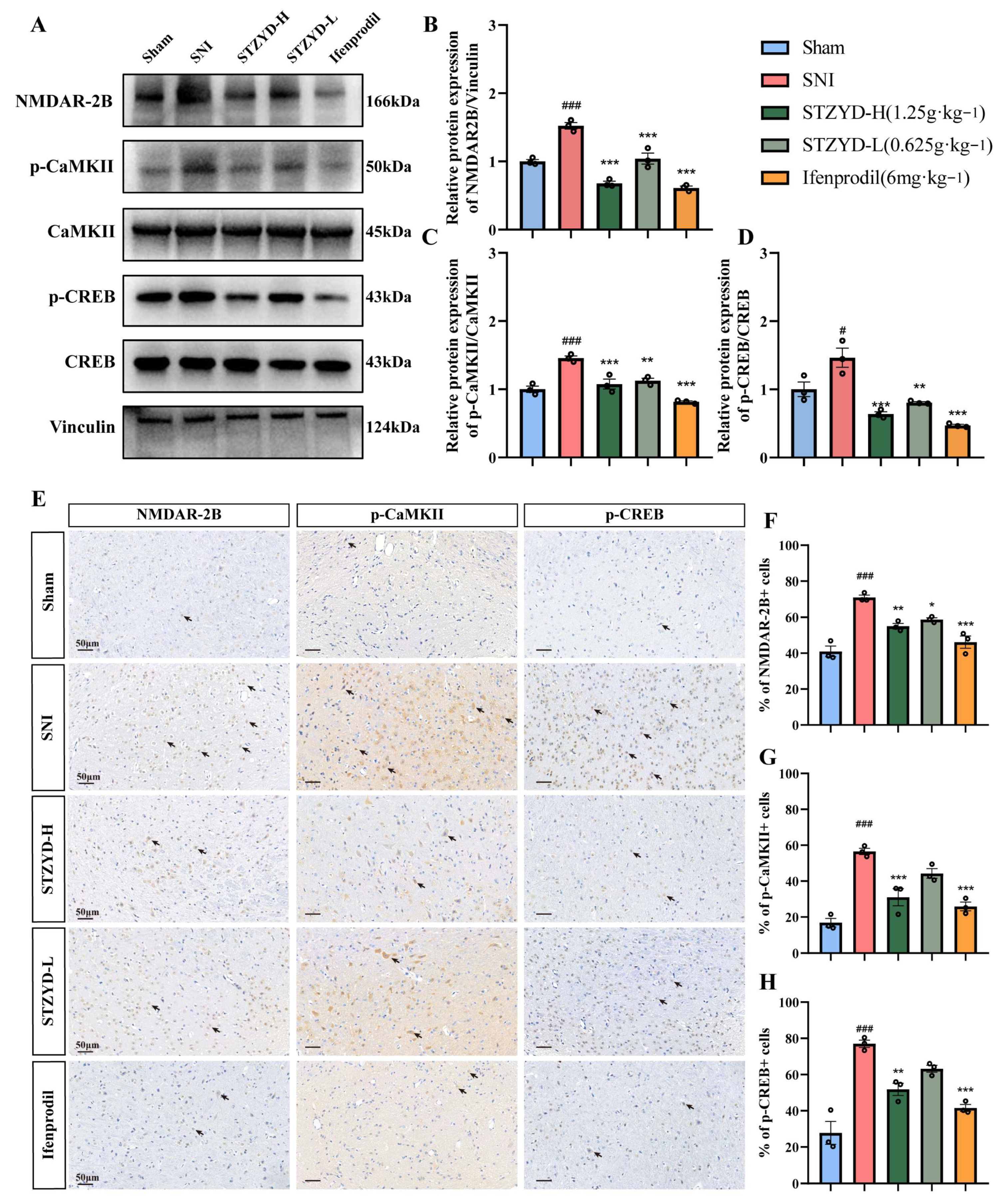
Electrochemical signaling plays a pivotal role in the central nervous system. As ligand-gated, voltage-dependent ion channels, hyperactivated NMDARs mediate pathological Ca
2+ influx. When an action potential reaches the axonal terminal, the Ca
2+/CaM complex binds to the autoinhibitory domain of CaMKII, relieving its autoinhibition and triggering autophosphorylation-mediated activation [
53]. Activated CaMKII phosphorylates CREB at Ser133 [
39], enabling its binding to cAMP response elements (CREs) and the subsequent recruitment of RNA polymerase II to form transcriptional complexes that regulate downstream gene expression [
54]. CREB activation promotes the transcription of multiple pain-related genes, including c-Fos, c-Jun, NK-1, COX-2, and BDNF [
39,
54]. The upregulation of these genes contributes to central sensitization—the neurobiological foundation underlying the transition from acute injury to chronic pain.
