Sulfated Laminarin Polysaccharides Reduce the Adhesion of Nano-COM Crystals to Renal Epithelial Cells by Inhibiting Oxidative and Endoplasmic Reticulum Stress
Abstract
1. Introduction
2. Results
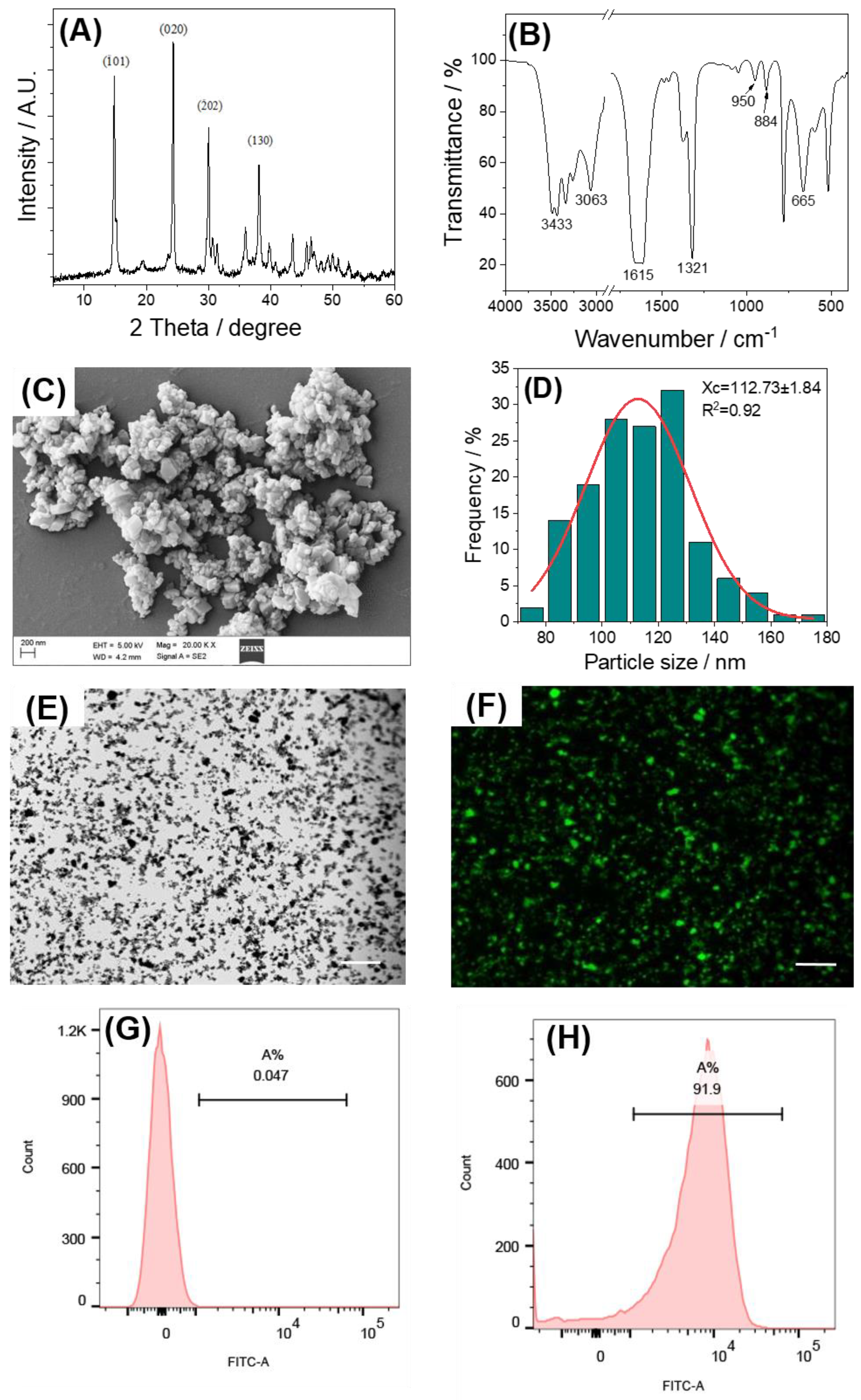
2.1. Characterization of Nano-COM and FITC-COM
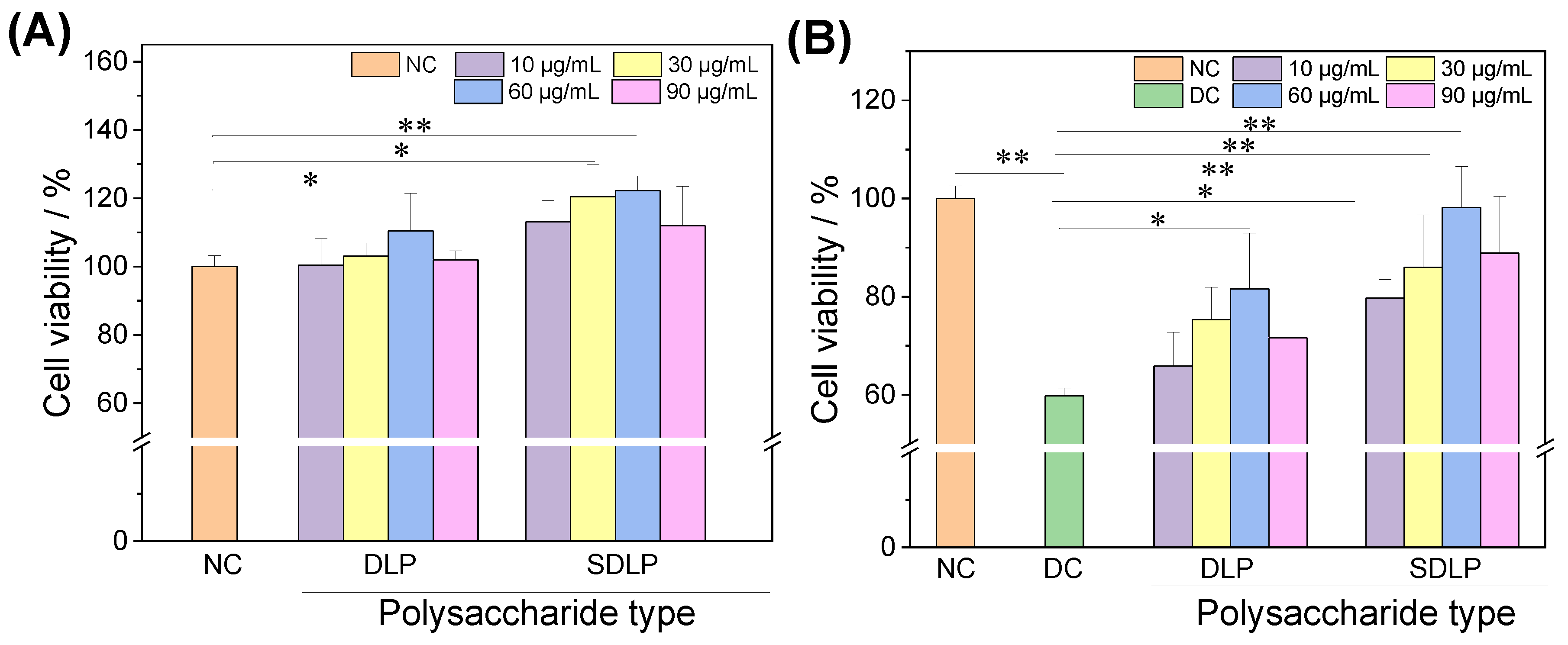
2.2. Cytotoxic and Protective Effects of Polysaccharides
2.3. Reduction in Cellular ROS Levels by Polysaccharides
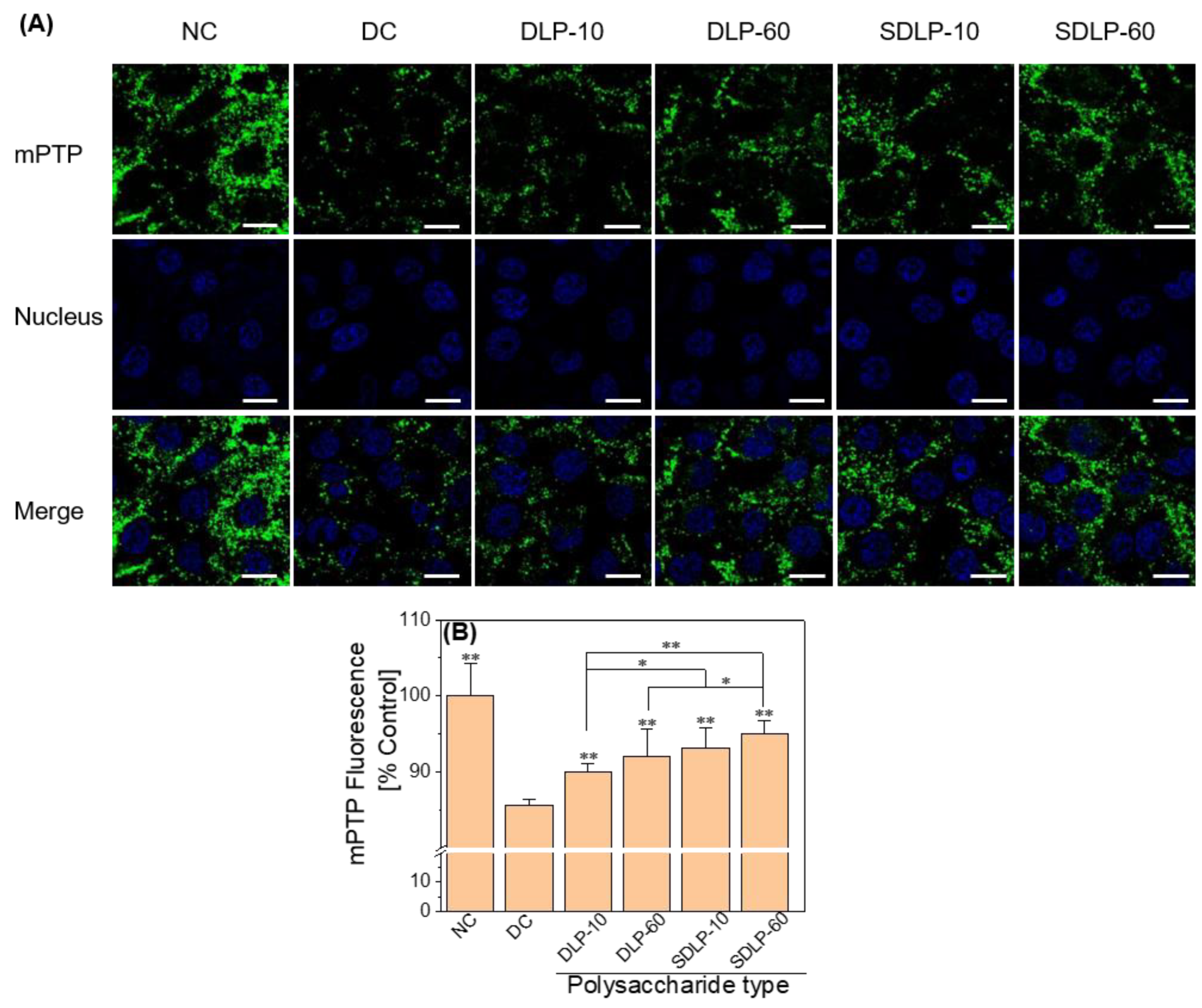
2.4. Polysaccharides Reduce Mitochondrial Permeability Transition pore (mPTP) Opening Levels
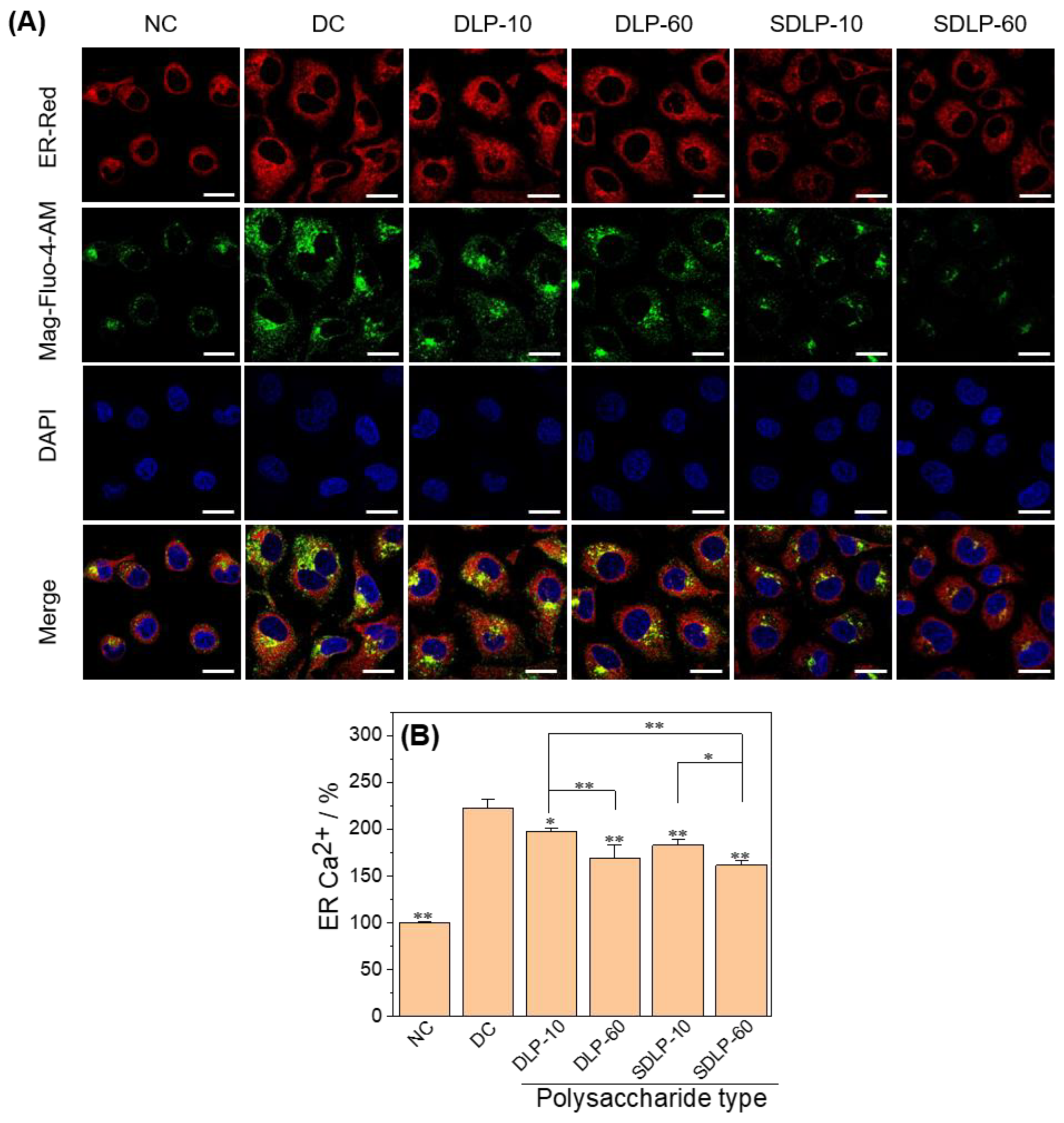
2.5. Polysaccharide Reduces ER Stress Level and ER Free Ca2+ Ion Concentration
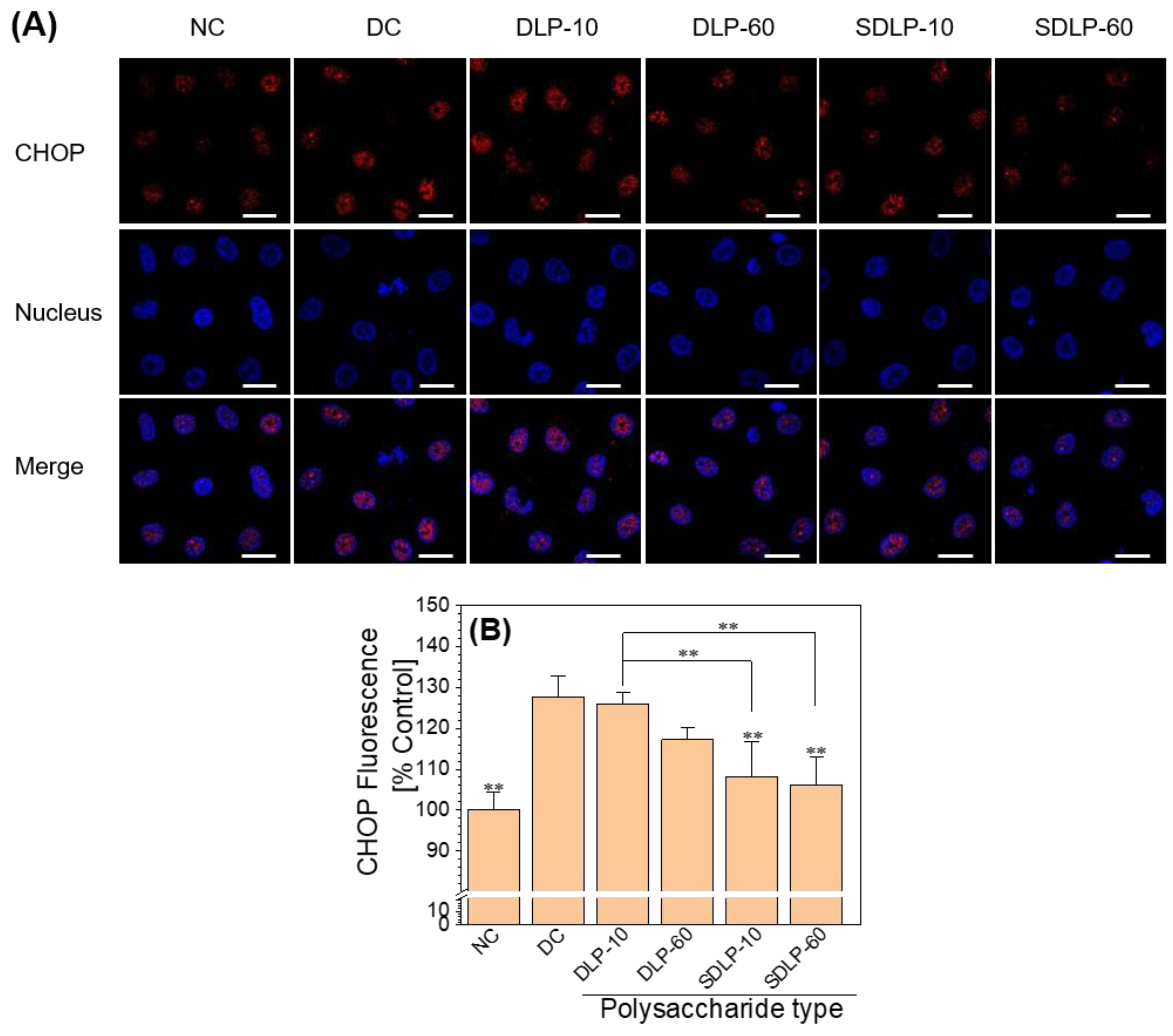
2.6. Polysaccharides Reduce Transcription Factor CHOP Expression
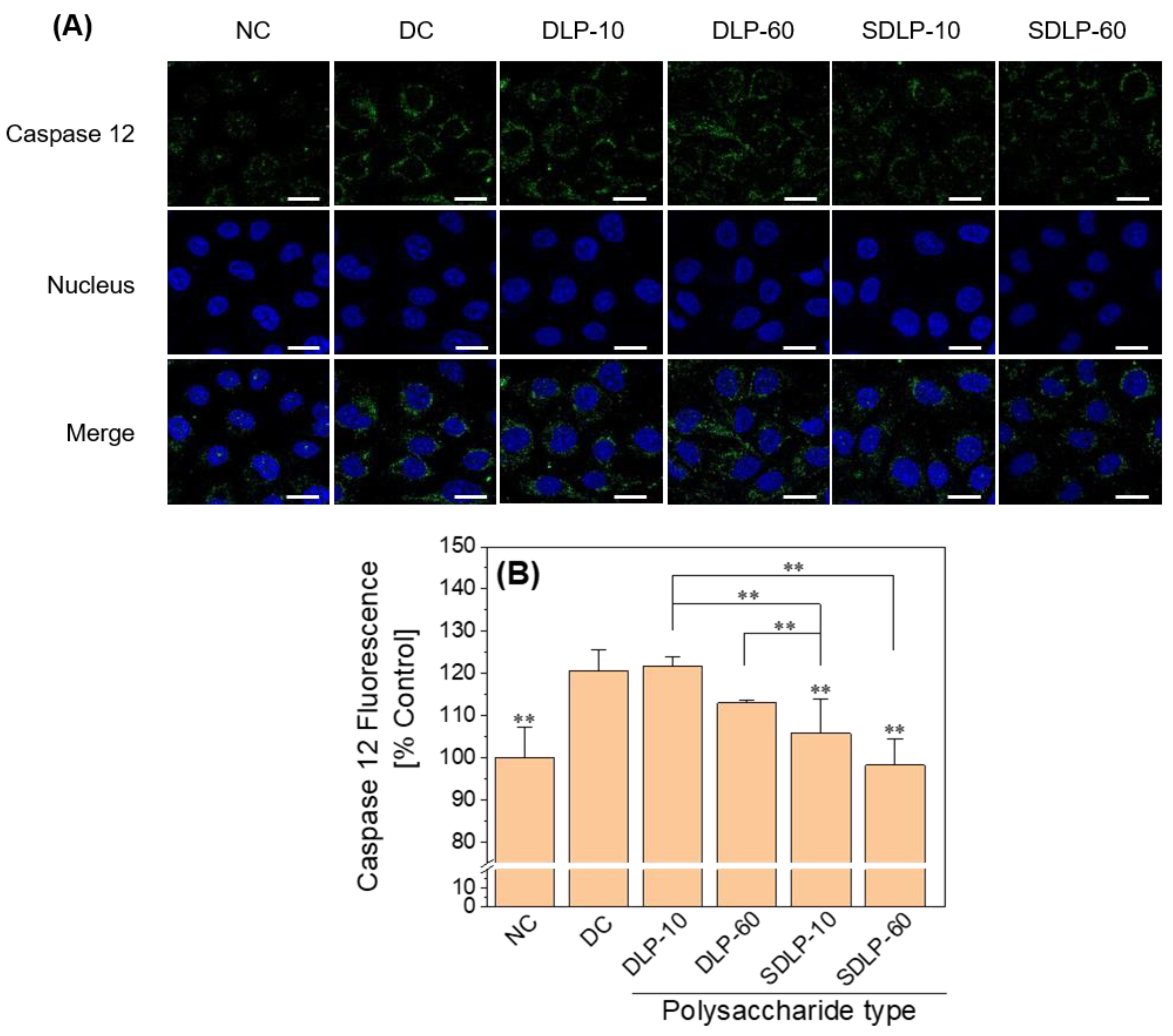
2.7. Caspase 12 Polysaccharide Reduces Caspase 12 Expression Levels
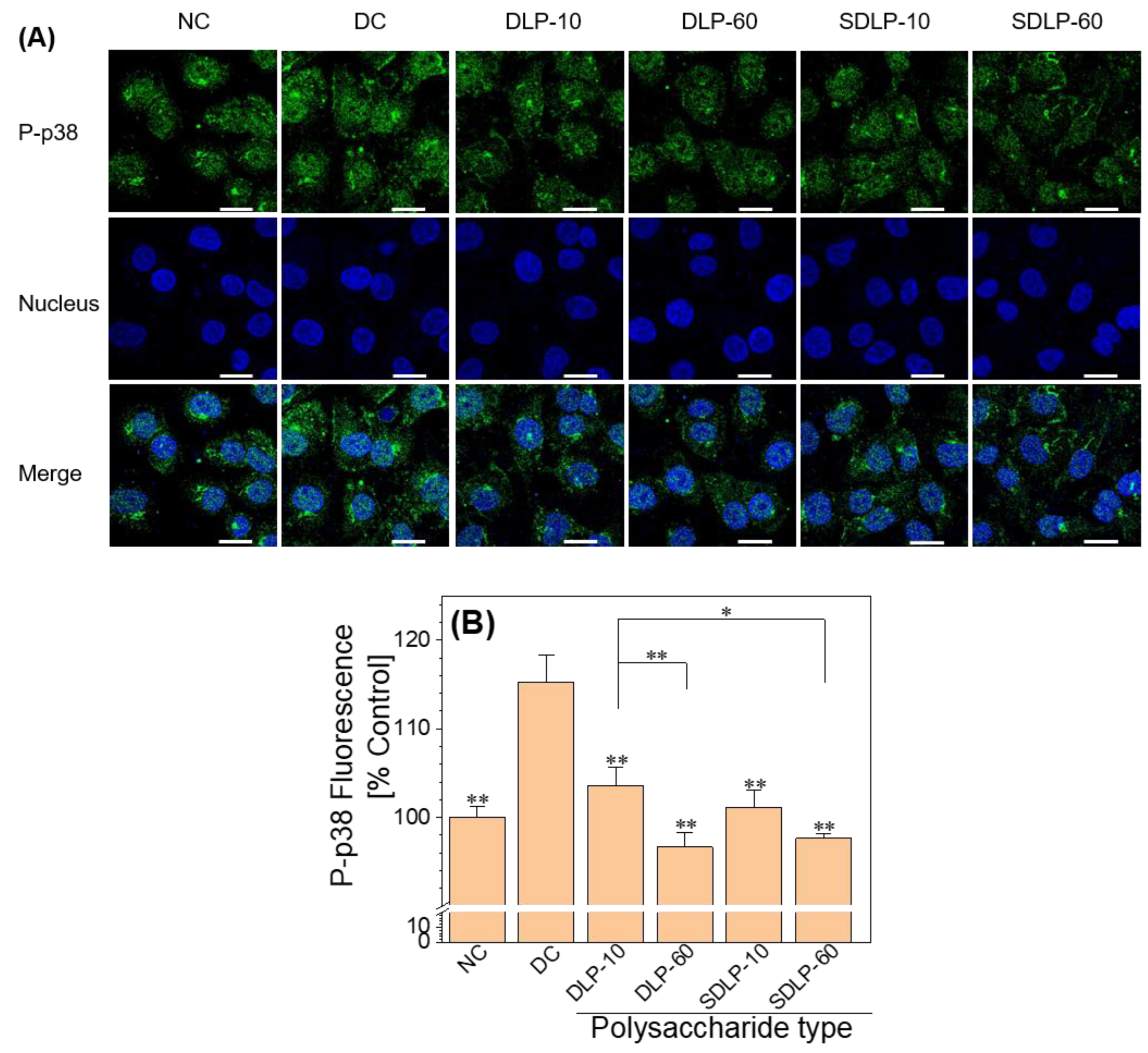
2.8. Polysaccharides Reduce the Phosphorylation of p38 (P-p38) Expression Levels
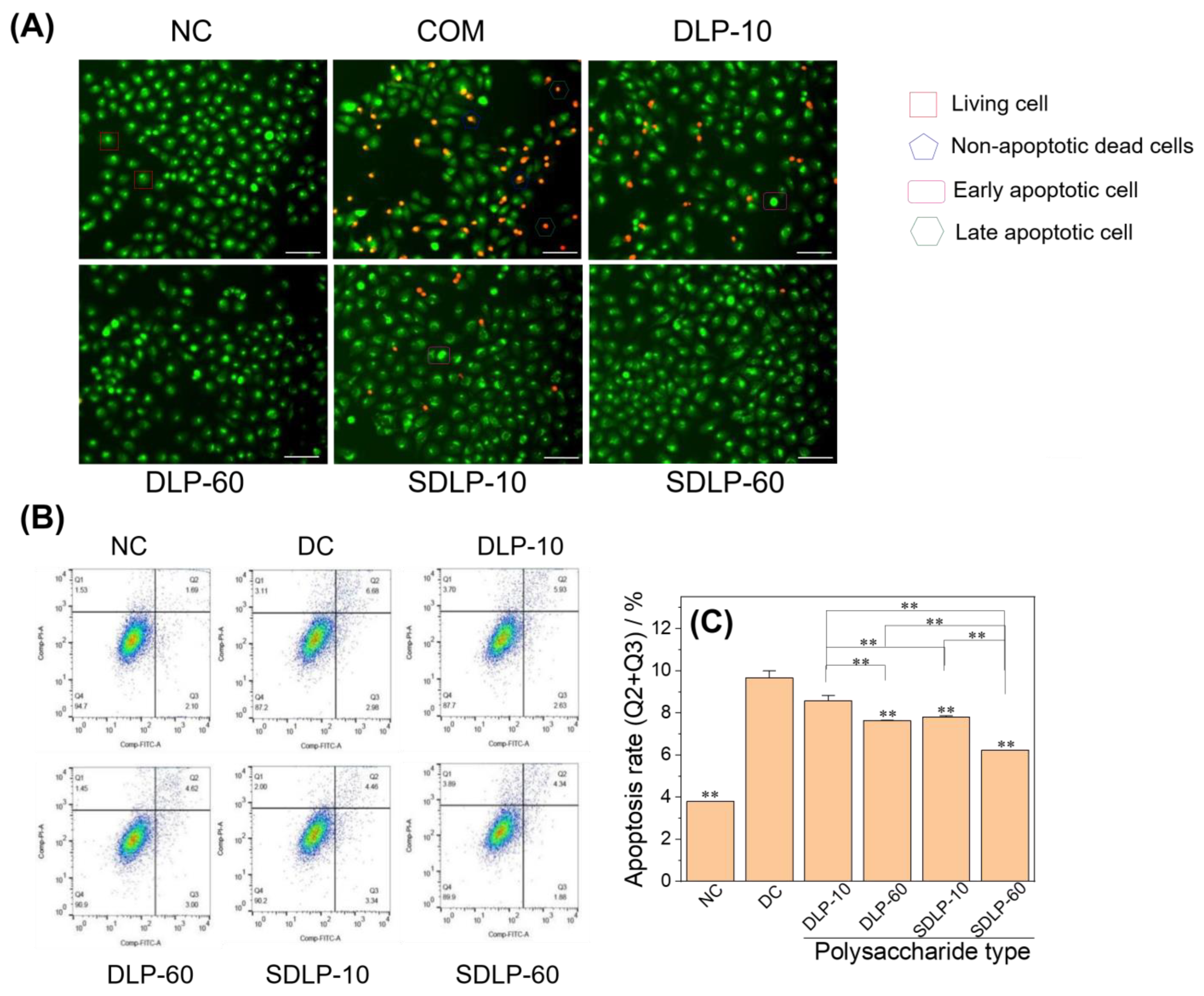
2.9. Qualitative and Quantitative Analyses of Apoptosis Reduction by Polysaccharides
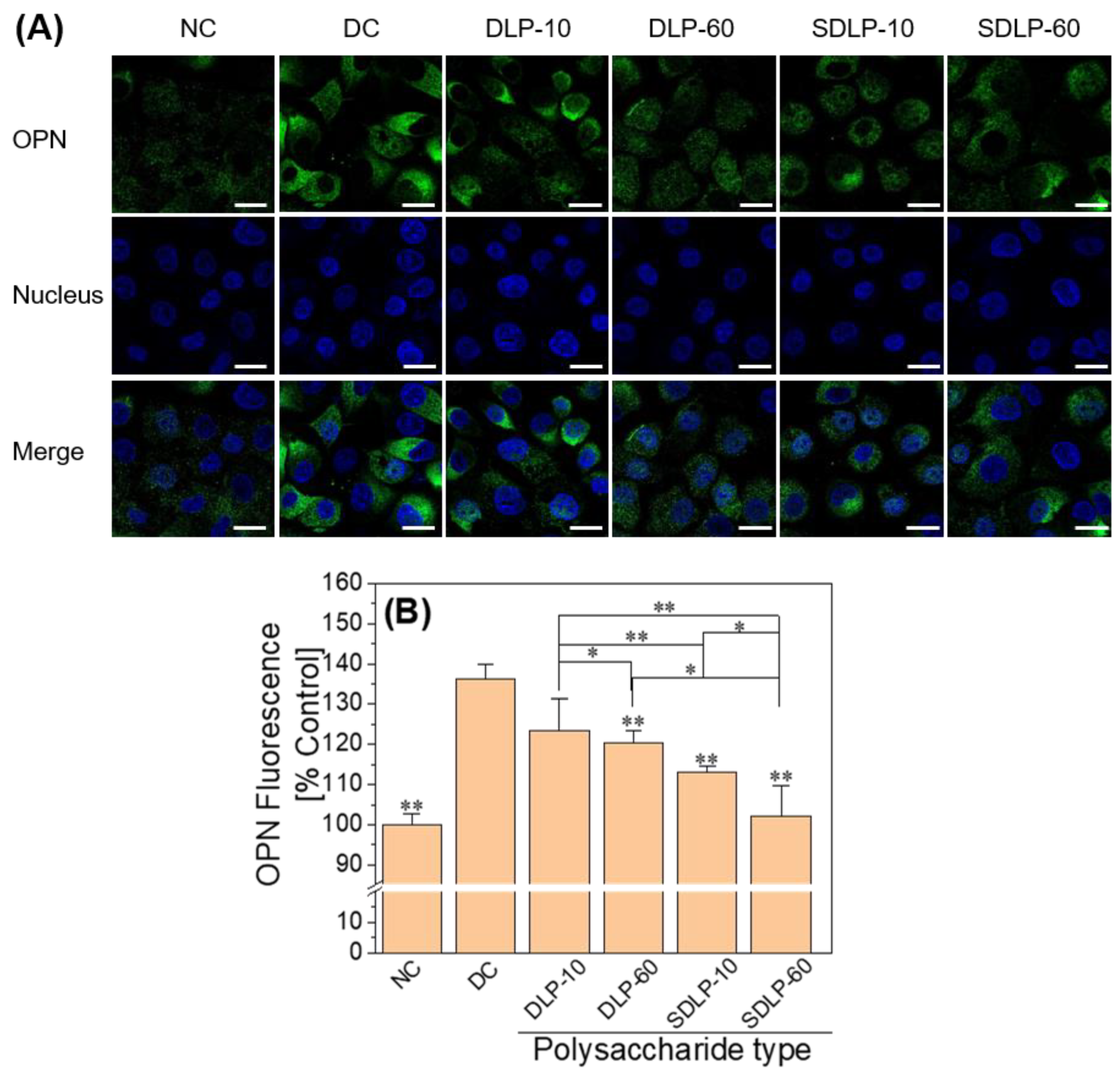
2.10. Polysaccharide Reduces Cell Surface Osteopontin (OPN) Expression
2.11. Qualitative Assay of Polysaccharide to Reduce Crystal Adhesion on Cell Surface
2.12. Quantification of the Proportion of Cells Adhering to the Crystals
3. Discussion
3.1. Characterization of the Laminarin Polysaccharides
3.2. Nano-COM Causes Mitochondrial Dysfunction and ER Stress by Promoting ROS Overproduction
3.3. DLP and SDLP Inhibit Apoptosis by Regulating ER Stress
3.4. DLP and SDLP Polysaccharides Inhibit Crystal Adhesion on the Cell Surface by Reducing the Expression of Adhesion Molecules
4. Materials and Methods
4.1. Materials and Equipment
4.2. Experimental Methods
4.2.1. Synthesis and Characterization of Nano-COM and FITC-Nano-COM Crystals
4.2.2. Cell Culture
4.2.3. Cell Viability Detected by CCK-8
Cytotoxic Effect of Polysaccharides
Cell Protection by Polysaccharides
4.2.4. Polysaccharide Inhibits Oxidative Stress
DCFH-DA Staining to Detect Cellular Reactive Oxygen Species (ROS) Levels
Mitochondrial Permeability Transition Pore (mPTP) Assay
4.2.5. Polysaccharide Inhibits ER Stress
Detection of ER Stress
Fluorescence Detection of Calcium Ion Concentration in the Endoplasmic Reticulum
Immunofluorescence Staining and Imaging of CHOP
Immunofluorescence Staining and Imaging of Caspase 12
Immunofluorescence Staining and Imaging of P-p38
4.2.6. Polysaccharide Inhibited the Adhesion of Nano-COM Crystal
