Alleviation of Autophagic Deficits and Neuroinflammation by Histamine H3 Receptor Antagonist E159 Ameliorates Autism-Related Behaviors in BTBR Mice
Abstract
1. Introduction
2. Results
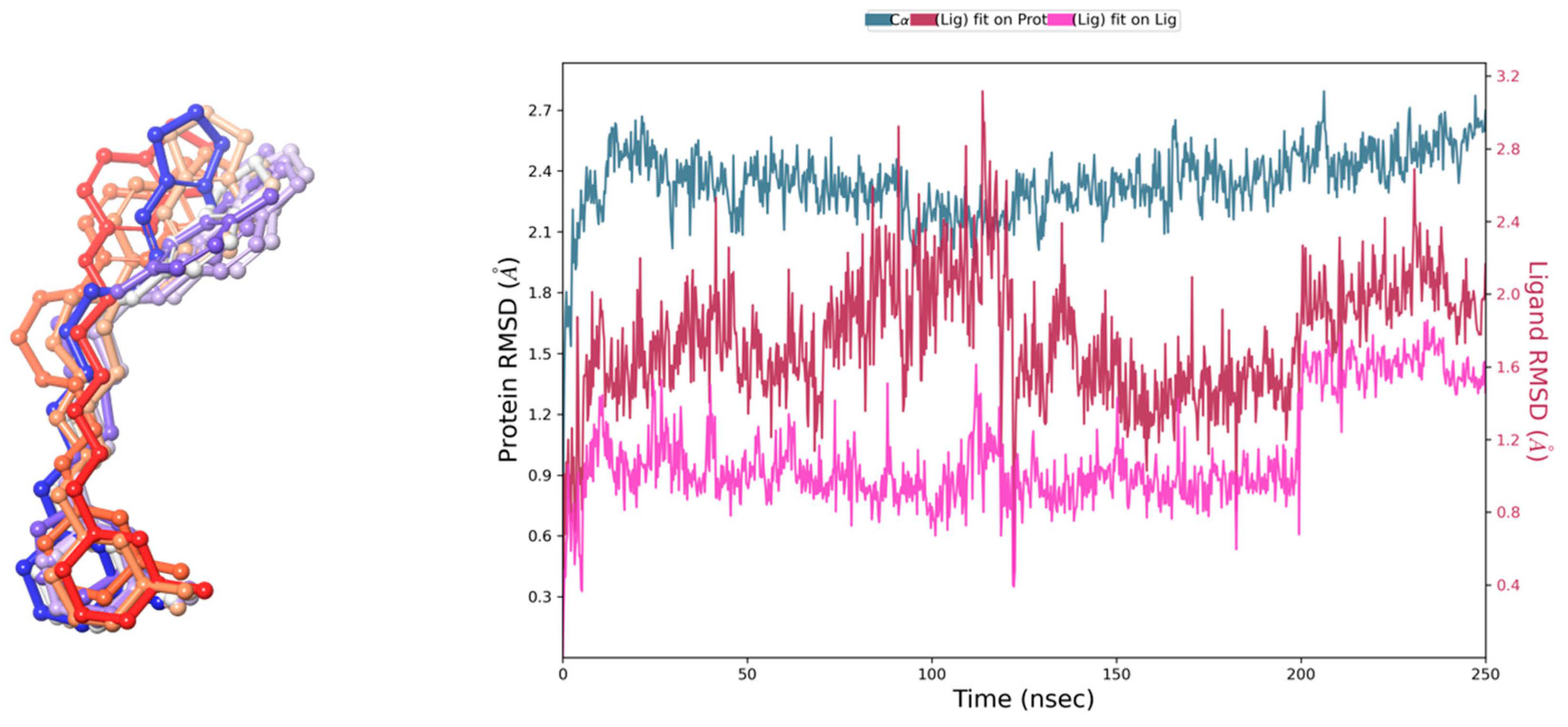
2.1. Docking Studies of E159
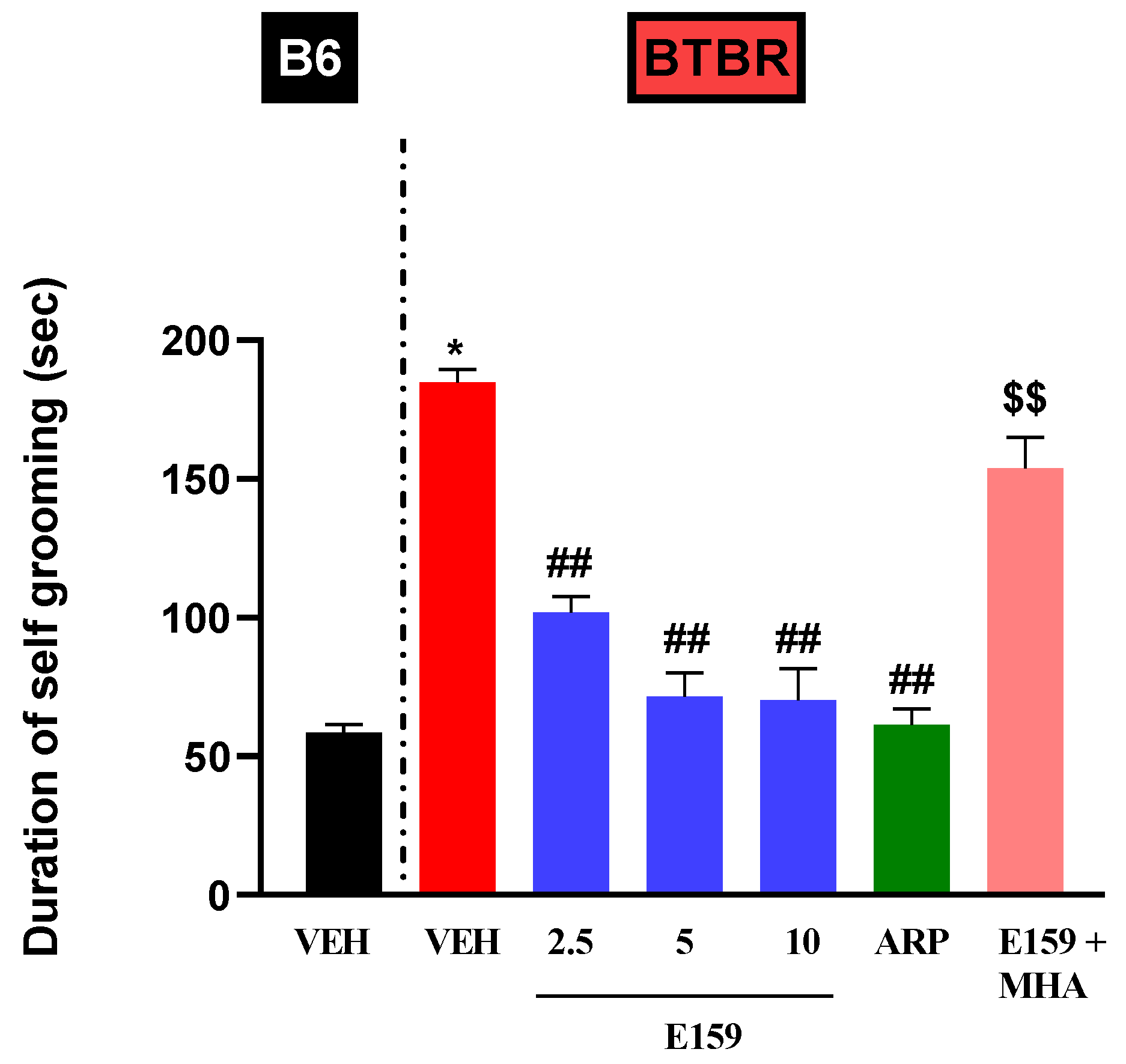
2.2. Effects of E159 on Stereotypical Repetitive Self-Grooming Behaviors in Mice
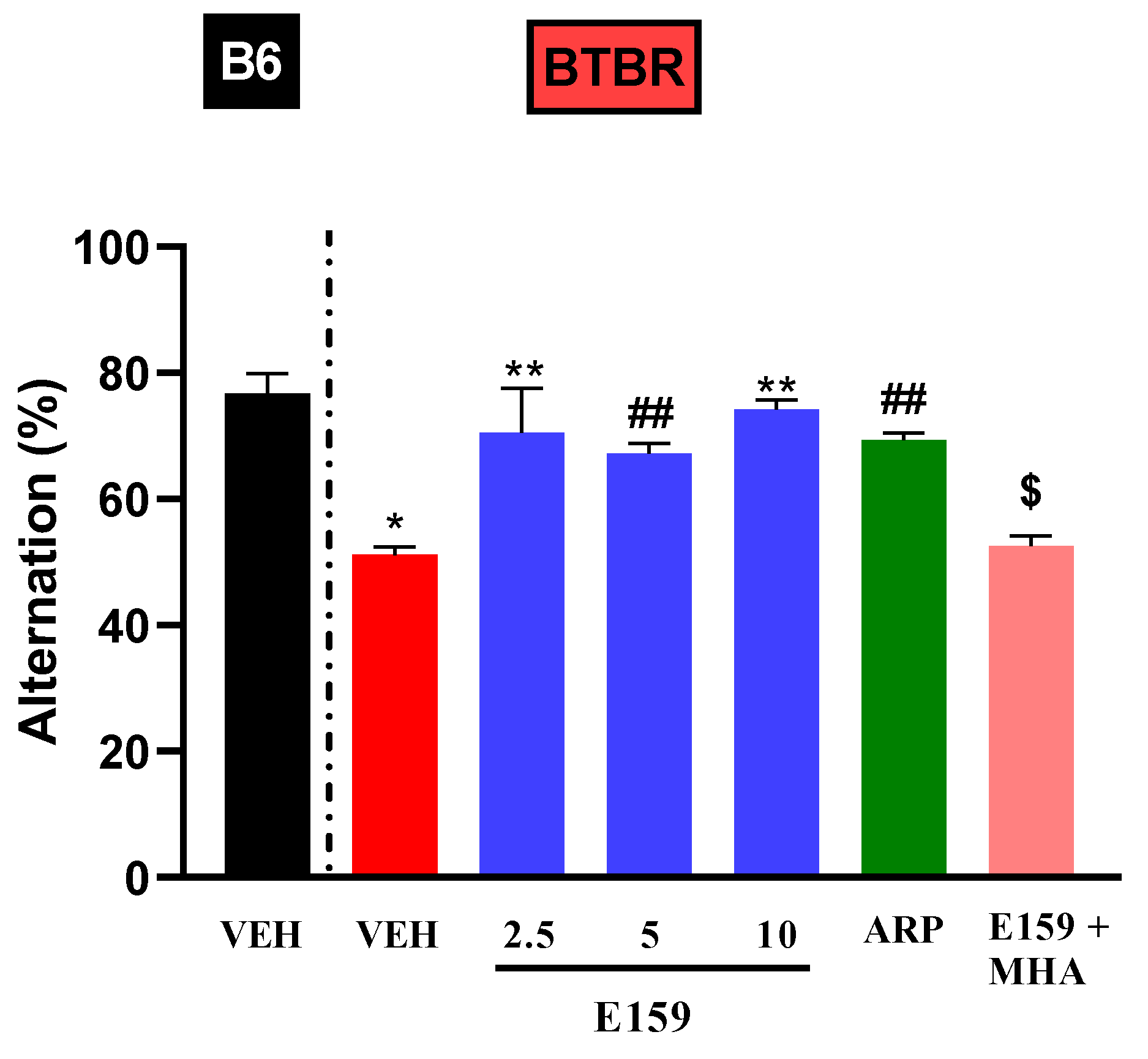
2.3. Effects of E159 on Spontaneous Alternation in BTBR Mice
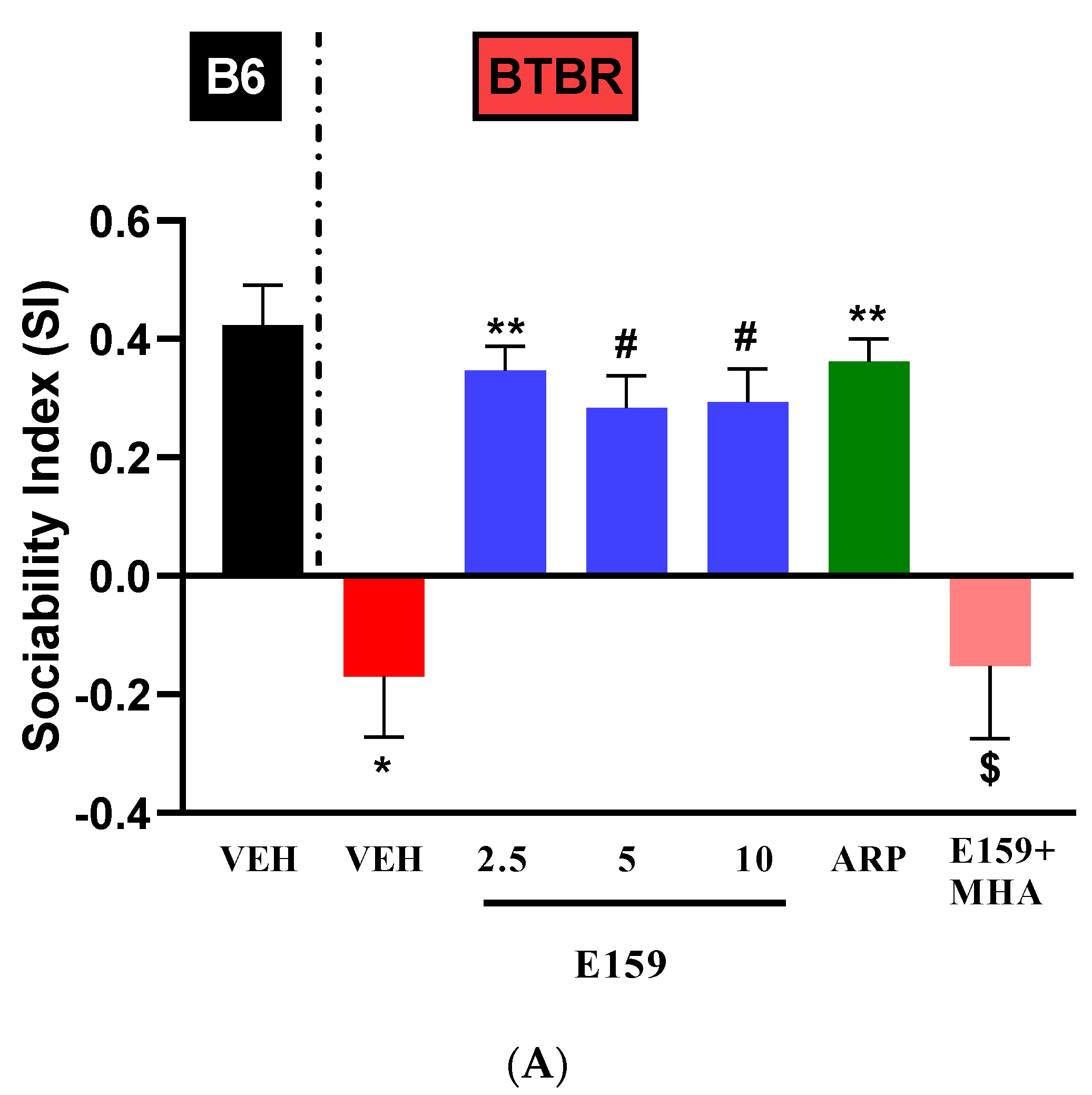
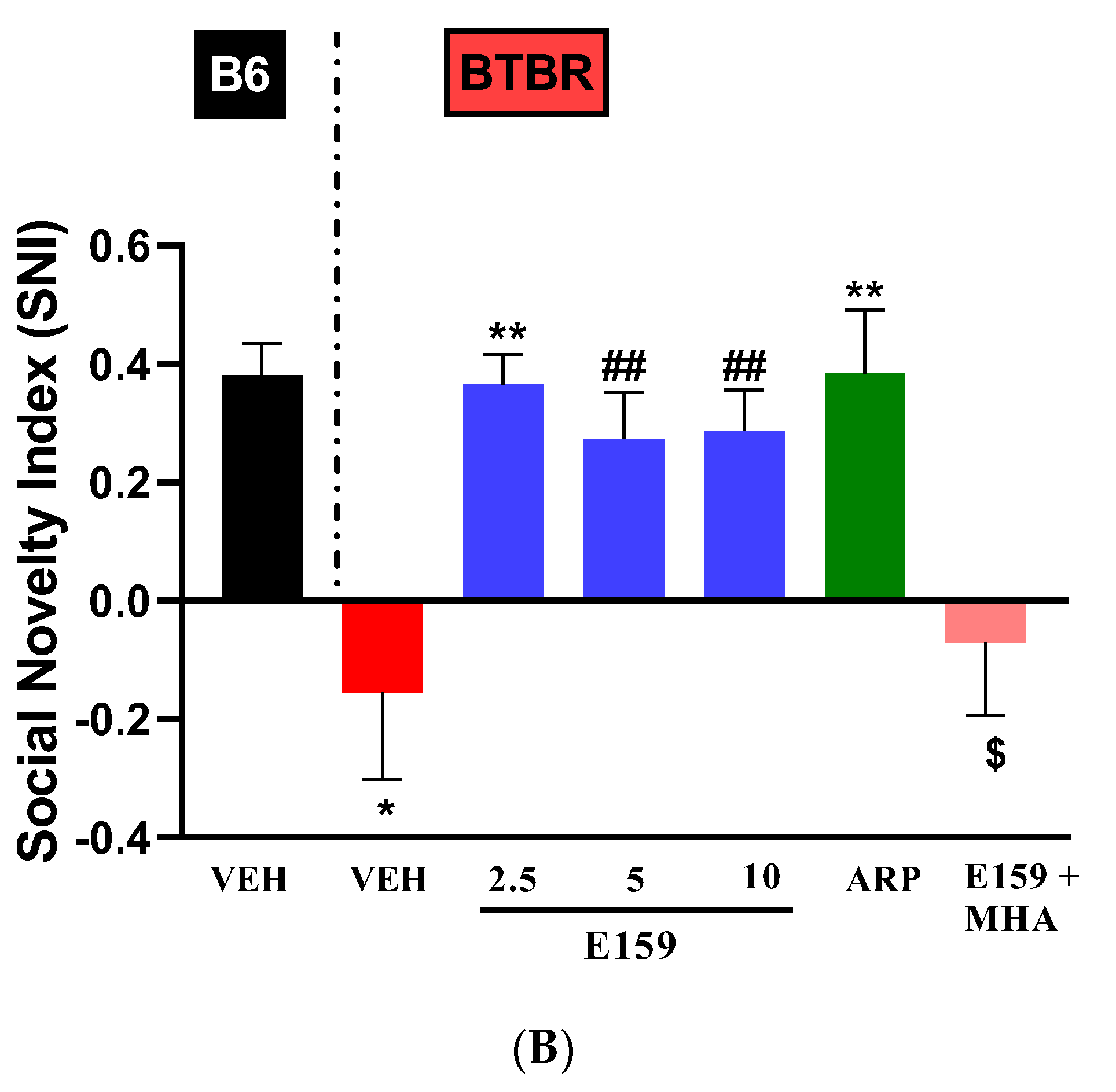
2.4. Effects of E159 on Sociability and Social Novelty Preference of BTBR Mice in the Three-Chambered Task
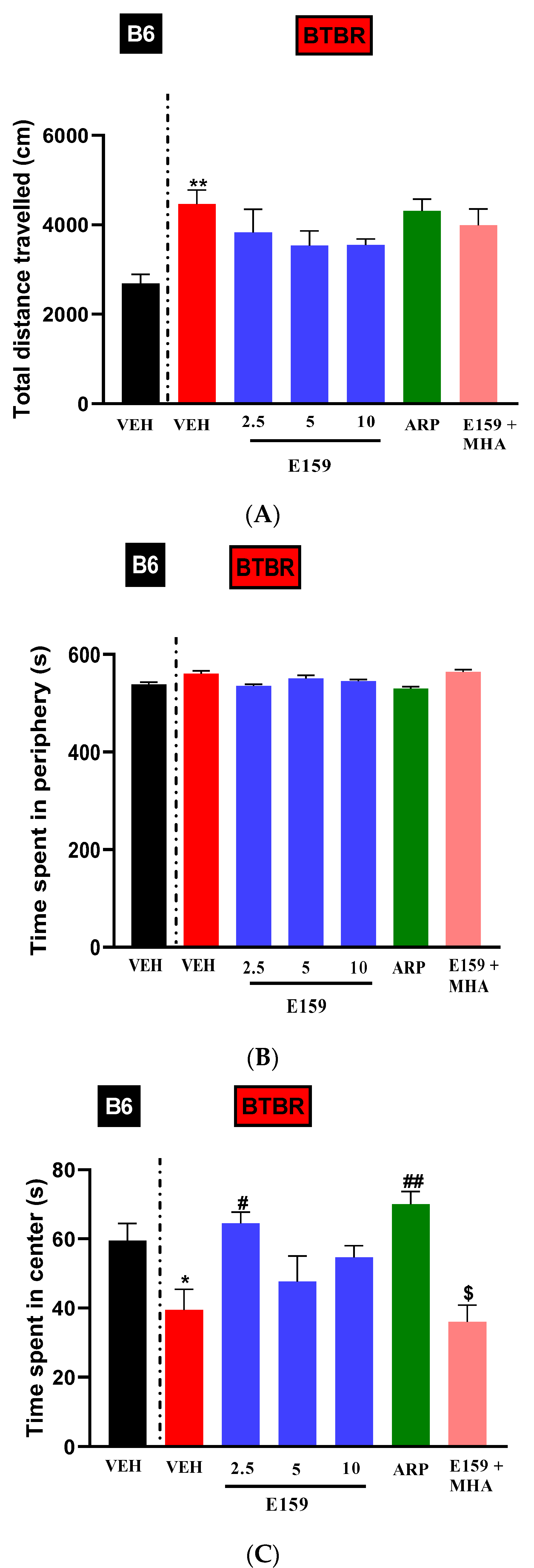
2.5. Impact of E159 on Anxiety and Locomotor Activity
2.6. Effects of E159 on the Level of Proinflammatory Cytokines in Cerebellum and Hippocampus of BTBR Mice
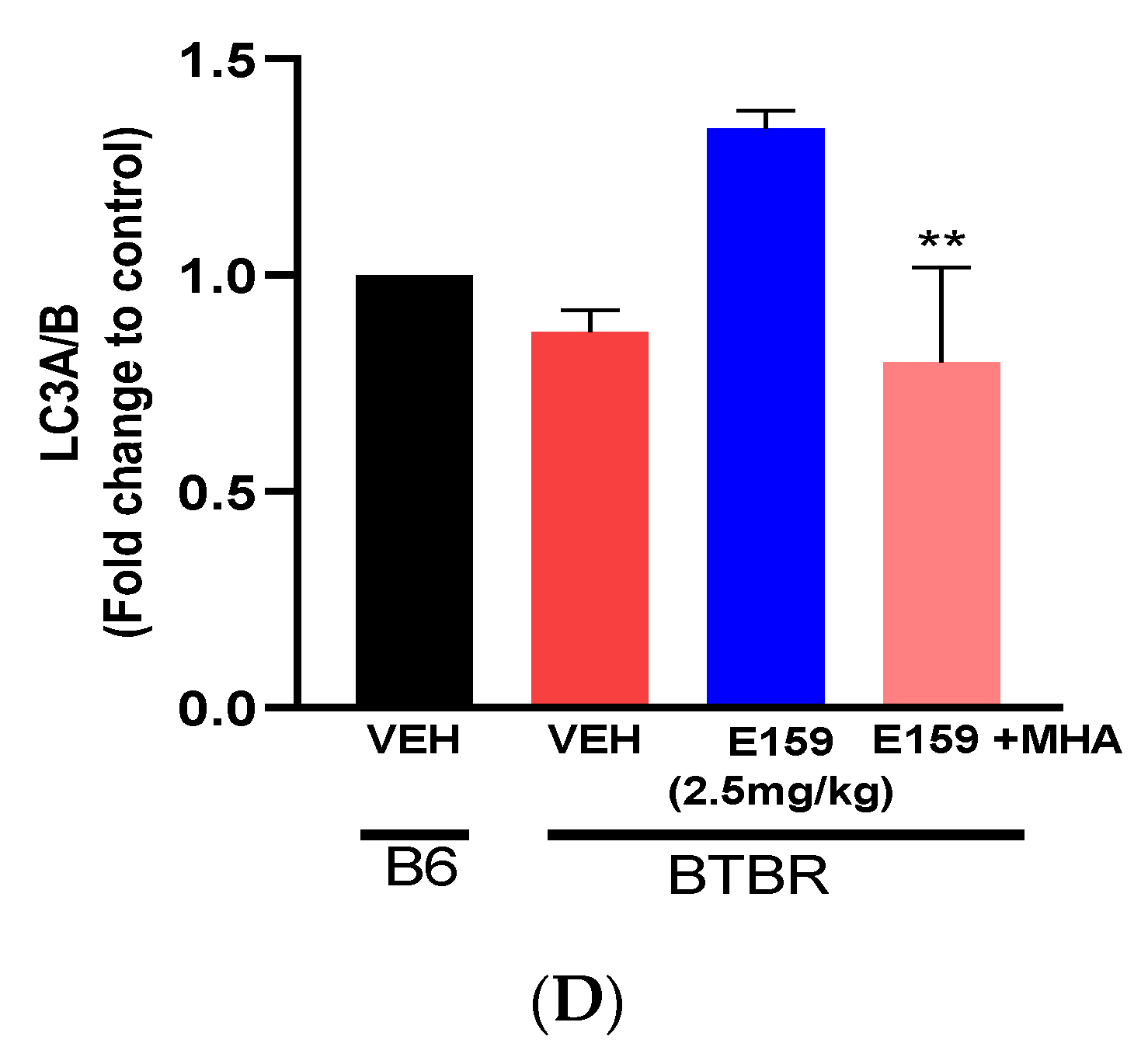
2.7. Effect of E159 on Autophagy
3. Discussion
4. Materials and Methods
4.1. Molecular Docking Studies
4.2. In Vivo Studies
4.2.1. Animals
4.2.2. Drug Compounds and Biochemical Materials
4.2.3. Study Design and Treatment
4.2.4. Behavioral Assessments
Self-Grooming
Y-Maze or Spontaneous Alteration
Three Chamber Social Test (TCT)
Open Field Test (OFT)
4.3. Biochemical Investigations
4.3.1. Brain Collection and Tissue Processing
4.3.2. Pro-Inflammatory Cytokine Assessments
4.3.3. Western Blot
4.4. Statistical Analyses
5. Conclusions
Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, S.D.; Jha, N.K.; Ojha, S.; Sadek, B. mTOR Signaling Disruption and Its Association with the Development of Autism Spectrum Disorder. Molecules 2023, 28, 1889. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Reynolds, K.; Ji, Y.; Gu, R.; Rai, S.; Zhou, C.J. Impaired Neurodevelopmental Pathways in Autism Spectrum Disorder: A Review of Signaling Mechanisms and Crosstalk. J. Neurodev. Disord. 2019, 11, 10. [Google Scholar] [CrossRef]
- Dana, H.; Tahtasakal, R.; Sener, E.F. Animal Models of Autism: A Perspective from Autophagy Mechanisms. J. Transl. Genet. Genom. 2020, 4, 251–262. [Google Scholar] [CrossRef]
- Jiang, P.; Zhou, L.; Zhao, L.; Fei, X.; Wang, Z.; Liu, T.; Tang, Y.; Li, D.; Gong, H.; Luo, Y.; et al. Puerarin Attenuates Valproate-Induced Features of ASD in Male Mice via Regulating Slc7a11-Dependent Ferroptosis. Neuropsychopharmacology 2024, 49, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Saad, K.; Chirumbolo, S.; Kern, J.K.; Geier, D.A.; Geier, M.R.; Urbina, M.A. Immune Dysfunction and Neuroinflammation in Autism Spectrum Disorder. Acta Neurobiol. Exp. 2016, 76, 257–268. [Google Scholar] [CrossRef]
- Siniscalco, D.; Schultz, S.; Brigida, A.L.; Antonucci, N. Inflammation and Neuro-Immune Dysregulations in Autism Spectrum Disorders. Pharmaceuticals 2018, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Oxidative Stress and Immune System Dysfunction in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 3293. [Google Scholar] [CrossRef]
- Jiang, C.-C.; Lin, L.-S.; Long, S.; Ke, X.-Y.; Fukunaga, K.; Lu, Y.-M.; Han, F. Signalling Pathways in Autism Spectrum Disorder: Mechanisms and Therapeutic Implications. Signal Transduct. Target. Ther. 2022, 7, 229. [Google Scholar] [CrossRef]
