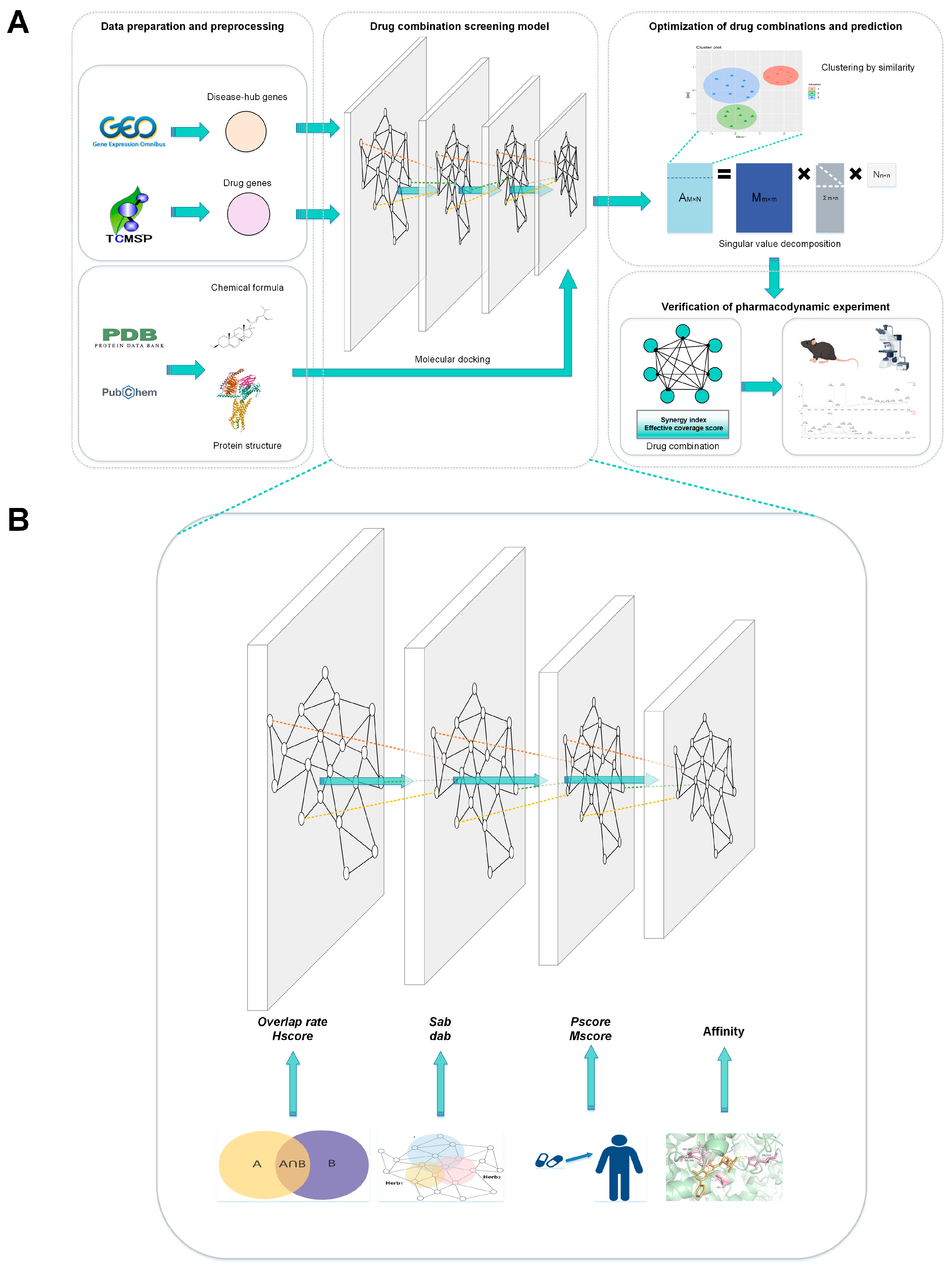
Syn-COM: A Multi-Level Predictive Synergy Framework for Innovative Drug Combinations
Abstract
1. Introduction
2. Results
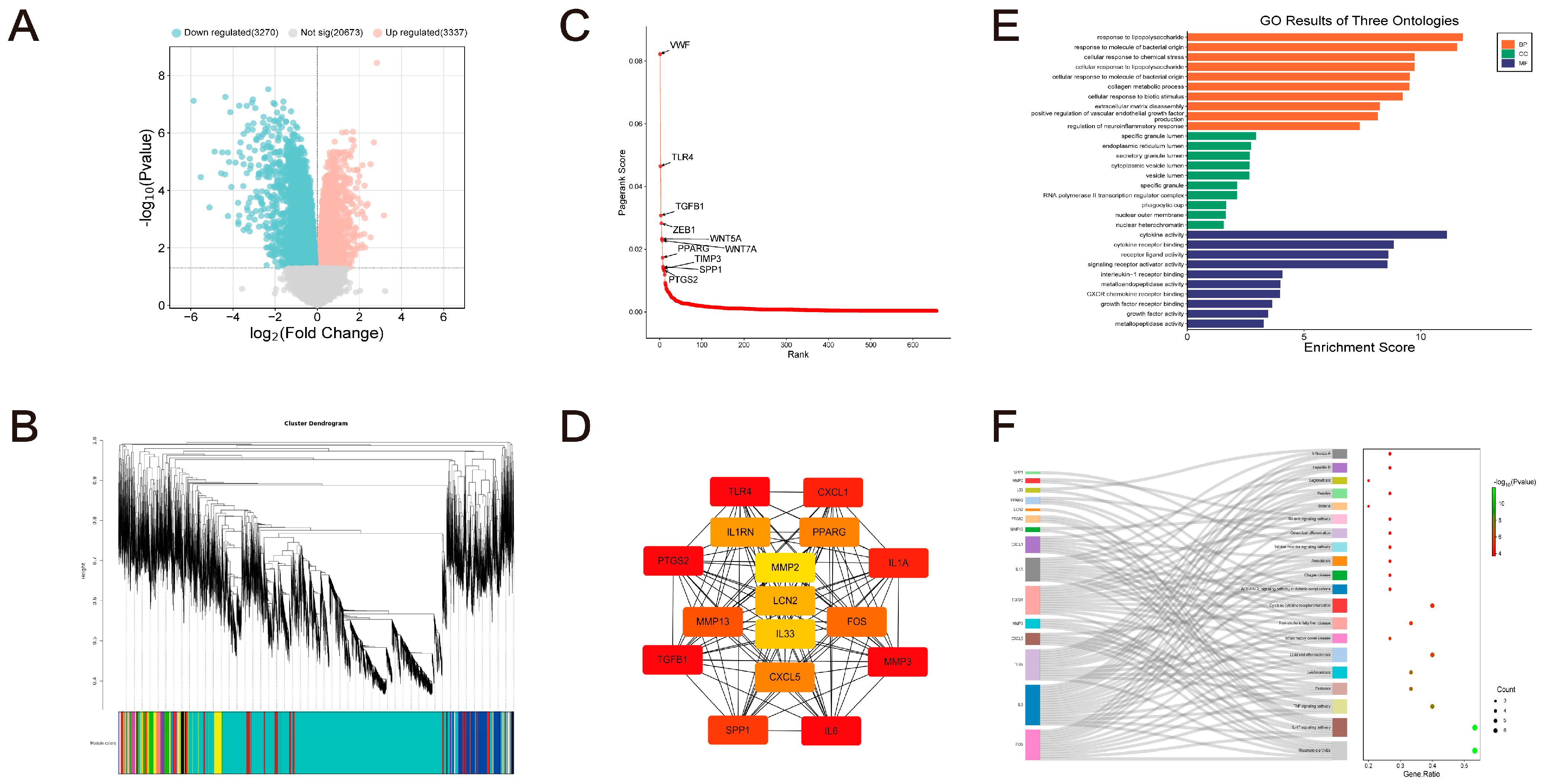
2.1. Discovering Advantage Genes
2.2. Identification of Hub-GA Based on MCODE
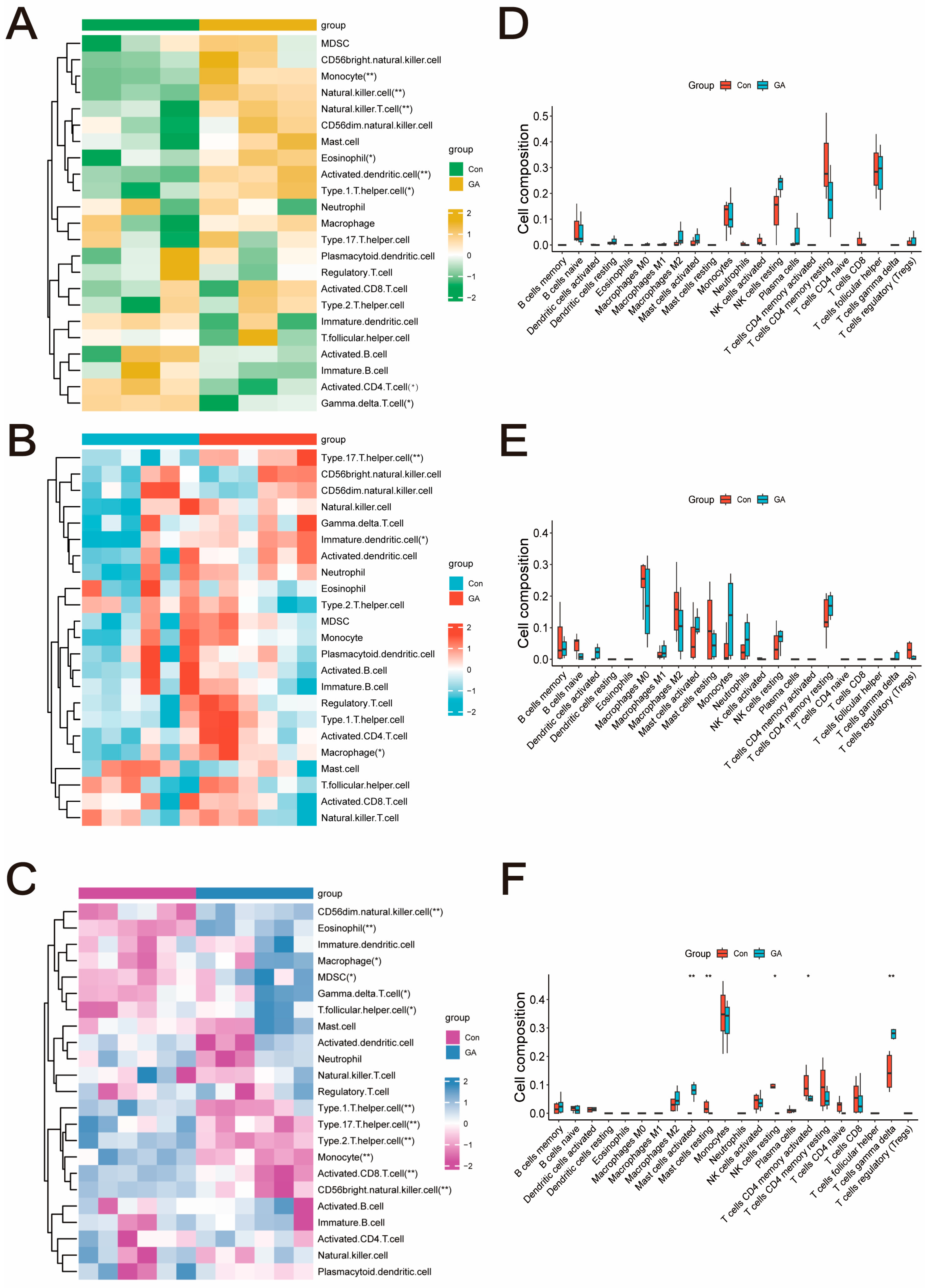
2.3. Functional Enrichment and Immune Infiltration Analysis
2.4. Dataset Validation
2.5. Multiple-Angle TCM Group Filtration for GA Therapy
2.6. TCM Combination Based on Molecular Virtual Screening and Clustering
2.7. Building Multi-Level Networks and Discovering Critical Compounds
2.8. Synergistic Association among TCM Combinations
2.9. Method Comparison
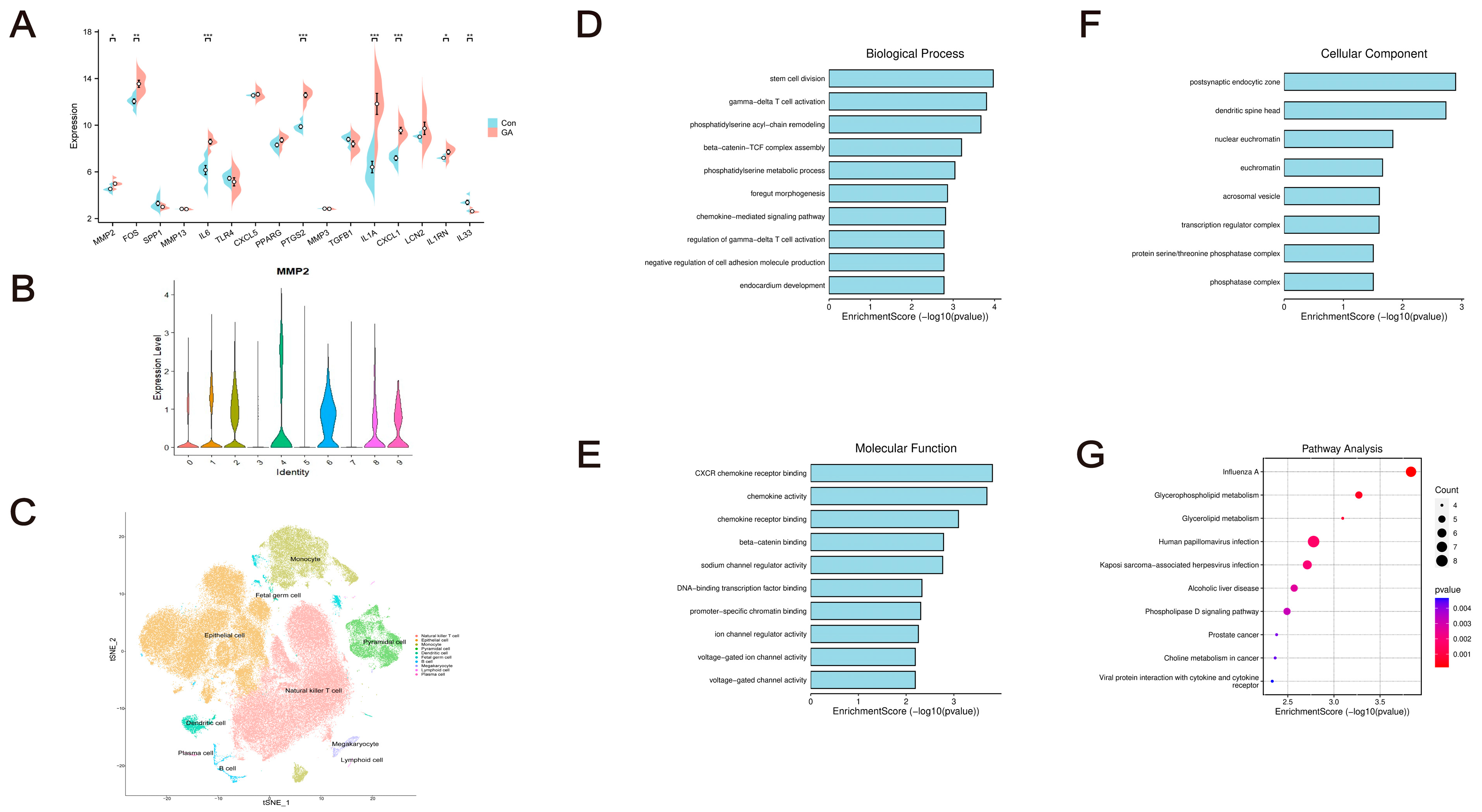
2.10. Pharmacodynamic Verification of GAD
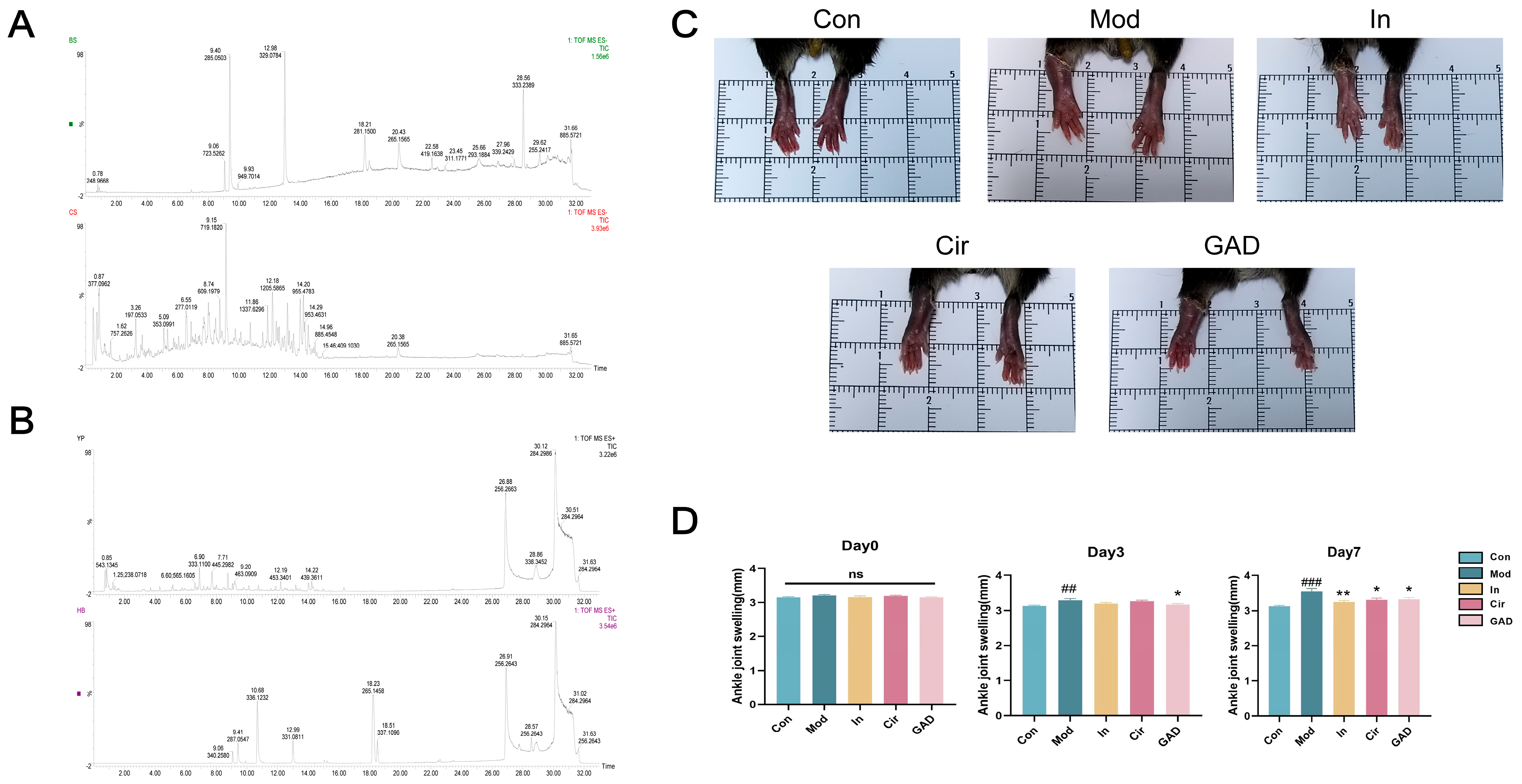
2.10.1. Identification of Components in Chinese Herbal Medicine
2.10.2. Effects on Ankle Joint Swelling in GA Mice
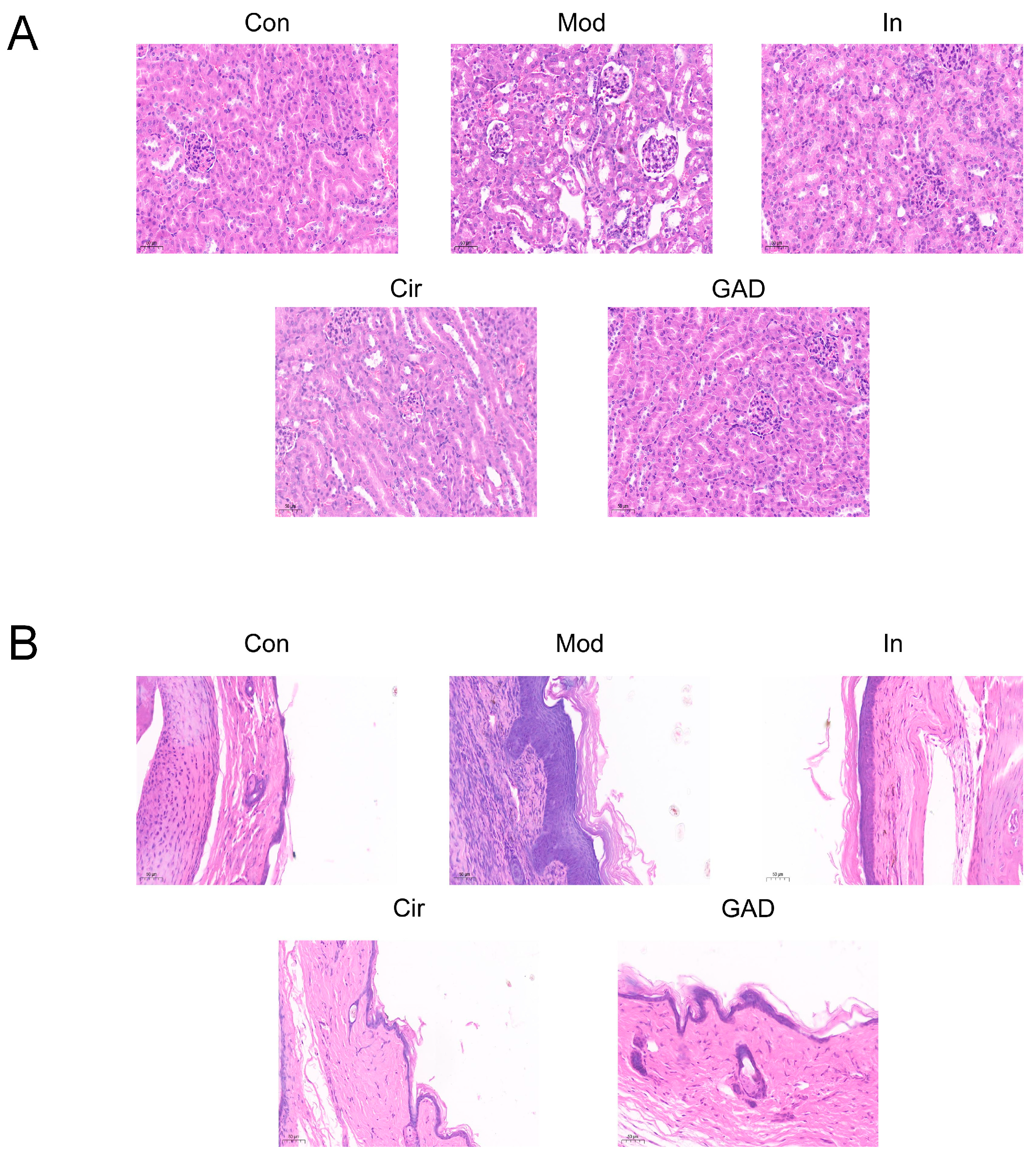
2.10.3. Effects on Renal and Joint Morphological Changes in GA Mice
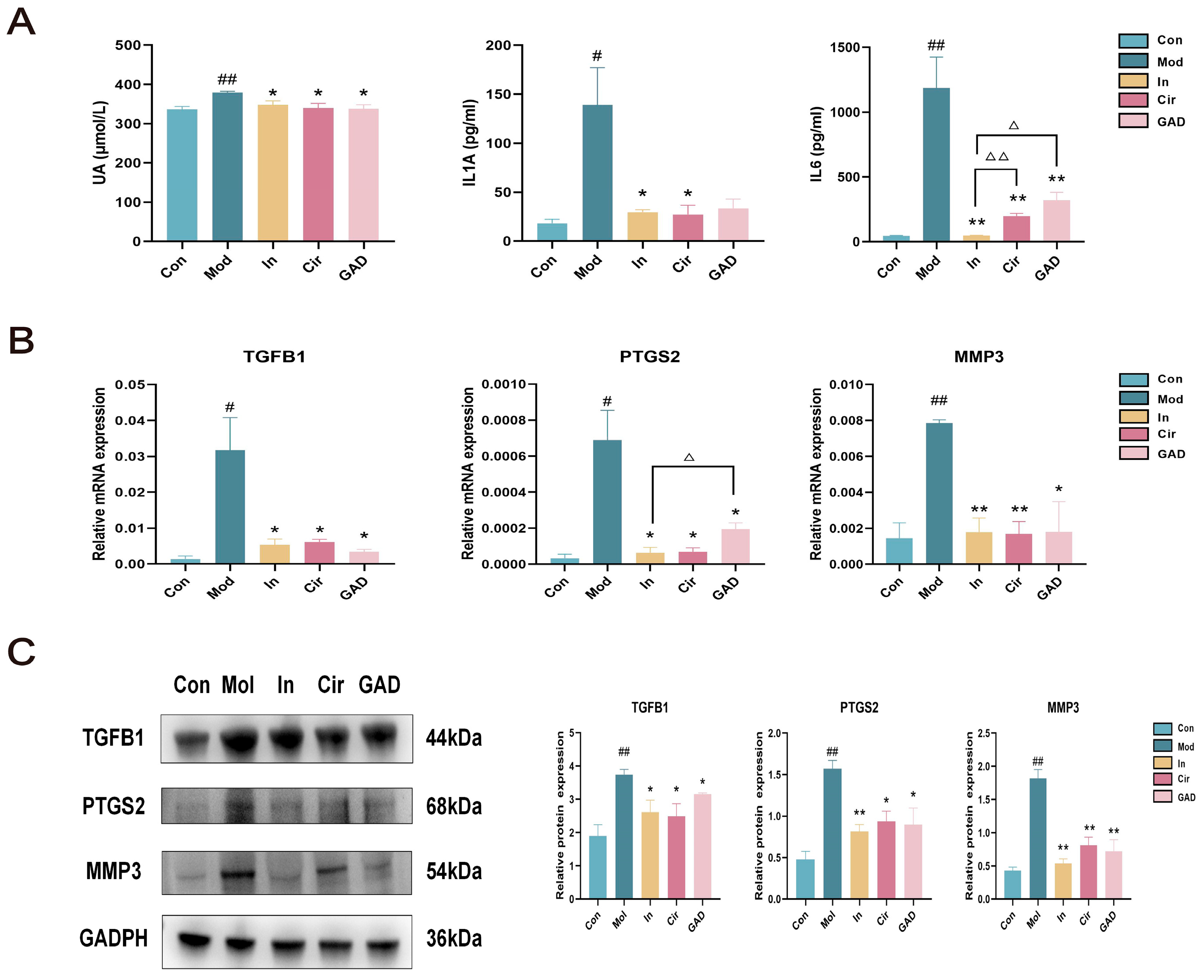
2.10.4. Effects on UA and Abnormal Inflammation in GA Mice
2.10.5. Effects on the Expression Levels of TGFB1, PTGS2, and MMP3 in Ankle Joints of GA Mice
3. Discussion
4. Materials and Methods
4.1. Thorough Screening for Crucial Genes
4.1.1. Screening for Differentially Expressed Genes (DEGs)
4.1.2. Weighted Gene Co-Expression Network Analysis (WGCNA)
4.1.3. Prioritizing Potential Genes
4.1.4. Construction of PPI Network and Module Optimization
4.2. Verification with Hub Genes
4.3. Functional Enrichment Analysis
4.4. Immune Infiltration Analysis
4.5. Effective Drug Combination Screening
4.5.1. The Relationship between Chinese Herbal Medicines and Disease under Effectiveness and Overlap
4.5.2. The Relationship between Chinese Herbal Medicines and Disease under the Network Module
4.5.3. The Relationship between Chinese Herbal Medicines and Disease under Clinical Medication Experience
4.5.4. Molecular Docking
4.5.5. Identification of Representative Compounds and TCM Combination Refinement with Similarity Clustering
4.5.6. Cooperative Association of Herbal Combination Based on SVD
4.6. Method Comparison
4.7. Drug Combinations Analysis Utilizing UPLC-Q-TOF-MS/MS