Detection of Cell Surface Osteopontin (OPN) Expression
Qualitative and Quantitative Observation of Cell Adhesion
4.2.7. Detection of Cell Death and Apoptosis
Qualitative Detection of Cell Death by AO/EB Double Staining
Annexin V/PI Double Staining for Quantitative Detection of Apoptosis
4.2.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szarka, A.; Lőrincz, T.; Hajdinák, P. Friend or Foe: The relativity of (anti) oxidative agents and pathways. Int. J. Mol. Sci. 2022, 23, 5188. [Google Scholar] [CrossRef]
- Bao, B.; Liu, H.; Han, Y.; Xu, L.; Xing, W.; Li, Z. Simultaneous elimination of reactive oxygen species and activation of Nrf2 by ultrasmall nanoparticles to relieve acute kidney injury. ACS Appl. Mater. Interfaces 2023, 15, 16460–16470. [Google Scholar] [CrossRef]
- Ho, H.J.; Shirakawa, H. Oxidative stress and mitochondrial dysfunction in chronic kidney disease. Cells 2022, 12, 88. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, J.; Guan, X.; Xu, H.; Wang, X.; Deng, Y. Regulation of endoplasmic reticulum stress on the damage and apoptosis of renal tubular epithelial cells induced by calcium oxalate crystals. Urolithiasis 2021, 49, 291–299. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Feng, D.; Fu, Y.; Wu, H.; Lu, J.; Bao, J. Calcium-sensing receptor promotes calcium oxalate crystal adhesion and renal injury in Wistar rats by promoting ROS production and subsequent regulation of PS ectropion, OPN, KIM-1, and ERK expression. Ren. Fail. 2021, 43, 465–476. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057. [Google Scholar] [CrossRef]
- Tian, M.; Dong, B.; Zhang, Z.; Yin, J.; Lin, W. Permeability-controlled probe for directly visualizing the opening of mitochondrial permeability transition pore in Native Status. Anal. Chem. 2022, 94, 5255–5264. [Google Scholar] [CrossRef]
- Randhawa, R.; Bhardwaj, R.; Kaur, T. Amelioration of hyperoxaluria-induced kidney dysfunction by chemical chaperone 4-phenylbutyric acid. Urolithiasis 2019, 47, 171–179. [Google Scholar] [CrossRef]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef]
- Krols, M.; Van Isterdael, G.; Asselbergh, B.; Kremer, A.; Lippens, S.; Timmerman, V.; Janssens, S. Mitochondria-associated membranes as hubs for neurodegeneration. Acta Neuropathol. 2016, 131, 505–523. [Google Scholar] [CrossRef]
- Yang, B.; Lu, X.; Li, Y.; Li, Y.; Yu, D.; Zhang, W.; Duan, C.; Taguchi, K.; Yasui, T.; Kohri, K. A proteomic network approach across the kidney stone disease reveals endoplasmic reticulum stress and crystal-cell interaction in the kidney. Oxidative Med. Cell. Longev. 2019, 2019, 9307256. [Google Scholar] [CrossRef]
