Potential Molecular Mechanism of Illicium simonsii Maxim Petroleum Ether Fraction in the Treatment of Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
2.1. LC-MS/MS Analyzed the Chemical Substances of PEIM
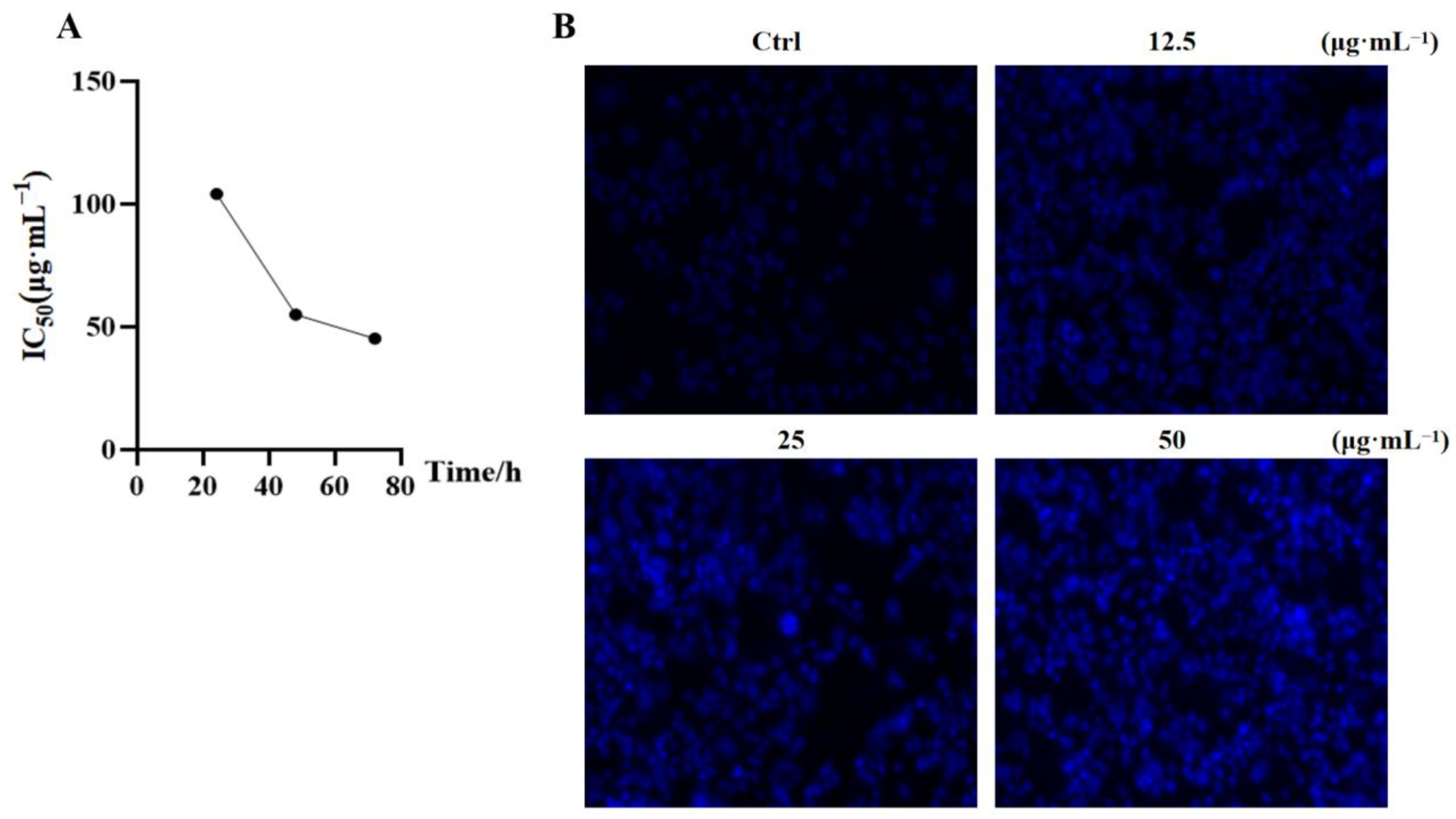
2.2. PEIM Affected the Proliferation and Morphology in HepG2 Cells
2.3. PEIM Decreased the Content of TNF-α, IL-1β and IL-6 in HepG2 Cells
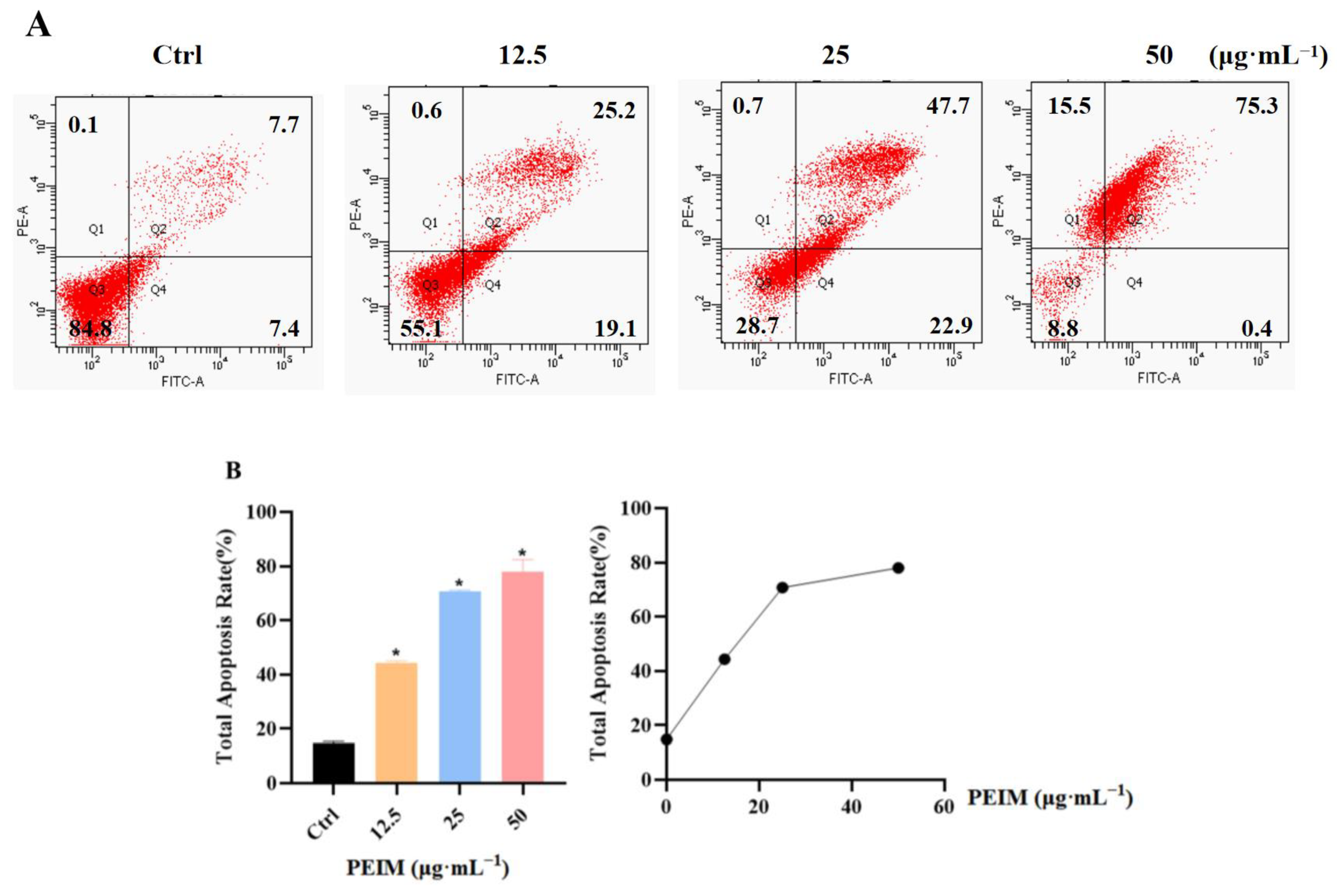
2.4. PEIM Promoted Apoptosis in HepG2 Cells
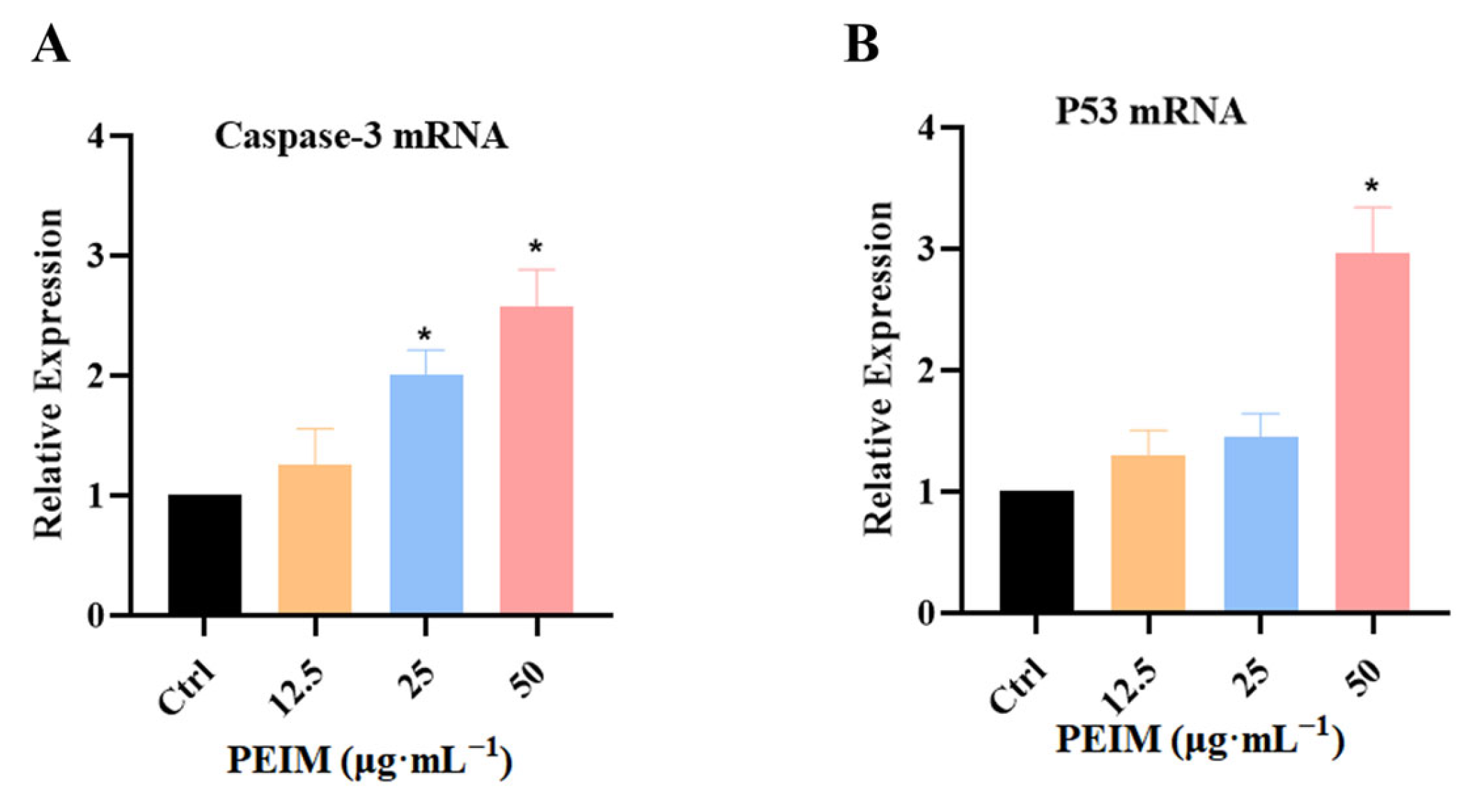
2.5. PEIM Increased the Expression of P53 mRNA and Caspase-3 mRNA in HepG2 Cells
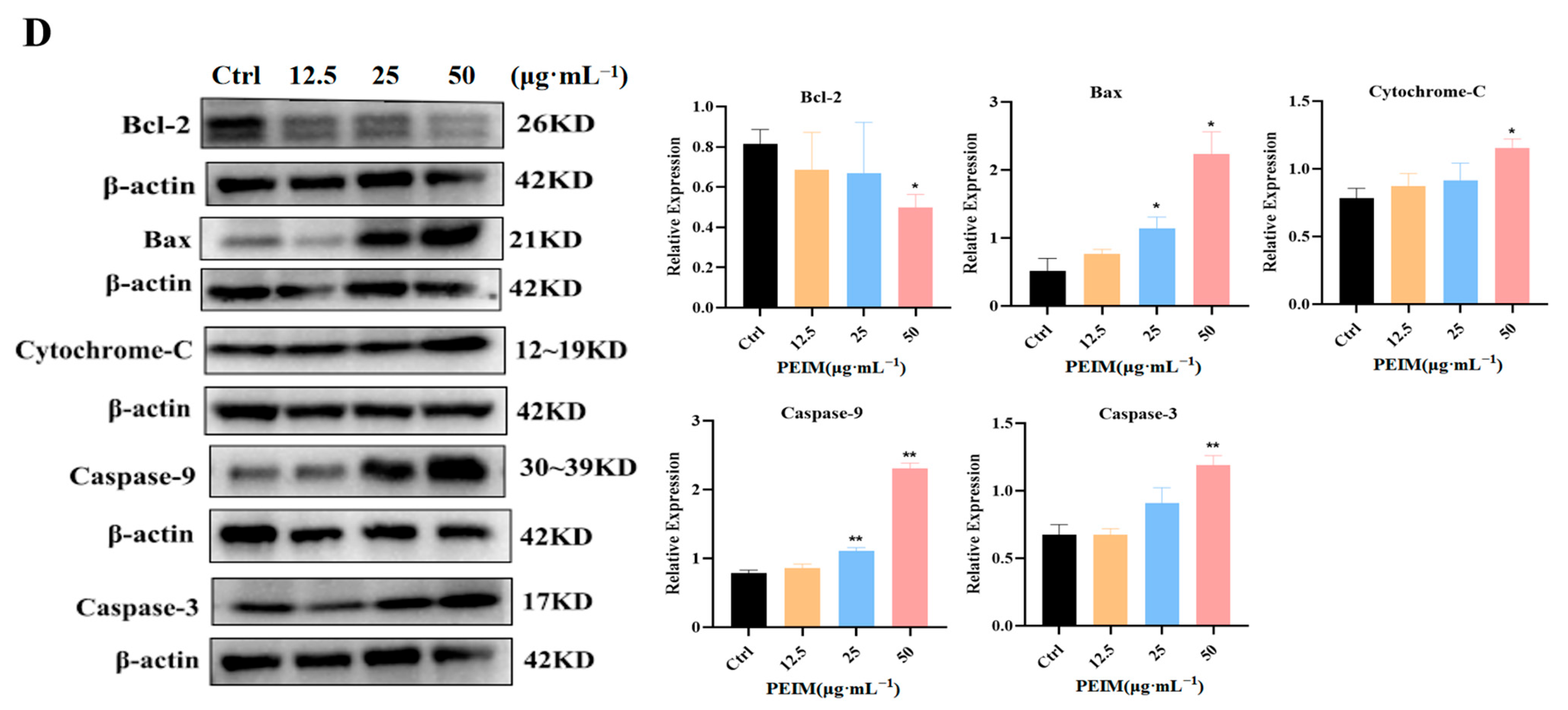
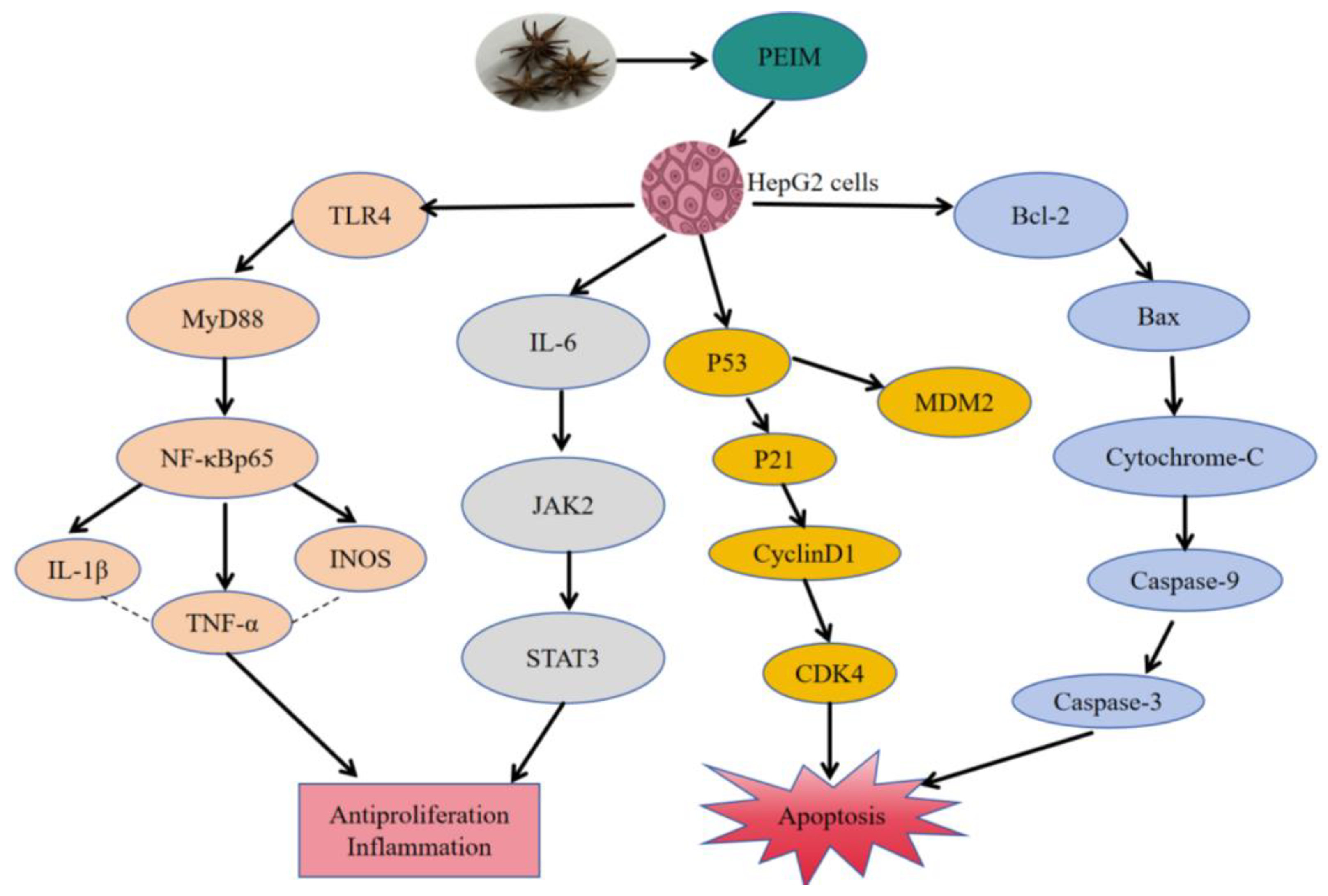
2.6. PEIM Affected the Expression of TLR4/MyD88/NF-κB, JAK2/STAT3, P53/P21/MDM2, and Mitochondrial Apoptosis Pathway-Related Proteins in HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. Drug Preparation
4.2. Liquid Chromatography-Mass Spectroscopy Study
4.3. Cell Culture and Treatment
4.4. Cell Viability Assay
4.5. Enzyme-Linked Immunosorbent Assay
4.6. Hoechst33342 Staining
4.7. FACS Analysis
4.8. RT-qPCR Assay
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemalet, A. Global Cancer Statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Cheng, Z.; Lin, X.; Ding, J. Characteristics of hepatocellular carcinoma stem cells and clinical correlations. Cancer Lett. 2016, 379, 230. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, P.H.; Gao, W.H.; Tian, X.F.; Tian, S.; Wu, R.X.; Huang, X.D.; Zheng, P.; Huang, Z.; Guo, Y.M. To explore the relationship between pyroptosis and hepatocellular carcinoma based on the theory of deficiency toxin and blood stasis pathogenesis. Chin. J. Basic Med. Tradit. Chin. Med. 2021, 27, 818–820+852. [Google Scholar]
- Jin, H.; Shi, L.Y. EGFR activation limits the response of liver cancer to lenvatinib. Nature 2021, 595, 730–734. [Google Scholar] [CrossRef]
- Miller, A.B.; Hoogstraten, B.; Staquet, M. Reporting results of cancer treatment. Cancer 1981, 47, 207–214. [Google Scholar] [CrossRef]
