Azorella compacta Organic Extracts Exacerbate Metabolic Dysfunction-Associated Fatty Liver Disease in Mice Fed a High-Fat Diet
Abstract
1. Introduction
2. Results
2.1. Metabolomic Characterization of Azorella compacta Extracts
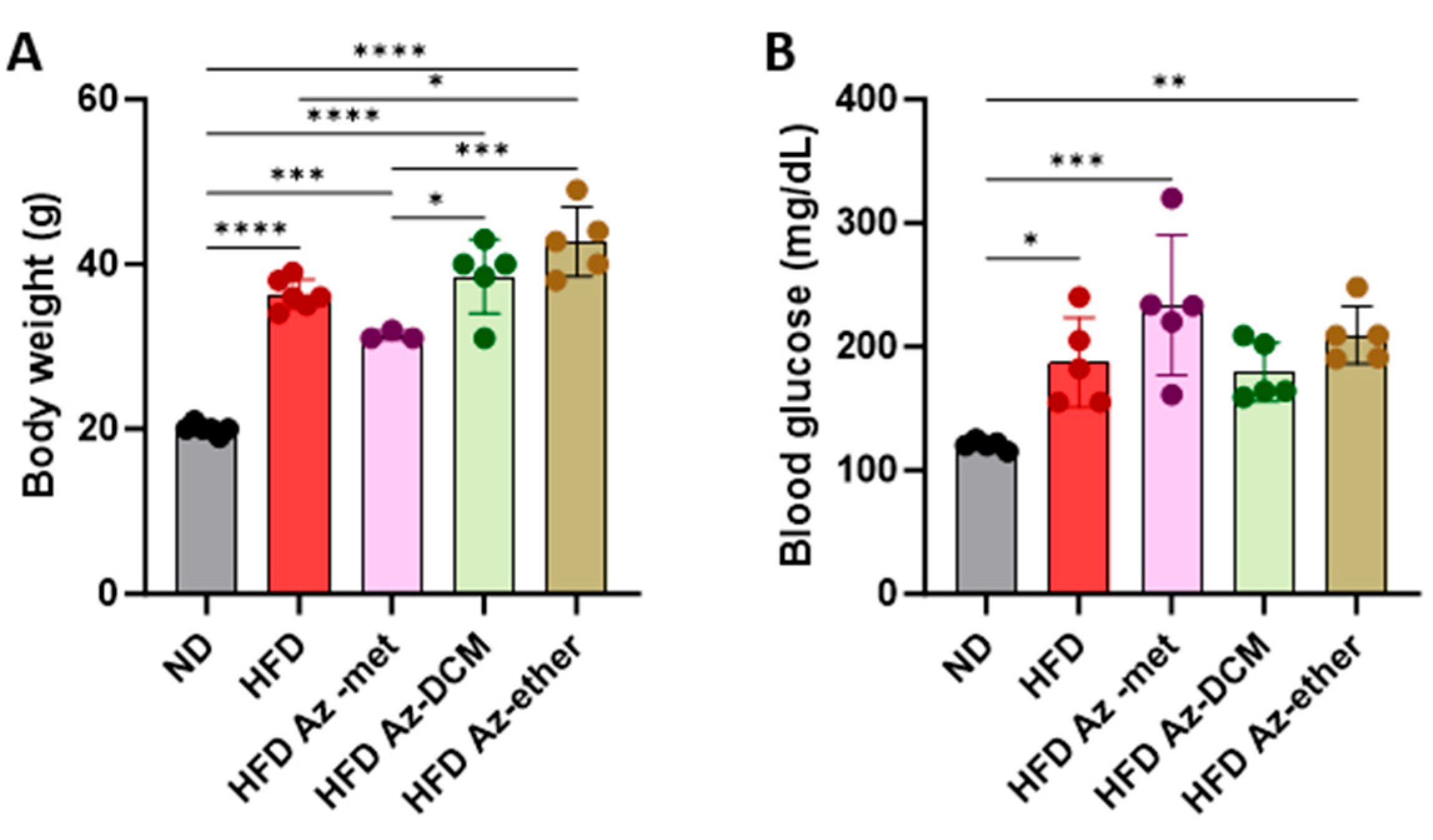
2.2. Impact of Azorella compacta Organic Fractions on Mice Fed a High-Fat Diet
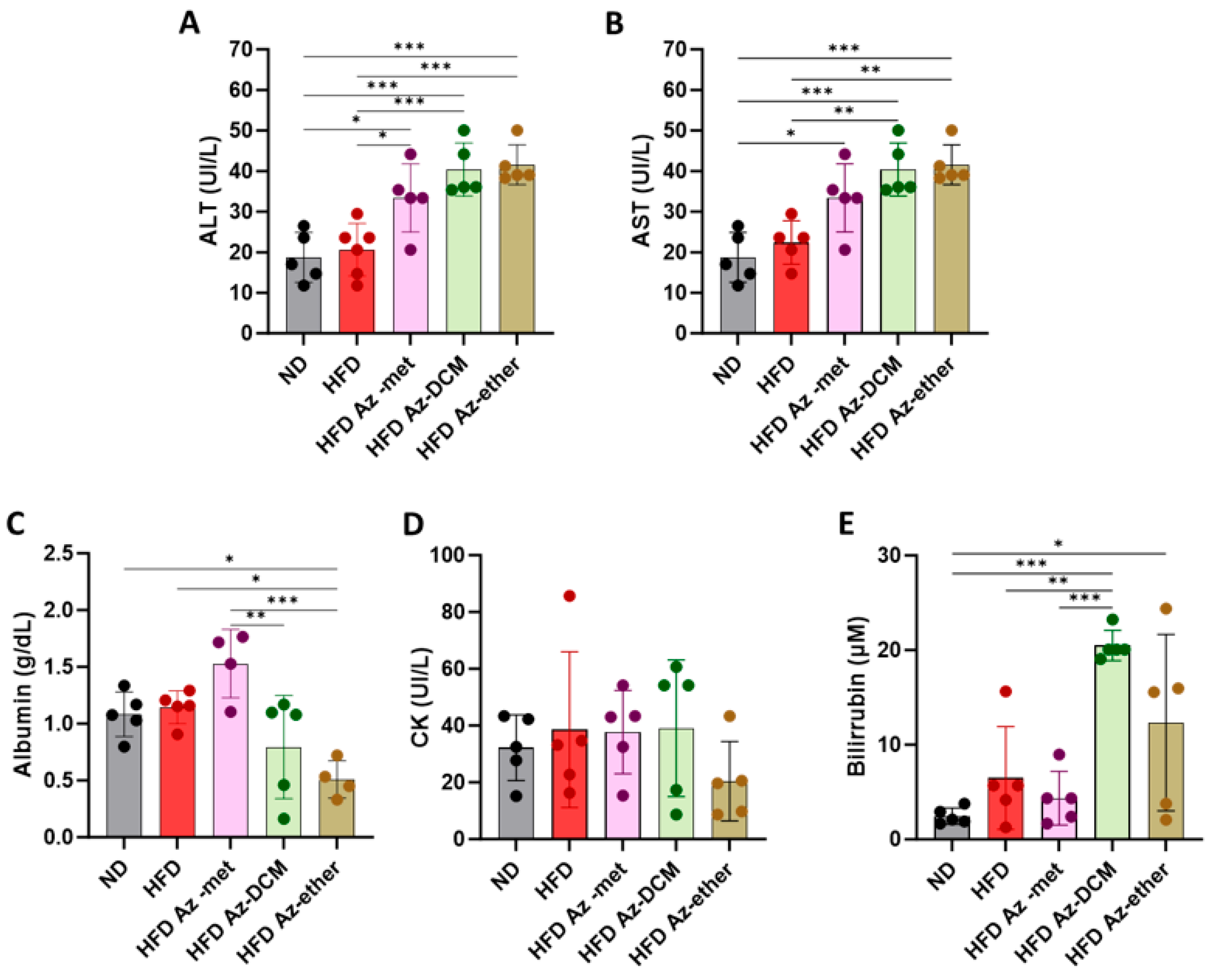
2.3. Effect of A. compacta Organic Extract Treatment on Hepatic Pathology
2.4. In Silico Analysis of Toxicity and Metabolism of A. compacta Diterpenoids
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Metabolomic Analysis
4.3. Mice and Diets
4.4. Determination of Biochemical Parameters
4.5. Liver Histopathology
4.6. In Silico Analysis of Metabolism and Toxicity
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleier, C.; Trenary, T.; Graham, E.A.; Stenzel, W.; Rundel, P.W. Size class structure, growth rates, and orientation of the central Andean cushion Azorella compacta. PeerJ 2015, 3, e843. [Google Scholar] [CrossRef] [PubMed]
- Pugnaire, F.I.; Morillo, J.A.; Armas, C.; Rodríguez-Echeverría, S.; Gaxiola, A. Azorella compacta: Survival champions in extreme, high-elevation environments. Ecosphere 2020, 11, e03031. [Google Scholar] [CrossRef]
- Wickens, G.E. Llareta (Azorella compacta, Umbelliferae): A review. Econ. Bot. 1995, 49, 207–212. [Google Scholar] [CrossRef]
- Fatima, C.; Ignazio, P.; Spadaro, V. Evaluación etnobotanica de la Yareta (Azorella compacta) en Arequipa (Perú) y sus posibles aplicaciones. Quad. Bot. Amb. Appl. 2013, 23, 15–30. [Google Scholar]
- Delporte, C.; Backhouse, N.; Salinas, P.; San-Martin, A.; Borquez, J.; Loyola, A. Pharmaco-toxicological study of diterpenoids. Bioorganic Med. Chem. 2003, 11, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Areche, C.; Rojas-Alvarez, F.; Campos-Briones, C.; Lima, C.; Perez, E.G.; Sepulveda, B. Further mulinane diterpenoids from Azorella compacta. J. Pharm. Pharmacol. 2013, 65, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Borquez, J.; Ardiles, A.; Loyola, L.A.; Pena-Rodriguez, L.M.; Molina-Salinas, G.M.; Vallejos, J.; Collado, I.G.; Simirgiotis, M.J. Further mulinane and azorellane diterpenoids isolated from Mulinum crassifolium and Azorella compacta. Molecules 2014, 19, 3898–3908. [Google Scholar] [CrossRef] [PubMed]
