Cytotoxicity of Benzofuran-Containing Simplified Viniferin Analogues
Abstract
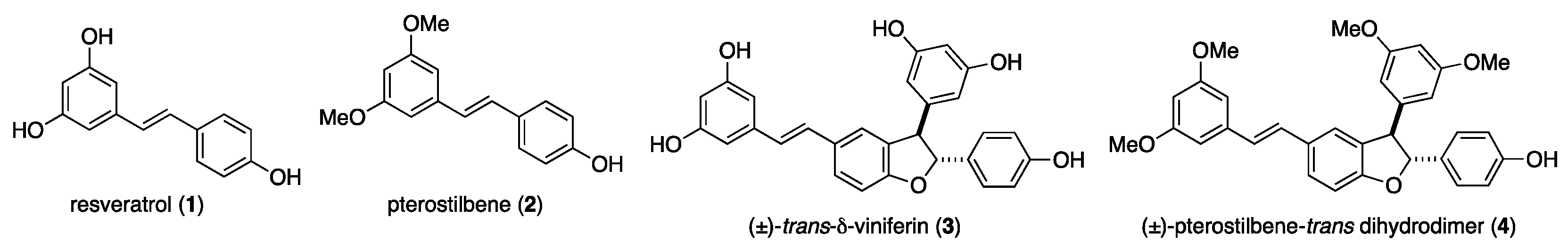
1. Introduction
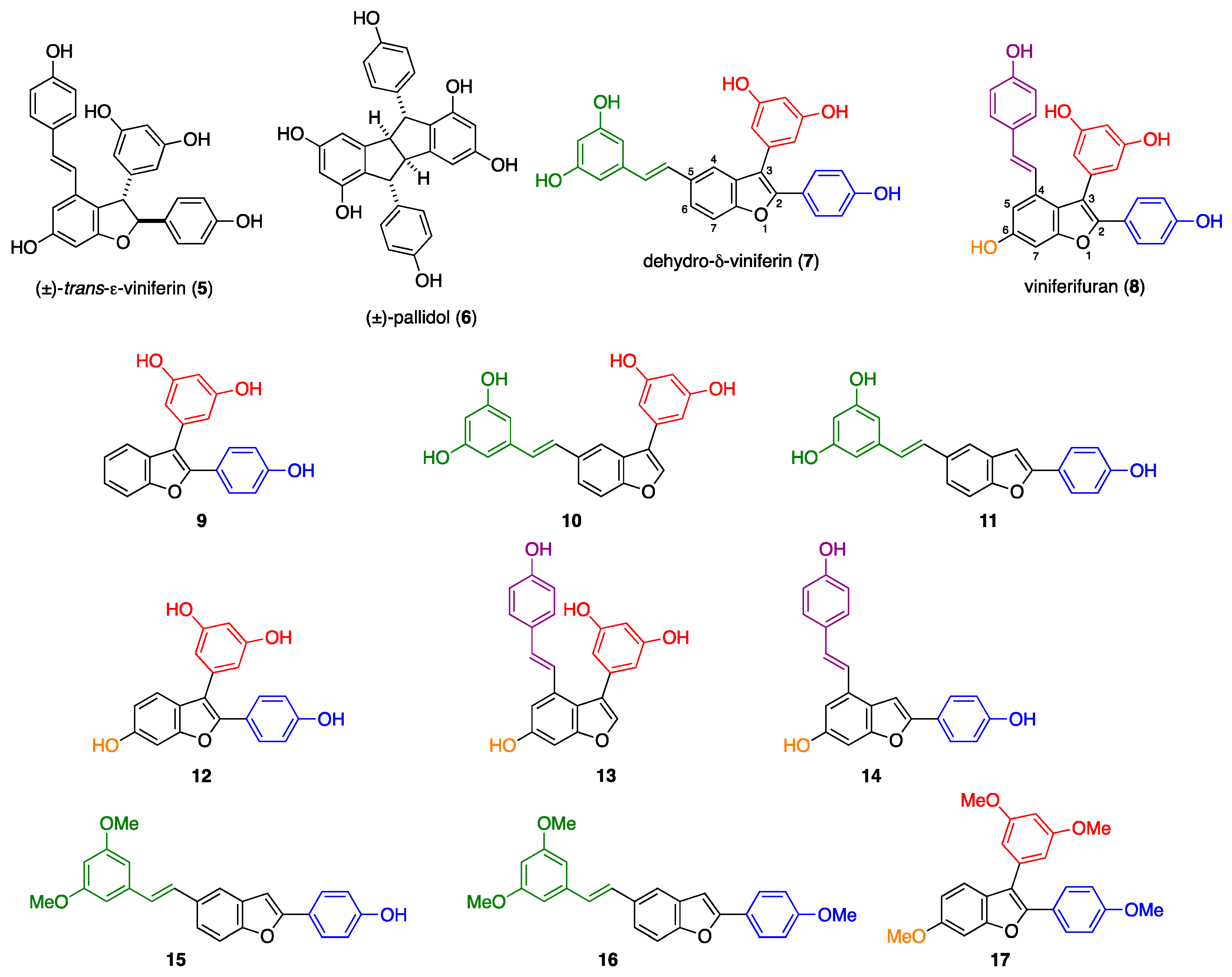
2. Results and Discussion
2.1. Cytotoxic Activity Evaluation
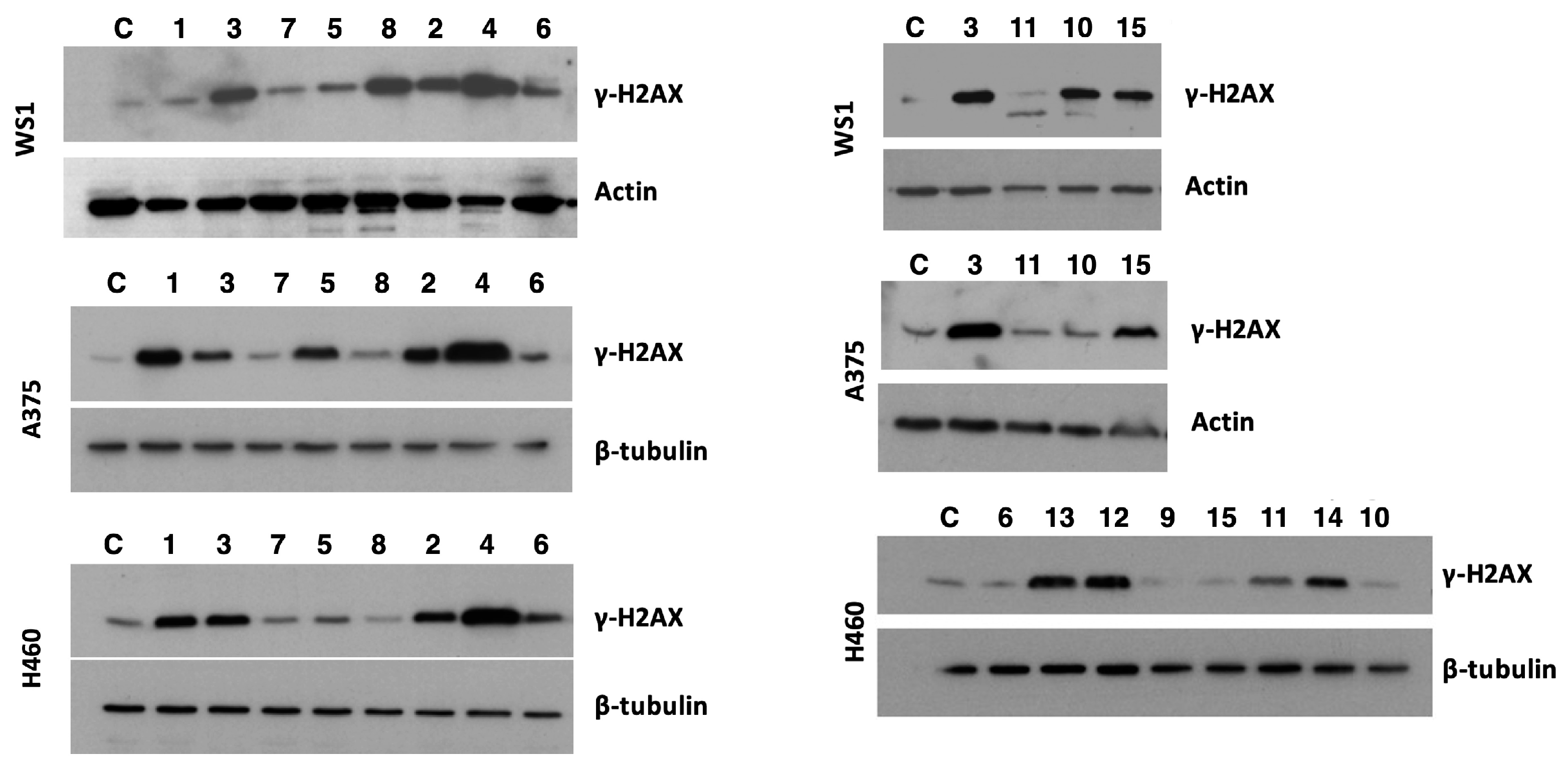
2.2. DNA Damage
3. Materials and Methods
3.1. Synthesis
3.2. Cell Lines
3.3. Cytotoxicity Assay
3.4. Western Blot Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duta-Bratu, C.-G.; Nitulescu, G.M.; Mihai, D.P.; Olaru, O.T. Resveratrol and Other Natural Oligomeric Stilbenoid Compounds and Their Therapeutic Applications. Plants 2023, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Akinwumi, B.; Bordun, K.-A.; Anderson, H. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-C.; Ho, C.-T.; Pan, M.-H. Recent Advances in Health Benefits of Stilbenoids. J. Agric. Food Chem. 2021, 69, 10036–10057. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, B.; Ammazzalorso, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R. Anticancer Activity of Stilbene-Based Derivatives. ChemMedChem 2017, 12, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Kursvietiene, L.; Kopustinskiene, D.M.; Staneviciene, I.; Mongirdiene, A.; Kubová, K.; Masteikova, R.; Bernatoniene, J. Anti-Cancer Properties of Resveratrol: A Focus on Its Impact on Mitochondrial Functions. Antioxidants 2023, 12, 2056. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.J.; Altaf, A.; Imran, M.; Amir, M.; Alsagaby, S.A.; Al Abdulmonem, W.; Mujtaba, A.; El-Ghorab, A.H.; Ghoneim, M.M.; Hussain, M.; et al. Anti-Cancer Perspectives of Resveratrol: A Comprehensive Review. Food Agric. Immunol. 2023, 34, 2265686. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; Jihad-Jebbar, A.; López-Blanch, R.; Dellinger, T.H.; Dellinger, R.W.; Estrela, J.M. Pterostilbene in Cancer Therapy. Antioxidants 2021, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Platella, C.; Mazzini, S.; Napolitano, E.; Mattio, L.M.; Beretta, G.L.; Zaffaroni, N.; Pinto, A.; Montesarchio, D.; Dallavalle, S. Plant-Derived Stilbenoids as DNA-Binding Agents: From Monomers to Dimers. Chem.–A Eur. J. 2021, 27, 8832–8845. [Google Scholar] [CrossRef] [PubMed]
- Catinella, G.; Mattio, L.M.; Musso, L.; Arioli, S.; Mora, D.; Beretta, G.L.; Zaffaroni, N.; Pinto, A.; Dallavalle, S. Structural Requirements of Benzofuran Derivatives Dehydro-δ- and Dehydro-ε-Viniferin for Antimicrobial Activity Against the Foodborne Pathogen Listeria Monocytogenes. Int. J. Mol. Sci. 2020, 21, 2168. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial Activity of Resveratrol-Derived Monomers and Dimers against Foodborne Pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef] [PubMed]
