Silibinin’s Effects against Methotrexate-Induced Hepatotoxicity in Adjuvant-Induced Arthritis Rat Model
Abstract
1. Introduction
2. Results
2.1. Effect of SIL on Disease Severity in AIA Rat Model
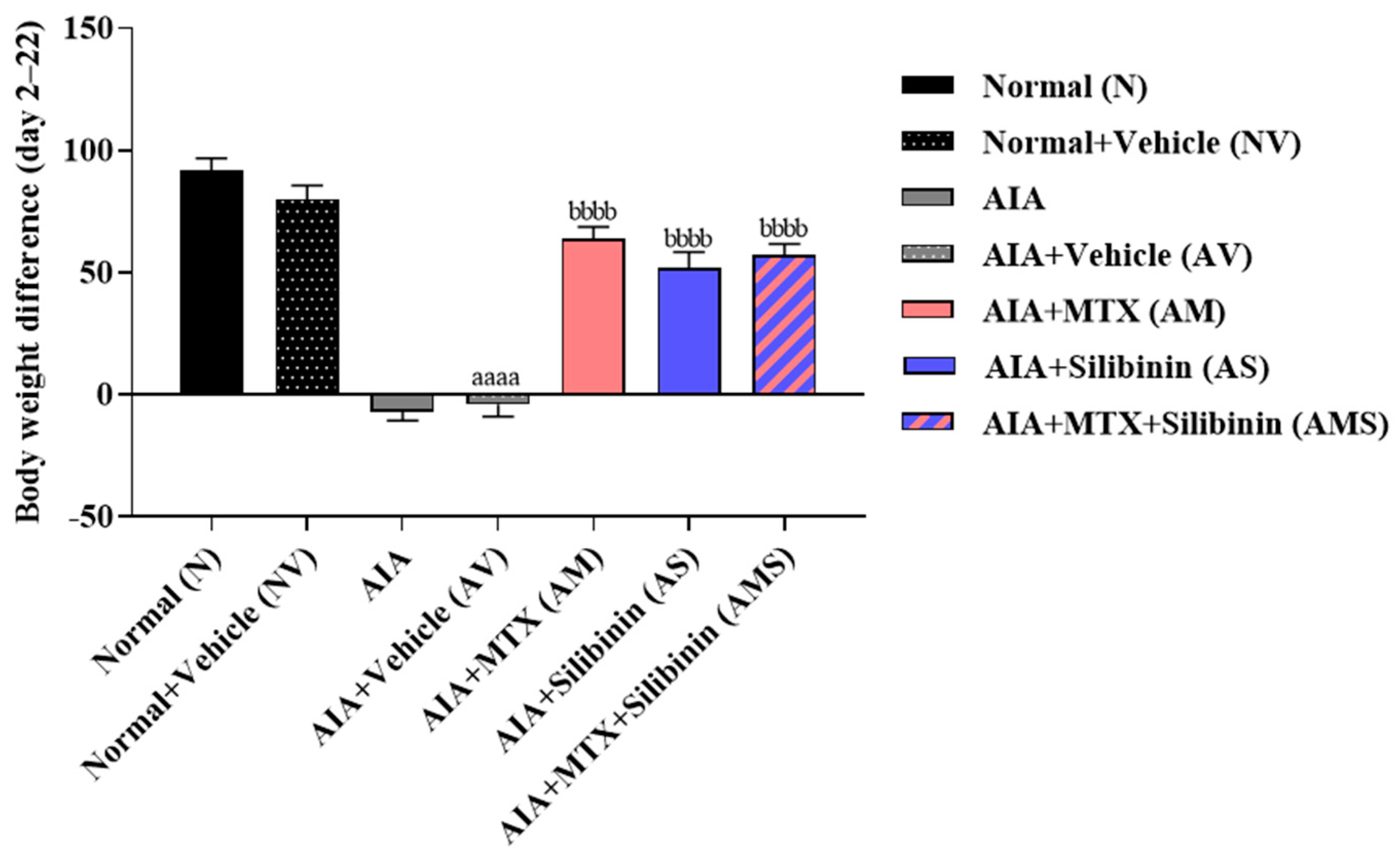
2.1.1. Body Weight
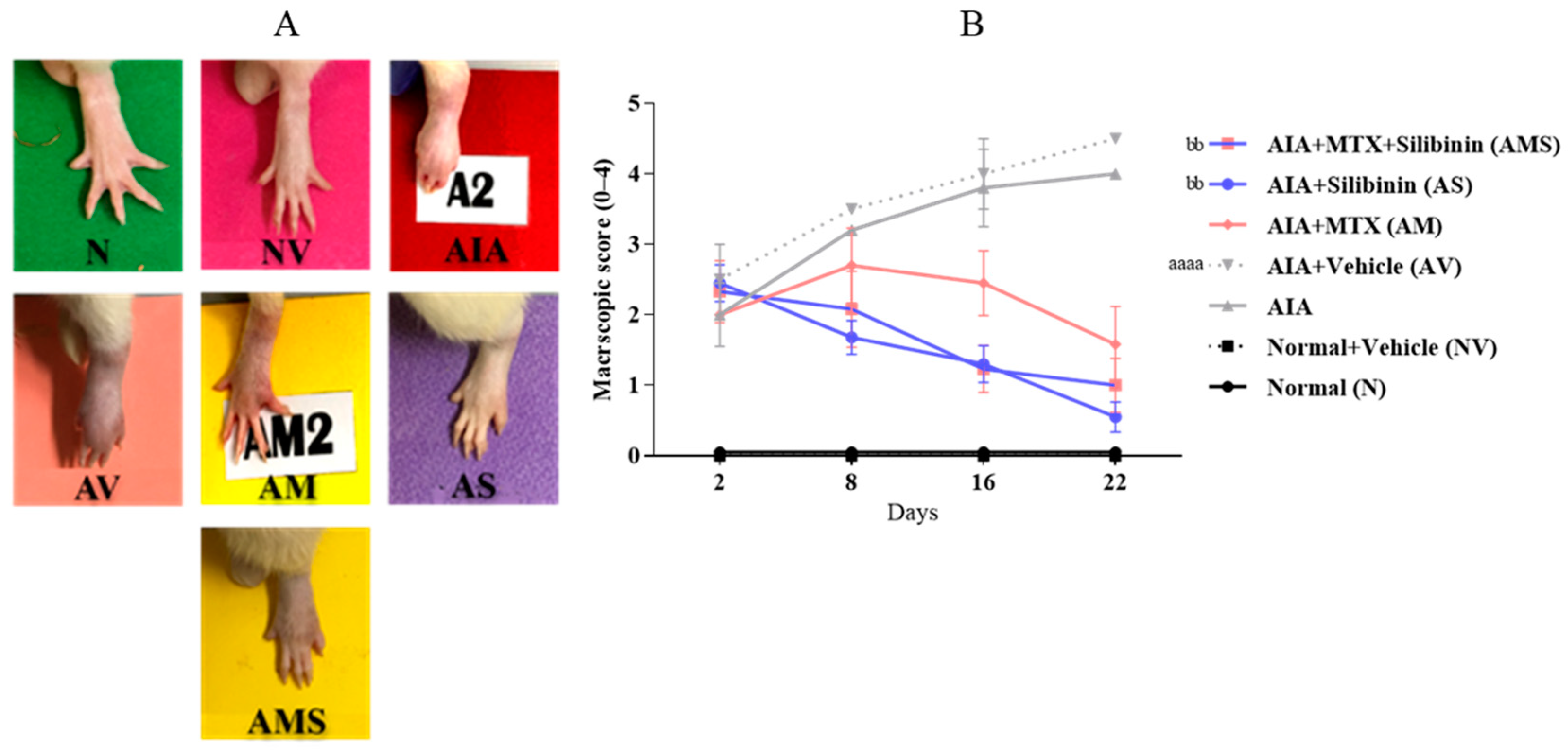
2.1.2. Macroscopic Scoring of the Injected Paw
2.1.3. Histological Examination of the Tibiotarsal Joint
2.1.4. ESR: Erythrocyte Sedimentation Rate Determination
2.2. Effect of SIL on Liver Injury Induced in AIA Rat Model and MTX Treatment
2.2.1. Histological Examination of Liver Sections
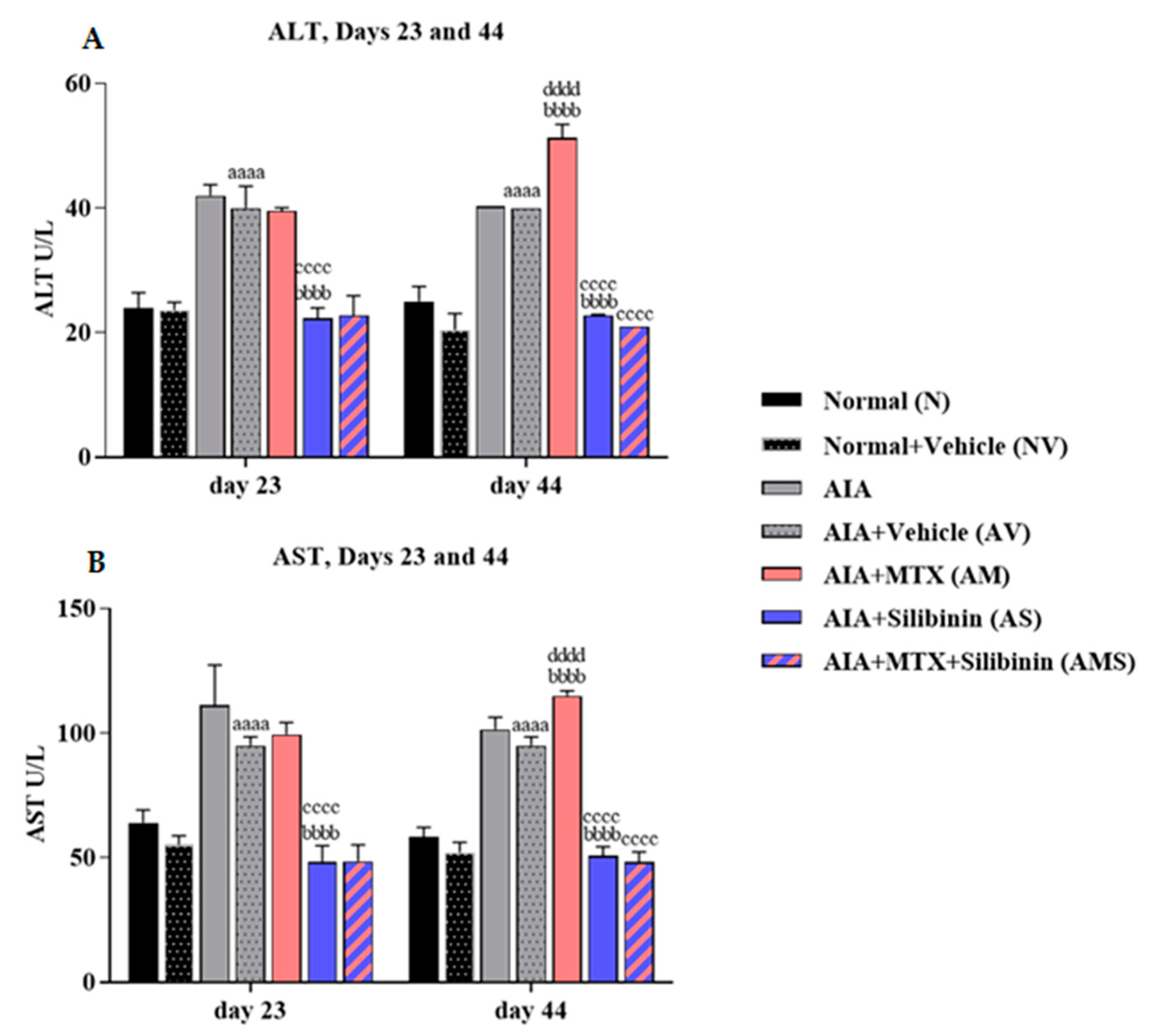
2.2.2. Serum ALT and AST Levels
2.3. Effect of SIL on Oxidative Stress and Antioxidant Defense System in the Liver of AIA Rats
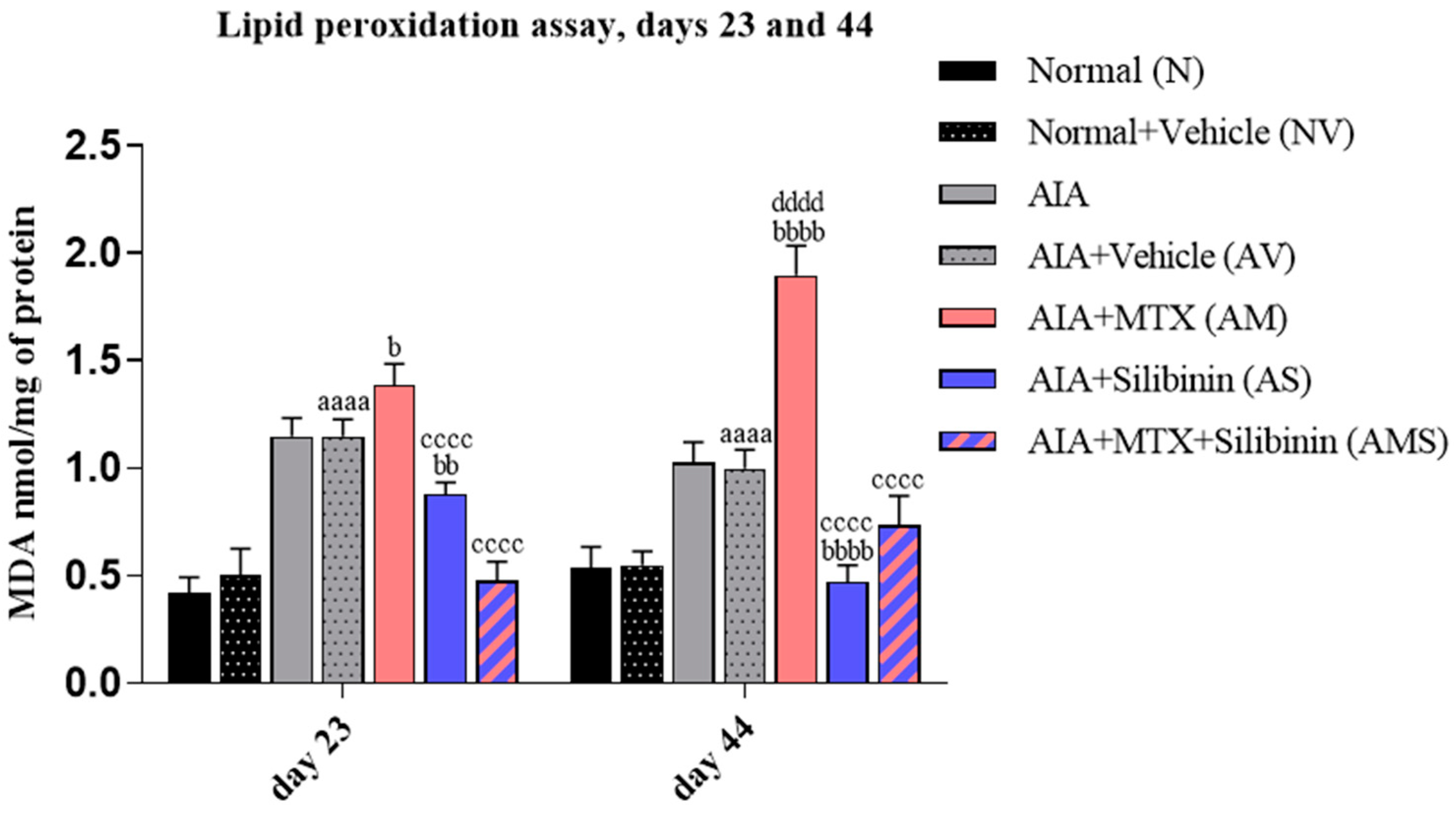
2.3.1. Malondialdehyde Level
2.3.2. Superoxide Dismutase and Catalase Activities and Reduced Glutathione Levels
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents Used
4.2. Animals
4.3. Induction of AIA Model
4.4. Drug Doses and Preparations
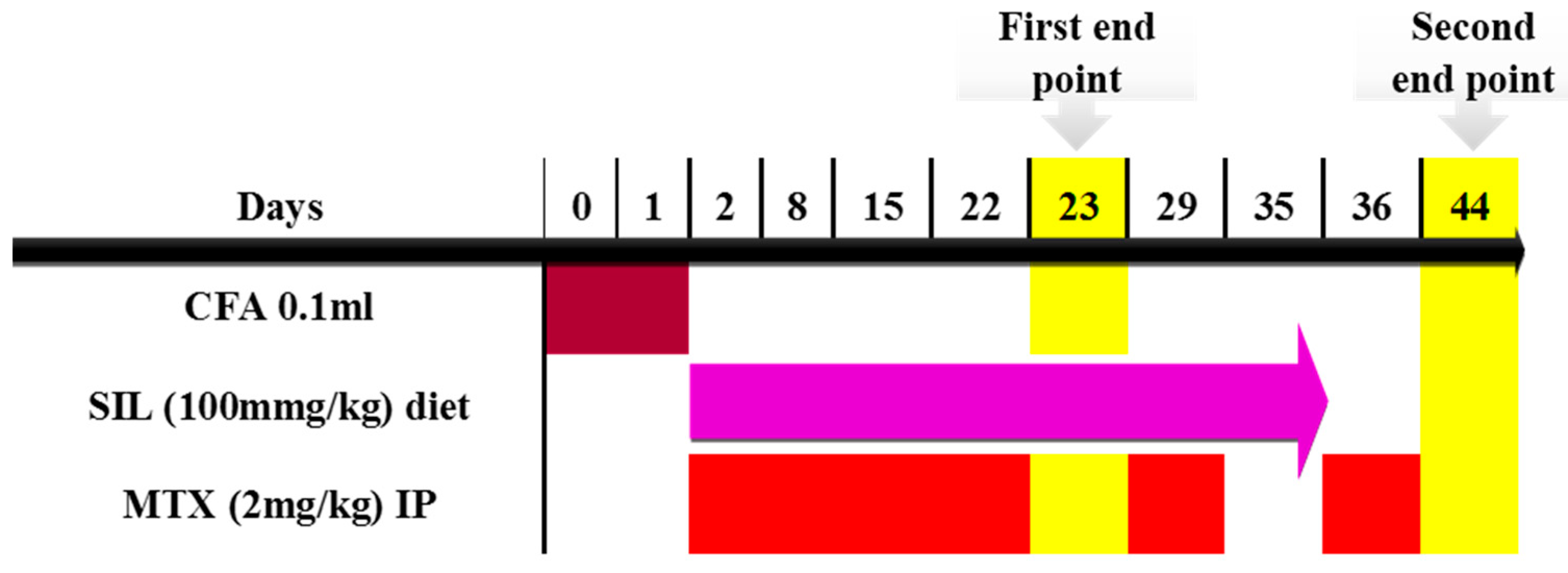
4.5. Timeline and Study Design
4.6. Experimental Groups
4.7. Experimental Model Assessment
4.7.1. Body Weight Measurement
4.7.2. Macroscopic Assessment of the Injected Paw
4.7.3. Tiobiotarsal Joint Histology
4.7.4. ESR: Erythrocyte Sedimentation Rate Determination
4.8. Biochemical Assessment
4.8.1. Hepatic Enzymes
4.8.2. Lipid Peroxidation Assay (MDA)
4.8.3. Superoxide Dismutase Assay (SOD)
4.8.4. Catalase Assay (CAT)
4.8.5. Reduced Glutathione Assay (GSH)
4.9. Liver Histology
4.10. Protein Quantification
4.11. Statistical Analysis
5. Limitations of this Study
6. Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Y.-J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Luo, Y.; Li, T.; Zhao, X.; Lv, T.; Fang, G.; Ou, P.; Li, H.; Luo, X.; Huang, A.; et al. Systemic complications of rheumatoid arthritis: Focus on pathogenesis and treatment. Front. Immunol. 2022, 13, 1051082. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kwon, E.-J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.D.; Holers, V.M. Rheumatoid Arthritis Pathogenesis, Prediction, and Prevention: An Emerging Paradigm Shift. Arthritis Rheumatol. 2021, 73, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free. Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.-L.; Payandeh, Z.; Mohammadkhani, N.; Mubarak, S.M.H.; Zakeri, A.; Bahrami, A.A.; Brockmueller, A.; Shakibaei, M. Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: New Treatment Strategies. Cells 2021, 10, 3017. [Google Scholar] [CrossRef]