- Salgado, F.; Areche, C.; Sepulveda, B.; Simirgiotis, M.J.; Caceres, F.; Quispe, C.; Quispe, L.; Cano, T. A new mulinane diterpenoid from the cushion shrub Azorella compacta growing in Peru. Pharmacogn. Mag. 2014, 10, S543–S548. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Oros, D.R.; Jaffe, R.; Didyk-Pena, A.; Areche, C.; Sepulveda, B.; Didyk, B.M. Mulinane and Azorellane Diterpenoid Biomarkers by GC-MS from a Representative Apiaceae (Umbelliferae) Species of the Andes. Molecules 2019, 24, 684. [Google Scholar] [CrossRef] [PubMed]
- Dzul-Beh, A.J.; Uc-Cachon, A.H.; Borquez, J.; Loyola, L.A.; Pena-Rodriguez, L.M.; Molina-Salinas, G.M. Mulinane- and Azorellane-Type Diterpenoids: A Systematic Review of Their Biosynthesis, Chemistry, and Pharmacology. Biomolecules 2020, 10, 1333. [Google Scholar] [CrossRef]
- Fuentes, N.L.; Sagua, H.; Morales, G.; Borquez, J.; San Martin, A.; Soto, J.; Loyola, L.A. Experimental antihyperglycemic effect of diterpenoids of llareta Azorella compacta (Umbelliferae) Phil in rats. Phytother. Res. 2005, 19, 713–716. [Google Scholar] [CrossRef] [PubMed]
- San-Martín, A.; Bacho, M.; Cretton, S.; Christen, P.; Olea, A.; Muñoz, D.; Guillen, A.; Balcazar, N. Evaluation of the hypoglycemic effects of extracts and diterpenoids from Azorella compacta (llareta). Planta Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Pafili, K.; Roden, M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef] [PubMed]
- Gofton, C.; Upendran, Y.; Zheng, M.H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Scorletti, E.; Mosca, A.; Alisi, A.; Byrne, C.D.; Targher, G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism 2020, 111, 154170. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rodas, M.C.; Valenzuela, R.; Videla, L.A. Relevant Aspects of Nutritional and Dietary Interventions in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2015, 16, 25168–25198. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Sabaj, M.; Tolosa, G.; Herrera Vielma, F.; Zuniga, M.J.; Gonzalez, D.R.; Zuniga-Hernandez, J. Maresin-1 Prevents Liver Fibrosis by Targeting Nrf2 and NF-kappaB, Reducing Oxidative Stress and Inflammation. Cells 2021, 10, 3406. [Google Scholar] [CrossRef]
- Videla, L.A.; Valenzuela, R.; Del Campo, A.; Zuniga-Hernandez, J. Omega-3 Lipid Mediators: Modulation of the M1/M2 Macrophage Phenotype and Its Protective Role in Chronic Liver Diseases. Int. J. Mol. Sci. 2023, 24, 15528. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Liu, Y. Terpenoids: Natural Compounds for Non-Alcoholic Fatty Liver Disease (NAFLD) Therapy. Molecules 2022, 28, 272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ou, Y.; Hu, G.; Wen, C.; Yue, S.; Chen, C.; Xu, L.; Xie, J.; Dai, H.; Xiao, H.; et al. Naringenin attenuates non-alcoholic fatty liver disease by down-regulating the NLRP3/NF-κB pathway in mice. Br. J. Pharmacol. 2020, 177, 1806–1821. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.; Islam, M.T.; Tabrez, S.; Firoz, C.K.; Jabir, N.R.; Kamal, M.A.; Melo-Cavalcante, A.A.C.; Almeida, F.R.C. Effect of Diterpenes on Hepatic System. Curr. Pharm. Des. 2018, 24, 4093–4100. [Google Scholar] [CrossRef]
- Chen, P.; Huang, Y.; Zhang, X.; Zhao, Z.; Sun, Z.; Cui, H.; Lin, C.; Peng, G.; Wu, A.; Zhu, C. New chemical structures and liver-protective activity of the diterpenoids from Callicarpa rubella. Fitoterapia 2023, 165, 105394. [Google Scholar] [CrossRef]
- Park, M.Y.; Sung, M.K. Carnosic acid attenuates obesity-induced glucose intolerance and hepatic fat accumulation by modulating genes of lipid metabolism in C57BL/6J-ob/ob mice. J. Sci. Food Agric. 2015, 95, 828–835. [Google Scholar] [CrossRef]
