Current Utilization and Research Status of Herbal Medicine Sipjeondaebotang for Anemia: A Scoping Review
Abstract
1. Introduction
2. Results
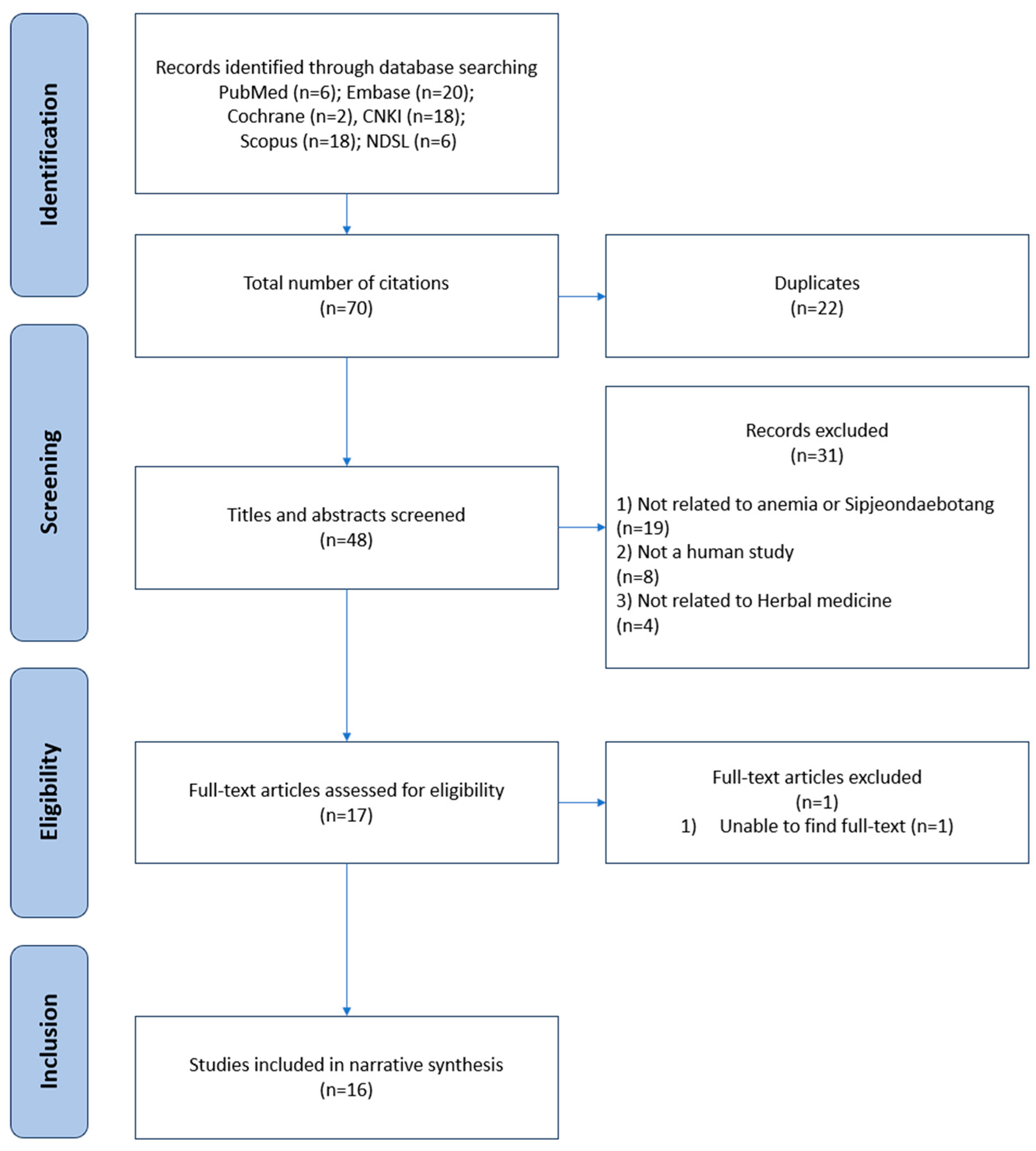
2.1. Literature Search and Selection Process
2.2. General Characteristics of the Identified Literature
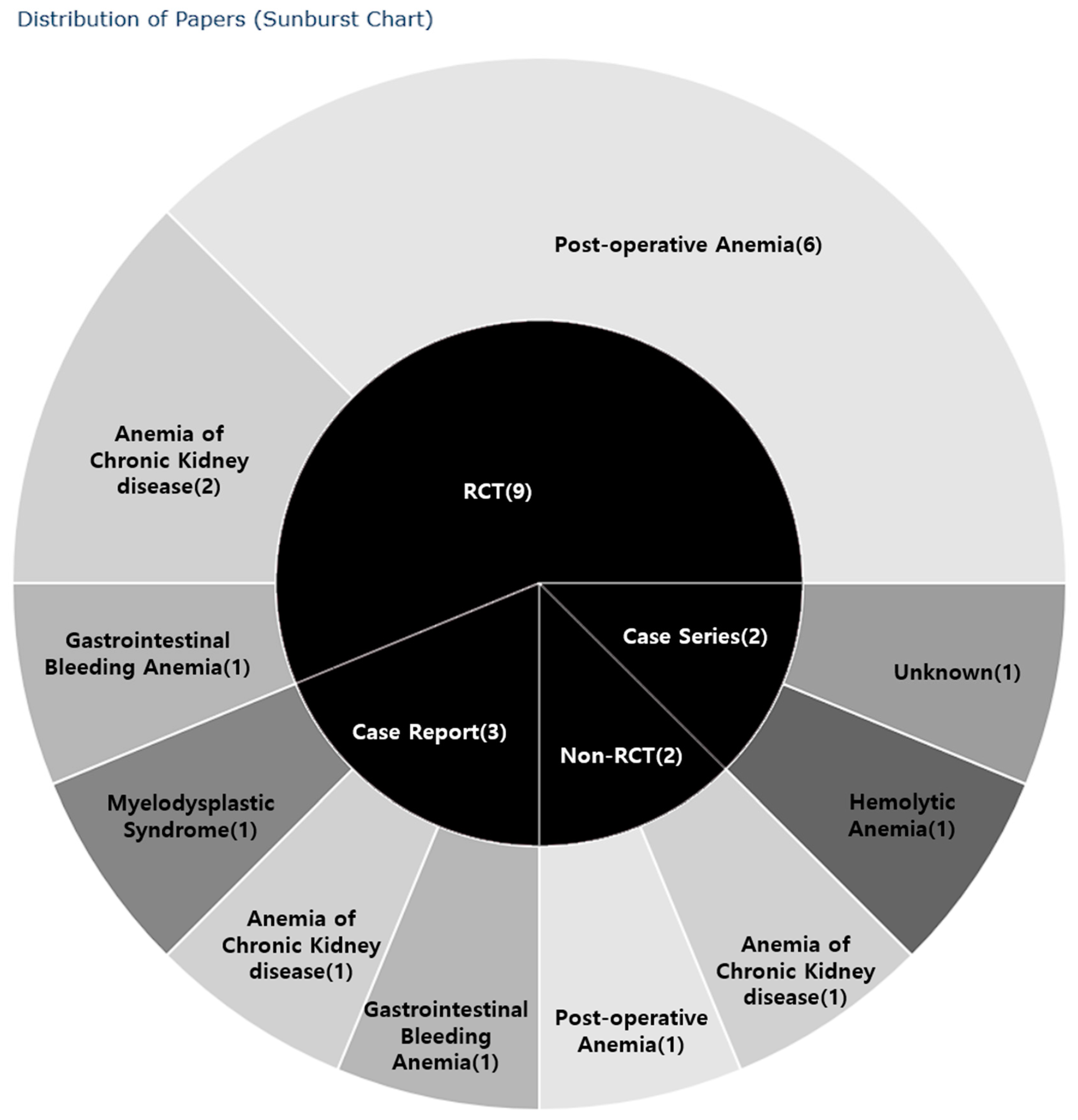
2.3. Study Designs
2.4. Research Regions
2.5. Demographic Characteristics of Study Participants
2.5.1. Participant Number, Sex, and Age
2.5.2. Types of Anemia
2.5.3. Types of Treatment and Medication
2.6. Details in Intervention
2.6.1. Dosage, Frequency, and Treatment Period of Herbal Medicine
2.6.2. Composition of SDT and Herbal Medicines Included in Its Variants
2.7. Evaluation Methods
Evaluation Tools
2.8. Treatment Outcomes
2.9. Safety
3. Discussion
3.1. Research Status
3.2. Types of Anemia
3.3. Significance of SDT Treatment
3.4. Proposed Treatment Mechanisms for SDT in Anemia
3.5. Limitations of This Study and Suggestions for Further Studies
4. Materials and Methods
4.1. Identifying the Research Questions
4.2. Literature Search
4.3. Literature Selection
4.4. Data Extraction and Schematization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Key Words Used in Searches
- (Anemia[MeSH Terms])
- (((((((((Sipjeondaebo-tang[Title/Abstract]) OR (sipjeondaebotang[Title/Abstract])) OR (sibjeondaebo-tang[Title/Abstract])) OR (sibjeondaebo-tang[Title/Abstract])) OR (Juzentaiho-to[Title/Abstract])) OR (Juzen-taiho-to[Title/Abstract])) OR (Juzentaihoto[Title/Abstract])) OR (shiquandabutang[Title/Abstract])) OR (shi-quan-da-bu-tang[Title/Abstract])) OR (shiquandabu-tang[Title/Abstract])4 OR 5
- #1 AND #2
- ‘anemia’/exp OR anemia
- ‘sipjeondaebo tang’:ab,ti OR ‘sipjeondaebotang:ab,ti OR ‘sibjeondaebo tang’:ab,ti OR ‘juzentaiho to’:ab,ti OR ‘juzen taiho to’:ab,ti OR juzentaihoto:ab,ti OR shiquandabutang:ab,ti OR ‘Shi quan da bu tang’:ab,ti
- #1 AND #2
- MeSH descriptor: [Anemia] explode all trees
- Sipjeondaebo-tang OR Sipjeondaebotang OR sibjeondaebo-tang OR Juzentaiho-to OR Juzen-taiho-to OR Juzentaihoto OR Shiquandabutang OR Shi-quan-da-bu-tang
- #1 AND #2
- TKA = Anemia OR TKA = 貧血
- TI = ‘Juzentaiho-to’ OR TI = ‘Juzen-taiho-to’ OR TI = ‘Juzentaihoto’ OR TI = Shiquandabutang OR TI = ‘Shi-quan-da-bu-tang’ OR TI = ‘十全大補湯’ OR TI = ‘十全大补汤’
- #1 AND #2
- TITLE-ABS-KEY (“anemia”)
- INDEXTERMS (“anemia”)
- #1 OR #2
- INDEXTERMS (“Sipjeondaebo-tang” OR “sipjeondaebotang” OR “sibjeondaebo-tang” OR “sibjeondaebo-tang” OR “Juzentaiho-to” OR “Juzen-taiho-to” OR “Juzentaihoto” OR “shiquandabutang” OR “shi-quan-da-bu-tang”)
- TITLE-ABS-KEY (“Sipjeondaebo-tang” OR “sipjeondaebotang” OR “sibjeondaebo-tang” OR “sibjeondaebo-tang” OR “Juzentaiho-to” OR “Juzen-taiho-to” OR “Juzentaihoto” OR “shiquandabutang” OR “shi-quan-da-bu-tang”)
- #4 OR #5
- #3 AND #6
References
