Musa paradisiaca L. Inflorescence Abrogates Neutrophil Activation by Downregulating TLR4/NF-KB Signaling Pathway in LPS-Induced Acute Lung Injury Model
Abstract
1. Introduction
2. Results
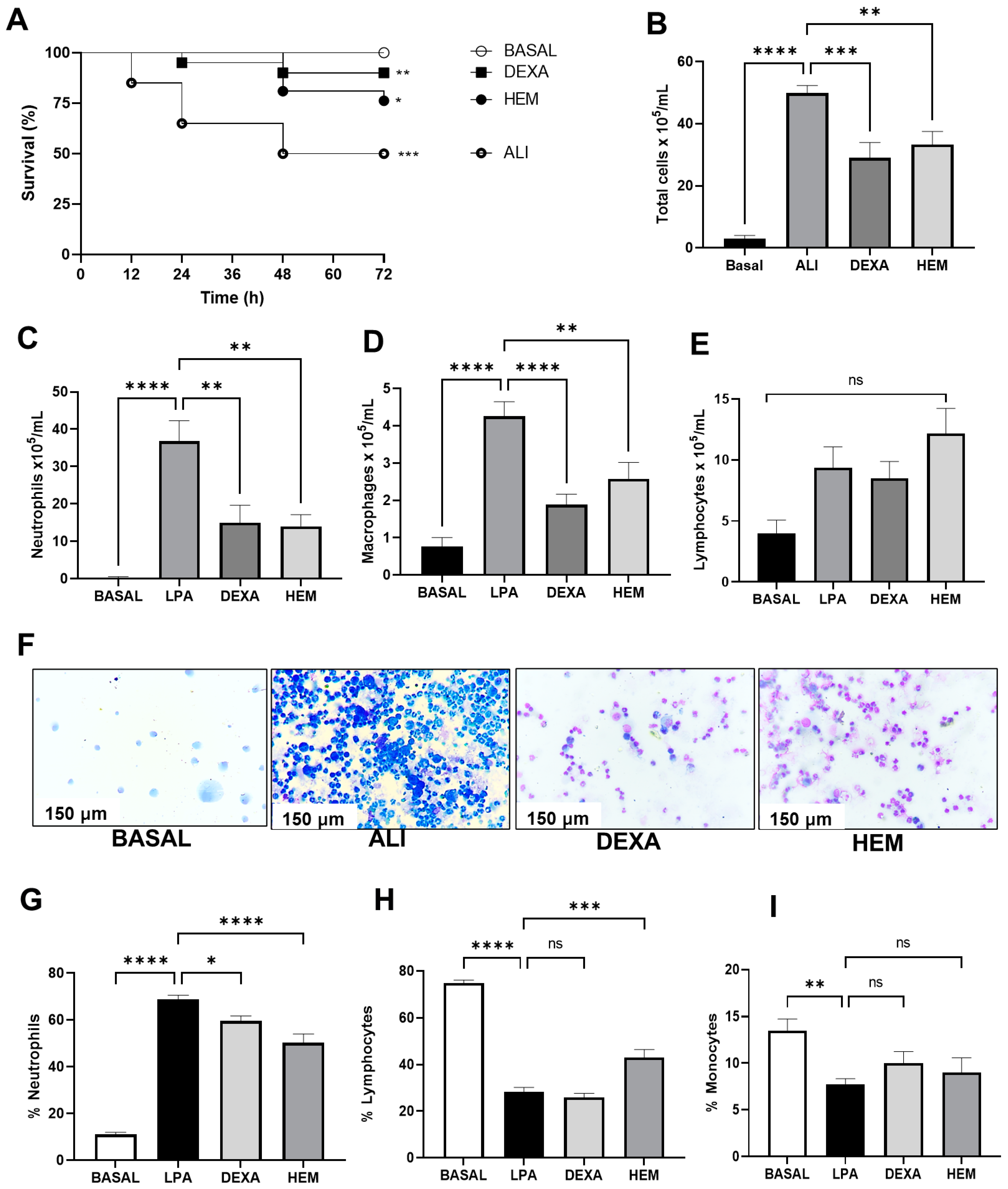
2.1. HEM Improves Animal Survival and Reduces Inflammatory Cell Migration in the BALF in LPS-Induced ALI
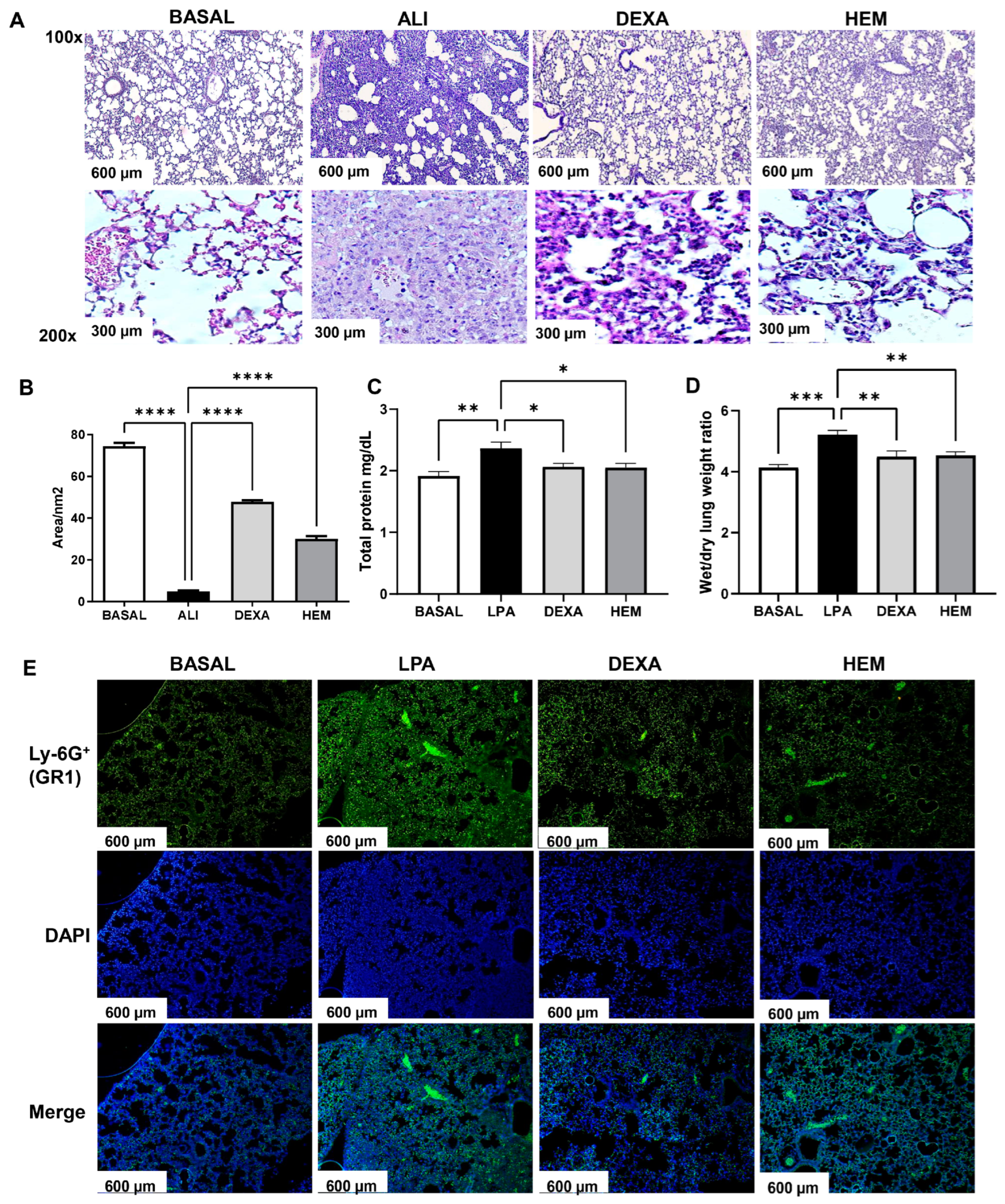
2.2. HEM Decreases Pulmonary Edema and Inflammation in LPS-Induced ALI
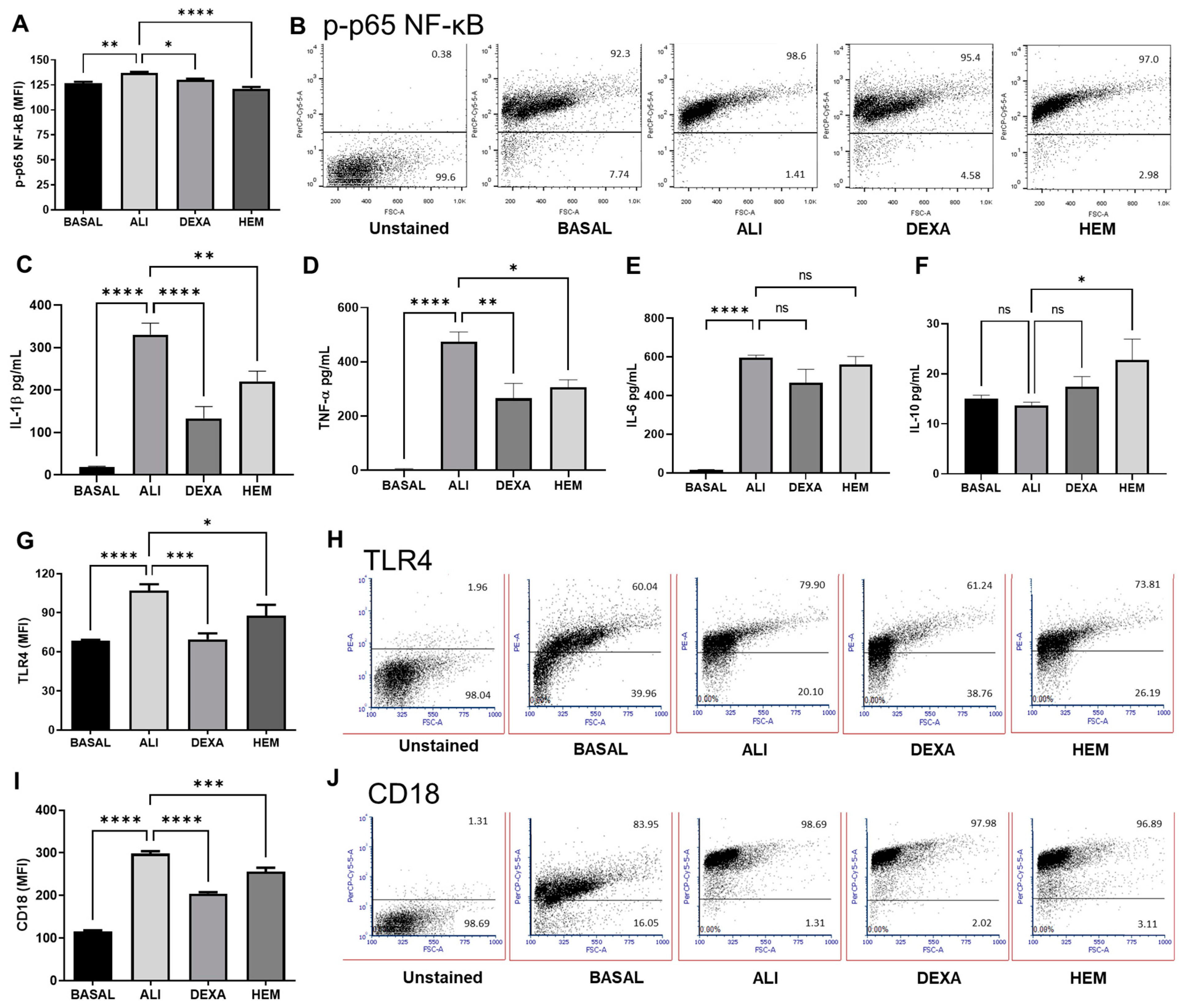
2.3. HEM Impairs NF-κB Phosphorylation and Cytokine Production and Decreases TLR4 and CD18 Expression in Cells of the BALF in LPS-Induced ALI
2.4. HEM Downregulates Neutrophil Activity in LPS-Induced ALI Mice Without Affecting Cell Viability
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Hydroalcoholic Extract of Musa paradisiaca L. Inflorescence
4.3. Murine Model of Lipopolysaccharide (LPS)-Induced Acute Lung Injury (ALI)
4.4. Animal Survival Analysis
4.5. Inflammatory Cells in Bronchoalveolar Lavage Fluids (BALFs) and Blood
4.6. Protein Concentration in the BALF
4.7. Cytokine Quantification in the BALF
4.8. Cell Analysis by Flow Cytometry
4.9. Histological Analysis
4.10. Wet Weight/Dry Weight Lung Ratio
4.11. Free DNA Quantification
4.12. Cell Death
4.13. Immunofluorescence in Lung Tissue
4.14. Myeloperoxidase (MPO) Activity in Lung Tissue
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kligerman, S. Pathogenesis, Imaging, and Evolution of Acute Lung Injury. Radiol. Clin. N. Am. 2022, 60, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.J.; Ware, L.B. Acute Respiratory Distress Syndrome 2022 1 Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef]
- Zhang, F.B.; Guo, F.F.; Zhang, Y.; Xu, H.; Liu, Y.L.; Lin, L.F.; Li, H.; Yang, H.J.; Huang, L.Q. Huashibaidu formula attenuates sepsis-induced acute lung injury suppressing cytokine storm: Implications for treatment of COVID-19. Phytomedicine 2023, 109, 154549. [Google Scholar] [CrossRef] [PubMed]
- Grommes, J.; Soehnlein, O. Contribution of Neutrophils to Acute Lung Injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Hussaniy, H.A.; Al-Harcan, N.A.H.; Alexiou, A.; Batiha, G.E. Neutrophil Extracellular Traps (NETs) and Covid-19: A new frontiers for therapeutic modality. Int. Immunopharmacol. 2022, 104, 108516. [Google Scholar] [CrossRef]
