Myrtus communis L. Essential Oil Exhibits Antiviral Activity against Coronaviruses
Abstract
1. Introduction
2. Results
2.1. Chemical Compositions of MEO
2.2. Antioxidant Activity
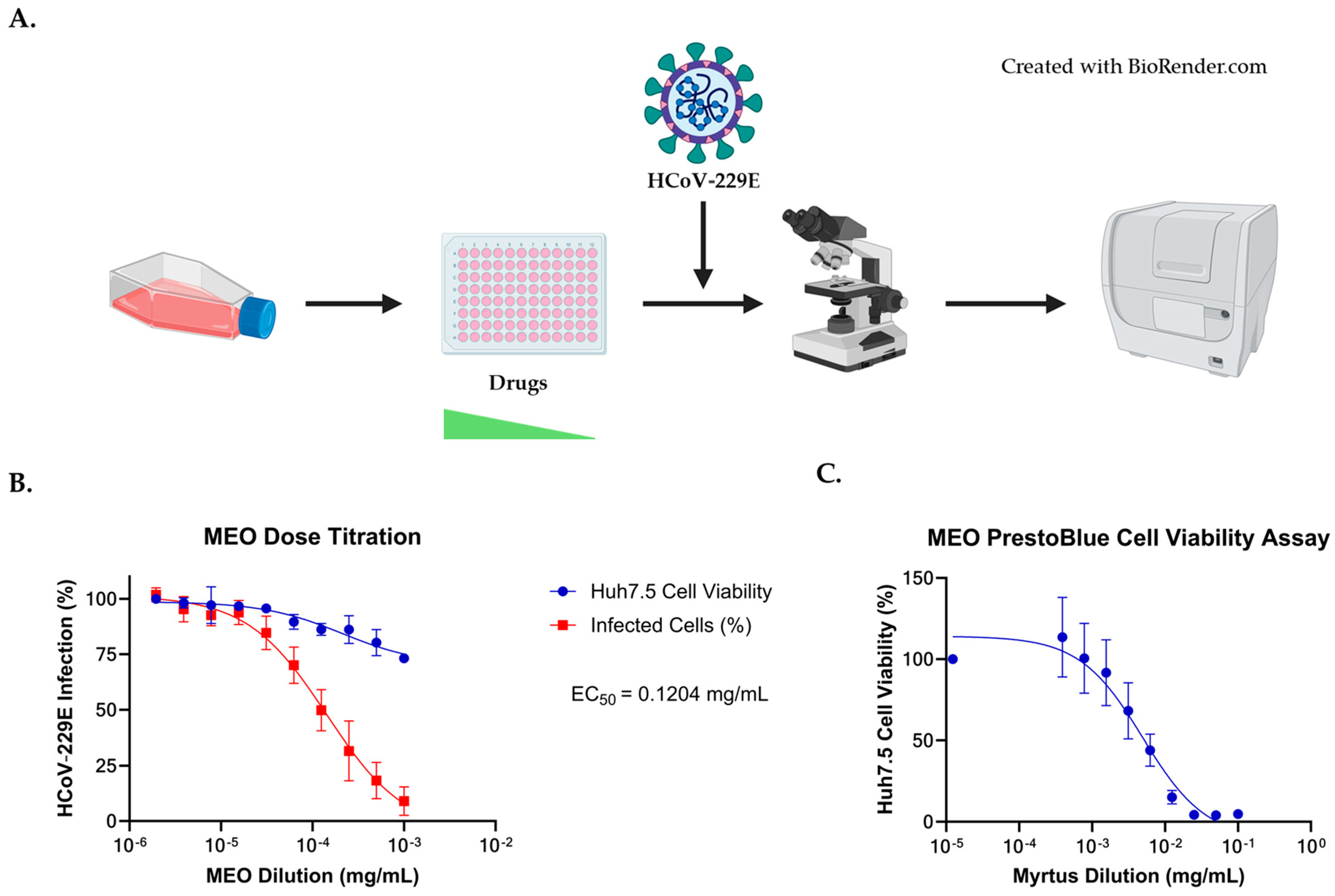
2.3. Antiviral Activity against HCoV-229E Coronavirus
2.4. Comparison of EC50 and CC50 with HCoV-229E Infection for MEO, Remdesivir, and Nirmatrelvir
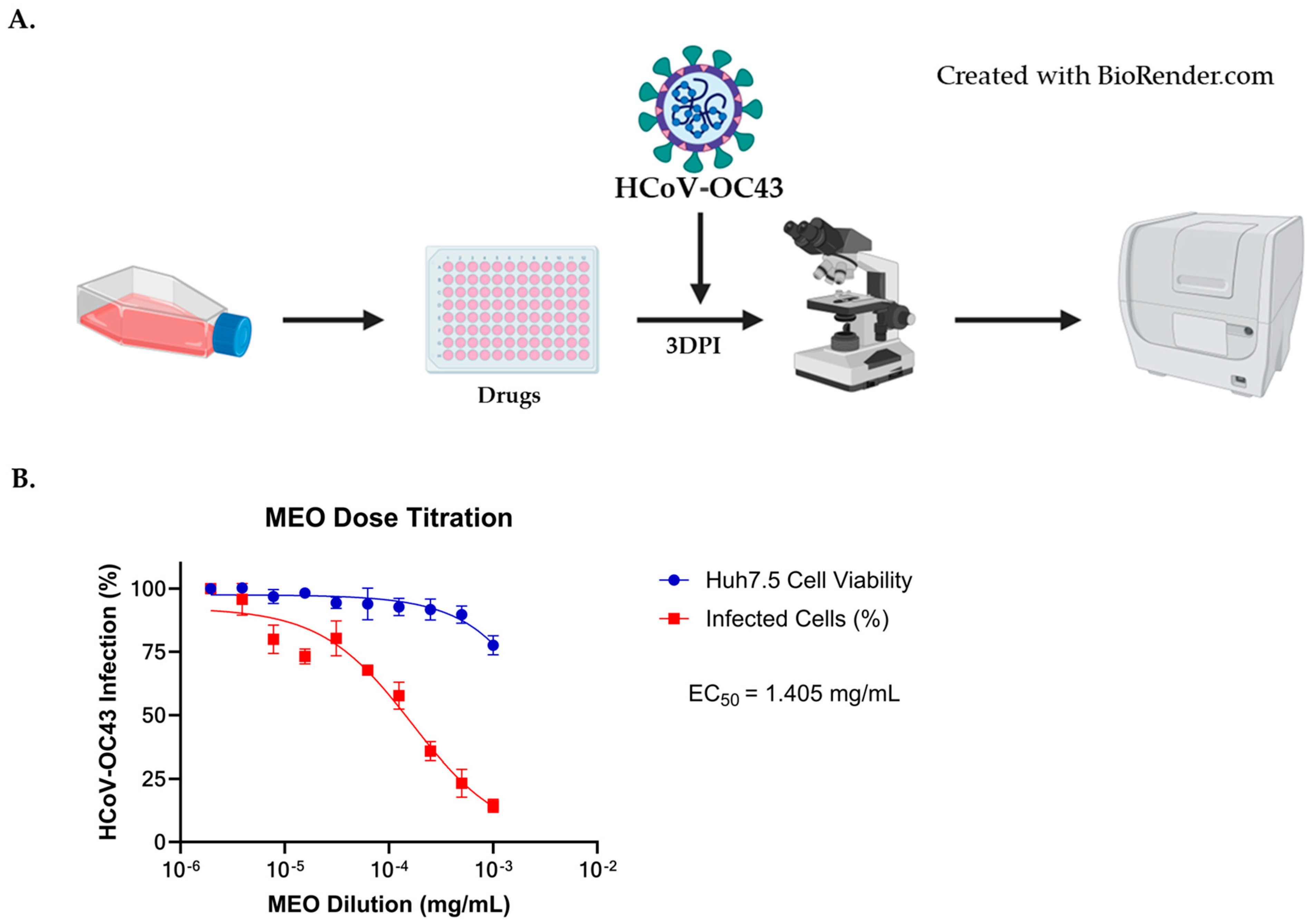
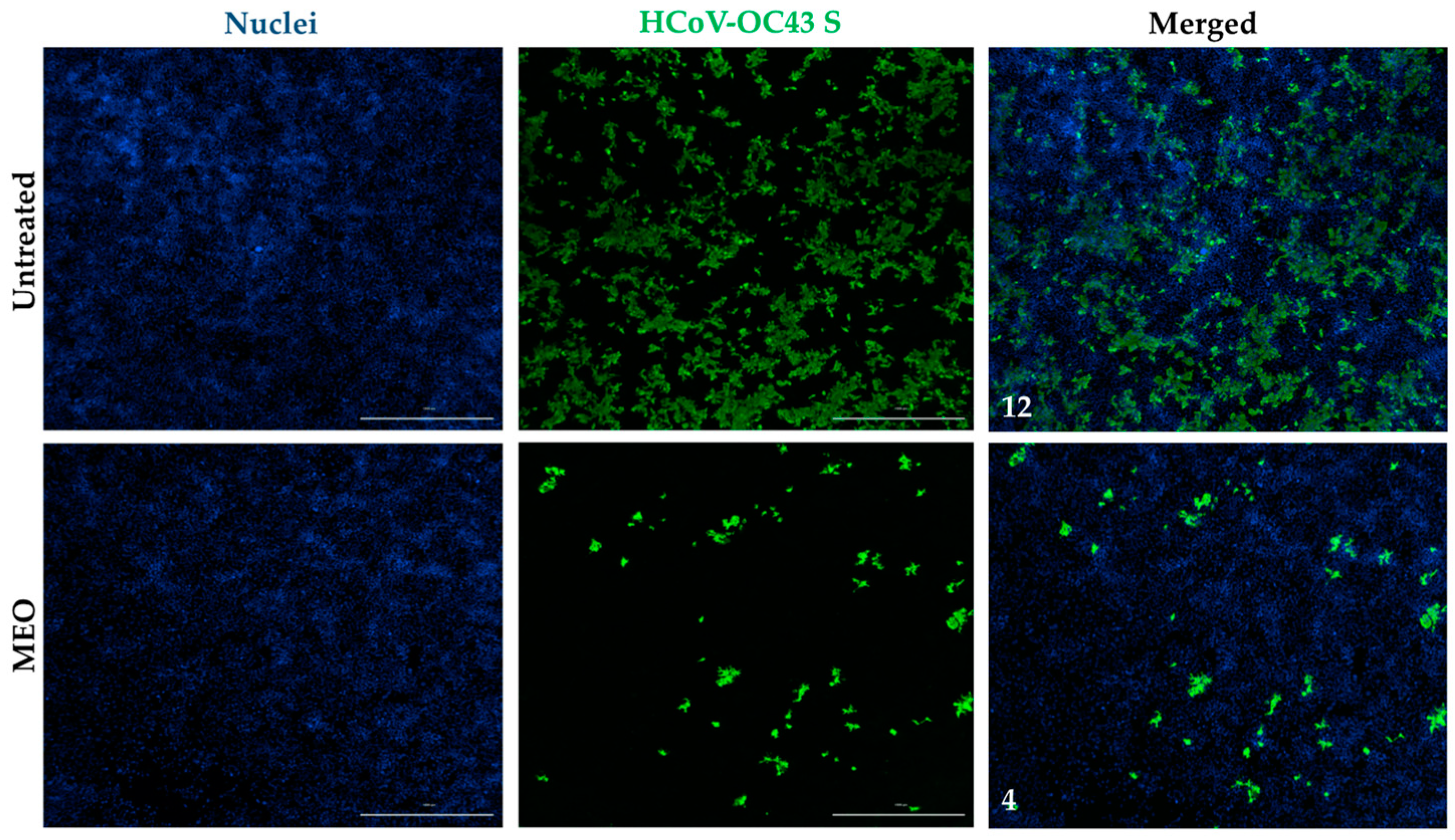
2.5. Antiviral Activity against HCoV-OC43 Coronavirus
2.6. Comparison of EC50 and CC50 with HCoV-OC43E Infection for MEO, Remdesivir, and Nirmatrelvir
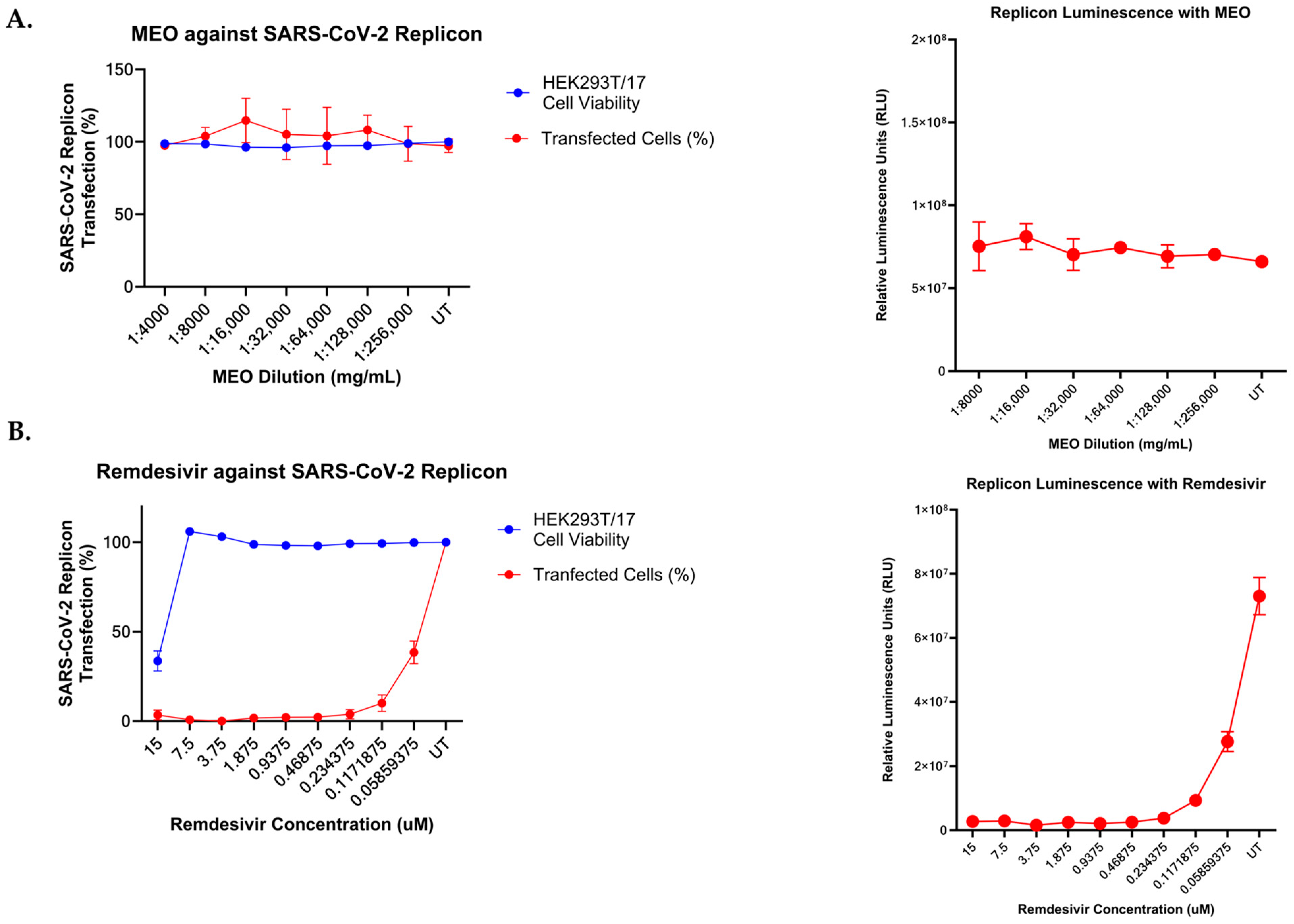
2.7. Effect of MEO and Remdesivir on SARS-CoV-2 Subgenomic Replicon System
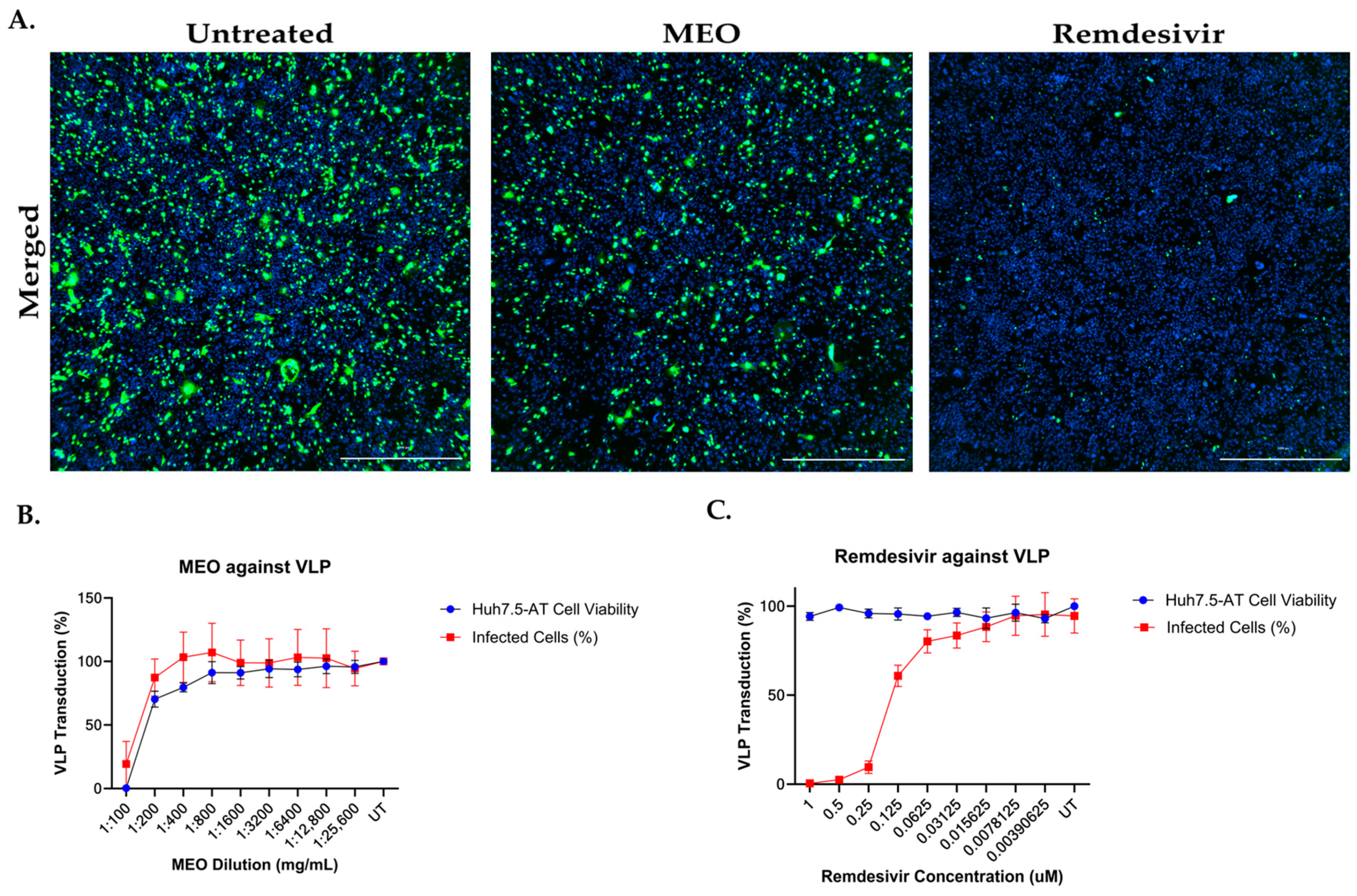
2.8. SARS-CoV-2 Virus-like Particles System on MEO and Remdesivir
3. Discussion
4. Materials and Methods
4.1. Myrtus Essential Oil Extraction
4.2. Gas Chromatography—Mass Spectrometry (GC-MS) Analysis
4.3. Antiradical Activity of Essential Oil from Myrtus
4.4. Cell Culture
4.5. HCoV-229E and HCoV-OC43 Infection and Inhibition Assays
4.6. HCoV-229E and HCoV-OC43 Immunofluorescence Staining and Imaging
4.7. PrestoBlue Cell Viability Assay for Huh7.5
4.8. SARS-CoV-2 MEO Replicon Assay and Luminescence
4.9. MEO Transfection Efficiency Assay
4.10. SARS-CoV-2 Virus-like Particles Production and Titration