- Shander, A.; Goodnough, L.T.; Javidroozi, M.; Auerbach, M.; Carson, J.; Ershler, W.B.; Ghiglione, M.; Glaspy, J.; Lew, I. Iron Deficiency Anemia-Bridging the Knowledge and Practice Gap. Transfus. Med. Rev. 2014, 28, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.C.A.; Lam, M.J.; Wong, H.C.A.; Hon Kam, L.; Li, X. Iron Deficiency Anemia: An Updated Review. Curr. Pediatr. Rev. 2024, 20, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Motta, I. Anemia in Clinical Practice—Definition and Classification: Does Hemoglobin Change with Aging? Semin. Hematol. 2015, 52, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Pasaribu, R.D.; Aritonang, E.; Sudaryati, E.; Zuska, F. Anemia in Pregnancy: Study Phenomenology. Port. J. Public Health 2023, 42, 6–14. [Google Scholar] [CrossRef]
- Stauder, R.; Thein, S.L. Anemia in the elderly: Clinical implications and new therapeutic concepts. Haematologica 2014, 99, 1127–1130. [Google Scholar] [CrossRef]
- Savarese, G.; von Haehling, S.; Butler, J.; Cleland, J.G.F.; Ponikowski, P.; Anker, S.D. Iron deficiency and cardiovascular disease. Eur. Heart J. 2023, 44, 14–27. [Google Scholar] [CrossRef]
- Chamoli, S.C. Clinical Evaluation of Different Types of Anemia. World J. Anemia 2018, 2, 26–30. [Google Scholar] [CrossRef]
- Koh, J.S.; Song, I.-C. Pharmacotherapeutics for iron deficiency anemia in adults. J. Korean Med. Assoc. 2024, 67, 48–53. [Google Scholar] [CrossRef]
- Jo, D.Y. Current Clinical Practice: Treatment of pernicious anemia. Korean J. Med. 2006, 71, 237. [Google Scholar]
- Oak, C.Y.; Kim, N.H. Anemia and nutrition in end stage renal disease patient. J. Korean Med. Assoc. 2013, 56, 592–599. [Google Scholar] [CrossRef][Green Version]
- Fujimoto, D.; Adachi, M.; Miyasato, Y.; Hata, Y.; Inoue, H.; Oda, A.; Kakizoe, Y.; Nakagawa, T.; Shimasaki, A.; Nakamura, K.; et al. Efficacy of continuous erythropoietin receptor activator for end-stage renal disease patients with renal anemia before and after peritoneal dialysis initiation. Clin. Exp. Nephrol. 2021, 25, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, H.; Li, Q.; Dong, W.; Wang, S.; Wang, A.; Wang, X.; Gu, Y. Shengxuebao Mixture for Iron Deficiency Anemia: A Meta-Analysis and Systematic Review. Complement. Med. Res. 2022, 29, 249–256. [Google Scholar] [CrossRef]
- Ding, L.; Xu, L.; Jin, Y.; Wei, Y.; Pan, Y.; Sattar, S.; Tan, Y.; Yang, T.; Zhou, F. Efficacy of SXN in the Treatment of Iron Deficiency Anemia: A Phase IV Clinical Trial. Evid.-Based Complement. Altern. Med. 2019, 2019, 8796234. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Liu, X.; Wang, X.; Li, M.; Jiang, Y.; Wang, X.; Yang, Z. Comparative effectiveness and safety of traditional Chinese medicine supporting Qi and enriching blood for cancer related anemia in patients not receiving chemoradiotherapy: A meta-analysis and systematic review. Drug Des. Dev. Ther. 2019, 13, 221–230. [Google Scholar] [CrossRef]
- Kim, M.; Han, C.-H. Effects of Bojungikgi-tang and its modifications for Anemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Korean Med. 2023, 44, 181–200. [Google Scholar] [CrossRef]
