Quercetin Intake and Absolute Telomere Length in Patients with Type 2 Diabetes Mellitus: Novel Findings from a Randomized Controlled Before-and-After Study
Abstract
1. Introduction
2. Results
3. Discussion
Study Strengths and Limitations
4. Materials and Methods
4.1. Study Design, Participants, Phytonutrient
Sample Size Estimation
4.2. Telomere Length Evaluation
4.2.1. DNA Extraction
4.2.2. Absolute Quantification of Telomere Length
4.3. Statistical Analysis
4.4. Ethical Compliance and Approvals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gramatges, M.M.; Bertuch, A.A. Short Telomeres: From Dyskeratosis Congenita to Sporadic Aplastic Anemia and Malignancy. Transl. Res. 2013, 162, 353–363. [Google Scholar] [CrossRef]
- Dhillon, V.; Bull, C.; Fenech, M. Chapter 10—Telomeres, Aging, and Nutrition. In Molecular Basis of Nutrition and Aging; Malavolta, M., Mocchegiani, E., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 129–140. ISBN 978-0-12-801816-3. [Google Scholar]
- Maleki, M.; Khelghati, N.; Alemi, F.; Bazdar, M.; Asemi, Z.; Majidinia, M.; Sadeghpoor, A.; Mahmoodpoor, A.; Jadidi-Niaragh, F.; Targhazeh, N.; et al. Stabilization of Telomere by the Antioxidant Property of Polyphenols: Anti-Aging Potential. Life Sci. 2020, 259, 118341. [Google Scholar] [CrossRef]
- Ruiz, A.; Flores-Gonzalez, J.; Buendia-Roldan, I.; Chavez-Galan, L. Telomere Shortening and Its Association with Cell Dysfunction in Lung Diseases. Int. J. Mol. Sci. 2022, 23, 425. [Google Scholar] [CrossRef] [PubMed]
- Verdun, R.E.; Karlseder, J. Replication and Protection of Telomeres. Nature 2007, 447, 924–931. [Google Scholar] [CrossRef]
- Shammas, M.A. Telomeres, Lifestyle, Cancer, and Aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef]
- Oeseburg, H.; de Boer, R.A.; van Gilst, W.H.; van der Harst, P. Telomere Biology in Healthy Aging and Disease. Pflug. Arch. 2010, 459, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Eppard, M.; Passos, J.F.; Victorelli, S. Telomeres, Cellular Senescence, and Aging: Past and Future. Biogerontology 2024, 25, 329–339. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Daniali, L.; Benetos, A.; Susser, E.; Kark, J.D.; Labat, C.; Kimura, M.; Desai, K.; Granick, M.; Aviv, A. Telomeres Shorten at Equivalent Rates in Somatic Tissues of Adults. Nat. Commun. 2013, 4, 1597. [Google Scholar] [CrossRef] [PubMed]
- Fragkiadaki, P.; Renieri, E.; Kalliantasi, K.; Kouvidi, E.; Apalaki, E.; Vakonaki, E.; Mamoulakis, C.; Spandidos, D.A.; Tsatsakis, A. Τelomerase Inhibitors and Activators in Aging and Cancer: A Systematic Review. Mol. Med. Rep. 2022, 25, 158. [Google Scholar] [CrossRef] [PubMed]
- Kakridonis, F.; Pneumatikos, S.G.; Vakonaki, E.; Berdiaki, A.; Tzatzarakis, M.N.; Fragkiadaki, P.; Spandidos, D.A.; Baliou, S.; Ioannou, P.; Hatzidaki, E.; et al. Telomere Length as a Predictive Biomarker in Osteoporosis (Review). Biomed. Rep. 2023, 19, 87. [Google Scholar] [CrossRef]
- Fragkiadaki, P.; Nikitovic, D.; Kalliantasi, K.; Sarandi, E.; Thanasoula, M.; Stivaktakis, P.; Nepka, C.; Spandidos, D.; Theodoros, T.; Tsatsakis, A. Telomere Length and Telomerase Activity in Osteoporosis and Osteoarthritis (Review). Exp. Ther. Med. 2019, 19, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.P.; Zakharenko, A.M.; Golokhvast, K.S.; Thanasoula, M.; Sarandi, E.; Nikolouzakis, K.; Fragkiadaki, P.; Tsoukalas, D.; Spandidos, D.A.; Tsatsakis, A. Telomerase and Telomeres in Aging Theory and Chronographic Aging Theory (Review). Mol. Med. Rep. 2020, 22, 1679–1694. [Google Scholar] [CrossRef] [PubMed]
- Vakonaki, E.; Tsiminikaki, K.; Plaitis, S.; Fragkiadaki, P.; Tsoukalas, D.; Katsikantami, I.; Vaki, G.; Tzatzarakis, M.N.; Spandidos, D.A.; Tsatsakis, A.M. Common Mental Disorders and Association with Telomere Length. Biomed. Rep. 2018, 8, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Fragkiadaki, P.; Tsoukalas, D.; Fragkiadoulaki, I.; Psycharakis, C.; Nikitovic, D.; Spandidos, D.A.; Tsatsakis, A.M. Telomerase Activity in Pregnancy Complications (Review). Mol. Med. Rep. 2016, 14, 16–21. [Google Scholar] [CrossRef]
- Apetroaei, M.-M.; Fragkiadaki, P.; Velescu, B.Ș.; Baliou, S.; Renieri, E.; Dinu-Pirvu, C.E.; Drăgănescu, D.; Vlăsceanu, A.M.; Nedea, M.I.; Udeanu, D.I.; et al. Pharmacotherapeutic Considerations on Telomere Biology: The Positive Effect of Pharmacologically Active Substances on Telomere Length. Int. J. Mol. Sci. 2024, 25, 7694. [Google Scholar] [CrossRef]
- Bonfigli, A.R.; Spazzafumo, L.; Prattichizzo, F.; Bonafè, M.; Mensà, E.; Micolucci, L.; Giuliani, A.; Fabbietti, P.; Testa, R.; Boemi, M.; et al. Leukocyte Telomere Length and Mortality Risk in Patients with Type 2 Diabetes. Oncotarget 2016, 7, 50835–50844. [Google Scholar] [CrossRef]
- Palmer, A.K.; Tchkonia, T.; LeBrasseur, N.K.; Chini, E.N.; Xu, M.; Kirkland, J.L. Cellular Senescence in Type 2 Diabetes: A Therapeutic Opportunity. Diabetes 2015, 64, 2289–2298. [Google Scholar] [CrossRef]
- Mantadaki, A.E.; Linardakis, M.; Tsakiri, M.; Baliou, S.; Fragkiadaki, P.; Vakonaki, E.; Tzatzarakis, M.N.; Tsatsakis, A.; Symvoulakis, E.K. Benefits of Quercetin on Glycated Hemoglobin, Blood Pressure, PiKo-6 Readings, Night-Time Sleep, Anxiety, and Quality of Life in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 3504. [Google Scholar] [CrossRef]