- Antunes, G.L.; Matzenbacher, L.S.; Costa, B.P.; de Sousa Basso, B.; Levorse, V.G.S.; Antunes, K.H.; Costa-Ferro, Z.S.M.; de Oliveira, J.R. Methoxyeugenol Protects Against Lung Inflammation and Suppresses Neutrophil Extracellular Trap Formation in an LPS-Induced Acute Lung Injury Model. Inflammation 2022, 45, 1534–1547. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.F.; Kong, K.W.; Leong, K.H.; Sun, J.; He, X.; Wang, Z.; Mustafa, M.R.; Ling, T.C.; Ismail, A. Banana inflorescence: Its bio-prospects as an ingredient for functional foods. Trends Food Sci. Technol. 2020, 97, 14–28. [Google Scholar] [CrossRef]
- Motta, G.E.; Angonese, M.; Valencia, G.A.; Ferreira, S.R.S. Beyond the peel: Biorefinery approach of other banana residues as a springboard to achieve the United Nations’ sustainable development goals. Sustain. Chem. Pharm. 2022, 30, 100893. [Google Scholar] [CrossRef]
- Nisha, P.; Mini, S. Flavanoid rich ethyl acetate fraction of Musa paradisiaca inflorescence down-regulates the streptozotocin induced oxidative stress, hyperglycaemia and mRNA levels of selected inflammatory genes in rats. J. Funct. Foods 2013, 5, 1838–1847. [Google Scholar] [CrossRef]
- Correa, M.; Bombardelli, M.C.M.; Fontana, P.D.; Bovo, F.; Messias-Reason, I.J.; Maurer, J.B.B.; Corazza, M.L. Bioactivity of extracts of Musa paradisiaca L. obtained with compressed propane and supercritical CO2. J. Supercrit. Fluid. 2017, 122, 63–69. [Google Scholar] [CrossRef]
- Arun, K.B.; Thomas, S.; Reshmitha, T.R.; Akhil, G.C.; Nisha, P. Dietary fibre and phenolic-rich extracts from Musa paradisiaca inflorescence ameliorates type 2 diabetes and associated cardiovascular risks. J. Funct. Foods 2017, 31, 198–207. [Google Scholar] [CrossRef]
- Correa, M.; Mesomo, M.C.; Pianoski, K.E.; Torres, Y.R.; Corazza, M.L. Extraction of inflorescences of Musa paradisiaca L. using supercritical CO 2 and compressed propane. J. Supercrit. Fluids 2016, 113, 128–135. [Google Scholar] [CrossRef]