- Bai, B.; Li, D.; Xue, G.; Feng, P.; Wang, M.; Han, Y.; Wang, Y.; Hölscher, C. The novel GLP-1/GIP dual agonist DA3-CH is more effective than liraglutide in reducing endoplasmic reticulum stress in diabetic rats with cerebral ischemia-reperfusion injury. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 333–343. [Google Scholar] [CrossRef]
- Peerapen, P.; Thongboonkerd, V. p38 MAPK mediates calcium oxalate crystal-induced tight junction disruption in distal renal tubular epithelial cells. Sci. Rep. 2013, 3, srep01041. [Google Scholar] [CrossRef]
- Jiang, W.; Lin, Y.; Qian, L.; Lu, S.; Shen, H.; Ge, X.; Miao, L. Mulberry Leaf Polysaccharides Attenuate Oxidative Stress Injury in Peripheral Blood Leukocytes by Regulating Endoplasmic Reticulum Stress. Antioxidants 2024, 13, 136. [Google Scholar] [CrossRef]
- Hu, W.; Li, Z.; Wang, W.; Song, M.; Dong, R.; Zhou, Y.; Li, Y.; Wang, D. Structural characterization of polysaccharide purified from Amanita caesarea and its pharmacological basis for application in Alzheimer’s disease: Endoplasmic reticulum stress. Food Funct. 2021, 12, 11009–11023. [Google Scholar] [CrossRef]
- Sun, S.; Yang, S.; An, N.; Wang, G.; Xu, Q.; Liu, J.; Mao, Y. Astragalus polysaccharides inhibits cardiomyocyte apoptosis during diabetic cardiomyopathy via the endoplasmic reticulum stress pathway. J. Ethnopharmacol. 2019, 238, 111857. [Google Scholar] [CrossRef]
- Huang, L.-Q.; Zhang, Y.-Z.; Zheng, B.; He, Y. Lycium barbarum polysaccharide attenuates cisplatininduced apoptosis in ovary granulosa cells via alleviation of endoplasmic reticulum stress. Trop. J. Pharm. Res. 2017, 16, 827–835. [Google Scholar] [CrossRef][Green Version]
- Zhou, B.; Tan, J.; Zhang, C.; Wu, Y. Neuroprotective effect of polysaccharides from Gastrodia elata blume against corticosterone-induced apoptosis in PC12 cells via inhibition of the endoplasmic reticulum stress-mediated pathway. Mol. Med. Rep. 2018, 17, 1182–1190. [Google Scholar] [CrossRef]
- Shirani, M.; Arjaki, D.; Kheiri, S.; Bijad, E.; Mohammadi, S.; Lorigooini, Z. An in vitro screening potential traditional medicinal plants for nephrolithiasis. Clin. Phytosci. 2020, 6, 66. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Bao, J.; Chen, S. Protective potential of Angelica sinensis polysaccharide extract against ethylene glycol-induced calcium oxalate urolithiasis. Ren. Fail. 2018, 40, 618–627. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Tian, J.; Wang, Y.; Xing, L.; Wang, J. Chemical modification, antioxidant and α-amylase inhibitory activities of corn silk polysaccharides. Carbohydr. Polym. 2013, 98, 428–437. [Google Scholar] [CrossRef]
