Phytochemical Analysis, Biological Activities, and Molecular Docking Studies of Root Extracts from Paeonia Species in Serbia
Abstract
1. Introduction
2. Results and Discussion
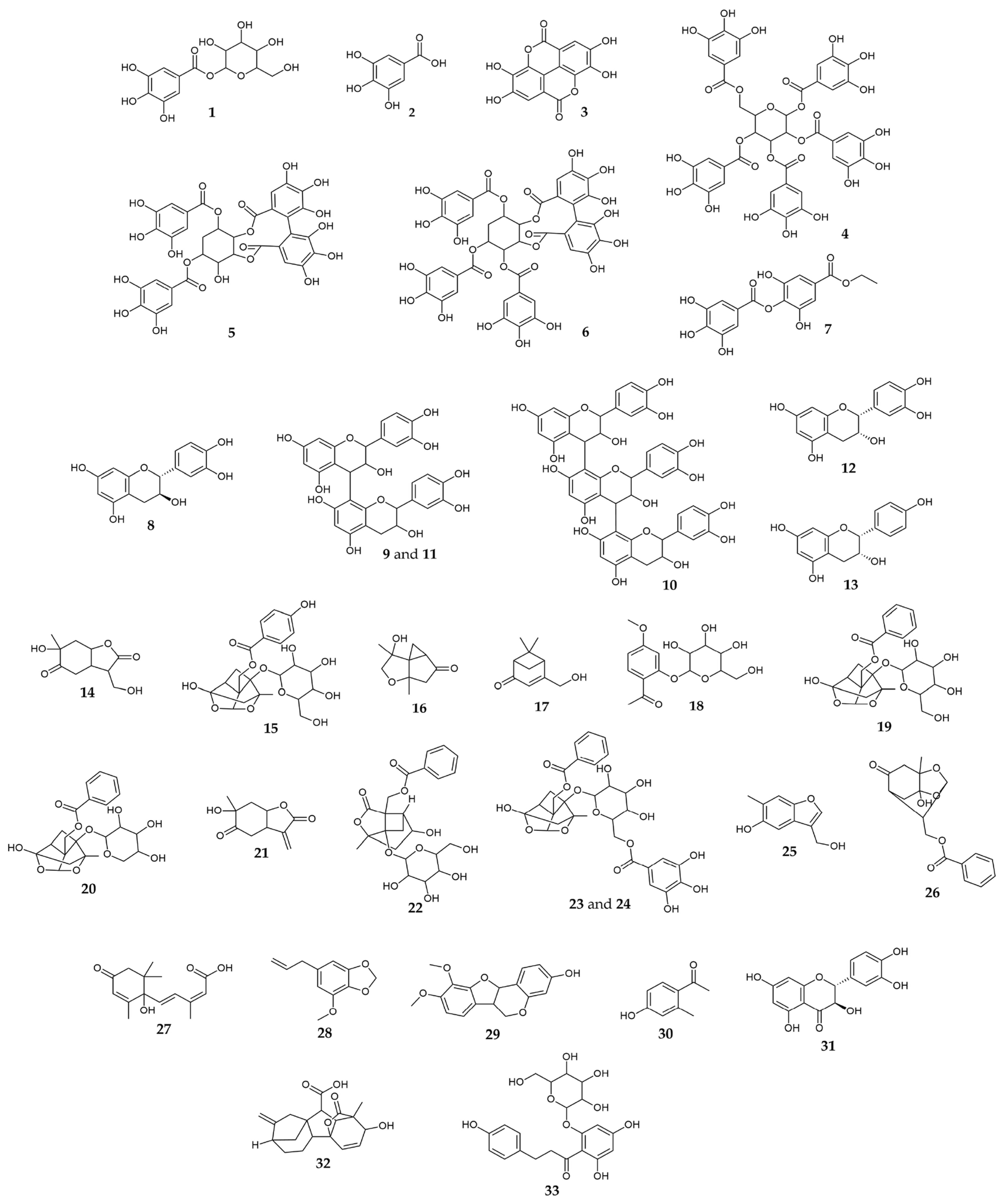
2.1. Chemical Characterization
| No | Compound Name | tR, min | Molecular Formula, [M ± H]± | Calculated Mass, m/z | Exact Mass, m/z | Δ ppm | MS2 Fragments, (% Base Peak) | MS3 Fragments, (% Base Peak) | MS4 Fragments, (% Base Peak) | PT | PP | PO | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallic acid derivatives | |||||||||||||
| 1 | Galloyl-hexose | 0.59 | C13H15O10− | 331.06707 | 331.06588 | 3.61 | 125 (19), 169 (100), 193 (10), 211 (24), 271 (50) | 125 (100) | 67 (58), 73 (59), 107 (100) | + | – | + | [19] |
| 2 | Gallic acid | 0.91 | C7H5O5− | 169.01425 | 169.01355 | 4.14 | 125 (100) | 53 (57), 63 (50), 97 (100) | NA | + | + | + | [20] |
| 3 | Ellagic acid | 4.62 | C14H5O8− | 300.99899 | 300.99744 | 5.16 | 185 (45), 201 (25), 229 (100), 257 (77), 284 (44) | 129 (28), 157 (34), 173 (44), 185 (84), 201 (100) | NA | + | + | + | [15] |
| 4 | Pentagalloyl-hexose | 5.00 | C41H31O26− | 939.11091 | 939.10833 | 2.74 | 617 (6), 769 (100), 787 (9) | 429 (16), 447 (29), 599 (34), 601 (22), 617 (100) | 193 (10), 313 (15), 429 (9), 447 (40), 465 (100) | + | – | + | [25] |
| 5 | Digalloyl-HHDP-protoquercitol | 5.23 | C34H27O21+ | 771.10394 | 771.10271 | 1.59 | 233 (29), 261 (75), 279 (50), 305 (100), 413 (14), 431 (60) | 153 (100) | 125 (100), 143 (25) | + | + | + | [24] |
| 6 | Trigalloyl-HHDP-protoquercitol | 5.57 | C41H31O25+ | 923.11489 | 923.11375 | 1.23 | 305 (100), 431 (53), 457 (30), 601 (22), 771 (42) | 153 (100) | 125 (100), 143 (26) | + | + | + | [41] |
| 7 | Ethyl-digallate | 5.93 | C16H13O9− | 349.05651 | 349.05455 | 5.61 | 197 (100) | 125 (14), 169 (100) | 125 (100) | + | – | + | [21] |
| Flavan-3-ols | |||||||||||||
| 8 | Catechin | 3.47 | C15H15O6+ | 291.08632 | 291.08529 | 3.52 | 123 (100), 139 (97), 147 (8), 151 (21), 165 (43), 273 (15) | 67 (73), 77 (53), 95 (55), 105 (100), 199 (12) | NA | + | + | + | [25] |
| 9 | B-type procyanidin dimer 1 | 3.53 | C30H27O12+ | 579.14970 | 579.14788 | 3.14 | 247 (28), 289 (33), 291 (66), 301 (20), 409 (60), 427 (100) | 247 (14), 275 (62), 287 (18), 301 (69), 409 (100) | 257 (20), 287 (100), 299 (10), 391 (11) | + | + | + | [42,43] |
| 10 | B-type procyanidin trimer | 3.79 | C45H39O18+ | 867.21309 | 867.2118 | 1.48 | 425 (19), 559 (20), 577 (88), 579 (100), 715 (19) | 289 (23), 291 (48), 301 (27), 409 (75), 427 (100) | 247 (23), 275 (43), 287 (8), 301 (43), 409 (100) | + | – | + | [42,43] |
| 11 | B-type procyanidin dimer 2 | 4.02 | C30H27O12+ | 579.14970 | 579.14836 | 2.32 | 247 (23), 289 (38), 291 (71), 301 (17), 409 (37), 427 (100) | 247 (19), 275 (67), 287 (14), 301 (68), 409 (100) | 257 (19), 287 (100), 299 (7), 391 (11) | + | + | + | [42,43] |
| 12 | Epicatechin | 4.23 | C15H15O6+ | 291.08632 | 291.08547 | 2.9 | 123 (98), 139 (100), 147 (8), 151 (21), 165 (42), 273 (10) | 67 (19), 83 (13), 93 (14), 111 (100) | 65 (26), 69 (70), 83 (100), 93 (73), 111 (24) | + | + | + | [25] |
| 13 | Epiafzelechin | 5.92 | C15H15O5+ | 275.09140 | 275.09057 | 3.03 | 107 (100), 127 (15), 149 (22), 169 (33) | 53 (80), 77 (46), 79 (100), 93 (33), 99 (36) | NA | + | + | + | [26] |
| Paeonia terpenes | |||||||||||||
| 14 | 9-Hydroxypaeonilactone A | 2.42 | C10H15O5+ | 215.09140 | 215.09072 | 3.16 | 155 (100), 158 (26), 169 (19), 173 (14), 197 (81) | 95 (26), 109 (42), 113 (26), 127 (28), 137 (100) | 69 (47), 81 (25), 95 (39), 106 (7), 109 (100) | + | + | – | [29] |
| 15 | Oxypaeoniflorin | 3.28 | C23H27O12− | 495.15080 | 495.14796 | 5.74 | 331 (20), 333 (22), 427 (32), 449 (27), 465 (100) | NA | NA | + | – | + | [44] |
| 16 | Paeonisothujone | 3.71 | C10H15O3+ | 183.10157 | 183.10085 | 3.96 | 137 (14), 147 (69), 165 (100) | 123 (18), 137 (24), 147 (100) | 119 (100), 129 (20) | + | + | – | [30] |
| 17 | Paeonisuffrone C | 3.94 | C10H15O2+ | 167.10666 | 167.10622 | 2.63 | 109 (22), 121 (27), 125 (17), 149 (100) | 93 (11), 107 (24), 109 (8), 121 (100), 131 (81) | 64 (14), 77 (15), 91 (40), 93 (100), 105 (30) | + | + | + | [29] |
| 18 | Paeonoside | 4.28 | C15H19O8− | 327.10854 | 327.10743 | 3.39 | 123 (14), 165 (100), 309 (12) | 95 (29), 121 (19), 123 (100) | NA | + | – | – | [22] |
| 19 | Paeoniflorin + FA | 4.39 | C24H29O13− | 525.16137 | 525.15874 | 5.00 | 449 (100), 479 (33) | 165 (42), 309 (8), 327 (100) | 123 (10), 165 (100), 309 (16) | + | + | + | [44] |
| 20 | Paeoniflorigenin 1-O-pentoside | 4.40 | C22H25O10− | 449.14532 | 449.14346 | 4.13 | 165 (25), 309 (3), 327 (100) | 123 (15), 165 (100), 309 (28) | 123 (100), 147 (21), 150 (14) | + | + | + | [45] |
| 21 | Paeonilactone B | 4.53 | C10H13O4+ | 197.08084 | 197.07993 | 4.57 | 125 (41), 138 (100), 179 (20) | 69 (13), 83 (7), 110 (100) | 69 (94), 83 (100), 94 (30) | + | + | + | [25] |
| 22 | Albiflorin + FA | 4.91 | C24H29O13− | 525.16137 | 525.15859 | 5.29 | 449 (100), 479 (41) | 165 (38), 309 (7), 327 (100) | 123 (11), 165 (100), 309 (11) | + | – | + | [44,45] |
| 23 | Galloyl-paeoniflorin | 5.02 | C30H31O15− | 631.16684 | 631.16434 | 3.96 | 271 (20), 313 (10), 399 (10), 479 (12), 491 (23), 613 (100) | 271 (100), 313 (33), 375 (16), 399 (27), 491 (67) | 169 (8), 210 (7), 211 (100) | + | – | + | [44,45] |
| 24 | Galloyl-paeoniflorin isomer | 5.32 | C30H31O15− | 631.16684 | 631.16451 | 3.70 | 313 (100), 463 (9), 481 (25), 483 (6), 509 (12) | 125 (37), 151 (31), 169 (100), 209 (11), 223 (12) | 125 (100) | + | – | + | [44,45] |
| 25 | Paeoveitol D | 5.38 | C10H11O3+ | 179.07027 | 179.06977 | 2.82 | 93 (21), 107 (32), 135 (97), 151 (100), 161 (14) | 95 (21), 105 (19), 109 (100), 123 (80), 133 (31) | 65 (3), 69 (10), 79 (6), 81 (100), 91 (14) | + | + | + | [46] |
| 26 | Paeoniflorigenone | 8.13 | C17H19O6+ | 319.11762 | 319.11663 | 3.07 | 179 (15), 197 (100), 259 (8), 273 (10), 301 (8) | 119 (21), 137 (33), 155 (59), 161 (14), 179 (100) | 85 (13), 133 (21), 137 (100), 151 (10), 161 (24) | + | + | + | [32] |
| Other metabolites | |||||||||||||
| 27 | Abscisic acid | 3.80 | C15H21O4+ | 265.14344 | 265.14258 | 3.22 | 175 (13), 203 (17), 229 (12), 235 (8), 247 (100) | 157 (52), 187 (36), 201 (48), 217 (56), 229 (100) | 159 (27), 173 (21), 187 (40), 201 (100), 211 (68) | – | + | + | [33] |
| 28 | Myristicin | 3.96 | C11H13O3+ | 193.08592 | 193.08535 | 2.98 | 138 (18), 161 (100), 166 (14) | 105 (22), 133 (100) | 105 (100) | + | + | + | [35] |
| 29 | 3-Hydroxy-9,10-dimethoxypterocarpan | 4.53 | C17H17O5+ | 301.10705 | 301.10602 | 3.40 | 187 (18), 243 (40), 283 (100), 285 (16) | 211 (31), 235 (19), 239 (100), 247 (24), 265 (23) | 211 (100), 213 (3) | + | + | + | [36] |
| 30 | 4-Hydroxy-2-methylacetophenone | 4.58 | C9H11O2+ | 151.07536 | 151.07499 | 2.40 | 95 (30), 105 (25), 109 (100), 123 (93), 133 (38) | 69 (8), 79 (8), 81 (100), 91 (12) | NA | + | + | + | [37] |
| 31 | Taxifolin | 4.80 | C15H13O7+ | 305.06558 | 305.06445 | 3.69 | 153 (99), 195 (35), 259 (87), 287 (100) | 105 (9), 259 (100), 269 (3) | 149 (76), 231 (100), 241 (10) | + | + | + | [40] |
| 32 | Gibberellin A7 | 5.06 | C19H23O5+ | 331.15400 | 331.15286 | 3.45 | 151 (35), 189 (18), 285 (13), 287 (47), 313 (100) | 137 (8), 151 (100), 189 (76), 249 (10), 281 (28) | 91 (40), 95 (20), 119 (100), 123 (4), 136 (8) | + | + | + | [39] |
| 33 | Phloridzin | 5.66 | C21H23O10− | 435.12967 | 435.12705 | 6.03 | 205 (4), 271 (5), 273 (100), 293 (5), 399 (5) | 123 (15), 167 (100) | 123 (100) | + | – | + | [47] |
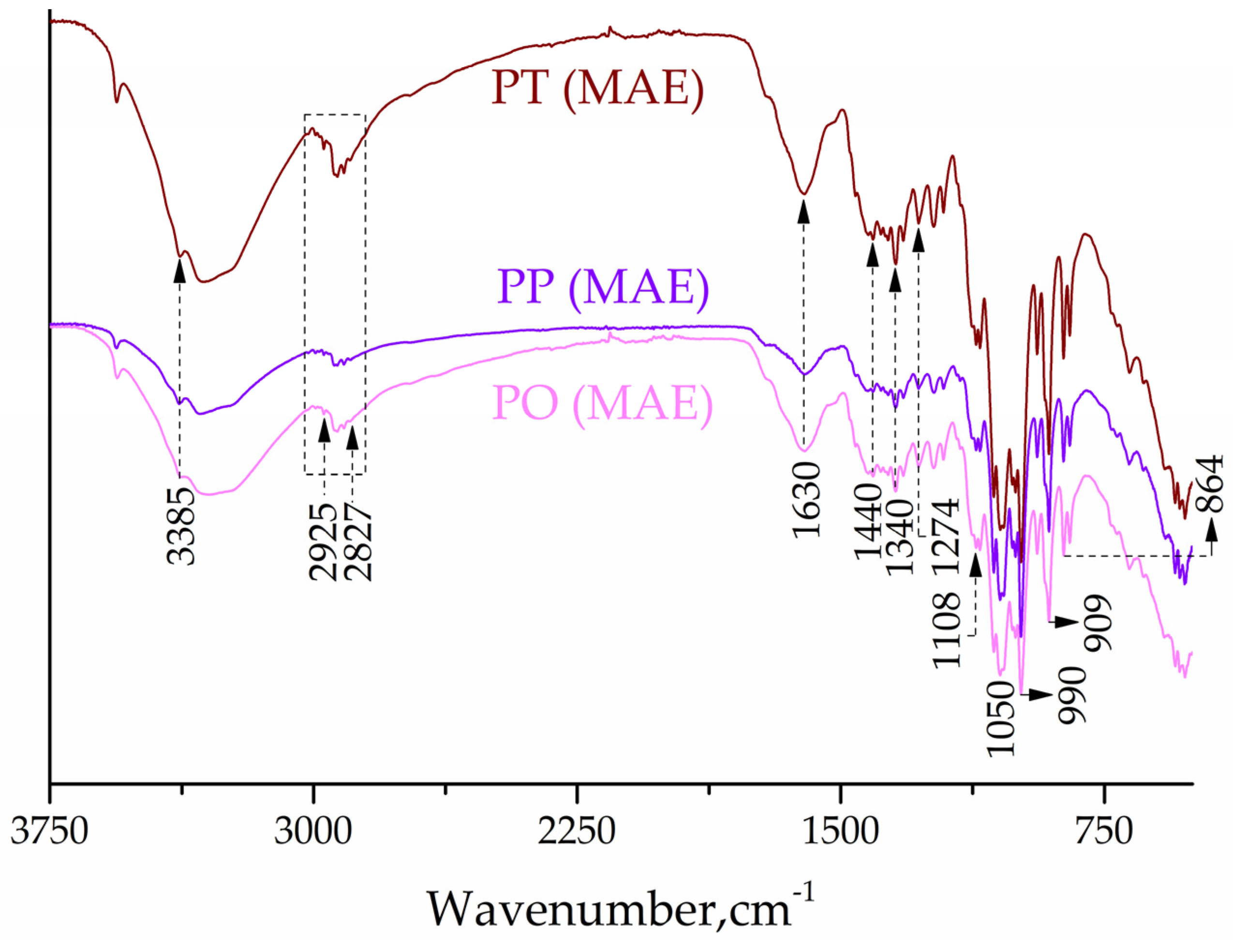
2.2. FTIR Study
2.3. Optimization of the Polyphenol Extraction Process from Paeonia Roots
2.4. Total Flavonoid Content (TFC) of Root Extracts