- Eftekharian, M.M.; Ghafouri-Fard, S.; Noroozi, R.; Omrani, M.D.; Arsang-jang, S.; Ganji, M.; Gharzi, V.; Noroozi, H.; Komaki, A.; Mazdeh, M.; et al. Cytokine Profile in Autistic Patients. Cytokine 2018, 108, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int. J. Mol. Sci. 2023, 24, 5487. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial Activation and Neuroinflammation in the Brain of Patients with Autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Lin, Y.-K.; Lai, J.-H.; Lo, Y.-C.; Yang, Y.-C.S.H.; Ye, S.-Y.; Lee, C.-J.; Wang, C.-C.; Chiang, Y.-H.; Tseng, S.-H. Maternal Immune Activation Causes Social Behavior Deficits and Hypomyelination in Male Rat Offspring with an Autism-Like Microbiota Profile. Brain Sci. 2021, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Onore, C.; Yang, H.; Van de Water, J.; Ashwood, P. Dynamic Akt/mTOR Signaling in Children with Autism Spectrum Disorder. Front. Pediatr. 2017, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yang, M.; Wang, Y.; Ren, J.; Lin, P.; Cui, C.; Song, J.; He, Q.; Hu, H.; Wang, K.; et al. Melatonin Prevents Diabetes-Associated Cognitive Dysfunction from Microglia-Mediated Neuroinflammation by Activating Autophagy via TLR4/Akt/mTOR Pathway. FASEB J. 2021, 35, e21485. [Google Scholar] [CrossRef]
- Han, H.-E.; Kim, T.-K.; Son, H.-J.; Park, W.J.; Han, P.-L. Activation of Autophagy Pathway Suppresses the Expression of iNOS, IL6 and Cell Death of LPS-Stimulated Microglia Cells. Biomol. Ther. 2013, 21, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, K. Autophagy and Apoptosis in Liver Injury. Cell Cycle 2015, 14, 1631–1642. [Google Scholar] [CrossRef]
- Matsuzawa-Ishimoto, Y.; Hwang, S.; Cadwell, K. Autophagy and Inflammation. Annu. Rev. Immunol. 2018, 36, 73–101. [Google Scholar] [CrossRef]
- Deng, Z.; Zhou, X.; Lu, J.-H.; Yue, Z. Autophagy Deficiency in Neurodevelopmental Disorders. Cell Biosci. 2021, 11, 214. [Google Scholar] [CrossRef]
- Lieberman, O.J.; Cartocci, V.; Pigulevskiy, I.; Molinari, M.; Carbonell, J.; Broseta, M.B.; Post, M.R.; Sulzer, D.; Borgkvist, A.; Santini, E. mTOR Suppresses Macroautophagy During Striatal Postnatal Development and Is Hyperactive in Mouse Models of Autism Spectrum Disorders. Front. Cell. Neurosci. 2020, 14, 70. [Google Scholar] [CrossRef]
- Huber, K.M.; Klann, E.; Costa-Mattioli, M.; Zukin, R.S. Dysregulation of Mammalian Target of Rapamycin Signaling in Mouse Models of Autism. J. Neurosci. 2015, 35, 13836–13842. [Google Scholar] [CrossRef]
- Agam, G.; Taylor, Z.; Vainer, E.; Golan, H.M. The Influence of Choline Treatment on Behavioral and Neurochemical Autistic-like Phenotype in Mthfr-Deficient Mice. Transl. Psychiatry 2020, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Sadeq, A.; Sasse, A.; Sadek, B. Role of Neuroinflammation in Autism Spectrum Disorder and the Emergence of Brain Histaminergic System. Lessons Also for BPSD? Front. Pharmacol. 2020, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Baronio, D.; Castro, K.; Gonchoroski, T.; de Melo, G.M.; Nunes, G.D.F.; Bambini-Junior, V.; Gottfried, C.; Riesgo, R. Effects of an H3R Antagonist on the Animal Model of Autism Induced by Prenatal Exposure to Valproic Acid. PLoS ONE 2015, 10, e0116363. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Shin, J.H.; Rajpurohit, A.; Deep-Soboslay, A.; Collado-Torres, L.; Brandon, N.J.; Hyde, T.M.; Kleinman, J.E.; Jaffe, A.E.; Cross, A.J.; et al. Altered Expression of Histamine Signaling Genes in Autism Spectrum Disorder. Transl. Psychiatry 2017, 7, e1126. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Azimullah, S.; Jayaprakash, P.; Jayaraj, R.L.; Reiner, D.; Ojha, S.K.; Beiram, R.; Stark, H.; Łażewska, D.; Kieć-Kononowicz, K.; et al. The Dual-Active Histamine H3 Receptor Antagonist and Acetylcholine Esterase Inhibitor E100 Ameliorates Stereotyped Repetitive Behavior and Neuroinflammmation in Sodium Valproate Induced Autism in Mice. Chem. Biol. Interact. 2019, 312, 108775. [Google Scholar] [CrossRef] [PubMed]
