Decreased Risk of Osteoporosis Incident in Subjects Receiving Chinese Herbal Medicine for Sjögren syndrome Treatment: A Retrospective Cohort Study with a Nested Case-Control Analysis
Abstract
1. Introduction
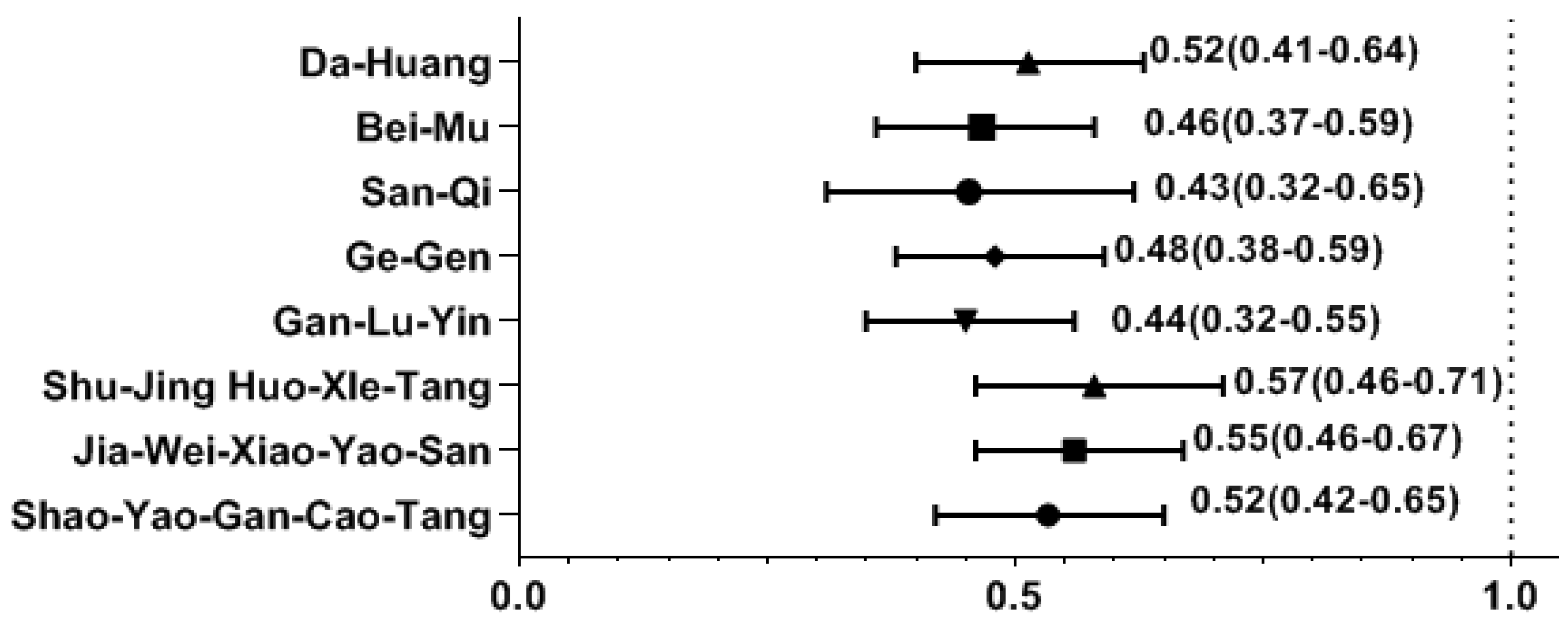
2. Results
3. Discussion
4. Methods
4.1. Data Source
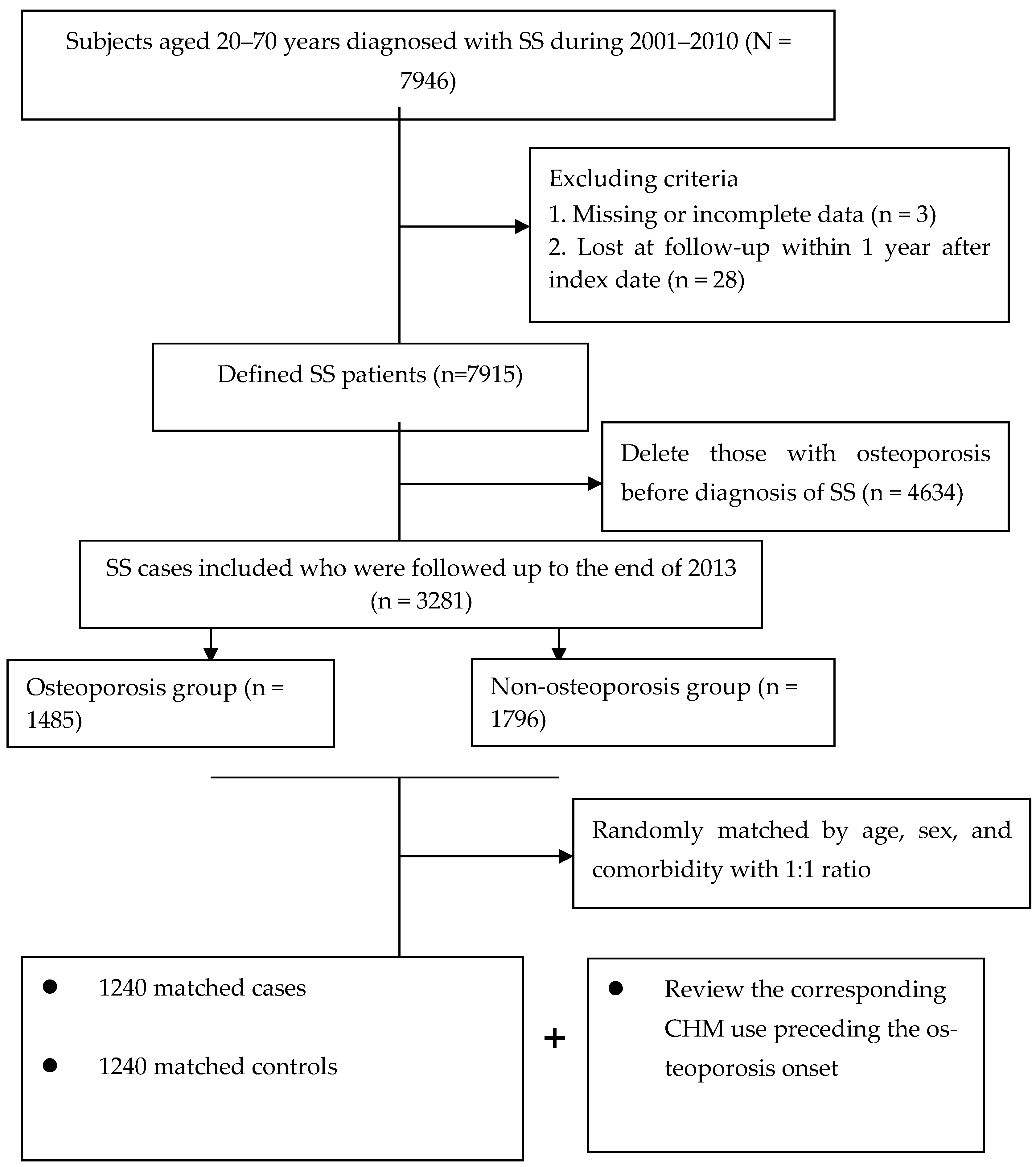
4.2. Underlying Cohort Establishment
4.3. Ascertainment of Case and Control Groups
4.4. Categorization of Chinese Herbal Medicine Use
4.5. Definition of Covariates
4.6. Evaluation of Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thurtle, E.; Grosjean, A.; Steenackers, M.; Strege, K.; Barcelos, G.; Goswami, P. Epidemiology of Sjögren’s: A systematic literature review. Rheumatol. Ther. 2023, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjögren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Ma, L.; Punwaney, R.; Ramachandran, S. Clinical and Cost Burden of Primary Sjögren’s Syndrome: Descriptive Analysis Using a US Administrative Claims Database. J. Health Econ. Outcomes Res. 2018, 5, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Skarlis, C.; Palli, E.; Nezos, A.; Koutsilieris, M.; Mavragani, C.P. Study of the incidence of osteoporosis in patients with Sjögren’s syndrome (pSS) and investigation of activation of the RANKL/RANK and osteoprotegerin (OPG) system. Mediterr. J. Rheumatol. 2018, 29, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Benchabane, S.; Boudjelida, A.; Toumi, R.; Belguendouz, H.; Youinou, P.; Touil-Boukoffa, C. A case for IL-6, IL-17A, and nitric oxide in the pathophysiology of Sjögren’s syndrome. Int. J. Immunopathol. Pharmacol. 2016, 29, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ming, B.; Zhang, C.; Deng, X.; Li, P.; Wei, Z.; Xia, Y.; Jiang, K.; Ye, H.; Ma, W.; et al. IL-2 Inhibition of Th17 Generation Rather Than Induction of Treg Cells Is Impaired in Primary Sjögren’s Syndrome Patients. Front. Immunol. 2018, 9, 1755. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N. Bone and the immune system. Toxicol. Pathol. 