The Effectiveness of a Dietary Supplement with Honey, Propolis, Pelargonium sidoides Extract, and Zinc in Children Affected by Acute Tonsillopharyngitis: An Open, Randomized, and Controlled Trial
Abstract
1. Introduction
2. Results
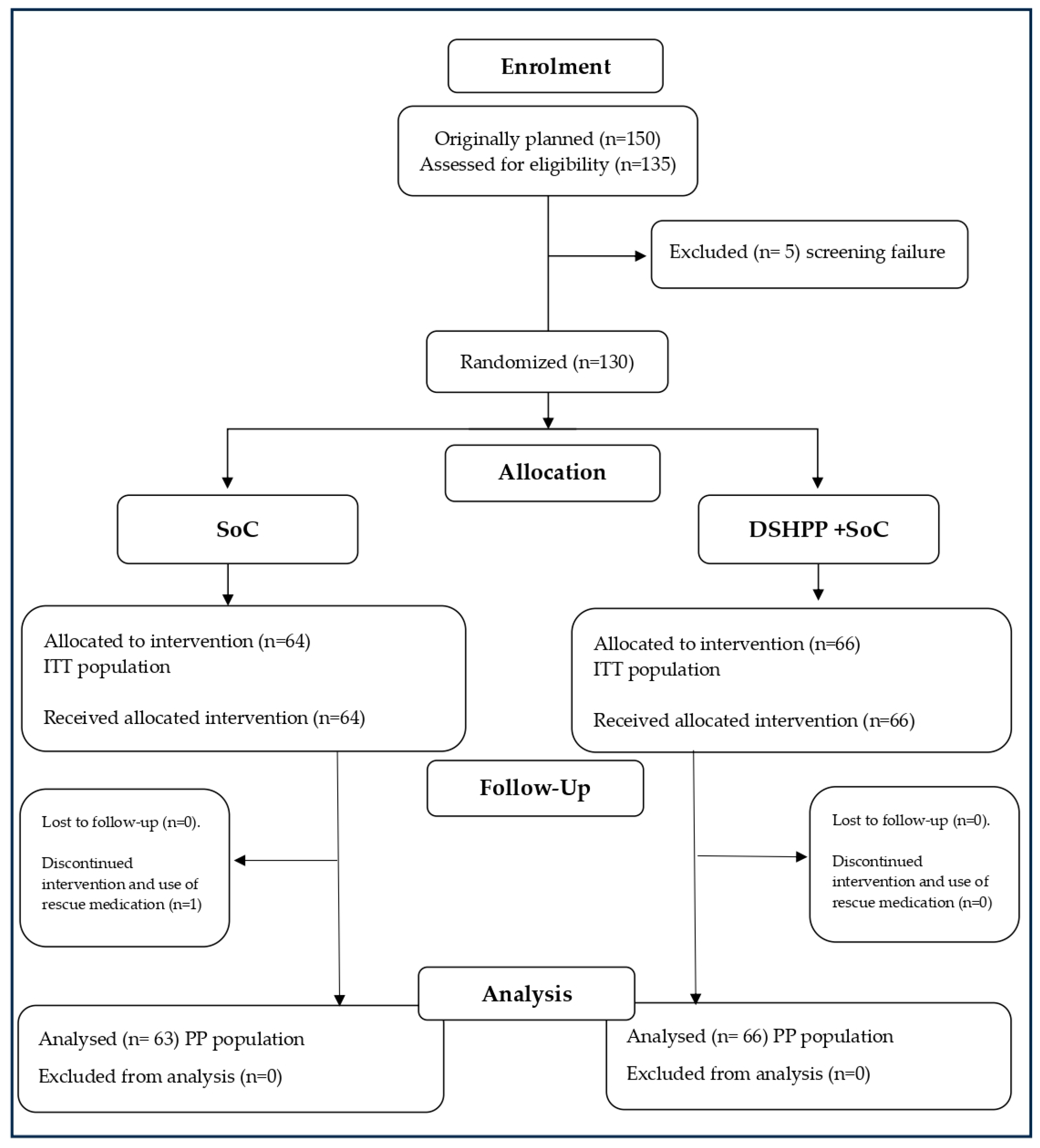
2.1. Patient Disposition
2.2. Administered Treatments and Compliance
2.3. Efficacy
2.3.1. Primary Outcomes
Tonsillitis Severity Score
TSS Total Score
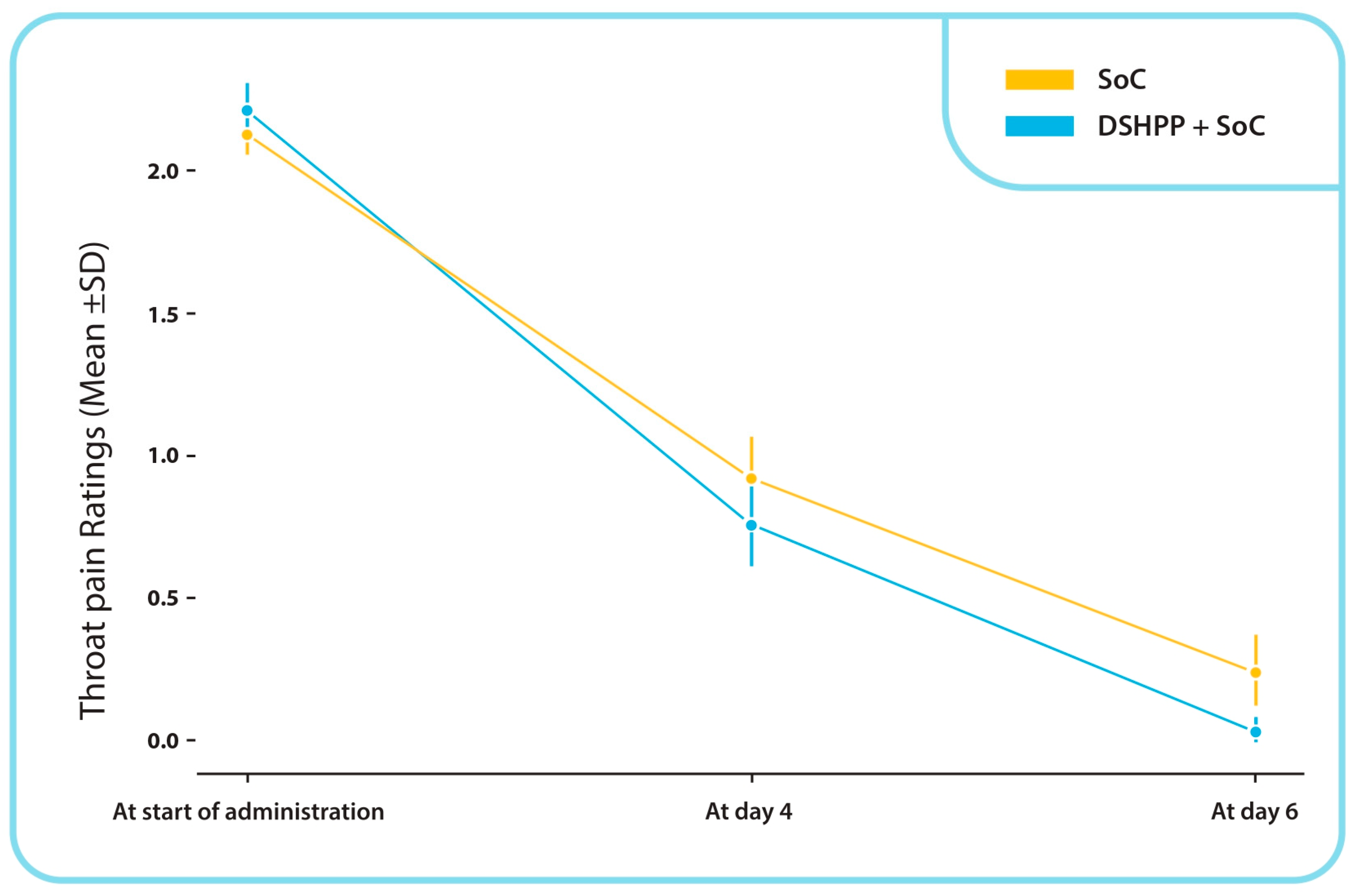
TSS Throat Pain Sub-Score
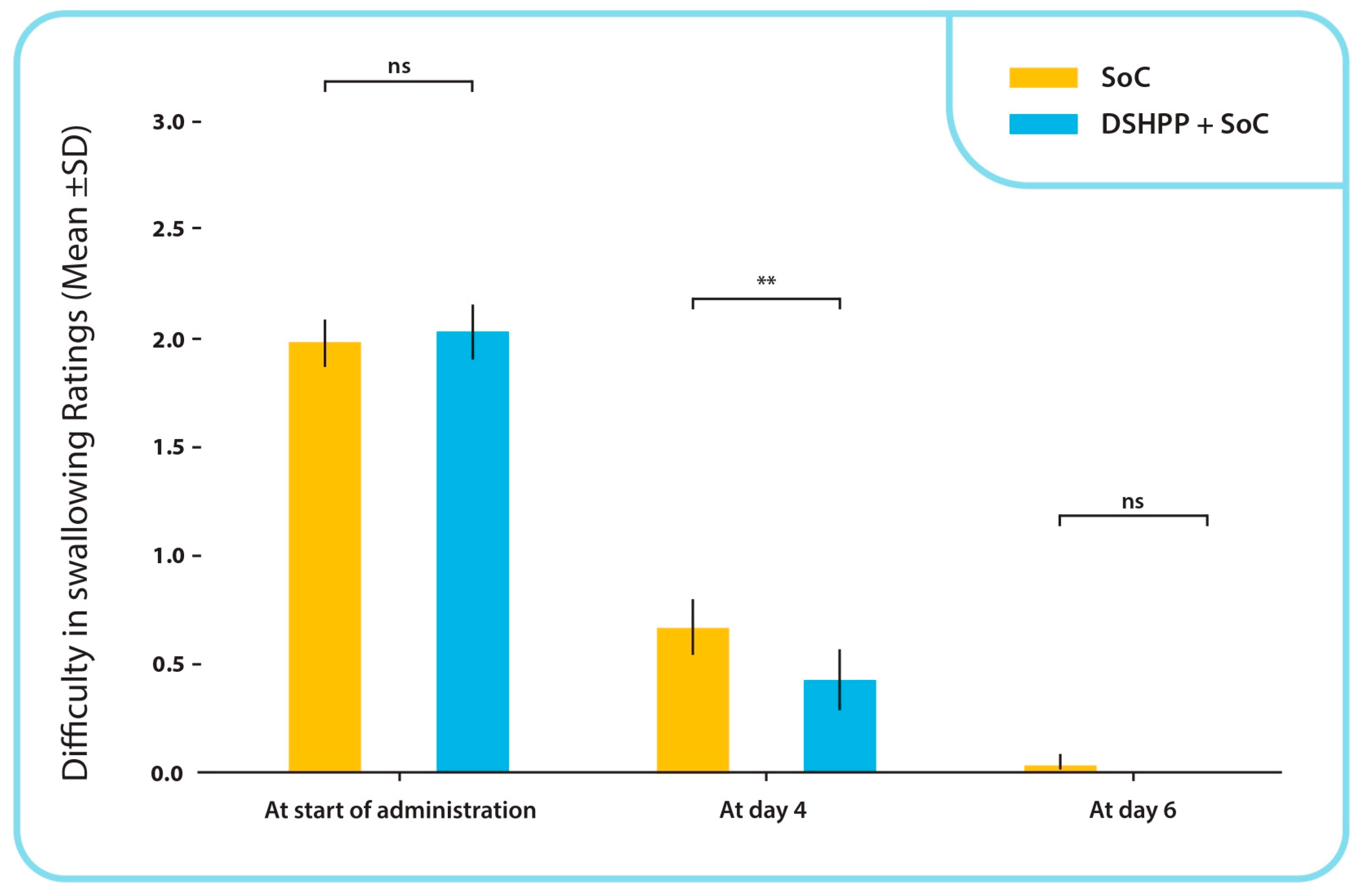
TSS Difficulty in Swallowing Sub-Score
TSS Salivation and Fever Sub-Score
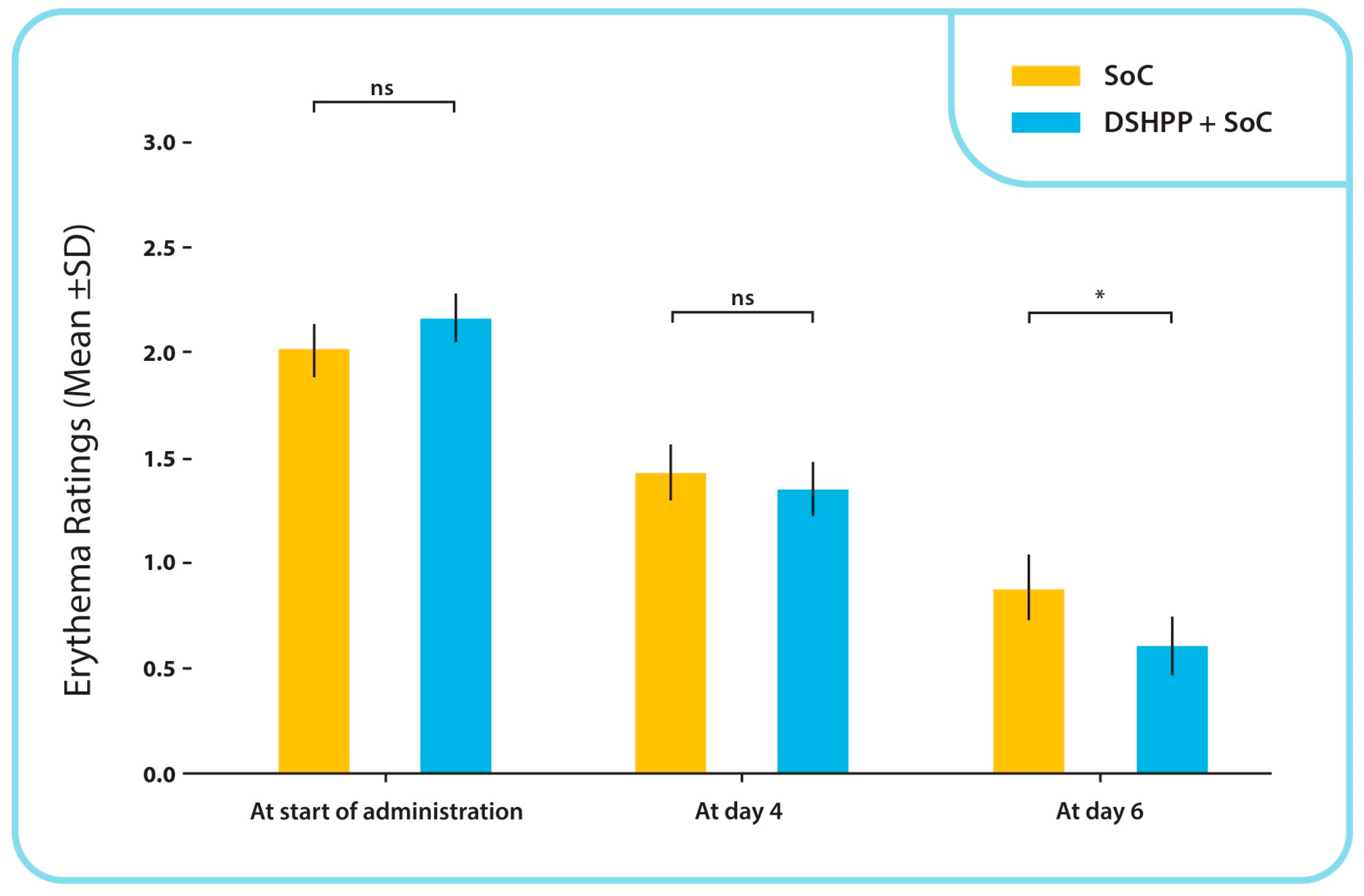
TSS Erythema Sub-Score
Use of Rescue Medications
2.3.2. Secondary Outcomes
IGA
PGA
2.4. Safety
3. Discussion
- (1)