2.5. Antioxidant Activity of Root Extracts
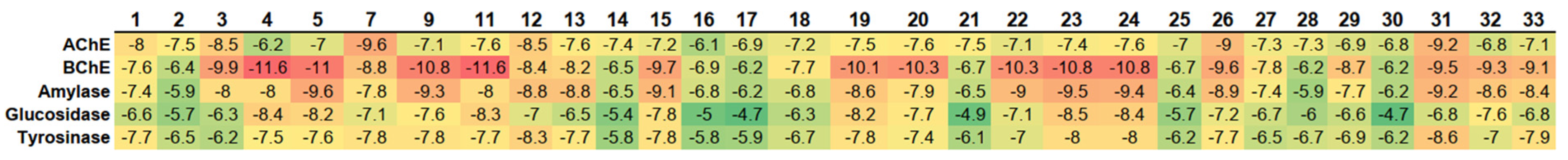
2.6. Enzyme Inhibitory Activity of Root Extracts
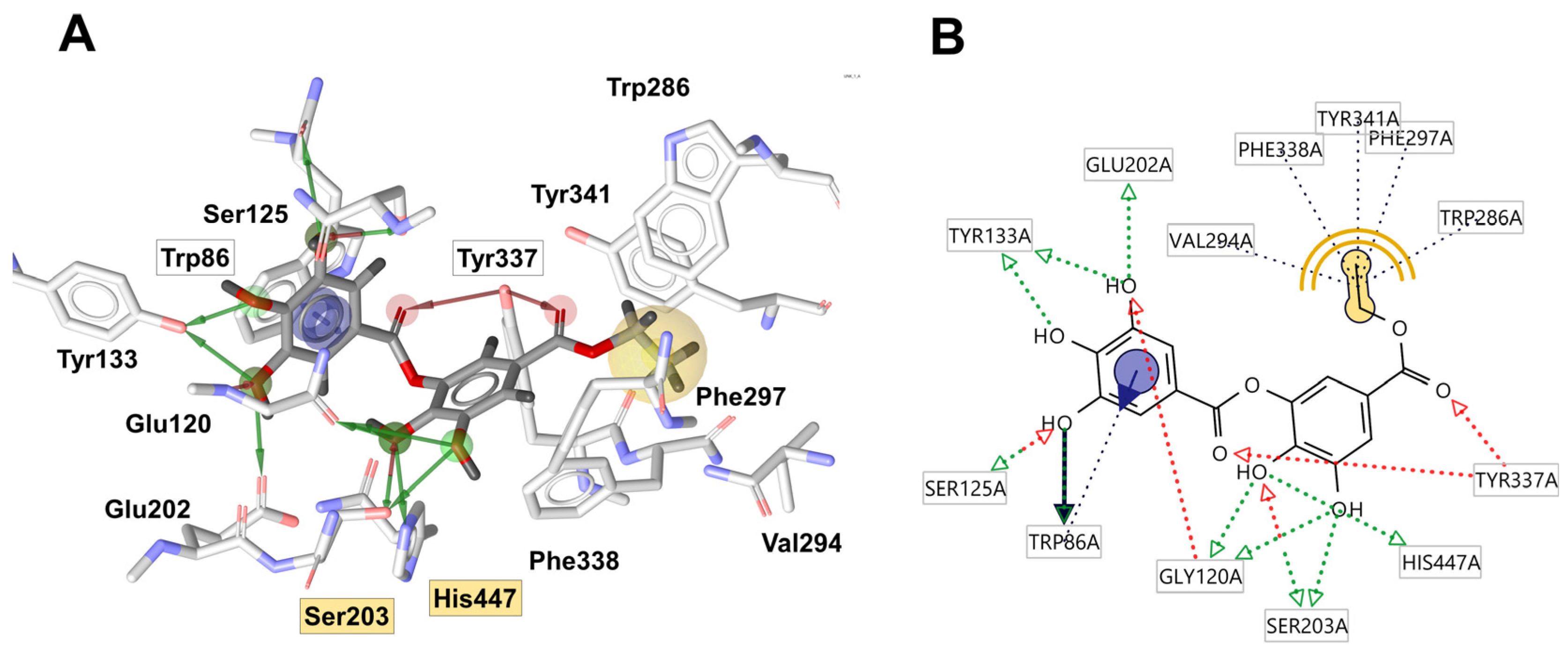
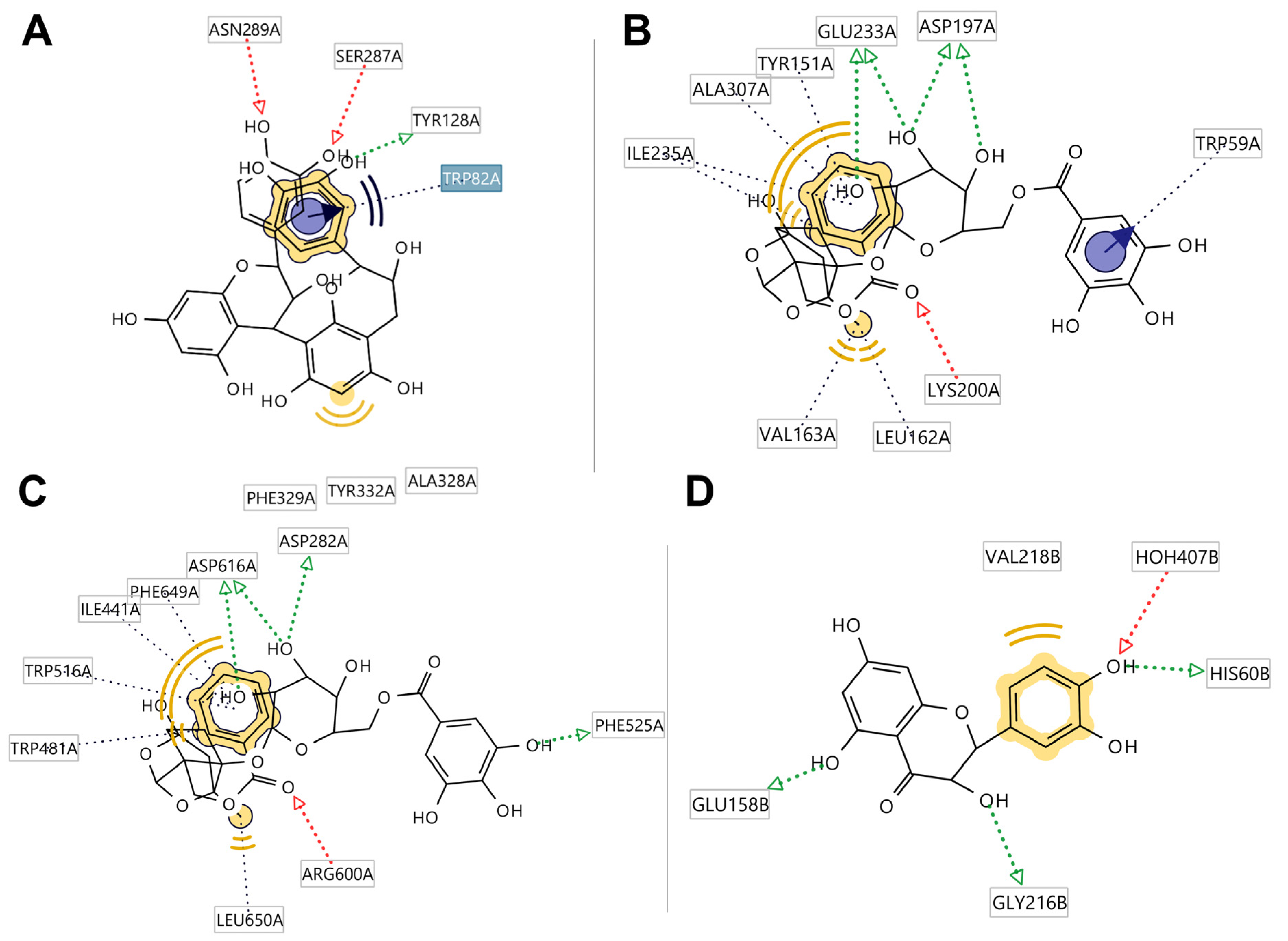
2.7. Molecular Docking
2.8. Antibacterial Activity of Root Extracts
2.9. Simulated In Vitro GID and Bioaccessibility
3. Materials and Methods
3.1. Origin of Plant Material
3.2. Chemicals and Reagents
3.3. Extraction
3.3.1. MAE
3.3.2. UAE
3.3.3. M Method
3.3.4. Preparation of Hot Water Extracts (Teas)
3.4. Chemical and Structural Analysis
3.4.1. UHPLC-LTQ-OrbiTrap MS Analysis
3.4.2. FTIR Analysis
3.4.3. Determination of TPC
3.4.4. Determination of TFC
3.5. Determination of Free Radical Scavenging Activity
ABTS and DPPH Assays
3.6. Determination of Enzyme Inhibitory Activities
3.6.1. AChE and BuChE Enzymatic Assays
3.6.2. HPA Enzymatic Assay
3.6.3. HIG Enzymatic Assay
3.6.4. Tyrosinase Enzymatic Assay
3.7. Molecular Docking
3.8. Determination of Antibacterial Activity
3.8.1. Selection of Microorganisms and Culture Conditions
3.8.2. Broth Microdilution Method
3.9. Simulated In Vitro GID and Bioaccessibility
UHPLC Q-ToF MS Analysis Digested Samples
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, P.; Shen, J.; Wang, Z.; Liu, S.; Liu, Q.; Li, Y.; He, C.; Xiao, P. Genus Paeonia: A Comprehensive Review on Traditional Uses, Phytochemistry, Pharmacological Activities, Clinical Application, and Toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; da Silva, J.A.T.; Yu, X.; Wang, L. Characterization of Phytochemicals in the Roots of Wild Herbaceous Peonies from China and Screening for Medicinal Resources. Phytochemistry 2020, 174, 112331. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; May, B.; Zhang, C.; Zhang, A.L.; Lu, C.; Xue, C.C. A Pharmacological Review of Bioactive Constituents of Paeonia lactiflora Pallas and Paeonia veitchii Lynch. Phytother. Res. 2016, 30, 1445–1473. [Google Scholar] [CrossRef] [PubMed]
- Oancea, S.; Perju, M.; Olosutean, H. Influence of Enzyme-Aided Extraction and Ultrasonication on the Phenolics Content and Antioxidant Activity of Paeonia officinalis L. Petals. J. Serbian Chem. Soc. 2020, 85, 845–856. [Google Scholar] [CrossRef]
- Oancea, S.; Perju, M.; Coman, D.; Olosutean, H. Optimization of Conventional and Ultrasound-Assisted Extraction of Paeonia officinalis Anthocyanins, as Natural Alternative for a Green Technology of Cotton Dyeing. Rom. Biotechnol. Lett. 2021, 26, 2527–2534. [Google Scholar] [CrossRef]
- Jovanović, A.; Petrović, P.; Đorđević, V.; Zdunić, G.; Šavikin, K.; Bugarski, B. Polyphenols Extraction from Plant Sources. Lek. Sirovine 2017, 37, 45–49. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Mosurović, M.; Bugarski, B.; Batinić, P.; Čutović, N.; Gordanić, S.; Marković, T. Melissa officinalis Extracts Obtained Using Maceration, Ultrasoundand Microwave-Assisted Extractions: Chemical Composition, Antioxidant Capacity, and Physical Characteristics. Lek. Sirovine 2022, 51–59. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the Extraction Process of Polyphenols from Thymus serpyllum L. Herb Using Maceration, Heat- and Ultrasound-Assisted Techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Vajić, U.-J.V.; Mijin, D.Z.; Zdunić, G.M.; Šavikin, K.P.; Branković, S.; Kitić, D.; Bugarski, B.M. Polyphenol Extraction in Microwave Reactor Using By-Product of Thymus serpyllum L. and Biological Potential of the Extract. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100417. [Google Scholar] [CrossRef]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on Phenolic Compounds Stability during Microwave-Assisted Extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef]
- Ahmad, F.; Tabassum, N.; Rasool, S. Medicinal Uses and Phytoconstituents of Paeonia officinalis. Int. Res. J. Pharm. 2012, 3, 85–87. [Google Scholar]
- Ahmad, F.; Tabassum, N. Effect of 70% Ethanolic Extract of Roots of Paeonia officinalis Linn. on Hepatotoxicity. J. Acute Med. 2013, 3, 45–49. [Google Scholar] [CrossRef]
- Kostova, I.N.; Simeonov, M.F.; Todorova, D.I. A Monoterpene Glucoside from Paeonia peregrina Roots. Phytochemistry 1998, 47, 1303–1307. [Google Scholar] [CrossRef]
- Nikolova, P.; Ivanovska, N. Estimation of Immunological Properties of Flower and Root Extracts from Paeonia peregrina. J. Herbs Spices Med. Plants 2000, 6, 1–9. [Google Scholar] [CrossRef]
- Marković, T.; Čutović, N.; Carević, T.; Gašić, U.; Stojković, D.; Xue, J.; Jovanović, A. Paeonia peregrina Mill Petals as a New Source of Biologically Active Compounds: Chemical Characterization and Skin Regeneration Effects of the Extracts. Int. J. Mol. Sci. 2023, 24, 11764. [Google Scholar] [CrossRef]
- Čutović, N.; Marković, T.; Kostić, M.; Gašić, U.; Prijić, Ž.; Ren, X.; Lukić, M.; Bugarski, B. Chemical Profile and Skin-Beneficial Activities of the Petal Extracts of Paeonia tenuifolia L. from Serbia. Pharmaceuticals 2022, 15, 1537. [Google Scholar] [CrossRef] [PubMed]
- Batinić, P.; Cutovic, N.; Jovanović, A.; Stojković, D.; Zengin, G.; Carević, T.; Prijić, Ž.; Marinković, A.; Marković, T. Antioxidant, Antibacterial and Anti-Enzymatic Activity of the Leaf Extracts of Paeonia daurica Andrews Grown in Serbia. Lek. Sirovine 2023, 43, 1–8. [Google Scholar] [CrossRef]
- Robin, T.; Reuveni, S.; Urbakh, M. Single-Molecule Theory of Enzymatic Inhibition. Nat. Commun. 2018, 9, 779. [Google Scholar] [CrossRef]
- Sut, S.; Zengin, G.; Dall’Acqua, S.; Gazdová, M.; Šmejkal, K.; Bulut, G.; Dogan, A.; Haznedaroglu, M.Z.; Aumeeruddy, M.Z.; Maggi, F. Paeonia arietina and Paeonia kesrounansis Bioactive Constituents: NMR, LC-DAD-MS Fingerprinting and in vitro Assays. J. Pharm. Biomed. Anal. 2019, 165, 1–11. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, Z.; Huang, X.-Y.; Chen, J.-J.; Geng, C.-A. Chemical and Biological Comparison of Different Parts of Paeonia suffruticosa (Mudan) Based on LCMS-IT-TOF and Multi-Evaluation in vitro. Ind. Crops. Prod. 2020, 144, 112028. [Google Scholar] [CrossRef]
- Liu, J.; Sun, H.; Zhang, A.; Yan, G.; Han, Y.; Xue, C.; Zhou, X.; Shi, H.; Wang, X. Serum Pharmacochemistry Combined with Multiple Data Processing Approach to Screen the Bioactive Components and Their Metabolites in Mutan Cortex by Ultra-performance Liquid Chromatography Tandem Mass Spectrometry. Biomed. Chromatogr. 2014, 28, 500–510. [Google Scholar] [CrossRef]
- Li, B.; Ge, J.; Liu, W.; Hu, D.; Li, P. Unveiling Spatial Metabolome of Paeonia suffruticosa and Paeonia lactiflora Roots Using MALDI MS Imaging. New Phytol. 2021, 231, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, M.; Yamagishi, T.; Nonaka, G.; Nishioka, I.; Nagasawa, T.; Oura, H. Tannins and Related Compounds. XII. Isolation and Characterization of Galloylglucoses from Paeoniae Radix and Their Effect on Urea-Nitrogen Concentration in Rat Serum. Chem. Pharm. Bull. 1983, 31, 2593–2600. [Google Scholar] [CrossRef]
- Yan, Z.; Xie, L.; Li, M.; Yuan, M.; Tian, Y.; Sun, D.; Zhang, Y.; Niu, L. Phytochemical Components and Bioactivities of Novel Medicinal Food–Peony Roots. Food Res. Int. 2021, 140, 109902. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Zhang, Y.; Jin, Q.; Zhang, S.; Wu, G.; Chen, L.; Zhang, H.; Wang, X. Identification and Characterisation of Bioactive Compounds from the Seed Kernels and Hulls of Paeonia lactiflora Pall by UPLC-QTOF-MS. Food Res. Int. 2021, 139, 109916. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-Y.; Huang, C.-S.; Tong, Y.-L.; Guo, H.-W.; Zhou, S.-J.; Ye, J.-H.; Gong, S.-Y. Widely Targeted Metabolomics Analysis of White Peony Teas with Different Storage Time and Association with Sensory Attributes. Food Chem. 2021, 362, 130257. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, P.-L.; Chen, X.-L.; Gong, M.-J.; Zhang, L.; Qiu, X.-H.; Zhang, J.; Huang, Z.-H.; Xu, W. The Impact of Citrus-Tea Cofermentation Process on Chemical Composition and Contents of Pu-Erh Tea: An Integrated Metabolomics Study. Front Nutr. 2021, 8, 737539. [Google Scholar] [CrossRef]