- Jeong, H.S.; Kim, K.H.; Lee, I.S.; Park, J.Y.; Kim, Y.; Kim, K.S.; Jang, H.J. Ginkgolide A ameliorates non-alcoholic fatty liver diseases on high fat diet mice. Biomed. Pharmacother. 2017, 88, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, Y.; Wang, D.; Zhao, Y.; Wang, Y.; Li, F.; Fang, J.; Chen, H.; Fan, S.; Huang, C. Ginkgolide B lowers body weight and ameliorates hepatic steatosis in high-fat diet-induced obese mice correlated with pregnane X receptor activation. RSC Adv. 2017, 7, 37858–37866. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Thawabteh, A.; Lelario, F.; Bufo, S.A.; Scrano, L. Classification, Toxicity and Bioactivity of Natural Diterpenoid Alkaloids. Molecules 2021, 26, 4103. [Google Scholar] [CrossRef]
- Chan, T.Y. Incidence and Causes of Aconitum Alkaloid Poisoning in Hong Kong from 1989 to 2010. Phytother. Res. 2015, 29, 1107–1111. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, J.-L.; Li, X.; Guo, M.-Q.; Leung, E.L.-H.; Zhou, H.; Liu, L.; Li, N. Anti-cancer and anti-inflammatory new vakognavine-type alkaloid from the roots of Aconitum carmichaelii. Tetrahedron Lett. 2016, 57, 5881–5884. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, C.; Wang, K.; Takahashi, S.; Krausz, K.W.; Lu, D.; Wang, Q.; Luo, Y.; Gong, X.; Mu, X.; et al. Comprehensive analysis of transcriptomics and metabolomics to understand triptolide-induced liver injury in mice. Toxicol. Lett. 2020, 333, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Chen, L.; Fang, P.; Cai, H.; Tang, H.; Peng, Y.; Deng, Y.; Cao, L.; Li, H.; Zhang, B.; et al. Mechanisms of Triptolide-Induced Hepatotoxicity and Protective Effect of Combined Use of Isoliquiritigenin: Possible Roles of Nrf2 and Hepatic Transporters. Front. Pharmacol. 2018, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Guo, Q.; Shen, J.; Zheng, K.; Lu, C.; Zhang, G.; Lu, A.; He, X. The Effect of Triptolide in Rheumatoid Arthritis: From Basic Research towards Clinical Translation. Int. J. Mol. Sci. 2018, 19, 376. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Monteiro, C.S.J.; Dos Santos, J.L. Herb-Induced Liver Injury—A Challenging Diagnosis. Healthcare 2022, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K.-S.; Tujios, S.R. Hidden Dangers: Herbal and Dietary Supplement Induced Hepatotoxicity. Livers 2023, 3, 618–636. [Google Scholar] [CrossRef]