4.11. SARS-CoV-2 Virus-like Particles Transduction Assay and Immunofluorescence Staining
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 22 July 2024).
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous System Involvement after Infection with COVID-19 and Other Coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Chan, J.F.W.; Azhar, E.I.; Hui, D.S.C.; Yuen, K.-Y. Coronaviruses—Drug Discovery and Therapeutic Options. Nat. Rev. Drug Discov. 2016, 15, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.D.; Subramanian, A.; Chakraborty, R.; Cotton, S.A.; Herrera, M.D.P.G.; Huang, Y.; Lambert, N.; Pinto, M.D.; Rahmani, A.M.; Sierra, C.J.; et al. The Effect of SARS-CoV-2 Variant on Respiratory Features and Mortality. Sci. Rep. 2023, 13, 4503. [Google Scholar] [CrossRef] [PubMed]
- Tortorici, M.A.; Walls, A.C.; Lang, Y.; Wang, C.; Li, Z.; Koerhuis, D.; Boons, G.-J.; Bosch, B.-J.; Rey, F.A.; De Groot, R.J.; et al. Structural Basis for Human Coronavirus Attachment to Sialic Acid Receptors. Nat. Struct. Mol. Biol. 2019, 26, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Breslin, J.J.; Mørk, I.; Smith, M.K.; Vogel, L.K.; Hemmila, E.M.; Bonavia, A.; Talbot, P.J.; Sjöström, H.; Norén, O.; Holmes, K.V. Human Coronavirus 229E: Receptor Binding Domain and Neutralization by Soluble Receptor at 37 °C. J. Virol. 2003, 77, 4435–4438. [Google Scholar] [CrossRef][Green Version]