- Chiu, M.L.; Hsu, Y.L.; Chen, C.J.; Li, T.M.; Chiou, J.S.; Tsai, F.J.; Lin, T.H.; Liao, C.C.; Huang, S.M.; Chou, C.H.; et al. Chinese Herbal Medicine Therapy Reduces the Risks of Overall and Anemia-Related Mortalities in Patients with Aplastic Anemia: A Nationwide Retrospective Study in Taiwan. Front. Pharmacol. 2021, 12, 730776. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Kim, J.H.; Lee, S.H.; Bang, M.; Chang, G.T. Safety and efficacy of East Asian herbal medicine for iron deficiency anemia in children and adolescents: A systematic review and meta-analysis. Front. Pharmacol. 2024, 15, 1339486. [Google Scholar] [CrossRef]
- Chen, Y.; Ai, J.; Yang, J.; Qi, X.; Zhang, Z. Shiquan Dabu Decoction Combined with Iron Sucrose in Treatment of Perioperative Anemia in Total Knee Arthroplasty. Acta Chin. Med. 2024, 39, 1098–1103. [Google Scholar]
- Ai, J.; Han, Y.; Yao, P.; Zhang, Z.; Zhang, D.; Cao, Y.; Wang, S.; Liu, R. The Effects of Shiquandabu-tang on Postoperative Anemia in Elderly Patients with Intertrochanteric Femur Fractures. Tradit. Chin. Med. Res. 2022, 35, 54–58. [Google Scholar]
- Wang, C.; Yuan, A.; Liu, S. Forty-two cases of moderate-to-severe anemia treated with Shiquandabu-tang. J. Med. Theory Pract. 1999, 286–287. [Google Scholar]
- Sho, Y.; Fujisaki, K.; Sakashita, H.; Yamaguchi, K.; Tahara, K.; Kubozono, O.; Ido, A.; Tsubouchi, H. Orally administered Kampo medicine, Juzen-taiho-to, ameliorates anemia during interferon plus ribavirin therapy in patients with chronic hepatitis C. J. Gastroenterol. 2004, 39, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, H.; Mimura, T.; Honda, N. Orally administrated Juzen-taiho-to/TJ-48 ameliorates erythropoietin (rHuEPO)-resistant anemia in patients on hemodialysis. Hemodial. Int. 2008, 12, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Kishida, Y.; Nishii, T.; Inoue, T.; Nishida, S.; Arimitsu, J.; Yoshikawa, H.; Sugano, N. Juzentaihoto (TJ-48), a traditional Japanese herbal medicine, influences hemoglobin recovery during preoperative autologous blood donation and after hip surgery. Int. J. Clin. Pharmacol. Ther. 2009, 47, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Kainuma, M.; Mukae, H.; Furusyo, N.; Unno, M.; Hayashi, J. A Case of Anemia and Thrombocytopenia with Myelodysplastic Syndrome Successfully Treated with Juzentaihoto and Malt Sugar. Jpn. J. Orient. Med. 2011, 62, 363–368. [Google Scholar]
- Yao, S.; Wang, Z.; Ji, H. Clinical observation on treating renal anemia with the Shiquan Dabu decoction plus erythropoietin. Clin. J. Chin. Med. 2012, 4, 2. [Google Scholar]
- Yao, Z. The Effect of Jiawei Shiquandabu Decoction Combined with Erythropoietin in the Treatment of Renal Anemia. Mod. Diagn. Treat. 2016, 27, 1202–1204. [Google Scholar]
- Sheng, L.; Zhou, H.; Fang, Y.; Peng, L.; Dong, G. Effect of Prescription Named “Jiawei Shiquan Dabu Tang” on Hidden Blood Loss After Total Hip Arthroplasty Surgery. Chin. J. Mod. Appl. Pharm. 2017, 34, 420–422. [Google Scholar]
- Li, X. Shiquan Dabu Decoction combined with Western Medicine in the Treatment of Anemia in Late stage of Acute Upper Gastrointestinal Bleeding(Qixue Liangxu) Randomized Parallel Control Study. J. Pract. Tradit. Chin. Intern. Med. 2018, 32, 46–49. [Google Scholar]