- Singh, J.A. Treatment Guidelines in Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2022, 48, 679–689. [Google Scholar] [CrossRef]
- Friedman, B.; Cronstein, B. Methotrexate mechanism in treatment of rheumatoid arthritis. Jt. Bone Spine 2019, 86, 301–307. [Google Scholar] [CrossRef]
- Torres, R.P.; Santos, F.P.; Branco, J.C. Methotrexate: Implications of pharmacogenetics in the treatment of patients with Rheumatoid Arthritis. ARP Rheumatol. 2022, 1, 225–229. [Google Scholar] [PubMed]
- Zhao, Z.; Hua, Z.; Luo, X.; Li, Y.; Yu, L.; Li, M.; Lu, C.; Zhao, T.; Liu, Y. Application and pharmacological mechanism of methotrexate in rheumatoid arthritis. Biomed. Pharmacother. 2022, 150, 113074. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, V.; Verhoeven, D.W.; Verhoeven, F.; Aubin, F.; Avouac, J.; Vuitton, L.; Lioté, F.; Thévenot, T.; Wendling, D. Busting the myth of methotrexate chronic hepatotoxicity. Nat. Rev. Rheumatol. 2023, 19, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, K.; Pahwa, R.; Kumar, M.; Kumar, S.; Sharma, P.C.; Singh, G.; Verma, R.; Mittal, V.; Singh, I.; Kaushik, D.; et al. Mechanistic Insights into the Pharmacological Significance of Silymarin. Molecules 2022, 27, 5327. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, A.; Schmidt, H.H.-J. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef] [PubMed]
- Fanoudi, S.; Alavi, M.S.; Karimi, G.; Hosseinzadeh, H. Milk thistle (Silybum Marianum) as an antidote or a protective agent against natural or chemical toxicities: A review. Drug Chem. Toxicol. 2020, 43, 240–254. [Google Scholar] [CrossRef]
- Saxena, N.; Dhaked, R.K.; Nagar, D. Silibinin ameliorates abrin induced hepatotoxicity by attenuating oxidative stress, inflammation and inhibiting Fas pathway. Environ. Toxicol. Pharmacol. 2022, 93, 103868. [Google Scholar] [CrossRef]
- Trouillas, P.; Marsal, P.; Svobodova, A.; Vostalova, J.; Gažák, R.; Hrbač, J.; Sedmera, P.; Křen, V.; Lazzaroni, R.; Duroux, J.L.; et al. Mechanism of the Antioxidant Action of Silybin and 2,3-Dehydrosilybin Flavonolignans: A Joint Experimental and Theoretical Study. J. Phys. Chem. A 2008, 112, 1054–1063. [Google Scholar] [CrossRef]
- Neumann, U.P.; Biermer, M.; Eurich, D.; Neuhaus, P.; Berg, T. Successful prevention of hepatitis C virus (HCV) liver graft reinfection by silibinin monotherapy. J. Hepatol. 2010, 52, 951–952. [Google Scholar] [CrossRef]