- Mah, L.-J.; El-Osta, A.; Karagiannis, T.C. ΓH2AX: A Sensitive Molecular Marker of DNA Damage and Repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Platella, C.; Raucci, U.; Rega, N.; D’Atri, S.; Levati, L.; Roviello, G.N.; Fuggetta, M.P.; Musumeci, D.; Montesarchio, D. Shedding Light on the Interaction of Polydatin and Resveratrol with G-Quadruplex and Duplex DNA: A Biophysical, Computational and Biological Approach. Int. J. Biol. Macromol. 2020, 151, 1163–1172. [Google Scholar] [CrossRef]
- Platella, C.; Guida, S.; Bonmassar, L.; Aquino, A.; Bonmassar, E.; Ravagnan, G.; Montesarchio, D.; Roviello, G.N.; Musumeci, D.; Fuggetta, M.P. Antitumour Activity of Resveratrol on Human Melanoma Cells: A Possible Mechanism Related to Its Interaction with Malignant Cell Telomerase. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 2843–2851. [Google Scholar] [CrossRef]
| Cpd | WS1 | A375 | H460 | PC3 |
|---|---|---|---|---|
| IC50 (µM) b | ||||
| 1 c | >200 | 44.5 ± 3.5 | 25 ± 0.77 | >100 |
| 2 c | 57 ± 10 | 33 ± 0.7 | 25 ± 0.4 | 97 ± 4.9 |
| 3 c | 69 ± 5.6 | 95 ± 7 | 81 ± 4 | 120 ± 7.8 |
| 4 c | 82.7 ± 1.1 | 25.5 ± 2.1 | 24.7 ± 0.3 | 46.7 ± 3.3 |
| 5 | 82 ± 2.8 | 95 ± 2.8 | 61.3 ± 17 | 95 ± 0.2 |
| 6 | >100 | 93 ± 2.8 | 73 ± 0.3 | >200 |
| 7 | 37 ± 1.4 d | 42.3 ± 8.0 | 27 ± 0.3 | 46 ± 0.5 |
| 8 | 33 ± 1.4 d | 46.5 ± 3.5 | 26 ± 1.0 | 43.3 ± 0.4 |
| 9 | 98.7 ± 1.8 d | 58.2 ± 0.8 | 50 ± 3.5 | 101 ± 1.4 |
| 10 | 96.8 ± 4.5 d | 24 ± 1.8 | 28 ± 2.8 | 100 ± 0.7 |
