The Properties of the Transient Outward, Inward Rectifier and Acetylcholine-Sensitive Potassium Currents in Atrial Myocytes from Dogs in Sinus Rhythm and Experimentally Induced Atrial Fibrillation Dog Models
Abstract
1. Introduction
2. Results
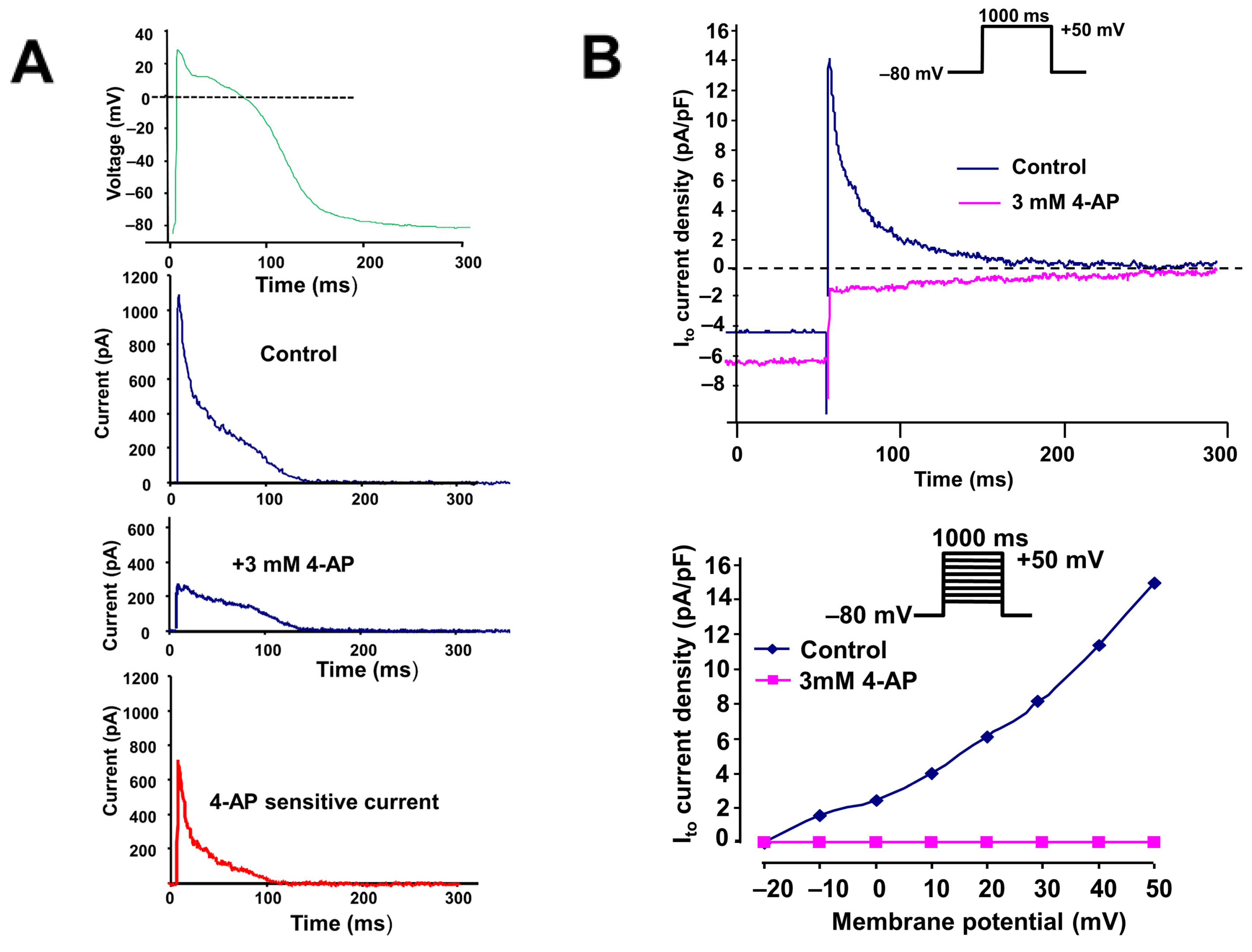
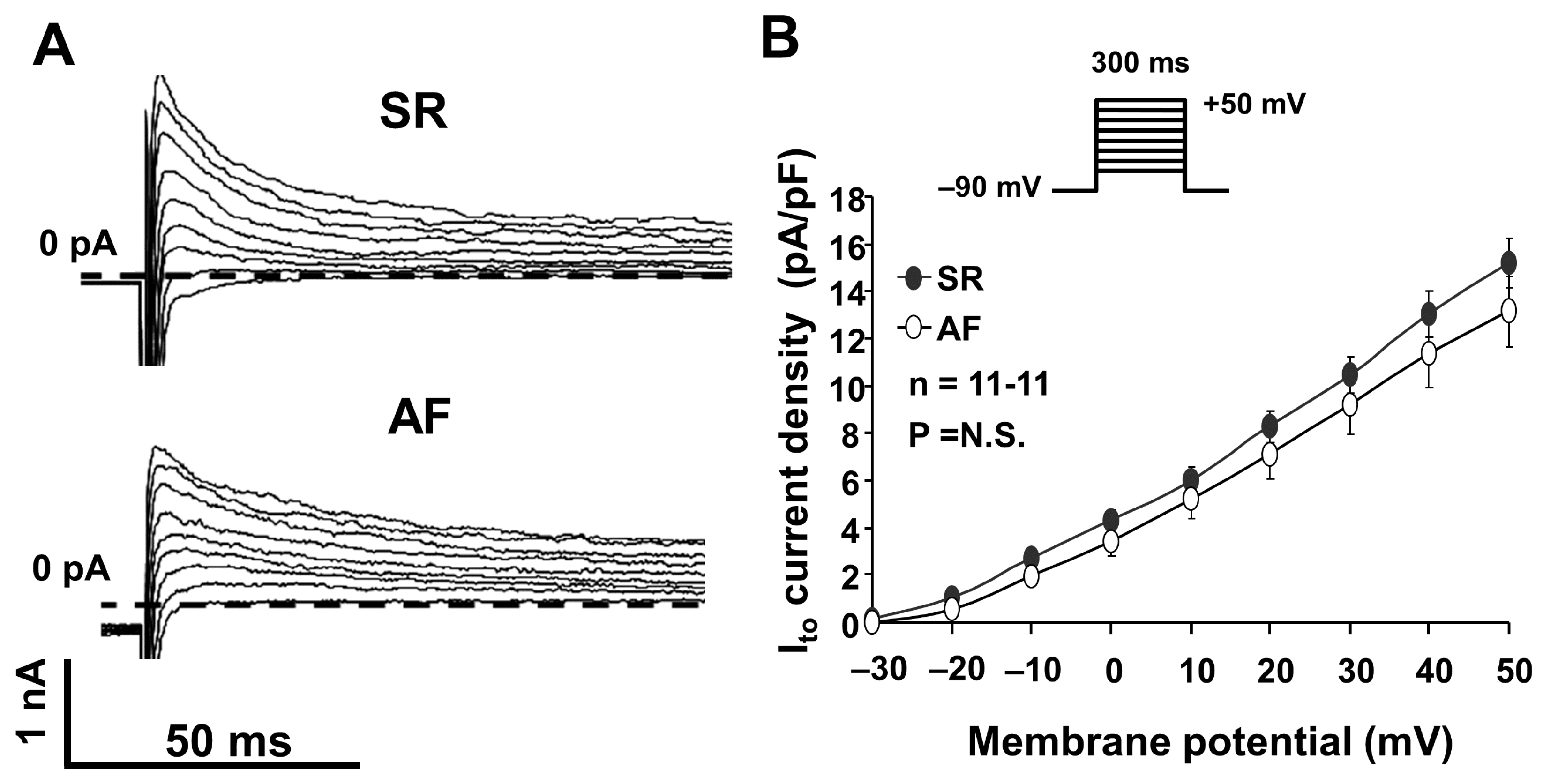
2.1. The Transient Outward Potassium Current (Ito)
2.2. The Inward Rectifier Potassium Current (IK1)
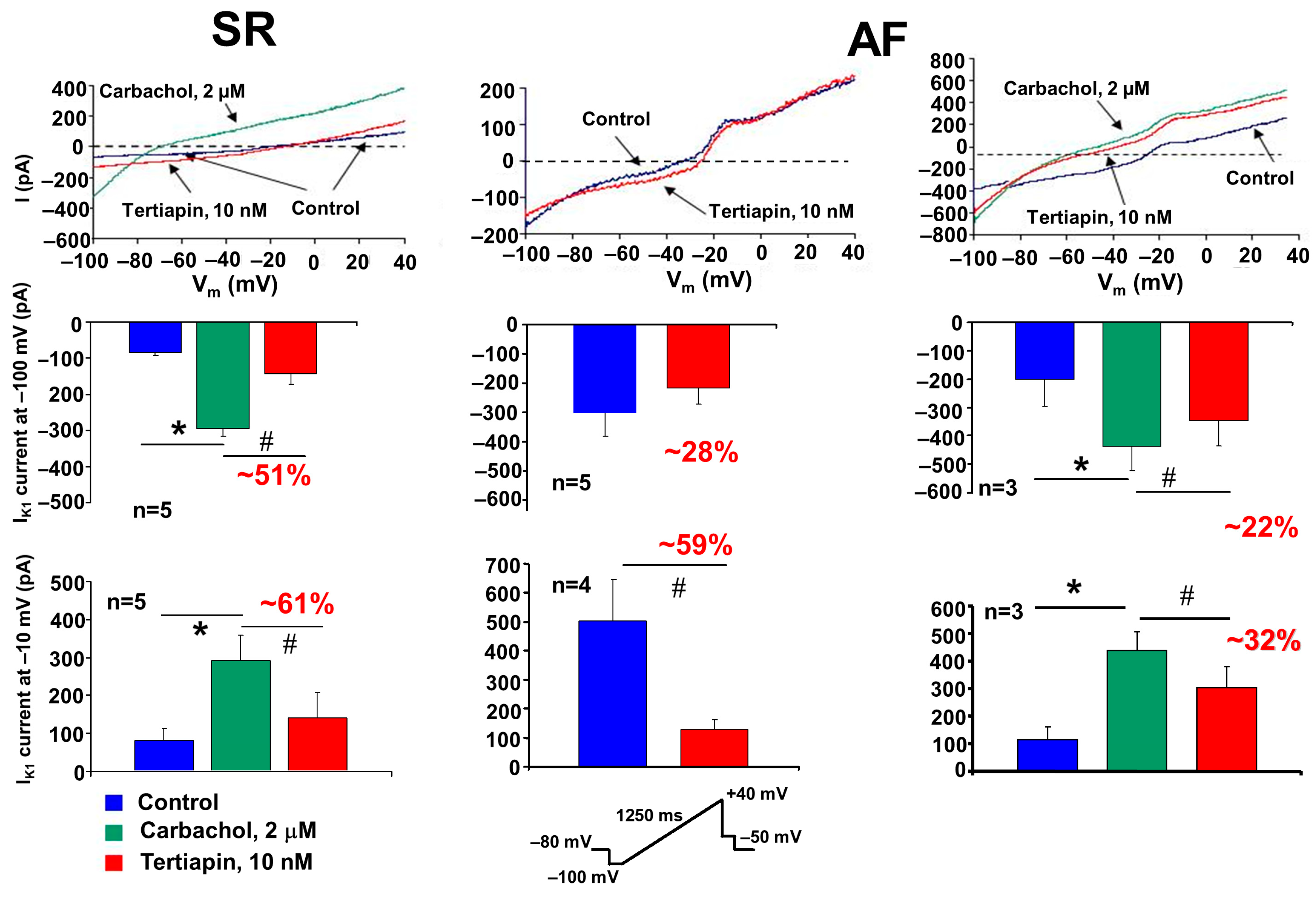
2.3. The Acetylcholine-Sensitive Potassium Current (IK,ACh)
3. Discussion
3.1. The Transient Outward Potassium Current (Ito)
3.2. The Inward Rectifier Potassium Current (IK1)
3.3. Acetylcholine-Activated Potassium Current (IK,ACh)
4. Materials and Methods
4.1. Atrial Tachypacing-Induced AF in Conscious Dogs
4.2. Isolation of Right Atrial Myocytes
4.3. Electrophysiological Measurements
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| AF | atrial fibrillation |
| 4-AP | 4-aminopyridine |
| AP | action potential |
| APD | action potential duration |
| ATR | atrial tachycardia-induced remodeling |
| CCh | carbachol |
| ERP | effective refractory period |
| ES | extra systole |
| HP | holding potential |
| ICaL | L-type calcium current |
| IK,ACh | acetylcholine-sensitive potassium current |
| IK1 | inward rectifier potassium current |
| Ito | transient outward potassium current |
| SR | sinus rhythm |
References
- Jost, N.; Christ, T.; Magyar, J. New strategies for the treatment of atrial fibrillation. Pharmaceuticals 2021, 14, 926. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Heijman, J.; Zhou, L.; Dobrev, D. Molecular Basis of Atrial Fibrillation Pathophysiology and Therapy: A Translational Perspective. Circ. Res. 2020, 127, 51–72. [Google Scholar] [CrossRef]