- Sadek, B.; Saad, A.; Sadeq, A.; Jalal, F.; Stark, H. Histamine H3 Receptor as a Potential Target for Cognitive Symptoms in Neuropsychiatric Diseases. Behav. Brain Res. 2016, 312, 415–430. [Google Scholar] [CrossRef]
- Guilloux, J.-P.; Samuels, B.A.; Mendez-David, I.; Hu, A.; Levinstein, M.; Faye, C.; Mekiri, M.; Mocaer, E.; Gardier, A.M.; Hen, R.; et al. S 38093, a Histamine H3 Antagonist/Inverse Agonist, Promotes Hippocampal Neurogenesis and Improves Context Discrimination Task in Aged Mice. Sci. Rep. 2017, 7, 42946. [Google Scholar] [CrossRef]
- Eissa, N.; Khan, N.; Ojha, S.K.; Łazewska, D.; Kieć-Kononowicz, K.; Sadek, B. The Histamine H3 Receptor Antagonist DL77 Ameliorates MK801-Induced Memory Deficits in Rats. Front. Neurosci. 2018, 12, 42. [Google Scholar] [CrossRef]
- Brown, J.W.; Whitehead, C.A.; Basso, A.M.; Rueter, L.E.; Zhang, M. Preclinical Evaluation of Non-Imidazole Histamine H3 Receptor Antagonists in Comparison to Atypical Antipsychotics for the Treatment of Cognitive Deficits Associated with Schizophrenia. Int. J. Neuropsychopharmacol. 2013, 16, 889–904. [Google Scholar] [CrossRef]
- Zhang, M.; Jiao, J.; Hu, X.; Yang, P.; Huang, Y.; Situ, M.; Guo, K.; Cai, J.; Huang, Y. Exploring the Spatial Working Memory and Visual Perception in Children with Autism Spectrum Disorder and General Population with High Autism-like Traits. PLoS ONE 2020, 15, e0235552. [Google Scholar] [CrossRef]
- Griebel, G.; Pichat, P.; Pruniaux, M.-P.; Beeské, S.; Lopez-Grancha, M.; Genet, E.; Terranova, J.-P.; Castro, A.; Sánchez, J.A.; Black, M.; et al. SAR110894, a Potent Histamine H3-Receptor Antagonist, Displays Procognitive Effects in Rodents. Pharmacol. Biochem. Behav. 2012, 102, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Jayaprakash, P.; Azimullah, S.; Ojha, S.K.; Al-Houqani, M.; Jalal, F.Y.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. The Histamine H3R Antagonist DL77 Attenuates Autistic Behaviors in a Prenatal Valproic Acid-Induced Mouse Model of Autism. Sci. Rep. 2018, 8, 13077. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Azimullah, S.; Jayaprakash, P.; Jayaraj, R.L.; Reiner, D.; Ojha, S.K.; Beiram, R.; Stark, H.; Łażewska, D.; Kieć-Kononowicz, K.; et al. The Dual-Active Histamine H3 Receptor Antagonist and Acetylcholine Esterase Inhibitor E100 Alleviates Autistic-Like Behaviors and Oxidative Stress in Valproic Acid Induced Autism in Mice. Int. J. Mol. Sci. 2020, 21, 3996. [Google Scholar] [CrossRef]
- Thomas, S.D.; Abdalla, S.; Eissa, N.; Akour, A.; Jha, N.K.; Ojha, S.; Sadek, B. Targeting Microglia in Neuroinflammation: H3 Receptor Antagonists as a Novel Therapeutic Approach for Alzheimer’s Disease, Parkinson’s Disease, and Autism Spectrum Disorder. Pharmaceuticals 2024, 17, 831. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Eissa, N.; Awad, M.A.; Jayaprakash, P.; Zhong, S.; Stölting, F.; Stark, H.; Sadek, B. The Histamine H3R and Dopamine D2R/D3R Antagonist ST-713 Ameliorates Autism-like Behavioral Features in BTBR T+tf/J Mice by Multiple Actions. Biomed. Pharmacother. 2021, 138, 111517. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, X.; Hu, W.; Ma, J.; Hou, W.; Zhang, X.; Wang, X.; Gao, J.; Shen, Y.; Lv, J.; et al. Histamine H3 Receptors Aggravate Cerebral Ischaemic Injury by Histamine-Independent Mechanisms. Nat. Commun. 2014, 5, 3334. [Google Scholar] [CrossRef]
- Meyza, K.Z.; Blanchard, D.C. The BTBR Mouse Model of Idiopathic Autism—Current View on Mechanisms. Neurosci. Biobehav. Rev. 2017, 76, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, G.; Liu, S.; Zhang, Q.; Wang, P.; Cao, Y.; Wu, L. Supplementation with Selenium Attenuates Autism-like Behaviors and Improves Oxidative Stress, Inflammation and Related Gene Expression in an Autism Disease Model. J. Nutr. Biochem. 2022, 107, 109034. [Google Scholar] [CrossRef]