2017, 45, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Takakura, N.; Hiura, F.; Nakamura, I.; Hirata-Tsuchiya, S. The role of NF-κB in physiological bone development and inflammatory bone diseases: Is NF-κB inhibition “Killing two birds with one stone”? Cells 2019, 8, 1636. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.I.; Lin, T.M.; Chang, Y.S.; Hou, T.Y.; Hsu, H.C.; Lin, S.H.; Chen, W.S.; Lin, Y.C.; Wang, L.H.; Chang, C.C.; et al. Primary Sjogren syndrome increases the risk of bisphosphonate-related osteonecrosis of the jaw. Sci. Rep. 2021, 11, 1612. [Google Scholar] [CrossRef]
- Kanis, J.A.; Oden, A.; Johnell, O.; De Laet, C.; Jonsson, B.; Oglesby, A.K. The components of excess mortality after hip fracture. Bone 2003, 32, 468–473. [Google Scholar] [CrossRef]
- Luo, H.; Li, X.; Liu, J.; Andrew, F.; George, L. Chinese Herbal medicine in treating primary sjögren’s syndrome: A systematic review of randomized trials. Evid.-Based Complement. Altern. Med. Ecam 2012, 2012, 640658. [Google Scholar] [CrossRef]
- Chen, H.H.; Lai, J.N.; Yu, M.C.; Chen, C.Y.; Hsieh, Y.T.; Hsu, Y.F.; Wei, J.C. Traditional Chinese medicine in patients with primary sjogren’s syndrome: A randomized, double-blind, placebo-controlled clinical trial. Front. Med. 2021, 8, 744194. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Wu, P.C.; Lin, J.R.; Jan Wu, Y.J.; Luo, S.F.; Hsue, Y.T.; Lan, J.L.; Pan, T.L.; Wu, Y.T.; Yu, K.H.; et al. Herbal formula SS-1 increases tear secretion for sjögren’s syndrome. Front. Pharmacol. 2021, 12, 645437. [Google Scholar] [CrossRef] [PubMed]
- Doss, H.M.; Samarpita, S.; Ganesan, R.; Rasool, M. Ferulic acid, a dietary polyphenol suppresses osteoclast differentiation and bone erosion via the inhibition of RANKL dependent NF-κB signalling pathway. Life Sci. 2018, 207, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.F.; Lin, Y.J.; Tsai, F.J.; Li, T.M.; Lin, T.H.; Liao, C.C.; Huang, S.M.; Liu, X.; Li, M.J.; Ban, B.; et al. Effects of Chinese herbal medicines on the risk of overall mortality, readmission, and reoperation in hip fracture patients. Front. Pharmacol. 2019, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Liao, C.C.; Su, Y.C.; Tsai, C.C.; Lin, J.G. Gender differences in traditional Chinese medicine use among adults in Taiwan. PLoS ONE 2012, 7, e32540. [Google Scholar] [CrossRef] [PubMed]