- Nattel, S. Atrial electrophysiological remodeling caused by rapid atrial activation: Underlying mechanisms and clinical relevance to atrial fibrillation. Cardiovasc. Res. 1999, 42, 298–308. [Google Scholar] [CrossRef]
- Allessie, M.; Ausma, J.; Schotten, U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc. Res. 2002, 54, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Maguy, A.; Le Bouter, S.; Yeh, Y.H. Arrhythmogenic ion-channel remodeling in the heart: Heart failure, myocardial infarction, and atrial fibrillation. Physiol. Rev. 2007, 87, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, D.; Ravens, U. Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic. Res. Cardiol. 2003, 98, 137–148. [Google Scholar] [CrossRef]
- Yue, L.; Feng, J.; Gaspo, R.; Li, G.R.; Wang, Z.; Nattel, S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ. Res. 1997, 81, 512–525. [Google Scholar] [CrossRef]
- Christ, T.; Boknik, P.; Wöhrl, S.; Wettwer, E.; Graf, E.M.; Bosch, R.F.; Knaut, M.; Schmitz, W.; Ravens, U.; Dobrev, D. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation 2004, 110, 2651–2657. [Google Scholar] [CrossRef]
- Dobrev, D.; Graf, E.; Wettwer, E.; Himmel, H.M.; Hála, O.; Doerfel, C.; Christ, T.; Schüler, S.; Ravens, U. Molecular basis of downregulation of G-protein-coupled inward rectifying K(+) current (I(K,ACh) in chronic human atrial fibrillation: Decrease in GIRK4 mRNA correlates with reduced I(K,ACh) and muscarinic receptor-mediated shortening of action potentials. Circulation 2001, 104, 2551–2557. [Google Scholar] [CrossRef]
- Dobrev, D.; Friedrich, A.; Voigt, N.; Jost, N.; Wettwer, E.; Christ, T.; Knaut, M.; Ravens, U. The G-protein gated potassium current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation 2005, 112, 3697–3706. [Google Scholar] [CrossRef]