- Trappoliere, M.; Caligiuri, A.; Schmid, M.; Bertolani, C.; Failli, P.; Vizzutti, F.; Novo, E.; di Manzano, C.; Marra, F.; Loguercio, C.; et al. Silybin, a component of sylimarin, exerts anti-inflammatory and anti-fibrogenic effects on human hepatic stellate cells. J. Hepatol. 2009, 50, 1102–1111. [Google Scholar] [CrossRef]
- Wang, L.; Huang, B.; Zeng, Y.; Yang, J.; Li, Z.; Ng, J.P.L.; Xu, X.; Su, L.; Yun, X.; Qu, L.; et al. N-Acetylcysteine overcomes epalrestat-mediated increase of toxic 4-hydroxy-2-nonenal and potentiates the anti-arthritic effect of epalrestat in AIA model. Int. J. Biol. Sci. 2023, 19, 4082–4102. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, Y.; Zeng, Y.; Huang, X.; Liu, D.; Ye, L.; Li, Y.; Chen, X.; Liu, T.; Li, H.; et al. Tanshinone IIA Suppresses Proliferation and Inflammatory Cytokine Production of Synovial Fibroblasts from Rheumatoid Arthritis Patients Induced by TNF-α and Attenuates the Inflammatory Response in AIA Mice. Front. Pharmacol. 2020, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Jia, X.; Wei, F.; Wang, C.; Sun, X.; Xu, S.; Yang, X.; Zhao, Y.; Chen, J.; Wu, H.; et al. CP-25, a novel compound, protects against autoimmune arthritis by modulating immune mediators of inflammation and bone damage. Sci. Rep. 2016, 6, 26239. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.K. Combination of coenzyme Q10 with methotrexate suppresses Freund’s complete adjuvant-induced synovial inflammation with reduced hepatotoxicity in rats: Effect on oxidative stress and inflammation. Int. Immunopharmacol. 2015, 24, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Yanasoglu, E.; Buyukavci, M.; Cetinkaya, A.; Turan, G.; Koroglu, M.; Yazar, H.; Buyukokuroglu, M.E. Silibinin Effect on Methotrexate-Induced Hepatotoxicity in Rats. Eurasian J. Med. 2022, 54, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhu, Y.; Ding, C.; Zhang, W.; Guan, H.; Li, C.; Lin, X.; Zhang, Y.; Huang, C.; Zhang, L.; et al. LncRNA H19 regulates macrophage polarization and promotes Freund’s complete adjuvant-induced arthritis by upregulating KDM6A. Int. Immunopharmacol. 2021, 93, 107402. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.S.; Neog, M.K.; Rasool, M.; Kumar, G.S.; Hemshekhar, M.; Kemparaju, K.; Girish, K.S. Guggulipid ameliorates adjuvant-induced arthritis and liver oxidative damage by suppressing inflammatory and oxidative stress mediators. Phytomedicine 2019, 64, 152924. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.; Hou, H.; Zou, W.; Hu, L.; Gong, L.; Fan, W.; Wang, R.; Ibrahim, I.A.A.; Fan, S. Xanthorrhizol Ameliorates Oxidative Stress and Inflammation in Freund’s Complete Adjuvant-Induced Rheumatoid Arthritis in Rats. Appl. Biochem. Biotechnol. 2022, 194, 6423–6437. [Google Scholar] [CrossRef]