- Palmer, A.K.; Gustafson, B.; Kirkland, J.L.; Smith, U. Cellular Senescence: At the Nexus between Ageing and Diabetes. Diabetologia 2019, 62, 1835–1841. [Google Scholar] [CrossRef]
- Narasimhan, A.; Flores, R.R.; Robbins, P.D.; Niedernhofer, L.J. Role of Cellular Senescence in Type II Diabetes. Endocrinology 2021, 162, bqab136. [Google Scholar] [CrossRef] [PubMed]
- Prattichizzo, F.; de Candia, P.; Ceriello, A. Diabetes and Kidney Disease: Emphasis on Treatment with SGLT-2 Inhibitors and GLP-1 Receptor Agonists. Metabolism 2021, 120, 154799. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Inagaki, N.; Kondoh, H. Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells. Front. Endocrinol. 2022, 13, 869414. [Google Scholar] [CrossRef]
- Iwasaki, K.; Abarca, C.; Aguayo-Mazzucato, C. Regulation of Cellular Senescence in Type 2 Diabetes Mellitus: From Mechanisms to Clinical Applications. Diabetes Metab. J. 2023, 47, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ojcius, D.M.; Wu, C.-Y.; Peng, H.-H.; Voisin, L.; Perfettini, J.-L.; Ko, Y.-F.; Young, J.D. Emerging Use of Senolytics and Senomorphics against Aging and Chronic Diseases. Med. Res. Rev. 2020, 40, 2114–2131. [Google Scholar] [CrossRef]
- Boccardi, V.; Paolisso, G. Telomerase Activation: A Potential Key Modulator for Human Healthspan and Longevity. Ageing Res. Rev. 2014, 15, 1–5. [Google Scholar] [CrossRef]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of Telomeres and Telomerase in Cancer, and Advances in Telomerase-Targeted Therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms Underlying the Activation of TERT Transcription and Telomerase Activity in Human Cancer: Old Actors and New Players. Oncogene 2019, 38, 6172–6183. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular Senescence: A Key Therapeutic Target in Aging and Diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A Flavonol with Multifaceted Therapeutic Applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Mbara, K.C.; Devnarain, N.; Owira, P.M.O. Potential Role of Polyphenolic Flavonoids as Senotherapeutic Agents in Degenerative Diseases and Geroprotection. Pharm. Med. 2022, 36, 331–352. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, B.; Shi, Y.; Xie, C.; Huang, C.; Chen, B.; Zhang, H.; Zeng, G.; Liang, H.; Wu, Y.; et al. Senolytic Agent Quercetin Ameliorates Intervertebral Disc Degeneration via the Nrf2/NF-κB Axis. Osteoarthr. Cartil. 2021, 29, 413–422. [Google Scholar] [CrossRef]
- Kim, S.R.; Jiang, K.; Ogrodnik, M.; Chen, X.; Zhu, X.-Y.; Lohmeier, H.; Ahmed, L.; Tang, H.; Tchkonia, T.; Hickson, L.J.; et al. Increased Renal Cellular Senescence in Murine High-Fat Diet: Effect of the Senolytic Drug Quercetin. Transl. Res. 2019, 213, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-C.; Tsai, T.-Y.; Wang, C.-J. The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. Int. J. Mol. Sci. 2023, 24, 6328. [Google Scholar] [CrossRef]
- Ioannou, P.; Baliou, S. The Molecular Mechanisms and Therapeutic Potential of Cranberry, D-Mannose, and Flavonoids against Infectious Diseases: The Example of Urinary Tract Infections. Antibiotics 2024, 13, 593. [Google Scholar] [CrossRef]
- Mantadaki, A.E.; Linardakis, M.; Vafeiadi, M.; Anastasiou, F.; Tsatsakis, A.; Symvoulakis, E.K. The Impact of Three-Month Quercetin Intake on Quality of Life and Anxiety in Patients with Type II Diabetes Mellitus: An Early Data Analysis from a Randomized Controlled Trial. Cureus 2024, 16, e58219. [Google Scholar] [CrossRef] [PubMed]
- Shatylo, V.; Antoniuk-Shcheglova, I.; Naskalova, S.; Bondarenko, O.; Havalko, A.; Krasnienkov, D.; Zabuga, O.; Kukharskyy, V.; Guryanov, V.; Vaiserman, A. Cardio-Metabolic Benefits of Quercetin in Elderly Patients with Metabolic Syndrome. PharmaNutrition 2021, 15, 100250. [Google Scholar] [CrossRef]
- O’Callaghan, N.J.; Fenech, M. A Quantitative PCR Method for Measuring Absolute Telomere Length. Biol. Proced. Online 2011, 13, 3. [Google Scholar] [CrossRef]
- Lai, T.-P.; Wright, W.E.; Shay, J.W. Comparison of Telomere Length Measurement Methods. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160451. [Google Scholar] [CrossRef]
- O’Callaghan, N.J.; Dhillon, V.S.; Thomas, P.; Fenech, M. A Quantitative Real-Time PCR Method for Absolute Telomere Length. BioTechniques 2008, 44, 807–809. [Google Scholar] [CrossRef]
- Kahl, V.F.S.; Allen, J.A.M.; Nelson, C.B.; Sobinoff, A.P.; Lee, M.; Kilo, T.; Vasireddy, R.S.; Pickett, H.A. Telomere Length Measurement by Molecular Combing. Front. Cell Dev. Biol. 2020, 8, 493. [Google Scholar] [CrossRef] [PubMed]
- Lindrose, A.R.; McLester-Davis, L.W.Y.; Tristano, R.I.; Kataria, L.; Gadalla, S.M.; Eisenberg, D.T.A.; Verhulst, S.; Drury, S. Method Comparison Studies of Telomere Length Measurement Using qPCR Approaches: A Critical Appraisal of the Literature. PLoS ONE 2021, 16, e0245582. [Google Scholar] [CrossRef] [PubMed]
- ISRCTN—ISRCTN13131584: Study on the Benefit of Quercetin Intake in Diabetic Patients Treated with Antidiabetic Tablets. Available online: https://www.isrctn.com/ISRCTN13131584 (accessed on 14 July 2024).