- Johnson, E.K.; Springer, S.J.; Wang, W.; Dranoff, E.J.; Zhang, Y.; Kanter, E.M.; Yamada, K.A.; Nerbonne, J.M. Differential expression and remodeling of transient outward potassium currents in human left ventricles. Circ. Arrhythm. Electrophysiol. 2018, 11, e005914. [Google Scholar] [CrossRef]
- Jin, W.; Lu, Z. A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry 1998, 37, 13291–13299. [Google Scholar] [CrossRef]
- Bosch, R.F.; Zeng, X.; Grammer, J.B.; Popovic, K.; Mewis, C.; Kühlkamp, V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc. Res. 1999, 44, 121–131. [Google Scholar] [CrossRef]
- Caballero, R.; de la Fuente, M.G.; Gómez, R.; Barana, A.; Amorós, I.; Dolz-Gaitón, P.; Osuna, L.; Almendral, J.; Atienza, F.; Fernández-Avilés, F.; et al. In humans, chronic atrial fibrillation decreases the transient outward current and ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. J. Am. Coll. Cardiol. 2010, 55, 2346–2354. [Google Scholar] [CrossRef]
- Zicha, S.; Xiao, L.; Stafford, S.; Cha, T.J.; Han, W.; Varro, A.; Nattel, S. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J. Physiol. 2004, 561 Pt 3, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Anumonwo, C.J.M.B.; Lopatin, A.N. Cardiac strong inward rectifier potassium channels. J. Mol. Cell Cardiol. 2010, 48, 45–54. [Google Scholar] [CrossRef]
- Reilly, L.; Eckhardt, L.L. Cardiac potassium inward rectifier Kir2: Review of structure, regulation, pharmacology, and arrhythmogenesis. Heart Rhythm. 2021, 18, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Heidbüchel, H.; Vereecke, J.; Carmeliet, E. Three different potassium channels in human atrium: Contribution to the basal potassium conductance. Circ. Res. 1990, 66, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Inanobe, A.; Kurachi, Y. G protein regulation of potassium ion channels. Pharmacol. Rev. 1998, 50, 723–757. [Google Scholar]