- Łażewska, D.; Ligneau, X.; Schwartz, J.-C.; Schunack, W.; Stark, H.; Kieć-Kononowicz, K. Ether Derivatives of 3-Piperidinopropan-1-Ol as Non-Imidazole Histamine H3 Receptor Antagonists. Bioorg. Med. Chem. 2006, 14, 3522–3529. [Google Scholar] [CrossRef]
- Alachkar, A.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. The Histamine H3 Receptor Antagonist E159 Reverses Memory Deficits Induced by Dizocilpine in Passive Avoidance and Novel Object Recognition Paradigm in Rats. Front. Pharmacol. 2017, 8, 709. [Google Scholar] [CrossRef]
- McFarlane, H.G.; Kusek, G.K.; Yang, M.; Phoenix, J.L.; Bolivar, V.J.; Crawley, J.N. Autism-like Behavioral Phenotypes in BTBR T+tf/J Mice. Genes Brain Behav. 2008, 7, 152–163. [Google Scholar] [CrossRef]
- Amodeo, D.A.; Jones, J.H.; Sweeney, J.A.; Ragozzino, M.E. Differences in BTBR T+ Tf/J and C57BL/6J Mice on Probabilistic Reversal Learning and Stereotyped Behaviors. Behav. Brain Res. 2012, 227, 64–72. [Google Scholar] [CrossRef]
- Shimamura, T.; Shiroishi, M.; Weyand, S.; Tsujimoto, H.; Winter, G.; Katritch, V.; Abagyan, R.; Cherezov, V.; Liu, W.; Han, G.W.; et al. Structure of the Human Histamine H1 Receptor Complex with Doxepin. Nature 2011, 475, 65–70. [Google Scholar] [CrossRef]
- Peng, X.; Yang, L.; Liu, Z.; Lou, S.; Mei, S.; Li, M.; Chen, Z.; Zhang, H. Structural Basis for Recognition of Antihistamine Drug by Human Histamine Receptor. Nat. Commun. 2022, 13, 6105. [Google Scholar] [CrossRef]
- Im, D.; Kishikawa, J.; Shiimura, Y.; Hisano, H.; Ito, A.; Fujita-Fujiharu, Y.; Sugita, Y.; Noda, T.; Kato, T.; Asada, H.; et al. Structural Insights into the Agonists Binding and Receptor Selectivity of Human Histamine H4 Receptor. Nat. Commun. 2023, 14, 6538. [Google Scholar] [CrossRef] [PubMed]
- Jończyk, J.; Malawska, B.; Bajda, M. Hybrid Approach to Structure Modeling of the Histamine H3 Receptor: Multi-Level Assessment as a Tool for Model Verification. PLoS ONE 2017, 12, e0186108. [Google Scholar] [CrossRef]
- Kas, M.J.; Glennon, J.C.; Buitelaar, J.; Ey, E.; Biemans, B.; Crawley, J.; Ring, R.H.; Lajonchere, C.; Esclassan, F.; Talpos, J.; et al. Assessing Behavioural and Cognitive Domains of Autism Spectrum Disorders in Rodents: Current Status and Future Perspectives. Psychopharmacology 2014, 231, 1125–1146. [Google Scholar] [CrossRef]
- Karagiannidis, I.; Dehning, S.; Sandor, P.; Tarnok, Z.; Rizzo, R.; Wolanczyk, T.; Madruga-Garrido, M.; Hebebrand, J.; Nöthen, M.M.; Lehmkuhl, G.; et al. Support of the Histaminergic Hypothesis in Tourette Syndrome: Association of the Histamine Decarboxylase Gene in a Large Sample of Families. J. Med. Genet. 2013, 50, 760–764. [Google Scholar] [CrossRef]
- Rapanelli, M.; Frick, L.; Pogorelov, V.; Ohtsu, H.; Bito, H.; Pittenger, C. Histamine H3R Receptor Activation in the Dorsal Striatum Triggers Stereotypies in a Mouse Model of Tic Disorders. Transl. Psychiatry 2017, 7, e1013. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Immune Mediators in the Brain and Peripheral Tissues in Autism Spectrum Disorder. Nat. Rev. Neurosci. 2015, 16, 469–486. [Google Scholar] [CrossRef]
- Onore, C.E.; Careaga, M.; Babineau, B.A.; Schwartzer, J.J.; Berman, R.F.; Ashwood, P. Inflammatory Macrophage Phenotype in BTBR T+tf/J Mice. Front. Neurosci. 2013, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Alshammari, M.A.; Khan, M.R.; Alsaad, A.M.S.; Attia, S.M. S3I-201, a Selective Stat3 Inhibitor, Restores Neuroimmune Function through Upregulation of Treg Signaling in Autistic BTBR T+ Itpr3tf/J Mice. Cell. Signal. 2018, 52, 127–136. [Google Scholar] [CrossRef] [PubMed]