- Ali, E.A.I.; Barakat, B.M.; Hassan, R. Antioxidant and Angiostatic Effect of Spirulina platensis Suspension in Complete Freund’s Adjuvant-Induced Arthritis in Rats. PLoS ONE 2015, 10, e0121523. [Google Scholar] [CrossRef]
- Cascão, R.; Vidal, B.; Raquel, H.; Neves-Costa, A.; Figueiredo, N.; Gupta, V.; Fonseca, J.; Moita, L. Effective treatment of rat adjuvant-induced arthritis by celastrol. Autoimmun. Rev. 2012, 11, 856–862. [Google Scholar] [CrossRef]
- Darwish, S.F.; El-Bakly, W.M.; Arafa, H.M.; El-Demerdash, E. Targeting TNF-alpha and NF-kappaB activation by bee venom: Role in suppressing adjuvant induced arthritis and methotrexate hepatotoxicity in rats. PLoS ONE 2013, 8, e79284. [Google Scholar] [CrossRef]
- Gardi, C.; Bauerova, K.; Stringa, B.; Kuncirova, V.; Slovak, L.; Ponist, S.; Drafi, F.; Bezakova, L.; Tedesco, I.; Acquaviva, A.; et al. Quercetin reduced inflammation and increased antioxidant defense in rat adjuvant arthritis. Arch. Biochem. Biophys. 2015, 583, 150–157. [Google Scholar] [CrossRef]
- Suke, S.G.; Negi, H.; Mediratta, P.; Banerjee, B.; Sharma, K. Anti-arthritic and anti-inflammatory activity of combined pioglitazone and prednisolone on adjuvant-induced arthritis. Eur. J. Pharmacol. 2013, 718, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, Y.; Dong, H.; Zhang, W.; Li, F. Investigation of the effect of phlomisoside F on complete Freund’s adjuvant-induced arthritis. Exp. Ther. Med. 2017, 13, 710–716. [Google Scholar] [CrossRef] [PubMed]
- de Arruda, J.A.A.; Corrêa, J.D.; Singh, Y.; Oliveira, S.R.; Machado, C.C.; Schneider, A.H.; Medeiros, J.D.; Fernandes, G.R.; Macari, S.; Barrioni, B.R.; et al. Methotrexate promotes recovery of arthritis-induced alveolar bone loss and modifies the composition of the oral-gut microbiota. Anaerobe 2022, 75, 102577. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Mei, L.; Wang, M.; Huang, Q.; Huang, R. Anti-inflammatory and Pro-apoptotic Effects of 18beta-Glycyrrhetinic Acid In Vitro and In Vivo Models of Rheumatoid Arthritis. Front. Pharmacol. 2021, 12, 681525. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, J.M.; Wan, J.; Zhang, H.; Shin, D.B.; Ogdie, A.; Syed, M.N.; Egeberg, A. Risk of liver disease in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis receiving methotrexate: A population-based study. J. Am. Acad. Dermatol. 2021, 84, 1636–1643. [Google Scholar] [CrossRef]