- Liu, L.; Nattel, S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: Role of refractoriness heterogeneity. Am. J. Physiol. 1997, 273, H805–H816. [Google Scholar] [CrossRef]
- Yeh, Y.-H.; Lemola, K.; Nattel, S. Vagal atrial fibrillation. Acta Cardiol. Sin. 2007, 23, 1–12. [Google Scholar]
- Coumel, P.; Attuel, P.; Lavallée, J.; Flammang, D.; Leclercq, J.F.; Slama, R. Syndrome d’arythmie auriculaire d’origine vagale. Arch. Mal. Coeur 1978, 6, 643–656. [Google Scholar]
- Voigt, N.; Maguay, A.; Yeh, Y.H.; Qi, X.; Ravens, U.; Dobrev, D.; Nattel, S. Changes in I K,ACh single channel activity with atrial tachycardia remodeling in canine atrial cardiomyocytes. Cardiovasc. Res. 2008, 77, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Juhász, V.; Hornyik, T.; Benák, A.; Nagy, N.; Husti, Z.; Pap, R.; Sághy, L.; Virág, L.; Varró, A.; Baczkó, I. Comparison of the effects of IK,ACh, IKr and INa block in conscious dogs with atrial fibrillation and on action potentials in remodeled atrial trabeculae. Can. J. Physiol. Pharmacol. 2018, 96, 18–25. [Google Scholar] [CrossRef]
- Blaauw, Y.; Schotten, U.; van Hunnik, A.; Neuberger, H.R.; Allessie, M.R. Cardioversion of persistent atrial fibrillation by a combination of atrial specific and non-specific class III drugs in the goat. Cardiovasc. Res. 2007, 75, 89–98. [Google Scholar] [CrossRef]
- Nagy, N.; Márton, Z.; Kiss, L.; Varró, A.; Nánási, P.P.; Tóth, A. Role of Ca2+-sensitive K+ currents in controlling ventricular repolarization: Possible implications for future antiarrhytmic drug therapy. Curr. Med. Chem. 2011, 18, 3622–3639. [Google Scholar] [CrossRef]


| Parameter | τfast (ms) | τslow (ms) | DensAmpfast (pA/pF) | DensAmpslow (pA/pF) |
|---|---|---|---|---|