- Behrens, Y.L.; Thomay, K.; Hagedorn, M.; Ebersold, J.; Henrich, L.; Nustede, R.; Schlegelberger, B.; Göhring, G. Comparison of Different Methods for Telomere Length Measurement in Whole Blood and Blood Cell Subsets: Recommendations for Telomere Length Measurement in Hematological Diseases. Genes Chromosomes Cancer 2017, 56, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Tilburt, J.; DeVries, A.; Muralidhar, B.; Aue, G.; Hedges, L.; Atkinson, J.; Schwartz, H. Comparison of Chromosome Telomere Integrity in Multiple Tissues from Subjects at Different Ages. Cancer Genet. Cytogenet. 1998, 105, 138–144. [Google Scholar] [CrossRef]
- Arai, Y.; Martin-Ruiz, C.M.; Takayama, M.; Abe, Y.; Takebayashi, T.; Koyasu, S.; Suematsu, M.; Hirose, N.; von Zglinicki, T. Inflammation, But Not Telomere Length, Predicts Successful Ageing at Extreme Old Age: A Longitudinal Study of Semi-Supercentenarians. EBioMedicine 2015, 2, 1549–1558. [Google Scholar] [CrossRef]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2021, 11, 630186. [Google Scholar] [CrossRef]
- Shin, D.-Y.; Lim, K.M.; Park, H.S.; Kwon, S.; Yoon, S.-S.; Lee, D.-S. The Importance of Critically Short Telomere in Myelodysplastic Syndrome. Biomark. Res. 2022, 10, 79. [Google Scholar] [CrossRef]
- Whittemore, K.; Vera, E.; Martínez-Nevado, E.; Sanpera, C.; Blasco, M.A. Telomere Shortening Rate Predicts Species Life Span. Proc. Natl. Acad. Sci. USA 2019, 116, 15122–15127. [Google Scholar] [CrossRef]
- Yan, X.; Yang, P.; Li, Y.; Liu, T.; Zha, Y.; Wang, T.; Zhang, J.; Feng, Z.; Li, M. New Insights from Bidirectional Mendelian Randomization: Causal Relationships between Telomere Length and Mitochondrial DNA Copy Number in Aging Biomarkers. Aging 2024, 16, 7387. [Google Scholar] [CrossRef]
- Cheng, F.; Carroll, L.; Joglekar, M.V.; Januszewski, A.S.; Wong, K.K.; Hardikar, A.A.; Jenkins, A.J.; Ma, R.C.W. Diabetes, Metabolic Disease, and Telomere Length. Lancet Diabetes Endocrinol. 2021, 9, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Luk, A.O.; Shi, M.; Huang, C.; Jiang, G.; Yang, A.; Wu, H.; Lim, C.K.P.; Tam, C.H.T.; Fan, B.; et al. Shortened Leukocyte Telomere Length Is Associated With Glycemic Progression in Type 2 Diabetes: A Prospective and Mendelian Randomization Analysis. Diabetes Care 2022, 45, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Salpea, K.D.; Humphries, S.E. Telomere Length in Atherosclerosis and Diabetes. Atherosclerosis 2010, 209, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics Decrease Senescent Cells in Humans: Preliminary Report from a Clinical Trial of Dasatinib plus Quercetin in Individuals with Diabetic Kidney Disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ Heel of Senescent Cells: From Transcriptome to Senolytic Drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Molinuevo, J.-L.; Sala-Vila, A. Plant-Rich Dietary Patterns, Plant Foods and Nutrients, and Telomere Length. Adv. Nutr. 2019, 10, S296–S303. [Google Scholar] [CrossRef]
- Rahman, S.T.; Waterhouse, M.; Pham, H.; Romero, B.D.; Baxter, C.; McLeod, D.S.A.; English, D.R.; Ebeling, P.R.; Hartel, G.; Armstrong, B.K.; et al. Effects of Vitamin D Supplementation on Telomere Length: An Analysis of Data from the Randomised Controlled D-Health Trial. J. Nutr. Health Aging 2023, 27, 609–616. Available online: https://link.springer.com/article/10.1007/s12603-023-1948-3 (accessed on 18 July 2024). [CrossRef]
- Kiecolt-Glaser, J.K.; Epel, E.S.; Belury, M.A.; Andridge, R.; Lin, J.; Glaser, R.; Malarkey, W.B.; Hwang, B.S.; Blackburn, E. Omega-3 Fatty Acids, Oxidative Stress, and Leukocyte Telomere Length: A Randomized Controlled Trial. Brain Behav. Immun. 2013, 28, 16–24. [Google Scholar] [CrossRef]
- O’Callaghan, N.; Parletta, N.; Milte, C.M.; Benassi-Evans, B.; Fenech, M.; Howe, P.R.C. Telomere Shortening in Elderly Individuals with Mild Cognitive Impairment May Be Attenuated with ω-3 Fatty Acid Supplementation: A Randomized Controlled Pilot Study. Nutrition 2014, 30, 489–491. [Google Scholar] [CrossRef]
- Freitas-Simoes, T.-M.; Cofán, M.; Blasco, M.A.; Soberón, N.; Foronda, M.; Serra-Mir, M.; Roth, I.; Valls-Pedret, C.; Doménech, M.; Ponferrada-Ariza, E.; et al. Walnut Consumption for Two Years and Leukocyte Telomere Attrition in Mediterranean Elders: Results of a Randomized Controlled Trial. Nutrients 2018, 10, 1907. [Google Scholar] [CrossRef]
- Pusceddu, I.; Herrmann, M.; Kirsch, S.H.; Werner, C.; Hübner, U.; Bodis, M.; Laufs, U.; Widmann, T.; Wagenpfeil, S.; Geisel, J.; et al. One-Carbon Metabolites and Telomere Length in a Prospective and Randomized Study of B- and/or D-Vitamin Supplementation. Eur. J. Nutr. 2017, 56, 1887–1898. [Google Scholar] [CrossRef]
- Exploring the Effects of Dasatinib, Quercetin, and Fisetin on DNA Methylation Clocks: A Longitudinal Study on Senolytic Interventions—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10929829/ (accessed on 20 July 2024).