- Osuntokun, O.T.; Idowu, T.O.; Gamberini, M.C. Bio-Guided Isolation, Purification and Chemical Characterization of Epigallocatechin; Epicatechin, Stigmasterol, Phytosterol from of Ethyl Acetate Stem Bark Fraction of Spondias mombin (Linn.). Biochem. Pharmacol. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Z.-M.; Li, T.; Zhang, Y.-B.; Sze, S.C.W.; Wang, G.-C.; Li, Y.-L.; Ye, W.-C. Monoterpene Derivatives from the Roots of Paeonia lactiflora and Their Anti-Proliferative Activity. Fitoterapia 2014, 98, 124–129. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Harada, E.; Minematsu, T.; Muraoka, O.; Yamahara, J.; Murakami, N.; Kitagawa, I. Absolute Stereostructures of Paeonisothujone, a Novel Skeletal Monoterpene Ketone, and Deoxypaeonisuffrone, and Isopaeonisuffral, Two New Monoterpenes, from Moutan Cortex. Chem. Pharm. Bull. 1994, 42, 736–738. [Google Scholar] [CrossRef]
- Shimizu, M.; Hayashi, T.; Morita, N.; Kimura, I.; Kimura, M.; Kiuchi, F.; Noguchi, H.; Iitaka, Y.; Sankawa, U. Paeoniflorigenone, a New Monoterpene from Paeony Roots. Tetrahedron Lett. 1981, 22, 3069–3070. [Google Scholar] [CrossRef]
- Chen, P.-C.; Dlamini, B.S.; Chen, C.-R.; Shih, W.-L.; Lee, C.-H.; Chang, C.-I. Inhibitory Potential of Chemical Constituents from Paeonia suffruticosa Against α-glucosidase and α-amylase. Pharm. Chem. J. 2022, 56, 821–826. [Google Scholar] [CrossRef]
- Xie, A.; Lv, M.; Zhang, D.; Shi, Y.; Yang, L.; Yang, X.; Du, J.; Sun, L.; Sun, X. Effects of Slight Shading in Summer on the Leaf Senescence and Endogenous Hormone and Polyamine Contents in Herbaceous Peony. Sci. Rep. 2023, 13, 18714. [Google Scholar] [CrossRef] [PubMed]
- Seneme, E.F.; Dos Santos, D.C.; Silva, E.M.R.; Franco, Y.E.M.; Longato, G.B. Pharmacological and Therapeutic Potential of Myristicin: A Literature Review. Molecules 2021, 26, 5914. [Google Scholar] [CrossRef]
- Yayli, N.; Yaşar, A.; Yayli, N.; Albay, M.; Coşkunçelebi, K. Essential Oil Analysis and Antimicrobial Activity of Paeonia mascula from Turkey. Nat. Prod. Commun. 2008, 3, 1934578X0800300624. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, R.; An, L.; Guo, J.; Hou, X.; Tang, J.; Wang, F.; Du, Q. Exploring the Role and Mechanism of Astragalus Membranaceus and Radix Paeoniae Rubra in Idiopathic Pulmonary Fibrosis through Network Pharmacology and Experimental Validation. Sci. Rep. 2023, 13, 10110. [Google Scholar] [CrossRef]
- Sun, L.; Kong, C.; Zhao, H.; Wu, D.; Xu, F. Two New Disaccharide Glycosides from the Root Cortex of Paeonia ostii. Rec. Nat. Prod. 2022, 35, 2564–2568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Si, F.; Zhang, Y.; Gai, S. Effect of Exogenous Gibberellin on DNA Methylation Level and Expression of Related Enzyme Genes in Tree Peony Floral Buds. Sci. Agric. Sin. 2018, 51, 3561–3569. [Google Scholar]
- Yuxi, Z.; Yanchao, Y.; Zejun, L.; Tao, Z.; Feng, L.; Chunying, L.; Shupeng, G. GA 3 Is Superior to GA 4 in Promoting Bud Endodormancy Release in Tree Peony (Paeonia suffruticosa) and Their Potential Working Mechanism. BMC Plant. Biol. 2021, 21, 323. [Google Scholar] [CrossRef]
- Bai, Z.-Z.; Ni, J.; Tang, J.-M.; Sun, D.-Y.; Yan, Z.-G.; Zhang, J.; Niu, L.-X.; Zhang, Y.-L. Bioactive Components, Antioxidant and Antimicrobial Activities of Paeonia rockii Fruit during Development. Food Chem. 2021, 343, 128444. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, H.-M.; Wang, P.-L.; Ma, Z.-Q.; Zhang, Y.; Wu, J.-J.; Nie, J.; Chen, S.-J.; Han, W.-J.; Wang, Q. Bioactive Components from Qingwen baidu Decoction against LPS-Induced Acute Lung Injury in Rats. Molecules 2017, 22, 692. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Xie, L.; Tian, Y.; Li, M.; Ni, J.; Zhang, Y.; Niu, L. Insights into the Phytochemical Composition and Bioactivities of Seeds from Wild Peony Species. Plants 2020, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kataoka, M.; Tsuboi, N.; Kouno, I. New Monoterpene Glycoside Esters and Phenolic Constituents of Paeoniae Radix, and Increase of Water Solubility of Proanthocyanidins in the Presence of Paeoniflorin. Chem. Pharm. Bull. 2000, 48, 201–207. [Google Scholar] [CrossRef]
- Shi, Y.-H.; Zhu, S.; Ge, Y.-W.; Toume, K.; Wang, Z.; Batkhuu, J.; Komatsu, K. Characterization and Quantification of Monoterpenoids in Different Types of Peony Root and the Related Paeonia Species by Liquid Chromatography Coupled with Ion Trap and Time-of-Flight Mass Spectrometry. J. Pharm. Biomed. Anal. 2016, 129, 581–592. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y.; Pang, Q.; Mei, Y.; Su, Z.; Yao, X.-S.; Yu, Y. Monoterpene Glycosides with Anti-Inflammatory Activity from Paeoniae Radix. Fitoterapia 2019, 138, 104290. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.-J.; Ma, Y.-B.; Geng, C.-A.; Huang, X.-Y.; Xu, H.-B.; Zhang, X.-M.; Chen, J.-J. Paeoveitols A–E from Paeonia veitchii. Fitoterapia 2015, 106, 36–40. [Google Scholar] [CrossRef]
- Bai, Z.-Z.; Tang, J.-M.; Ni, J.; Zheng, T.-T.; Zhou, Y.; Sun, D.-Y.; Li, G.-N.; Liu, P.; Niu, L.-X.; Zhang, Y.-L. Comprehensive Metabolite Profile of Multi-Bioactive Extract from Tree Peony (Paeonia ostii and Paeonia rockii) Fruits Based on MS/MS Molecular Networking. Food Res. Int. 2021, 148, 110609. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Vunduk, J.; Todorovic, N.; Jakovljevic, D.; Zizak, Z.; Pavlovic, V.; Levic, S.; Niksic, M.; Van Griensven, L.J.L.D. Biological Potential of Extracts of the Wild Edible Basidiomycete Mushroom Grifola Frondosa. Food Res. Int. 2015, 67, 272–283. [Google Scholar] [CrossRef]
- Mathlouthi, M.; Koenig, J.L. Vibrational Spectra of Carbohydrates. Adv. Carbohydr. Chem. Biochem. 1987, 44, 7–89. [Google Scholar]
- Jovanović, A.; Đorđević, V.; Zdunić, G.; Šavikin, K.; Pljevljakusić, D.; Bugarski, B. Ultrasound-Assisted Extraction of Polyphenols from Thymus serpyllum and Its Antioxidant Activity. Hem. Ind. 2016, 70, 391–398. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Petrović, P.M.; Zdunić, G.M.; Šavikin, K.P.; Kitić, D.; Đorđević, V.B.; Bugarski, B.M.; Branković, S. Influence of Lyophilized Thymus Serpyllum L. Extracts on the Gastrointestinal System: Spasmolytic, Antimicrobial and Antioxidant Properties. S. Afr. J. Bot. 2021, 142, 274–283. [Google Scholar] [CrossRef]
- Čutović, A.; Marković, T.; Žugić, A.; Batinić, P.; Bugarski;, B.; Jovanović, A. Antioxidant Activity of Paeonia tenuifolia L. Petal Extract-Loaded Liposomes. Lekovite sirovine 2023, 43, e167. [Google Scholar] [CrossRef]
- Hidalgo, M.; Sánchez-Moreno, C.; de Pascual-Teresa, S. Flavonoid–Flavonoid Interaction and Its Effect on Their Antioxidant Activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent Advances in Extraction of Nutraceuticals from Plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Hirano, R.; Sasamoto, W.; Matsumoto, A.; Itakura, H.; Igarashi, O.; Kondo, K. Antioxidant Ability of Various Flavonoids against DPPH Radicals and LDL Oxidation. J. Nutr. Sci. Vitaminol. 2001, 47, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-F.; Zhang, H.-Y. Theoretical Evaluation of Flavonoids as Multipotent Agents to Combat Alzheimer’s Disease. J. Mol. Struct. THEOCHEM 2006, 767, 3–9. [Google Scholar] [CrossRef]
- Karim, N.; Khan, H.; Khan, I.; Guo, O.; Sobarzo-Sánchez, E.; Rastrelli, L.; Kamal, M.A. An Increasing Role of Polyphenols as Novel Therapeutics for Alzheimer’s: A Review. Med. Chem. 2020, 16, 1007–1021. [Google Scholar] [CrossRef]