- ATR should be diagnosed early (possibly in the last 48 h) through clinical evaluation of symptoms, including sore throat and catarrhal angina.
- (2)
- A negative result from a rapid test for group A beta-hemolytic Streptococcus (GABHS) or culture and the identification of nasal and/or pharyngeal exudate tests were mandatory.
- (3)
- The severity of ATR should be clearly defined with the exclusion of cases with lacunar or follicular angina, indications for antibiotic therapy (i.e., abscess and septic tonsillitis), immunodeficiencies, and chronic illnesses.
- (4)
- The exclusion of children under 3 years of age. This point was added because of the potential difficulty in justifying the exclusion of antibiotic therapy with the parents of infants of this age.
- The target was the medical doctor visiting and treating children affected by URTI.
- The objective was to modify therapeutic strategies based on antibiotic prescription that have been proved to be not justified in the majority of cases
- The final result should be that thousands of medical doctors understand situations when antibiotics may not be indicated, and this should reduce the consumption of antibiotics inadequately administered to children.
4. Materials and Methods
4.1. Study Design
4.2. Study Population
4.3. Intervention Population
4.3.1. Tested Product: DSHPP
4.3.2. Comparator: SoC
- Nasopharyngeal lavage through the administration of hydrating fluids to facilitate the elimination of body fluids, along with the aspiration of secretions, saline solution for nasal irrigation and nasal sprays containing seawater.
- Paracetamol (acetaminophen) (120 mg/5 mL): as antipyretic (fever is defined as body temperature > 38.5 °C), as needed, 10 mg/kg/dose. The dosage was to be administered every 6 to 8 h, according to the leaflet. The maximum dosage was 30 mg/kg/day.
- Benzydamine hydrochloride was to be administered by throat sprays for 6 days in children under 6 years of age; the dosage was to be 1 spray per 4 kg of body weight, up to a maximum of 4 sprays at one time, 2 to 6 times daily. In children from 6 to 12 years, the benzydamine hydrochloride dosage was to be 4 sprays, administered two to six times daily. Each spray corresponds to 0.17 mL of solution.
4.3.3. Rescue Medication and Treatment Not Permitted
- ibuprofen (100 mg/5 mL) orally;
- high dose (>30 mg/kg/day) paracetamol (120 mg/5 mL) administered orally.