Our results demonstrate that STZYD exerts potent, dose-dependent antinociception in the SNI model by silencing the peripheral NMDAR-2B/CaMKII/CREB axis. The rapid upregulation of NMDAR-2B and the concomitant phosphorylation of CaMKII and CREB observed in the IPN following nerve injury mirror the cascade reported in diabetic and chemotherapy-induced neuropathy models, where this pathway drives mechanical hypersensitivity [
48,
55,
56,
57]. STZYD reversed these molecular changes at both the protein and phosphorylation levels, indicating that it disrupts the entire receptor kinase–transcription factor signaling axis rather than acting at a single node. The high-dose regimen produced a complete suppression of p-CREB, a finding rarely achieved by classic NMDAR antagonists that merely attenuate channel gating. This downstream shut-off suggests that STZYD may prevent the transcription of pronociceptive genes (c-Fos, BDNF, COX-2), a mechanism recently highlighted in chronic pain epigenetic studies [
58]. In contrast, the low-dose group inhibited NMDAR-2B expression without altering p-CaMKII/p-CREB, implying that additional anti-inflammatory or antioxidant constituents of STZYD become indispensable for full pathway blockade at higher concentrations. Such multitarget synergy is reminiscent of kaempferol, which simultaneously modulates Nrf2 and NF-κB to dampen neuropathic pain [
58,
59]. In contrast to conventional NMDAR antagonists, which predominantly target the central nervous system and can induce cognitive or motor side effects, STZYD, as a traditional orally administered Chinese medicine, demonstrates a more favorable safety profile [
60]. Collectively, whereas GABA_B agonists (e.g., baclofen) only partially dampen CREB phosphorylation, STZYD achieves the comprehensive shut-down of the NMDAR-2B/CaMKII/CREB signaling chain.
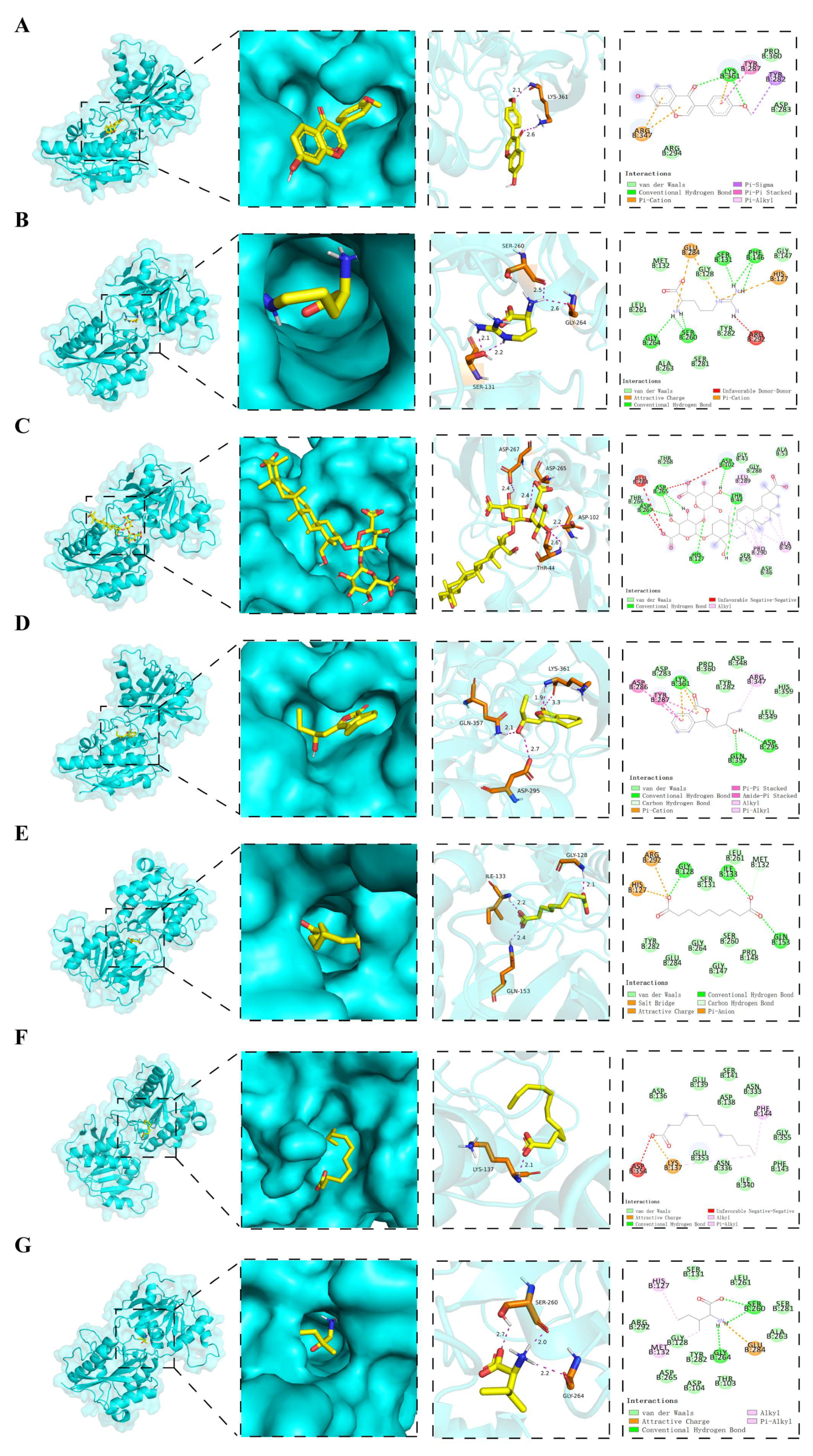
Using HPLC-Q-TOF-MS/MS, we identified seven constituents that crossed the blood–brain barrier (BBB) and accumulated in the IPN: L-arginine, azelaic acid, formononetin, licoricesaponin K2, senkyunolide F, L-isoleucine, and lauric acid. Given that the NMDAR-2B/CaMKII/CREB axis is a key mediator of central sensitization, we focused on investigating whether these BBB-penetrant compounds modulate NMDAR-2B to contribute to STZYD’s analgesic effects. Consistent with this hypothesis, ifenprodil, a selective NMDAR-2B antagonist, replicated STZYD’s analgesic efficacy, validating NMDAR-2B as a viable target for pain modulation. The molecular docking of the seven compounds to NMDAR-2B revealed that licoricesaponin K2 and senkyunolide F exhibited the strongest binding affinities, suggesting stable receptor–ligand engagement. Both compounds formed key hydrogen bonds and hydrophobic and π–π stacking interactions, stabilizing NMDAR-2B in an inactive conformation and preventing its activation at the glutamate- and glycine-binding sites. This high-affinity binding impedes NMDAR-2B channel opening, thereby reducing pathological Ca
2+ influx. Licoricesaponin K2, with a binding energy of −8.2 kJ/mol, demonstrated stable binding via hydrogen bonds and hydrophobic interactions, with its glycosidic moiety deeply penetrating the receptor’s binding cavity, enhancing its therapeutic potential for pain. Similarly, senkyunolide F, with a binding energy of −7.8 kJ/mol, formed multiple interactions, including hydrogen bonds, van der Waals forces, and π–cation interactions, supporting its potential as a potent NMDAR-2B modulator for pain management. The diverse nature of its binding interactions suggests that senkyunolide F could exert a more stable and effective influence on receptor activity, making it a promising candidate for further preclinical evaluation. These interactions are consistent with the mechanism proposed for ifenprodil and related NMDAR-2B selective antagonists, which prevent glutamate/glycine co-agonist binding, and thus block Ca
2+ influx and downstream CaMKII/CREB signaling [
60,
61,
62]. Integrating in vivo data, including the complete suppression of the NMDAR-2B/CaMKII/CREB axis and dose-dependent analgesia, we hypothesize that licoricesaponin K2 is the primary active constituent, with the other compounds acting as allosteric modulators to enhance the overall inhibitory potency. This multicomponent, multitarget binding mode underpins the superior efficacy of STZYD compared with single-target NMDAR-2B blockers.