- Fang, Y.; Zhou, H. Analysis of Shiquan Dabu decoction in treating recessive blood loss after joint replacement. China Mod. Dr. 2018, 56, 131–134. [Google Scholar]
- Kim, S.-H.; Kim, J.-H.; Lee, H.-K.; Cho, K.-H.; Mun, S.-K.; Jung, W.-S.; Kwon, S.-W. Case of Anemia with Chronic Kidney Disease Using Shipjeondaebo-tang. J. Intern. Korean Med. 2019, 40, 173–180. [Google Scholar] [CrossRef]
- Kyong-lim, K.; Seung-eun, L.; Kyoung-min, K. A Case Report of Elderly Patients with Anemia that Improved with Shipjeondaebo-tang-gami. J. Intern. Korean Med. 2020, 41, 508–514. [Google Scholar]
- Ling, J.; Li, J.; Li, C.; Huang, G. Effects of Jiawei Shiquan Dabu decoction on recessive blood loss and blood glucose levels after operation in the elderly patients with femoral intertrochanteric fracture. Shaanxi J. Tradit. Chin. Med. 2020, 41, 1254–1257. [Google Scholar]
- Tian, H. Clinical Study of Shiquandabu Decoction Combined with Tranexamic Acid on Reducing Perioperative Blood Loss (Syndrome of Qi-Blood Deficiency) in Total Hip Arthroplasty. Master’s Thesis, Henan University of Chinese Medicine, Zhengzhou, China, 2020. [Google Scholar]
- Wei, D. New Advances in Hidden Blood Loss After Hip Replacement Surgery. Shenzhen J. Integr. Tradit. Chin. West. Med. 2017, 27, 193–194. [Google Scholar]
- Lo, B.D.; Qayum, O.; Penberthy, K.K.; Gyi, R.; Lester, L.C.; Hensley, N.B.; Sciubba, D.M.; Frank, S.M.; Cho, B.C. Dose-dependent effects of red blood cell transfusion and case mix index on venous thromboembolic events in spine surgery. Vox Sang. 2023, 118, 76–83. [Google Scholar] [CrossRef]
- Shi, Y.-P.; Zhu, B.-D.; Huang, Q.; Zheng, T.-F.; Zhao, J.-H. Effect of Shi-Quan-Da-Bu-Granule on routine blood and erythropoietin on myelosuppressed mice. Lab. Med. Clin. 2009, 6, 1221–1223. [Google Scholar]
- Hisha, H.; Yamada, H.; Sakurai, M.H.; Kiyohara, H.; Li, Y.; Yu, C.; Takemoto, N.; Kawamura, H.; Yamaura, K.; Shinohara, S.; et al. Isolation and identification of hematopoietic stem cell-stimulating substances from Kampo (Japanese herbal) medicine, Juzen-taiho-to. Blood 1997, 90, 1022–1030. [Google Scholar] [CrossRef]
- Tian, Y.; Xiang, Y.; Wan, G.; Wan, D.; Zhu, H.; Wang, T.; Yang, X. Effects and mechanisms of Bazhen decoction, Siwu decoction, and Sijunzi decoction on 5-fluorouracil-induced anemia in mice. J. Tradit. Chin. Med. 2016, 36, 486–495. [Google Scholar] [CrossRef][Green Version]
- Lee, H.W.; Kim, H.; Ryuk, J.A.; Kil, K.J.; Ko, B.S. Hemopoietic effect of extracts from constituent herbal medicines of Samul-tang on phenylhydrazine-induced hemolytic anemia in rats. Int. J. Clin. Exp. Pathol. 2014, 7, 6179–6185. [Google Scholar] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). NICE Process and Methods Guides. In Methods for the Development of NICE Public Health Guidance. Available online: https://www.nice.org.uk/process/pmg4/ (accessed on 8 September 2024).