- Hachmo, Y.; Hadanny, A.; Hamed, R.A.; Daniel-Kotovsky, M.; Catalogna, M.; Fishlev, G.; Lang, E.; Polak, N.; Doenyas, K.; Friedman, M.; et al. Hyperbaric Oxygen Therapy Increases Telomere Length and Decreases Immunosenescence in Isolated Blood Cells: A Prospective Trial. Aging 2020, 12, 22445–22456. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.E.; Esquivel, S.; Goldberg, A.; Seeman, T.E.; Effros, R.B.; Dock, J.; Olmstead, R.; Breen, E.C.; Irwin, M.R. Insomnia and Telomere Length in Older Adults. Sleep 2016, 39, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Tempaku, P.; Hirotsu, C.; Mazzotti, D.; Xavier, G.; Maurya, P.; Brietzke, E.; Belangero, S.; Poyares, D.; Bittencourt, L.; Tufik, S. Long Sleep Duration, Insomnia, and Insomnia with Short Objective Sleep Duration Are Independently Associated With Short Telomere Length. J. Clin. Sleep Med. 2018, 14, 2037–2045. [Google Scholar] [CrossRef]
- Jin, J.-H.; Kwon, H.S.; Choi, S.H.; Koh, S.-H.; Lee, E.-H.; Jeong, J.H.; Jang, J.-W.; Park, K.W.; Kim, E.-J.; Kim, H.J.; et al. Association between Sleep Parameters and Longitudinal Shortening of Telomere Length. Aging 2022, 14, 2930–2944. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Chang, Y.K.; Lee, D.H.; Ko, J.-H.; Lim, I.; Bang, H.; Kim, J.-H. Gender-Specific Associations between Quality of Life and Leukocyte Telomere Length. Maturitas 2018, 107, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Huzen, J.; van der Harst, P.; de Boer, R.A.; Lesman-Leegte, I.; Voors, A.A.; van Gilst, W.H.; Samani, N.J.; Jaarsma, T.; van Veldhuisen, D.J. Telomere Length and Psychological Well-Being in Patients with Chronic Heart Failure. Age Ageing 2010, 39, 223–227. [Google Scholar] [CrossRef]
- Tsur, N.; Levin, Y.; Abumock, H.; Solomon, Z. One ‘Knows’: Self-Rated Health and Telomere Length among Ex-Prisoners of War. Psychol. Health 2018, 33, 1503–1518. [Google Scholar] [CrossRef]
- Tellechea, M.L.; Pirola, C.J. The Impact of Hypertension on Leukocyte Telomere Length: A Systematic Review and Meta-Analysis of Human Studies. J. Hum. Hypertens. 2017, 31, 99–105. [Google Scholar] [CrossRef]
- Lung, F.-W.; Ku, C.-S.; Kao, W.-T. Telomere Length May Be Associated with Hypertension. J. Hum. Hypertens. 2008, 22, 230–232. [Google Scholar] [CrossRef]
- Monickaraj, F.; Aravind, S.; Gokulakrishnan, K.; Sathishkumar, C.; Prabu, P.; Prabu, D.; Mohan, V.; Balasubramanyam, M. Accelerated Aging as Evidenced by Increased Telomere Shortening and Mitochondrial DNA Depletion in Patients with Type 2 Diabetes. Mol. Cell. Biochem. 2012, 365, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Rodriguez, A.; Morell-Azanza, L.; Alonso-Pedrero, L.; del Moral, A.M. Chapter 12—Aging, Telomere Integrity, and Antioxidant Food. In Obesity; del Moral, A.M., Aguilera García, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 241–261. ISBN 978-0-12-812504-5. [Google Scholar] [CrossRef]
- Prasad, K.N.; Wu, M.; Bondy, S.C. Telomere Shortening during Aging: Attenuation by Antioxidants and Anti-Inflammatory Agents. Mech. Ageing Dev. 2017, 164, 61–66. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S. Diet and Aging: The Role of Polyphenol-Rich Diets in Slow Down the Shortening of Telomeres: A Review. Antioxidants 2023, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- Heger, V.; Tyni, J.; Hunyadi, A.; Horáková, L.; Lahtela-Kakkonen, M.; Rahnasto-Rilla, M. Quercetin Based Derivatives as Sirtuin Inhibitors. Biomed. Pharmacother. 2019, 111, 1326–1333. [Google Scholar] [CrossRef]
- Ungurianu, A.; Zanfirescu, A.; Margină, D. Exploring the Therapeutic Potential of Quercetin: A Focus on Its Sirtuin-Mediated Benefits. Phytother. Res. 2024, 38, 2361–2387. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic Application of Quercetin in Aging-Related Diseases: SIRT1 as a Potential Mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef] [PubMed]
- Rahnasto-Rilla, M.; Tyni, J.; Huovinen, M.; Jarho, E.; Kulikowicz, T.; Ravichandran, S.; Bohr, V.A.; Ferrucci, L.; Lahtela-Kakkonen, M.; Moaddel, R. Natural Polyphenols as Sirtuin 6 Modulators. Sci. Rep. 2018, 8, 4163. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Peng, M.; Zhang, Y.; Xu, W.; Darko, K.O.; Tao, T.; Huang, Y.; Tao, X.; Yang, X. Quercetin Induces Bladder Cancer Cells Apoptosis by Activation of AMPK Signaling Pathway. Am. J. Cancer Res. 2016, 6, 498–508. [Google Scholar]
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin Induces Pro-Apoptotic Autophagy via SIRT1/AMPK Signaling Pathway in Human Lung Cancer Cell Lines A549 and H1299 In Vitro. Thorac. Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef]
- Shen, Y.; Croft, K.D.; Hodgson, J.M.; Kyle, R.; Lee, I.-L.E.; Wang, Y.; Stocker, R.; Ward, N.C. Quercetin and Its Metabolites Improve Vessel Function by Inducing eNOS Activity via Phosphorylation of AMPK. Biochem. Pharmacol. 2012, 84, 1036–1044. [Google Scholar] [CrossRef]
- Jacczak, B.; Rubiś, B.; Totoń, E. Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging. Int. J. Mol. Sci. 2021, 22, 6381. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.C.; Nabavi, S.M.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int. J. Mol. Sci. 2018, 19, 13. [Google Scholar] [CrossRef]
- Parekh, N.; Garg, A.; Choudhary, R.; Gupta, M.; Kaur, G.; Ramniwas, S.; Shahwan, M.; Tuli, H.S.; Sethi, G. The Role of Natural Flavonoids as Telomerase Inhibitors in Suppressing Cancer Growth. Pharmaceuticals 2023, 16, 605. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wu, X.; Wang, Z.; Guo, Y.; Xu, R. Quercetin Regulates Telomere-Binding Proteins Expression of POT1, TRF1, TRF2 TO Inhibit Proliferation and Induce Apoptosis in AML THP-1 Cells. Available online: https://library.ehaweb.org/eha/2017/22nd/182385/ruirong.xu.quercetin.regulates.telomere-binding.proteins.expression.of.pot1.html (accessed on 22 July 2024).