- Aljefree, N.M.; Almoraie, N.M.; Althaiban, M.A.; Hanbazaza, M.A.; Wazzan, H.A.; Shatwan, I.M. Gender differences in knowledge, attitudes, and practices with respect to type 1 diabetes among Saudi public-school teachers. BMC Public Health 2023, 23, 118. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Horie-Inoue, K.; Inoue, S. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J. Steroid Biochem. Mol. Biol. 2019, 191, 105375. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Pescinini-Salzedas, L.M.; Minniti, G.A.D.S.; Laurindo, L.F.; Barbalho, S.M.; Vargas Sinatora, R.; Sloan, L.A.; Haber, R.S.; AraAojo, A.C.; Quesada, K.; et al. Organokines, sarcopenia, and metabolic repercussions: The vicious cycle and the interplay with exercise. Int. J. Mol. Sci. 2022, 23, 13452. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.; Hong, S.; Kwon, B.; Song, M.W.; Song, K.; Kim, E.Y.; Jung, H.S.; Sohn, Y. Anti-inflammatory effects of Fritillaria thunbergii Miquel extracts in LPS-stimulated murine macrophage RAW 264.7 cells. Exp. Ther. Med. 2021, 21, 429. [Google Scholar] [CrossRef]
- Laksmitawati, D.R.; Widyastuti, A.; Karami, N.; Afifah, E.; Rihibiha, D.D.; Nufus, H.; Widowati, W. Anti-inflammatory effects of Anredera cordifolia and Piper crocatum extracts on lipopolysaccharide-stimulated macrophage cell line. Bangladesh J. Pharmacol. 2017, 12, 35–40. [Google Scholar] [CrossRef]
- Hu, W.; Yang, X.; Zhe, C.; Zhang, Q.; Sun, L.; Cao, K. Puerarin inhibits iNOS, COX-2 and CRP expression via suppression of NF-κB activation in LPS-induced RAW264.7 macrophage cells. Pharmacol. Rep. PR 2011, 63, 781–789. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Han, F.; Hu, Y.; Liu, Y.; Li, J.; Wang, L. Curcumin inhibits polyethylene-induced osteolysis via repressing NF-κB signaling pathway activation. Cell Physiol. Biochem. 2018, 50, 1100–1112. [Google Scholar] [CrossRef]
- Shrimali, D.; Shanmugam, M.K.; Kumar, A.P.; Zhang, J.; Tan, B.K.; Ahn, K.S.; Sethi, G. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013, 341, 139–149. [Google Scholar] [CrossRef]