4.4. Statistical Methods
4.4.1. Trial Hypothesis
4.4.2. Sample Size Calculation
4.4.3. Statistical Analyses
4.5. Evaluation Outcomes
4.5.1. Primary Outcomes
4.5.2. Secondary Outcomes
4.5.3. Safety
4.5.4. Schedule of Examinations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ESCMID Sore Throat Guideline Group; Pelucchi, C.; Grigoryan, L.; Galeone, C.; Esposito, S.; Huovinen, P.; Little, P.; Verheij, T. Guideline for the management of acute sore throat. Clin. Microbiol. Infect. 2012, 18 (Suppl. S1), 1–28. [Google Scholar] [CrossRef]
- Hersh, A.L.; Jackson, M.A.; Hicks, L.A.; American Academy of Pediatrics Committee on Infectious Diseases. Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics 2013, 132, 1146–1154. [Google Scholar] [CrossRef]
- Vicentini, C.; Vola, L.; Previti, C.; Brescia, V.; Dal Mas, F.; Zotti, C.M.; Bert, F. Antimicrobial Stewardship Strategies Including Point-of-Care Testing (POCT) for Pediatric Patients with Upper-Respiratory-Tract Infections in Primary Care: A Systematic Review of Economic Evaluations. Antibiotics 2022, 11, 1139. [Google Scholar] [CrossRef]
- Yildiz, I.; Gonullu, E.; Soysal, A.; Oner, C.N.; Karabocuoglu, M. The Epidemiology of Influenza Virus Infection and Group A Streptococcal Pharyngitis in Children between 2011 and 2018 in an Outpatient Pediatric Clinic. Cureus 2023, 15, e33492. [Google Scholar] [CrossRef]
- Hersh, A.L.; Shapiro, D.J.; Pavia, A.T.; Shah, S.S. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics 2011, 128, 1053–1061. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M., Jr.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef]
- Al-Waili, N.; Al-Ghamdi, A.; Ansari, M.J.; Al-Attal, Y.; Salom, K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int. J. Med. Sci. 2012, 9, 793–800. [Google Scholar] [CrossRef]
- Dimitriu, L.; Constantinescu-Aruxandei, D.; Preda, D.; Nichițean, A.-L.; Nicolae, C.-A.; Faraon, V.A.; Ghiurea, M.; Ganciarov, M.; Băbeanu, N.E.; Oancea, F. Honey and Its Biomimetic Deep Eutectic Solvent Modulate the Antioxidant Activity of Polyphenols. Antioxidants 2022, 11, 2194. [Google Scholar] [CrossRef]
- Oduwole, O.; Udoh, E.E.; Oyo-Ita, A.; Meremikwu, M.M. Honey for acute cough in children. Cochrane Database Syst. Rev. 2018, 4, CD007094. [Google Scholar] [CrossRef]
- Abuharfeil, N.; Al-Oran, R.; Abo-Shehada, M. The effect of bee honey on the proliferative activity of human B- and T lymphocytes and the activity of phagocytes. Food Agric. Immunol. 1999, 11, 169–177. [Google Scholar] [CrossRef]
- Khan, R.U.; Naz, S.; Abudabos, A.M. Towards a better understanding of the therapeutic applications and corresponding mechanisms of action of honey. Environ. Sci. Pollut. Res. Int. 2017, 24, 27755–27766. [Google Scholar] [CrossRef]
- Llor, C.; Moragas, A.; Ouchi, D.; Monfà, R.; Garcia-Sangenís, A.; Gómez-Lumbreras, A.; Pera, H.; Pujol, J.; Morros, R. Effectiveness of antitussives, anticholinergics, and honey versus usual care in adults with uncomplicated acute bronchitis: A multiarm randomized clinical trial. Fam. Pr. 2023, 40, 407–413. [Google Scholar] [CrossRef]
- Nazari-Bonab, H.; Jamilian, P.; Radkhah, N.; Zarezadeh, M.; Ebrahimi-Mameghani, M. The effect of propolis supplementation in improving antioxidant status: A systematic review and meta-analysis of controlled clinical trials. Phytother. Res. 2023, 37, 3712–3723. [Google Scholar] [CrossRef]
- Esposito, C.; Garzarella, E.U.; Bocchino, B.; D’Avino, M.; Caruso, G.; Buonomo, A.R.; Sacchi, R.; Galeotti, F.; Tenore, G.C.; Zaccaria, V.; et al. A standardized polyphenol mixture extracted from poplar-type propolis for remission of symptoms of uncomplicated upper respiratory tract infection (URTI): A monocentric, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2021, 80, 153368. [Google Scholar] [CrossRef]