- Avci, C.B.; Yilmaz, S.; Dogan, Z.O.; Saydam, G.; Dodurga, Y.; Ekiz, H.A.; Kartal, M.; Sahin, F.; Baran, Y.; Gunduz, C. Quercetin-Induced Apoptosis Involves Increased hTERT Enzyme Activity of Leukemic Cells. Hematology 2011, 16, 303–307. [Google Scholar] [CrossRef]
- Bhatiya, M.; Pathak, S.; Jothimani, G.; Duttaroy, A.K.; Banerjee, A. A Comprehensive Study on the Anti-Cancer Effects of Quercetin and Its Epigenetic Modifications in Arresting Progression of Colon Cancer Cell Proliferation. Arch. Immunol. Ther. Exp. 2023, 71, 6. [Google Scholar] [CrossRef]
- Zhu, J.; Cheng, X.; Naumovski, N.; Hu, L.; Wang, K. Epigenetic Regulation by Quercetin: A Comprehensive Review Focused on Its Biological Mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 1–20. [Google Scholar] [CrossRef]
- Canela, A.; Vera, E.; Klatt, P.; Blasco, M.A. High-Throughput Telomere Length Quantification by FISH and Its Application to Human Population Studies. Proc. Natl. Acad. Sci. USA 2007, 104, 5300–5305. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Tsoukalas, D.; Fragkiadaki, P.; Vakonaki, E.; Tzatzarakis, M.; Sarandi, E.; Nikitovic, D.; Tsilimidos, G.; Alegakis, A.K. Developing BIOTEL: A Semi-Automated Spreadsheet for Estimating Telomere Length and Biological Age. Front. Genet. 2019, 10, 84. [Google Scholar] [CrossRef]
- Dweck, A.; Maitra, R. The Advancement of Telomere Quantification Methods. Mol. Biol. Rep. 2021, 48, 5621–5627. [Google Scholar] [CrossRef]
- Vera, E.; Blasco, M.A. Beyond Average: Potential for Measurement of Short Telomeres. Aging 2012, 4, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Joksic, G.; Joksic, I.; Filipović, J.; Liehr, T. Telomere Length Measurement by FISH. In Fluorescence In Situ Hybridization (FISH): Application Guide; Liehr, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 147–152. ISBN 978-3-662-52959-1. [Google Scholar]
- Heritier, S.R.; Gebski, V.J.; Keech, A.C. Inclusion of Patients in Clinical Trial Analysis: The Intention-to-Treat Principle. Med. J. Aust. 2003, 179, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Matacchione, G.; Perugini, J.; Di Mercurio, E.; Sabbatinelli, J.; Prattichizzo, F.; Senzacqua, M.; Storci, G.; Dani, C.; Lezoche, G.; Guerrieri, M.; et al. Senescent Macrophages in the Human Adipose Tissue as a Source of Inflammaging. Geroscience 2022, 44, 1941–1960. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R.; Malarkey, W.B.; Hwang, B.S.; Glaser, R. Omega-3 Supplementation Lowers Inflammation in Healthy Middle-Aged and Older Adults: A Randomized Controlled Trial. Brain Behav. Immun. 2012, 26, 988–995. [Google Scholar] [CrossRef]
- Harrigan, M.; Cartmel, B.; Loftfield, E.; Sanft, T.; Chagpar, A.B.; Zhou, Y.; Playdon, M.; Li, F.; Irwin, M.L. Randomized Trial Comparing Telephone Versus In-Person Weight Loss Counseling on Body Composition and Circulating Biomarkers in Women Treated for Breast Cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. J. Clin. Oncol. 2016, 34, 669–676. [Google Scholar] [CrossRef]
- Sanft, T.; Usiskin, I.; Harrigan, M.; Cartmel, B.; Lu, L.; Li, F.-Y.; Zhou, Y.; Chagpar, A.; Ferrucci, L.M.; Pusztai, L.; et al. Randomized Controlled Trial of Weight Loss versus Usual Care on Telomere Length in Women with Breast Cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. Breast Cancer Res. Treat. 2018, 172, 105–112. [Google Scholar] [CrossRef]
- Opstad, T.B.; Alexander, J.; Aaseth, J.O.; Larsson, A.; Seljeflot, I.; Alehagen, U. Selenium and Coenzyme Q10 Intervention Prevents Telomere Attrition, with Association to Reduced Cardiovascular Mortality—Sub-Study of a Randomized Clinical Trial. Nutrients 2022, 14, 3346. [Google Scholar] [CrossRef]
- Ward, S.J.; Hill, A.M.; Buckley, J.D.; Banks, S.; Dhillon, V.S.; Holman, S.L.; Morrison, J.L.; Coates, A.M. Minimal Changes in Telomere Length after a 12-Week Dietary Intervention with Almonds in Mid-Age to Older, Overweight and Obese Australians: Results of a Randomised Clinical Trial. Br. J. Nutr. 2022, 127, 872–884. [Google Scholar] [CrossRef]
- Denham, J.; O’Brien, B.J.; Prestes, P.R.; Brown, N.J.; Charchar, F.J. Increased Expression of Telomere-Regulating Genes in Endurance Athletes with Long Leukocyte Telomeres. J. Appl. Physiol. 2016, 120, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Manchia, M.; Paribello, P.; Arzedi, C.; Bocchetta, A.; Caria, P.; Cocco, C.; Congiu, D.; Cossu, E.; Dettori, T.; Frau, D.V.; et al. A Multidisciplinary Approach to Mental Illness: Do Inflammation, Telomere Length and Microbiota Form a Loop? A Protocol for a Cross-Sectional Study on the Complex Relationship between Inflammation, Telomere Length, Gut Microbiota and Psychiatric Disorders. BMJ Open 2020, 10, e032513. [Google Scholar] [CrossRef]
- Franzoni, L.T.; Garcia, E.L.; Motta, S.B.; Ahner, M.M.; Bertoletti, O.A.; Saffi, M.A.L.; da Silveira, A.D.; Pereira, A.A.; Pereira, A.H.; Danzmann, L.C.; et al. Aerobic Exercise and Telomere Length in Patients with Systolic Heart Failure: Protocol Study for a Randomized Controlled Trial. Trials 2022, 23, 283. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, D.; Fragkiadaki, P.; Docea, A.O.; Alegakis, A.K.; Sarandi, E.; Vakonaki, E.; Salataj, E.; Kouvidi, E.; Nikitovic, D.; Kovatsi, L.; et al. Association of Nutraceutical Supplements with Longer Telomere Length. Int. J. Mol. Med. 2019, 44, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Renieri, E.; Tsoukalas, D.; Buga, A.M.; Sarandi, E.; Vakonaki, E.; Fragkiadaki, P.; Alegakis, A.; Nikitovic, D.; Calina, D.; et al. A Novel Nutraceutical Formulation Increases Telomere Length and Activates Telomerase Activity in Middle-aged Rats. Mol. Med. Rep. 2023, 28, 1–11. [Google Scholar] [CrossRef]
- Chung, S.S.; Dutta, P.; Chard, N.; Wu, Y.; Chen, Q.-H.; Chen, G.; Vadgama, J. A Novel Curcumin Analog Inhibits Canonical and Non-Canonical Functions of Telomerase through STAT3 and NF-κB Inactivation in Colorectal Cancer Cells. Oncotarget 2019, 10, 4516–4531. [Google Scholar] [CrossRef]
- Precision in qPCR—GR. Available online: https://www.thermofisher.com/tr/en/home/life-science/pcr/real-time-pcr/real-time-pcr-learning-center/gene-expression-analysis-real-time-pcr-information/precision-qpcr.html (accessed on 20 July 2024).