- Ballotin, V.R.; Bigarella, L.G.; Brandao, A.B.M.; Balbinot, R.A.; Balbinot, S.S.; Soldera, J. Herb-induced liver injury: Systematic review and meta-analysis. World J. Clin. Cases 2021, 9, 5490–5513. [Google Scholar] [CrossRef] [PubMed]
- Fandohan, P.; Gnonlonfin, B.; Laleye, A.; Gbenou, J.D.; Darboux, R.; Moudachirou, M. Toxicity and gastric tolerance of essential oils from Cymbopogon citratus, Ocimum gratissimum and Ocimum basilicum in Wistar rats. Food Chem. Toxicol. 2008, 46, 2493–2497. [Google Scholar] [CrossRef]
- Pimkaew, P.; Suksen, K.; Somkid, K.; Chokchaisiri, R.; Jariyawat, S.; Chuncharunee, A.; Suksamrarn, A.; Piyachaturawat, P. Zederone, a sesquiterpene from Curcuma elata Roxb, is hepatotoxic in mice. Int. J. Toxicol. 2013, 32, 454–462. [Google Scholar] [CrossRef]
- Li, H.; Peng, Y.; Zheng, J. Dioscorea bulbifera L.-induced hepatotoxicity and involvement of metabolic activation of furanoterpenoids. Drug Metab. Rev. 2020, 52, 568–584. [Google Scholar] [CrossRef]
- Tumova, L.; Ducaiova, Z.; Cheel, J.; Vokral, I.; Sepulveda, B.; Vokurkova, D. Azorella compacta Infusion Activates Human Immune Cells and Scavenges Free Radicals In vitro. Pharmacogn. Mag. 2017, 13, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.H.; Kwon, O.K.; Oh, S.R.; Lee, J.; Park, S.H.; Han, S.B.; Ahn, K.S. Azorella compacta methanolic extract induces apoptosis via activation of mitogen-activated protein kinase. Mol. Med. Rep. 2015, 12, 6821–6828. [Google Scholar] [CrossRef] [PubMed]
- Borquez, J.; Bartolucci, N.L.; Echiburu-Chau, C.; Winterhalter, P.; Vallejos, J.; Jerz, G.; Simirgiotis, M.J. Isolation of cytotoxic diterpenoids from the Chilean medicinal plant Azorella compacta Phil from the Atacama Desert by high-speed counter-current chromatography. J. Sci. Food Agric. 2016, 96, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- San-Martín, A.; Bacho, M.; Núñez, S.; Rovirosa, J.; Soler, A.; Blanc, V.; León, R.; Olea, A.F. A novel normulinane isolated from Azorella compacta and assessment of its antibacterial activity. J. Chil. Chem. Soc. 2018, 63, 4082–4085. [Google Scholar] [CrossRef]