- Huang, S.; Chen, F.; Cheng, H.; Huang, G. Modification and application of polysaccharide from traditional Chinese medicine such as Dendrobium officinale. Int. J. Biol. Macromol. 2020, 157, 385–393. [Google Scholar] [CrossRef]
- Xie, J.-H.; Wang, Z.-J.; Shen, M.-Y.; Nie, S.-P.; Gong, B.; Li, H.-S.; Zhao, Q.; Li, W.-J.; Xie, M.-Y. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocoll. 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Hussein, U.K.; Mahmoud, H.M.; Farrag, A.G.; Bishayee, A. Chemoprevention of diethylnitrosamine-initiated and phenobarbital-promoted hepatocarcinogenesis in rats by sulfated polysaccharides and aqueous extract of Ulva lactuca. Integr. Cancer Ther. 2015, 14, 525–545. [Google Scholar] [CrossRef]
- Feng, G.; Laijin, S.; Chen, S.; Teng, W.; Dejian, Z.; Yin, C.; Shoudong, G. In vitro and in vivo immunoregulatory activity of sulfated fucan from the sea cucumber A. leucoprocta. Int. J. Biol. Macromol. 2021, 187, 931–938. [Google Scholar] [CrossRef]
- Devillé, C.; Damas, J.; Forget, P.; Dandrifosse, G.; Peulen, O. Laminarin in the dietary fibre concept. J. Sci. Food Agric. 2004, 84, 1030–1038. [Google Scholar] [CrossRef]
- Choi, J.-I.; Kim, H.-J.; Lee, J.-W. Structural feature and antioxidant activity of low molecular weight laminarin degraded by gamma irradiation. Food Chem. 2011, 129, 520–523. [Google Scholar] [CrossRef]
- Moroney, N.C.; O’grady, M.N.; Lordan, S.; Stanton, C.; Kerry, J.P. Seaweed polysaccharides (laminarin and fucoidan) as functional ingredients in pork meat: An evaluation of anti-oxidative potential, thermal stability and bioaccessibility. Mar. Drugs 2015, 13, 2447–2464. [Google Scholar] [CrossRef]
- Liu, J.-H.; Ouyang, J.-M. Synergistic inhibition of calcium oxalate crystal formation and synergistic protection of HK-2 cells from crystal damage by sulfated Laminarin polysaccharide and potassium citrate. Biomater. Sci. 2023, 11, 3524–3546. [Google Scholar] [CrossRef]
- Thanasekaran, P.; Liu, C.-M.; Cho, J.-F.; Lu, K.-L. Melamine-promoted crystal growth of calcium oxalate monohydrate from calcium nitrate and oxalic acid. Inorg. Chem. Commun. 2012, 17, 84–87. [Google Scholar] [CrossRef]
- Bhatt, P.A.; Paul, P. Analysis of urinary stone constituents using powder X-ray diffraction and FT-IR. J. Chem. Sci. 2008, 120, 267–273. [Google Scholar] [CrossRef]
- Conti, C.; Casati, M.; Colombo, C.; Realini, M.; Brambilla, L.; Zerbi, G. Phase transformation of calcium oxalate dihydrate–monohydrate: Effects of relative humidity and new spectroscopic data. Spectrochim. Acta Part A 2014, 128, 413–419. [Google Scholar] [CrossRef]