We acknowledge that the 12-herb STZYD composition was not analytically standardized across batches and that analgesic end-points were assessed only within 24 h after the last daily dose. Consequently, the metabolite variability, long-term PK, and effect duration remain undetermined and will be addressed in future marker-controlled extract studies.
We posit that STZYD should serve as an adjuvant to standard NMDA antagonists or GABA mimetics, rather than as a monotherapy, to reduce their required doses. High-dose STZYD completely suppressed the GluN2B/CaMKII/CREB axis with broader reach than ifenprodil. We hypothesize that when combined, STZYD and traditional NMDA antagonists may achieve the same level of inhibition at lower doses, benefiting from both direct blockade and allosteric synergy. This could limit adverse CNS events, such as cognitive impairment, which are common with high-dose NMDA antagonists. Unlike the existing GluN2B-selective drugs that target nociception only, STZYD simultaneously alleviates pain and comorbid anxiety, offering a dual analgesic–anxiolytic profile aligned with multidimensional pain management. Bioactive constituents (licoricesaponin K2, senkyunolide F) are small, BBB-permeant GluN2B ligands that can be engineered into targeted probes for optimized co-formulations. Regarding the pharmacokinetics of STZYD, while 40 compounds entered murine plasma and 7 reached the IPN, their human pharmacokinetics remain uncharacterized. The concentration of a single component in the body is insufficient to produce a significant effect. However, the seven components achieve the transformation from a low-dose combination to efficient pathway regulation through additive or synergistic effects, which represents the core advantage of the multi-mechanistic properties of compound traditional Chinese medicine. We recognize that future studies should focus on the human oral bioavailability, steady-state brain exposure, major metabolites, drug–drug interaction potential, and chronic toxicology before clinical use. Furthermore, we will include a more detailed discussion on the possible synergistic effects of these compounds in STZYD, as polypharmacology may contribute significantly to its therapeutic efficacy.
4. Materials and Methods
4.1. Animals
Male C57BL/6J mice (19–22 g) were obtained from Beijing Huafukang Biotechnology Co., Ltd. (Beijing, China), and maintained in the lab animal center of the Institute of Basic Theory for Chinese Medicine, China Academy of Chinese Medical Sciences. Animals were kept at 20–24 °C and 40–50% relative humidity under a 12 h light–dark cycle with ad libitum access to food and water. All animal procedures were approved by the Animal Care and Use Committee of the Experimental Research Center of the China Academy of Chinese Medical Sciences (Approval No. ERCCACMS21-2405-01) and were performed in strict accordance with the institutional guidelines for laboratory animal welfare.
4.2. Network Pharmacology Analysis
The active ingredients of STZYD were identified through the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP,
https://old.tcmsp-e.com/tcmsp.php, accessed on 24 September 2024). The chemical constituents of the 12 herbs in STZYD were retrieved, and potential bioactive compounds were selected based on two key pharmacokinetic parameters: their oral bioavailability (OB ≥ 30%) and drug-likeness (DL ≥ 0.18). Target proteins for these active compounds were identified via TCMSP and further cross-referenced with the UniProt database (
http://www.uniprot.org/, accessed on 22 August 2024) to standardize the gene names and ensure accuracy.
Disease targets associated with NPP were gathered from the GeneCards database (
https://www.genecards.org/, accessed on 24 August 2024), the Human Mendelian Inheritance Database (OMIM,
http://www.omim.org, accessed on 24 August 2024), and the DrugBank database (
https://www.drugbank.ca, accessed on 24 August 2024). Redundant targets were removed, leaving only unique disease-related targets for further analysis.
The intersection of the STZYD active compound targets and NPP-related disease targets was determined using jvenn (
https://jvenn.toulouse.inra.fr/app/index.html, accessed on 24 August 2024), resulting in the identification of overlapping targets between the drug and disease.
