Efficacy Assessment of Five Policosanol Brands and Damage to Vital Organs in Hyperlipidemic Zebrafish by Six-Week Supplementation: Highlighting the Toxicity of Red Yeast Rice and Safety of Cuban Policosanol (Raydel®)
Abstract
1. Introduction
2. Results
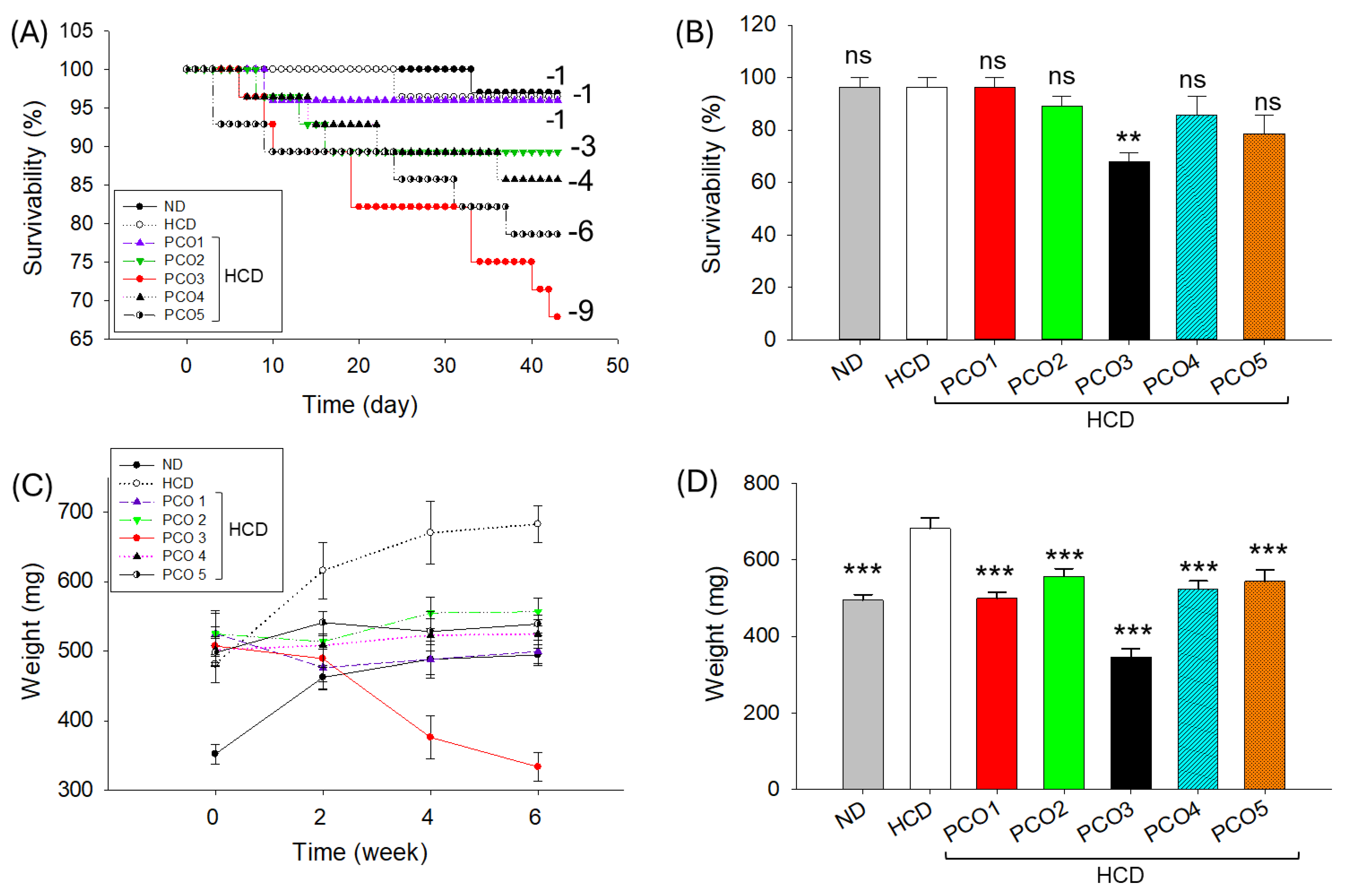
2.1. Survivability and Body Weight of Zebrafish
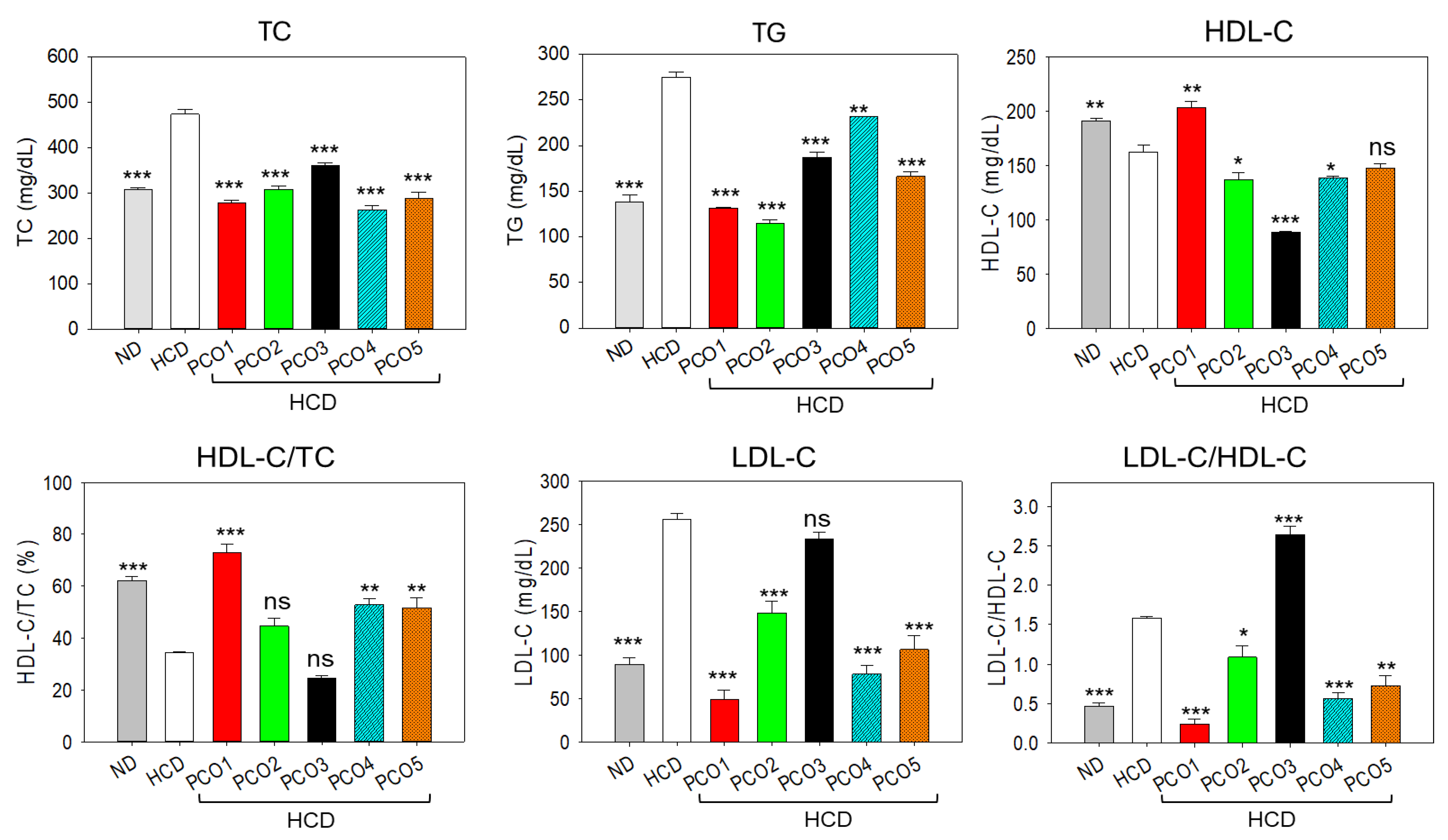
2.2. Blood Lipid Analysis
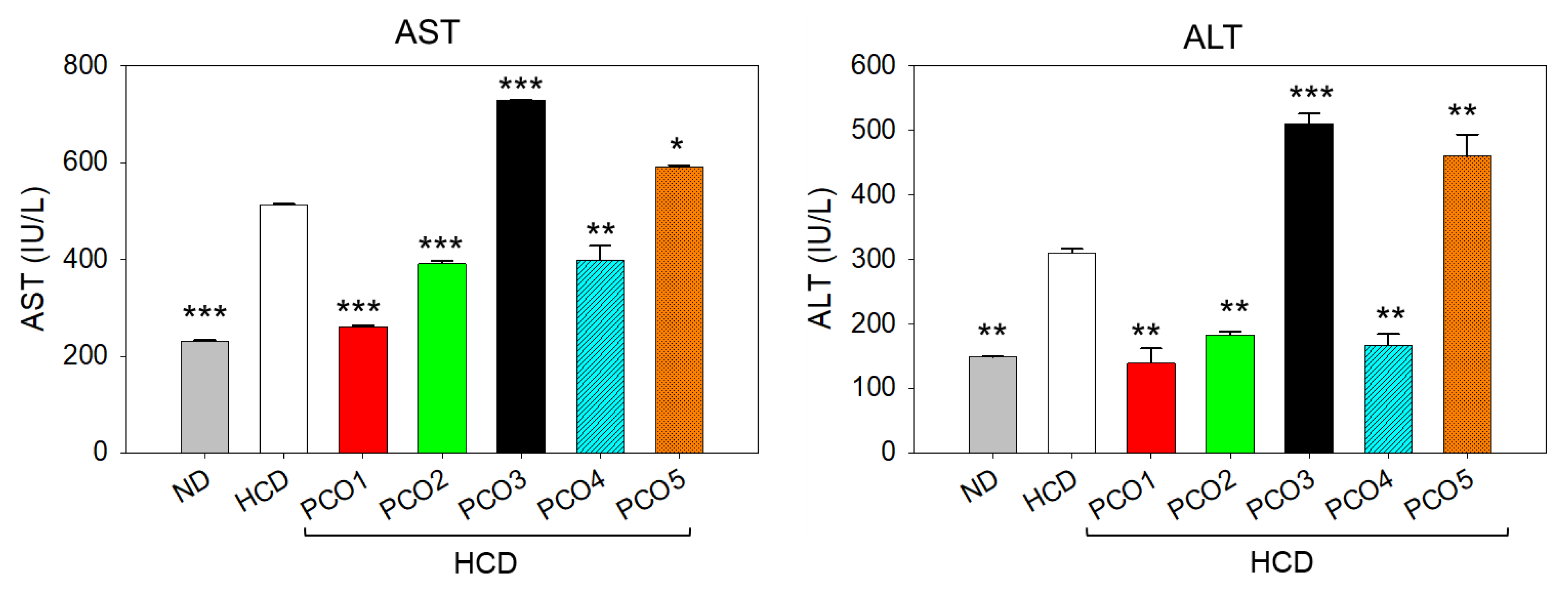
2.3. Plasma Hepatic Function Biomarker Assessment
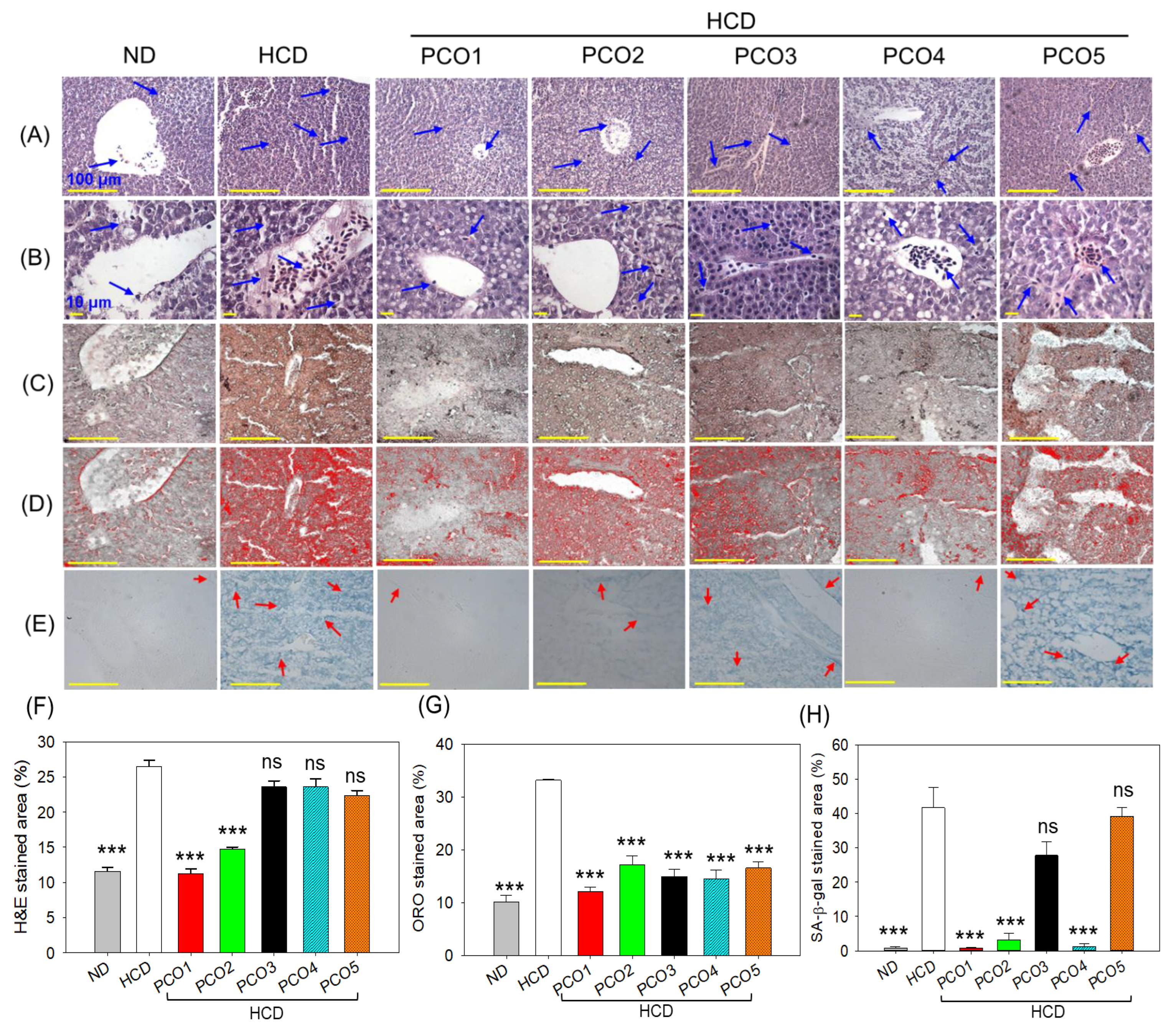
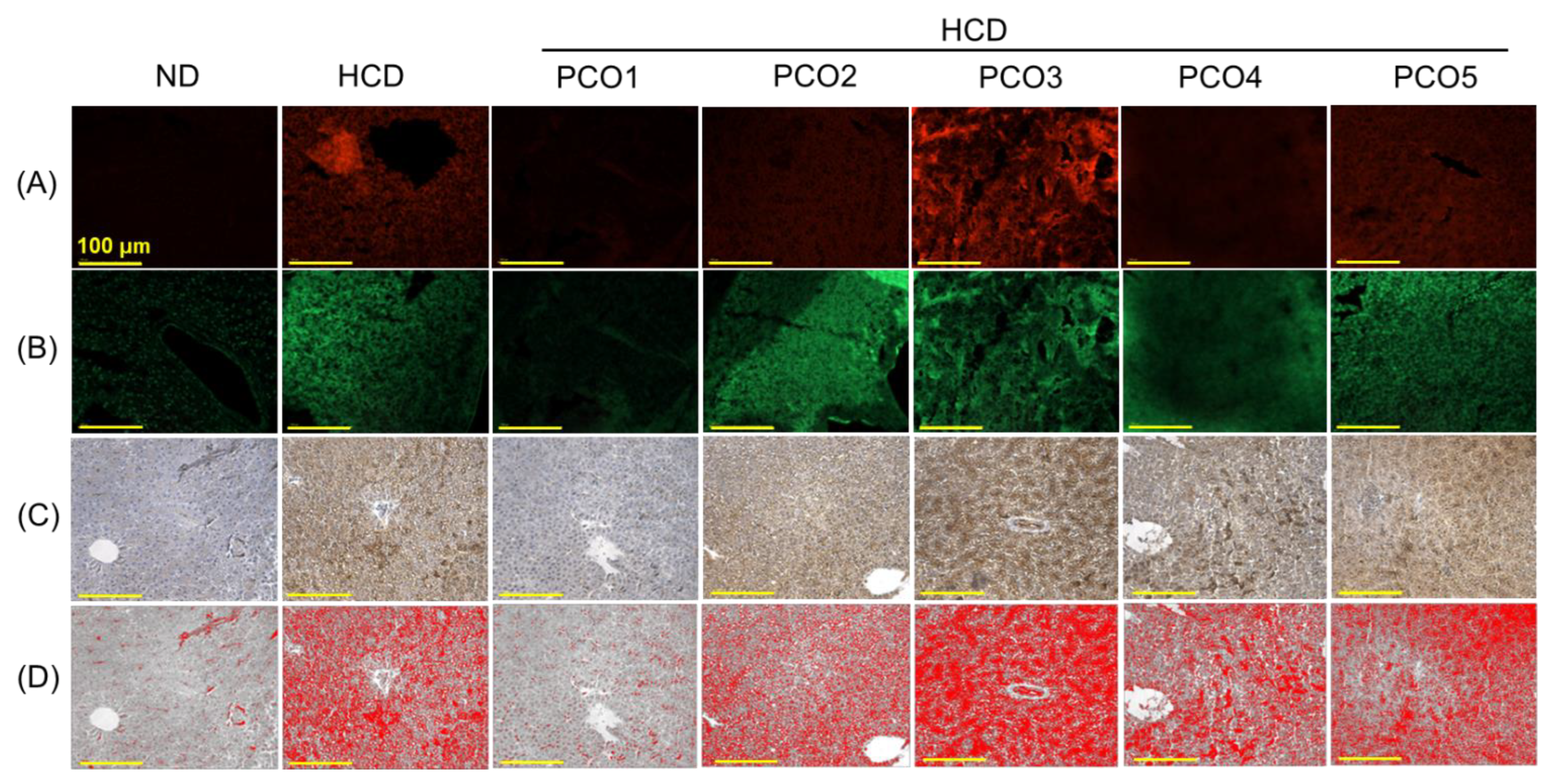
2.4. Evaluation of the Hepatic Section
2.5. Assessment of Oxidative Stress, Apoptosis, and Inflammation in the Liver
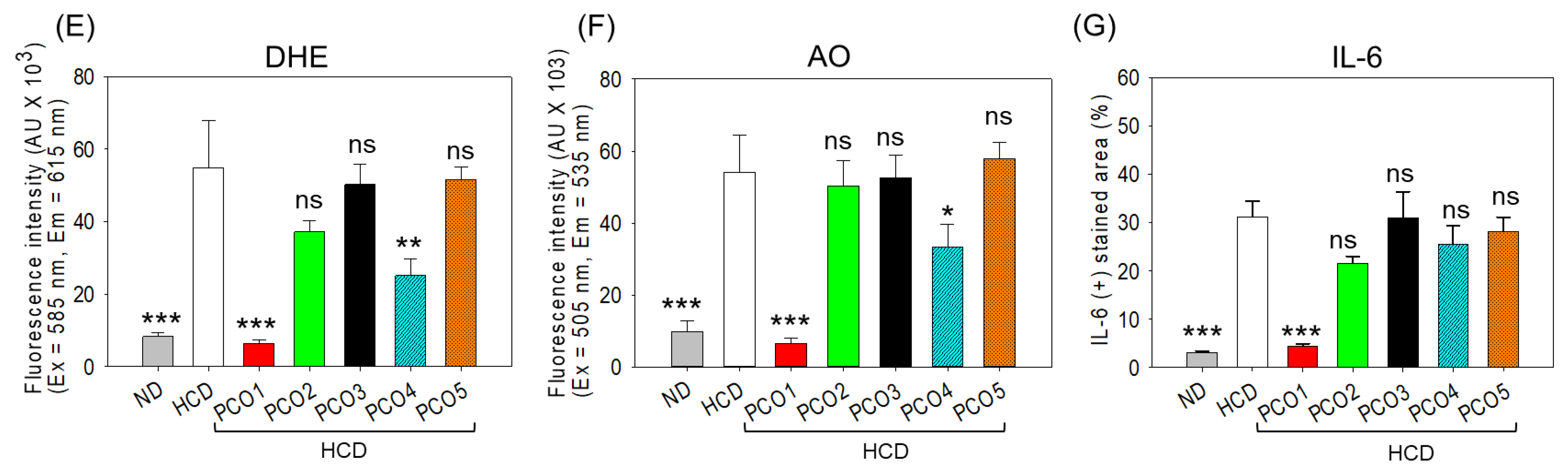
2.6. Examination of the Kidney Section
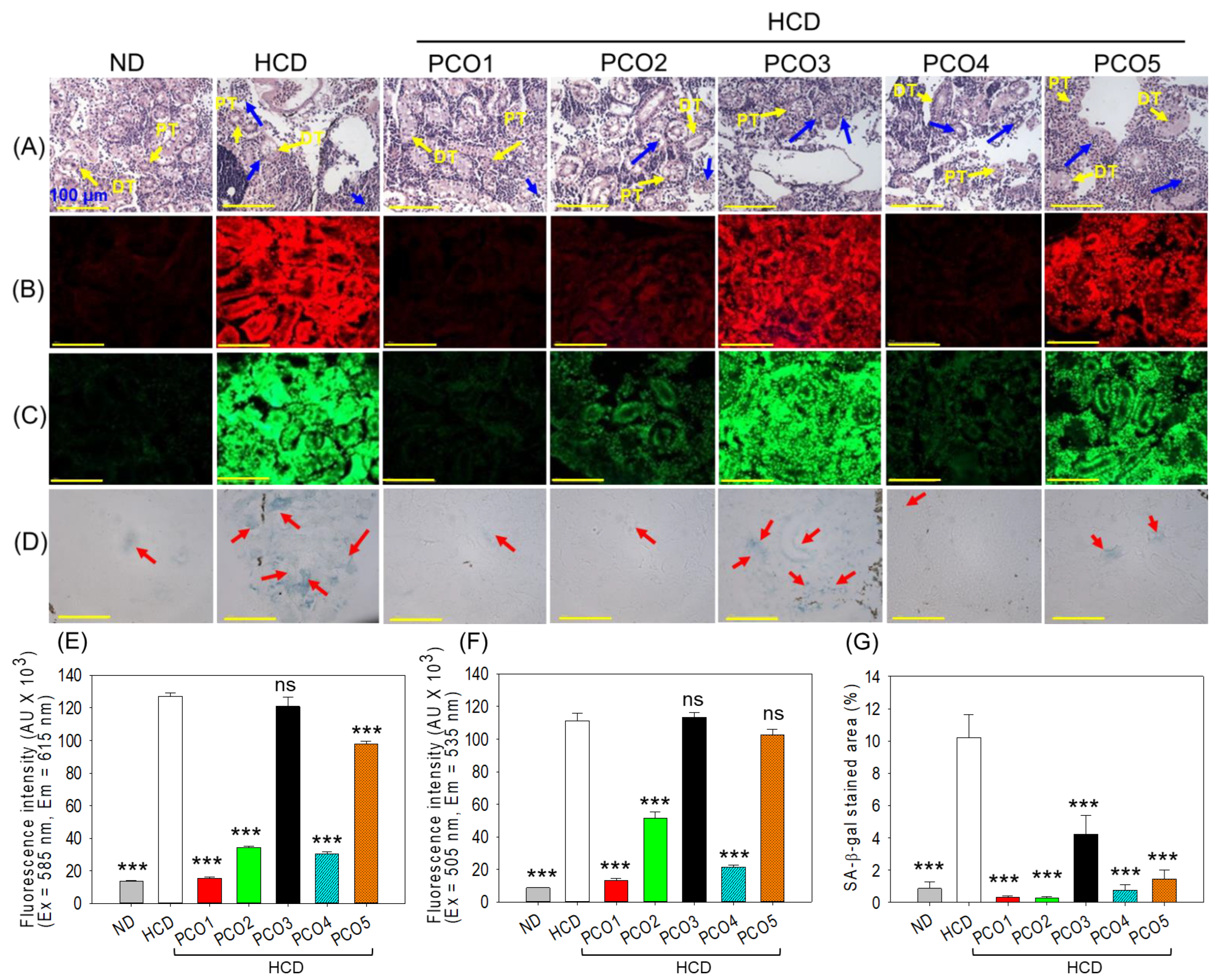
2.7. Examination of the Ovary Section
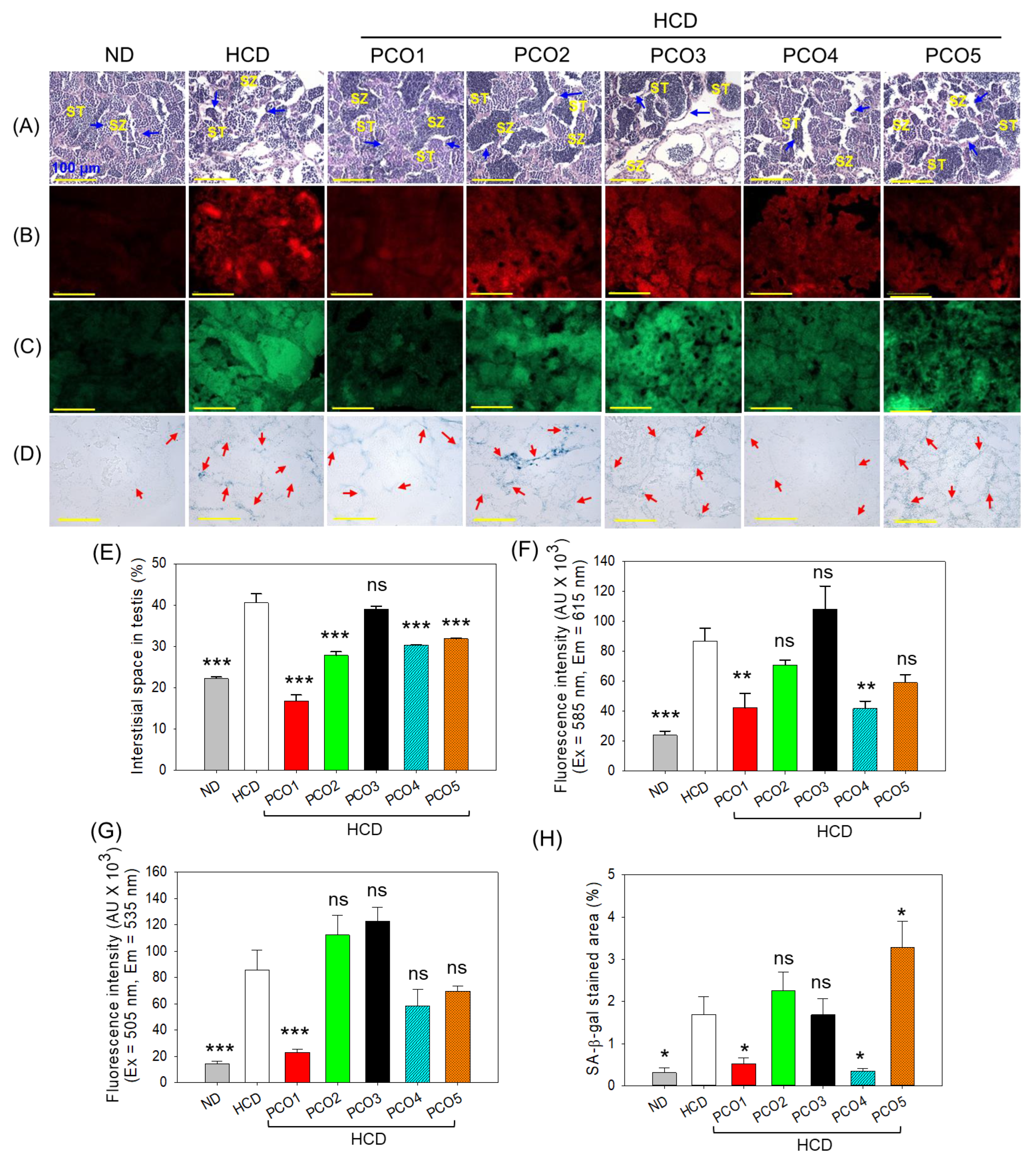
2.8. Examination of the Testis Section
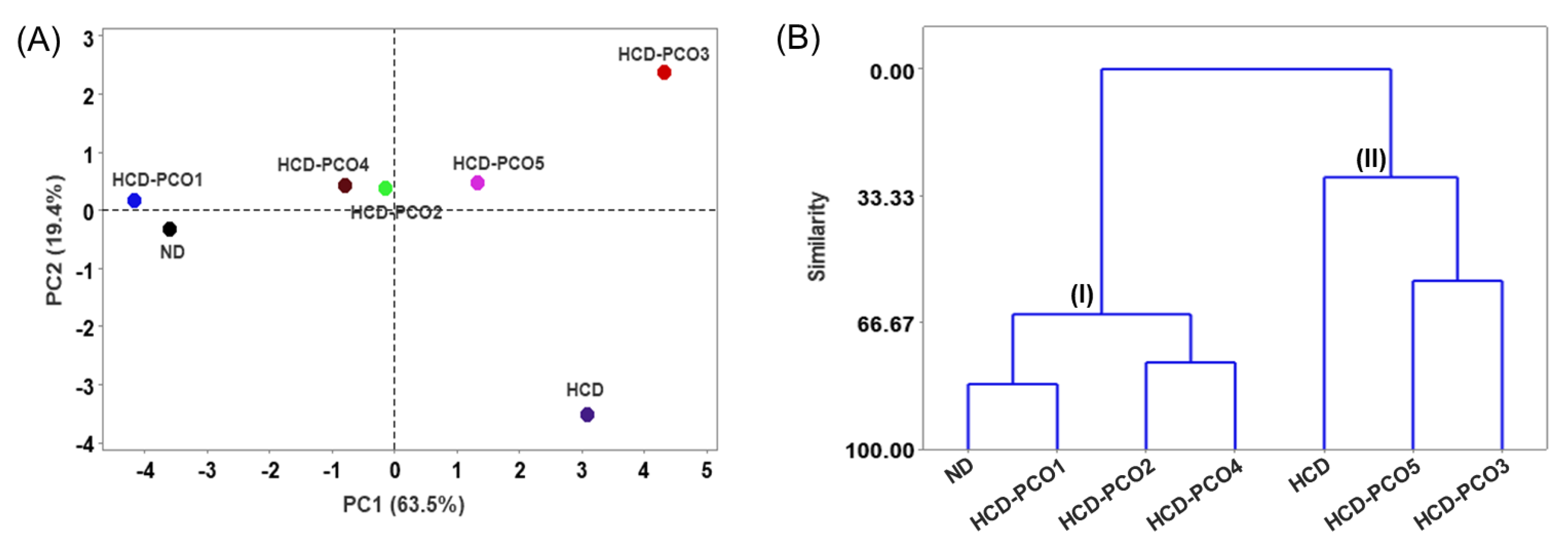
2.9. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Zebrafish Husbandry
4.3. Preparation of High-Cholesterol Diet (HCD) and Policosanol-Supplemented HCD
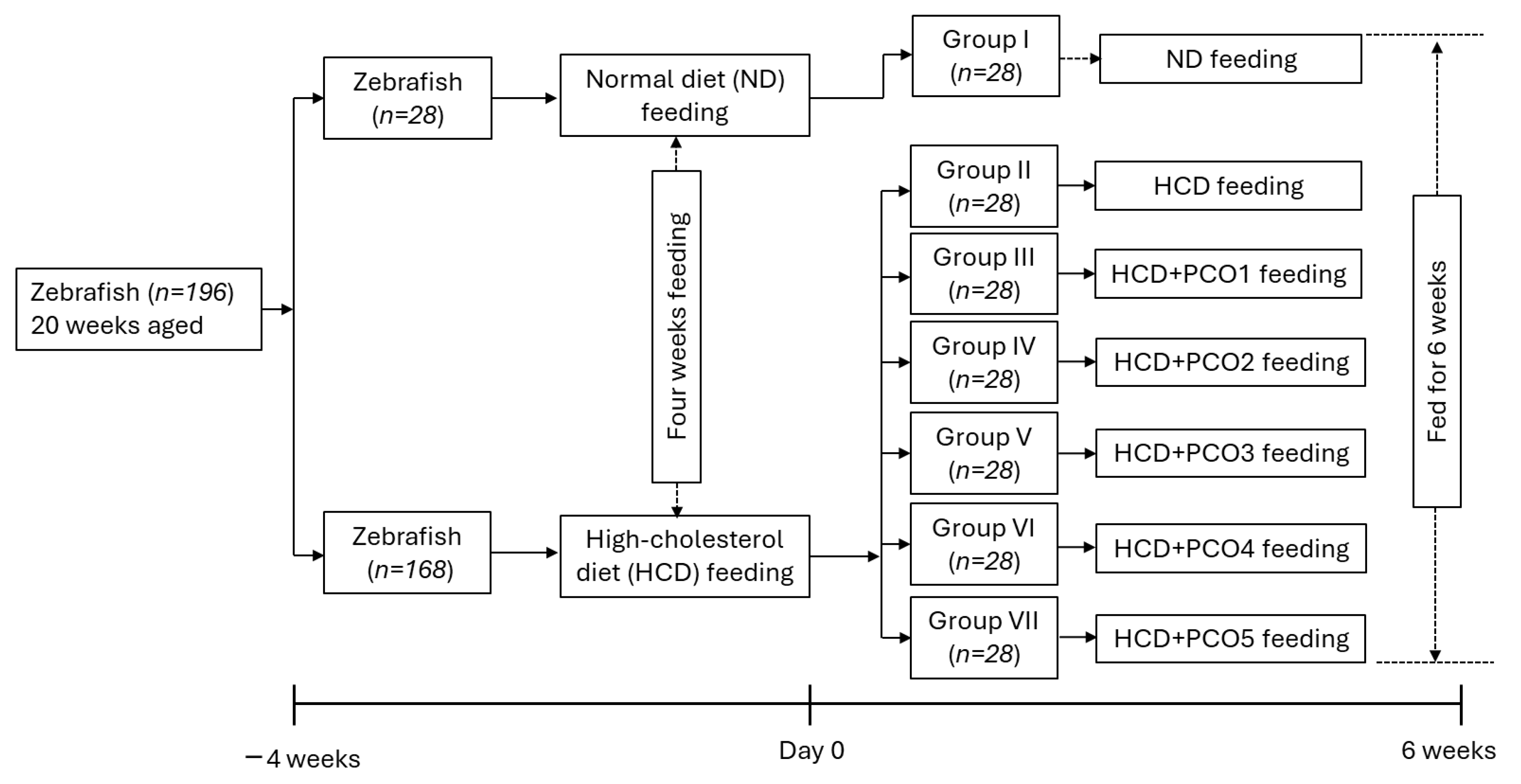
4.4. Zebrafish Fed with Different Policosanol-Supplemented High-Cholesterol Diets (HCDs)
4.5. Analysis of Plasma
4.6. Histology and Immunohistochemistry (IHC)
4.7. Senescent-Associated β-Galactosidase Imaging
4.8. Fluorescence Imaging of the Tissue Section
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Oliveira, L.L.H.; de Assis, A.C.R.; Giraldez, V.Z.R.; Scudeler, T.L.; Soares, P.R. Dyslipidemia: A Narrative Review on Pharmacotherapy. Pharmaceuticals 2024, 17, 289. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Alkerwi, A. Physical activity, sedentary behavior time and lipid levels in the Observation of Cardiovascular Risk Factors in Luxembourg study. Lipids Health Dis. 2015, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, A.; Petersen, K.S.; Jafari, F.; Kris-Etherton, P.M. Dietary management of dyslipidemia and the impact of dietary patterns on lipid disorders. Prog. Cardiovasc. Dis. 2022, 75, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef] [PubMed]
- Mahalle, N.; Garg, M.; Naik, S.S.; Kulkarni, M.V. Study of pattern of dyslipidemia and its correlation with cardiovascular risk factors in patients with proven coronary artery disease. Indian J. Endocrinol. Metab. 2014, 18, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, K.I.; Gloviczki, P.; Antignani, P.L.; Comerota, A.J.; Dardik, A.; Davies, A.H.; Eckstein, H.-H.; Faggioli, G.; Fernandes, J.F.E.; Fraedrich, G. Benefits and drawbacks of statins and non-statin lipid lowering agents in carotid artery disease. Prog. Cardiovasc. Dis. 2022, 73, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahman, T.; Bukhari, S.M.A.; Herrera, E.C.; Awuah, W.A.; Lawrence, J.; de Andrade, H.; Patel, N.; Shah, R.; Shaikh, R.; Capriles, C.A.A. Lipid lowering therapy: An era beyond statins. Curr. Probl. Cardiol. 2022, 47, 101342. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Guo, M.; Chai, H.; Wang, W.T.; Gao, Z.Y.; Shi, D.Z. Effects of coenzyme Q10 on statin-induced myopathy: An updated meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2018, 7, e009835. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Mikhailidis, D.P. Statin intolerance: Some practical hints. Cardiol. Clin. 2018, 36, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Rizzo, M.; Toth, P.P.; Farnier, M.; Davidson, M.H.; Al-Rasadi, K.; Aronow, W.S.; Athyros, V.; Djuric, D.M.; Ezhov, M.V. Statin intolerance—An attempt at a unified definition. Position paper from an International Lipid Expert Panel: This paper is also published in parallel in Archives of Medical Science [Banach M, Rizzo M, Toth PP; et al. Statin intolerance–an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci 2015; 11 (1): 1–23]. Expert Opin. Drug Saf. 2015, 14, 935–955. [Google Scholar] [PubMed]
- Chen, Z.-Y.; Jiao, R.; Ma, K.Y. Cholesterol-lowering nutraceuticals and functional foods. J. Agric. Food Chem. 2008, 56, 8761–8773. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Qin, X.; Yuan, F.; Hu, M.; Chen, G.; Fang, K.; Wang, D.; Jiang, S.; Li, J.; Zhao, Y. Efficacy and safety of sugarcane policosanol on dyslipidemia: A meta-analysis of randomized controlled trials. Mol. Nutr. Food Res. 2018, 62, 1700280. [Google Scholar] [CrossRef] [PubMed]
- Askarpour, M.; Ghaedi, E.; Roshanravan, N.; Hadi, A.; Mohammadi, H.; Symonds, M.E.; Miraghajani, M. Policosanol supplementation significantly improves blood pressure among adults: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 45, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, L.K.; Jimoh, A.O.; Tukur, U.M.; Imam, M.U. A review of the effects of policosanol on metabolic syndrome. Clin. Complement. Med. Pharmacol. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Guo, Y.-L.; Xu, R.-X.; Zhu, C.-G.; Wu, N.-Q.; Cui, Z.-P.; Li, J.-J. Policosanol attenuates statin-induced increases in serum proprotein convertase subtilisin/kexin type 9 when combined with atorvastatin. Evid.-Based Complement. Altern. Med. 2014, 2014, 926087. [Google Scholar]