- Maurice-Estepa, L.; Levillain, P.; Lacour, B.; Daudon, M. Advantage of zero-crossing-point first-derivative spectrophotometry for the quantification of calcium oxalate crystalline phases by infrared spectrophotometry. Clin. Chim. Acta 2000, 298, 1–11. [Google Scholar] [CrossRef]
- Shen, Y.; Yue, W.; Xie, A.; Li, S.; Qian, Z. Effects of amino acids on crystal growth of CaC2O4 in reverse microemulsion. Colloids Surf. B 2005, 45, 120–124. [Google Scholar] [CrossRef]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef]
- Huang, M.; Xu, A.; Wu, X.; Zhang, Y.; Guo, Y.; Guo, F.; Pan, Z.; Kong, L. Japanese encephalitis virus induces apoptosis by the IRE1/JNK pathway of ER stress response in BHK-21 cells. Arch. Virol. 2016, 161, 699–703. [Google Scholar] [CrossRef]
- Zuo, M.-Y.; Tang, T.-J.; Zhou, P.; Wang, X.; Ding, R.; Gu, J.-F.; Chen, J.; Wang, L.; Yao, J.; Li, X.-Y.; et al. Mechanism of atractylenolide III in alleviating H9c2 cell apoptosis through ROS/GRP78/caspase-12 signaling pathway based on molecular docking. China J. Chin. Mater. Med. 2022, 47, 4436–4445. [Google Scholar]
- Whitaker, R.H.; Cook, J.G. Stress Relief Techniques: p38 MAPK determines the balance of cell cycle and apoptosis pathways. Biomolecules 2021, 11, 1444. [Google Scholar] [CrossRef]
- Aluko, O.M.; Lawal, S.A.; Ijomone, O.M.; Aschner, M. Perturbed MAPK signaling in ASD: Impact of metal neurotoxicity. Curr. Opin. Toxicol. 2021, 26, 1–7. [Google Scholar] [CrossRef]
- Vega, G.G.; Aviles-Salas, A.; Ramon Chalapud, J.; Martinez-Paniagua, M.; Pelayo, R.; Mayani, H.; Hernandez-Pando, R.; Martinez-Maza, O.; Huerta-Yepez, S.; Bonavida, B.; et al. P38 MAPK expression and activation predicts failure of response to CHOP in patients with Diffuse Large B-Cell Lymphoma. BMC Cancer 2015, 15, 722. [Google Scholar] [CrossRef]
- Khan, S.R. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl. Androl. Urol. 2014, 3, 256. [Google Scholar]
- Renschler, M.F. The emerging role of reactive oxygen species in cancer therapy. Eur. J. Cancer 2004, 40, 1934–1940. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Okada, A.; Hamamoto, S.; Taguchi, K.; Unno, R.; Sugino, T.; Ando, R.; Mizuno, K.; Tozawa, K.; Kohri, K.; Yasui, T. Kidney stone formers have more renal parenchymal crystals than non-stone formers, particularly in the papilla region. BMC Urol. 2018, 18, 19. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Niimi, K.; Yasui, T.; Hirose, M.; Hamamoto, S.; Itoh, Y.; Okada, A.; Kubota, Y.; Kojima, Y.; Tozawa, K.; Sasaki, S. Mitochondrial permeability transition pore opening induces the initial process of renal calcium crystallization. Free Radic. Biol. Med. 2012, 52, 1207–1217. [Google Scholar] [CrossRef]