To construct a “traditional Chinese medicine–active-ingredient–target–disease “ network, the identified active components and NPP-related targets were imported into Cytoscape software. Additionally, potential target proteins for STZYD in the treatment of NPP were predicted using the STRING database (
https://string-db.org/, accessed on 26 August 2024), with the species set to Homo sapiens and a confidence threshold of 0.400. The resulting protein–protein interaction (PPI) network was visualized, and the degree centrality (node connectivity) of each protein was calculated using the “Analyze Network” plugin in Cytoscape to identify core targets.
The intersecting drug–disease targets were further analyzed using the Metascape platform (
https://metascape.org, accessed on 26 August 2024). Gene Ontology (GO) enrichment analysis was performed, focusing on biological processes (BPs), molecular functions (MFs), and cellular components (CCs), to elucidate the role of STZYD’s target proteins in treating NPP. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was conducted to identify key signaling pathways involved in the treatment of NPP via STZYD. The most significant gene functions and pathways were visualized through bar and bubble charts.
4.3. Spared Nerve Injury Surgery
The spared nerve injury (SNI) surgery strictly followed the requirements of the International Association for the Study of Pain for conducting pain experiments on awake animals. Male C57BL/6J mice were first induced with 4% isoflurane and then maintained at 1.1% isoflurane (0.15 L·min−1) via a gas anesthesia machine. All surgical instruments were sterilized via high temperature. After the mice were completely anesthetized, the hair on the left knee joints of the mice was shaved off, and a 0.5–1 cm incision was made with scissors at the muscle bulge to expose the sciatic nerve bundle comprised the tibial nerve, common peroneal nerve, and sural nerve. The tibial and common peroneal nerves were ligated with surgical suture and then cut 1–2 mm distally, leaving only the sural nerve. In the Sham group, the mice underwent a similar procedure but without nerve injury. After the surgery, gentamicin (0.01 mL·10 g−1) was injected into the mice to prevent infection, and then the mice were returned to the cage. Seven days after surgery, mice were randomly assigned to one of five groups (n = 6 per group): the Sham, SNI, SNI + low-dose STZYD (0.625 g·mL−1), SNI + high-dose STZYD (1.25 g·mL−1, expressed as crude drug), or SNI + ifenprodil (6 mg·kg−1, i.p.) groups. Treatments were administered daily for seven consecutive days; the Sham and SNI groups received equivalent volumes of normal saline.
4.4. STZYD Preparation
STZYD was administered to mice by oral gavage once daily for 7 days at 0.625 g·mL
−1 (0.2 mL·10
−1 g body weight). The dosing regimen was based on the Chinese Pharmacopoeia recommendation of 15 g crude drug/60 kg adult (0.25 g·kg
−1). According to the FDA interspecies body–surface area conversion (mouse factor: 12.3), the equivalent mouse dose was 0.625 g·kg
−1 (low-dose group), and the high-dose group received 1.25 g·kg
−1 (2× clinical exposure). STZYD consists of the following twelve traditional Chinese medicinal herbs, all of which were purchased from Beijing Tongrentang (Beijing, China) to ensure consistent quality for the subsequent experimental procedures:
Gentiana straminea Maxim. (Qinjiao, QJ), 3 g;
Ligusticum chuanxiong Hort. (Chuanxiong, CX), 6 g;
Prunus davidiana (Carr.) Franch. (Taoren, TR), 9 g;
Carthamus tinctorius L. (Honghua, HH), 9 g;
Glycyrrhiza uralensis Fisch. (Gancao, GC), 6 g;
Notopterygium incisum Ting ex H. T. Chang (Qianghuo, QH), 3 g;
Commiphora molmol Engl. (Moyao, MY), 6 g;
Angelica sinensis (Oliv.) Diels (Danggui, DG), 9 g;
Trogopterus xanthipes Milne-Edwards (Wulingzhi, WLZ), 6 g;
Cyperus rotundus L. (Xiangfu, XF), 3 g;