- Onishi, A.; Kamitsuji, S.; Nishida, M.; Uemura, Y.; Takahashi, M.; Saito, T.; Yoshida, Y.; Kobayashi, M.; Kawate, M.; Nishimura, K.; et al. Genetic and clinical prediction models for the efficacy and hepatotoxicity of methotrexate in patients with rheumatoid arthritis: A multicenter cohort study. Pharmacogenomics J. 2019, 20, 433–442. [Google Scholar] [CrossRef]
- Anvari, B. Methotrexate Hepatotoxicity in Rheumatoid Arthritis: An Analysis of the Physicians’ Policy. Curr. Rheumatol. Rev. 2020, 16, 67–73. [Google Scholar] [CrossRef]
- Malayeri, A.; Badparva, R.; Mombeini, M.A.; Khorsandi, L.; Goudarzi, M. Naringenin: A potential natural remedy against methotrexate-induced hepatotoxicity in rats. Drug Chem. Toxicol. 2022, 45, 491–498. [Google Scholar] [CrossRef]
- Ahmadvand, H.; Jafaripour, L.; Naserzadeh, R.; Alizamani, E.; Mashhadi, S.J.; Moghadam, E.; Nouryazdan, N. Effects of rosmarinic acid on methotrexate-induced nephrotoxicity and hepatotoxicity in wistar rats. Indian J. Nephrol. 2021, 31, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Messner, C.J.; Gaiser, C.; Hämmerli, C.; Suter-Dick, L. Methotrexate-Induced Liver Injury Is Associated with Oxidative Stress, Impaired Mitochondrial Respiration, and Endoplasmic Reticulum Stress In Vitro. Int. J. Mol. Sci. 2022, 23, 15116. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Abdel-Latif, R.; Hafez, S.M.N.A.; Kandeel, M.; Abdel-Gaber, S.A. Paeonol Protects against Methotrexate Hepatotoxicity by Repressing Oxidative Stress, Inflammation, and Apoptosis—The Role of Drug Efflux Transporters. Pharmaceuticals 2022, 15, 1296. [Google Scholar] [CrossRef] [PubMed]
- Alfwuaires, M.A. Galangin mitigates oxidative stress, inflammation, and apoptosis in a rat model of methotrexate hepatotoxicity. Environ. Sci. Pollut. Res. Int. 2022, 29, 20279–20288. [Google Scholar] [CrossRef] [PubMed]
- Sırmalı, M.; Solak, O.; Tezel, C.; Sırmalı, R.; Ginis, Z.; Atik, D.; Agackıran, Y.; Koylu, H.; Delibas, N. Comparative analysis of the protective effects of caffeic acid phenethyl ester (CAPE) on pulmonary contusion lung oxidative stress and serum copper and zinc levels in experimental rat model. Biol. Trace Elem. Res. 2013, 151, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Rukmini, M.S.; D’Souza, B.; D’Souza, V. Superoxide dismutase and catalase activities and their correlation with malondialdehyde in schizophrenic patients. Indian J. Clin. Biochem. 