- Qin, M.R.; Chen, R.H.; Liu, L.H.; Xie, T.Z.; Wang, P.; Wang, X.W. Mechanism Exploration of Polyphyllin I in Inhibiting Proliferation, Invasion, Migration Hepatocellular Carcinoma via PI3K/AKT Signaling Pathway. Liaoning J. Tradit. Chin. Med. 2024, 51, 144–147+221. [Google Scholar]
- Yuan, Z.; Liang, Z.; Yi, J. Koumine Promotes ROS Production to Suppress Hepatocellular Carcinoma Cell Proliferation via NF-κB and ERK/p38 MAPK Signaling. Biomolecules 2019, 9, 559. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhuang, P.y.; Gou, L.X.; Li, X.Q. Research on the chemical components of wild anise fruits. Chin. Patent Med. 2019, 41, 1867–1871. [Google Scholar] [CrossRef]
- Lin, Q. Medicinal plant resources of Illicium L. Chin. Tradit. Herb. Drugs 2021, 33, 81–84. [Google Scholar]
- Zhang, Q.Z.; Wang, J.; Liang, Z.C. Experimental study on anti-inflammatory and analgesic effects of illicium S.M. J. China Prescr. Drug 2018, 16, 39–40. [Google Scholar]
- Li, H.J.; Song, X.H.; Li, H.R.; Zhu, L.F.; Cao, S.B.; Liu, J.F. Sesquiterpenes and Monoterpenes from the Leaves and Stems of Illicium simonsii and Their Antibacterial Activity. Molecules 2022, 27, 1115. [Google Scholar] [CrossRef]
- Su, G.Z.; Li, M.; Wang, X.J.; Wang, R.B.; Ma, S.G.; Zhang, D.; Wang, X.L.; Li, L.; Liu, Y.B.; Qu, J.; et al. Chemical constituents from the fruits of Illicium simonsii and their antiviral activity and neuroprotective effect. Phytochemistry 2022, 202, 113323. [Google Scholar] [CrossRef]
- Yin, P.J.; Wang, J.S.; Wang, P.R.; Kong, L.Y. Sesquiterpenes and lignans from the fruits of Illicium simonsii and their cytotoxicities. Chin. J. Nat. Med. 2012, 10, 383–387. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Luo, S.Q.; Wan, Z.; Chen, C.Y.; Zhang, X.N.; Li, B.B.; Huang, G.L.; Chen, L.Y.; He, Z.W.; Huang, Z.N. Glabridin arrests cell cycle and inhibits proliferation of hepatocellular carcinoma by suppressing braf/MEK signaling pathway. Tumor Biol. 2016, 37, 5837–5846. [Google Scholar] [CrossRef]