Achyranthes bidentata Bl. (Niuxi, NX), 9 g;
Pheretima aspergillum (E. Perrier) (Dilong, DL), 6 g. First, 135 g of QJ, QH, and XF, 270 g of CX, GC, MY, WLZ, and DL, and 405 g of TR, HH, DG, and NX were taken. Then, they were added to 10 times the volume of distilled water (31 L), soaked for 1 h, and heated in a sealed container, and after boiling, the extract was refluxed for 1 h [
18,
19,
20]. For the second time, 8 times the volume of distilled water (24.84 L) was added. After the pot boiled, it was sealed and refluxed for 1 h. The two extracts were combined, filtered, and concentrated in a low-temperature cycle. The solution was then made up to 5.2 L. An amount of 1.5 L of concentrate was reserved for the low-dose decoction (0.625 g·mL
−1), and the remainder was concentrated to obtain the high-dose decoction (1.25 g·mL
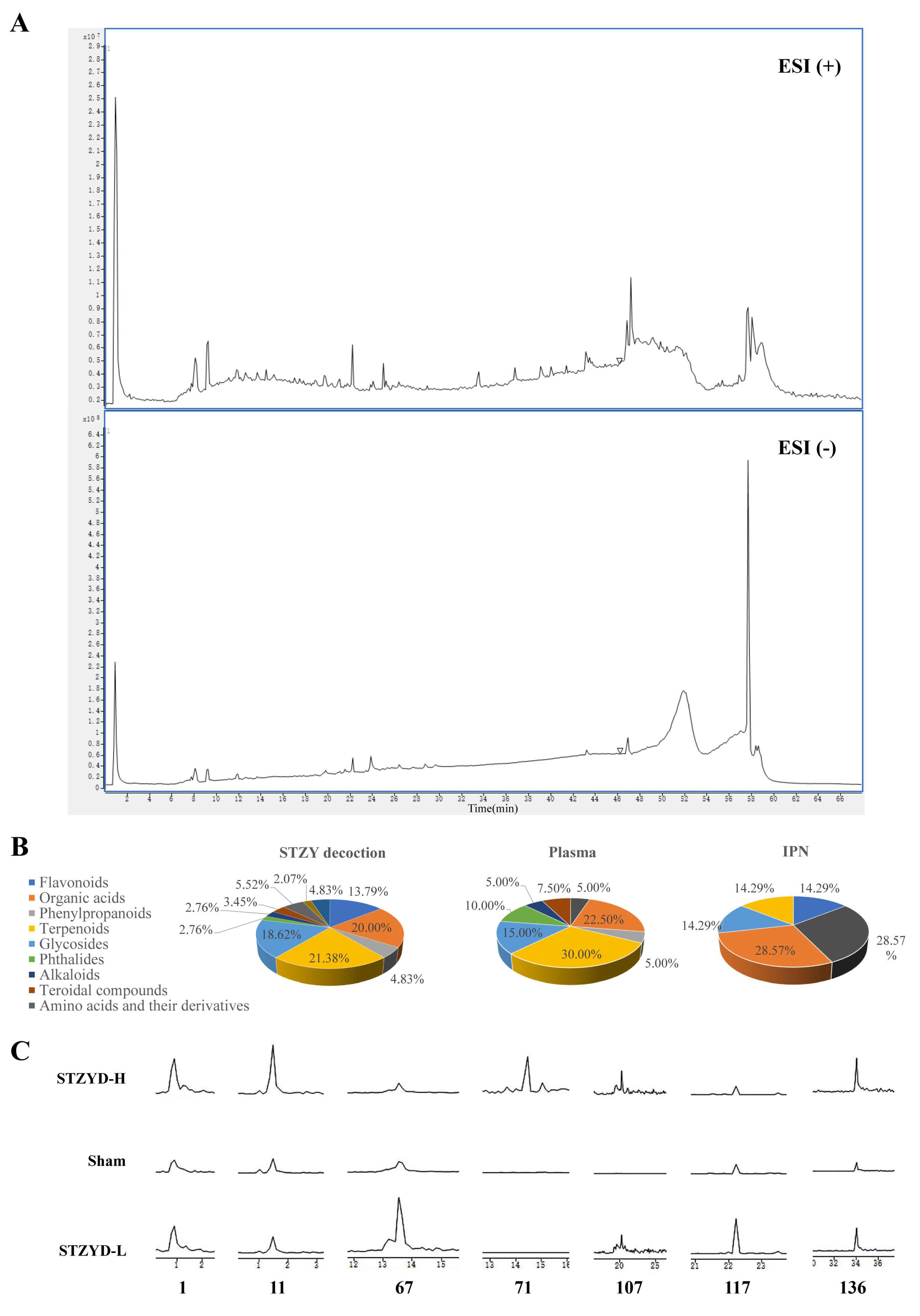
−1). For chemical profiling, 5 g of each herb was finely powdered and extracted with 70% methanol via sonication for 30 min. After centrifugation at 13,000 rpm for 15 min, the supernatant was collected and diluted appropriately with 50% methanol, and a 50 μL aliquot was analyzed via HPLC-Q-TOF-MS/MS.
4.5. Preparation of Mouse Serum and Brain Tissue Samples
The SNI mice in the high- and low-dose STZYD groups were administered the STZYD via gavage (0.2 mL·10 g
−1), while the Sham group was given the same volume of normal saline via gavage. The doses were continuously administered for 3 d to ensure that the marker components reached steady-state plasma levels and to avoid potential metabolite degradation or nonspecific tissue interference due to long-term administration [
63]. On the last day, blood was collected from the orbital vein, and the brain was removed 2 h after the administration. The blood samples were left to stand at room temperature for 2 h, the samples were then centrifuged at 3500 rpm and 4 °C for 15 min, and the supernatant was taken. The brain tissue was sliced using a mouse brain mold, and the IPN brain region was extracted. The IPN tissue was precisely weighed and homogenized with 2 times the volume of normal saline. The aforementioned prepared samples were subjected to HPLC-Q-TOF-MS/MS analysis.
For in vivo pharmacodynamic studies, all tissue samples were obtained after 7 days to ensure that the therapeutic effect of the drug had stabilized. After the behavioral test, three mice from each group were selected for the Western blotting and RT-qPCR experiments to detect proteins and mRNA, and the remaining three mice were used for immunohistochemical staining. After the microdialysis experiment, the brain tissues of the mice were taken and fixed with 4% paraformaldehyde for HE staining to assess the probe implantation site. The c-Fos protein detection was carried out after the behavioral test, and the brain tissue was removed via cardiac perfusion and fixed.
4.6. Behavioral Tests
All mice were acclimated to the testing room environment for 3 h prior to behavioral testing. To ensure consistency, the same experimenter conducted all the procedures.
4.6.1. Von Frey Hairs Test
The mice were placed in transparent plastic boxes (10 cm × 5.8 cm × 10 cm) on the wire mesh for at least 30 min to familiarize themselves with the experimental environment. When Von Frey filaments were applied vertically to the metacarpal and calcaneal junction of the left hind paw, painful reactions such as brisk paw withdrawal, flinching, licking, or shaking were considered positive. The minimum fiber gram value when the mouse positive reaction reached three out of five stimulation times was identified as the mechanical pain threshold response value, and the interval of each measurement was at least 1 min. If there was no positive reaction, a higher gram weight fiber was used for stimulation until a positive reaction occurred. The positive values were denoted as the paw withdrawal threshold (PWT).