- Korourian, S.; Hakkak, R.; Ronis, M.J.; Shelnutt, S.R.; Waldron, J.; Ingelman-Sundberg, M.; Badger, T.M. Diet and risk of ethanol-induced hepatotoxicity: Carbohydrate-fat relationships in rats. Toxicol. Sci. 1999, 47, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]

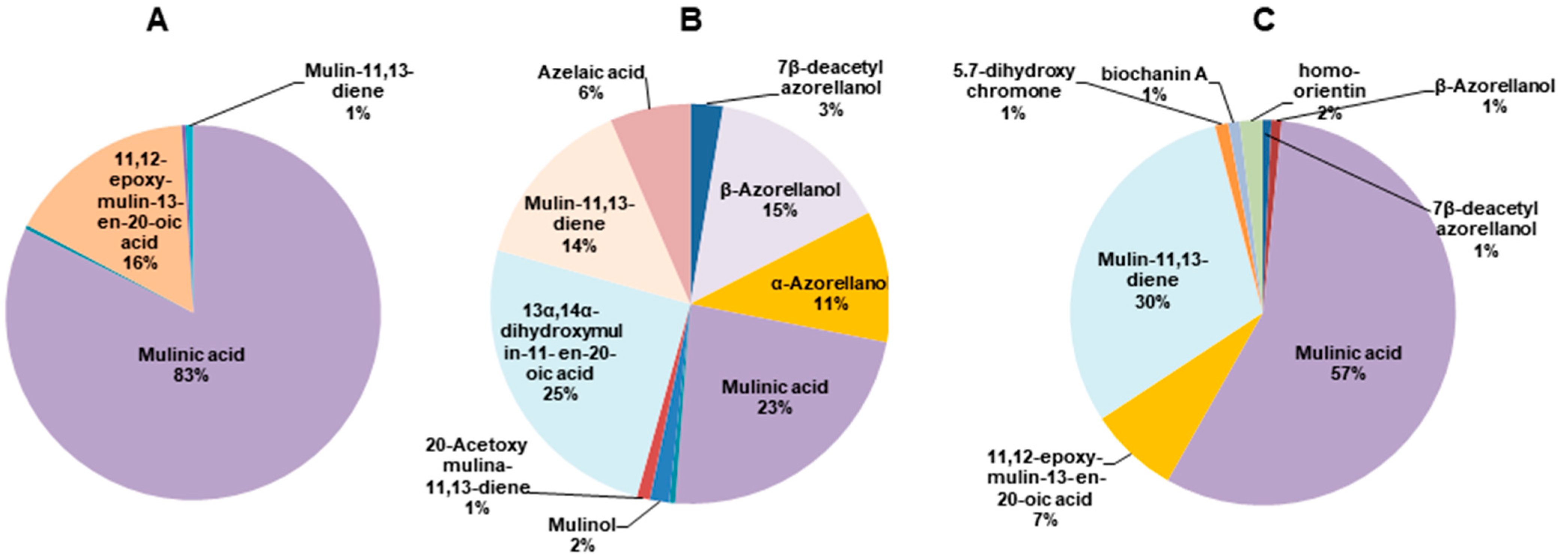

| Molecule | Name | RT (min) | Mass m/z | Petroleum Ether Fraction (s. Intensity) | DCM Fraction (s. Intensity) | MeOH Fraction (s. Intensity) |
|---|---|---|---|---|---|---|
| 1 | 7β-deacetylazorellanol (azorellan-13α,7β-diol) | 6.03 | 306.2 | 0 | 2790 | 1518 |
| 2a | β-azorellanol (azorellan-13β-hydroxy-7β-yl acetate) | 6.15 | 348.2 | 0 | 15,769 | 1811 |
| 2b | α-azorellanol (azorellan-13α-hydroxy-7β-yl acetate) | 5.45 | 348.2 | 0 | 11,261 | 0 |
| 3 | Mulinic acid (mulin-11,14-peroxi-12-en-20-oic acid) | 8.49 | 334.2 | 276,146 | 24,558 | 121,212 |
| 4 | Mulin-11,13-dien-20-oic acid | 10.41 | 302.2 | 1162 | 493 | 0 |
| 5 | 11,12-epoxymulin-13-en-20-oic acid (mulin-11,12-epoxy-13-en-20-oic acid) | 7.7 | 318.2 | 54,656 | 0 | 16,065 |
| 6 | Mulinol (mulin-11-en-13α,20-diol) | 8.3 | 306.2 | 0 | 1694 | 0 |
| 7 | 20-acetoxymulina-11,13-diene (20-hydroxymulin-11,13-dienyl acetate) | 7.83 | 330.2 | 0 | 1121 | 0 |
| 8 | 13α,14α-dihydroxymulin-11-en-20-oic acid (mulin-13α,14α-dihydroxy-11-ene-20-oic acid) | 5.38 | 336.2 | 0 | 26,551 | 0 |
| 9 | 7β-acetoxymulin-9,12-diene (mulin-9,12-dien-7β-yl acetate) | 7.6 | 330.2 | 1099 | 0 | 0 |
| 10 | Mulin-11,13-diene | 10.58 | 272.2 | 2198 | 15,021 | 64,755 |
| 11 | 5,7-dihydroxychromone | 2.7 | 178.0 | 0 | 0 | 2361 |
| 12 | Biochanin A | 8.86 | 284.2 | 0 | 0 | 2018 |
| 13 | Azelaic acid | 2.42 | 188.1 | 0 | 6919 | 0 |
| 14 | Homoorientin | 0.71 | 448.1 | 0 | 0 | 4113 |
| Molecule | H-HT (p) | DILI (p) | CYP1A2 Substrate | CYP2C19 Substrate | CYP2D6 Inhibitor | CYP2D6 Substrate | CYP2C9 Substrate | CYP3A4 Inhibitor | CYP3A4 Substrate | SR-MMP | SR-p53 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.371 | 0.018 | + | +++ | --- | --- | --- | + | + | +++ | + |