- Sun, Y.L.; Pang, B.; Wang, Y.Z.; Xiao, J.L.; Jiang, D.C. Baohuoside I Inhibits the Proliferation of Hepatocellular Carcinoma Cells via Apoptosis Signaling and NF-kB Pathway. Chem. Biodivers. 2021, 18, e2100063. [Google Scholar] [CrossRef]
- Nair, B.; Anto, R.J.; Sabitha, M.; Nath, L.R. Kaempferol-Mediated Sensitization Enhances Chemotherapeutic Efficacy of Sorafenib Against Hepatocellular Carcinoma: An In Silico and In Vitro Approach. Adv. Pharm. Bull. 2020, 10, 472–476. [Google Scholar] [CrossRef]
- Choi, Y.H. Isorhamnetin induces ROS-dependent cycle arrest at G2/M phase and apoptosis in human hepatocarcinoma Hep3B cells. Gen. Physiol. Biophys 2019, 38, 473–484. [Google Scholar] [CrossRef]
- Singh, M.P.; Cho, H.J.; Kim, J.T.; Baek, K.E.; Lee, H.G.; Kang, S.C. Morin Hydrate Reverses Cisplatin Resistance by Impairing PARP1/HMGB1-Dependent Autophagy in Hepatocellular Carcinoma. Cancers 2019, 11, 986. [Google Scholar] [CrossRef]
- Ku, W.C.; Sridharan, B.; Chen, J.Y.; Li, J.Y.; Yang, S.Y.; Lee, M.J. Kaempferitrin-Treated HepG2 Differentially Expressed Exosomal Markers and Affect Extracellular Vesicle Sizes in the Secretome. Biomolecules 2021, 11, 187. [Google Scholar] [CrossRef]
- Zhang, Q.; Bao, J.; Yang, J.H. Genistein-triggered anticancer activity against liver cancer cell line HepG2 involves ROS generation, mitochondrial apoptosis, G2/M cell cycle arrest and inhibition of cell migration. Arch. Med. Sci. 2019, 15, 1001–1009. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.Z.; Yang, H.; Zhang, Q.L.; Chen, M.Z. Pectolinarigenin flavonoid exhibits selective anti-proliferative activity in cisplatin-resistant hepatocellular carcinoma, autophagy activation, inhibiting cell migration and invasion, G2/M phase cell cycle arrest and targeting ERK1/2 MAP kinases. J. BUON 2020, 25, 415–420. [Google Scholar]
- Liu, W.T.; Chen, D.Y.; Su, J.Y.; Zheng, R.L.; Kong, R.; Zhu, B.; Dong, H.; Li, Y.H. Quercetin induced HepG2 cells apoptosis through ATM/JNK/STAT3 signaling pathway. Biocell 2023, 47, 187–194. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, Y.H. Regulation of apoptosis and autophagy by luteolin in human hepatocellular cancer Hep3B cells. Biochem. Biophys. Res. Commun. 2019, 517, 617–622. [Google Scholar] [CrossRef]