4.6.2. Hargreaves Test
Thermal sensitivity was assessed using the Hargreaves and hot-plate tests. During testing, an infrared radiant heat source (55 W) (Ugo Basile 21036, Gemonio, VA, Italy) was applied to the central plantar surface of the left hind paw. The latency to paw withdrawal or licking in response to thermal stimulation was recorded as the paw withdrawal latency (PWL). Thermal laser stimulation was limited to a maximum duration of 20 s to prevent tissue damage. Each animal was tested at 10 min intervals for at least three trials, and the average value of the three measurements was calculated as the final PWL for each mouse.
4.6.3. Hot-Plate Test
The hot-plate test was used to assess the thermal nociception by measuring the PWLs in the mice. Animals were individually confined in transparent acrylic cylinders on a heated surface maintained at 55.0 ± 0.1 °C. The PWL was defined as the time elapsed until the manifestation of nociceptive behaviors, including hind paw licking, shaking, or lifting. An automatic 30 s cutoff was implemented to prevent tissue injury, with the manual removal of non-responsive mice at this threshold. Tests were conducted at 10 min intervals to allow for recovery between trials.
4.6.4. Light–Dark-Box Test
The light–dark-box test was conducted using a 21 cm × 21 cm × 45 cm box. The left side of the box was black and the right side was a white chamber with illumination. The mice were allowed to move freely between the two compartments for 5 min. A motion-tracking system recorded the light-box residence time (LBRT) and the number of transitions between the light and dark compartments. Data analysis was performed using Shanghai Jiliang software (version 2021, Shanghai, China).
4.7. Stereotaxic Surgery
Mice were deeply anesthetized with intraperitoneal tribromoethanol (0.2 mL·10 g−1, i.p.). After shaving the scalps, animals were positioned in a stereotaxic frame (RWD Life Science Co., Ltd., Shenzhen, China) on a thermostatically controlled heating pad (KEL-2000, Nanjing, China) to maintain their core temperatures. After leveling the skull, we targeted the interpeduncular nucleus (coordinates: AP: −3.52 mm; ML: −1.25 mm; DV: −4.53 mm; 16° right angle), drilled a craniotomy with a skull drill (RWD Life Science Co., Ltd., Shenzhen, China), and secured the microdialysis probe guide cannula to the skull using screws and dental cement. Postoperatively, each mouse received gentamicin (0.01 mL per 10 g, s.c.) and was housed individually.
In the fiber photometry experiment, viral preparations were injected into the IPN using a Hamilton 10 μL microsyringe (1701RN; Hamilton Company, Reno, NV, USA) and a Harvard Apparatus (PUMP 11 ELITE Nanomite, Harvard, MA, USA). A dual-virus strategy was employed, using rAAV-GAD67-CRE-mCherry-WPRE-hGH polyA and rAAV-EF1 a-DIO-GCaMp6s-WPRE-hGH polyA (virus titers: 5.55E + 12 vg·mL−1 and 5.27E + 12 vg·mL−1, respectively) (BrainVTA Technology Co., Ltd., Wuhan, China), mixed in a 1:2 ratio and injected at 300 nL per mouse.
In the chemogenetic experiment, 300 nL of rAAV GAD67-hM4D(Gi)-mCherry-WPREs or rAAV GAD67-hM3D(Gq)-mCherry-WPREs (virus titers: 5.27 × 1012 vg·mL−1 and 6.04 × 1012 vg·mL−1, respectively) (BrainVTA Technology Co., Ltd., Wuhan, China) was injected into the interpeduncular nucleus (IPN) of C57BL/6 mice. The IPN coordinates were as follows: AP: −3.4 mm; ML: −1.3 mm; DV: −4.85 mm; a 14° angle. After the viral injection, the glass electrode was left in place for 10 min to prevent viral leakage. Optic fibers (Ø1.25 mm/5.5 mm; Thinker Tech Nanjing Bioscience, Nanjing, China) were implanted and secured with dental cement. After surgery, the mice were injected with gentamicin (0.01 mL·10 g−1) to prevent infection and were kept in a single cage. The viral injections were performed 3 weeks prior to the start of the experiment to ensure sufficient transgene expression.
4.8. Microdialysis Experiment
Prior to implantation, the mouse brain microdialysis probes were soaked in pure water for 30 min and subsequently placed in a mixed standard solution containing glutamate (Glu), D-serine (D-Ser), aspartate (Asp), glycine (Gly), and gamma-aminobutyric acid (GABA). Probes were perfused with saline (1.0 μL·min−1) for 30 min to determine the in vitro recovery rates.
On the experimental day, the probes were implanted and perfused with compound sodium chloride (1 μL·min−1) for 2 h to achieve equilibration. Baseline dialysate was collected at 30 min intervals over 120 min. Following intraperitoneal ifenprodil administration and the oral gavage of low-/high-dose STZYD, post-administration dialysate was collected continuously for 240 min (12 tubes) under identical conditions. Post-experiment, brains were fixed in 4% tissue fixative, sectioned, and microscopically verified for probe placement, with data included only if the dialysis membrane position deviated ≤ 30% from the target site’s dorsal/ventral boundaries.