2004, 19, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T. Protection against cancer by plant phenyl propenoids: Induction of mammalian anticarcinogenic enzymes. Mini Rev. Med. Chem. 2002, 2, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Mobasheri, A. Oxidative Stress in Applied Basic Research and Clinical Practice. In Studies of Arthritis and Joint Disorders; Maria, M.J., Gualillo, O., Sanchez-Pernaute, O., Eds.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany; Dordrecht, The Netherlands; London, UK, 2013; p. 411. [Google Scholar]
- Aouacheri, O.; Saka, S.; Krim, M.; Messaadia, A.; Maidi, I. The investigation of the oxidative stress-related parameters in type 2 diabetes mellitus. Can. J. Diabetes 2015, 39, 44–49. [Google Scholar] [CrossRef]
- Kehili, N.; Saka, S.; Aouacheri, O. L’effet phytoprotecteur de la nigelle (Nigella sativa) contre la toxicité induite par le cadmium chez les rats. Phytothérapie 2018, 16, 194–203. [Google Scholar] [CrossRef]
- Fallah, M.; Davoodvandi, A.; Nikmanzar, S.; Aghili, S.; Mirazimi, S.M.A.; Aschner, M.; Rashidian, A.; Hamblin, M.R.; Chamanara, M.; Naghsh, N.; et al. Silymarin (milk thistle extract) as a therapeutic agent in gastrointestinal cancer. Biomed. Pharmacother. 2021, 142, 112024. [Google Scholar] [CrossRef]
- Dupuis, M.L.; Conti, F.; Maselli, A.; Pagano, M.T.; Ruggieri, A.; Anticoli, S.; Fragale, A.; Gabriele, L.; Gagliardi, M.C.; Sanchez, M.; et al. The Natural Agonist of Estrogen Receptor β Silibinin Plays an Immunosuppressive Role Representing a Potential Therapeutic Tool in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 1903. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasan, D.; Karthikeyan, S.; Vivekanandan, P. Ameliorative effect of silibinin against N-nitrosodimethylamine-induced hepatic fibrosis in rats. Environ. Toxicol. Pharmacol. 2012, 34, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.O.; Peraçoli, M.T.S.; Weel, I.C.; Bannwart, C.F.; Romão, M.; Nakaira-Takahagi, É.; de Medeiros, L.T.L.; da Silva, M.G.; Peraçoli, J.C. Hepatoprotective and anti-inflammatory effects of silibinin on experimental preeclampsia induced by L-NAME in rats. Life Sci. 2012, 91, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, T.; Gnanasekaran, N.; Mehare, T. Hepatoprotective effect of silymarin on fructose induced nonalcoholic fatty liver disease in male albino wistar rats. BMC Complement. Med. Ther. 2021, 21, 104. [Google Scholar]