| Herbal Medicine | Scientific Name (Family) |
|---|---|
| Ginseng Radix | Panax ginseng C. A. Meyer (Araliaceae) |
| Astragali Radix | Astragalus membranaceus Bunge (Fabaceae) |
| Hoelen alba | Poria cocos Wolf (Polyporaceae) |
| Atractylodis Rhizoma Alba | Atractylodes japonica Koidzumi (Asteraceae) |
| Glycyrrhizae Radix | Glycyrrhiza uralensis Fischer (Fabaceae) |
| Rehmanniae Radix preparate * | Rehmannia glutinosa (Liboschitz ex Steudel) (Plantaginaceae) |
| Angelicae gigantis Radix | Angelica gigas Nakai (Apiaceae) |
| Cnidii Rhizoma | Cnidium officinale Makino (Apiaceae) |
| Paeoniae Radix alba | Paeonia lactiflora Pallas (Paeoniaceae) |
| Cinnamomi Cortex | Cinnamomum cassia Presl (Lauraceae) |
| Variables | Categories | N (%) |
|---|---|---|
| Publication year | 1990–1999 | 1 (6.25%) |
| 2000–2009 | 3 (18.75%) | |
| 2010–2024 | 12 (75%) | |
| Research Methodology | Case series | 2 (12.5%) |
| Case report | 3 (18.75%) | |
| Randomized controlled trial | 9 (56.25) | |
| Non-randomized controlled trial | 2 (12.5%) | |
| Location | China | 10 (62.5%) |
| South Korea | 2 (12.5%) | |
| Japan | 4 (25%) |
| Location/ Design | Sample Size (Male/Female) | Age (Treatment/Control) | Types of Anemia | Author (Year) |
|---|---|---|---|---|
| South Korea/ Case report | 1(0/1) | 76 | Gastrointestinal bleeding anemia | Kim (2020) [31] |
| South Korea/ Case report | 1(1/0) | 47 | CKD anemia | Kim (2019) [30] |
| China/RCT | 54 | Not mentioned | CKD anemia | Yao (2012) [25] |
| China/RCT | 120(70/50) | 40.3 ± 2.8 | CKD anemia | Yao (2016) [26] |
| China/RCT | 60(31/29) | (64.21 ± 2.29 /66.21 ± 1.86) | Post-operative anemia (knee joint replacement) | Fang (2018) [29] |
| China/RCT | 120(73/47) | 71.47 ± 4.66 | Post-operative anemia (proximal femur nail antirotation) | Li (2020) [32] |
| China/RCT | 100(57/43) | (48.46 ± 3.66 /48.14 ± 3.27) | Gastrointestinal bleeding anemia | Li (2018) [28] |
| China/ Case series | 42(23/19) | 18~67 | Unknown (no mention) | Wang (1999) [20] |
| China/RCT | 45(22/23) | (66.32 ± 8.09 /63.57 ± 6.32) | Post-operative anemia (total hip arthroplasty) | Tian (2020) [33] |
| China/RCT | 40(13/27) | 65~82 (68.7 ± 5.33 /70.7 ± 4.68) | Post-operative anemia (total hip arthroplasty) | Sheng (2017) [27] |
| China/RCT | 80(34/46) | 75~95(83.6 ± 7.2) /76~93(82.4 ± 5.8) | Post-operative anemia (proximal femur nail antirotation) | Ai (2022) [19] |
| Japan/ Case report | 1(0/1) | 76 | Myelodysplastic syndrome | Beinu (2011) [24] |
| China/RCT | 72(26/46) | (67.36 ± 6.38 /67.89 ± 7.47) | Post-operative anemia (total knee arthroplasty) | Chen (2024) [18] |
| Japan/ Controlled clinical trial | 18(0/18) | (49.4 ± 9.4 /52.1 ± 3.1) | Post-operative anemia (total hip arthroplasty or rotational acetabular osteotomy) | Kishida (2009) [23] |
| Japan/ Controlled clinical trial | 42(33/9) | (67 ± 12/62 ± 12) | CKD anemia | Nakamoto (2008) [22] |
| Japan/ Case series | 67(42/25) | (54.8 ± 9.0/54.6 ± 7.8) | Hemolytic anemia | Sho (2004) [21] |
| Intervention | Other Treatment | Other Medications | Outcomes | Significant Findings | Adverse Events | References |
|---|---|---|---|---|---|---|
| SDT variants 1 | Acupuncture | Beta-blocker Vasodilator Analgesics Antiemetic Iron supplements Statin Proton pump inhibitor | Hb RBC WBC Hct Intensity of dizziness (VAS) | The major blood parameters returned to normal ranges Hb 8.8 g/dL → 11.8 g/dL RBC 2.68 × 106/μL → 3.86 × 106/μL WBC 2.21 × 103/μL → 3.16 × 103/μL Hct 26% → 35.9% The patient’s subjective symptom of dizziness VAS 5 → 1 | Not mentioned | Kim (2020) [31] |
| SDT | Acupuncture | Antacid Amino acid rHuEPO | Hb Fatigue severity scale(FSS) | The patient’s hemoglobin levels improved after taking the medicine Hb 9.6 g/dL → 12.7 g/dL The patient’s fatigue severity scale (FSS) was improved 35 → 15 | Not mentioned | Kim (2019) [30] |
| SDT variants 2 | (-) | rHuEPO Iron Folic acid Vitamin B12 | Hb Hct | The patient’s hemoglobin and hematocrit level of the treatment group were significantly increased Treatment group: Hb 64.68 ± 9.11 → 105.82 ± 13.66 Hct 20.87 ± 2.18 → 32.51 ± 3.11 Control group: Hb 64.42 ± 7.50 → 95.23 ± 12.50 Hct 20.47 ± 2.23 → 28.57 ± 2.68 | Not mentioned | Yao (2012) [25] |
| SDT variants 3 | (-) | rHuEPO Iron Folic acid Vitamin B12 | Treatment effective rate Hb Hct | Total effective rate of treatment group was significantly higher and the patient’s hemoglobin and hematocrit level of the treatment group were significantly increased Treatment group: Total effective rate Hb 62.46 ± 12.64 → 106.21 ± 11.61 Hct 21.52 ± 3.20 → 31.51 ± 4.21 Control group Total effective rate 81.67 Hb 63.13 ± 14.01 → 87.07 ± 12.21 Hct 20.17 ± 3.27 → 25.81 ± 4.67 | Not mentioned | Yao (2016) [26] |