4.8. Verification of Pharmacodynamic Experiment
4.8.1. Animals and Drugs
4.8.2. Animal Modeling, Grouping, and Drug Administration
4.8.3. Ankle Joint Swelling Index
4.8.4. Hematoxylin and Eosin (HE) Staining
4.8.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8.6. RNA Extraction and Quantitative Polymerase Chain Reaction (q-PCR)
4.8.7. Western Blotting
4.9. Data Sources and Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.Y.; Yang, H.B.; Chen, L.X.; Jafari, M.; Tang, J. Network-based modeling of herb combinations in traditional Chinese medicine. Brief. Bioinform. 2021, 22, 106. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.Y.; Liu, Y.C.; Wang, M.; Dong, X.; Sun, X.; Luo, Y.; Sun, X.B. Identification and validation of platelet-related diagnostic markers and potential drug screening in ischemic stroke by integrating comprehensive bioinformatics analysis and machine learning. Front. Immunol. 2024, 14, 1320475. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Yu, H.; Chen, G.L.; Yang, Q.C.; Wang, Z.C.; Niu, N.; Han, L.; Zhao, D.Y.; Wang, M.J.; Liu, Y.Y.; et al. An herbal drug combination identified by knowledge graph alleviates the clinical symptoms of plasma cell mastitis patients: A nonrandomized controlled trial. eLife 2023, 12, 84414. [Google Scholar] [CrossRef]
- Huo, X.; Gu, Y.; Zhang, Y. The discovery of multi-target compounds with anti-inflammation activity from traditional Chinese medicine by TCM-target effects relationship spectrum. J. Ethnopharmacol. 2022, 293, 115289. [Google Scholar] [CrossRef]
- Wu, X.L.; Cao, S.J.; Zou, Y.; Wu, F.X. Traditional Chinese Medicine studies for Alzheimer’s disease via network pharmacology based on entropy and random walk. PLoS ONE 2023, 18, 0294772. [Google Scholar] [CrossRef]
- Han, K.; Zhang, L.; Wang, M.; Zhang, R.; Wang, C.Y.; Zhang, C.Z. Prediction Methods of Herbal Compounds in Chinese Medicinal Herbs. Molecules 2018, 23, 2303. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, M.; Xu, Q.; Song, F.; Wang, L.B.; Gai, S.C.; Tang, H.F.; Wang, S.W.; Zhou, L.Y.; Li, H. Exploration of molecular targets and mechanisms of Chinese medicinal formula Acacia Catechu -Scutellariae Radix in the treatment of COVID-19 by a systems pharmacology strategy. Phytother. Res. 2022, 36, 4210–4229. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ni, Q.H.; Jiang, F.Y.; Liu, B.C.; Wang, J.Y.; Zhang, L.; Huang, J.H. Discovery and Validation of Traditional Chinese and Western Medicine Combination Antirheumatoid Arthritis Drugs Based on Machine Learning (Random Forest Model). BioMed Res. Int. 2023, 2023, 6086388. [Google Scholar] [CrossRef]
- Keller, S.F.; Mandell, B.F. Management and Cure of Gouty Arthritis. Med. Clin. N. Am. 2021, 105, 297–310. [Google Scholar] [CrossRef]
- Wen, P.F.; Luo, P.; Zhang, B.; Zhang, Y.M. Mapping Knowledge Structure and Global Research Trends in Gout: A Bibliometric Analysis From 2001 to 2021. Front. Public Health 2022, 10, 924676. [Google Scholar] [CrossRef]
- Bardin, T.; Richette, P. New ACR guidelines for gout management hold some surprises. Nat. Rev. Rheumatol. 2013, 9, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Xu, Y.; Ye, J.X.; Tan, J.R.; Hou, J.; Wang, Y.Z.; Li, J.W.; Cui, W.Z.; Wang, S.Y.; Zhao, Q.Y. Qingre Huazhuo Jiangsuan Decoction promotes autophagy by inhibiting PI3K/AKT/mTOR signaling pathway to relieve acute gouty arthritis. J. Ethnopharmacol. 2023, 302, 115875. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Liu, W.; Wei, B.W.; Wang, A.H.; Wang, Y.W.; Wang, W.; Gao, J.Y.; Jin, Y.; Lu, H.; Ka, Y.X.; et al. Traditional herbal medicine: Therapeutic potential in acute gouty arthritis. J. Ethnopharmacol. 2024, 330, 118182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Xu, Y.; Tan, J.R.; Ye, J.X.; Cui, W.Z.; Hou, J.; Liu, P.Y.; Li, J.W.; Wang, S.Y.; Zhao, Q.Y. Anti-inflammation is an important way that Qingre-Huazhuo-Jiangsuan recipe treats acute gouty arthritis. Front. Pharmacol. 2023, 14, 1268641. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Wang, Y.H.; Lin, S.B.; Guan, W.; Liang, H.; Shen, J.J. Neutrophil autophagy induced by monosodium urate crystals facilitates neutrophil extracellular traps formation and inflammation remission in gouty arthritis. Front. Endocrinol. 2023, 14, 1071630. [Google Scholar] [CrossRef]