- Al-Miahy, A.J.; Alkalby, J.M. Effect of Policosanol Extract and Simvastatin On Some Liver Enzymes And Histopathology In Hypercholestrolemic Female Rats During Lactation. J. Educ. Pure Sci. 2018, 8, 43–56. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Jiao, Q.-P.; Chen, S.-Y.; Sheng, J.; Jiang, H.; Lu, J.; Zheng, S.-B.; Fang, N.-Y. Efficacy and safety of Policosanol plus Fenofibrate combination therapy in elderly patients with mixed Dyslipidemia: A randomized, controlled clinical study. Am. J. Med. Sci. 2018, 356, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Nam, H.-S.; Kim, N.-Y.; Lee, M.-S.; Kang, D.-J. Combination Therapy of Cuban Policosanol (Raydel®, 20 mg) and Intensive Exercise for 12 Weeks Resulted in Improvements in Obesity, Hypertension, and Dyslipidemia without a Decrease in Serum Coenzyme Q10: Enhancement of Lipoproteins Quality and Antioxidant Functionality in Obese Participants. Pharmaceuticals 2024, 17, 132. [Google Scholar] [PubMed]
- Cho, K.-H.; Kim, S.-J.; Yadav, D.; Kim, J.-Y.; Kim, J.-R. Consumption of Cuban policosanol improves blood pressure and lipid profile via enhancement of HDL functionality in healthy women subjects: Randomized, double-blinded, and placebo-controlled study. Oxidative Med. Cell. Longev. 2018, 2018, 4809525. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-Y.; Yoo, J.-A.; Lim, S.-M.; Cho, K.-H. Anti-aging and tissue regeneration ability of policosanol along with lipid-lowering effect in hyperlipidemic zebrafish via enhancement of high-density lipoprotein functionality. Rejuvenation Res. 2016, 19, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-M.; Yoo, J.-A.; Lee, E.