- Lu, K.H.; Lee, H.Y.; Chu, Y.L.; Ho, C.T.; Sheen, L.Y. Bitter orange peel extract induces endoplasmic reticulum-mediated autophagy in human hepatoma cells. J. Funct. Foods 2019, 60, 103404. [Google Scholar] [CrossRef]
- Liu, Y.; Piao, X.J.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Li, Y.N.; Zuo, W.B.; Sun, G.; Fu, Z.R.; et al. Calycosin induces mitochondrial-dependent apoptosis and cell cycle arrest, and inhibits cell migration through a ROS-mediated signaling pathway in HepG2 hepatocellular carcinoma cells. Toxicol. In Vitro 2021, 70, 105052. [Google Scholar] [CrossRef]
- Jiang, S.; Xiong, H.S.; Wu, H.K.; Wen, J.; Liang, L. Regulatory effect of hyperoside on proliferation and apoptosis of hepaticcarcinoma cell HepG2 via mitochondrial P53/Caspase signaling pathway. Chin. J. Immunol. 2018, 34, 1832–1836. [Google Scholar]
- Wei, S.; Zhou, H.; Wang, Q.; Zhou, S.; Li, C.; Liu, R.; Qiu, J.; Shi, C.; Lu, L. RIP3 deficiency alleviates liver fibrosis by inhibiting ROCK1-TLR4-NF-kB pathway in macrophages. FASEB J. 2019, 33, 11180–11193. [Google Scholar] [CrossRef]
- Pan, Z.Q.; Zhang, X.H.; Ai, Y.Q.; Wu, X.P.; Shi, J.S. Correlation analysis of TLR4/MyD88/NF-κB expression in hepatocellular carcinoma tissue and prognosis after radical resection. Clin. Exp. Med. 2022, 21, 1836–1840. [Google Scholar]
- Yang, J.; Wang, L.J.; Miu, X.; Yang, F. B-elemene inhibits the proliferation, migration and invasion of hepatocellular carcinoma cells through JAK2/STAT3 pathway. J. Harbin Med. Univ. 2023, 57, 594–598. [Google Scholar]
- Serrya, M.S.; Zaghloul, M.S. Mycophenolate mofetil attenuates concanavalin A-induced acute liver injury throughmodulation of TLR4/NF-kB and Nrf2HO-1 pathways. Pharmacol. Rep. 2020, 72, 945–955. [Google Scholar] [CrossRef]
- Hin Tang, J.J.; Hao Thng, D.K.; Lim, J.J.; Toh, T.B. JAK/STAT signaling in hepatocellular carcinoma. Hepat. Oncol. 2020, 7, HEP18. [Google Scholar] [CrossRef]