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 2018, 69, 62–72. [Google Scholar] [CrossRef]
- Albert, A.; Paul, E.; Rajakumar, S.; Saso, L. Oxidative stress and endoplasmic stress in calcium oxalate stone disease: The chicken or the egg? Free Radic. Res. 2020, 54, 244–253. [Google Scholar] [CrossRef]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef]
- Liao, B.; Luo, F.; Zhang, S.; Deng, Z.; Cai, L. Rehmanniae Radix-Induced apoptosis via inhibition of PI3K/AKT/mTOR signaling pathways in human hepatocellular carcinoma cell lines SMMC-7721. Pharmacogn. Mag. 2022, 18, 4–9. [Google Scholar]
- Shen, T.; Huang, Z.; Shi, C.; Pu, X.; Xu, X.; Wu, Z.; Ding, G.; Cao, L. Pancreatic cancer-derived exosomes induce apoptosis of T lymphocytes through the p38 MAPK-mediated endoplasmic reticulum stress. FASEB J. 2020, 34, 8442–8458. [Google Scholar] [CrossRef]
- Koul, H.K.; Menon, M.; Chaturvedi, L.S.; Koul, S.; Sekhon, A.; Bhandari, A.; Huang, M. COM crystals activate the p38 mitogen-activated protein kinase signal transduction pathway in renal epithelial cells. J. Biol. Chem. 2002, 277, 36845–36852. [Google Scholar] [CrossRef]
- Chaturvedi, L.S.; Koul, S.; Sekhon, A.; Bhandari, A.; Menon, M.; Koul, H.K. Oxalate Selectively Activates p38 Mitogen-activated Protein Kinase and c-Jun N-terminal Kinase Signal Transduction Pathways in Renal Epithelial Cells. J. Biol. Chem. 2002, 277, 13321–13330. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, Y.; Liu, Y.-C.; Wang, J.-Y.; Su, Q.; Tang, Z.-L.; Li, L. Coronary Microembolization induces Cardiomyocyte apoptosis through the LOX-1–dependent endoplasmic reticulum stress pathway involving JNK/P38 MAPK. Can. J. Cardiol. 2015, 31, 1272–1281. [Google Scholar] [CrossRef]
- Wei, H.; Li, Z.; Hu, S.; Chen, X.; Cong, X. Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J. Cell. Biochem. 2010, 111, 967–978. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Green, D.R.; Llambi, F. Cell death signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef]
- Park, H.S.; Han, C.R.; Woo, H.J.; Kim, Y.H. Proteasome inhibitor MG132-induced apoptosis via ER stress-mediated apoptotic pathway and its potentiation by protein tyrosine kinase p56lck in human Jurkat T cells. Biochem. Pharmacol. 2011, 82, 1110–1125. [Google Scholar] [CrossRef]