-Y.; Cho, K.-H. Enhancement of high-density lipoprotein cholesterol functions by encapsulation of policosanol exerts anti-senescence and tissue regeneration effects via improvement of anti-glycation, anti-apoptosis, and cholesteryl ester transfer inhibition. Rejuvenation Res. 2016, 19, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Nam, H.-S.; Baek, S.-H.; Kang, D.-J.; Na, H.; Komatsu, T.; Uehara, Y. Beneficial effect of Cuban policosanol on blood pressure and serum lipoproteins accompanied with lowered glycated hemoglobin and enhanced high-density lipoprotein functionalities in a randomized, placebo-controlled, and double-blinded trial with healthy Japanese. Int. J. Mol. Sci. 2023, 24, 5185. [Google Scholar] [PubMed]
- Uehara, Y.; Komatsu, T.; Sasaki, K.; Abe, S.; Nakashima, S.; Yamamoto, T.; Kim, J.-E.; Cho, K.-H. Cuban policosanol improves high-density lipoprotein cholesterol efflux capacity in healthy Japanese subjects. Front. Nutr. 2024, 10, 1297008. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.-E.; Yun, J.-M.; Kim, D.; Kim, O.-K. Policosanol attenuates cholesterol synthesis via AMPK activation in hypercholesterolemic rats. J. Med. Food 2019, 22, 1110–1117. [Google Scholar] [CrossRef]
- Canavaciolo, V.L.G.; Gómez, C.V. “Copycat-policosanols” versus genuine policosanol. Rev. CENIC Cienc. Quím. 2007, 38, 207–213. [Google Scholar]
- Cho, K.-H.; Baek, S.H.; Nam, H.-S.; Kim, J.-E.; Kang, D.-J.; Na, H.; Zee, S. Cuban Sugar Cane Wax Alcohol Exhibited Enhanced Antioxidant, Anti-Glycation and Anti-Inflammatory Activity in Reconstituted High-Density Lipoprotein (rHDL) with Improved Structural and Functional Correlations: Comparison of Various Policosanols. Int. J. Mol. Sci. 2023, 24, 3186. [Google Scholar] [CrossRef] [PubMed]