- Qiao, X.; Xu, S.H.; Wang, Y.W.; Feng, B.; Peng, P.K.; Shen, K.K. Da Chaihutang Inhibits Hepatocellular Carcinoma by Regulating p38MAPK/IL-6/STAT3 Signaling Pathway. Chin. J. Exp. Tradit. Med. Formul. 2022, 28, 19–31. [Google Scholar]
- Hu, C.; Chen, Z.; Wu, J.; Zhang, Y.; Xu, H.; Yang, W.M.; Ye, Z.Q. Up-regulation of Tumor Suppressor Gene p21WAF1/ClP1 in Human Cells by Small Double Strand RNA. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012, 41, 60–64. [Google Scholar]
- Wang, Y.; Duan, H.C. Angelica Sinensis and Hedysarum Polysaccharides in human hepatoma SMMC-7721 cell radiation sensitizing effect and its molecular mechanis. Gansu Med. J. 2023, 42, 485–488+496. [Google Scholar]
- Shao, X.T.; Chen, Q.X.; Dou, X.X.; Lei, J.X.; Zhang, W. Lower range of molecular weight of xanthan gum inhibits cartilage matrixdestruction via intrinsic Bax-mitochondria cytochrome C-caspase pathway. Carbohydr. Polym. 2018, 198, 354–363. [Google Scholar] [CrossRef]
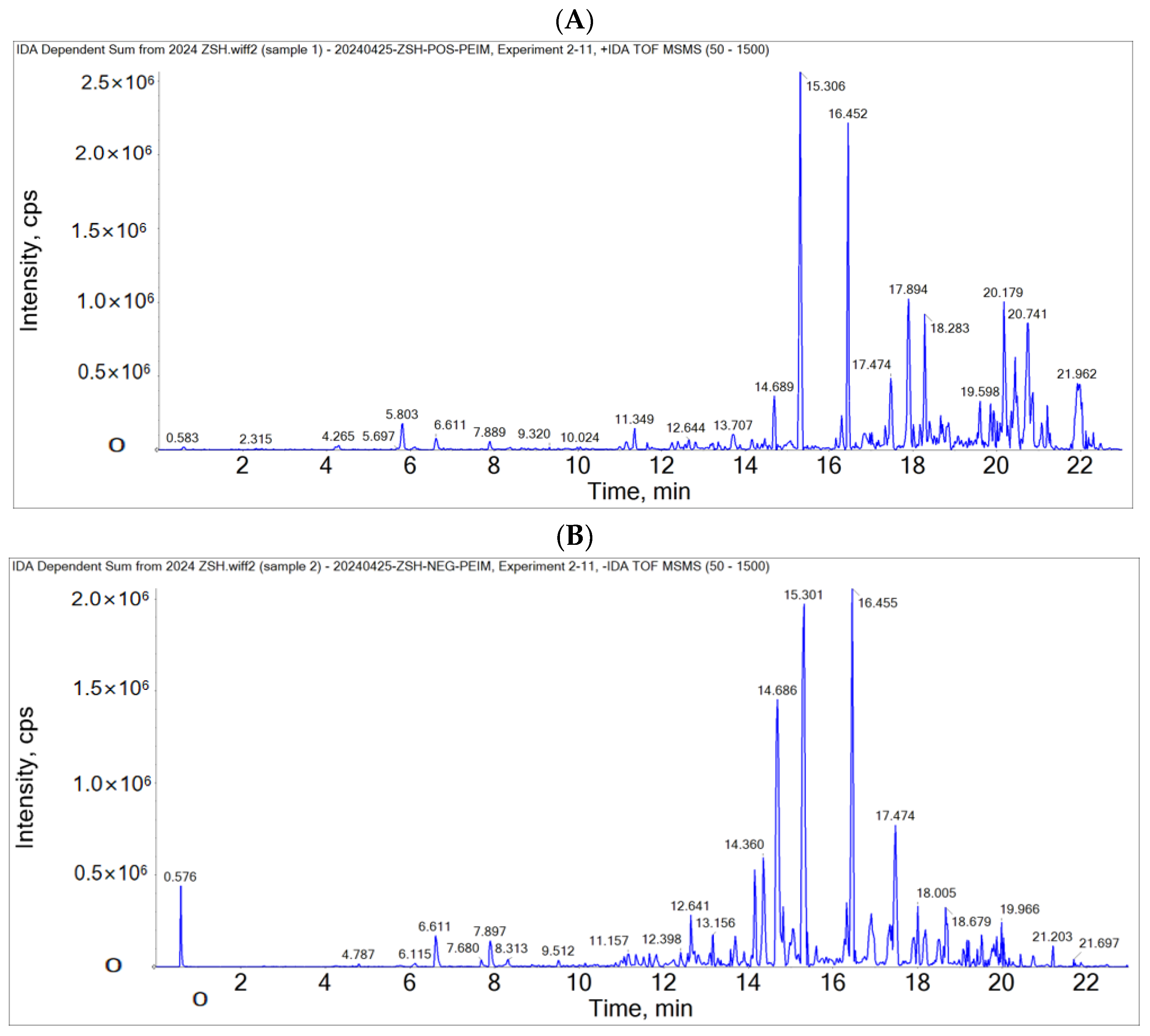
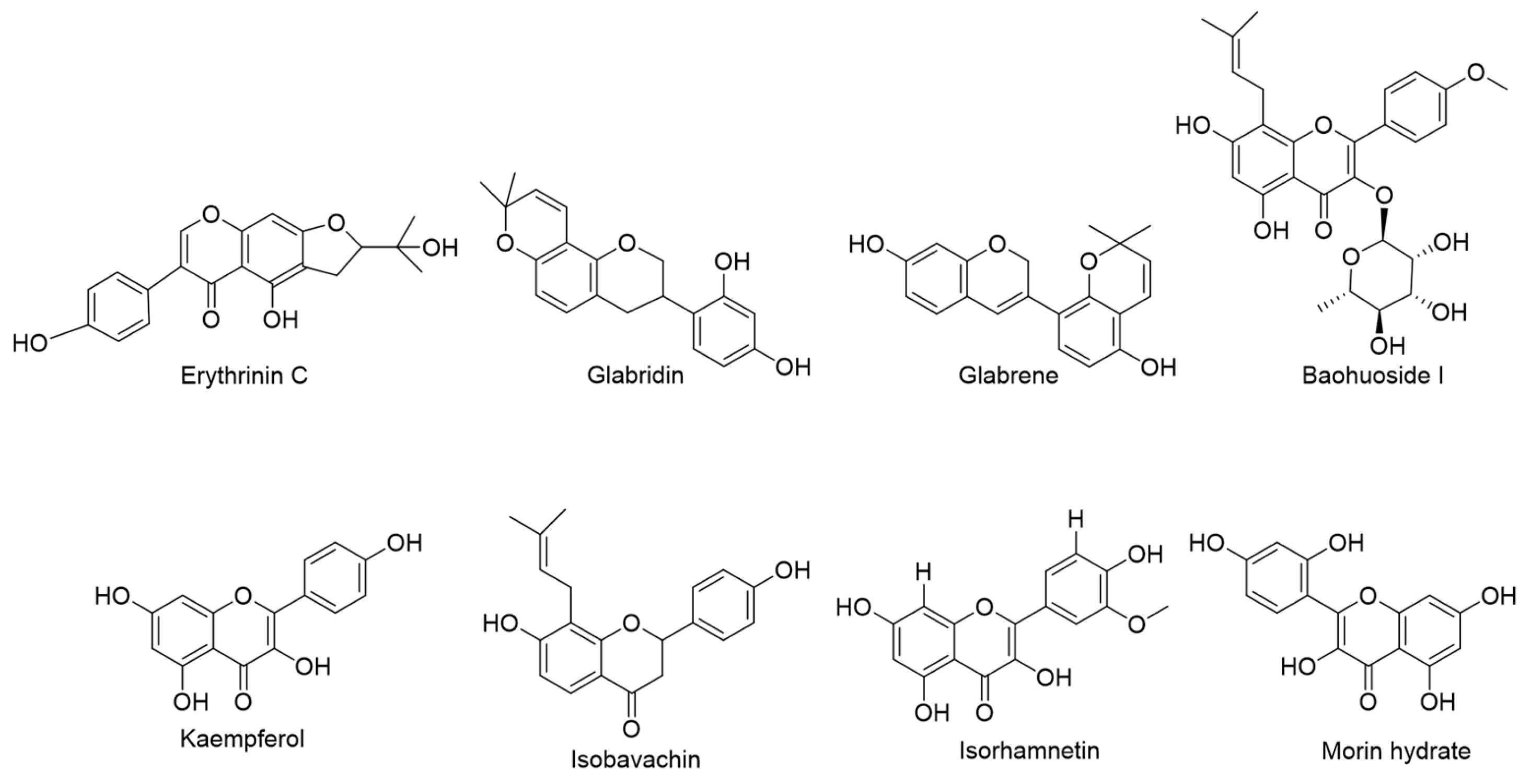
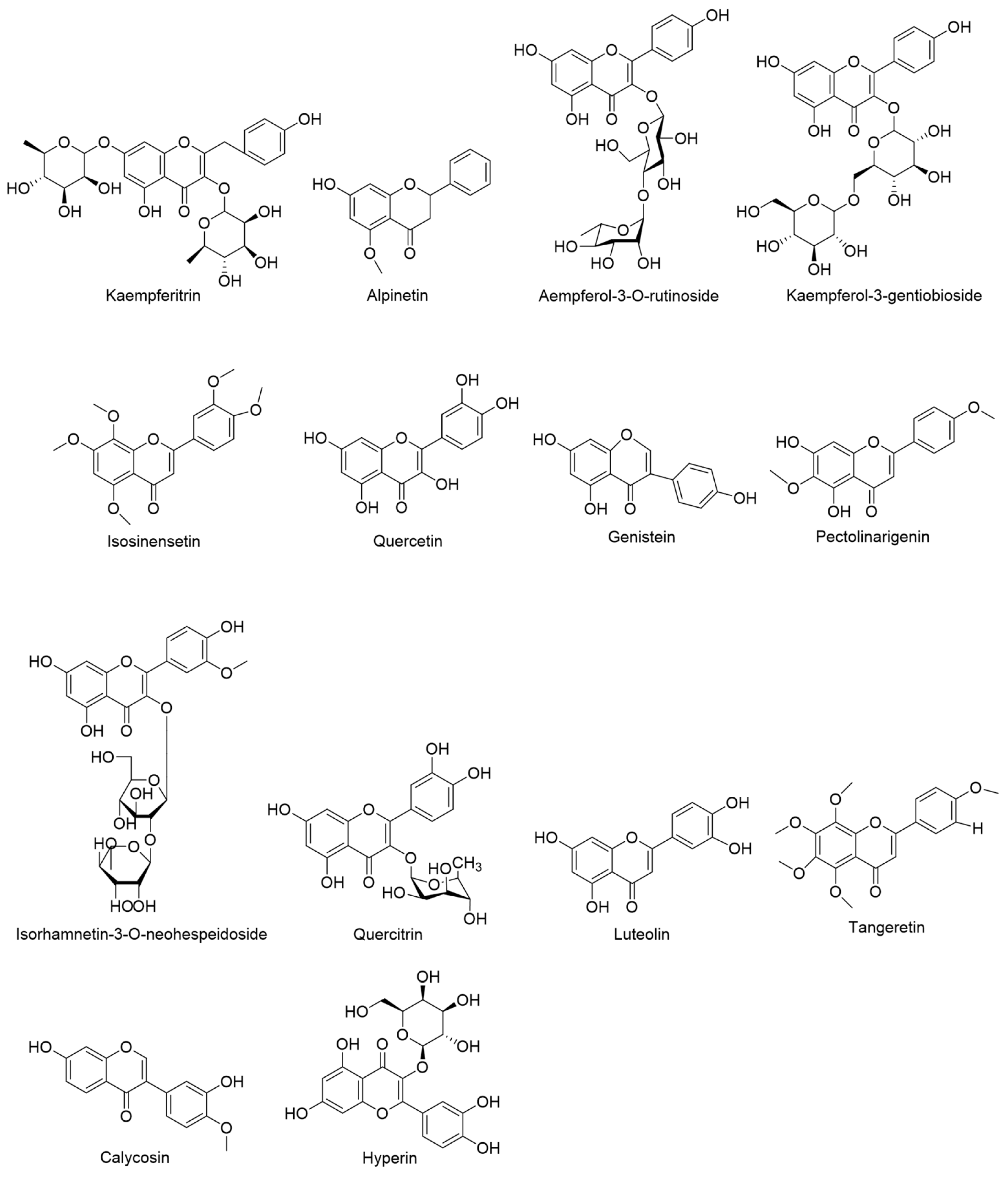


| NO. | RT (min.) | Precursor m/z | Fragmentation | Molecular Formula | Possible Compounds Identified |
|---|---|---|---|---|---|
| [M+H]+ [M−H]− | |||||
| Flavonoids | |||||
| 1 | 11.36 | 355.1180 | 355.1201, 135.0449 | C20H18O6 | Erythrinin C |
| 2 | 14.05 | 325.1432 | 131.0511, 103.0567 | C20H20O4 | Glabridin |
| 3 | 9.39 | 323.1278 | 323.1294, 161.0605, 135.0456 | C20H18O4 | Glabrene |
| 4 | 5.76 | 515.1910 | 515.1897 | C27H30O10 | Baohuoside I |
| 5 | 7.69 | 287.0552 | 287.0571, 153.0202 | C15H10O6 | Kaempferol |
| 6 | 14.05 | 325.1434 | 131.0511, 103.0567 | C20H20O4 | Isobavachin |
| 7 | 7.90 | 317.0660 | 317.0658, 302.0441, 153.0203, | C16H12O7 | Isorhamnetin |
| 8 | 6.62 | 303.0500 | 303.0498, 229.0508, 153.0200 | C15H10O7 | Morin hydrate |
| 9 | 7.21 | 577.1562 | 369.0970, 346.9870, 297.1123, 108.0188 | C27H30O14 | Kaempferitrin |
| 10 | 9.80 | 269.0820 | 269.1349, 225.0925, 149.0614 | C16H14O4 | Alpinetin |