- Navarrete-Yañez, V.; Garate-Carrillo, A.; Rodriguez, A.; Mendoza-Lorenzo, P.; Ceballos, G.; Calzada-Mendoza, C.; Hogan, M.C.; Villarreal, F.; Ramirez-Sanchez, I. Effects of (−)-Epicatechin on Neuroinflammation and Hyperphosphorylation of Tau in the Hippocampus of Aged Mice. Food Funct. 2020, 11, 10351–10361. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, K.M.; Thayumanavan, B.; Palvannan, T.; Rajaguru, P. Inhibitory Effect of Gymnema Montanum Leaves on α-glucosidase Activity and α-amylase Activity and Their Relationship with Polyphenolic Content. Med. Chem. Res. 2010, 19, 948–961. [Google Scholar] [CrossRef]
- Xiao, J. Natural Polyphenols and Diabetes: Understanding Their Mechanism of Action. Curr. Med. Chem. 2015, 22, 2–3. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; Abd El-Twab, S.M.; Yousef, A.I.; Ashour, M.B.; Reheim, E.S.A.; Hamed, M.A.A. New Insights into the in vitro, in situ and in vivo Antihyperglycemic Mechanisms of Gallic Acid and p-coumaric Acid. Arch. Physiol. Biochem. 2022, 128, 1188–1194. [Google Scholar] [CrossRef]
- Sourris, K.C.; Watson, A.; Jandeleit-Dahm, K. Inhibitors of Advanced Glycation End Product (AGE) Formation and Accumulation. In Reactive Oxygen Species: Network Pharmacology and Therapeutic Applications; Springer: Cham, Switzerland, 2021; pp. 395–423. [Google Scholar] [CrossRef]
- Zengin, G.; Bulut, G.; Mollica, A.; Haznedaroglu, M.Z.; Dogan, A.; Aktumsek, A. Bioactivities of Achillea phrygia and Bupleurum croceum Based on the Composition of Phenolic Compounds: In vitro and in silico Approaches. Food Chem. Toxicol. 2017, 107, 597–608. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Dogra, S.; Sarangal, R. Pigmentary Disorders: An Insight. Pigment Int. 2014, 1, 5–7. [Google Scholar] [CrossRef]
- Talebi, M.; Majidi, K.; Bassam, K.; Abdi, M.; Daneshvar, M.; Moayedi, S.S.; Pourhesabi, S.; Attarroshan, M.; Boumi, S.; Kabiri, M. Synthesis, Biological Evaluation, and Molecular Docking Analysis of Novel 1, 3, 4-Thiadiazole-Based Kojic Acid Derivatives as Tyrosinase Inhibitors. J. Mol. Struct. 2022, 1268, 133707. [Google Scholar] [CrossRef]
- Chaita, E.; Lambrinidis, G.; Cheimonidi, C.; Agalou, A.; Beis, D.; Trougakos, I.; Mikros, E.; Skaltsounis, A.-L.; Aligiannis, N. Anti-Melanogenic Properties of Greek Plants. A Novel Depigmenting Agent from Morus Alba Wood. Molecules 2017, 22, 514. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; O’Brien, A.D. Overview and Historical Perspectives. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Mühldorfer, I.; Ziebuhr, W.; Hacker, J. Escherichia coli in Urinary Tract Infections. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2002; pp. 1515–1540. [Google Scholar]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.-L.; Recio, M.C. Medicinal Plants and Antimicrobial Activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Ngan, L.T.M.; Moon, J.-K.; Kim, J.-H.; Shibamoto, T.; Ahn, Y.-J. Growth-Inhibiting Effects of Paeonia lactiflora Root Steam Distillate Constituents and Structurally Related Compounds on Human Intestinal Bacteria. World J. Microbiol. Biotechnol. 2012, 28, 1575–1583. [Google Scholar] [CrossRef]
- Davidson, P.M.; Taylor, T.M.; Schmidt, S.E. Chemical Preservatives and Naturally Antimicrobial Compounds. In Food Microbiology: Fundamentals and Frontiers; ASM Press: Washington, DC, USA, 2012. [Google Scholar]
- Stojković, D.; Soković, M.; Glamočlija, J.; Džamić, A.; Ćirić, A.; Ristić, M.; Grubišić, D. Chemical Composition and Antimicrobial Activity of Vitex agnus-castus L. Fruits and Leaves Essential Oils. Food Chem. 2011, 128, 1017–1022. [Google Scholar] [CrossRef]
- Yi, S.; Zhu, J.; Fu, L.; Li, J. Tea Polyphenols Inhibit Pseudomonas aeruginosa through Damage to the Cell Membrane. Int. J. Food Microbiol. 2010, 144, 111–117. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Widyaningrum, I.; Wibisono, N.; Kusumawati, A.H. Effect of Extraction Method on Antimicrobial Activity against Staphylococcus aureus of Tapak Liman (Elephantopus scaber L.) Leaves. Int. J. Med. Health Sci. 2020, 3, 105–110. [Google Scholar]
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and Antimicrobial Activity of the Essential Oils of Two Origanum Species. J. Agric. Food Chem. 2001, 49, 4168–4170. [Google Scholar] [CrossRef]
- Qin, W.; Ketnawa, S.; Ogawa, Y. Effect of Digestive Enzymes and pH on Variation of Bioavailability of Green Tea during Simulated in vitro Gastrointestinal Digestion. Food Sci. Hum. Wellness 2022, 11, 669–675. [Google Scholar] [CrossRef]
- Koláčková, T.; Sumczynski, D.; Minařík, A.; Yalçin, E.; Orsavová, J. The Effect of in vitro Digestion on Matcha Tea (Camellia sinensis) Active Components and Antioxidant Activity. Antioxidants 2022, 11, 889. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Xie, Y.-Q.; Chen, F.; Zhao, Y.-Y.; Luo, C.-X.; Gao, Y.-Q. Total Phenolic, Flavonoid and Antioxidant Activity of 23 Edible Flowers Subjected to in vitro Digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Huang, W.; Mao, S.; Zhang, L.; Lu, B.; Zheng, L.; Zhou, F.; Zhao, Y.; Li, M. Phenolic Compounds, Antioxidant Potential and Antiproliferative Potential of 10 Common Edible Flowers from China Assessed Using a Simulated in vitro Digestion–Dialysis Process Combined with Cellular Assays. J. Sci. Food Agric. 2017, 97, 4760–4769. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Sasmaz, H.K.; Aksay, O.; Selli, S.; Kahraman, O.; Fields, C. Exploring the Impact of Infusion Parameters and in vitro Digestion on the Phenolic Profile and Antioxidant Capacity of Guayusa (Ilex guayusa Loes.) Tea Using Liquid Chromatography, Diode Array Detection, and Electrospray Ionization Tandem Mass Spectrometry. Foods 2024, 13, 694. [Google Scholar] [CrossRef] [PubMed]
- Ćirkovic Veličković, T.D.; Stanić-Vučinić, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of Polyphenols with Carbohydrates, Lipids and Proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A. In vitro Digestion of Meat-and Cereal-Based Food Matrix Enriched with Grape Extracts: How Are Polyphenol Composition, Bioaccessibility and Antioxidant Activity Affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Huang, Q.; Zou, L.; Wei, P.; Lu, J.; Zhang, Y. Methyl Gallate: Review of Pharmacological Activity. Pharmacol. Res. 2023, 194, 106849. [Google Scholar] [CrossRef] [PubMed]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Gašić, U.M.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Bioaccessibility of Phenolic Compounds and Antioxidant Properties of Goat-Milk Powder Fortified with Grape-Pomace-Seed Extract after in vitro Gastrointestinal Digestion. Antioxidants 2022, 11, 2164. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-H.; Lee, C.-Y.; Lee, Y.-E.; Yoo, S.-H.; Chung, J.-O.; Rha, C.-S.; Park, M.-Y.; Hong, Y.-D.; Shim, S.-M. Profiling of in vitro Bioaccessibility and Intestinal Uptake of Flavonoids after Consumption of Commonly Available Green Tea Types. Molecules 2021, 26, 1518. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-B.; Wang, Y. Gut Microbiota-Based Pharmacokinetics and the Antidepressant Mechanism of Paeoniflorin. Front. Pharmacol. 2019, 10, 442126. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Jiang, Z.H.; Liu, L.; Hu, M. Mechanisms Responsible for Poor Oral Bioavailability of Paeoniflorin: Role of Intestinal Disposition and Interactions with Sinomenine. Pharm. Res. 2006, 23, 2768–2780. [Google Scholar] [CrossRef]
- Institute for Medicinal Plant Research Dr. Josif Pančić Čaj Od Korena Belog Sleza (Tea of Root of Althaea officinalis). Available online: https://www.mocbilja.rs/proizvod/caj-od-korena-belog-sleza/ (accessed on 1 April 2024).