- Heidarian, E.; Nouri, A. Hepatoprotective effects of silymarin against diclofenac-induced liver toxicity in male rats based on biochemical parameters and histological study. Arch. Physiol. Biochem. 2019, 127, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Betsou, A.; Lambropoulou, M.; Georgakopoulou, A.E.; Kostomitsopoulos, N.; Konstandi, O.; Anagnostopoulos, K.; Tsalikidis, C.; Simopoulos, C.E.; Valsami, G.; Tsaroucha, A.K. The hepatoprotective effect of silibinin after hepatic ischemia/reperfusion in a rat model is confirmed by immunohistochemistry and qRT-PCR. J. Pharm. Pharmacol. 2021, 73, 1274–1284. [Google Scholar] [CrossRef]
- Keshavarz-Maleki, R.; Shalmani, A.A.; Gholami, M.; Sabzevari, S.; Rahimzadegan, M.; Jeivad, F.; Sabzevari, O. The Ameliorative Effect of Monomethyl Fumarate and Silymarin Against Valproic Acid Induced Hepatotoxicity in Rats. Pharm. Chem. J. 2021, 55, 240–245. [Google Scholar] [CrossRef]
- Navarro, V.J.; Belle, S.H.; D’Amato, M.; Adfhal, N.; Brunt, E.M.; Fried, M.W.; Reddy, K.R.; Wahed, A.S.; Harrison, S.; Silymarin in NASH and C Hepatitis (SyNCH) Study Group. Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: A randomized, double-blind, placebo controlled trial. PLoS ONE 2019, 14, e0221683. [Google Scholar]
- Federico, A.; Dallio, M.; Masarone, M.; Gravina, A.G.; Di Sarno, R.; Tuccillo, C.; Cossiga, V.; Lama, S.; Stiuso, P.; Morisco, F.; et al. Evaluation of the Effect Derived from Silybin with Vitamin D and Vitamin E Administration on Clinical, Metabolic, Endothelial Dysfunction, Oxidative Stress Parameters, and Serological Worsening Markers in Nonalcoholic Fatty Liver Disease Patients. Oxidative Med. Cell. Longev. 2019, 2019, 8742075. [Google Scholar] [CrossRef]
- Tong, W.W.; Zhang, C.; Hong, T.; Liu, D.H.; Wang, C.; Li, J.; He, X.K.; Xu, W.D. Silibinin alleviates inflammation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes and has a therapeutic effect on arthritis in rats. Sci. Rep. 2018, 8, 3241. [Google Scholar] [CrossRef]
- Gazák, R.; Sedmera, P.; Vrbacky, M.; Vostálová, J.; Drahota, Z.; Marhol, P.; Walterová, D.; Kren, V. Molecular mechanisms of silybin and 2,3-dehydrosilybin antiradical activity—Role of individual hydroxyl groups. Free. Radic. Biol. Med. 2009, 46, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Erlejman, A.G.; Verstraeten, S.V.; Fraga, C.G.; Oteiza, P.I. The interaction of flavonoids with membranes: Potential determinant