- Taylor, S.; Wakem, M.; Dijkman, G.; Alsarraj, M.; Nguyen, M. A Practical Approach to RT-qPCR—Publishing Data That Conform to the MIQE Guidelines. Methods 2010, 50, S1–S5. [Google Scholar] [CrossRef]
- Kitchen, R.R.; Kubista, M.; Tichopad, A. Statistical Aspects of Quantitative Real-Time PCR Experiment Design. Methods 2010, 50, 231–236. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Linardakis, M.; Papadaki, A.; Smpokos, E.; Kafatos, A.; Lionis, C. Prevalence of Multiple Behavioral Risk Factors for Chronic Diseases in Medical Students and Associations with Their Academic Performance. J. Public Health 2020, 28, 383–392. [Google Scholar] [CrossRef]
- Linardakis, M.; Papadaki, A.; Smpokos, E.; Micheli, K.; Vozikaki, M.; Philalithis, A. Relationship of Behavioral Risk Factors for Chronic Diseases and Preventive Health Services Utilization among Adults, Aged 50+, from Eleven European Countries. J. Public Health 2015, 23, 257–265. [Google Scholar] [CrossRef]
- Symvoulakis, E.K.; Stachteas, P.; Smyrnakis, E.; Volkos, P.; Mantadaki, A.E.; Karelis, A.; Petraki, C.; Nioti, K.; Mastronikolis, S.; Antoniou, A.M.; et al. Multiple Behavioral Risk Factors As Assets for Chronic Disease Prevention: Observations From Urban Primary Care Settings in Crete, Greece. Cureus 2024, 16, e56711. [Google Scholar] [CrossRef]
- Nagelkerke, N.J.D. A Note on a General Definition of the Coefficient of Determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- ICH Harmonised Guideline Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice ICH E6(R2) ICH Consensus Guideline. Available online: https://ichgcp.net/home (accessed on 25 April 2024).
- European Medicines Agency Guideline for Good Clinical Practice E6 (R2). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-good-clinical-practice-e6r2-4-step-2b_en.pdf (accessed on 22 March 2024).
- European Parliament and the Council of the European Union Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on Clinical Trials on Medicinal Products for Human Use, and Repealing Directive 2001/20/EC. Available online: https://health.ec.europa.eu/document/download/f724d198-9ec8-4cad-9ce7-b6d2ac1ec44e_en (accessed on 22 March 2024).
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- World Health Organization. Handbook for Good Clinical Research Practice (GCP): Guidance for Implementation; World Health Organization: Geneva, Switzerland, 2005; Available online: https://iris.who.int/bitstream/handle/10665/43392/924159392X_eng.pdf (accessed on 14 July 2024).
- International Council For Harmonisation (ICH). Harmonised Guideline on Genomic Sampling and Management of Genomic Data E18. Available online: https://database.ich.org/sites/default/files/E18_Guideline.pdf (accessed on 14 July 2024).
- European Medicines Agency (EMA). ICH Guideline E18 on Genomic Sampling and Management of Genomic Data. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-e18-genomic-sampling-and-management-genomic-data-step-3_en.pdf (accessed on 14 July 2024).