- Ding, D.; Yan, J.; Feng, G.; Zhou, Y.; Ma, L.; Jin, Q. Dihydroartemisinin attenuates osteoclast formation and bone resorption via inhibiting the NF-κB, MAPK and NFATc1 signaling pathways and alleviates osteoarthritis. Int. J. Mol. Med. 2022, 49, 4. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.R.; Jagga, S.; Chakraborty, C.; Lee, S.-S. Fibroblast-like-synoviocytes mediate secretion of pro-inflammatory cytokines via ERK and JNK MAPKs in ti-particle-induced osteolysis. Materials 2020, 13, 3628. [Google Scholar] [CrossRef]
- Tsai, F.J.; Ho, T.J.; Cheng, C.F.; Shiao, Y.T.; Chien, W.K.; Chen, J.H.; Liu, X.; Tsang, H.; Lin, T.H.; Liao, C.C.; et al. Characteristics of Chinese herbal medicine usage in ischemic heart disease patients among type 2 diabetes and their protection against hydrogen peroxide-mediated apoptosis in H9C2 cardiomyoblasts. Oncotarget 2017, 8, 15470–15489. [Google Scholar] [CrossRef]
- Cortés-Vieyra, R.; Silva-García, O.; Gómez-García, A.; Gutiérrez-Castellanos, S.; Álvarez-Aguilar, C.; Baizabal-Aguirre, V.M. Glycogen synthase kinase 3β modulates the inflammatory response activated by bacteria, viruses, and parasites. Front. Immunol. 2021, 12, 675751. [Google Scholar] [CrossRef]
- Yasui, T.; Yamada, M.; Uemura, H.; Ueno, S.-i.; Numata, S.; Ohmori, T.; Tsuchiya, N.; Noguchi, M.; Yuzurihara, M.; Kase, Y. Changes in circulating cytokine levels in midlife women with psychological symptoms with selective serotonin reuptake inhibitor and Japanese traditional medicine. Maturitas 2009, 62, 146–152. [Google Scholar] [CrossRef]
- Guo, R.B.; Wang, G.F.; Zhao, A.P.; Gu, J.; Sun, X.L.; Hu, G. Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses. PLoS ONE 2012, 7, e49701. [Google Scholar] [CrossRef]
- Inagaki, Y.; Kido, J.-i.; Nishikawa, Y.; Kido, R.; Sakamoto, E.; Bando, M.; Naruishi, K.; Nagata, T.; Yumoto, H. Gan-lu-yin (kanroin), traditional Chinese herbal extracts, reduces osteoclast differentiation in vitro and prevents alveolar bone resorption in rat experimental periodontitis. J. Clin. Med. 2021, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhang, B.; Kong, L.; Gong, Y.; Feng, M.; Gao, X.; Wang, D.; Yan, L. Receptor activator of nuclear factor-κB ligand-mediated osteoclastogenesis signaling pathway and related therapeutic natural compounds. Front. Pharmacol. 