Synthesis and Characterization of Multifunctional Chitosan–Silver Nanoparticles: An In-Vitro Approach for Biomedical Applications
Abstract
1. Introduction
2. Results
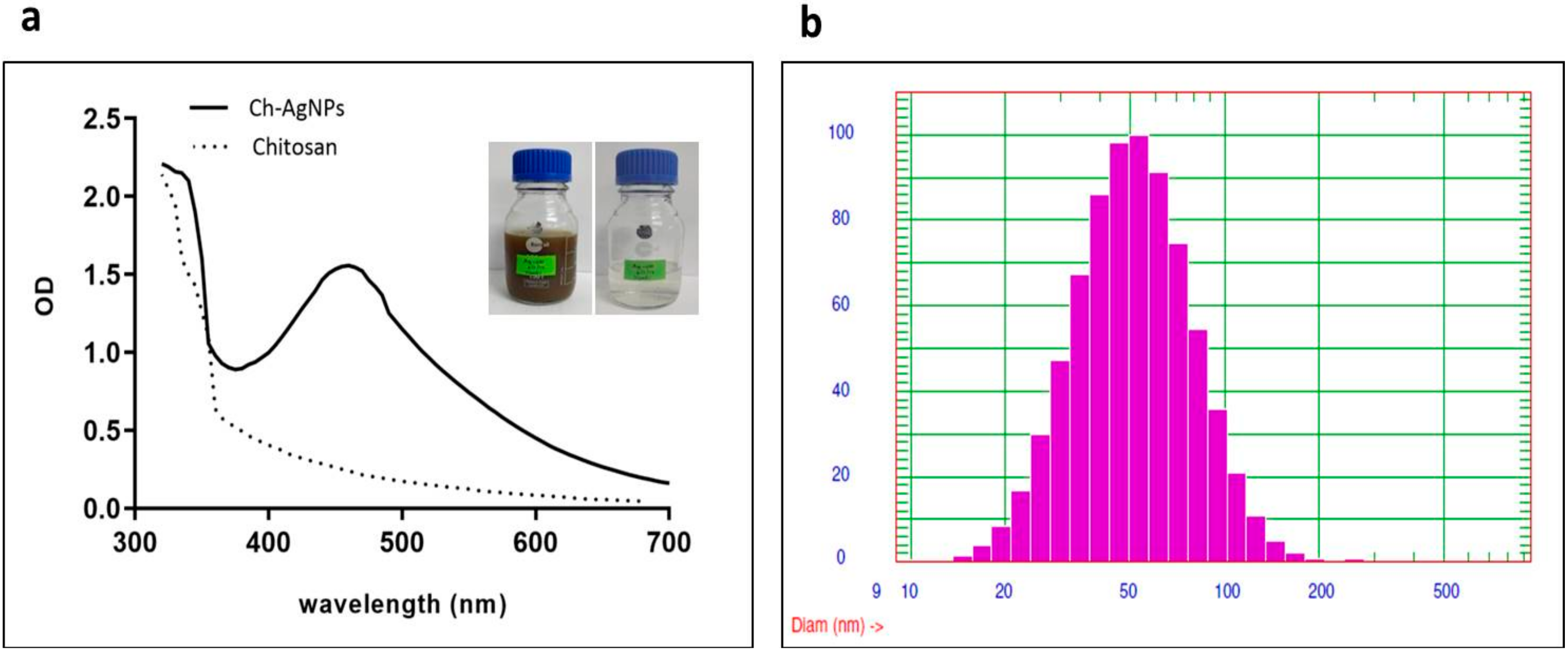
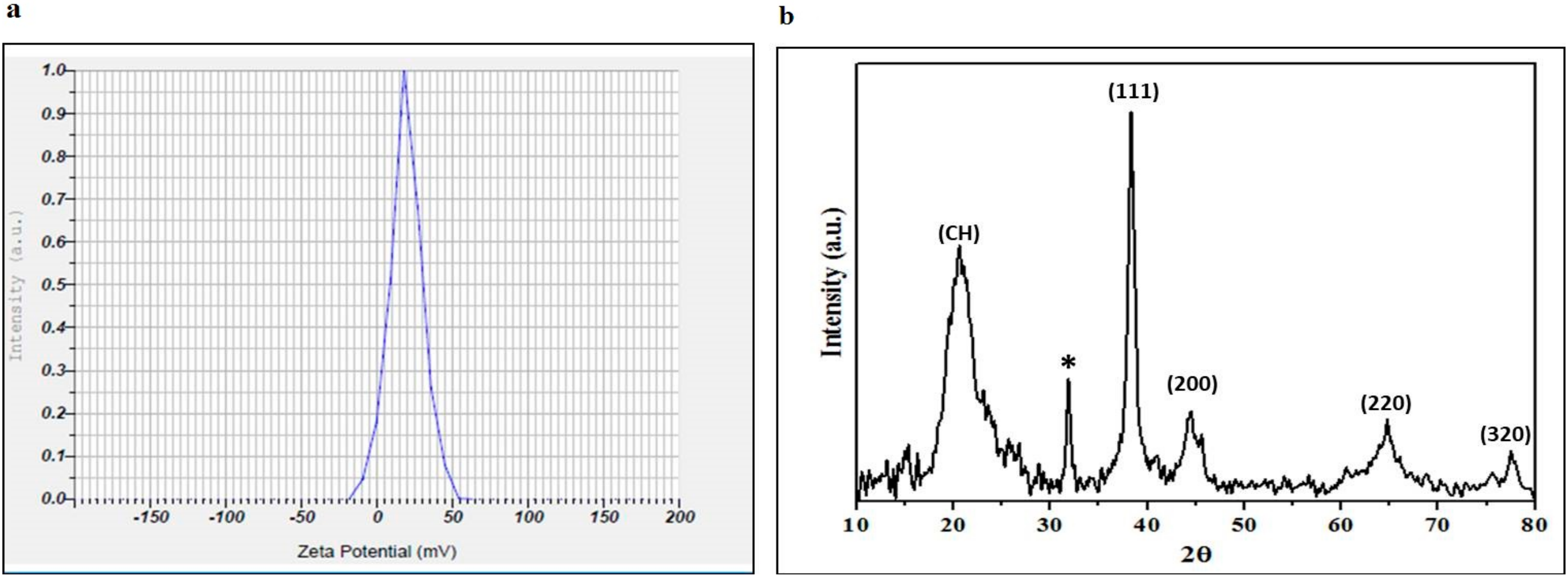
2.1. Nanoparticles Characterization
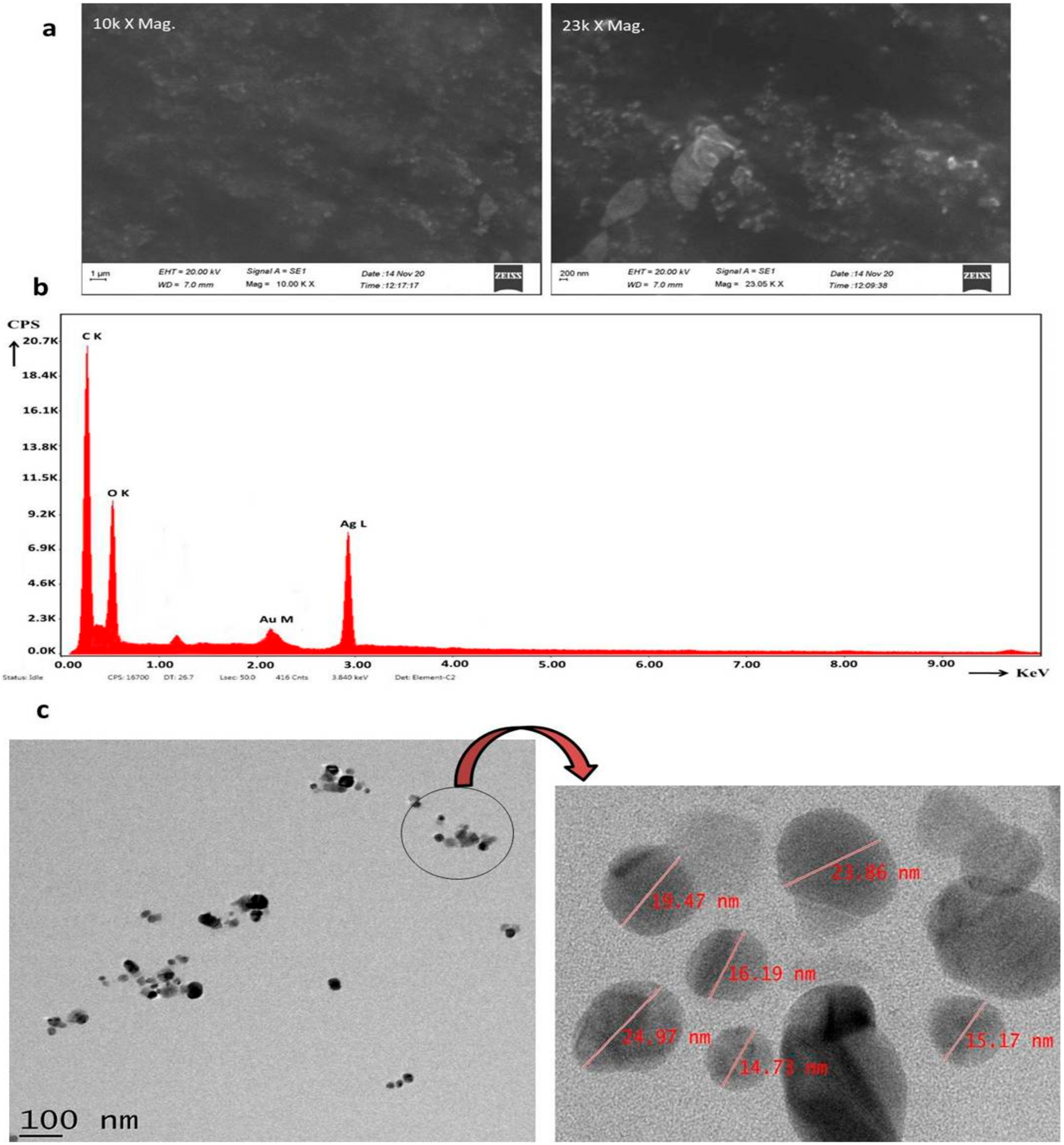
2.2. Electron Microscopy and EDS Analysis of NPs
2.3. Efficacy of NPs on Clinical Pathogens
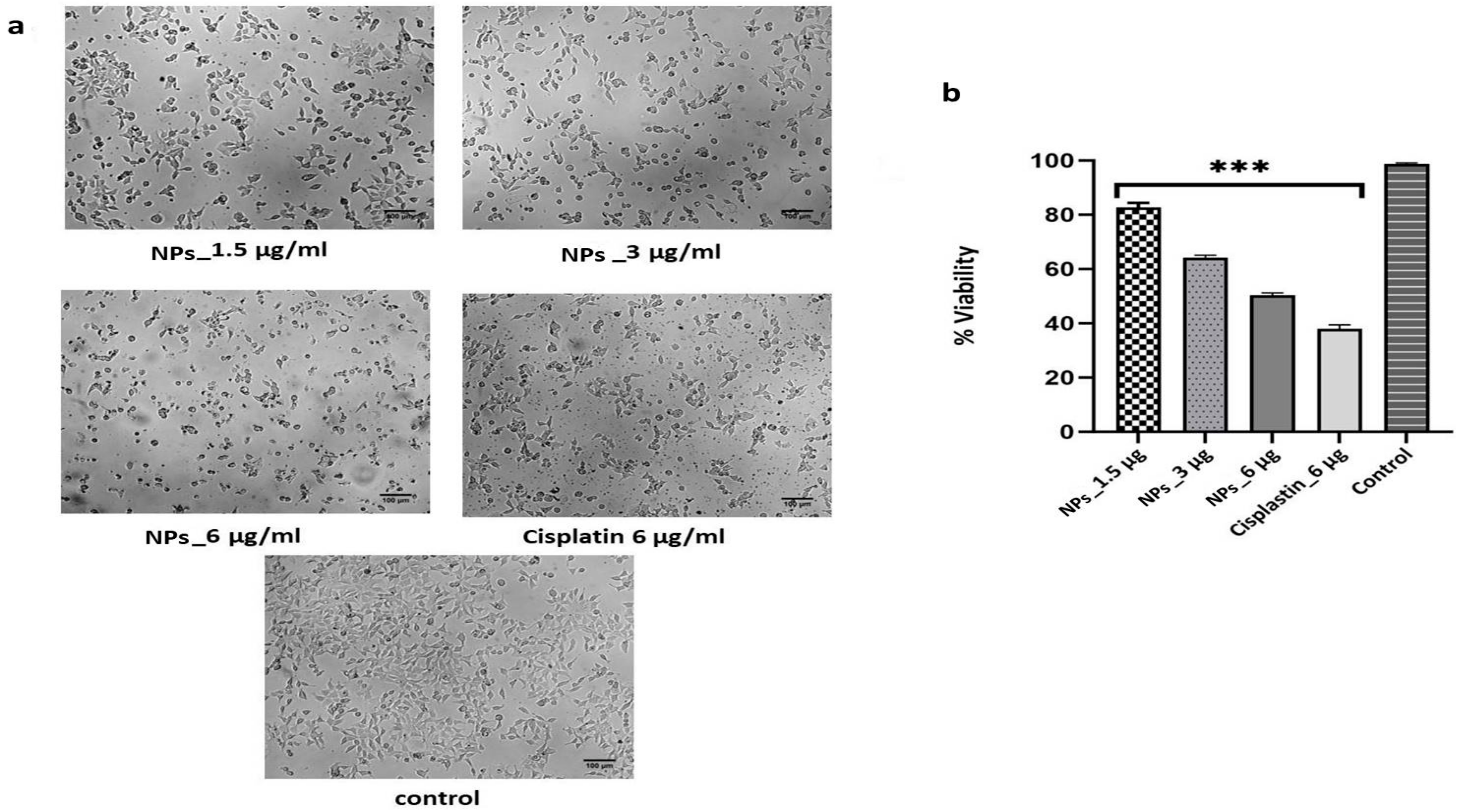
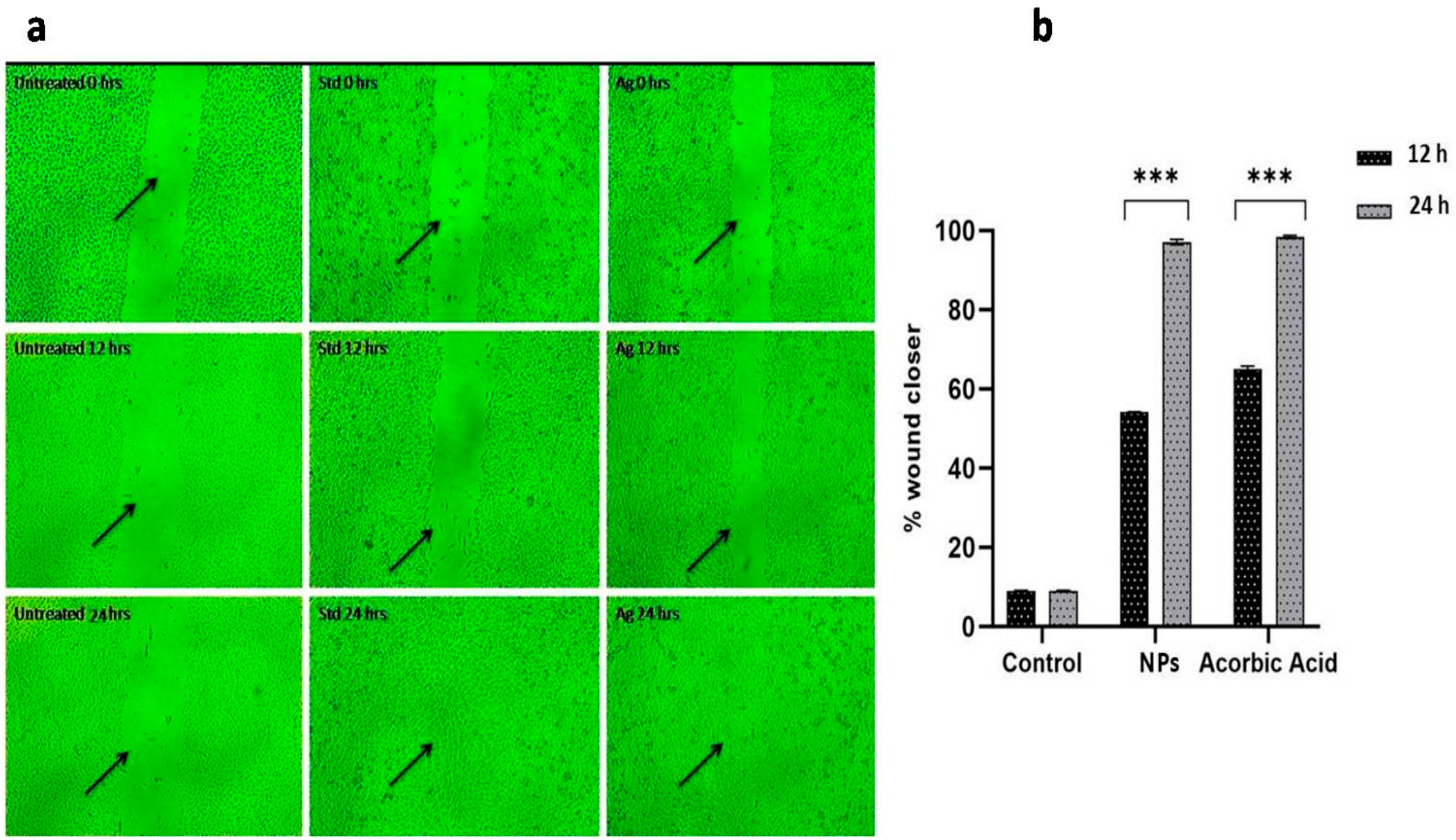
2.4. Toxicity and WoundHealing Assay
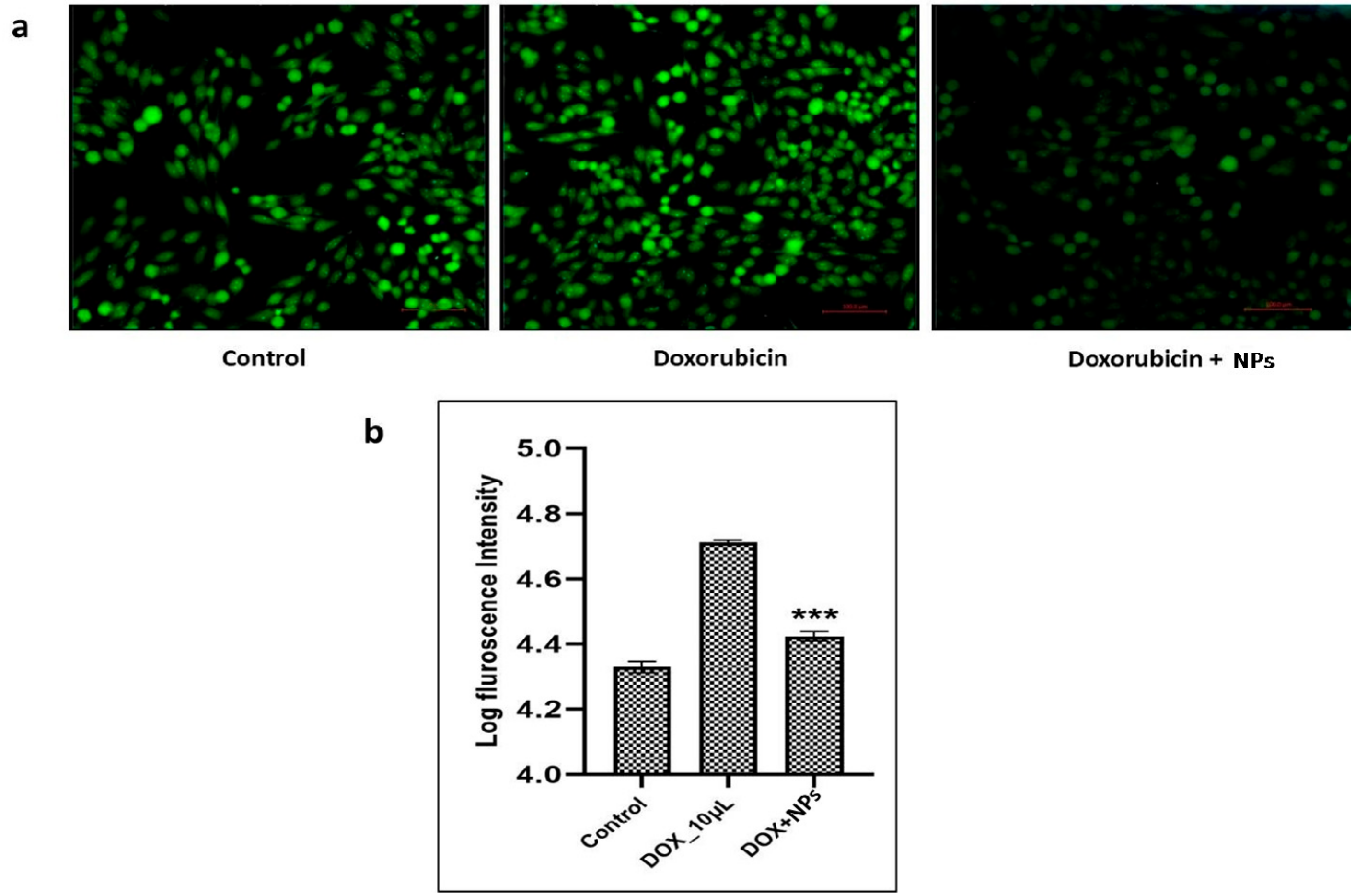
2.5. Antioxidant Activity of NPs
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Synthesis of Chitosan–Silver Nanoparticles (Ch-AgNPs)
5.2. Characterization of the Nanoparticles
5.2.1. Spectroscopic Studies
5.2.2. Electron Microscopy Studies
5.3. Antibacterial Activity of Ch-AgNPs
5.4. Cytotoxicity Assay of Ch-AgNPs by MTT
5.5. Wound Healing Property of Ch-AgNPs
5.6. Antioxidant Property of Ch-AgNPs
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Browne, A.J.; Chipeta, M.G.; Haines-Woodhouse, G.; Kumaran, E.P.; Hamadani, B.H.; Zaraa, S.; Henry, N.J.; Deshpande, A.; Reiner, R.C.; Day, N.P.; et al. Global antibiotic consumption and usage in humans, 2000–2018: A spatial modelling study. Lancet Planet. Health 2021, 5, e893–e904. [Google Scholar] [CrossRef] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and wound healing: From bench to bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Hajji, S.; Khedir, S.B.; Hamza-Mnif, I.; Hamdi, M.; Jedidi, I.; Kallel, R.; Nasri, M. Biomedical potential of chitosan-silver nanoparticles with special reference to antioxidant, antibacterial, hemolytic and in vivo cutaneous wound healing effects. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 241–254. [Google Scholar] [CrossRef]
- Kharaghani, D.; Khan, M.Q.; Tamada, Y.; Ogasawara, H.; Inoue, Y.; Saito, Y.; Kim, I.S. Fabrication of electrospun antibacterial PVA/Cs nanofibers loaded with CuNPs and AgNPs by an in-situ method. Polym. Test. 2018, 72, 315–321. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 20, 385–393. [Google Scholar] [CrossRef]
- Ran, L.; Zou, Y.; Cheng, J.; Lu, F. Silver nanoparticles in situ synthesized by polysaccharides from Sanghuangporussanghuang and composites with chitosan to prepare scaffolds for the regeneration of infected full-thickness skin defects. Int. J. Biol. Macromol. 2019, 125, 392–403. [Google Scholar] [CrossRef]
- Wichai, S.; Chuysinuan, P.; Chaiarwut, S.; Ekabutr, P.; Supaphol, P. Development of bacterial cellulose/alginate/chitosan composites incorporating copper (II) sulfate as an antibacterial wound dressing. Int. J. Drug Deliv. Technol. 2019, 51, 662–671. [Google Scholar] [CrossRef]
- Shah, A.; Yameen, M.A.; Fatima, N.; Murtaza, G. Chemical synthesis of chitosan/silver nanocomposites films loaded with moxifloxacin: Their characterization and potential antibacterial activity. Int. J. Pharm. 