- Wang, X.; Xiong, H.; Wang, S.; Zhang, Y.; Song, Z.; Zhang, X. Physicochemical Analysis, Sensorial Evaluation, Astringent Component Identification and Aroma-Active Compounds of Herbaceous Peony (Paeonia lactiflora Pall.) Black Tea. Ind. Crops Prod. 2023, 193, 116159. [Google Scholar] [CrossRef]
- Classics Lowry, O.; Rosebrough, N.; Farr, A.; Randall, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of Total Flavonoid Content by Aluminum Chloride Assay: A Critical Evaluation. Lwt 2021, 150, 111932. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro Enzyme Inhibitory Properties, Antioxidant Activities, and Phytochemical Profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Brus, B.; Kosak, U.; Turk, S.; Pislar, A.; Coquelle, N.; Kos, J.; Stojan, J.; Colletier, J.-P.; Gobec, S. Discovery, Biological Evaluation, and Crystal Structure of a Novel Nanomolar Selective Butyrylcholinesterase Inhibitor. J. Med. Chem. 2014, 57, 8167–8179. [Google Scholar] [CrossRef] [PubMed]
- Gilles, C.; Astier, J.; Marchis-Mouren, G.; Cambillau, C.; Payan, F. Crystal Structure of Pig Pancreatic α-amylase Isoenzyme II, in Complex with the Carbohydrate Inhibitor Acarbose. Eur. J. Biochem. 1996, 238, 561–569. [Google Scholar] [CrossRef]
- Roig-Zamboni, V.; Cobucci-Ponzano, B.; Iacono, R.; Ferrara, M.C.; Germany, S.; Bourne, Y.; Parenti, G.; Moracci, M.; Sulzenbacher, G. Structure of Human Lysosomal Acid α-glucosidase–a Guide for the Treatment of Pompe Disease. Nat. Commun. 2017, 8, 1111. [Google Scholar] [CrossRef] [PubMed]
- Sendovski, M.; Kanteev, M.; Ben-Yosef, V.S.; Adir, N.; Fishman, A. First Structures of an Active Bacterial Tyrosinase Reveal Copper Plasticity. J. Mol. Biol. 2011, 405, 227–237. [Google Scholar] [CrossRef]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Fumagalli, L.; Vistoli, G. The VEGA Suite of Programs: An Versatile Platform for Cheminformatics and Drug Design Projects. Bioinformatics 2021, 37, 1174–1175. [Google Scholar] [CrossRef]
- Halgren, T.A. MMFF VI. MMFF94s Option for Energy Minimization Studies. J. Comput. Chem. 1999, 20, 720–729. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. MOPAC: A Semiempirical Molecular Orbital Program. J. Comput. Aided Mol. Des. 1990, 4, 1–103. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.-M.; Härkönen, H.; Fabritius, M.; Poutanen, K. Development of An in vitro Enzymic Digestion Method for Removal of Starch and Protein and Assessment of Its Performance Using Rye And Wheat Breads. J. Cereal Sci. 1999, 29, 139–152. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Špirović Trifunović, B.; Nedić, N.; Gašić, U.M.; Tešić, Ž.L.; Stanojević, S.P.; Pešić, M.B. Monofloral Corn Poppy Bee-Collected Pollen—A Detailed Insight into Its Phytochemical Composition and Antioxidant Properties. Antioxidants 2023, 12, 1424. [Google Scholar] [CrossRef]
| Plant Species (Root Extract) | Locality | Extraction Method | TPC [mg GAE/g] |
|---|---|---|---|
| PT | Gulenovci | MAE | 77.20 ± 2.56 |
| Deliblato sand | 67.64 ± 0.75 | ||
| Bogovo gumno | 31.14 ± 1.38 | ||
| Gulenovci | UAE | 87.89 ± 1.38 | |
| Deliblato sand | 99.64 ± 0.25 | ||
| Bogovo gumno | 36.45 ± 3.94 | ||
| Gulenovci | M | 53.70 ± 2.94 | |
| Deliblato sand | 47.76 ± 1.00 | ||
| Bogovo gumno | 72.58 ± 2.56 | ||
| PP | Pirot | MAE | 161.2 ± 2.19 |
| Južni Kučaj | 110.7 ± 4.63 | ||
| Golina | 82.20 ± 3.94 | ||
| Bogovo gumno | 72.45 ± 0.94 | ||
| Krivi vir | 48.20 ± 0.81 | ||
| Pančevo | 91.14 ± 4.63 | ||
| Pirot | UAE | 93.58 ± 0.56 | |
| Južni Kučaj | 32.39 ± 0.13 | ||
| Golina | 57.01 ± 6.63 | ||
| Bogovo gumno | 43.20 ± 4.19 | ||
| Krivi vir | 69.01 ± 0.63 | ||
| Pančevo | 143.7 ± 2.56 | ||
| Pirot | M | 105.9 ± 15.8 | |
| Južni Kučaj | 81.01 ± 5.63 | ||
| Golina | 83.89 ± 6.13 | ||
| Bogovo gumno | 54.26 ± 0.00 | ||
| Krivi vir | 95.08 ± 1.06 | ||
| Pančevo | 109.3 ± 3.06 | ||
| PO | Rujevica | MAE | 35.01 ± 2.50 |
| Božurna | 82.58 ± 8.56 | ||
| Rujevica | UAE | 49.26 ± 2.63 | |
| Božurna | 77.58 ± 3.44 | ||
| Rujevica | M | 165.6 ± 5.00 | |
| Božurna | 101.2 ± 3.81 |
| TPC [mg GAE/g] | Effect. | Std. Err. | Effect Estimates | Coeff. | Std. Err. Coeff. | p | |
|---|---|---|---|---|---|---|---|
| PT | Constant | 82.910 | 0.449 | 0.000 | |||
| Locality (1) | 1.267 | 0.898 | 1.410 | 0.633 | 0.449 | 0.196 | |
| Extraction procedure (2) | −21.527 | 0.898 | −23.965 | −10.763 | 0.449 | 0.000 | |
| 1 by 2 | −10.573 | 0.898 | −11.771 | −5.287 | 0.449 | 0.000 | |
| PP | Constant | 123.727 | 1.530 | 0.000 | |||
| Locality (1) | 13.342 | 3.061 | 4.358 | 6.671 | 1.530 | 0.002 | |
| Extraction procedure (2) | 56.908 | 3.061 | 18.591 | 28.454 | 1.530 | 0.000 | |
| 1 by 2 | 4.555 | 3.061 | 1.488 | 2.277 | 1.530 | 0.175 | |
| PO | Constant | 71.032 | 0.417 | 0.000 | |||
| Locality (1) | 41.532 | 0.835 | 49.731 | 20.766 | 0.417 | 0.000 | |
| Extraction procedure (2) | 24.535 | 0.835 | 29.379 | 12.267 | 0.417 | 0.000 | |
| 1 by 2 | −5.998 | 0.835 | −7.182 | −2.999 | 0.417 | 0.000 |
| Plant Species (Root Extract) | Locality | Extraction Method | TFC [mg CE/g] |
|---|---|---|---|
| PT | Gulenovci | MAE | 2.30 ± 0.38 d |
| Deliblato sand | 1.84 ± 0.28 de | ||
| Bogovo gumno | 0.94 ± 0.45 fg | ||
| Gulenovci | UAE | 2.97 ± 0.85 d | |
| Deliblato sand | 3.27 ± 0.55 cd | ||
| Bogovo gumno | 1.09 ± 0.27 f | ||
| Gulenovci | M | 1.29 ± 0.23 f | |
| Deliblato sand | 0.99 ± 0.50 fg | ||
| Bogovo gumno | 2.24 ± 0.12 d | ||
| PP | Pirot | MAE | 5.75 ± 0.37 ab |
| Južni Kučaj | 4.10 ± 0.35 c | ||
| Golina | 2.34 ± 0.28 d | ||
| Bogovo gumno | 2.14 ± 0.22 d | ||
| Krivi vir | 1.10 ± 0.17 fg | ||
| Pančevo | 2.99 ± 0.42 d | ||
| Pirot | UAE | 3.03 ± 0.65 d | |
| Južni Kučaj | 0.99 ± 0.15 gh | ||
| Golina | 1.53 ± 0.10 ef | ||
| Bogovo gumno | 0.74 ± 0.15 h | ||
| Krivi vir | 1.93 ± 0.30 e | ||
| Pančevo | 5.35 ± 0.03 b | ||
| Pirot | M | 3.99 ± 0.53 cd | |
| Južni Kučaj | 2.14 ± 0.85 d | ||
| Golina | 2.32 ± 0.13 d | ||
| Bogovo gumno | 1.37 ± 0.18 f | ||
| Krivi vir | 3.14 ± 0.65 cd | ||
| Pančevo | 4.00 ± 0.92 c | ||
| PO | Rujevica | MAE | 1.01 ± 0.10 g |
| Božurna | 2.39 ± 0.15 d | ||
| Rujevica | UAE | 1.01 ± 0.06 g | |
| Božurna | 2.39 ± 0.07 d | ||
| Rujevica | M | 5.92 ± 0.33 a | |
| Božurna | 3.72 ± 0.23 cd |
| Plant Species (Root Extract) | Locality | Extraction Method | Antioxidant Activity | |
|---|---|---|---|---|
| ABTS [µg TE/mL] | DPPH [IC50, mg/mL] | |||
| PT | Gulenovci | MAE | 15.0 ± 0.5 e | 0.207 ± 0.054 ab |
| Deliblato sand | 8.0 ± 0.2 j | 0.203 ± 0.022 b | ||
| Bogovo gumno | 15.0 ± 0.2 e | 0.250 ± 0.000 c | ||
| Gulenovci | UAE | 19.5 ± 0.5 bc | 0.179 ± 0.052 ab | |
| Deliblato sand | 12.0 ± 1.0 fg | 0.168 ± 0.056 ab | ||
| Bogovo gumno | 8.0 ± 0.5 ij | 0.199 ± 0.048 ab | ||
| Gulenovci | M | 28.5 ± 1.8 a | 0.190 ± 0.0843 ab | |
| Deliblato sand | 10.5 ± 0.8 gh | 0.188 ± 0.055 ab | ||
| Bogovo gumno | 13.0 ± 0.9 f | 0.184 ± 0.034 ab | ||
| PP | Pirot | MAE | 8.5 ± 0.5 ij | 0.221 ± 0.014 b |
| Južni Kučaj | 10.5 ± 0. 5 gh | 0.188 ± 0.039 ab | ||
| Golina | 6.0 ± 0.5 k | 0.244 ± 0.068 bc | ||
| Bogovo gumno | 9.0 ± 0.5 i | 0.235 ± 0.037 bc | ||