| SDT | Knee joint replacement | Tranexamic Acid Sodium Chloride Morphine Epinephrine Ropivacaine | Amount of bleeding and drainage Hb Knee resting pain score(VAS) | The Hb in the treatment group was significantly improved compared to the control group The knee resting pain score (VAS) of treatment group was significantly lower than that of control group Treatment group: The patient satisfaction of treatment group was significantly higher than that of control group Treatment group: Hb 104.36 ± 8.83 → 117.72 ± 6.07 VAS 6.31 ± 0.52 Satisfaction 96.0 Control group: Hb 107.83 ± 9.08 → 109.62 ± 5.38 VAS 8.02 ± 0.81 Satisfaction 82.0 | Not mentioned | Fang (2018) [29] |
| SDT variants 4 | Proximal femur nail antirotation | Not mentioned | Hb Hct Hidden blood loss Fasting blood glucose level Hospitalization and fracture healing times Hip joint function recovery rates | The Hb, Hct was significantly improved and hidden blood loss was reduced, and hospitalization and fracture healing time were shorter than control group. Treatment group Hb 122.85 ± 15.24 → 100.85 ± 13.28 Hct 33.65 ± 5.41 → 27.96 ± 3.25 Hidden blood loss 245.74 ± 88.26 → 188.26 ± 70.12 Hospitalization time 8.72 ± 2.37 Fracture healing time 12.65 ± 1.34 Control group Hb 120.96 ± 16.31 → 90.41 ± 11.27 Hct 32.98 ± 6.13 → 25.47 ± 4.52 Hidden blood loss 288.67 ± 90.94 → 245.94 ± 75.36 Hospitalization time 15.12 ± 3.41 Fracture healing time 15.27 ± 5.78 | Not mentioned | Li (2020) [32] |
| SDT | No treatment | Erythropoietin Vitamin B12 Folic acid Somatostatin Pantoprazole sodium | Hb Reticulocytes Platelets White blood cells | The Hb was significantly improved and reticulocytes, platelets, white blood cell counts were significantly increased. Treatment group Hb 42.86 ± 5.41 → 95.47 ± 6.98 Reticulocytes 0.95 ± 0.25 → 2.48 ± 1.63 Platelets: 28.95 ± 5.42 → 59.74 ± 9.85 White blood cells: 2.44 ± 0.52 → 4.85 ± 1.6 | Total adverse event rate Treatment group: 4.00% Control group: 34.00% | Li (2018) [28] |
| SDT | No treatment | Iron Folic acid Vitamin B12 | Hb | The total effect rates were 95.2% | Not mentioned | Wang (1999) [20] |
| SDT | Total hip arthroplasty | Tranexamic acid | RBC Hb Hct D-dimer Total blood loss Patient blood volume Dominant blood loss Hidden blood loss Transfusion rate and average transfusion volume Harris hip score Traditional Chinese medicine (TCM) Syndrome score and efficacy | The treatment group’s RBC, Hb, Hct were significantly improved and D-dimer, total blood loss, hidden blood loss were significantly lower than control group. The Harris hip score and TCM syndrome efficacy were significantly higher in treatment group. | Total adverse event rate Treatment group: 13.64% Control group: 30.43% | Tian (2020) [33] |
| SDT variants 5 | Total hip arthroplasty | Not mentioned | Hb Hct Total blood loss Intra-operative blood loss Post-operative drainage Hidden blood loss Transfusion rate | The treatment group’s Hb and Hct were significantly improved and total, post-operative drainage, hidden blood loss were significantly reduced. Transfusion rate was also lower in treatment group. Treatment group Hb 131.5 ± 15.1 → 93.5 ± 17.9 Hct 0.42 ± 0.05 → 0.29 ± 0.05 Post-operative drainage 456.2 ± 147.3 Hidden blood loss 796.7 ± 354.6 Transfusion rate 15% Control group Hb 130.5 ± 13.9 → 83.5 ± 9.6 Hct 0.41 ± 0.06 → 0.24 ± 0.04 Post-operative drainage 612.4 ± 161.5 Hidden blood loss 1064.6 ± 335.7 Transfusion rate 35% | No adverse effect | Sheng (2017) [27] |
| SDT | Proximal femur nail antirotation | Antibiotics Ribaroxaban Albumin | Hb Serum albumin Transfusion rate Transfusion volume Serum albumin infusion rate Serum albumin infusion volume | The treatment group’s Hb level was significant increased and transfusion rates and volume were significantly lower than control group. Treatment group Hb 115.24± 6.13 → 80.45 ± 9.48 Transfusion rate 20.0% Transfusion volume 1.94 ± 0.44 Control group Hb 116.43 ± 3.64 → 71.81 ± 12.43 Transfusion rate 40.0% Transfusion volume 3.86 ± 2.62 | Total adverse event rate Treatment group: 30% Control group: 47.5% | Ai (2022) [19] |
| ① SDT ② SDT variants 6 ③ SDT variants 7 | Transfusion | Vitamin K2 Hwangigunjung-tang Samul-tang | WBC RBC Hb Platelet | After adding malt sugar to Sipjeondaebotang, hemoglobin and platelet levels significantly increased. Bone marrow findings showed a reduction in erythroblasts. | No adverse effect | Beinu (2011) [24] |