2019, 561, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Cheng, J.; Yu, K. In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int. J. Biol. Macromol. 2019, 121, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional silver nanoparticles based on chitosan: Antibacterial, antibiofilm, antifungal, antioxidant, and wound-healing activities. J. Fungi. 2022, 8, 612. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 1–21. [Google Scholar] [CrossRef]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and challenges in the use of chitosan and its derivatives in biomedical fields: A Review. Carbohydr. Polym. 2023, 26, 100323. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front. Pharmacol. 2021, 12, 601–626. [Google Scholar] [CrossRef]
- Bravo-Osuna, I.; Vauthier, C.; Farabollini, A.; Palmieri, G.; Ponchel, G. Mucoadhesion mechanism of chitosan and thiolated chitosan-poly (isobutyl cyanoacrylate) core-shell nanoparticles. Biomater. Res. 2007, 28, 2233–2243. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S.A. Review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. J. Mater. 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Mirda, E.; Idroes, R.; Khairan, K.; Tallei, T.E.; Ramli, M.; Earlia, N.; Maulana, A.; Idroes, G.M.; Muslem, M.; Jalil, Z. Synthesis of chitosan-silver nanoparticle composite spheres and their antimicrobial activities. Polymers 2021, 13, 3990. [Google Scholar] [CrossRef] [PubMed]