- McTighe, S.M.; Neal, S.J.; Lin, Q.; Hughes, Z.A.; Smith, D.G. The BTBR Mouse Model of Autism Spectrum Disorders Has Learning and Attentional Impairments and Alterations in Acetylcholine and Kynurenic Acid in Prefrontal Cortex. PLoS ONE 2013, 8, e62189. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Tolu, S.S.; Barkan, C.L.; Crawley, J.N. Repetitive Self-Grooming Behavior in the BTBR Mouse Model of Autism Is Blocked by the mGluR5 Antagonist MPEP. Neuropsychopharmacology 2010, 35, 976–989. [Google Scholar] [CrossRef]
- Choi, H.J.; Im, S.J.; Park, H.R.; Park, S.; Kim, C.-E.; Ryu, S. Long-Term Effects of Aripiprazole Treatment during Adolescence on Cognitive Function and Dopamine D2 Receptor Expression in Neurodevelopmentally Normal Rats. Clin. Psychopharmacol. Neurosci. 2019, 17, 400–408. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [CrossRef]
- Eissa, N.; Venkatachalam, K.; Jayaprakash, P.; Falkenstein, M.; Dubiel, M.; Frank, A.; Reiner-Link, D.; Stark, H.; Sadek, B. The Multi-Targeting Ligand ST-2223 with Histamine H3 Receptor and Dopamine D2/D3 Receptor Antagonist Properties Mitigates Autism-Like Repetitive Behaviors and Brain Oxidative Stress in Mice. Int. J. Mol. Sci. 2021, 22, 1947. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Xu, Y.; Luan, H.; Wang, C.; Yang, M.; Zhao, R.; Song, M.; Liu, J.; Sun, L.; et al. Thioperamide Attenuates Neuroinflammation and Cognitive Impairments in Alzheimer’s Disease via Inhibiting Gliosis. Exp. Neurol. 2022, 347, 113870. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.Y.; Kim, H.-S.; Cha, K.-H.; Won, D.H.; Lee, J.-Y.; Jang, S.W.; Sohn, U.D. The Effects of Donepezil, an Acetylcholinesterase Inhibitor, on Impaired Learning and Memory in Rodents. Biomol. Ther. 2018, 26, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, H.; Schreiweis, C.; Mondragón-González, L.S.; Rebbah, S.; Lavielle, O.; Mallet, L.; Burguière, E. The Sapap3−/− Mouse Reconsidered as a Comorbid Model Expressing a Spectrum of Pathological Repetitive Behaviours. Transl. Psychiatry 2023, 13, 26. [Google Scholar] [CrossRef]
- Mostofsky, S.H.; Goldberg, M.C.; Landa, R.J.; Denckla, M.B. Evidence for a Deficit in Procedural Learning in Children and Adolescents with Autism: Implications for Cerebellar Contribution. J. Int. Neuropsychol. Soc. 2000, 6, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Marko, M.K.; Crocetti, D.; Hulst, T.; Donchin, O.; Shadmehr, R.; Mostofsky, S.H. Behavioural and Neural Basis of Anomalous Motor Learning in Children with Autism. Brain 2015, 138, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated Plasma Cytokines in Autism Spectrum Disorders Provide Evidence of Immune Dysfunction and Are Associated with Impaired Behavioral Outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The Influence of Neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.-M.; Ji, L.; Brown, T.; Malik, M. Elevated Immune Response in the Brain of Autistic Patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef]
- Goines, P.E.; Ashwood, P. Cytokine Dysregulation in Autism Spectrum Disorders (ASD): Possible Role of the Environment. Neurotoxicol. Teratol. 2013, 36, 67–81. [Google Scholar] [CrossRef]
- Barata-Antunes, S.; Cristóvão, A.C.; Pires, J.; Rocha, S.M.; Bernardino, L. Dual Role of Hista mine on Microglia-Induced Neurodegeneration. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 764–769. [Google Scholar] [CrossRef]
- Saraiva, C.; Barata-Antunes, S.; Santos, T.; Ferreiro, E.; Cristóvão, A.C.; Serra-Almeida, C.; Ferreira, R.; Bernardino, L. Histamine Modulates Hippocampal Inflammation and Neurogenesis in Adult Mice. Sci. Rep. 2019, 9, 8384. [Google Scholar] [CrossRef]