4.9. Fiber Photometry
The fiber photometry system was provided by Thinker Tech Nanjing Bioscience Inc. A blue excitation light at 470 nm was emitted from an LED source, reflected by a dichroic mirror, and coupled through an objective lens into a multimode optical fiber. The tip of the fiber delivered the excitation light into the IPN region, where it activated GCaMP calcium-sensitive fluorescent proteins expressed in neurons. The light intensity at the fiber tip was maintained at approximately 30 μW to minimize photobleaching. Mechanical and thermal nociceptive stimuli were applied to the left hind paws of mice using a Von Frey filament (0.4 g, Danmic, San Jose, CA, USA) and radiant heat source, respectively, to monitor calcium signal fluctuations in GABAergic neurons within the interpeduncular nucleus (IPN) during pain-related tests. Each stimulus event was time-stamped within the recording window, and the resulting calcium signals were analyzed using MATLAB software (version 2017b; MathWorks, Natick, MA, USA) in conjunction with a fiber photometry analysis package (Thinker Tech Nanjing Bioscience, Nanjing, China). The relative change in the fluorescence intensity (ΔF/F
0) was calculated by subtracting the baseline fluorescence (F
0) from the recorded fluorescence (F) and then dividing by F
0, i.e., (F − F
0)/F
0. Here, F
0 was defined as the mean GCaMP6s signal during the 5 s baseline period immediately preceding either the Von Frey stimulation or the thermal radiant heat stimulus. The fiber photometry data were further processed using custom-written MATLAB scripts. Quantitative parameters, including the area under the curve (AUC) (determined via trapezoidal integration) and peak fluorescence amplitude, were extracted from a 5 s peri-event window, as previously described [
64].
4.10. Chemogenetic Manipulation
In the chemogenetic experiment, 300 nL of rAAV GAD67-hM4D(Gi)-mCherry-WPREs or rAAV GAD67-hM3D(Gq)-mCherry-WPREs (BrainVTA Technology Co., Ltd., Wuhan, China) was injected into the IPNs of C57BL/6 mice. The IPN coordinates were as follows: AP: −3.4 mm; ML: −1.3 mm; DV: −4.85 mm; a 14° angle. In the chemogenetic inhibition experiment, the hM4D(Gi) vector was injected, and one week later, a SNI model was induced. Behavioral testing was conducted two weeks post-surgery. One hour following the intraperitoneal injection of clozapine-N-oxide (CNO) (5 mg·kg−1), the PWT and PWL were measured. In the chemogenetic activation experiment, behavioral testing was performed three weeks after the hM3D(Gq) injection, with CNO (1 mg·kg−1) administered one hour prior to the test.
4.11. HPLC-FLD Detection Conditions
The ortho-phthalaldehyde (OPA) derivatization reagent was prepared by dissolving 5 mg OPA in 120 μL methanol, followed by the addition of 10 μL β-mercaptoethanol and 1 mL borate buffer (0.2 mol·L−1, pH 9.2). The OPA reagent was prepared fresh daily and stored protected from light on ice until use. This solution was mixed thoroughly and stored protected from light. The mobile phase consisted of Liquid A (20 mmol·L−1 sodium acetate buffer (pH 7.2)/methanol/tetrahydrofuran at 400:95:5) and Liquid B (buffer/methanol at 120:380). Chromatographic separation was performed at a 0.8 mL·min−1 flow rate and 40 °C column temperature using gradient elution: initial column conditioning with 80% methanol/20% water (30 min) followed by 50% methanol/50% water (30 min) and 20% methanol/80% water (30 min). After column pressure stabilization, the analytical gradient was applied: 0–10 min (0–63% B), 10–12 min (63% B), 12–17 min (100% B), 17–18 min (100–0% B), and 18–21 min (0% B). Amino acid analytes underwent automated pre-column derivatization with OPA before injection. Fluorescence detection employed 340 nm excitation and 455 nm emission wavelengths at a photomultiplier tube gain of 12. Blank and sample analyses were conducted following system equilibration.
4.12. HPLC-Q-TOF-MS/MS Analysis Conditions
Two hundred microliters of serum or brain homogenate from each mouse was transferred into a 5 mL EP tube, and the proteins were precipitated by adding three volumes of ice-cold methanol. After vortexing for 3 min, samples were centrifuged (8000 rpm, 4 °C, 10 min), the supernatant was dried with nitrogen gas at 40 °C, and then 50% methanol was added to redissolve it. The mixture was vortexed for 3 min and centrifuged at 4 °C and 10,000 r·min−1 for 10 min, and 3 μL of the supernatant was injected.
4.12.1. Chromatographic Conditions
Agilent 6520 Q-TOF LC-MS was used for analysis, consisting of an Agilent 1200 liquid chromatograph and an Agilent 6520 Q-TOF time-of-flight mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). For HPLC separation, the sample solution was injected into an ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm, Waters, Milford, MA, USA). The mobile phase consisted of phase A (0.1% formic acid in water) and phase B (ACN), with a linear gradient elution (0–4 min, 95% A; 4–51 min, 95–0% A; 51–56 min, 0% A; 56–58 min, 0–95% A; 58–68 min, 95% A), at a flow rate of 0.4 mL·min−1. The column temperature was 40 °C, and the injection volume was 3 μL.
4.12.2. Mass Spectrometric Conditions
The mass spectrometric conditions were as follows: electrospray ionization source (ESI); positive-ion mode; capillary voltage: 3500 V; auto MS/MS mode scanning; scan range: m/z 50–1750; collision voltages: 10, 20, 40 V; drying gas nitrogen, flow rate: 10 L·min−1; nebulizer gas pressure: 40 psi; desolvation gas temperature: 350 °C. All data were processed using MassHunter software (Agilent, v10.0).