| 11 | 5.01 | 593.1510 | 593.1449, 285.0322 | C27H30O15 | Aempferol-3-O-rutinoside |
| 12 | 4.52 | 609.1464 | 609.1445, 300.0280, 255.0291, 179.0019, 151.0070 | C27H30O16 | Kaempferol-3-gentiobioside |
| 13 | 7.14 | 371.1140 | 371.1179, 327.1230, 297.1129, 267.0613, 160.0536 | C20H20O7 | Isosinensetin |
| 14 | 6.61 | 301.0351 | 301.0355, 178.9978, 151.0025, 107.0136 | C15H10O7 | Quercetin |
| 15 | 7.54 | 269.0458 | 269.0475, 228.9901, 151.0048, 117.0352 | C15H10O5 | Genistein |
| 16 | 10.48 | 313.0719 | 313.1500, 283.0253, 255.0330, 216.9912, 145.0307 | C17H14O6 | Pectolinarigenin |
| 17 | 5.13 | 623.1620 | 623.1631, 315.0606, 299.0172, 259.0542 | C28H32O16 | Isorhamnetin-3-O-neohespeidoside |
| 18 | 5.19 | 447.0932 | 447.0894, 284.0345, 227.0324, 174.9554, 146.9589 | C21H20O11 | Quercitrin |
| 19 | 7.67 | 285.0401 | 285.0390, 257.0452, 229.0486, 151.0040 | C15H10O6 | Luteolin |
| 20 | 7.14 | 371.1139 | 371.1179, 327.1230, 282.0891, 267.0653, 160.0536 | C20H20O7 | Tangeretin |
| 21 | 9.93 | 283.0610 | 283.0603, 268.0371, 242.9949, 152.9947 | C16H12O5 | Calycosin |
| 22 | 4.70 | 463.0881 | 463.0875, 300.0278, 271.0220, 174.9579 | C21H20O12 | Hyperin |
| Diterpenoids | |||||
| 23 | 6.60 | 557.1960 | 111.3465, 81.0746 | C36H28O6 | Neoprzewaquine A |
| 24 | 16.58 | 351.2170 | 351.1984, 207.1384, 161.1346, 105.0723 | C20H30O5 | Andrographolide |
| 25 | 17.49 | 533.2384 | 533.3047, 356.1428, 135.1185, 107.0874 | C28H36O10 | Butanedioicacid |
| 26 | 12.86 | 297.1487 | 297.1535, 279.2671, 256.1101, 227.0734 | C19H20O3 | Cryptotanshinone |
| 27 | 12.93 | 331.1900 | 313.2739, 271.2097, 149.0977, 133.1014 | C20H26O4 | Carnosol |
| 28 | 12.13 | 333.2059 | 333.2167, 315.1934, 269.1915, 119.0892 | C20H28O4 | Carnosic acid |
| 29 | 7.58 | 357.1342 | 357.1323, 342.1085, 232.9816, 137.0604, 83.0139 | C20H22O6 | Triptonide |
| 30 | 6.11 | 359.1496 | 326.1166, 300.1245, 269.0821, 208.0739, 180.0745 | C20H24O6 | Triptolide |
| Triterpenoids | |||||
| 31 | 16.78 | 439.3571 | 439.3638, 191.1792, 135.1175 | C30H46O2 | Ganoderiol A |