- Sartori, P.; Delamare, A.P.; Machado, G.; Devine, D.M.; Crespo, J.S.; Giovanela, M. Synthesis and characterization of silver nanoparticles for the preparation of chitosan pellets and their application in industrial wastewater disinfection. Water 2023, 15, 190. [Google Scholar] [CrossRef]
- Vanti, G.L.; Masaphy, S.; Kurjogi, M.; Chakrasali, S.; Nargunda, V.B. Synthesis and application of chitosan-copper nanoparticle on dampping off causing plant pathogenic Fungi. Int. J. Biol. Macromol. 2020, 156, 1387–1395. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Lotfy, T.M.R.; Shawir, S.M.S. Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull Natl. Res. Cent. 2019, 43, 83. [Google Scholar] [CrossRef]
- Singha, D.; Sahu, D.; Sahu, K. Coupling of molecular transition with the surface plasmon resonance of silver nanoparticles inside the restricted environment of reverse micelles. ACS Omega. 2017, 2, 5494–5503. [Google Scholar] [CrossRef] [PubMed]
- Sanpui, P.; Murugadoss, A.; Prasad, P.D.; Ghosh, S.S.; Chattopadhyay, A. The antibacterial properties of a novel chitosan-ag nanoparticle composite. Int. J. Food. Microbiol. 2008, 124, 142–146. [Google Scholar] [CrossRef]
- Govindan, S.; Nivethaa, E.A.; Saravanan, R.; Narayanan, V.; Stephen, A. Synthesis and of chitosan-silver nanocomposite. Appl. Nanosci. 2012, 2, 299–303. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Giri, A.K.; Jena, B.; Biswal, B.; Pradhan, A.K.; Arakha, M.; Acharya, S.; Acharya, L. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci. Rep. 2022, 12, 8383. [Google Scholar] [CrossRef]