- Rai, R.; Kumar, S.; Singh, K.B.; Khanka, S.; Singh, Y.; Arya, K.R.; Kanojiya, S.; Maurya, R.; Singh, D. Extract and fraction of flower have osteogenic effect and prevent ovariectomy induced osteopenia. Phytomedicine 2021, 93, 153750. [Google Scholar] [CrossRef]
- Gadelha, F.A.A.F.; Cavalcanti, R.F.P.; Vieira, G.C.; Ferreira, L.K.D.P.; de Sousa, G.R.; Filho, J.M.B.; Barbosa, M.A.; dos Santos, S.G.; Piuvezam, M.R. Immunomodulatory properties of Musa paradisiaca L. inflorescence in Combined Allergic Rhinitis and Asthma Syndrome (CARAS) model towards NFκB pathway inhibition. J. Funct. Foods 2021, 83, 104540. [Google Scholar] [CrossRef]
- do Nascimento Xavier, B.M.; Ferreira, L.; Ferreira, L.; Gadelha, F.; Monteiro, T.M.; de Araujo Silva, L.A.; Rodrigues, L.C.; Piuvezam, M.R. MHTP, a synthetic tetratetrahydroisoquinoline alkaloid, attenuates lipopolysaccharide-induced acute lung injury via p38MAPK/p65NF-kappaB signaling pathway-TLR4 dependent. Inflamm. Res. 2019, 68, 1061–1070. [Google Scholar] [CrossRef]
- Ni, J.W.; Li, G.; Dai, N.F.; Quan, Z.J.; Tong, H.B.; Liu, Y. Esculin alleviates LPS-induced acute lung injury via inhibiting neutrophil recruitment and migration. Int. Immunopharmacol. 2023, 119, 110177. [Google Scholar] [CrossRef]
- Sang, A.M.; Wang, Y.; Wang, S.; Wang, Q.Y.; Wang, X.H.; Li, X.Y.; Song, X.M. Quercetin attenuates sepsis-induced acute lung injury via suppressing oxidative stress-mediated ER stress through activation of SIRT1/AMPK pathways. Cell. Signal. 2022, 96, 110363. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Yang, J.J.; Yang, M.L.; Li, Y.C.; Kuan, Y.H. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-kappa B pathway. Free Radic. Bio. Med. 2014, 69, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Xiang, H.X.; Liu, Z.; Xiao, H.; Li, X.; Gong, W.Q.; Pan, L.H.; Zhao, L.Z.; Yao, J.C.; Sun, C.H.; et al. Jingfang Granules alleviates bleomycin-induced acute lung injury through regulating PI3K/Akt/mTOR signaling pathway. J. Ethnopharmacol. 2024, 318, 116946. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.M.P.; Ferreira, L.K.D.P.; Monteiro, T.M.; Gadelha, F.A.A.F.; de Lima, L.M.; Maia, M.D.; Scotti, M.T.; Ribeiro, J.; Dias, C.D.; Piuvezam, M.R. Curine Ameliorates Lipopolysaccharide-Induced Acute Lung Injury by Downregulating the TLR4/MD-2/NF-kappa B(p65) Signaling Pathway. Rev. Bras. Farmacogn. 2022, 32, 111–121. [Google Scholar] [CrossRef]