- Zhou, Z.; An, Q.; Zhang, W.; Li, Y.; Zhang, Q.; Yan, H. Histamine and Receptors in Neuroinflammation: Their Roles on Neurodegenerative Diseases. Behav. Brain Res. 2024, 465, 114964. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Sun, F.; Xu, Y.; Luan, H.; Yang, M.; Wang, C.; Zhang, T.; Zhou, Z.; Yan, H. Histamine H3R Antagonist Counteracts the Impaired Hippocampal Neurogenesis in Lipopolysaccharide-Induced Neuroinflammation. Int. Immunopharmacol. 2022, 110, 109045. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Codogno, P.; Levine, B. Autophagy Modulation as a Potential Therapeutic Target for Diverse Diseases. Nat. Rev. Drug Discov. 2012, 11, 709–730. [Google Scholar] [CrossRef]
- Nixon, R.A.; Cataldo, A.M. Lysosomal System Pathways: Genes to Neurodegeneration in Alzheimer’s Disease. J. Alzheimers Dis. 2006, 9, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Bowling, H.; Klann, E. Shaping Dendritic Spines in Autism Spectrum Disorder: mTORC1-Dependent Macroautophagy. Neuron 2014, 83, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Gudsnuk, K.; Kuo, S.-H.; Cotrina, M.L.; Rosoklija, G.; Sosunov, A.; Sonders, M.S.; Kanter, E.; Castagna, C.; Yamamoto, A.; et al. Loss of mTOR-Dependent Macroautophagy Causes Autistic-like Synaptic Pruning Deficits. Neuron 2014, 83, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.-X.; Zhang, Q.-L. PI3K/AKT/mTOR-Mediated Autophagy in the Development of Autism Spectrum Disorder. Brain Res. Bull. 2016, 125, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.S.; Roy, S.K.; Hasan, R.; Pk, M.M.U. The Crucial Role of the Cerebellum in Autism Spectrum Disorder: Neuroimaging, Neurobiological, and Anatomical Insights. Health Sci. Rep. 2024, 7, e2233. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, P.; Zhao, L.; Fei, X.; Tang, Y.; Luo, Y.; Gong, H.; Wang, X.; Li, X.; Li, S.; et al. Ligustilide Inhibits Purkinje Cell Ferritinophagy via the ULK1/NCOA4 Pathway to Attenuate Valproic Acid-Induced Autistic Features. Phytomedicine 2024, 126, 155443. [Google Scholar] [CrossRef]
- Mapelli, L.; Soda, T.; D’Angelo, E.; Prestori, F. The Cerebellar Involvement in Autism Spectrum Disorders: From the Social Brain to Mouse Models. Int. J. Mol. Sci. 2022, 23, 3894. [Google Scholar] [CrossRef]
- Sydnor, L.M.; Aldinger, K.A. Structure, Function, and Genetics of the Cerebellum in Autism. J. Psychiatry Brain Sci. 2022, 7, e220008. [Google Scholar] [CrossRef]
- Su, L.-D.; Xu, F.-X.; Wang, X.-T.; Cai, X.-Y.; Shen, Y. Cerebellar Dysfunction, Cerebro-Cerebellar Connectivity and Autism Spectrum Disorders. Neuroscience 2021, 462, 320–327. [Google Scholar] [CrossRef]
- Giordano, S.; Darley-Usmar, V.; Zhang, J. Autophagy as an Essential Cellular Antioxidant Pathway in Neurodegenerative Disease. Redox Biol. 2014, 2, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.J.R.; Elder, K.; Dale, B. Link Between Increased Prevalence of Autism Spectrum Disorder Syndromes and Oxidative Stress, DNA Methylation, and Imprinting: The Impact of the Environment. JAMA Pediatr. 2015, 169, 1066–1067. [Google Scholar] [CrossRef]
- He, Y.; She, H.; Zhang, T.; Xu, H.; Cheng, L.; Yepes, M.; Zhao, Y.; Mao, Z. P38 MAPK Inhibits Autophagy and Promotes Microglial Inflammatory Responses by Phosphorylating ULK1. J. Cell Biol. 2018, 217, 315–328. [Google Scholar] [CrossRef]
- Sato, A.; Ikeda, K. Genetic and Environmental Contributions to Autism Spectrum Disorder Through Mechanistic Target of Rapamycin. Biol. Psychiatry Glob. Open Sci. 2022, 2, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, M. mTOR Inhibition: From Aging to Autism and Beyond. Scientifica 2013, 2013, 849186. [Google Scholar] [CrossRef]