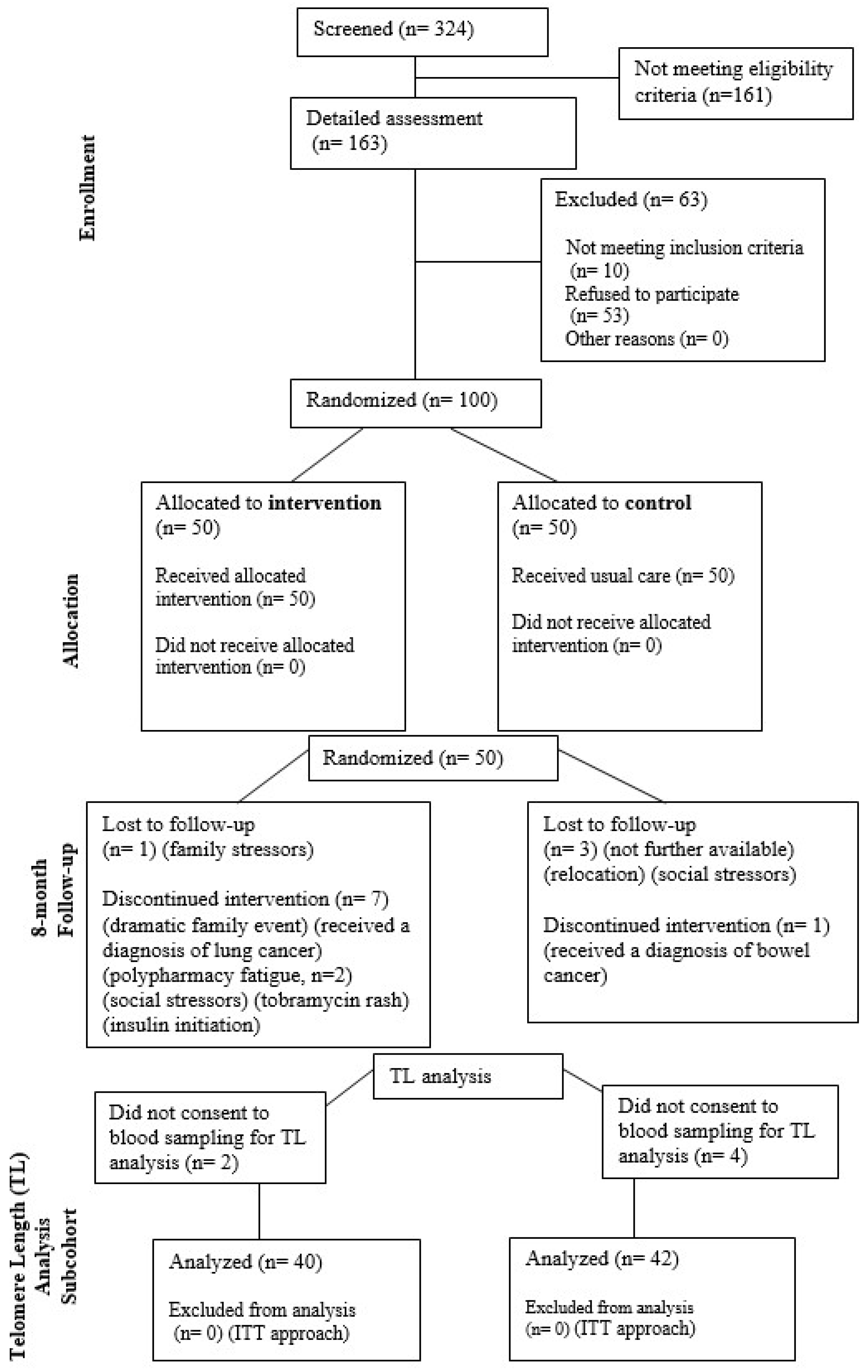
| Groups | |||
|---|---|---|---|
| Intervention (n = 40) | Control (n = 42) | ||
| n (%) | |||
| Sex | male | 22 (55.0) | 23 (54.8) |
| female | 18 (45.0) | 19 (45.2) | |
| Age, years | mean ± stand. dev. | 66.3 ± 7.4 | 67.8 ± 6.2 |
| Nationality | Greek | 36 (90.0) | 42 (100.0) |
| Lifestyle Risk Factors | smoking | 13 (32.5) | 15 (35.7) |
| alcohol consumption | 28 (70.0) | 28 (66.7) | |
| high body weight | 35 (87.5) | 39 (92.9) | |
| physical inactivity | 16 (40.0) | 21 (50.0) | |
| not consuming fruits | 2 (5.0) | 2 (4.8) | |
| 3+ factors | 16 (40.0) | 23 (54.8) | |
| Multimorbidity | 3+ chronic conditions | 32 (80.0) | 28 (66.7) |
| Polypharmacy | 4+ medications | 28 (70.0) | 29 (69.0) |
| Years diagnosed with T2DM | mean ± stand. dev. | 11.0 ± 7.1 | 10.3 ± 6.6 |
| Groups | |||||
|---|---|---|---|---|---|
| Intervention | Control | Cohen’s d Effect Size | |||
| Mean ± Stand. Dev. | p-Value | ||||
| Night-time sleep (hours) | beginning | 6.2 ± 1.7 | 6.6 ± 1.5 | ||
| 8 months | 7.0 ± 1.3 | 6.1 ± 1.7 | |||
| Δ-change | +0.8 | −0.5 | <0.001 | 0.93 | |
| p-value | 0.002 | 0.011 | |||
| Health self-assessment (scale 0 to 10, 10: excellent) | beginning | 6.0 ± 2.1 | 5.9 ± 2.1 | ||
| 8 months | 7.5 ± 1.7 | 5.3 ± 2.0 | |||
| Δ-change | +1.5 | −0.6 | <0.001 | 1.56 | |
| p-value | <0.001 | 0.005 | |||
| Body mass index (kg/m2) | beginning | 31.4 ± 5.1 | 30.6 ± 5.4 | ||
| 8 months | 31.0 ± 5.1 | 30.3 ± 5.2 | |||
| Δ-change | −0.4 | −0.3 | 0.780 | 0.05 | |
| p-value | 0.012 | 0.089 | |||
| Systolic blood pressure (mmHg) | beginning | 130.7 ± 14.2 | 128.4 ± 13.8 | ||
| 8 months | 124.4 ± 13.8 | 128.9 ± 16.5 | |||
| Δ-change | −6.3 | +0.5 | 0.020 | 0.52 | |
| p-value | 0.003 | 0.804 | |||
| Diastolic blood pressure (mmHg) | beginning | 76.0 ± 8.8 | 75.4 ± 10.7 | ||
| 8 months | 77.4 ± 9.1 | 77.3 ± 8.8 | |||
| Δ-change | +1.4 | +1.9 | 0.822 | 0.05 | |
| p-value | 0.276 | 0.196 | |||
| Total cholesterol (mg/dL) | beginning | 154.9 ± 35.9 | 163.7 ± 37.6 | ||
| 8 months | 160.4 ± 36.4 | 163.9 ± 44.4 | |||
| Δ-change | +5.5 | +0.2 | 0.460 | 0.17 | |
| p-value | 0.122 | 0.966 | |||
| Glycosylated hemoglobin (HbA1c) (%) | beginning | 7.03 ± 1.12 | 6.71 ± 0.79 | ||
| 8 months | 6.75 ± 0.89 | 6.74 ± 0.94 | |||
| Δ-change | −0.28 | +0.03 | 0.008 | 0.60 | |
| p-value | 0.005 | 0.655 | |||
| Cholesterol ratio | beginning | 3.937 ± 1.962 | 3.567 ± 0.927 | ||
| 8 months | 3.855 ± 1.403 | 4.109 ± 2.147 | |||
| Δ-change | −0.072 | +0.752 | 0.070 | 0.41 | |
| p-value | 0.802 | 0.035 | |||
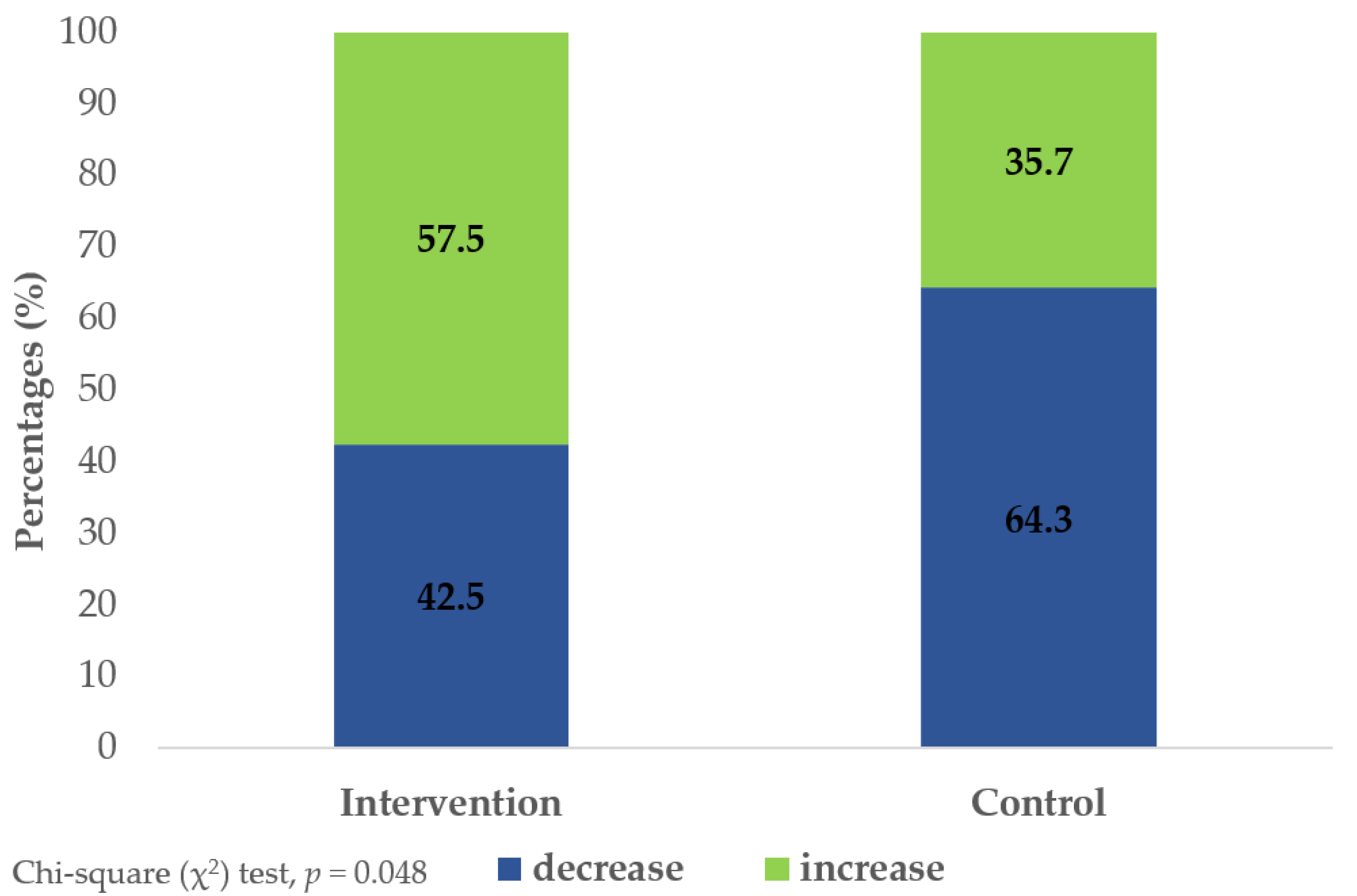
| Telomere length (kb) | beginning | 5.11 ± 1.35 | 5.19 ± 1.76 | ||
| 8 months | 5.63 ± 2.08 | 4.88 ± 1.19 | |||
| Δ-change | +0.52 | −0.31 | 0.048 | 0.44 | |
| p-value | 0.120 | 0.235 | |||