- Zhang, R.H.; Ai, X.; Duan, Y.J.; Xue, M.; He, W.X.; Wang, C.L.; Xu, T.; Xu, M.J.; Liu, B.J.; Li, C.H.; et al. Kaempferol ameliorates H9N2 swine influenza virus-induced acute lung injury by inactivation of TLR4/MyD88-mediated NF-kappa B and MAPK signaling pathways. Biomed. Pharmacother. 2017, 89, 660–672. [Google Scholar] [CrossRef]
- Wang, W.M.; Liu, Y.; Zhang, H.; Ling, D.D.; Yan, Q.C.; Wu, Y.; Jin, Y.G.; Xie, F. Preparation of inhalable quercetin-B-cyclodextrin inclusion complexes using the supercritical antisolvent process for the prevention of smoke inhalation-induced acute lung injury. J. CO2 Util. 2023, 69, 102414. [Google Scholar] [CrossRef]
- Matsuda, M.; Inaba, M.; Hamaguchi, J.; Tomita, H.; Omori, M.; Shimora, H.; Sakae, H.; Kitatani, K.; Nabe, T. Local IL-10 replacement therapy was effective for steroid-insensitive asthma in mice. Int. Immunopharmacol. 2022, 110, 109037. [Google Scholar] [CrossRef]
- Miao, F.J.; Geng, S.X.; Ning, D.L. Hydroxytyrosol ameliorates LPS-induced acute liver injury (ALI) in mice by modulating the balance between M1/M2 phenotype macrophage and inhibiting TLR4/NF-?B activation. J. Funct. Foods 2023, 102, 105455. [Google Scholar] [CrossRef]
- Shokry, A.A.; El-Shiekh, R.A.; Kamel, G.; Bakr, A.F.; Ramadan, A. Bioactive phenolics fraction of Hedera helix L. (Common Ivy Leaf) standardized extract ameliorates LPS-induced acute lung injury in the mouse model through the inhibition of proinflammatory cytokines and oxidative stress. Heliyon 2022, 8, e09477. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Afzal, O.; Almalki, W.H.; Kazmi, I.; Shaikh, M.A.J.; Thangavelu, L.; Gulati, M.; Singh, S.K.; Jha, N.K.; Gupta, P.K.; et al. Nuclear factor-kappa B (NF-Kappa B) inhibition as a therapeutic target for plant nutraceuticals in mitigating inflammatory lung diseases. Chem-Biol. Interact. 2022, 354, 109842. [Google Scholar] [CrossRef]