| SDT | Total knee arthroplasty | Iron sucrose injection | Hb RBC Hct Platelet Interleukin-6 Tumor necrosis factor-alpha C-reactive protein Hospital for special surgery knee score(HSS) Visual analogue scale (VAS) Perioperative blood loss and transfusion rate Post-operative complication rate | The effective rate of the treatment group and the HSS score were significantly higher than that of the control group, and the levels of Hb, RBC, Hct were significantly increased. The levels of protein and CRP and the VAS score, and the perioperative blood transfusion rate were significantly lower than that in the control group. Treatment group Effective rate 91.7% Hb 130.44 ± 7.13 → 121.39 ± 6.05 RBC 4.30 ± 0.25 → 3.96 ± 0.19 Hct 39.88 ± 3.30 IL-6 97.52 ± 5.89 → 31.80 ± 2.58 TNF-a 17.51 ± 2.18 → 7.16 ± 1.11 CRP 26.87 ± 3.38 → 11.70 ± 1.87 HSS score 61.92 ± 2.87 → 85.86 ± 2.65 VAS score 6.87 ± 1.10 → 1.17 ± 0.43 Perioperative transfusion rate 8.33% Control group Hb 130.44 ± 7.13 → 121.39 ± 6.05 RBC 4.30 ± 0.25 → 3.96 ± 0.19 Hct 39.88 ± 3.30 IL-6 98.22 ± 6.08 → 34.52 ± 2.79 TNF-a 17.32 ± 2.39 → 8.31 ± 1.30 CRP 26.34 ± 5.53 → 13.37 ± 2.11 Perioperative transfusion rate 27.78% HSS score 61.53 ± 3.08 → 84.67 ± 2.08 VAS score 6.81 ± 1.12 → 1.93 ± 0.47 | Total adverse event rate Treatment group: 5.56% Control group: 27.78% | Chen (2024) [18] |
| SDT | Total hip arthroplasty or rotational acetabular osteotomy Autologous donation | Not mentioned | Hb blood loss post-operative recovery rate laboratory tests (serum creatinine, AST, ALT, Na, K) | The Hb levels were significantly improved during the preoperative period. At the last autologous donation, the levels of Hb were higher in treatment group than control group. Treatment group Reduction of Hb at last autologous donation 0.7 g/dL Control group Reduction of Hb at last autologous donation 1.5 g/dL | No adverse effect | Kishida (2009) [23] |
| SDT | Not mentioned | Erythropoietin | Hb CRP | The Hb levels of treatment group were significantly increased, and the CRP levels were significantly decreased compared to the control group. Treatment group Hb 8.4 ± 1.10 → 9.5 ± 1.3 CRP 1.4 ± 1.7 → 0.6 ± 0.8 Control group Hb 8.3 ± 0.7 → 8.5 ± 0.5 CRP did not significantly change | No adverse effect in treatment group | Nakamoto (2008) [22] |
| SDT | Not mentioned | Interferon plus ribavirin(IFN/Rib) | Hb ALT | The Hb levels of treatment group showed significant reduction in HB decrease, and requirement for ribavirin dose reduction or withdrawal were significantly lower than that of the control group. Treatment group Requirement for ribavirin dose reduction/withdrawal 13% (4/32) Control group Requirement for ribavirin dose reduction/withdrawal 13% (4/32) | Not mentioned | Sho (2004) [21] |
| Intervention | Composition | Dosage | Frequency | Treatment Duration | References |
|---|---|---|---|---|---|
| SDT variants 1 | Panax ginseng C. A. Meyer (Araliaceae) 6 g, Astragalus membranaceus Bunge (Fabaceae) 3 g, Wolfiporia extensa (Peck) Ginns (as Poria cocos Wolf) (Polyporaceae) 6 g, Atractylodes japonica Koidzumi (Asteraceae) 6 g, Glycyrrhiza uralensis Fischer (Fabaceae) 6 g, Rehmannia glutinosa (Liboschitz ex Steudel) (Plantaginaceae) 3 g, Angelica gigas Nakai (Apiaceae) 3 g, Cnidium officinale Makino (Apiaceae) 3 g, Paeonia lactiflora Pallas (Paeoniaceae) 3 g, Cinnamomum cassia Presl (Lauraceae) 3 g, Zingiber officinale Roscoe (Zingiberaceae) 6 g, Ziziphus jujuba Mill. (Rhamnaceae) 4 g, Pinellia ternata (Thunb.) Makino ex Breitenbach (Araceae) 4 g, Citrus reticulata Blanco (Rutaceae) 4 g. | 2 doses per day | Three times per day p.o | 41 days | Kim (2020) [31] |
| SDT | P. ginseng 6 g, A. membranaceus 6 g, W. extensa 5 g, A. japonica 5 g, G. uralensis 5 g, R. glutinosa 5 g, A. gigas 5 g, C. officinale 5 g, P. lactiflora 5 g, C. cassia 6 g, Z. jujuba 6 g, Z. officinale 6 g | 2 doses per day | Three times per day p.o | 16 days | Kim (2019) [30] |
| SDT variants 2 | P. ginseng 9 g, A. membranaceus 30 g, W. extensa 12 g, A. japonica 10 g, G. uralensis 6 g, R. glutinosa 20 g, A. gigas 15 g, C. officinale 9 g, P. lactiflora 10 g, C. cassia 5 g, Rheum palmatum L. (Polygonaceae) 9 g. | 1 dose per day | Two times per day p.o | 3 months | Yao (2012) [25] |
| SDT variants 3 | A. membranaceus 30 g, W. extensa 12 g, A. japonica 10 g, G. uralensis 6 g, R. glutinosa 20 g, A. gigas 15 g, C. officinale 9 g, P. lactiflora 10 g, C. cassia 5 g, R. palmatum 9 g. | 1 dose per day | Two times per day p.o | 3 months | Yao (2016) [26] |
| SDT | A. membranaceus 50 g, W. extensa 10 g, A. japonica 13 g, G. uralensis 6 g, A. gigas 10 g, C. officinale 8 g, P. lactiflora 13 g, Equus asinus L. (Equidae) (as Asini Corri Colla) 10 g. | 1 dose per day | Two times per day p.o | 1 day | Fang (2018) [29] |