- Bachar, S.C.; Mazumder, K.; Bachar, R.; Aktar, A.; Al Mahtab, M. A Review of Medicinal Plants with Antiviral Activity Available in Bangladesh and Mechanistic Insight into Their Bioactive Metabolites on SARS-CoV-2, HIV and HBV. Front. Pharmacol. 2021, 12, 732891. [Google Scholar] [CrossRef]
- Ali, S.I.; Sheikh, W.M.; Rather, M.A.; Venkatesalu, V.; Muzamil Bashir, S.; Nabi, S.U. Medicinal Plants: Treasure for Antiviral Drug Discovery. Phytother. Res. 2021, 35, 3447–3483. [Google Scholar] [CrossRef]
- Barac, A.; Donadu, M.; Usai, D.; Spiric, V.T.; Mazzarello, V.; Zanetti, S.; Aleksic, E.; Stevanovic, G.; Nikolic, N.; Rubino, S. Antifungal Activity of Myrtus Communis against Malassezia Sp. Isolated from the Skin of Patients with Pityriasis Versicolor. Infection 2018, 46, 253–257. [Google Scholar] [CrossRef]
- Mazzarello, V.; Gavini, E.; Rassu, G.; Donadu, M.G.; Usai, D.; Piu, G.; Pomponi, V.; Sucato, F.; Zanetti, S.; Montesu, M.A. Clinical Assessment of New Topical Cream Containing Two Essential Oils Combined with Tretinoin in the Treatment of Acne. Clin. Cosmet. Investig. Dermatol. 2020, 13, 233–239. [Google Scholar] [CrossRef]
- Barhouchi, B.; Menacer, R.; Bouchkioua, S.; Mansour, A.; Belattar, N. Compounds from Myrtle Flowers as Antibacterial Agents and SARS-CoV-2 Inhibitors: In-Vitro and Molecular Docking Studies. Arab. J. Chem. 2023, 16, 104939. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, V.; Knezevic, P. Antimicrobial and Antioxidative Activity of Extracts and Essential Oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, R.; Pan, W.; Huang, W.; Liu, B.; Xie, Y.; Wang, Z.; Li, C.; Jiang, H.; Huang, J.; et al. Phillyrin (KD-1) Exerts Anti-Viral and Anti-Inflammatory Activities against Novel Coronavirus (SARS-CoV-2) and Human Coronavirus 229E (HCoV-229E) by Suppressing the Nuclear Factor Kappa B (NF-κB) Signaling Pathway. Phytomedicine 2020, 78, 153296. [Google Scholar] [CrossRef]
- Ojha, D.; Jessop, F.; Bosio, C.M.; Peterson, K.E. Effective Inhibition of HCoV-OC43 and SARS-CoV-2 by Phytochemicals In Vitro and In Vivo. Int. J. Antimicrob. Agents 2023, 62, 106893. [Google Scholar] [CrossRef]
- Bouzabata, A.; Castola, V.; Bighelli, A.; Abed, L.; Casanova, J.; Tomi, F. Chemical Variability of Algerian Myrtus communis L. Chem. Biodivers. 2013, 10, 129–137. [Google Scholar] [CrossRef]
- Bouzabata, A.; Cabral, C.; Gonçalves, M.J.; Cruz, M.T.; Bighelli, A.; Cavaleiro, C.; Casanova, J.; Tomi, F.; Salgueiro, L. Myrtus communis L. as Source of a Bioactive and Safe Essential Oil. Food Chem. Toxicol. 2015, 75, 166–172. [Google Scholar] [CrossRef]
- Chalchat, J.-C.; Garry, R.-P.; Michet, A. Essential Oils of Myrtle (Myrtus communis L.) of the Mediterranean Littoral. J. Essent. Oil Res. 1998, 10, 613–617. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I.; Maccioni, S.; Baldini, R. Phytochemical Typologies in Some Populations of Myrtus communis L. on Caprione Promontory (East Liguria, Italy). Food Chem. 2004, 85, 599–604. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Mirzakhani, M.; Pirbalouti, A.G. Essential Oil Variation among 21 Wild Myrtle (Myrtus communis L.) Populations Collected from Different Geographical Regions in Iran. Ind. Crops Prod. 2013, 51, 328–333. [Google Scholar] [CrossRef]
- Cherrat, L.; Espina, L.; Bakkali, M.; García-Gonzalo, D.; Pagán, R.; Laglaoui, A. Chemical Composition and Antioxidant Properties of Laurus nobilis L. and Myrtus communis L. Essential Oils from Morocco and Evaluation of Their Antimicrobial Activity Acting Alone or in Combined Processes for Food Preservation. J. Sci. Food Agric. 2014, 94, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Gardeli, C.; Vassiliki, P.; Athanasios, M.; Kibouris, T.; Komaitis, M. Essential Oil Composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of Antioxidant Capacity of Methanolic Extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Boelens, M.H.; Jimenez, R. The Chemical Composition of Spanish Myrtle Oils. Part II. J. Essent. Oil Res. 1992, 4, 349–353. [Google Scholar] [CrossRef]