| Krivi vir | 18.0 ± 1.5 bc | 0.266 ± 0.047 bc | ||
| Pančevo | 30.0 ± 1.0 a | 0.196 ± 0.037 ab | ||
| Pirot | UAE | 12.0 ± 0.5 g | 0.186 ± 0.056 ab | |
| Južni Kučaj | 8.5 ± 0.5 ij | 0.190 ± 0.019 ab | ||
| Golina | 16.5 ± 0.5 cd | 0.269 ± 0.048 bc | ||
| Bogovo gumno | 14.0 ± 1.0 ef | 0.235 ± 0.034 bc | ||
| Krivi vir | 15.0 ± 1.0 de | 0.224 ± 0.014 bc | ||
| Pančevo | 11.5 ± 0.5 g | 0.186 ± 0.044 ab | ||
| Pirot | M | 13.5 ± 0.5 f | 0.191 ± 0.033 ab | |
| Južni Kučaj | 10.5 ± 0.9 gh | 0.187 ± 0.057 ab | ||
| Golina | 11.0 ± 0.7 g | 0.233 ± 0.039 bc | ||
| Bogovo gumno | 20.5 ± 0.8 b | 0.171 ± 0.058 ab | ||
| Krivi vir | 16.0 ± 0.3 d | 0.199 ± 0.059 ab | ||
| Pančevo | 18.5 ± 0.9 c | 0.165 ± 0.055 ab | ||
| PO | Rujevica | MAE | 13.0 ± 0.5 c | 0.170 ± 0.039 ab |
| Božurna | 10.5 ± 0. 5 gh | 0.207 ± 0.015 b | ||
| Rujevica | UAE | 10.5 ± 0.7 gh | 0.190 ± 0.032 ab | |
| Božurna | 20.0 ± 1.0 bc | 0.213 ± 0.021 b | ||
| Rujevica | M | 19.5 ± 0.8 bc | 0.168 ± 0.054 ab | |
| Božurna | 12.0 ± 0.8 g | 0.145 ± 0.0350 a | ||
| Plant Species (Root Extract) | Locality | Extraction Method | Ache Inhibition | Buche Inhibition | HPA Inhibition | HIG Inhibition | Tyrosinase Inhibition |
|---|---|---|---|---|---|---|---|
| [mg GALAE/g] | [mmol ACAE/g] | [mg KAE/g] | |||||
| PT | Gulenovci | MAE | 1.14 ± 0.02 j | 0.90 ± 0.14 cd | 0.24 ± 0.00 b | 1.21 ± 0.01 f | 24.02 ± 0.66 e |
| Deliblato sand | 1.78 ± 0.07 cd | 1.41 ± 0.18 b | 0.17 ± 0.01 f | 1.02 ± 0.02 j | 28.73 ± 2.26 cd | ||
| Bogovo gumno | 2.43 ± 0.03 a | 2.94 ± 0.23 a | 0.15 ± 0.01 g | 1.23 ± 0.01 f | 24.29 ± 0.55 e | ||
| Gulenovci | UAE | 1.35 ± 0.02 h | 1.01 ± 0.19 c | 0.22 ± 0.00 c | 1.28 ± 0.01 bc | 29.59 ± 2.36 c | |
| Deliblato sand | 1.81 ± 0.05 c | 0.78 ± 0.06 de | 0.20 ± 0.00 e | 1.19 ± 0.01 g | 29.99 ± 1.76 c | ||
| Bogovo gumno | 1.62 ± 0.02 e | 0.98 ± 0.12 cd | 0.18 ± 0.00 f | 1.13 ± 0.02 h | 24.83 ± 1.57 e | ||
| Gulenovci | M | 1.22 ± 0.06 i | 0.96 ± 0.06 cd | 0.24 ± 0.00 b | 1.28 ± 0.01 bc | 28.63 ± 0.51 d | |
| Deliblato sand | 1.92 ± 0.07 bc | 1.09 ± 0.07 c | 0.16 ± 0.00 fg | 1.16 ± 0.03 g | 32.68 ± 1.33 b | ||
| Bogovo gumno | 1.70 ± 0.09 de | 1.14 ± 0.04 c | 0.15 ± 0.01 g | 1.09 ± 0.01 i | 24.12 ± 1.38 e | ||
| PP | Pirot | MAE | 1.63 ± 0.02 e | 1.01 ± 0.04 c | 0.15 ± 0.01 g | 1.25 ± 0.00 f | 31.05 ± 1.63 bc |
| Južni Kučaj | 1.69 ± 0.06 de | 1.18 ± 0.28 bc | 0.18 ± 0.01 ef | 1.29 ± 0.00 b | 32.34 ± 1.05 b | ||
| Golina | 1.25 ± 0.03 i | 1.10 ± 0.08 c | 0.18 ± 0.00 f | 0.62 ± 0.01 m | 25.37 ± 2.05 de | ||
| Bogovo gumno | 1.41 ± 0.02 g | 1.05 ± 0.20 c | 0.20 ± 0.01 de | 1.22 ± 0.02 ef | 26.07 ± 1.32 de | ||
| Krivi vir | 0.48 ± 0.08 k | 1.11 ± 0.14 c | 0.16 ± 0.01 fg | 0.90 ± 0.04 k | 22.95 ± 1.43 f | ||
| Pančevo | 1.35 ± 0.04 gh | 1.14 ± 0.17 c | 0.21 ± 0.00 d | 1.27 ± 0.01 cd | 25.68 ± 0.22 e | ||
| Pirot | UAE | 1.67 ± 0.05 e | 0.68 ± 0.06 e | 0.18 ± 0.00 f | 1.29 ± 0.00 b | 33.99 ± 0.48 b | |
| Južni Kučaj | 1.63 ± 0.03 e | 0.74 ± 0.07 de | 0.25 ± 0.02 a | 1.29 ± 0.00 b | 35.50 ± 1.91 ab | ||
| Golina | 1.23 ± 0.02 i | 0.74 ± 0.07 de | 0.24 ± 0.01 ab | 0.71 ± 0.06 l | 22.18 ± 0.28 f | ||
| Bogovo gumno | 1.53 ± 0.04 f | 0.67 ± 0.05 e | 0.21 ± 0.00 d | 1.24 ± 0.02 ef | 25.36 ± 1.43 e | ||
| Krivi vir | 1.82 ± 0.04 c | 1.03 ± 0.19 c | 0.23 ± 0.01 b | 1.03 ± 0.02 j | 27.44 ± 0.77 d | ||
| Pančevo | 1.54 ± 0.03 f | 1.26 ± 0.16 bc | 0.18 ± 0.00 f | 1.14 ± 0.03 h | 30.22 ± 0.68 c | ||
| Pirot | M | 2.00 ± 0.01 b | 1.38 ± 0.03 b | 0.20 ± 0.00 e | 1.28 ± 0.00 c | 35.33 ± 1.71 ab | |
| Južni Kučaj | 1.73 ± 0.02 d | 1.17 ± 0.04 c | 0.23 ± 0.01 b | 1.29 ± 0.00 b | 30.57 ± 0.60 c | ||
| Golina | 1.31 ± 0.02 h | 0.81 ± 0.02 d | 0.18 ± 0.00 f | 0.66 ± 0.05 lm | 23.28 ± 1.22 ef | ||
| Bogovo gumno | 1.40 ± 0.01 g | 0.82 ± 0.05 d | 0.26 ± 0.01 a | 1.28 ± 0.01 bc | 27.61 ± 2.67 cd | ||
| Krivi vir | 1.42 ± 0.02 g | 1.10 ± 0.04 c | 0.20 ± 0.01 de | 1.01 ± 0.01 j | 23.16 ± 1.16 ef | ||
| Pančevo | 1.46 ± 0.08 fg | 1.10 ± 0.08 c | 0.19 ± 0.01 ef | 1.26 ± 0.00 e | 27.97 ± 2.13 cd | ||
| PO | Rujevica | MAE | 1.69 ± 0.02 de | 1.21 ± 0.09 bc | 0.23 ± 0.01 b | 1.26 ± 0.00 e | 33.67 ± 1.19 b |
| Božurna | 1.65 ± 0.05 de | 0.79 ± 0.06 d | 0.21 ± 0.00 d | 1.27 ± 0.00 d | 30.08 ± 0.90 c | ||
| Rujevica | UAE | 1.85 ± 0.06 c | 1.42 ± 0.17 b | 0.24 ± 0.00 b | 1.29 ± 0.00 b | 35.96 ± 1.05 ab | |
| Božurna | 1.82 ± 0.03 c | 0.66 ± 0.04 e | 0.21 ± 0.00 d | 1.29 ± 0.00 b | 30.44 ± 1.18 c | ||
| Rujevica | M | 1.72 ± 0.02 d | 1.34 ± 0.07 b | 0.24 ± 0.01 ab | 1.30 ± 0.00 a | 37.06 ± 1.33 a | |
| Božurna | 1.62 ± 0.03 e | 1.20 ± 0.09 bc | 0.20 ± 0.00 e | 1.28 ± 0.00 c | 26.80 ± 0.63 d | ||
| Plant Species (Root Extract) | Locality | Extraction Method | Salmonella Typhimurium | Listeria monocytogenes | Bacillus cereus | Pseudomonas aeruginosa | Staphylococcus aureus | Escherichia coli | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |||
| PT | Gulenovci | MAE | 2.0 | 4.0 | 4.0 | 8.0 | 2.0 | 4.0 | 4.0 | 8.0 | 4.0 | 8.0 | 2.0 | 4.0 |
| Deliblato sand | 1.0 | 2.0 | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 4.0 | ||
| Bogovo gumno | 4.0 | 8.0 | 4.0 | 8.0 | 4.0 | 8.0 | 4.0 | 8.0 | 4.0 | 8.0 | 2.0 | 4.0 | ||
| Gulenovci | UAE | 1.0 | 2.0 | 1.0 | 2.0 | 0.5 | 1.0 | 1.0 | 2.0 | 2.0 | 4.0 | 1.0 | 2.0 | |
| Deliblato sand | 2.0 | 4.0 | 1.0 | 2.0 | 1.0 | 2.0 | 2.0 | 4.0 | 2.0 | 4.0 | 1.0 | 2.0 | ||
| Bogovo gumno | 0.5 | 1.0 | 1.0 | 2.0 | 1.0 | 2.0 | 0.5 | 1.0 | 2.0 | 4.0 | 1.0 | 2.0 | ||
| Gulenovci | M | 1.0 | 2.0 | 2.0 | 4.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | |
| Deliblato sand | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 2.0 | 4.0 | 1.0 | 2.0 | ||
| Bogovo gumno | 2.0 | 4.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 2.0 | 4.0 | 0.5 | 1.0 | ||
| PP | Pirot | MAE | 4.0 | 8.0 | 4.0 | 8.0 | 4.0 | 8.0 | 4.0 | 8.0 | 4.0 | 8.0 | 4.0 | 8.0 |
| Južni Kučaj | 2.0 | 4.0 | 2.0 | 4.0 | 1.0 | 2.0 | 2.0 | 4.0 | 2.0 | 4.0 | 1.0 | 2.0 | ||
| Golina | 2.0 | 4.0 | 2.0 | 4.0 | 1.0 | 2.0 | 1.0 | 2.0 | 2.0 | 4.0 | 1.0 | 2.0 | ||
| Bogovo gumno | 4.0 | 8.0 | 4.0 | 8.0 | 2.0 | 4.0 | 4.0 | 8.0 | 2.0 | 4.0 | 2.0 | 4.0 | ||
| Krivi vir | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 4.0 | 1.0 | 2.0 | 2.0 | 4.0 | 2.0 | 4.0 | ||
| Pančevo | 4.0 | 8.0 | 2.0 | 4.0 | 1.0 | 2.0 | 2.0 | 4.0 | 2.0 | 4.0 | 0.5 | 1.0 | ||
| Pirot | UAE | 2.0 | 4.0 | 1.0 | 2.0 | 1.0 | 2.0 | 2.0 | 4.0 | 2.0 | 4.0 | 1.0 | 2.0 | |
| Južni Kučaj | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 2.0 | 4.0 | ||
| Golina | 4.0 | 8.0 | 2.0 | 4.0 | 1.0 | 2.0 | 2.0 | 4.0 | 2.0 | 4.0 | 1.0 | 2.0 | ||
| Bogovo gumno | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 2.0 | 4.0 | 1.0 | 2.0 | ||
| Krivi vir | 4.0 | 8.0 | 1.0 | 2.0 | 1.0 | 2.0 | 2.0 | 4.0 | 4.0 | 8.0 | 1.0 | 2.0 | ||
| Pančevo | 1.0 | 2.0 | 1.0 | 2.0 | 2.0 | 4.0 | 0.5 | 1.0 | 2.0 | 4.0 | 2.0 | 4.0 | ||
| Pirot | M | 1.0 | 2.0 | 0.5 | 1.0 | 0.5 | 1.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | |
| Južni Kučaj | 1.0 | 2.0 | 1.0 | 2.0 | 0.125 | 0.25 | 1.0 | 2.0 | 1.0 | 2.0 | 0.5 | 1.0 | ||
| Golina | 1.0 | 2.0 | 1.0 | 2.0 | 0.25 | 0.5 | 1.0 | 2.0 | 1.0 | 2.0 | 0.5 | 1.0 | ||