- Kläning, E.; Christensen, B.; Sørensen, E.S.; Vorup-Jensen, T.; Jensen, J.K. Osteopontin binds multiple calcium ions with high affinity and independently of phosphorylation status. Bone 2014, 66, 90–95. [Google Scholar] [CrossRef]
- Wesson, J.A.; Johnson, R.J.; Mazzali, M.; Beshensky, A.M.; Stietz, S.; Giachelli, C.; Liaw, L.; Alpers, C.E.; Couser, W.G.; Kleinman, J.G. Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J. Am. Soc. Nephrol. 2003, 14, 139–147. [Google Scholar] [CrossRef]
- Aggarwal, K.P.; Narula, S.; Kakkar, M.; Tandon, C. Nephrolithiasis: Molecular mechanism of renal stone formation and the critical role played by modulators. BioMed Res. Int. 2013, 2013, 292953. [Google Scholar] [CrossRef]
- Mckee, M.; Nancl, A.; Khan, S. Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi. J. Bone Miner. Res. 1995, 10, 1913–1929. [Google Scholar] [CrossRef]
- Okada, A.; Nomura, S.; Saeki, Y.; Higashibata, Y.; Hamamoto, S.; Hirose, M.; Itoh, Y.; Yasui, T.; Tozawa, K.; Kohri, K. Morphological conversion of calcium oxalate crystals into stones is regulated by osteopontin in mouse kidney. J. Bone Miner. Res. 2008, 23, 1629–1637. [Google Scholar] [CrossRef]
- Peng, Q.-L.; Li, C.-Y.; Zhao, Y.-W.; Sun, X.-Y.; Liu, H.; Ouyang, J.-M. Protective Effect of Degraded Porphyra yezoensis Polysaccharides on the Oxidative Damage of Renal Epithelial Cells and on the Adhesion and Endocytosis of Nanocalcium Oxalate Crystals. Oxid. Med. Cell. Longev. 2021, 2021, 6463281. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.-Y.; Ouyang, J.-M. Effects of Porphyra yezoensis polysaccharide with different molecular weights on the adhesion and endocytosis of nanocalcium oxalate monohydrate in repairing damaged HK-2 cells. ACS Biomater. Sci. Eng. 2019, 5, 3974–3986. [Google Scholar] [CrossRef]
- Evan, A.P.; Coe, F.L.; Rittling, S.R.; Bledsoe, S.M.; Shao, Y.; Lingeman, J.E.; Worcester, E.M. Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: Osteopontin localization. Kidney Int. 2005, 68, 145–154. [Google Scholar] [CrossRef]
- Chen, X.-W.; Huang, W.-B.; Sun, X.-Y.; Xiong, P.; Ouyang, J.-M. Antioxidant activity of sulfated Porphyra yezoensis polysaccharides and their regulating effect on calcium oxalate crystal growth. Mater. Sci. Eng. C 2021, 128, 112338. [Google Scholar] [CrossRef]
- Verkoelen, C.; Romijn, J.; Cao, L.; Boevé, E.; De Bruijn, W.; Schroder, F. Crystal-cell interaction inhibition by polysaccharides. J. Urol. 1996, 155, 749–752. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Sun, X.-Y.; Chen, X.-W.; Ouyang, J.-M. Degraded Porphyra yezoensis polysaccharide protects HK-2 cells and reduces nano-COM crystal toxicity, adhesion and endocytosis. J. Mater. Chem. B 2020, 8, 7233–7252. [Google Scholar] [CrossRef]