| SR (n = 10) | 11.70 ± 0.76 | 121.9 ± 8.82 | 5.80 ± 0.61 | 2.32 ± 0.38 |
| AF (n = 11) | 17.83 ± 2.97 * | 179.2 ± 25.42 * | 4.22 ± 0.7 | 2.71 ± 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohajda, Z.; Corici, C.; Kristóf, A.; Virág, L.; Husti, Z.; Baczkó, I.; Sághy, L.; Varró, A.; Jost, N. The Properties of the Transient Outward, Inward Rectifier and Acetylcholine-Sensitive Potassium Currents in Atrial Myocytes from Dogs in Sinus Rhythm and Experimentally Induced Atrial Fibrillation Dog Models. Pharmaceuticals 2024, 17, 1138. https://doi.org/10.3390/ph17091138
Kohajda Z, Corici C, Kristóf A, Virág L, Husti Z, Baczkó I, Sághy L, Varró A, Jost N. The Properties of the Transient Outward, Inward Rectifier and Acetylcholine-Sensitive Potassium Currents in Atrial Myocytes from Dogs in Sinus Rhythm and Experimentally Induced Atrial Fibrillation Dog Models. Pharmaceuticals. 2024; 17(9):1138. https://doi.org/10.3390/ph17091138
Chicago/Turabian StyleKohajda, Zsófia, Claudia Corici, Attila Kristóf, László Virág, Zoltán Husti, István Baczkó, László Sághy, András Varró, and Norbert Jost. 2024. "The Properties of the Transient Outward, Inward Rectifier and Acetylcholine-Sensitive Potassium Currents in Atrial Myocytes from Dogs in Sinus Rhythm and Experimentally Induced Atrial Fibrillation Dog Models" Pharmaceuticals 17, no. 9: 1138. https://doi.org/10.3390/ph17091138
APA StyleKohajda, Z., Corici, C., Kristóf, A., Virág, L., Husti, Z., Baczkó, I., Sághy, L., Varró, A., & Jost, N. (2024). The Properties of the Transient Outward, Inward Rectifier and Acetylcholine-Sensitive Potassium Currents in Atrial Myocytes from Dogs in Sinus Rhythm and Experimentally Induced Atrial Fibrillation Dog Models. Pharmaceuticals, 17(9), 1138. https://doi.org/10.3390/ph17091138