- Cheng, J.J.; Ma, X.D.; Ai, G.X.; Yu, Q.X.; Chen, X.Y.; Yan, F.; Li, Y.C.; Xie, J.H.; Su, Z.R.; Xie, Q.F. Palmatine Protects Against MSU-Induced Gouty Arthritis via Regulating the NF-κB/NLRP3 and Nrf2 Pathways. Drug Des. Dev. Ther. 2022, 16, 2119–2132. [Google Scholar] [CrossRef]
- Fu, W.; Ge, M.; Li, J. Phospholipase A2 regulates autophagy in gouty arthritis: Proteomic and metabolomic studies. J. Transl. Med. 2023, 21, 261. [Google Scholar] [CrossRef]
- Wu, C.G.; Chen, S.H.; Liu, Y.; Kong, B.; Yan, W.; Jiang, T.; Tian, H.; Liu, Z.Y.; Shi, Q.; Wang, Y.J.; et al. Cynarin suppresses gouty arthritis induced by monosodium urate crystals. Bioengineered 2022, 13, 11782–11793. [Google Scholar] [CrossRef]
- Nayarisseri, A.; Khandelwal, R.; Tanwar, P.; Madhavi, M.; Sharma, D.; Thakur, G.; Speck, A.; Singh, S.K. Artificial Intelligence, Big Data and Machine Learning Approaches in Precision Medicine & Drug Discovery. Curr. Drug Targets 2021, 22, 631–655. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Ding, H.D.; Hu, G.; Lu, Y. Simiaosan alleviates the symptoms of gouty arthritis via the NALP3/IL 1β pathway. Mol. Med. Rep. 2021, 23, 223. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, S.H.; Huang, Y.; Qin, K.; Chen, Z. MicroRNA-181a regulates Treg functions via TGF-β1/Smad axis in the spleen of mice with acute gouty arthritis induced by MSU crystals. Braz. J. Med. Biol. Res. 2022, 55, e12002. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liang, T.; Yang, F.; Zhu, F.F.; Liu, J.; Jiang, J.Q.; Wu, X.W.; Chen, A.S.; Yuan, D.P.; Liang, X.L. Matrix Metalloproteinase-3 induces proteoglycan degradation in gouty arthritis model. Gene 2021, 765, 145120. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jia, Q.Q.; Zhang, H.Q.; Yi, L.Y.; Hu, P.; Wang, R.; Zhang, H.Y. Tissue lipidomics, network pharmacology, and molecular docking to explore the therapeutic mechanism of anthocyanins from Lycium ruthenicum Murr. against gouty arthritis. Food Funct. 2023, 14, 7011–7023. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Lai, L.H. Associating 197 Chinese herbal medicine with drug targets and diseases using the similarity ensemble approach. Acta Pharmacol. Sin. 2020, 41, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, H.; Wang, Y.C.; Wang, S.F.; Xun, D.J.; Wang, Y.; Cheng, Y.Y.; Zhang, B.L. Multimodal Identification by Transcriptomics and Multiscale Bioassays of Active Components in Xuanfeibaidu Formula to Suppress Macrophage-Mediated Immune Response. Engineering 2023, 20, 63–76. [Google Scholar] [CrossRef]