- Crişan, I.; Zaharia, C.N.; Popovici, F.; Jucu, V.; Belu, O.; Dascălu, C.; Mutiu, A.; Petrescu, A. Natural propolis extract NIVCRISOL in the treatment of acute and chronic rhinopharyngitis in children. Rom. J. Virol. 1995, 46, 115–133. [Google Scholar]
- Khayyal, M.T.; El-Ghazaly, M.A.; El-Khatib, A.S.; Hatem, A.M.; De Vries, P.J.F.; El-Shafei, S.; Khattab, M.M. A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundam. Clin. Pharmacol. 2003, 17, 93–102. [Google Scholar] [CrossRef]
- Buha, I.; Mirić, M.; Agić, A.; Simic, M.; Stjepanovic, M.; Milenkovic, B.; Nagorni-Obradovic, L.; Skodric-Trifunovic, V.; Ilic, B.; Popevic, S.; et al. A randomized, double-blind, placebo-controlled study evaluating the efficacy of propolis and N-acetylcysteine in exacerbations of chronic obstructive pulmonary disease. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4809–4815. [Google Scholar] [CrossRef]
- Kolarov, V.; Kotur Stevuljević, J.; Ilić, M.; Bogdan, M.; Tusek, B.; Agic, A.; Dugajlic, M.; Veres, K.T.; Stevic, S.K.; Zvezdin, B. Factorial analysis of N-acetylcysteine and propolis treatment effects on symptoms, life quality and exacerbations in patients with Chronic Obstructive Pulmonary Disease (COPD): A randomized, double-blind, placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3192–3199. [Google Scholar] [CrossRef]
- Nault, D.; Machingo, T.A.; Shipper, A.G.; A Antiporta, D.; Hamel, C.; Nourouzpour, S.; Konstantinidis, M.; Phillips, E.; A Lipski, E.; Wieland, L.S. Zinc for prevention and treatment of the common cold. Cochrane Database Syst. Rev. 2024, 5, CD014914. [Google Scholar] [CrossRef]
- Careddu, D.; Pettenazzo, A. Pelargonium sidoides extract EPs 7630: A review of its clinical efficacy and safety for treating acute respiratory tract infections in children. Int. J. Gen. Med. 2018, 11, 91–98. [Google Scholar] [CrossRef]
- Bereznoy, V.V.; Riley, D.S.; Wassmer, G.; Heger, M. Efficacy of extract of Pelargonium sidoides in children with acute non-group A beta-hemolytic Streptococcus tonsillopharyngitis: A randomized, double-blind, placebo-controlled trial. Altern. Ther. Health Med 2003, 9, 68–79. [Google Scholar]
- Nöldner, M.; Schötz, K. Inhibition of lipopolysaccharid-induced sickness behavior by a dry extract from the roots of Pelargonium sidoides (EPs 7630) in mice. Phytomedicine 2007, 14 (Suppl. S6), 27–31. [Google Scholar] [CrossRef]
- Kolodziej, H. Antimicrobial, Antiviral and Immunomodulatory Activity Studies of Pelargonium sidoides (EPs® 7630) in the Context of Health Promotion. Pharmaceuticals 2011, 4, 1295–1314. [Google Scholar] [CrossRef]
- Shulman, S.T.; Bisno, A.L.; Clegg, H.W.; Gerber, M.A.; Kaplan, E.L.; Lee, G.; Martin, J.M.; Van Beneden, C. Infectious Diseases Society of America. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2012, 55, e86–e102, Erratum in Clin. Infect. Dis. 2014, 58, 1496. [Google Scholar] [CrossRef]
- Sauve, L.; Forrester, A.M.; Top, K.A. Group A streptococcal pharyngitis: A practical guide to diagnosis and treatment. Paediatr. Child Health 2021, 26, 319–320. [Google Scholar] [CrossRef]
- Baillie, E.J.; Merlo, G.; Magin, P.; Tapley, A.; Mulquiney, K.J.; Davis, J.S.; Fielding, A.; Davey, A.; Holliday, E.; Ball, J.; et al. Antibiotic prescribing for upper respiratory tract infections and acute bronchitis: A longitudinal analysis of general practitioner trainees. Fam. Pract. 2022, 39, 1063–1069. [Google Scholar] [CrossRef]
- Butler, C.C.; Simpson, S.A.; Dunstan, F.; Rollnick, S.; Cohen, D.; Gillespie, D.; Evans, M.R.; Alam, M.F.; Bekkers, M.J.; Evans, J.; et al. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: Practice based randomised controlled trial. BMJ 2012, 344, d8173. [Google Scholar] [CrossRef]