- Pan, H.D.; He, J.L.; Yang, Z.F.; Yao, X.J.; Zhang, H.; Li, R.F.; Xiao, Y.; Zhao, C.P.; Jiang, H.M.; Liu, Y.T.; et al. Myricetin possesses the potency against SARS-CoV-2 infection through blocking viral-entry facilitators and suppressing inflammation in rats and mice. Phytomedicine 2023, 116, 154858. [Google Scholar] [CrossRef]
- Scozzi, D.; Liao, F.Y.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The role of neutrophil extracellular traps in acute lung injury. Front. Immunol. 2022, 13, 953195. [Google Scholar] [CrossRef]
- Organization for Economic Co-Operation and Development. Acute Oral Toxicity, Guidelines for Chemical Testing No. 423. 2001. Available online: https://ntp.niehs.nih.gov/sites/default/files/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf (accessed on 17 October 2024).
- Yang, H.L.; Li, Y.; Huo, P.F.; Li, X.O.; Kong, D.L.; Mu, W.; Fang, W.; Li, L.X.; Liu, N.; Fang, L.; et al. Protective effect of Jolkinolide B on LPS-induced mouse acute lung injury. Int. Immunopharmacol. 2015, 26, 119–124. [Google Scholar] [CrossRef]
- Cavalcanti, R.F.P.; Gadelha, F.; Paiva Ferreira, L.K.D.; Paiva Ferreira, L.A.M.; Chaves Junior, J.V.; de Araujo Batista, R.S.; Melo, T.B.L.; de Souza, F.S.; Alves, A.F.; Maria Batista, L.; et al. Limosilactobacillus fermentum modulates the gut-airway axis by improving the immune response through FOXP3 activation on combined allergic rhinitis and asthma syndrome (CARAS). Immunobiology 2023, 228, 152721. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.D.F.; Formiga, R.D.; Lima, G.R.D.; de Jesus, N.Z.T.; Alves, E.B.; Marinho, A.F.; Tavares, J.F.; Santos, F.A.; Viana, A.F.S.C.; Araujo, A.A.; et al. Hyptis suaveolens (L.) Poit protects colon from TNBS-induced inflammation via immunomodulatory, antioxidant and anti-proliferative mechanisms. J. Ethnopharmacol. 2021, 265, 113153. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD–ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Zengina, G.; Llorent-Martínezb, E.J.; Córdovab, M.L.F.; Bahadoric, M.B.; Mocand, A.; Locatellif, M.; Aktumseka, A. Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillate subsp. Amasiaca. Ind. Crops Prod. 2018, 111, 11–21. [Google Scholar] [CrossRef]