| Telomere Length Changes over the 8-Month Study Period (Increase vs. Decrease) | |||||
|---|---|---|---|---|---|
| Model | Prognostic Factors | Odds Ratio, OR | 95% CIs | p-Value | |
| Crude | Groups | control | 1.00 (ref.) | ||
| intervention | 2.44 | 1.00, 5.93 | 0.050 | ||
| 1 | Baseline Telomeres (per unit change) | 0.52 | 0.35, 0.79 | 0.002 | |
| Groups | control | 1.00 (ref.) | |||
| intervention | 2.87 | 1.07, 7.70 | 0.036 | ||
| 2 | Sex | male | 1.00 (ref.) | ||
| female | 1.64 | 0.60, 4.52 | 0.336 | ||
| Age (per year change) | 0.95 | 0.88, 1.02 | 0.167 | ||
| Baseline Telomeres (per unit change) | 0.49 | 0.32, 0.76 | 0.002 | ||
| Groups | control | 1.00 (ref.) | |||
| intervention | 2.90 | 1.05, 8.00 | 0.040 | ||
| 3 | Sex | male | 1.00 (ref.) | ||
| female | 1.99 | 0.66, 5.96 | 0.221 | ||
| Age (per year change) | 0.94 | 0.86, 1.02 | 0.137 | ||
| Baseline Telomeres (per unit change) | 0.48 | 0.30, 0.76 | 0.002 | ||
| Lifestyle Risk Factors | 0–2 factors | 1.00 (ref.) | |||
| 3+ | 2.23 | 0.75, 6.63 | 0.148 | ||
| Multimorbidity | 0–2 chronic conditions | 1.00 (ref.) | |||
| 3+ | 0.61 | 0.17, 2.20 | 0.453 | ||
| Polypharmacy | 0–3 medications | 1.00 (ref.) | |||
| 4+ | 0.93 | 0.28, 3.10 | 0.909 | ||
| Years Diagnosed with T2DM (per year change) | 1.06 | 0.97, 1.15 | 0.186 | ||
| Groups | control | 1.00 (ref.) | |||
| intervention | 3.48 | 1.16, 10.41 | 0.026 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantadaki, A.E.; Baliou, S.; Linardakis, M.; Vakonaki, E.; Tzatzarakis, M.N.; Tsatsakis, A.; Symvoulakis, E.K. Quercetin Intake and Absolute Telomere Length in Patients with Type 2 Diabetes Mellitus: Novel Findings from a Randomized Controlled Before-and-After Study. Pharmaceuticals 2024, 17, 1136. https://doi.org/10.3390/ph17091136
Mantadaki AE, Baliou S, Linardakis M, Vakonaki E, Tzatzarakis MN, Tsatsakis A, Symvoulakis EK. Quercetin Intake and Absolute Telomere Length in Patients with Type 2 Diabetes Mellitus: Novel Findings from a Randomized Controlled Before-and-After Study. Pharmaceuticals. 2024; 17(9):1136. https://doi.org/10.3390/ph17091136
Chicago/Turabian StyleMantadaki, Aikaterini E., Stella Baliou, Manolis Linardakis, Elena Vakonaki, Manolis N. Tzatzarakis, Aristides Tsatsakis, and Emmanouil K. Symvoulakis. 2024. "Quercetin Intake and Absolute Telomere Length in Patients with Type 2 Diabetes Mellitus: Novel Findings from a Randomized Controlled Before-and-After Study" Pharmaceuticals 17, no. 9: 1136. https://doi.org/10.3390/ph17091136
APA StyleMantadaki, A. E., Baliou, S., Linardakis, M., Vakonaki, E., Tzatzarakis, M. N., Tsatsakis, A., & Symvoulakis, E. K. (2024). Quercetin Intake and Absolute Telomere Length in Patients with Type 2 Diabetes Mellitus: Novel Findings from a Randomized Controlled Before-and-After Study. Pharmaceuticals, 17(9), 1136. https://doi.org/10.3390/ph17091136