| Bogovo gumno | 1.0 | 2.0 | 0.5 | 1.0 | 1.0 | 2.0 | 0.5 | 1.0 | 2.0 | 4.0 | 1.0 | 2.0 | ||
| Krivi vir | 0.5 | 1.0 | 1.0 | 2.0 | 0.25 | 0.5 | 0.5 | 1.0 | 0.5 | 1.0 | 0.5 | 1.0 | ||
| Pančevo | 1.0 | 2.0 | 2.0 | 4.0 | 1.0 | 2.0 | 1.0 | 2.0 | 2.0 | 4.0 | 0.5 | 1.0 | ||
| PO | Rujevica | MAE | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 4.0 |
| Božurna | 2.0 | 4.0 | 1.0 | 2.0 | 0.5 | 1.0 | 2.0 | 4.0 | 2.0 | 4.0 | 0.5 | 1.0 | ||
| Rujevica | UAE | 2.0 | 4.0 | 1.0 | 2.0 | 0.5 | 1.0 | 0.5 | 1.0 | 2.0 | 4.0 | 1.0 | 2.0 | |
| Božurna | 1.0 | 2.0 | 1.0 | 2.0 | 0.5 | 1.0 | 0.5 | 1.0 | 2.0 | 4.0 | 1.0 | 2.0 | ||
| Rujevica | M | 1.0 | 2.0 | 0.25 | 0.5 | 0.5 | 1.0 | 0.25 | 0.5 | 1.0 | 2.0 | 0.5 | 1.0 | |
| Božurna | 1.0 | 2.0 | 1.0 | 2.0 | 0.5 | 1.0 | 0.5 | 1.0 | 1.0 | 2.0 | 0.5 | 1.0 | ||
| No. | Compound Name | Relative Content (%) * | |||||
|---|---|---|---|---|---|---|---|
| PTc | DPT | PPc | DPP | POc | DPO | ||
| Phenolic acids and derivatives | |||||||
| Gallic acid and derivatives | |||||||
| 1 | Gallic acid | 0.69 | 0.27 | 1.10 | 0.19 | 2.24 | 0.38 |
| 2 | Methyl gallate | 0.09 | 7.14 | 0.18 | 3.80 | 2.34 | 21.30 |
| 3 | Gallic acid derivative | 0.79 | 0.14 | 0.14 | 0.03 | 1.05 | 0.08 |
| 4 | Digallic acid isomer I | 0.21 | 0.05 | - | - | 1.00 | - |
| 5 | Digallic acid isomer II | 0.52 | 0.09 | 0.19 | - | 1.58 | 0.05 |
| 6 | Gallic acid hexoside | 1.21 | 0.62 | 1.62 | 0.62 | 1.54 | 0.41 |
| 7 | Methyl digallate | 0.22 | 1.21 | - | 0.11 | 0.03 | 1.70 |
| 8 | Methyl gallic acid hexoside | 0.06 | - | 0.34 | 0.06 | 0.05 | - |
| 9 | Galloyl-vanilloyl-rhamoside | 0.31 | 0.11 | 0.06 | - | 2.82 | 1.57 |
| 10 | Gallic acid dihexoside | 7.98 | 2.44 | 4.26 | 1.21 | 13.70 | 5.01 |
| 11 | Galloyl-HHDP-hexose isomer II | 0.97 | 0.11 | 0.44 | 0.04 | 1.58 | 0.15 |
| 12 | Galloyl-HHDP-hexose isomer I | 0.78 | 0.10 | 0.50 | - | 1.21 | 0.08 |
| 13 | Trigalloyl-hexoside | 0.33 | 0.08 | 0.26 | 0.09 | - | - |
| 14 | Digalloyl-HHDP-protoquercitol | 0.14 | - | 0.20 | 0.12 | 0.75 | 0.53 |
| 15 | Tetragalloyl-hexoside | 0.17 | 0.12 | 0.35 | 0.19 | 1.31 | 0.61 |
| 16 | Pentagalloyl-hexoside | 0.39 | 0.33 | 0.43 | 0.30 | 2.40 | 1.50 |
| ∑ | 14.66 | 12.83 | 10.07 | 6.64 | 33.59 | 33.38 | |
| Ellagic acid and derivatives | |||||||
| 17 | Ellagic acid | 0.83 | 0.29 | 2.37 | 0.73 | 4.18 | 1.53 |
| 18 | Methyl ellagic acid | 0.41 | 0.19 | 0.80 | 0.28 | 1.25 | 0.43 |
| 19 | Dimethyl ellagic acid | 0.13 | 0.04 | 0.20 | 0.19 | 1.19 | 1.49 |
| 20 | Trimethyl ellagic acid | - | - | 1.48 | 1.12 | 0.06 | 0.07 |
| 21 | Ellagic acid hexoside | 0.35 | 0.03 | 0.45 | 0.06 | 0.82 | 0.11 |
| 22 | Methyl ellagic acid hexoside | 0.46 | 0.18 | 0.28 | 0.12 | 0.52 | 0.22 |
| ∑ | 2.18 | 0.73 | 5.58 | 2.50 | 8.03 | 3.86 | |
| Other phenolic acids and their glycosides | |||||||
| 23 | Hydroxybenzoic acid | 1.26 | 0.84 | 1.46 | 1.10 | 1.98 | 1.33 |
| 24 | Dihydroxybenzoic acid isomer I | 0.38 | - | 0.50 | - | 0.29 | - |
| 25 | Dihydroxybenzoic acid isomer II | 0.15 | 0.0.04 | - | - | 0.45 | 0.25 |
| 26 | Dihydroxybenzoic acid hexoside | 0.23 | 0.18 | 0.45 | 0.27 | 0.77 | 0.25 |
| 27 | Vanillic acid hexoside | 0.39 | 0.10 | 1.12 | 0.33 | 0.16 | - |
| ∑ | 2.42 | 1.16 | 3.53 | 1.70 | 3.64 | 1.83 | |
| ∑RC of total phenolic acid | 19.26 | 14.71 | 19.18 | 10.84 | 45.26 | 39.06 | |
| Flavonoids and derivatives | |||||||
| Flavan-3-ols and procyanidins | |||||||
| 28 | Catechin | 13.86 | 12.97 | 16.28 | 12.58 | 5.08 | 2.57 |
| 29 | Epicatechin | 1.10 | 0.74 | 2.57 | 1.74 | 0.65 | 0.27 |
| 30 | Methyl epigallocatechin | 0.14 | 0.02 | 0.13 | - | 0.12 | - |
| 31 | Epicatechin-gallate | 0.27 | 0.21 | 0.16 | - | 0.04 | - |
| 32 | Catechin hexoside | 0.81 | 0.56 | 3.92 | 3.66 | 4.35 | 3.00 |
| 33 | Epicatechin-hexoside | 0.38 | 0.22 | 0.55 | 0.41 | 0.21 | - |
| 34 | B-type procyanidin dimer isomer I | 22.20 | 18.55 | 8.88 | 1.94 | 6.02 | 2.35 |
| 35 | B-type procyanidin dimer isomer II | 1.12 | 0.82 | 2.58 | 2.35 | 0.67 | 0.43 |
| 36 | Chalcan flavan-3-ols dimer isomer II | 0.20 | 0.16 | 0.87 | 0.50 | - | - |
| 37 | Chalcan flavan-3-ol dimer isomer I | 6.41 | 6.08 | 7.11 | 4.88 | 0.71 | 0.23 |
| 38 | Methyl B-type prodelphinidin | 0.27 | - | 0.26 | - | 0.21 | - |
| 39 | Procyanidin trimer B-type isomer I | 2.48 | 1.85 | 2.72 | 2.39 | 0.50 | 0.38 |
| 40 | Procyanidin trimer B-type isomer II | 0.58 | 0.18 | 0.37 | - | 0.08 | - |
| ∑ | 49.82 | 42.35 | 46.39 | 42.02 | 18.65 | 9.24 | |
| Other detected flavonoids | |||||||
| 41 | Kaempferol | 0.06 | - | 0.08 | 0.04 | 0.04 | 0.02 |
| 42 | Naringenin | 0.07 | 0.07 | 0.12 | 0.12 | 0.14 | 0.17 |
| 43 | Phloridzin | 0.26 | 0.33 | 0.36 | 0.45 | 1.25 | 1.22 |
| ∑ | 0.39 | 0.40 | 0.56 | 0.61 | 1.43 | 1.41 | |
| ∑RC of total flavonoids | 50.21 | 42.75 | 46.94 | 31.06 | 20.07 | 10.65 | |
| Peonia root terpenoids | |||||||
| 44 | Nor-paeonilactone | - | - | 0.08 | 0.03 | 0.18 | - |
| 45 | Paeoveitol D | 0.91 | 0.58 | 0.71 | - | 0.64 | - |
| 46 | Paeonisothujone | 0.57 | 0.03 | 0.44 | - | 1.44 | - |
| 47 | Paeonilactone B | 0.34 | 0.09 | 0.46 | 0.21 | 0.93 | 0.23 |
| 48 | Paeonilactone A | - | - | 0.39 | 0.22 | 1.73 | - |
| 49 | 9-Hydroxypaeonilactone A | 0.09 | - | 2.55 | 0.67 | 1.07 | - |
| 50 | Paeoniflorigenone | 0.31 | 0.18 | 4.22 | 7.18 | 2.66 | 1.96 |
| 51 | Oxypaeoniflorin | 6.18 | 5.53 | 4.81 | 4.14 | 5.02 | 4.66 |
| 52 | Mudanpioside D | - | - | 0.23 | 0.22 | - | - |
| 53 | Paeoniflorin + HCOOH | 12.91 | 8.61 | 14.17 | 13.28 | 15.72 | 10.24 |
| 54 | Albiflorin + HCOOH | 3.64 | 4.36 | 3.29 | 3.40 | 1.96 | 2.19 |
| 55 | Galloyl desbenzoyl paeoniflorin | 0.82 | 0.39 | 0.84 | 0.26 | - | - |
| 56 | Benzoyl paeoniflorin | - | - | - | - | 0.21 | 0.11 |
| 57 | Mudanpioside J | 1.03 | 1.10 | 0.26 | 0.24 | 1.39 | 1.30 |
| 58 | Galloyl-paeoniflorin | 3.74 | 3.58 | 1.44 | 0.73 | 1.72 | 1.32 |
| ∑RC of total terpenoids | 30.53 | 24.45 | 33.88 | 30.58 | 34.66 | 22.03 | |
| 100 | - | 100 | - | 100 | - | ||
| ** | 100 | - | 92.37 | - | 79.00 | - | |
| No | RT | Compound Name | Formula | m/z Exact Mass | Recovery after In Vitro GID (%) | Recovery Range (%) | ||
|---|---|---|---|---|---|---|---|---|
| DPT | DPP | DPO | ||||||
| Phenolic acids and derivatives | ||||||||
| Gallic acid and derivatives | ||||||||
| 1 | 1.14 | Gallic acid | C7H5O5− | 169.01443 | 39.18 | 17.11 | 17.02 | 17.02–39.18 |
| 2 | 4.24 | Methyl gallate | C8H7O5− | 183.02825 | 7538.45 | 2060.68 | 910.95 | 910.95–7538.45 |
| 3 | 1.75 | Gallic acid derivative | C10H11O7− | 243.05244 | 17.62 | 20.88 | 7.57 | 7.57–20.88 |
| 4 | 3.77 | Digallic acid isomer I | C14H9O9− | 321.02654 | 23.72 | - | 0 | 0–23.72 |
| 5 | 5.59 | Digallic acid isomer II | C14H9O9− | 321.02743 | 17.21 | 0 | 3.11 | 3.11–17.21 |
| 6 | 0.90 | Gallic acid hexoside | C13H15O10− | 331.06817 | 50.75 | 38.39 | 26.64 | 26.64–50.75 |
| 7 | 8.02 | Methyl digallate | C15H11O9− | 335.04267 | 5484 | ** | 5686.48 | >5484 |
| 8 | 1.75 | Methyl gallic acid hexoside | C14H17O10− | 345.08617 | 0 | 16.97 | 0 | 0–16.97 |
| 9 | 7.34 | Galloyl-vanilloyl-rhamoside | C21H21O12− | 465.10780 | 35.54 | 0 | 55.84 | 0–55.84 |