of flavonoid antioxidant effects. Free Radic. Res. 2004, 38, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Gazak, R.; Walterova, D.; Kren, V. Silybin and silymarin—New and emerging applications in medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Kalemci, S.; Topal, Y.; Celik, S.Y.; Yilmaz, N.; Beydilli, H.; Kosar, M.I.; Dirican, N.; Altuntas, I. Silibinin attenuates methotrexate-induced pulmonary injury by targeting oxidative stress. Exp. Ther. Med. 2015, 10, 503–507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banji, O.J.; Banji, D.; Soumya, N.; Chilipi, K.K.; Kalpana, C.H.; Kumar, C.K.; Annamalai, A.R. Combination of carvacrol with methotrexate suppresses Complete Freund’s Adjuvant induced synovial inflammation with reduced hepatotoxicity in rats. Eur. J. Pharmacol. 2014, 723, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Jawed, H.; Shah, S.U.A.; Jamall, S.; Simjee, S.U. N-(2-hydroxy phenyl) acetamide inhibits inflammation-related cytokines and ROS in adjuvant-induced arthritic (AIA) rats. Int. Immunopharmacol. 2010, 10, 900–905. [Google Scholar] [CrossRef]
- Wang, D.; Hu, S.; Zhu, J.; Yuan, J.; Wu, J.; Zhou, A.; Wu, Y.; Zhao, W.; Huang, Q.; Chang, Y.; et al. Angiotensin II type 2 receptor correlates with therapeutic effects of losartan in rats with adjuvant-induced arthritis. J. Cell. Mol. Med. 2013, 17, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. In Methods in Enzymology; Flesicher, S., Packer, L., Eds.; Academic Press: New York, NY, USA, 1978; pp. 302–310. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2009, 5, 51–66. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khawaja, G.; El-Orfali, Y. Silibinin’s Effects against Methotrexate-Induced Hepatotoxicity in Adjuvant-Induced Arthritis Rat Model. Pharmaceuticals 2024, 17, 431. https://doi.org/10.3390/ph17040431
Khawaja G, El-Orfali Y. Silibinin’s Effects against Methotrexate-Induced Hepatotoxicity in Adjuvant-Induced Arthritis Rat Model. Pharmaceuticals. 2024; 17(4):431. https://doi.org/10.3390/ph17040431
Chicago/Turabian StyleKhawaja, Ghada, and Youmna El-Orfali. 2024. "Silibinin’s Effects against Methotrexate-Induced Hepatotoxicity in Adjuvant-Induced Arthritis Rat Model" Pharmaceuticals 17, no. 4: 431. https://doi.org/10.3390/ph17040431
APA StyleKhawaja, G., & El-Orfali, Y. (2024). Silibinin’s Effects against Methotrexate-Induced Hepatotoxicity in Adjuvant-Induced Arthritis Rat Model. Pharmaceuticals, 17(4), 431. https://doi.org/10.3390/ph17040431




