Pichia pastoris Mediated Digestion of Water-Soluble Polysaccharides from Cress Seed Mucilage Produces Potent Antidiabetic Oligosaccharides
Abstract
1. Introduction
2. Results and Discussion
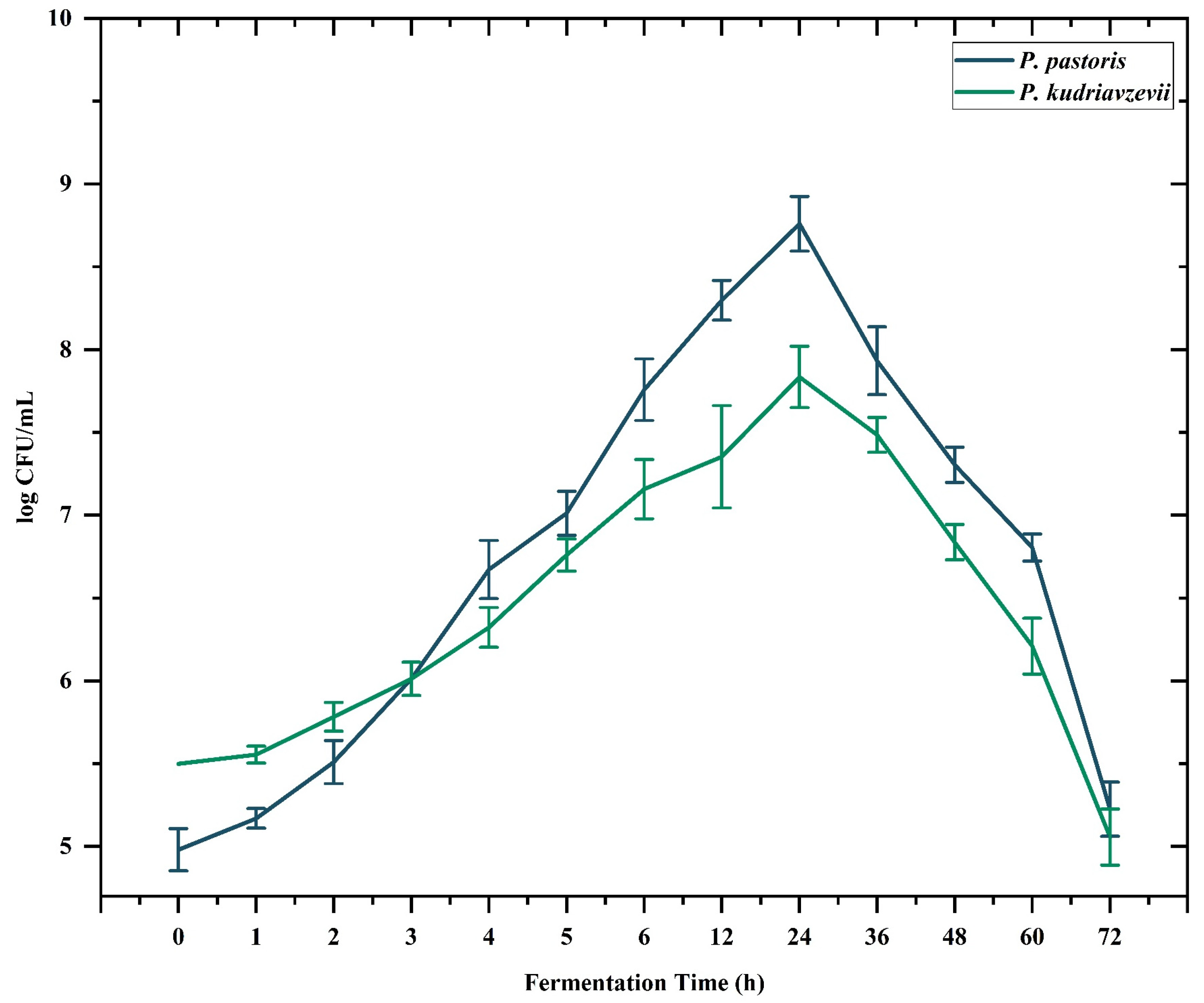