- Shah, M.Z.; Guan, Z.H.; Din, A.U.; Ali, A.; Rehman, A.U.; Jan, K.; Faisal, S.; Saud, S.; Adnan, M.; Wahid, F.; et al. Synthesis of silver nanoparticles using Plantago lanceolata extract and assessing their antibacterial and antioxidant activities. Sci. Rep. 2021, 11, 20754. [Google Scholar] [CrossRef] [PubMed]
- Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of silver nanoparticles using Cucumis prophetarum aqueous leaf extract and their antibacterial and antiproliferative activity against cancer cell lines. ACS Omega. 2020, 5, 5520. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-calculated IR spectrum amide I, II, and III band contributions of N-methylacetamide fine components. ACS Omega. 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Folliero, V.; Chianese, A.; Zannella, C.; De Filippis, A.; Rosati, L.; Prisco, M.; Falanga, A.; Mali, A.; Galdiero, M.; et al. Synthesis of chitosan-coated silver nanoparticle bioconjugates and their antimicrobial activity against multidrug-resistant bacteria. Appl. Sci. 2021, 11, 9340. [Google Scholar] [CrossRef]
- Jogaiah, S.; Kurjogi, M.; Abdelrahman, M.; Hanumanthappa, N.; Tran, L.S. Ganoderma applanatum- mediated green synthesis of silver nanoparticles: Structural characterisation, and in vitro and in vivo biomedical and agrochemical properties. Arab. J. Chem. 2019, 12, 1108–1120. [Google Scholar] [CrossRef]