- Alipour, G.; Dashti, S.; Hosseinzadeh, H. Review of Pharmacological Effects of Myrtus communis L. and Its Active Constituents: Myrtus communis L. and Its Active Constituents. Phytother. Res. 2014, 28, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Yadegarinia, D.; Gachkar, L.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Biochemical Activities of Iranian Mentha piperita L. and Myrtus communis L. Essential Oils. Phytochemistry 2006, 67, 1249–1255. [Google Scholar] [CrossRef]
- Romani, A.; Coinu, R.; Carta, S.; Pinelli, P.; Galardi, C.; Vincieri, F.F.; Franconi, F. Evaluation of Antioxidant Effect of Different Extracts of Myrtus communis L. Free Radic. Res. 2004, 38, 97–103. [Google Scholar] [CrossRef]
- Nicoliche, T.; Bartolomeo, C.S.; Lemes, R.M.R.; Pereira, G.C.; Nunes, T.A.; Oliveira, R.B.; Nicastro, A.L.M.; Soares, É.N.; Da Cunha Lima, B.F.; Rodrigues, B.M.; et al. Antiviral, Anti-Inflammatory and Antioxidant Effects of Curcumin and Curcuminoids in SH-SY5Y Cells Infected by SARS-CoV-2. Sci. Rep. 2024, 14, 10696. [Google Scholar] [CrossRef]
- Lan, S.; Tedbury, P.R.; Ong, Y.T.; Shah, R.; Slack, R.L.; Cilento, M.E.; Zhang, H.; Du, H.; Lulkin, N.; Le, U.; et al. Subgenomic SARS-CoV-2 Replicon and Reporter Replicon Cell Lines Enable Ultrahigh Throughput Antiviral Screening and Mechanistic Studies with Antivirals, Viral Mutations or Host Factors That Affect COVID-19 Replication. bioRxiv 2021, bioRxiv:12.29.474471. [Google Scholar]
- CDC. COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 2 December 2023).