| SDT variants 4 | A. membranaceus 15 g, A. japonica 15 g, C. officinale 10 g, R. glutinosa 10 g, A. gigas 10 g, W. extensa 10 g, P. lactiflora 10 g, C. cassia 3 g, G. uralensis 3 g, Codonopsis pilosula (Franch.) Nannf. (Campanulaceae) 15 g. | 1 dose per day | Two times per day p.o | 3 days | Li (2020) [32] |
| SDT | P. ginseng 6 g, A. membranaceus 12 g, W. extensa 9 g, A. japonica 9 g, G. uralensis 3 g, R. glutinosa 12 g, A. gigas 9 g, C. officinale 6 g, P. lactiflora 9 g, C. cassia 3 g. | 1 dose per day | Two times per day p.o | 10 days | Li (2018) [28] |
| SDT | P. ginseng 6 g, A. membranaceus 20 g, W. extensa 15 g, A. japonica 20 g, G. uralensis 6 g, R. glutinosa 20 g, A. gigas 20 g, C. officinale 12 g, P. lactiflora 20 g, C. cassia 5 g, R. glutinosa 20 g, Leonurus japonicus (Lamiaceae) 15 g, Crataegus pinnatifida (Rosaceae) 10 g, Triticum aestivum L. 10 g, Hordeum vulgare (Poaceae) 10 g. | 1 dose per day | Not mentioned | 3 months~4 years (mean 18 months) | Wang (1999) [20] |
| SDT | A. membranaceus 12 g, W. extensa 9 g, A. japonica 9 g, G. uralensis 3 g, R. glutinosa 12 g, A. gigas 9 g, C. officinale 6 g, P. lactiflora 9 g, C. cassia 3 g, C. pilosula 9 g. | 1 dose per day | Two times per day p.o | 7 days | Tian (2020) [33] |
| SDT variants 5 | A. membranaceus 15 g, W. extensa 10 g, A. japonica 15 g, G. uralensis 3 g, R. glutinosa 15 g, A. gigas 10 g, C. officinale 10 g, P. lactiflora 10 g, C. cassia 3 g, C. pilosula 15 g, Selaginella doederleinii (Selaginellaceae) 15 g, Coix lacryma-jobi (Poaceae) 30 g, Salvia miltiorrhiza (Lamiaceae) 20 g, Polygonatum odoratum (Asparagaceae) 15 g, Polygonum multiflorum (Polygonaceae) 15 g, Ligustrum lucidum (Oleaceae) 10 g, Eucommia ulmoides (Eucommiaceae) 15 g. | 1 dose per day | Two times per day p.o | 14 days | Sheng (2017) [27] |
| SDT | A. membranaceus 20 g, W. extensa 15 g, A. japonica 20 g, G. uralensis 3 g, R. glutinosa 15 g, A. gigas 15 g, C. officinale 15 g, P. lactiflora 15 g, C. cassia 3 g, C. pilosula 20 g. | 1 dose per day | Two times per day p.o | 14 days | Ai (2022) [19] |
| ① SDT ② SDT variants 6 ③ SDT variants 7 | ①: P. ginseng 3 g, A. membranaceus 3 g, W. extensa 3 g, A. japonica 3 g, G. uralensis 1.5 g, R. glutinosa 3 g, A. gigas 5 g, C. officinale 3 g, P. lactiflora 3 g, C. cassia 3 g ②: in ①, R. glutinosa, C. officinale, P. lactiflora, A. gigas were changed to 6 g. ③: in ②, add Oryza sativa L. (Poaceae) 10 g | Not mentioned | Not mentioned | 31 months | Beinu (2011) [24] |
| SDT | P. ginseng 6 g, A. membranaceus 20 g, W. extensa 15 g, A. japonica 15 g, G. uralensis 6 g, R. glutinosa 15 g, A. gigas 15 g, C. officinale 6 g, P. lactiflora 6 g, C. cassia 6 g, Drynaria fortunei J.Smith (Polypodiaceae) 10 g, Pyritum 10 g | 1 dose per day | Two times per day p.o | 14 days | Chen (2024) [18] |
| SDT | P. ginseng 3 g, A. membranaceus 3 g, W. extensa 3 g, A. japonica 3 g, G. uralensis 1.5 g, R. glutinosa 3 g, A. gigas 5 g, C. officinale 3 g, P. lactiflora 3 g, C. cassia 3 g | 7.5 g per day | Not mentioned | 21 days | Kishida (2009) [23] |
| SDT | P. ginseng 3 g, A. membranaceus 3 g, W. extensa 3 g, A. japonica 3 g, G. uralensis 1.5 g, R. glutinosa 3 g, A. gigas 5 g, C. officinale 3 g, P. lactiflora 3 g, C. cassia 3 g | 7.5 g per day | Not mentioned | 12 weeks | Nakamoto (2008) [22] |
| SDT | P. ginseng 3 g, A. membranaceus 3 g, W. extensa 3 g, A. japonica 3 g, G. uralensis 1.5 g, R. glutinosa 3 g, A. gigas 5 g, C. officinale 3 g, P. lactiflora 3 g, C. cassia 3 g | 7.5 g per day | Not mentioned | 2 weeks | Sho (2004) [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Lee, H.-G.; Ha, W.J.; Kwon, S. Current Utilization and Research Status of Herbal Medicine Sipjeondaebotang for Anemia: A Scoping Review. Pharmaceuticals 2024, 17, 1192. https://doi.org/10.3390/ph17091192
Kim G, Lee H-G, Ha WJ, Kwon S. Current Utilization and Research Status of Herbal Medicine Sipjeondaebotang for Anemia: A Scoping Review. Pharmaceuticals. 2024; 17(9):1192. https://doi.org/10.3390/ph17091192
Chicago/Turabian StyleKim, Gyeongmuk, Han-Gyul Lee, Won Jung Ha, and Seungwon Kwon. 2024. "Current Utilization and Research Status of Herbal Medicine Sipjeondaebotang for Anemia: A Scoping Review" Pharmaceuticals 17, no. 9: 1192. https://doi.org/10.3390/ph17091192
APA StyleKim, G., Lee, H.-G., Ha, W. J., & Kwon, S. (2024). Current Utilization and Research Status of Herbal Medicine Sipjeondaebotang for Anemia: A Scoping Review. Pharmaceuticals, 17(9), 1192. https://doi.org/10.3390/ph17091192