- Li, R.; Xu, Z.; Jiang, Q.; Heng, Y.; Chen, Z.; Chen, X. Characterization and biological evaluation of a novel silver nanoparticle-loaded collagen-chitosan dressing. Regen. Biomat. 2020, 7, 371–380. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, T.; Zhang, B.; Gong, F.; Huang, Y.; Tang, M. Cytotoxicity and apoptosis induced by silver nanoparticles in human liver HepG2 cells in different dispersion media. J. Appl. Toxicol. 2016, 36, 352–360. [Google Scholar] [CrossRef]
- Freire, P.L.; Albuquerque, A.J.; Farias, I.A.; da Silva, T.G.; Aguiar, J.S.; Galembeck, A.; Flores, M.A.; Sampaio, F.C.; Stamford, T.C.M.; Rosenblatt, A. Antimicrobial and cytotoxicity evaluation of colloidal chitosan–silver nanoparticles–fluoride nanocomposites. Int. J. Biol. Macromol. 2016, 93, 896–903. [Google Scholar] [CrossRef]
- Wahab, S.; Ali, H.M.; Khan, M.; Khan, T.; Krishnaraj, C.; Yun, S.I. Green synthesis and antibacterial assessment of chitosan/silver nanocomposite conjugated with tobramycin against antibiotic resistant Pseudomonas aeruginosa. Arab. J. Ch. 2024, 17, 105458. [Google Scholar] [CrossRef]
- Potara, M.; Jakab, E.; Damert, A.; Popescu, O.; Canpean, V.; Astilean, S. Synergistic antibacterial activity of chitosan–silver nanocomposites on Staphylococcus aureus. Nanotechnology 2011, 22, 135101. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tondervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Inter. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, P.; Gupta, M.S.; Jayakumar, R.; Gowda, D.V. Prospection of chitosan and its derivatives in wound healing: Proof of patent analysis (2010–2020). Int. J. Biol. Macromol. 