- Takei, N.; Nawa, H. mTOR Signaling and Its Roles in Normal and Abnormal Brain Development. Front. Mol. Neurosci. 2014, 7, 28. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 Network Regulates Autophagy and Apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Qin, L.; Dai, X.; Yin, Y. Valproic Acid Exposure Sequentially Activates Wnt and mTOR Pathways in Rats. Mol. Cell. Neurosci. 2016, 75, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, X.; Cheng, X.; Lin, L.; Wang, Q.; Zhan, R.; Wu, Q.; Liu, S. Protective Effect of Ferulic Acid on Lipopolysaccharide-Induced BV2 Microglia Inflammation via AMPK/mTOR Signaling Pathway. Molecules 2023, 28, 3482. [Google Scholar] [CrossRef]
- Bongers, G.; Bakker, R.A.; Leurs, R. Molecular Aspects of the Histamine H3 Receptor. Biochem. Pharmacol. 2007, 73, 1195–1204. [Google Scholar] [CrossRef]
- Wang, L.; Fang, J.; Jiang, H.; Wang, Q.; Xue, S.; Li, Z.; Liu, R. 7-Pyrrolidinethoxy-4′-Methoxyisoflavone Prevents Amyloid β–Induced Injury by Regulating Histamine H3 Receptor-Mediated cAMP/CREB and AKT/GSK3β Pathways. Front. Neurosci. 2019, 13, 334. [Google Scholar] [CrossRef]
- Schrödinger. Release 2022-4: Schrödinger Suite 2022-4; Schrödinger, LLC: New York, NY, USA, 2017. [Google Scholar]
- Watts, K.S.; Dalal, P.; Murphy, R.B.; Sherman, W.; Friesner, R.A.; Shelley, J.C. ConfGen: A Conformational Search Method for Efficient Generation of Bioactive Conformers. J. Chem. Inf. Model. 2010, 50, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.F.; Honig, B.; Shaw, D.E.; Friesner, R.A. A Hierarchical Approach to All-Atom Protein Loop Prediction. Proteins Struct. Funct. Genet. 2004, 55, 351–367. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the 2006 ACM/IEEE Conference on Supercomputing (SC’06), Tampa, FL, USA, 11–17 November 2006. [Google Scholar] [CrossRef]
- Lomize, M.A.; Lomize, A.L.; Pogozheva, I.D.; Mosberg, H.I. OPM: Orientations of Proteins in Membranes Database. Bioinformatics 2006, 22, 623–625. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, L.; Xiao, R.; Li, X.; He, X.; Gao, J.; Xu, H.; Fan, X. Autism-like Behavior in the BTBR Mouse Model of Autism Is Improved by Propofol. Neuropharmacology 2017, 118, 175–187. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, Y.; Wu, X.; Liu, A.; Wang, J.; Yang, S.; Liu, B.; Kong, Y.; Wang, L.; Zhang, K.; et al. Excitatory SST Neurons in the Medial Paralemniscal Nucleus Control Repetitive Self-Grooming and Encode Reward. Neuron 2022, 110, 3356–3373.e8. [Google Scholar] [CrossRef]
- Yeshurun, S.; Rogers, J.; Short, A.K.; Renoir, T.; Pang, T.Y.; Hannan, A.J. Elevated Paternal Glucocorticoid Exposure Modifies Memory Retention in Female Offspring. Psychoneuroendocrinology 2017, 83, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cai, Y.; Fan, X. Metformin Administration during Early Postnatal Life Rescues Autistic-Like Behaviors in the BTBR T+ Itpr3tf/J Mouse Model of Autism. Front. Behav. Neurosci. 2018, 12, 290. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. J. Vis. Exp. 2015, 96, 52434. [Google Scholar] [CrossRef]
- Eissa, N.; Jayaprakash, P.; Stark, H.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. Simultaneous Blockade of Histamine H3 Receptors and Inhibition of Acetylcholine Esterase Alleviate Autistic-Like Behaviors in BTBR T+ Tf/J Mouse Model of Autism. Biomolecules 2020, 10, 1251. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The Open Field as a Paradigm to Measure the Effects of Drugs on Anxiety-like Behaviors: A Review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Abul Khair, S.B.; Ojha, S.; Haque, M.E. Neuroprotective Effect of Nerolidol against Neuroinflammation and Oxidative Stress Induced by Rotenone. BMC Neurosci. 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Awad, M.A.; Thomas, S.D.; Venkatachalam, K.; Jayaprakash, P.; Zhong, S.; Stark, H.; Sadek, B. Simultaneous Antagonism at H3R/D2R/D3R Reduces Autism-like Self-Grooming and Aggressive Behaviors by Mitigating MAPK Activation in Mice. Int. J. Mol. Sci. 2022, 24, 526. [Google Scholar] [CrossRef]