- Dissayabutra, T.; Kalpongnukul, N.; Chindaphan, K.; Srisa-Art, M.; Ungjaroenwathana, W.; Kaewwongse, M.; Iampenkhae, K.; Tosukhowong, P. Urinary sulfated glycosaminoglycan insufficiency and chondroitin sulfate supplement in urolithiasis. PLoS ONE 2019, 14, e0213180. [Google Scholar] [CrossRef]
- Ou, Y.; Xue, J.-F.; Tan, C.-Y.; Gui, B.-S.; Sun, X.-Y.; Ouyang, J.-M. Inhibition of urinary macromolecule heparin on aggregation of nano-COM and nano-COD crystals. Molecules 2015, 20, 1626–1642. [Google Scholar] [CrossRef]
- Zhao, Y.-W.; Da Guo, C.-Y.L.; Ouyang, J.-M. Comparison of the adhesion of calcium oxalate monohydrate to HK-2 cells before and after repair using tea polysaccharides. Int. J. Nanomed. 2019, 14, 4277. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Ouyang, J.-M.; Liu, A.-J.; Ding, Y.-M.; Gan, Q.-Z. Preparation, characterization, and in vitro cytotoxicity of COM and COD crystals with various sizes. Mater. Sci. Eng. C 2015, 57, 147–156. [Google Scholar] [CrossRef]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.T.Y.; Ng, K.W.; Loo, S.C.J. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2013, 87, 1037–1052. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.-Q.; Wang, Z.; Li, C.-Y.; Zhao, Y.-W.; Tong, X.-Y.; Liu, J.-H.; Ouyang, J.-M. Sulfated Laminarin Polysaccharides Reduce the Adhesion of Nano-COM Crystals to Renal Epithelial Cells by Inhibiting Oxidative and Endoplasmic Reticulum Stress. Pharmaceuticals 2024, 17, 805. https://doi.org/10.3390/ph17060805
He T-Q, Wang Z, Li C-Y, Zhao Y-W, Tong X-Y, Liu J-H, Ouyang J-M. Sulfated Laminarin Polysaccharides Reduce the Adhesion of Nano-COM Crystals to Renal Epithelial Cells by Inhibiting Oxidative and Endoplasmic Reticulum Stress. Pharmaceuticals. 2024; 17(6):805. https://doi.org/10.3390/ph17060805
Chicago/Turabian StyleHe, Tian-Qu, Zhi Wang, Chuang-Ye Li, Yao-Wang Zhao, Xin-Yi Tong, Jing-Hong Liu, and Jian-Ming Ouyang. 2024. "Sulfated Laminarin Polysaccharides Reduce the Adhesion of Nano-COM Crystals to Renal Epithelial Cells by Inhibiting Oxidative and Endoplasmic Reticulum Stress" Pharmaceuticals 17, no. 6: 805. https://doi.org/10.3390/ph17060805
APA StyleHe, T.-Q., Wang, Z., Li, C.-Y., Zhao, Y.-W., Tong, X.-Y., Liu, J.-H., & Ouyang, J.-M. (2024). Sulfated Laminarin Polysaccharides Reduce the Adhesion of Nano-COM Crystals to Renal Epithelial Cells by Inhibiting Oxidative and Endoplasmic Reticulum Stress. Pharmaceuticals, 17(6), 805. https://doi.org/10.3390/ph17060805