| 32 | 19.09 | 455.3521 | 455.3567, 437.3455, 237.2719, 161.1372 | C30H46O3 | Betulonicacid |
| 33 | 12.82 | 537.2980 | 537.3027 | C30H4lO6 | Senegenin |
| 34 | 12.82 | 517.3166 | 517.3328, 365.2006, 347.1898 | C30H46O7 | Ganoderic acid C2 |
| 35 | 16.73 | 467.3167 | 467.3342, 423.3413, 399.1739, 125.0998 | C30H44O4 | Ganoderic acid DM |
| 36 | 13.76 | 471.3478 | 471.3559 | C30H48O4 | Ganodermanontriol |
| 37 | 16.77 | 469.3323 | 469.3325, 451.3229, 425.3417 | C30H46O4 | Glycyrrhetinic Acid |
| 38 | 16.77 | 455.3530 | 455.3522, 407.3415, 155.0367 | C30H48O3 | Betulinic acid |
| 39 | 11.28 | 487.3430 | 487.3406, 229.0074 | C30H48O5 | Asiatic acid |
| Alkaloids | |||||
| 40 | 7.07 | 308.2221 | 308.2257, 290.2117, 136.0786, 122.0184 | C18H29NO3 | Dihydrocapsaicin |
| 41 | 14.16 | 213.1021 | 213.1322, 183.0993, 172.0899, 129.0718 | C13H12N2O | Harmine |
| 42 | 0.61 | 272.1282 | 148.9876, 126.0561, 108.0458 | C16H17NO3 | Higenamine |
| 43 | 13.66 | 457.2334 | 457.2371, 123.1173 | C25H32N2O6 | Vindoline |
| 44 | 20.19 | 623.3131 | 623.3101, 563.2927 | C38H42N2O6 | Tetrandrine |
| 45 | 3.38 | 286.1440 | 286.1455, 256.0915, 226.0412 | C17H19NO3 | Piperine |
| 46 | 4.13 | 326.1595 | 326.1576, 182.9536 | C16H23NO6 | Monocrotaline |
| 47 | 8.71 | 286.1082 | 286.0996, 196.0750, 168.0871, 107.0369 | C16H17NO4 | Lycorine |
| Lignans | |||||
| 48 | 9.81 | 403.2116 | 403.2069, 129.0178 | C23H30O6 | Schisanhenol |
| 49 | 6.12 | 539.2280 | 367.1435, 343.1610, 163.0772 | C30H34O9 | Schisantherin E |
| 50 | 9.52 | 343.1540 | 343.1588, 311.1283, 265.0868, 161.0607 | C20H22O5 | Arisantetralone A |
| 51 | 11.36 | 355.1175 | 355.1201, 337.1097, 135.0449 | C20H18O6 | Asarinin |
| 52 | 14.68 | 399.1086 | 399.1835, 381.1745 | C21H20O8 | 4′-Demethylepipodophyllotoxin |
| 53 | 11.77 | 383.1500 | 382.9986, 363.0072, 322.9807, 302.9933, 121.0280 | C22H24O6 | Schisandrin C |
| 54 | 9.70 | 535.1969 | 535.1869, 341.1375, 193.0507, 134.0367 | C30H32O9 | Schisantherin A |
| Sesquiterpenoids | |||||
| 55 | 13.53 | 285.1768 | 285.2242, 125.0979, 107.0864, 81.0711 | C15H24O5 | Dihydroartemisinin |