- Hou, L.W.; Yang, L.; Zhu, C.T.; Miao, J.Y.; Zhou, W.J.; Tang, Y.C.; Meng, H.W.; Liu, S.W. Cuscutae semen alleviates CUS-induced depression-like behaviors in mice via the gut microbiota-neuroinflammation axis. Front. Pharmacol. 2023, 14, 1107781. [Google Scholar] [CrossRef]
- Yue, G.G.; Chan, B.C.; Kwok, H.F.; To, M.H.; Hon, K.L.; Fung, K.P.; Lau, C.B.; Leung, P.C. Screening for anti-inflammatory and bronchorelaxant activities of 12 commonly used Chinese herbal medicines. Phytother. Res. 2012, 26, 915–925. [Google Scholar] [CrossRef]
- Gao, J.; Yang, X.; Yin, W. From Traditional Usage to Pharmacological Evidence: A Systematic Mini-Review of Spina Gleditsiae. Evid. Based Complement. Alternat. Med. 2016, 2016, 3898957. [Google Scholar] [CrossRef]
- Tran, M.N.; Baek, S.J.; Jun, H.J.; Lee, S.H. Identifying target organ location of Radix Achyranthis Bidentatae: A bioinformatics approach on active compounds and genes. Front. Pharmacol. 2023, 14, 1187896. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Li, W.; Sun, B.; Xie, J.; Cheng, W.M.; Zhang, Q.L. Achyranthis bidentatae radix enhanced articular distribution and anti-inflammatory effect of berberine in Sanmiao Wan using an acute gouty arthritis rat model. J. Ethnopharmacol. 2018, 221, 100–108. [Google Scholar] [CrossRef]
- Jiang, T.; Peng, L.L.; Wang, Q.; Huang, B.Y.; Peng, D.W.; Men, L.T.; Jiang, Y.; Zhu, M.Y.; Wang, M.; Lin, L.; et al. Cirsiliol regulates mitophagy in colon cancer cells via STAT3 signaling. Cancer Cell Int. 2022, 22, 304. [Google Scholar] [CrossRef] [PubMed]
- Fadul, E.; Nizamani, A.; Rasheed, S.; Adhukari, A.; Yousuf, S.; Parveen, S.; Goren, N.; Alhazmi, H.A.; Choudary, M.; Khalid, A. Anti-glycating and anti-oxidant compounds from traditionally used anti-diabetic plant Geigeria alata(DC) Oliv. & Hiern. Nat. Prod. Res. 2020, 34, 2456–2464. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Q.; Li, Y.D.; Chen, L.; Guan, S.; Li, R.; Li, B.; Yu, Y.N.; Liu, J.; Zhang, Y.Y.; et al. Optimizing Genomic Control in Hit Network-Target Set Model Associations with Lung Adenocarcinoma. J. Cancer 2023, 14, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Tanglay, O.; Young, I.M.; Dadario, N.B.; Taylor, H.M.; Nicholas, P.J.; Doyen, S.; Sughrue, M.E. Eigenvector PageRank difference as a measure to reveal topological characteristics of the brain connectome for neurosurgery. J. Neurooncol. 2022, 157, 49–61. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Zhang, X.X.; Li, B.; Zhang, Y.Y.; Liu, J.; Li, H.X.; Chen, Y.Y.; Wang, P.Q.; Kang, R.X.; Wu, H.L.; et al. Entropy-based divergent and convergent modular pattern reveals additive and synergistic anticerebral ischemia mechanisms. Exp. Biol. Med. 2016, 241, 2063–2074. [Google Scholar] [CrossRef]
- Yu, X.; Xie, L.; Ge, J.; Li, H.; Zhong, S.; Liu, X. Integrating single-cell RNA-seq and spatial transcriptomics reveals MDK-NCL dependent immunosuppressive environment in endometrial carcinoma. Front. Immunol. 2023, 14, 1145300. [Google Scholar] [CrossRef]
- Craven, K.E.; Gökmen-Polar, Y.; Badve, S.S. CIBERSORT analysis of TCGA and METABRIC identifies subgroups with better outcomes in triple negative breast cancer. Sci. Rep. 2021, 11, 4691. [Google Scholar] [CrossRef]
- Lau, E.S.; Paniagua, S.M.; Zarbafian, S.; Hoffman, U.; Long, M.T.; Hwang, S.J.; Courchesne, P.; Yao, C.; Ma, J.T.; Larson, M.G.; et al. Cardiovascular Biomarkers of Obesity and Overlap With Cardiometabolic Dysfunction. J. Am. Heart Assoc. 2021, 10, e020215. [Google Scholar] [CrossRef]
- Niu, Q.K.; Li, H.T.; Tong, L.; Liu, S.H.; Zong, W.J.; Zhang, S.Q.; Tian, S.W.; Wang, J.G.; Liu, J.; Li, B.; et al. TCMFP: A novel herbal formula prediction method based on network target’s score integrated with semi-supervised learning genetic algorithms. Brief. Bioinform. 2023, 24, 102. [Google Scholar] [CrossRef]
- Cheng, F.; Kovács, I.A.; Barabási, A.L. Network-based prediction of drug combinations. Nat. Commun. 2019, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Voicu, A.; Duteanu, N.; Voicu, M.; Vlad, D.; Dumitrascu, V. The rcdk and cluster R packages applied to drug candidate selection. J. Cheminform. 2020, 12, 3. [Google Scholar] [CrossRef]