2021, 184, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Wang, L.; Wang, Y.; Zhang, C.; Wang, C.; Yan, Y.; Fan, J.; Xu, G.; Zhang, Q. Amplification of oxidative stress via intracellular ROS production and antioxidant consumption by two natural drug-encapsulated nanoagents for efficient anticancer therapy. Nanoscale Adv. 2020, 2, 3872–3881. [Google Scholar] [CrossRef]
- Formentini, L.; Santacatterina, F.; de Arenas, C.N.; Stamatakis, K.; López-Martínez, D.; Logan, A.; Fresno, M.; Smits, R.; Murphy, M.P.; Cuezva, J.M. Mitochondrial ROS production protects the intestine from inflammation through functional M2 macrophage polarization. Cell Rep. 2017, 19, 1202–1213. [Google Scholar] [CrossRef]
- Lin, W.; Qi, X.; Guo, W. A barrier against reactive oxygen species: Chitosan/acellular dermal matrix scaffold enhances stem cell retention and improves cutaneous wound healing. Stem Cell Res. Ther. 2020, 11, 383. [Google Scholar] [CrossRef]
- Tamer, T.; Aacute, K.; Mohyeldin, M.; Soltes, L. Free radical scavenger activity of chitosan and its aminated derivative. J. Appl. Pharm. Sci. 2016, 6, 195–201. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Biochemical significance of exogenous chitins, chitosan in animals, patients. Carbohydr. Polym. 2019, 93, 7–16. [Google Scholar] [CrossRef]
- Hattori, H.; Ishihara, M. Changes in blood aggregation with differences in molecular weight and degree of deacetylation of chitosan. Biomed. Mater. 