- Harrabi, S.; Ferchichi, A.; Bacheli, A.; Fellah, H. Policosanol composition, antioxidant and anti-arthritic activities of milk thistle (Silybium marianum L.) oil at different seed maturity stages. Lipids Health Dis. 2018, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-E.; Lee, M.-S.; Bahuguna, A. Cuban Policosanol (Raydel®) Exerts Higher Antioxidant and Anti-Glycation Activities than Chinese Policosanol (BOC Sciences) in Reconstituted High-Density Lipoproteins: In Vivo Anti-Inflammatory Activities in Zebrafish and Its Embryos. Pharmaceuticals 2024, 17, 406. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-E.; Nam, H.-S.; Baek, S.-H.; Bahuguna, A. Consumption of policosanol (Raydel®) improves hepatic, renal, and reproductive functions in zebrafish: In Vivo comparison study among Cuban, Chinese, and American policosanol. Pharmaceuticals 2023, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.Y.; Kim, B.; Seo, W.D.; Jia, Y.; Wu, C.; Jun, H.-J.; Lee, S.-J. Barley sprout extract containing policosanols and polyphenols regulate AMPK, SREBP2 and ACAT2 activity and cholesterol and glucose metabolism in vitro and in vivo. Food Res. Int. 2015, 72, 174–183. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Choi, H.-Y.; Kang, Y.-R.; Chang, H.-B.; Chun, H.-S.; Lee, M.-S.; Kwon, Y.-I. Effects of long-term supplementation of policosanol on blood cholesterol/glucose levels and 3-hydroxy-3-methylglutaryl coenzyme a reductase activity in a rat model fed high cholesterol diets. Food Sci. Biotechnol. 