4.13. Western Blotting
IPN tissue was dissected on a chilled brain matrix, snap-frozen, and lysed in 2% SDS buffer supplemented with protease/phosphatase inhibitor (#78440, Thermo Fisher Scientific Inc., Waltham, MA, USA). Equal amounts of protein were resolved on SDS-PAGE gels and transferred to PVDF membranes. Membranes were blocked with 5% BSA and incubated overnight at 4 °C with the following primary antibodies: NMDAR2B (ab183942, Abcam, Cambridge, UK; 1:1000), CaMKII (ab52476, Abcam, Cambridge, UK; 1:1000), p-CaMKII (#12716, Cell Signaling Technology, Danvers, MA, USA; 1:1000), CREB (#9197, Cell Signaling Technology, Danvers, MA, USA; 1:1000), p-CREB (#9198, Cell Signaling Technology, Danvers, MA, USA; 1:1000), and Vinculin (#26520-1-AP, Proteintech, Rosemont, IL, USA; 1:10,000). After PBST washes, HRP-conjugated secondary antibodies were applied. Protein bands were visualized via chemiluminescence and quantified using ImageJ [
65] (version 1.50i, NIH, Bethesda, MD, USA).
4.14. mRNA Gene Expression Using RT-qPCR Analysis
Total RNA from IPN punches was extracted with the TRIzol™ Reagent (#15596026, Thermo Fisher Scientific, Waltham, MA, USA) and treated with gDNA Eraser prior to reverse transcription using the PrimeScript™ RT kit (#RR047A, Takara Bio Inc., Shiga, Japan), as detailed by Castellanos-Rizaldos et al. (2020) [
66]. The target gene expressions (
Table 1) were normalized to GAPDH and quantified using the 2
−ΔΔCt method [
67].
4.15. Immunofluorescence and Immunohistochemistry
For immunofluorescence staining, tissue sections were baked at 65 °C for 1 h and then processed for dewaxing, antigen retrieval, and antibody incubation essentially as described by Wang et al. (2024) [
68]: the slides were dewaxed in xylene, rehydrated through a graded ethanol series, and subjected to heat-induced epitope retrieval in 10 mM sodium-citrate buffer (pH 6.0) at 95 °C for 20 min. After blocking with 3% (
w/
v) BSA at room temperature for 30 min, the sections were incubated overnight at 4 °C with rabbit anti-c-Fos antibody (ab208942, Abcam, Cambridge, UK; 1:400) and were subsequently incubated for 1 h at room temperature with CoraLite 488-conjugated goat anti-rabbit IgG (H+L) (SA00013-1, Proteintech, Rosemont, IL, USA; 1:400). Nuclei were counterstained with DAPI-containing anti-fade mounting medium (Solarbio S2110) and coverslipped. Fluorescence images were acquired with a KFBIO KF-PRO-020 slide scanner and quantified using ImageJ (version 1.50i, NIH, Bethesda, MD, USA).
For the immunohistochemistry experiment, sections were routinely deparaffinized and subjected to heat-induced antigen retrieval in citrate buffer for 20 min. After blocking for 30 min, the slides were incubated with the primary antibody at room temperature for 2 h, and the appropriate secondary antibody was then applied and left for 30 min at room temperature. Chromogenic detection was performed with DAB, followed by counterstaining with hematoxylin for 5 min. Sections were dehydrated, cleared, mounted, and examined under a microscope. The positive cells were counted using ImageJ software. Primary antibodies used included anti-NMDAR2B (ab183942, Abcam, Cambridge, UK; 1:1000), phospho-CREB (#9198, Cell Signaling Technology, Danvers, MA, USA; 1:1000), and phospho-CaMKII (#12716; Cell Signaling Technology, Danvers, MA, USA; 1:1000).
After the completion of the fiber photometry and chemogenetic experiments, mice were anesthetized with tribromoethanol (0.2 mL·10 g−1, i.p.). The brains were removed, post-fixed in 4% PFA for 10 h at 4 °C, and then immersed in a 30% sucrose solution until fully sank. Coronal brain sections (40 μm) were prepared using a cryostat (Epredia™ CryoStar™ NX70, Kalamazoo, MI, USA). Sections were rinsed with PBS, blocked with 0.5% Triton X-100 and 5% BSA for 1 h, and stained with DAPI for nuclear visualization. Viral injection sites were visualized on a confocal microscope (VS120, Olympus, Tokyo, Japan).
4.16. Molecular Docking
The molecular docking of four drug-derived molecules in the IPN with the NMDAR-2B receptor was conducted. The protein information was obtained from the UniProt website (
https://www.uniprot.org/uniprotkb, accessed on 22 July 2025), and the 3D structure of the target protein was downloaded from the PDB database (
https://www.rcsb.org/, accessed on 22 July 2025). Then, hydrogen atoms and charges were added to the protein using Autodock vina 1.5.6, and they were set as the receptors. The files were saved as PDB files for future use. The structures of the drug molecules were obtained from the PubChem database (
https://pubchem.ncbi.nlm.nih.gov/, accessed on 22 July 2025), and energy minimization was performed using Discovery Studio 2019. Drug molecule structures were exported as PDB files. Hydrogen atoms and charges were added to the drug molecule structures, and they were set as ligands. The ligand files were exported in PDBQT format. Molecular docking was carried out in Autodock vina 1.5.6, and the results were visually analyzed using PyMol 3.1 and Discovery Studio 2019.
4.17. Statistical Analysis
All data represent at least three independent biological replicates, expressed as means ± SEMs. Statistical analyses were conducted using GraphPad Prism (v8.0.1). For the group comparisons, we employed unpaired or paired two-tailed Student’s
t-tests, one-way ANOVAs, or two-way ANOVAs, as appropriate. Statistical significance was defined as
p < 0.05 for all analyses. Exact
p-values for all comparisons are provided in
Supplementary Table S2.