2015, 10, 015014. [Google Scholar] [CrossRef]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Vanti, G.L.; Kurjogi, M.; Basavesha, K.; Teradal, N.L.; Masaphy, S.; Nargund, V.B. Synthesis and antibacterial activity of solanum torvum mediated silver nanoparticle against Xxanthomonasaxonopodis pv. Punicae and Ralstonia solanacearum. J. Biotech. 2020, 309, 20–28. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanti, G.; Poondla, N.; Manogaran, P.; Teradal, N.; S, V.; Kaulgud, R.; Kurjogi, M. Synthesis and Characterization of Multifunctional Chitosan–Silver Nanoparticles: An In-Vitro Approach for Biomedical Applications. Pharmaceuticals 2024, 17, 1229. https://doi.org/10.3390/ph17091229
Vanti G, Poondla N, Manogaran P, Teradal N, S V, Kaulgud R, Kurjogi M. Synthesis and Characterization of Multifunctional Chitosan–Silver Nanoparticles: An In-Vitro Approach for Biomedical Applications. Pharmaceuticals. 2024; 17(9):1229. https://doi.org/10.3390/ph17091229
Chicago/Turabian StyleVanti, Gulamnabi, Naresh Poondla, Prasath Manogaran, Nagappa Teradal, Veeresh S, Ram Kaulgud, and Mahantesh Kurjogi. 2024. "Synthesis and Characterization of Multifunctional Chitosan–Silver Nanoparticles: An In-Vitro Approach for Biomedical Applications" Pharmaceuticals 17, no. 9: 1229. https://doi.org/10.3390/ph17091229
APA StyleVanti, G., Poondla, N., Manogaran, P., Teradal, N., S, V., Kaulgud, R., & Kurjogi, M. (2024). Synthesis and Characterization of Multifunctional Chitosan–Silver Nanoparticles: An In-Vitro Approach for Biomedical Applications. Pharmaceuticals, 17(9), 1229. https://doi.org/10.3390/ph17091229