- WHO. COVID-19 Eliminated a Decade of Progress in Global Level of Life Expectancy. Available online: https://www.who.int/news/item/24-05-2024-covid-19-eliminated-a-decade-of-progress-in-global-level-of-life-expectancy (accessed on 27 June 2024).
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium. Remdesivir and Three Other Drugs for Hospitalised Patients with COVID-19: Final Results of the WHO Solidarity Randomised Trial and Updated Meta-Analyses. Lancet 2022, 399, 1941–1953. [Google Scholar] [CrossRef]
- Arbel, R.; Wolff Sagy, Y.; Hoshen, M.; Battat, E.; Lavie, G.; Sergienko, R.; Friger, M.; Waxman, J.G.; Dagan, N.; Balicer, R.; et al. Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge. N. Engl. J. Med. 2022, 387, 790–798. [Google Scholar] [CrossRef]
- Farooq, S.; Ngaini, Z. Natural and Synthetic Drugs as Potential Treatment for Coronavirus Disease 2019 (COVID-2019). Chem. Afr. 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Albalawi, M.A. Essential Oils and COVID-19. Molecules 2022, 27, 7893. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.X.; Liang, J.Q.; Fung, T.S. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae). In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 428–440. ISBN 978-0-12-814516-6. [Google Scholar]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Ben Hsouna, A.; Hamdi, N.; Miladi, R.; Abdelkafi, S. Myrtus communis Essential Oil: Chemical Composition and Antimicrobial Activities against Food Spoilage Pathogens. Chem. Biodivers. 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Proskurnina, E.V.; Izmailov, D.Y.; Sozarukova, M.M.; Zhuravleva, T.A.; Leneva, I.A.; Poromov, A.A. Antioxidant Potential of Antiviral Drug Umifenovir. Molecules 2020, 25, 1577. [Google Scholar] [CrossRef]
- Prevc, T.; Šegatin, N.; Poklar Ulrih, N.; Cigić, B. DPPH Assay of Vegetable Oils and Model Antioxidants in Protic and Aprotic Solvents. Talanta 2013, 109, 13–19. [Google Scholar] [CrossRef]


| Rt | KI Let | KI Sper | Components | % ± SD | IM |
|---|---|---|---|---|---|
| 12.242 | 892 | 892 | isobutyl isobutyrate | 0.25 ± 0.02 | MS-RI |
| 12.626 | 930 | 929 | artemisia triene | 0.10 ± 0.01 | MS-RI |
| 12.781 | 939 | 939 | endo-5-norbornene-2-ol | 0.12 ± 0.01 | MS-RI |
| 13.068 | 939 | 940 | α-pinene | 37.83 ± 0.15 | Std |
| 13.552 | 944 | 947 | 5-methyl-3-heptanone | 0.06 ± 0.02 | MS-RI |
| 13.617 | 954 | 956 | camphene | 0.60 ± 0.03 | Std |
| 14.492 | 979 | 977 | trans-para-menthane | 0.21 ± 0.02 | MS-RI |
| 14.640 | 979 | 979 | ß-pinene | 0.38 ± 0.02 | Std |
| 15.047 | 986 | 985 | cis-pinane | 0.20 ± 0.01 | MS-RI |
| 16.281 | 1025 | 1025 | p-cymene | 1.59 ± 0.05 | Std |
| 16.450 | 1029 | 1030 | limonene | 10.70 ± 0.09 | Std |
| 16.562 | 1031 | 1032 | 1,8-cineole | 27.17 ± 0.8 | Std |
| 17.463 | 1060 | 1058 | γ-terpinene | 0.19 ± 0.03 | Std |
| 18.759 | 1097 | 1097 | linalool | 7.24 ± 0.10 | Std |