- Barrera-Vázquez, O.S.; Escobar-Ramírez, J.L.; Santiago-Mejía, J.; Carrasco-Ortega, O.F.; Magos-Guerrero, G.A. Discovering Potential Compounds for Venous Disease Treatment through Virtual Screening and Network Pharmacology Approach. Molecules 2023, 28, 7937. [Google Scholar] [CrossRef] [PubMed]
- Baxter-Koenigs, A.R.; El, N.G.; Barrick, D. Singular value decomposition of protein sequences as a method to visualize sequence and residue space. Protein Sci. 2022, 31, 4422. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.Q.; Wen, Z.H.; Su, S.X.; Chen, Y.P.; Liu, W.C.; Guo, S.Q.; Li, X.F.; Zhang, X.; Li, R.; Xu, N.B.; et al. Elucidating the Synergistic Effect of Multiple Chinese Herbal Prescriptions in the Treatment of Post-stroke Neurological Damage. Front. Pharmacol. 2022, 13, 784242. [Google Scholar] [CrossRef]
- Hu, J.; Szymczak, S. A review on longitudinal data analysis with random forest. Brief. Bioinform. 2023, 24, bbad002. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Bleakley, K.; Yamanishi, Y. Supervised prediction of drug–target interactions using bipartite local models. Bioinformatics 2009, 25, 2397–2403. [Google Scholar] [CrossRef]
- Yuan, K.C.; Tsai, L.W.; Lee, K.H.; Cheng, Y.W.; Hsu, S.C.; Lo, Y.S.; Chen, R.J. The development an artificial intelligence algorithm for early sepsis diagnosis in the intensive care unit. Int. J. Med. Inform. 2020, 141, 104176. [Google Scholar] [CrossRef]
- Goel, R.; Misra, A.; Kondal, D.; Pandey, R.M.; Vikram, N.K.; Wasir, J.S.; Dhingra, V.; Luthra, K. Identification of insulin resistance in Asian Indian adolescents: Classification and regression tree (CART) and logistic regression based classification rules. Clin. Endocrinol. 2009, 70, 717–724. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, H.; Chen, Y.; Wang, X.Q.; Yang, Y.Q.; Tao, J.H.; Deng, X.M.; Liang, G.L.; Zhang, H.F.; Jiang, W.; et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 2018, 10, 8689. [Google Scholar] [CrossRef] [PubMed]

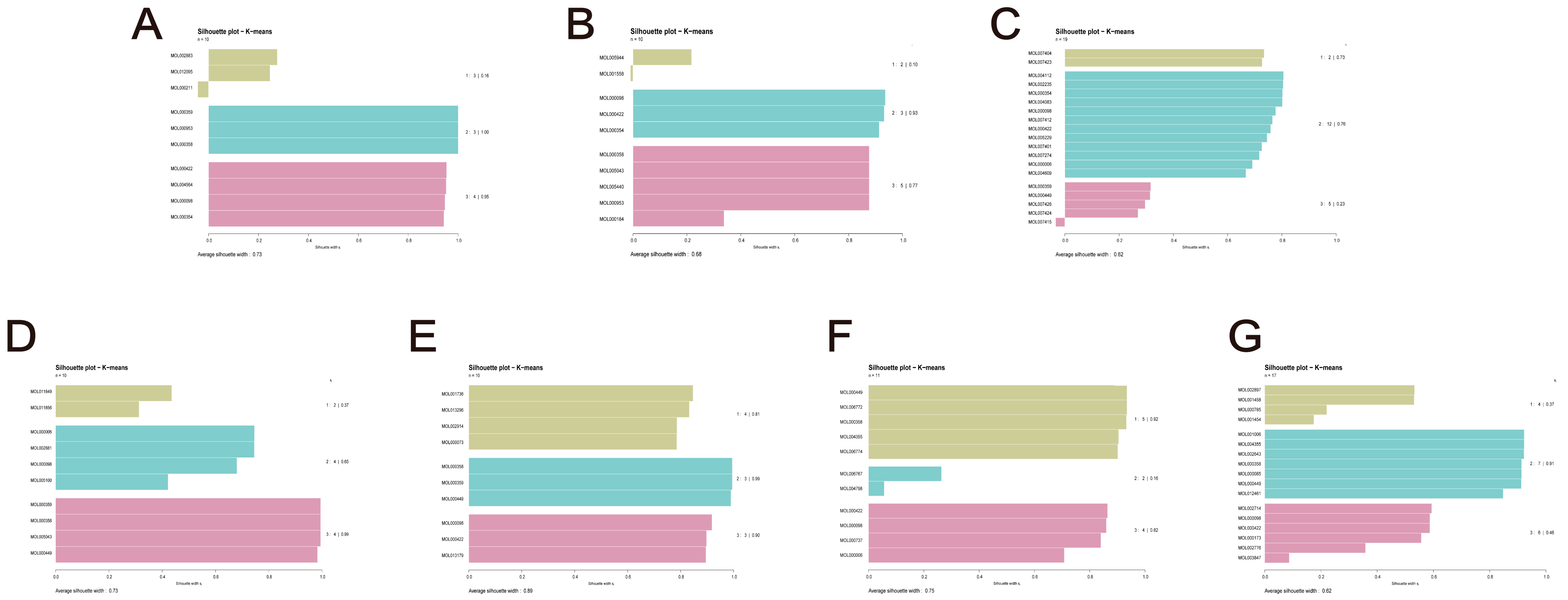
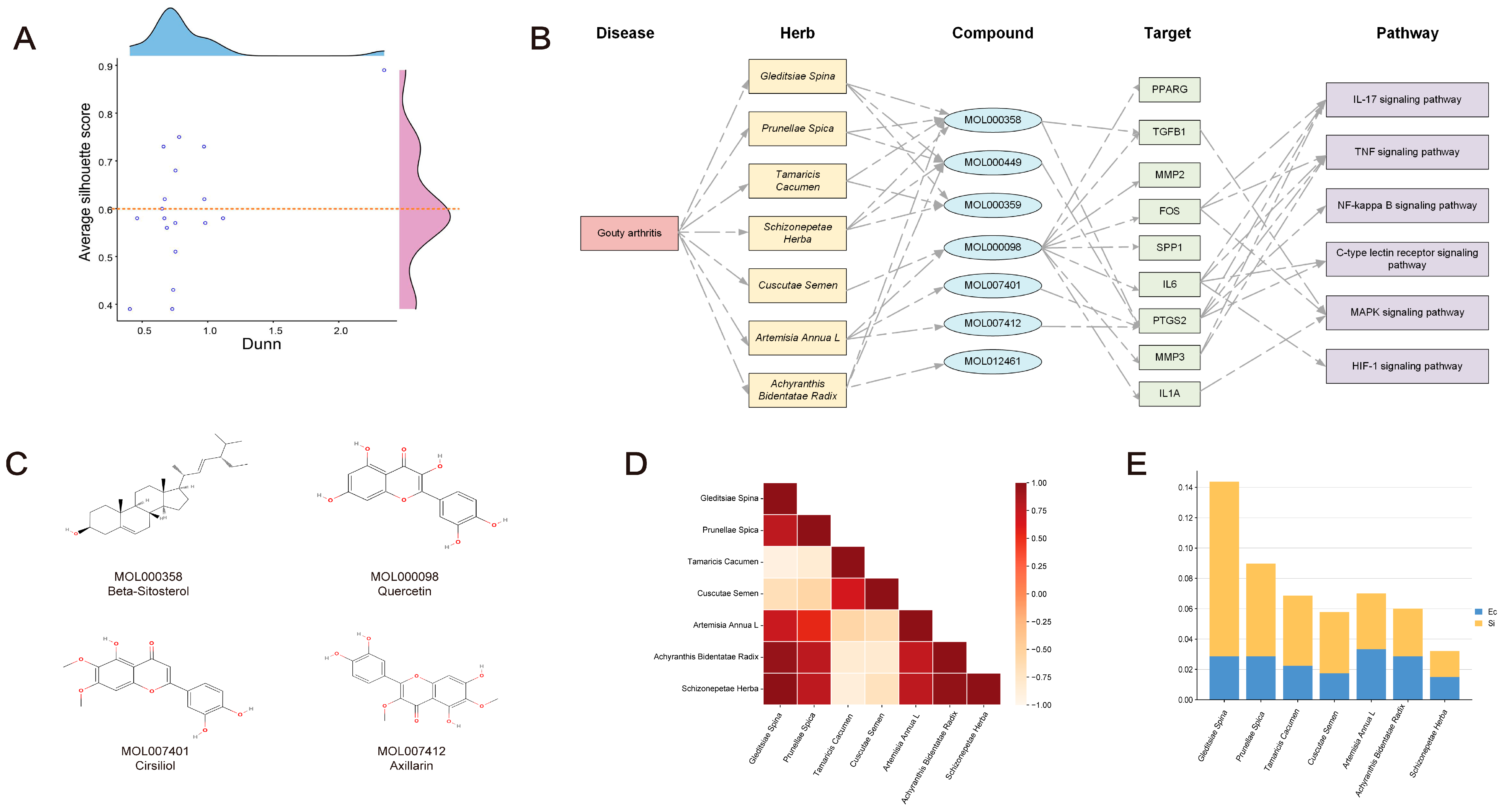
| Name | Overlap Rate | Hscore | dab | Zd | Mscore | Silhouette Score |
|---|---|---|---|---|---|---|
| Gleditsiae Spina | 0.0462 | 0.0023 | 1.7059 | −175.1157 | 3.0152 | 0.89 |
| Prunellae Spica | 0.0464 | 0.0016 | 1.7094 | −137.5972 | 4.4550 | 0.75 |