| 10 | 1.00 | Gallic acid dihexoside | C19H25O15− | 493.12260 | 30.64 | 28.33 | 36.54 | 28.33–36.54 |
| 11 | 5.93 | Galloyl-HHDP-hexose isomer II | C27H21O18− | 633.07382 | 11.28 | 9.49 | 9.24 | 9.24–11.28 |
| 12 | 2.89 | Galloyl-HHDP-hexose isomer I | C27H21O18− | 633.07577 | 13.45 | 0 | 6.82 | 0–13.45 |
| 13 | 6.80 | Trigalloyl-hexoside | C27H23O18− | 635.09260 | 25.11 | 34.26 | - | 25.11–34.26 |
| 14 | 8.15 | Digalloyl-HHDP-protoquercitol | C34H27O21+ | 771.10856 | 0 | 60.06 | 70.22 | 0–70.22 |
| 15 | 7.55 | Tetragalloyl-hexoside | C34H27O22− | 787.10066 | 70.80 | 54.59 | 46.96 | 46.96–70.80 |
| 16 | 7.95 | Pentagalloyl-hexoside | C41H31O26− | 939.11237 | 85.75 | 69.30 | 62.73 | 62.73–85.75 |
| ∑ | 87.49 | 65.93 | 99.37 | 65.93–99.37 | ||||
| Ellagic acid and derivatives | ||||||||
| 17 | 7.68 | Ellagic acid | C14H5O8− | 301.00097 | 34.42 | 31.04 | 36.58 | 31.04–36.58 |
| 18 | 8.42 | Methyl ellagic acid | C15H7O8− | 315.01710 | 46.31 | 35.33 | 34.48 | 34.48–46.31 |
| 19 | 9.57 | Dimethyl ellagic acid | C16H9O8− | 329.03194 | 31.94 | 95.14 | 125.18 | 31.94–125.18 |
| 20 | 10.85 | Trimethyl ellagic acid | C17H11O8− | 343.04668 | - | 75.52 | 111.78 | 75.52–111.78 |
| 21 | 6.87 | Ellagic acid hexoside | C20H15O13− | 463.05601 | 9.51 | 12.21 | 13.09 | 9.51–13.09 |
| 22 | 7.55 | Methyl ellagic acid hexoside | C21H17O13− | 477.07058 | 38.10 | 43.04 | 43.03 | 38.10–43.04 |
| ∑ | 33.32 | 44.81 | 48.03 | 33.32–48.03 | ||||
| Other phenolic acids and their glycosides | ||||||||
| 23 | 3.09 | Hydroxybenzoic acid | C7H5O3− | 137.02422 | 66.54 | 75.62 | 67.07 | 66.54–75.62 |
| 24 | 1.88 | Dihydroxybenzoic acid isomer I | C7H5O4− | 153.01946 | 0 | 0 | 0 | 0 |
| 25 | 5.12 | Dihydroxybenzoic acid isomer II | C7H5O4− | 153.01911 | 26.70 | - | 56.35 | 26.70–56.35 |
| 26 | 2.36 | Hydroxybenzoic acid hexoside | C13H15O9− | 315.07520 | 79.52 | 59.13 | 32.25 | 32.25–79.52 |
| 27 | 2.96 | Vanillic acid hexoside | C14H17O9− | 329.08958 | 25.02 | 29.84 | 0 | 0–29.84 |
| ∑ | 48.01 | 48.25 | 50.19 | 48.01–50.19 | ||||
| ∑ Total recovery of phenolic acid and derivatives | 76.40 | 56.53 | 86.30 | 56.53–86.30 | ||||
| Flavonoids and derivatives | ||||||||
| Flavan-3-ols and procyanidins | ||||||||
| 28 | 5.29 | Catechin | C15H13O6− | 289.07260 | 93.57 | 77.26 | 50.61 | 50.61–93.57 |
| 29 | 6.63 | Epicatechin | C15H13O6− | 289.07259 | 67.61 | 67.86 | 41.95 | 41.95–67.86 |
| 30 | 6.54 | Methyl epigallocatechin | C16H15O7− | 319.08293 | 12.38 | 0 | - | 0–12.38 |
| 31 | 7.68 | Epicatechin-gallate | C22H17O10− | 441.08941 | 80.00 | 0 | - | 0–80.00 |
| 32 | 4.18 | Catechin hexoside | C21H23O11− | 451.12584 | 68.83 | 93.38 | 69.09 | 69.09–93.38 |
| 33 | 5.73 | Epicatechin-hexoside | C21H25O11+ | 453.14384 | 56.80 | 75.10 | - | 56.80–75.10 |
| 34 | 4.51 | B-type procyanidin dimer isomer I | C30H25O12− | 577.13795 | 83.56 | 21.80 | 39.07 | 21.80–83.56 |
| 35 | 6.33 | B-type procyanidin dimer isomer II | C30H25O12− | 577.13723 | 73.12 | 91.33 | 63.74 | 63.74–91.33 |
| 36 | 6.63 | Chalcan flavan-3-ols dimer iso. II | C30H27O12− | 579.15511 | 76.98 | 57.41 | - | 57.41–76.98 |
| 37 | 5.29 | Chalcan flavan-3-ol dimer isomer I | C30H27O12− | 579.15287 | 94.87 | 68.67 | 33.12 | 33.12–94.87 |
| 38 | 6.19 | Methyl B-type prodelphinidin | C31H27O13− | 607.14904 | 0 | 0 | - | 0 |
| 39 | 6.02 | Procyanidin trimer B-type isomer I | C45H37O18− | 865.19890 | 74.33 | 87.76 | 75.93 | 74.33–87.76 |
| 40 | 6.93 | Procyanidin trimer B-type isomer II | C45H37O18− | 865.19733 | 31.38 | 0 | - | 0–31.38 |
| ∑ | 85.00 | 65.65 | 49.75 | 49.57–85.00 | ||||
| Other detected flavonoids | ||||||||
| 41 | 10.16 | Kaempferol | C15H9O6− | 285.04097 | 0 | 54.56 | 52.58 | 0–54.56 |
| 42 | 9.91 | Naringenin | C15H11O5− | 271.06201 | 102.19 | 103.78 | 116.53 | 102.19–116.53 |
| 43 | 8.49 | Phloridzin | C21H23O10− | 435.12768 | 128.75 | 122.32 | 98.08 | 98.08–128.75 |
| ∑ | 103.34 | 109.27 | 98.78 | 98.78–109.27 | ||||
| ∑ Total recovery of flavonoids and derivatives | 85.14 | 66.16 | 53.07 | 53.07–85.14 | ||||
| Paeonia root terpenoids | ||||||||
| 44 | 9.97 | Nor-paeonilactone | C9H15O2+ | 155.10880 | - | 37.72 | - | 37.72 |
| 45 | 6.40 | Paeoveitol D | C10H11O3+ | 179.07217 | 63.25 | 0 | - | 0–63.25 |
| 46 | 6.93 | Paeonisothujone | C10H15O3+ | 183.10219 | 4.85 | 0 | - | 0–4.85 |
| 47 | 6.60 | Paeonilactone B | C10H13O4+ | 197.08347 | 27.44 | 46.58 | 25.02 | 25.02–46.58 |
| 48 | 3.50 | Paeonilactone A | C10H15O4+ | 199.09852 | - | 56.27 | - | 56.27 |
| 49 | 2.62 | 9-Hydroxypaeonilactone A | C10H15O5+ | 215.09411 | 0 | 26.31 | - | 0–26.31 |
| 50 | 10.31 | Paeoniflorigenone | C17H19O6+ | 319.12118 | 56.97 | 170.17 | 73.95 | 56.97–170.17 |
| 51 | 5.82 | Oxypaeoniflorin | C23H27O12− | 495.15174 | 89.52 | 86.15 | 92.85 | 86.15–92.85 |
| 52 | 8.90 | Mudanpioside D | C24H29O12− | 509.17478 | - | 94.92 | - | 94.92 |
| 53 | 7.07 | Paeoniflorin + HCOOH | C24H29O13− | 525.16398 | 66.69 | 93.73 | 65.16 | 65.16–93.73 |
| 54 | 7.75 | Albiflorin + HCOOH | C24H29O13− | 525.16361 | 119.68 | 103.46 | 111.85 | 103.46–119.68 |
| 55 | 2.59 | Galloyl desbenzoyl paeoniflorin | C23H27O14− | 527.14182 | 47.83 | 30.60 | - | 30.60–47.83 |
| 56 | 10.17 | Benzoyl paeoniflorin | C30H33O12+ | 585.20263 | - | - | 53.04 | 53.04 |
| 57 | 9.91 | Mudanpioside J | C31H33O14− | 629.19505 | 107.32 | 91.87 | 93.54 | 91.87–107.32 |
| 58 | 7.87 | Galloyl-paeoniflorin | C30H31O15− | 631.16919 | 95.80 | 50.35 | 77.00 | 50.35–95.80 |
| ∑ Total recovery of terpenoids | 80.09 | 90.25 | 63.56 | 63.56–90.25 | ||||
| ∑∑ Total recovery of detected bioactive compounds | 81.91 | 72.47 | 71.75 | 71.75–81.91 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batinić, P.; Jovanović, A.; Stojković, D.; Zengin, G.; Cvijetić, I.; Gašić, U.; Čutović, N.; Pešić, M.B.; Milinčić, D.D.; Carević, T.; et al. Phytochemical Analysis, Biological Activities, and Molecular Docking Studies of Root Extracts from Paeonia Species in Serbia. Pharmaceuticals 2024, 17, 518. https://doi.org/10.3390/ph17040518
Batinić P, Jovanović A, Stojković D, Zengin G, Cvijetić I, Gašić U, Čutović N, Pešić MB, Milinčić DD, Carević T, et al. Phytochemical Analysis, Biological Activities, and Molecular Docking Studies of Root Extracts from Paeonia Species in Serbia. Pharmaceuticals. 2024; 17(4):518. https://doi.org/10.3390/ph17040518
Chicago/Turabian StyleBatinić, Petar, Aleksandra Jovanović, Dejan Stojković, Gökhan Zengin, Ilija Cvijetić, Uroš Gašić, Natalija Čutović, Mirjana B. Pešić, Danijel D. Milinčić, Tamara Carević, and et al. 2024. "Phytochemical Analysis, Biological Activities, and Molecular Docking Studies of Root Extracts from Paeonia Species in Serbia" Pharmaceuticals 17, no. 4: 518. https://doi.org/10.3390/ph17040518
APA StyleBatinić, P., Jovanović, A., Stojković, D., Zengin, G., Cvijetić, I., Gašić, U., Čutović, N., Pešić, M. B., Milinčić, D. D., Carević, T., Marinković, A., Bugarski, B., & Marković, T. (2024). Phytochemical Analysis, Biological Activities, and Molecular Docking Studies of Root Extracts from Paeonia Species in Serbia. Pharmaceuticals, 17(4), 518. https://doi.org/10.3390/ph17040518