- Little, P.; Stuart, B.; Francis, N.; Douglas, E.; Tonkin-Crine, S.; Anthierens, S.; Cals, J.W.; Melbye, H.; Santer, M.; Moore, M.; et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: A multinational, cluster, randomised, factorial, controlled trial. Lancet 2013, 382, 1175–1182. [Google Scholar] [CrossRef]
- Blair, P.S.; Young, G.J.; Clement, C.; Dixon, P.; Seume, P.; Ingram, J.; Taylor, J.; Horwood, J.; Lucas, P.J.; Cabral, C.; et al. A multifaceted intervention to reduce antibiotic prescribing among CHIldren with acute COugh and respiratory tract infection: The CHICO cluster RCT. Health Technol. Assess. 2023, 27, 1–110. [Google Scholar] [CrossRef]
- Kronman, M.P.; Gerber, J.S.; Grundmeier, R.W.; Zhou, C.; Robinson, J.D.; Heritage, J.; Stout, J.; Burges, D.; Hedrick, B.; Warren, L.; et al. Reducing Antibiotic Prescribing in Primary Care for Respiratory Illness. Pediatrics 2020, 146, e20200038. [Google Scholar] [CrossRef]
- Cardinale, F.; Barattini, D.F.; Morariu Bordea, M.; Herteg, D.; Matei, C.R. A Randomized, Open, Controlled Study to Evaluate the Efficacy and Safety of Pediaflù® (Dietary Supplement) Along with Standard of Care in Children with Acute Tonsillopharyngitis/Rhinopharyngitis versus Standard of Care Only V.1. 2023. Available online: https://www.protocols.io/view/a-randomized-open-controlled-study-to-evaluate-the-kqdg398oqg25/v1 (accessed on 3 June 2024).
- Saint-Raymond, A.; Hill, S.; Martines, J.; Bahl, R.; Fontaine, O.; Bero, L. CONSORT 2010. Lancet 2010, 376, 229–230. [Google Scholar] [CrossRef]
- Cardinale, F.; Barattini, D.F.; Sbrocca, F.; Centi, A.; Giuntini, G.; Morariu Bordea, M.; Herteg, D.; Rosu, S.; Matei, C.R. The Effects of a Dietary Supplement (PediaFlu) Plus Standard of Care in Children with Acute Tonsillopharyngitis/Rhinopharyngitis: Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2024, 13, e53703. [Google Scholar] [CrossRef]
| Characteristics | All no. = 129 | DSHPP + SoC no. = 66 | SoC no. = 63 | p-Value | |
|---|---|---|---|---|---|
| Age (years) | Mean (SD) | 5.52 (2.07) | 5.8 (2.01) | 5.22 (2.11) | ns |
| Median | 5 | 5.5 | 5 | ||
| Range | 3–10 | 3–10 | 3–10 | ||
| BMI (kg/m2) | Mean (SD) | 16.41 (3.06) | 16.71 (3.0) | 16.1 (3.11) | ns |
| Median | 15.54 | 15.97 | 15.42 | ||
| Range | 10.42–30.26 | 10.42–25.69 | 11.76–30.26 | ||
| Height (cm) | Mean (SD) | 114.51 (14.95) | 116.56 (15.36) | 112.37 (14.31) | ns |
| Median | 114 | 116 | 108 | ||
| Range | 89–160 | 89–160 | 90–148 | ||
| Weight (kg) | Mean (SD) | 22.1 (8.29) | 23.32 (8.74) | 20.83 (7.66) | ns |
| Median | 20 | 22 | 19 | ||
| Range | 11–60 | 12–60 | 11–54 |
| DSHPP + SoC | SoC | p-Value | ||
|---|---|---|---|---|
| Start of administration | no. | 66 | 63 | |
| Mean (SD) | 9.2 (1.4) | 8.9 (1.2) | ns | |
| Day 4 | no. | 66 | 63 | |
| Mean (SD) | 3.2 (1.4) | 3.8 (1.7) | * | |
| Day 6 | no. | 66 | 63 | |
| Mean (SD) | 0.7 (0.7) | 1.2 (1.0) | ** |
| IGA | Grade | DSHPP + SoC | SoC | p-Value |
|---|---|---|---|---|
| Subjects no. (%) | 66 (100.0%) | 63 (100.0%) | ||
| Very Good | 55 (83.3%) | 37 (58.7%) | 0.040 | |
| Good | 11 (16.7%) | 18 (28.6%) | ||
| Moderate | 0 (0.0%) | 8 (12.7%) | ||
| Poor | 0 (0.0%) | 0 (0.0%) |
| PGA | Grade | DSHPP + SoC | SoC | p-Value |
|---|---|---|---|---|
| Subjects no. (%) | 66 (100.0%) | 63 (100.0%) | ||
| Very Good | 53 (80.3%) | 35 (55.6%) | 0.013 | |
| Good | 12 (18.2%) | 19 (30.2%) | ||
| Moderate | 1 (1.5%) | 6 (9.5%) | ||
| Poor | 0 (0.0%) | 3 (4.8%) |
| Inclusion Criteria | |
| 1 | Male and female (children aged 3 to 10 years). |
| 2 | Acute tonsillopharyngitis/rhinopharyngitis (ATR; sore throat, catarrhal angina), duration of symptoms ≤ 48 h. |