2022, 13, 1043975. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- National Health Insurance Database, Taiwan. LHID 2000. Taiwan: Center for Biomedical Resources of NHRI; 2012. Available online: https://nhird.nhri.edu.tw//en/index.html (accessed on 1 January 2024).
- Huang, S.C.; Gau, S.Y.; Huang, J.Y.; Wu, W.J.; Wei, J.C. Increased risk of hypothyroidism in people with asthma: Evidence from a real-world population-based study. J. Clin. Med. 2022, 11, 2776. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Hung, Y.T.; Chuang, Y.L.; Chen, Y.J.; Weng, W.S.; Liu, J.S.; Liang, K.Y. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 2006, 4, 1–22. [Google Scholar] [CrossRef]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Austin, P.C. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat. Med. 2008, 27, 2037–2049. [Google Scholar] [CrossRef]
| Variables | Number (%) | Cases | Controls | Standardized Difference |
|---|---|---|---|---|
| N = 1240 (%) | N = 1240(%) | |||
| Age (Years) | 0.001 | |||
| ≤50 | 1286 (51.9) | 641 (51.7) | 645 (52.0) | |
| >50 | 1194 (48.1) | 599 (48.3) | 595 (48.0) | |
| Mean (SD) | 48.5 (14.0) | 48.2 (14.1) | 48.9 (14.0) | 0.004 |
| Gender | 0.003 | |||
| Male | 497 (20.0) | 255 (20.6) | 242 (19.5) | |
| Female | 1983 (80.0) | 985 (79.4) | 998 (80.5) | |
| Monthly income | 0.001 | |||
| 25th percentile | 1214 (49.0) | 606 (48.9) | 608 (49.0) | |
| 50th percentile | 1130 (45.6) | 573 (46.2) | 557 (44.9) | |
| 75th percentile | 136 (5.5) | 61 (4.9) | 75 (6.0) | |
| Residential typologies | 0.04 | |||
| Urban area | 1506 (60.7) | 737 (59.4) | 769 (62.0) | |
| Suburban area | 399 (16.1) | 209 (16.9) | 190 (15.3) | |
| Rural area | 575 (23.2) | 294 (23.7) | 281 (22.7) | |
| CCI | 2.7 (2.6) | 2.7 (2.3) | 2.6 (2.8) | 0.03 |
| Anti-osteoporotic medications | 1158 (46.7) | 586 (47.3) | 572 (46.1) | 0.04 |
| CHM Exposure | Subjects | Crude OR (95% CI) | Adjusted OR * (95% CI) | |||
|---|---|---|---|---|---|---|
| Cases n = 1240 | Controls n = 1240 | |||||