| 2a,b | 0.224 | 0.124 | -- | +++ | -- | --- | --- | - | + | ++ | +++ |
| 3 | 0.360 | 0.050 | ++ | +++ | --- | -- | + | -- | - | +++ | ++ |
| 4 | 0.614 | 0.011 | - | +++ | --- | - | ++ | - | + | ++ | + |
| 5 | 0.263 | 0.027 | + | +++ | --- | -- | - | --- | -- | ++ | + |
| 6 | 0.210 | 0.035 | - | ++ | --- | --- | --- | ++ | ++ | +++ | --- |
| 7 | 0.417 | 0.086 | -- | +++ | - | -- | --- | ++ | ++ | + | + |
| 8 | 0.246 | 0.015 | + | ++ | --- | --- | - | - | - | +++ | + |
| 9 | 0.173 | 0.798 | -- | +++ | --- | -- | - | + | + | -- | + |
| 10 | 0.466 | 0.023 | + | +++ | + | ++ | -- | ++ | ++ | + | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zúñiga-Hernandez, J.; Quiñones San Martin, M.; Figueroa, B.; Novoa, U.; Monsalve, F.A.; Bacho, M.; San-Martin, A.; González, D.R. Azorella compacta Organic Extracts Exacerbate Metabolic Dysfunction-Associated Fatty Liver Disease in Mice Fed a High-Fat Diet. Pharmaceuticals 2024, 17, 746. https://doi.org/10.3390/ph17060746
Zúñiga-Hernandez J, Quiñones San Martin M, Figueroa B, Novoa U, Monsalve FA, Bacho M, San-Martin A, González DR. Azorella compacta Organic Extracts Exacerbate Metabolic Dysfunction-Associated Fatty Liver Disease in Mice Fed a High-Fat Diet. Pharmaceuticals. 2024; 17(6):746. https://doi.org/10.3390/ph17060746
Chicago/Turabian StyleZúñiga-Hernandez, Jessica, Matías Quiñones San Martin, Benjamín Figueroa, Ulises Novoa, Francisco A. Monsalve, Mitchell Bacho, Aurelio San-Martin, and Daniel R. González. 2024. "Azorella compacta Organic Extracts Exacerbate Metabolic Dysfunction-Associated Fatty Liver Disease in Mice Fed a High-Fat Diet" Pharmaceuticals 17, no. 6: 746. https://doi.org/10.3390/ph17060746
APA StyleZúñiga-Hernandez, J., Quiñones San Martin, M., Figueroa, B., Novoa, U., Monsalve, F. A., Bacho, M., San-Martin, A., & González, D. R. (2024). Azorella compacta Organic Extracts Exacerbate Metabolic Dysfunction-Associated Fatty Liver Disease in Mice Fed a High-Fat Diet. Pharmaceuticals, 17(6), 746. https://doi.org/10.3390/ph17060746