- Lee, R.; Lee, V.S.Y.; Tzen, J.T.C.; Lee, M. Study of the release of gallic acid from (–)-epigallocatechin gallate in old oolong tea by mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Bouhafsoun, A.; Yilmaz, M.A.; Boukeloua, A.; Temel, H.; KAID Harche, M. Simultaneous quantification of phenolic acids and flavonoids in Chamaerops humilis L. using LC–ESI-MS/MS. Food Sci. Technol. 2018, 38, 242–247. [Google Scholar] [CrossRef]
- Dantas, C.A.G.; Abreu, L.S.; Cunha, H.N.; Veloso, C.A.G.; Souto, A.L.; Agra, M.F.; Costa, V.C.O.; Silva, M.S.; Tavares, J.F. Dereplication of phenolic derivatives of three Erythroxylum species using liquid chromatography coupled with ESI-MSn and HRESIMS. Phytochem. Anal. 2021, 32, 1011–1026. [Google Scholar] [CrossRef]
- He, Z.; Xia, W. Analysis of phenolic compounds in Chinese olive (Canarium album L.) fruit by RPHPLC–DAD–ESI–MS. Food Chem. 2007, 105, 1307–1311. [Google Scholar] [CrossRef]
- Souza, R.B.L.; Nascimento, Y.M.; Gouveia, R.G.; Souto, A.L.; Sobral, M.V.; Costa, V.C.O.; Melo, J.I.M.; Silva, M.S.; Tavares, J.F. Dereplication-guided isolation of a new flavonoid triglycoside from Macroptilium martii and its cytotoxicity evaluation. Phytochem. Lett. 2020, 39, 144–150. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Lewis, B.A.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Characterisation of phenolics by LC–UV/Vis, LC–MS/MS and sugars by GC in Melicoccus bijugatus Jacq. ‘Montgomery’ fruits. Food Chem. 2008, 111, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Dudley, E.; Plummer, S.; Tang, J.; Newton, R.P.; Brenton, A.G. Fingerprint profile of Ginkgo biloba nutritional supplements by LC/ESI-MS/MS. Phytochemistry 2008, 69, 1555–1564. [Google Scholar] [CrossRef]
- Riehle, P.; Vollmer, M.; Rohn, S. Phenolic compounds in Cistus incanus herbal infusions—Antioxidant capacity and thermal stability during the brewing process. Food Res. Int. 2013, 53, 891–899. [Google Scholar] [CrossRef]