| 19.077 | 1102 | 1101 | cis-thujone | 0.31 ± 0.04 | MS-RI |
| 19.425 | 1114 | 1114 | trans-thujone | 0.17 ± 0.02 | MS-RI |
| 19.962 | 1134 | 1137 | 1-terpineol | 0.17 ± 0.01 | Std |
| 20.288 | 1139 | 1139 | trans-ß-dihydro-terpineol | 0.40 ± 0.05 | MS-RI |
| 20.393 | 1146 | 1149 | camphor | 0.15 ± 0.01 | Std |
| 21.368 | 1177 | 1172 | terpinene-4-ol | 0.33 ± 0.02 | Std |
| 21.751 | 1189 | 1188 | α-terpineol | 2.84 ± 0.10 | MS-RI |
| 21.954 | 1194 | 1192 | dihydrocarveol | 0.94 ± 0.07 | MS-RI |
| 23.355 | 1243 | 1242 | carvone | 0.29 ± 0.02 | Std |
| 23.529 | 1267 | 1263 | geranial | 2.32 ± 0.07 | Std |
| 24.397 | 1349 | 1350 | terpinyl acetate | 0.12 ± 0.01 | MS-RI |
| 30.077 | 1495 | 1493 | methyl-isoeugenol | 1.18 ± 0.04 | MS-RI |
| Total | 95.77 |
| mg/mL—Time Points | MEO |
|---|---|
| 2 (60 min) | 36.2 ± 9.3 |
| 1 (60 min) | 28.7 ± 5.0 |
| 0.5 (60 min) | 18.9 ± 3.6 |
| 0.2 (60 min) | 1.3 ± 0.7 |
| 0.1 (60 min) | 0.4 ± 0.8 |
| 2 (180 min) | 39.1 ± 9.9 |
| 1 (180 min) | 33.0 ± 8.6 |
| 0.5 (180 min) | 24.1 ± 9.4 |
| 0.2 (180 min) | 1.3 ± 2.3 |
| 0.1 (180 min) | 0.2 ± 0.9 |
| 2 (300 min) | 40.7 ± 8.8 |
| 1 (300 min) | 34.1 ± 8.7 |
| 0.5 (300 min) | 26.3 ± 1.6 |
| 0.2 (300 min) | 1.4 ± 0.6 |
| 0.1 (300 min) | 1.8 ± 0.4 |
| Compound | EC50 (nM) | CC50 (nM) |
| Remdesivir | 0.003 | 9.662 |
| Nirmatrelvir | 1.243 | 337.9 |
| Compound | EC50 (mg/mL) | CC50 (mg/mL) |
| MEO | 0.1204 | 4.197 |
| Compound | EC50 (uM) | CC50 (nM) |
| Remdesivir | 0.021 | 9.662 |
| Nirmatrelvir | 0.012 | 337.9 |
| Compound | EC50 (mg/mL) | CC50 (mg/mL) |
| MEO | 1.405 | 4.197 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.-Y.; Donadu, M.G.; Shue, T.; Dangas, G.; Athanasiadis, A.; Lan, S.; Wen, X.; Battah, B.; Zanetti, S.; Mazzarello, V.; et al. Myrtus communis L. Essential Oil Exhibits Antiviral Activity against Coronaviruses. Pharmaceuticals 2024, 17, 1189. https://doi.org/10.3390/ph17091189
Li D-Y, Donadu MG, Shue T, Dangas G, Athanasiadis A, Lan S, Wen X, Battah B, Zanetti S, Mazzarello V, et al. Myrtus communis L. Essential Oil Exhibits Antiviral Activity against Coronaviruses. Pharmaceuticals. 2024; 17(9):1189. https://doi.org/10.3390/ph17091189
Chicago/Turabian StyleLi, Dar-Yin, Matthew G. Donadu, Taylor Shue, Georgios Dangas, Antonis Athanasiadis, Shuiyun Lan, Xin Wen, Basem Battah, Stefania Zanetti, Vittorio Mazzarello, and et al. 2024. "Myrtus communis L. Essential Oil Exhibits Antiviral Activity against Coronaviruses" Pharmaceuticals 17, no. 9: 1189. https://doi.org/10.3390/ph17091189
APA StyleLi, D.-Y., Donadu, M. G., Shue, T., Dangas, G., Athanasiadis, A., Lan, S., Wen, X., Battah, B., Zanetti, S., Mazzarello, V., Sarafianos, S. G., Ferrari, M., & Michailidis, E. (2024). Myrtus communis L. Essential Oil Exhibits Antiviral Activity against Coronaviruses. Pharmaceuticals, 17(9), 1189. https://doi.org/10.3390/ph17091189