| Non-CHM Users | 886 | 71.5% | 582 | 46.9% | 1 | 1 |
| CHM users | 334 | 28.5% | 658 | 53.1% | 0.49 (0.41–0.57) | 0.47 (0.39–0.57) |
| Level 1 (31–180 days) | 280 | 22.6% | 445 | 35.9% | 0.56 (0.46–0.66) | 0.56 (0.45–0.67) |
| Level 2 (181–365 days) | 63 | 5.1% | 160 | 12.9% | 0.35 (0.26–0.46) | 0.34 (0.24–0.47) |
| Level 3 (366 days or more) | 11 | 0.9% | 53 | 4.3% | 0.32 (0.19–0.55) | 0.29 (0.17–0.49) |
| Variables | Osteoporosis Cases, n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|
| Gender | |||
| Male | 255 (20.6) | 0.52 (0.35–0.76) | 0.53 (0.37–0.79) * |
| Female | 985 (79.4) | 0.32 (0.27–0.36) | 0.30 (0.24–0.36) * |
| Age (years) | |||
| ≤50 | 641 (49.8) | 0.31 (0.25–0.40) | 0.31 (0.25–0.38) ** |
| >50 | 599 (50.2) | 0.39 (0.37–0.50) | 0.37 (0.31–0.52) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.-T.; Livneh, H.; Huang, H.-L.; Lu, M.-C.; Chen, W.-J.; Tsai, T.-Y. Decreased Risk of Osteoporosis Incident in Subjects Receiving Chinese Herbal Medicine for Sjögren syndrome Treatment: A Retrospective Cohort Study with a Nested Case-Control Analysis. Pharmaceuticals 2024, 17, 745. https://doi.org/10.3390/ph17060745
Yen C-T, Livneh H, Huang H-L, Lu M-C, Chen W-J, Tsai T-Y. Decreased Risk of Osteoporosis Incident in Subjects Receiving Chinese Herbal Medicine for Sjögren syndrome Treatment: A Retrospective Cohort Study with a Nested Case-Control Analysis. Pharmaceuticals. 2024; 17(6):745. https://doi.org/10.3390/ph17060745
Chicago/Turabian StyleYen, Chieh-Tsung, Hanoch Livneh, Hua-Lung Huang, Ming-Chi Lu, Wei-Jen Chen, and Tzung-Yi Tsai. 2024. "Decreased Risk of Osteoporosis Incident in Subjects Receiving Chinese Herbal Medicine for Sjögren syndrome Treatment: A Retrospective Cohort Study with a Nested Case-Control Analysis" Pharmaceuticals 17, no. 6: 745. https://doi.org/10.3390/ph17060745
APA StyleYen, C.-T., Livneh, H., Huang, H.-L., Lu, M.-C., Chen, W.-J., & Tsai, T.-Y. (2024). Decreased Risk of Osteoporosis Incident in Subjects Receiving Chinese Herbal Medicine for Sjögren syndrome Treatment: A Retrospective Cohort Study with a Nested Case-Control Analysis. Pharmaceuticals, 17(6), 745. https://doi.org/10.3390/ph17060745