| Tamaricis Cacumen | 0.0484 | 0.0014 | 1.6821 | −177.3917 | 2.2708 | 0.73 |
| Schizonepetae Herba | 0.0474 | 0.0012 | 1.7186 | −126.7836 | 8.7373 | 0.73 |
| Cuscutae Semen | 0.0448 | 0.0028 | 1.7143 | −173.9090 | 14.7876 | 0.68 |
| Achyranthis Bidentatae Radix | 0.0455 | 0.0058 | 1.7053 | −150.4610 | 15.6081 | 0.62 |
| Artemisiae Annuae Herba | 0.0437 | 0.0057 | 1.7163 | −167.2389 | 3.3503 | 0.62 |
| Name | MSE | RMSE | MAE | MAPE |
|---|---|---|---|---|
| RF | 0.007 | 0.063 | 0.056 | 8.570 |
| GBDT | 0.010 | 0.101 | 0.088 | 13.787 |
| SVM | 0.009 | 0.096 | 0.077 | 13.687 |
| XGBoost | 0.010 | 0.099 | 0.090 | 14.086 |
| CART | 0.000 | 0.011 | 0.010 | 35.845 |
| Syn-COM | 0.001 | 0.037 | 0.025 | 0.981 |
| Gene | Primers |
|---|---|
| TGFB1 | Forward-5′-TGATACGCCTGAGTGGCTGTCT-3′ |
| Reverse-5′-CACAAGAGCAGTGAGCGCTGAA-3′ | |
| PTGS2 | Forward-5′-GATCCCCAGGGCTCAAACAT-3′ |
| Reverse-5′-GAAAAGGCGCAGTTTACGCT-3′ | |
| MMP3 | Forward-5′-CACTCACAGACCTGACTCGGTT-3′ |
| Reverse-5′-AAGCAGGATCACAGTTGGCTGG-3′ | |
| GAPDH | Forward-5′-TCTTGCTCAGTGTCCTTGC-3′ |
| Reverse-5′-CTTTGTCAAGCTCATTTCCTGG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Liu, J.; Guan, S.; Wang, S.; Yu, C.; Yu, Y.; Li, B.; Zhang, Y.; Yang, W.; Wang, Z. Syn-COM: A Multi-Level Predictive Synergy Framework for Innovative Drug Combinations. Pharmaceuticals 2024, 17, 1230. https://doi.org/10.3390/ph17091230
Shi Y, Liu J, Guan S, Wang S, Yu C, Yu Y, Li B, Zhang Y, Yang W, Wang Z. Syn-COM: A Multi-Level Predictive Synergy Framework for Innovative Drug Combinations. Pharmaceuticals. 2024; 17(9):1230. https://doi.org/10.3390/ph17091230
Chicago/Turabian StyleShi, Yinli, Jun Liu, Shuang Guan, Sicun Wang, Chengcheng Yu, Yanan Yu, Bing Li, Yingying Zhang, Weibin Yang, and Zhong Wang. 2024. "Syn-COM: A Multi-Level Predictive Synergy Framework for Innovative Drug Combinations" Pharmaceuticals 17, no. 9: 1230. https://doi.org/10.3390/ph17091230
APA StyleShi, Y., Liu, J., Guan, S., Wang, S., Yu, C., Yu, Y., Li, B., Zhang, Y., Yang, W., & Wang, Z. (2024). Syn-COM: A Multi-Level Predictive Synergy Framework for Innovative Drug Combinations. Pharmaceuticals, 17(9), 1230. https://doi.org/10.3390/ph17091230