| 3 | Negative with rapid test for group A beta-hemolytic Streptococcus (GABHS) or culture and identification of nasal and/or pharyngeal exudates; negative for SARS-CoV-2 infection. |
| 4 | Tonsillitis symptom score (TSS) ≥ 8 points. |
| 5 | Both parents are willing to provide written informed consent prior to participation in the clinical trial. |
| 6 | Children older than 6 years must also have the ability and willingness to provide written informed consent. |
| Exclusion criteria | |
| 1 | Evidence of lacunar or follicular angina. |
| 2 | More than 2 past episodes of tonsillitis in the previous 12 months. |
| 3 | Mandatory indication for antibiotic therapy (e.g., abscess, septic tonsillitis, status postrheumatic fever, poststreptococcal glomerulonephritis, and minor Sydenham chorea). |
| 4 | Treatment with antibiotics within 4 months prior to study enrollment. |
| 5 | Hemorrhagic diathesis increases and chronic illnesses (e.g., severe heart, kidney, or liver disease, and primary or secondary immunodeficiencies). |
| 6 | Close contact history with individuals infected with SARS-CoV-2 within 10 days before showing symptoms. |
| 7 | Known or suspected allergic reactions to any study medication. |
| 8 | Concomitant therapy that may affect study results or have known interactions with study medications (such as coumarin derivatives). |
| 9 | Participation in another clinical trial within 3 months before enrollment. |
| Time Point | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| Day −2 to −1 | Day 0 | Day 4 | Day 6 | |
| Informed consent | ✓ | |||
| Inclusion criteria | ✓ | |||
| Exclusion criteria | ✓ | ✓ | ✓ | ✓ |
| Demographics and medical history | ✓ | |||
| Physical examination | ✓ | ✓ | ✓ | ✓ |
| Disease assessment | ✓ | ✓ | ✓ | ✓ |
| Rapid test for GABHS a or nasal and/or pharyngeal exudate culture plus SARS-CoV-2 | ✓ | |||
| Concomitant treatments | ✓ | ✓ | ✓ | ✓ |
| TSS b | ✓ | ✓ | ✓ | ✓ |
| Product delivery | ✓ | |||
| Product return | ✓ | |||
| Subject diary delivery | ✓ | |||
| Patient’s diary verification | ✓ | |||
| Subject diary return | ✓ | |||
| Products accountability | ✓ | |||
| IGA c | ✓ | |||
| PGA d | ✓ | |||
| Global assessment of the safety | ✓ | |||
| Adverse events | ✓ | ✓ | ✓ | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardinale, F.; Barattini, D.F.; Martinucci, V.; Bordea, M.M.; Barattini, L.; Rosu, S. The Effectiveness of a Dietary Supplement with Honey, Propolis, Pelargonium sidoides Extract, and Zinc in Children Affected by Acute Tonsillopharyngitis: An Open, Randomized, and Controlled Trial. Pharmaceuticals 2024, 17, 804. https://doi.org/10.3390/ph17060804
Cardinale F, Barattini DF, Martinucci V, Bordea MM, Barattini L, Rosu S. The Effectiveness of a Dietary Supplement with Honey, Propolis, Pelargonium sidoides Extract, and Zinc in Children Affected by Acute Tonsillopharyngitis: An Open, Randomized, and Controlled Trial. Pharmaceuticals. 2024; 17(6):804. https://doi.org/10.3390/ph17060804
Chicago/Turabian StyleCardinale, Fabio, Dionisio Franco Barattini, Valentina Martinucci, Maria Morariu Bordea, Luca Barattini, and Serban Rosu. 2024. "The Effectiveness of a Dietary Supplement with Honey, Propolis, Pelargonium sidoides Extract, and Zinc in Children Affected by Acute Tonsillopharyngitis: An Open, Randomized, and Controlled Trial" Pharmaceuticals 17, no. 6: 804. https://doi.org/10.3390/ph17060804
APA StyleCardinale, F., Barattini, D. F., Martinucci, V., Bordea, M. M., Barattini, L., & Rosu, S. (2024). The Effectiveness of a Dietary Supplement with Honey, Propolis, Pelargonium sidoides Extract, and Zinc in Children Affected by Acute Tonsillopharyngitis: An Open, Randomized, and Controlled Trial. Pharmaceuticals, 17(6), 804. https://doi.org/10.3390/ph17060804