| 11 | 97.8 ± 3.0 d | 28 ± 9 | 57 ± 6.4 | 86 ± 2.8 |
| 12 | 98.5 ± 2.0 d | 67 ± 1.9 | 77 ± 0.42 | 99 ± 1.4 |
| 13 | 85 ± 4.6 d | 36 ± 1.1 | 69 ± 0.3 | 98 ± 2 |
| 14 | 45 ± 1.2 d | 46 ± 3.5 | 26.8 ± 0.3 | 42.7 ± 0.5 |
| 15 | 95 ± 2.3 d | 18 ± 2.8 | 25 ± 2.1 | 85 ± 21 |
| 16 | >100 d | >100 | >100 | >100 |
| 17 | >100 d | >100 | >100 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Princiotto, S.; Pinna, C.; Mattio, L.M.; Annunziata, F.; Beretta, G.L.; Pinto, A.; Dallavalle, S. Cytotoxicity of Benzofuran-Containing Simplified Viniferin Analogues. Pharmaceuticals 2024, 17, 1012. https://doi.org/10.3390/ph17081012
Princiotto S, Pinna C, Mattio LM, Annunziata F, Beretta GL, Pinto A, Dallavalle S. Cytotoxicity of Benzofuran-Containing Simplified Viniferin Analogues. Pharmaceuticals. 2024; 17(8):1012. https://doi.org/10.3390/ph17081012
Chicago/Turabian StylePrinciotto, Salvatore, Cecilia Pinna, Luce Micaela Mattio, Francesca Annunziata, Giovanni Luca Beretta, Andrea Pinto, and Sabrina Dallavalle. 2024. "Cytotoxicity of Benzofuran-Containing Simplified Viniferin Analogues" Pharmaceuticals 17, no. 8: 1012. https://doi.org/10.3390/ph17081012
APA StylePrinciotto, S., Pinna, C., Mattio, L. M., Annunziata, F., Beretta, G. L., Pinto, A., & Dallavalle, S. (2024). Cytotoxicity of Benzofuran-Containing Simplified Viniferin Analogues. Pharmaceuticals, 17(8), 1012. https://doi.org/10.3390/ph17081012