2016, 25, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Matsuzaka, T.; Kaushik, M.K.; Sugasawa, T.; Ohno, H.; Wang, Y.; Motomura, K.; Shimura, T.; Okajima, Y.; Mizunoe, Y. Octacosanol and policosanol prevent high-fat diet-induced obesity and metabolic disorders by activating brown adipose tissue and improving liver metabolism. Sci. Rep. 2019, 9, 5169. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Luo, J.; Ma, Z.; Sun, Q.; Wu, C.; Li, X. Quality and authenticity control of functional red yeast rice—A review. Molecules 2019, 24, 1944. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, X.; Duan, W.; Wang, X.; Du, J. Accelerated solvent extraction of monacolin K from red yeast rice and purification by high-speed counter-current chromatography. J. Chromatogr. B 2010, 878, 2881–2885. [Google Scholar] [CrossRef] [PubMed]
- Vrolijk, M.F.; van de Koppel, S.; van Hunsel, F. Red yeast rice (Monascus purpureus) supplements: Case series assessment of spontaneously reported cases to The Netherlands Pharmacovigilance Centre Lareb. Br. J. Clin. Pharmacol. 2021, 87, 2146–2151. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi Pharmaceutical Voluntarily Recalls Health Foods Containing “Red Koji” Ingredients. Available online: https://www3.nhk.or.jp/news/html/20240322/k10014399441000.html (accessed on 23 March 2024).
- Japan Recalls ‘Red Rice’ Health Products over Suspected Link to Five Deaths. Available online: https://edition.cnn.com/2024/03/28/asia/japan-red-rice-recall-hnk-intl/index.html (accessed on 29 March 2024).
- Japan Orders Recall of ‘Red Rice’ Health Products Amid Suspected Deaths. Available online: https://timesofindia.indiatimes.com/world/rest-of-world/japan-orders-recall-of-red-rice-health-products-amid-suspected-deaths/articleshow/108849589.cms (accessed on 28 March 2024).