- Engels, C.; Gräter, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; Freitas, A.S.; Barth, O.M.; Almeida-Muradian, L.B. Phenolic profile by HPLC-Ms, biological potential, and nutritional value of a promising food: Monofloral bee pollen. Food Biochem. 2018, 42, e12536. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Z.; Fang, J.; Liu, M.; Niu, Y.; Chen, S.; Wang, H. An on-line high-performance liquid chromatography–diode-array detector–electrospray ionization–ion-trap–time-of-flight–mass spectrometry–total antioxidant capacity detection system applying two antioxidant methods for activity evaluation of the edible flowers from Prunus mume. J. Chromatogr. A 2015, 1414, 88–102. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadelha, F.A.A.F.; Cavalcanti, R.F.P.; Vieira, C.I.D.; De Oliveira, J.B.; De Lima, L.M.; Alves, A.F.; Pessoa, M.M.B.; Batista, L.M.; Dejani, N.N.; Piuvezam, M.R. Musa paradisiaca L. Inflorescence Abrogates Neutrophil Activation by Downregulating TLR4/NF-KB Signaling Pathway in LPS-Induced Acute Lung Injury Model. Pharmaceuticals 2025, 18, 8. https://doi.org/10.3390/ph18010008
Gadelha FAAF, Cavalcanti RFP, Vieira CID, De Oliveira JB, De Lima LM, Alves AF, Pessoa MMB, Batista LM, Dejani NN, Piuvezam MR. Musa paradisiaca L. Inflorescence Abrogates Neutrophil Activation by Downregulating TLR4/NF-KB Signaling Pathway in LPS-Induced Acute Lung Injury Model. Pharmaceuticals. 2025; 18(1):8. https://doi.org/10.3390/ph18010008
Chicago/Turabian StyleGadelha, Francisco Allysson Assis Ferreira, Raquel Fragoso Pereira Cavalcanti, Cosmo Isaias Duvirgens Vieira, Joao Batista De Oliveira, Louíse Mangueira De Lima, Adriano Francisco Alves, Matheus Marley Bezerra Pessoa, Leônia Maria Batista, Naiara Naiana Dejani, and Marcia Regina Piuvezam. 2025. "Musa paradisiaca L. Inflorescence Abrogates Neutrophil Activation by Downregulating TLR4/NF-KB Signaling Pathway in LPS-Induced Acute Lung Injury Model" Pharmaceuticals 18, no. 1: 8. https://doi.org/10.3390/ph18010008
APA StyleGadelha, F. A. A. F., Cavalcanti, R. F. P., Vieira, C. I. D., De Oliveira, J. B., De Lima, L. M., Alves, A. F., Pessoa, M. M. B., Batista, L. M., Dejani, N. N., & Piuvezam, M. R. (2025). Musa paradisiaca L. Inflorescence Abrogates Neutrophil Activation by Downregulating TLR4/NF-KB Signaling Pathway in LPS-Induced Acute Lung Injury Model. Pharmaceuticals, 18(1), 8. https://doi.org/10.3390/ph18010008