| Behavioral Test | VEH | E159 (mg/kg, i.p.) | ARP (1 mg/kg, i.p.) | |||
|---|---|---|---|---|---|---|
| 2.5 | 5 | 10 | ||||
| Self-grooming (s) | 58.5 ± 2.89 | 57.5 ± 1.71 | 57.5 ± 2.17 | 55 ± 3.15 | 59.33 ± 3.93 | |
| Spontaneous alteration (%) | 76.75 ± 3.1 | 73.99 ± 3.78 | 72.54 ± 1.98 | 74.58 ± 1.55 | 78.69 ± 0.54 | |
| Open Field | Time in center (s) | 59.5 ± 4.94 | 56.93 ± 4.42 | 57.83 ± 2.81 | 54.5 ± 6.69 | 62.5 ± 2.43 |
| Time in periphery (s) | 538.8 ± 4.5 | 543 ± 4.36 | 542.2 ± 2.81 | 545.5 ± 6.69 | 537.5 ± 2.43 | |
| Total distance travelled (cm) | 2691 ± 203.6 | 2584 ± 219.5 | 2545 ± 213 | 2505 ± 215.5 | 2730 ± 95.66 | |
| Three Chamber Test | Sociability Index (SI) | 0.42 ± 0.07 | 0.41 ± 0.05 | 0.40 ± 0.03 | 0.39 ± 0.08 | 0.43 ± 0.05 |
| Social Novelty Index (SNI) | 0.38 ± 0.05 | 0.36 ± 0.09 | 0.37 ± 0.07 | 0.37 ± 0.04 | 0.35 ± 0.07 | |
| Treatment Groups | Cerebellum | Hippocampus | ||||
|---|---|---|---|---|---|---|
| Proinflammatory Cytokines | Proinflammatory Cytokines | |||||
| TNF-α | IL-6 | IL-1β | TNF-α | IL-6 | IL-1β | |
| B6 (Ctrl) (VEH) | 203.8 ± 4.9 | 67 ± 6.29 | 161.5 ± 3.13 | 191.4 ± 9.41 | 51.42 ± 1.9 | 70.66 ± 12.1 |
| BTBR (Ctrl) (VEH) | 275.7 ± 13.86 * | 118.1 ± 8.58 * | 340.9 ± 8.702 * | 259.9 ± 10.11 * | 95.08 ± 5.31 * | 166.2 ± 11.36 * |
| BTBR (E159, 2.5 mg/kg) | 224.6 ± 5.03 ### | 72.26 ± 5.86 ** | 198.3 ± 10.5 ## | 207.0 ± 11.81 ## | 58.52 ± 2.18 ** | 96.73 ± 7.93 ## |
| BTBR (ARP, 1 mg/kg) | 229.4 ± 6.5 ### | 77.54 ± 3.84 ## | 207.7 ± 10.84 ## | 199.8 ± 3.45 *** | 59.69 ± 4.83 *** | 94.80 ± 15.51 ## |
| BTBR (E159, 2.5 mg/kg) + MHA | 268.6 ± 15.49 $ | 100.3 ± 8.28 $ | 273.5 ± 15.29 $$ | 244.8 ± 6.99 $ | 90.46 ± 9.29 $$ | 147.2 ± 6.48 $ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.D.; Jayaprakash, P.; Marwan, N.Z.H.J.; Aziz, E.A.B.A.; Kuder, K.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. Alleviation of Autophagic Deficits and Neuroinflammation by Histamine H3 Receptor Antagonist E159 Ameliorates Autism-Related Behaviors in BTBR Mice. Pharmaceuticals 2024, 17, 1293. https://doi.org/10.3390/ph17101293
Thomas SD, Jayaprakash P, Marwan NZHJ, Aziz EABA, Kuder K, Łażewska D, Kieć-Kononowicz K, Sadek B. Alleviation of Autophagic Deficits and Neuroinflammation by Histamine H3 Receptor Antagonist E159 Ameliorates Autism-Related Behaviors in BTBR Mice. Pharmaceuticals. 2024; 17(10):1293. https://doi.org/10.3390/ph17101293
Chicago/Turabian StyleThomas, Shilu Deepa, Petrilla Jayaprakash, Nurfirzana Z. H. J. Marwan, Ezzatul A. B. A. Aziz, Kamil Kuder, Dorota Łażewska, Katarzyna Kieć-Kononowicz, and Bassem Sadek. 2024. "Alleviation of Autophagic Deficits and Neuroinflammation by Histamine H3 Receptor Antagonist E159 Ameliorates Autism-Related Behaviors in BTBR Mice" Pharmaceuticals 17, no. 10: 1293. https://doi.org/10.3390/ph17101293
APA StyleThomas, S. D., Jayaprakash, P., Marwan, N. Z. H. J., Aziz, E. A. B. A., Kuder, K., Łażewska, D., Kieć-Kononowicz, K., & Sadek, B. (2024). Alleviation of Autophagic Deficits and Neuroinflammation by Histamine H3 Receptor Antagonist E159 Ameliorates Autism-Related Behaviors in BTBR Mice. Pharmaceuticals, 17(10), 1293. https://doi.org/10.3390/ph17101293