- Ónody, A.; Csonka, C.; Giricz, Z.; Ferdinandy, P. Hyperlipidemia induced by a cholesterol-rich diet leads to enhanced peroxynitrite formation in rat hearts. Cardiovasc. Res. 2003, 58, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Luo, F.; Lin, Q. Policosanol: Extraction and biological functions. J. Funct. Foods 2019, 57, 351–360. [Google Scholar] [CrossRef]
- Ng, C.H.; Leung, K.Y.; Huang, Y.; Chen, Z.Y. Policosanol has no antioxidant activity in human low-density lipoprotein but increases excretion of bile acids in hamsters. J. Agric. Food Chem. 2005, 53, 6289–6293. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Yadav, D.; Jeong, D.-J.; Kim, S.-J.; Bae, M.-A.; Kim, J.-R.; Cho, K.-H. Short-term consumption of Cuban policosanol lowers aortic and peripheral blood pressure and ameliorates serum lipid parameters in healthy Korean participants: Randomized, double-blinded, and placebo-controlled study. Int. J. Environ. Res. Public Health 2019, 16, 809. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.J.; Sniderman, A.D.; Ditmarsch, M.; Dicklin, M.R.; Nicholls, S.J.; Davidson, M.H.; Kastelein, J.J.P. Cholesteryl Ester Transfer Protein Inhibition Reduces Major Adverse Cardiovascular Events by Lowering Apolipoprotein B Levels. Int. J. Mol. Sci. 2022, 23, 9417. [Google Scholar] [CrossRef] [PubMed]
- Teratani, T.; Tomita, K.; Suzuki, T.; Oshikawa, T.; Yokoyama, H.; Shimamura, K.; Tominaga, S.; Hiroi, S.; Irie, R.; Okada, Y. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology 2012, 142, 152–164.e10. [Google Scholar] [CrossRef] [PubMed]
- Loubser, L.; Weider, K.I.; Drake, S.M. Acute liver injury induced by red yeast rice supplement. BMJ Case Rep. CP 2019, 12, e227961. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Kim, J.-H.; Lim, D.-K.; Suh, Y.-H.; Chang, K.-A. Long-term treatment of Cuban policosanol attenuates abnormal oxidative stress and inflammatory response via amyloid plaques reduction in 5xFAD Mice. Antioxidants 2021, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zheng, X.-L.; Yang, L.; Shi, F.; Gao, L.-B.; Zhong, Y.-J.; Sun, H.; He, F.; Lin, Y.; Wang, X. Reactive oxygen species-mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin-induced cytotoxicity in cancer cells. J. Exp. Clin. Cancer Res. 2010, 29, 159. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, M.; Huang, Q.; Xie, L.; Huang, Z. Monacolin K Induces Apoptosis of Human Glioma U251 Cells by Triggering ROS-Mediated Oxidative Damage and Regulating MAPKs and NF-κB Pathways. ACS Chem. Neurosci. 2023, 14, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-E.; Baek, S.H. Cuban policosanol (Raydel®) potently protects the liver, ovary, and testis with an improvement in dyslipidemia in hyperlipidemic zebrafish: A comparative study with three Chinese policosanols. Molecules 2023, 28, 6609. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Flensted-Jensen, M.; Oró, D.; Rørbeck, E.A.; Zhang, C.; Madsen, M.R.; Madsen, A.N.; Norlin, J.; Feigh, M.; Larsen, S.; Hansen, H.H. Dietary intervention reverses molecular markers of hepatocellular senescence in the GAN diet-induced obese and biopsy-confirmed mouse model of NASH. BMC Gastroenterol. 2024, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Sahadevan, M.; Kasiske, B.L. Hyperlipidemia in kidney disease causes and consequences. Curr. Opin. Nephrol. Hypertens. 2002, 11, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Çiftci, G.; Tuna, E. Effects of cholesterol and Lactobacillus acidophilus on testicular function. Clin. Exp. Reprod. Med. 2021, 48, 229. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Chang, T.-C.; Lin, S.-H.; Wu, S.-T.; Cha, T.-L.; Tsao, C.-W. Metformin ameliorates testicular function and spermatogenesis in male mice with high-fat and high-cholesterol diet-induced obesity. Nutrients 2020, 12, 1932. [Google Scholar] [CrossRef] [PubMed]
- Elnagar, G.M.; Elseweidy, M.M.M.; Elkomy, N.M.; Keshawy, M.M.; Fathy, O.M.; Sobh, M.S.; Mahmoud, Y.K. Policosanol ameliorates renal inflammation and pyroptosis in hypercholesterolemic rabbits via modulation of HMGB1/PI3K/mTOR/NLRP3/Caspase-1 pathway. J. Funct. Foods 2022, 97, 105250. [Google Scholar] [CrossRef]
- Peterslund, P.; Christensen, H.D.; Urbahnke, J.; Cappeln, A. Red yeast rice as the presumed cause of acute kidney and liver failure. Ugeskr. Laeger 2019, 181, V02190107. [Google Scholar] [PubMed]
- Rodríguez, M.D.; García, H. Teratogenic and reproductive studies of policosanol in the rat and rabbit. Teratog. Carcinog. Mutagen. 1994, 14, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-E.; Bahuguna, A.; Kang, D.-J. Long-term supplementation of ozonated sunflower oil improves dyslipidemia and hepatic inflammation in hyperlipidemic zebrafish: Suppression of oxidative stress and inflammation against carboxymethyllysine toxicity. Antioxidants 2023, 12, 1240. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, pdb-prot4986. [Google Scholar] [CrossRef]
- Shull, L.C.; Sen, R.; Menzel, J.; Goyama, S.; Kurokawa, M.; Artinger, K.B. The conserved and divergent roles of Prdm3 and Prdm16 in zebrafish and mouse craniofacial development. Dev. Biol. 2020, 461, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Bahuguna, A.; Kang, D.-J.; Kim, J.-E. Prolonged Supplementation of Ozonated Sunflower Oil Bestows an Antiaging Effect, Improves Blood Lipid Profile and Spinal Deformities, and Protects Vital Organs of Zebrafish (Danio rerio) against Age-Related Degeneration: Two-Years Consumption Study. Antioxidants 2024, 13, 123. [Google Scholar] [CrossRef] [PubMed]
| Product Code | Product Manufacturer Name and Country | Country of Origin (Source Material) | Source Material | Major Dose | Additive |
|---|---|---|---|---|---|
| PCO1 | Raydel-policosanol, Thornleigh, NSW, Australia | Cuba | Sugarcane Wax | Policosanol 20 mg | None |
| PCO2 | Solgar-policosanol, Leonia, NJ, USA | USA | ND | Policosanol 20 mg | None |
| PCO3 | NutrioneLife-monacosanol, Seoul, South Korea | USA and India | ND | Octacosanol (1) 12 mg | RYR (2) 5 mg |
| PCO4 | Mothernest-policosanol, Seven Hills, NSW, Australia | Australia | Sugarcane | Wax alcohol 20 mg | None |
| PCO5 | Peter & John-policosanol, Auckland, New Zealand | New Zealand | ND | Policosanol 33.4 mg (octacosanol 20 mg) | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Bahuguna, A.; Kim, J.-E.; Lee, S.H. Efficacy Assessment of Five Policosanol Brands and Damage to Vital Organs in Hyperlipidemic Zebrafish by Six-Week Supplementation: Highlighting the Toxicity of Red Yeast Rice and Safety of Cuban Policosanol (Raydel®). Pharmaceuticals 2024, 17, 714. https://doi.org/10.3390/ph17060714
Cho K-H, Bahuguna A, Kim J-E, Lee SH. Efficacy Assessment of Five Policosanol Brands and Damage to Vital Organs in Hyperlipidemic Zebrafish by Six-Week Supplementation: Highlighting the Toxicity of Red Yeast Rice and Safety of Cuban Policosanol (Raydel®). Pharmaceuticals. 2024; 17(6):714. https://doi.org/10.3390/ph17060714
Chicago/Turabian StyleCho, Kyung-Hyun, Ashutosh Bahuguna, Ji-Eun Kim, and Sang Hyuk Lee. 2024. "Efficacy Assessment of Five Policosanol Brands and Damage to Vital Organs in Hyperlipidemic Zebrafish by Six-Week Supplementation: Highlighting the Toxicity of Red Yeast Rice and Safety of Cuban Policosanol (Raydel®)" Pharmaceuticals 17, no. 6: 714. https://doi.org/10.3390/ph17060714
APA StyleCho, K.-H., Bahuguna, A., Kim, J.-E., & Lee, S. H. (2024). Efficacy Assessment of Five Policosanol Brands and Damage to Vital Organs in Hyperlipidemic Zebrafish by Six-Week Supplementation: Highlighting the Toxicity of Red Yeast Rice and Safety of Cuban Policosanol (Raydel®). Pharmaceuticals, 17(6), 714. https://doi.org/10.3390/ph17060714