| 56 | 8.78 | 233.1536 | 233.1522, 175.1139, 147.1192 | C15H20O2 | Alantolactone |
| 57 | 8.36 | 251.1641 | 251.1280, 147.1217, 95.0863 | C15H22O3 | Nardosinone |
| 58 | 12.67 | 237.1850 | 237.2203, 201.1652, 149.1349, 71.0517 | C15H24O2 | Curdione |
| 59 | 13.29 | 235.1694 | 235.1711, 179.1091, 57.0713 | C15H22O2 | Curcumenol |
| Others: | |||||
| 60 | 7.69 | 287.0552 | 287.0571, 153.0202 | C15H10O6 | 3-Hydroxymorindone |
| 61 | 9.76 | 177.0545 | 149.0246, 65.0400 | C10H8O3 | Hymecromone |
| 62 | 4.27 | 365.1443 | 365.1316 | C15H24O10 | Harpagide |
| 63 | 20.42 | 401.3775 | 401.2042, 360.1636, 331.1258 | C28H48O | Campesterol |
| 64 | 14.05 | 337.1070 | 131.0511, 103.0567 | C20H16O5 | Psoralidin |
| Gene | Sense | Antisense |
|---|---|---|
| P53 | GCTTTCCACGACGGTGAC | GCTCGACGCTAGGATCTGAC |
| Caspase-3 | AGAGCTGGACTGCGCTATTGAG | GAACCATGACCCGTCCCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, S.; Wu, Y.; Wen, M.; Liu, J.; Chen, M.; Yuan, J.; Zhou, B. Potential Molecular Mechanism of Illicium simonsii Maxim Petroleum Ether Fraction in the Treatment of Hepatocellular Carcinoma. Pharmaceuticals 2024, 17, 806. https://doi.org/10.3390/ph17060806
Zou S, Wu Y, Wen M, Liu J, Chen M, Yuan J, Zhou B. Potential Molecular Mechanism of Illicium simonsii Maxim Petroleum Ether Fraction in the Treatment of Hepatocellular Carcinoma. Pharmaceuticals. 2024; 17(6):806. https://doi.org/10.3390/ph17060806
Chicago/Turabian StyleZou, Sihua, Yanchun Wu, Meiqi Wen, Jiao Liu, Minghui Chen, Jingquan Yuan, and Bei Zhou. 2024. "Potential Molecular Mechanism of Illicium simonsii Maxim Petroleum Ether Fraction in the Treatment of Hepatocellular Carcinoma" Pharmaceuticals 17, no. 6: 806. https://doi.org/10.3390/ph17060806
APA StyleZou, S., Wu, Y., Wen, M., Liu, J., Chen, M., Yuan, J., & Zhou, B. (2024). Potential Molecular Mechanism of Illicium simonsii Maxim Petroleum Ether Fraction in the Treatment of Hepatocellular Carcinoma. Pharmaceuticals, 17(6), 806. https://doi.org/10.3390/ph17060806