2.1. Microbial Digestion of Polysaccharides Obtained from Cress Seed Mucilage
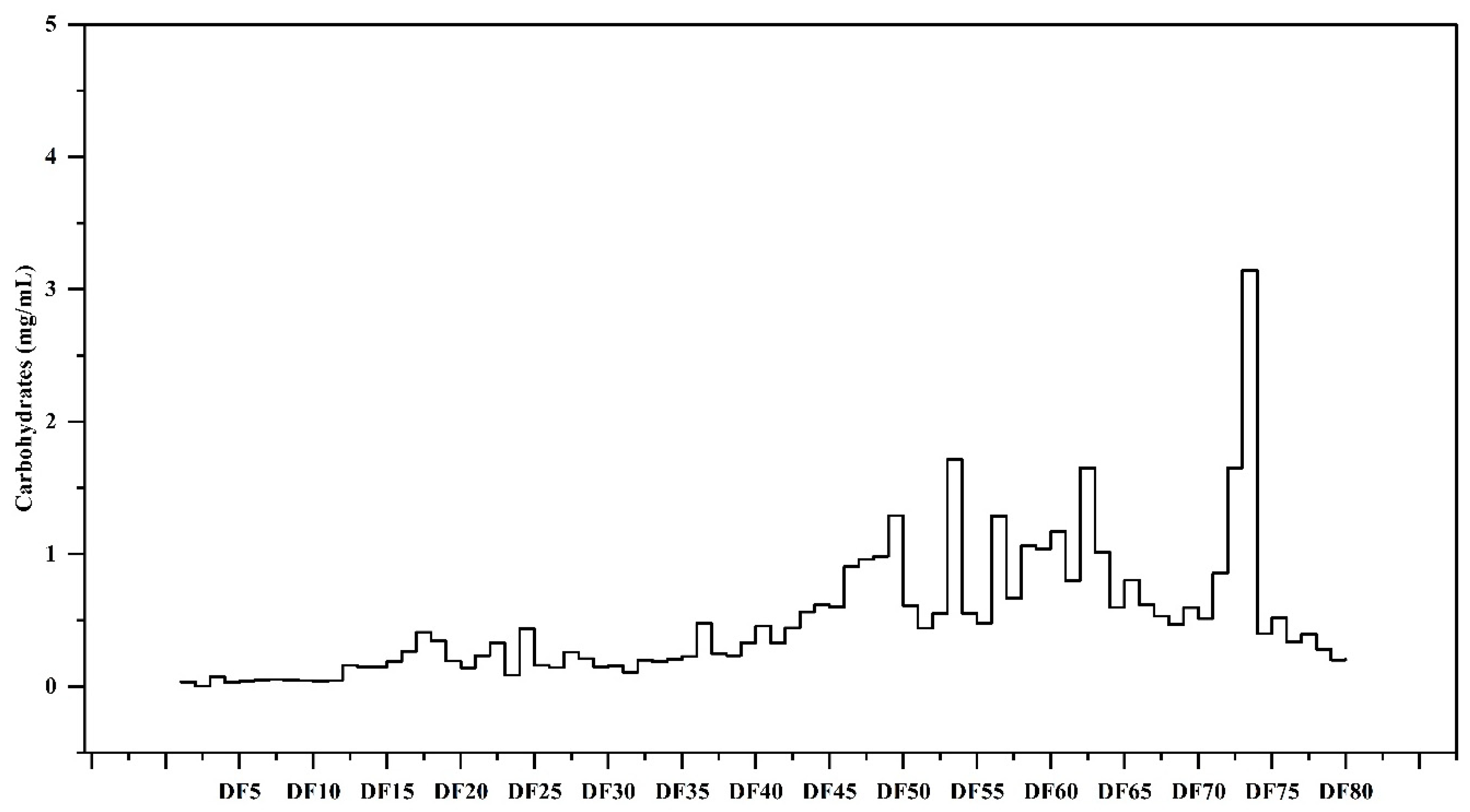
2.2. Total Carbohydrates Content of the GPC Fractionated Saccharides
2.3. Monosaccharides and Disaccharides Composition of Oligosaccharide Fractions
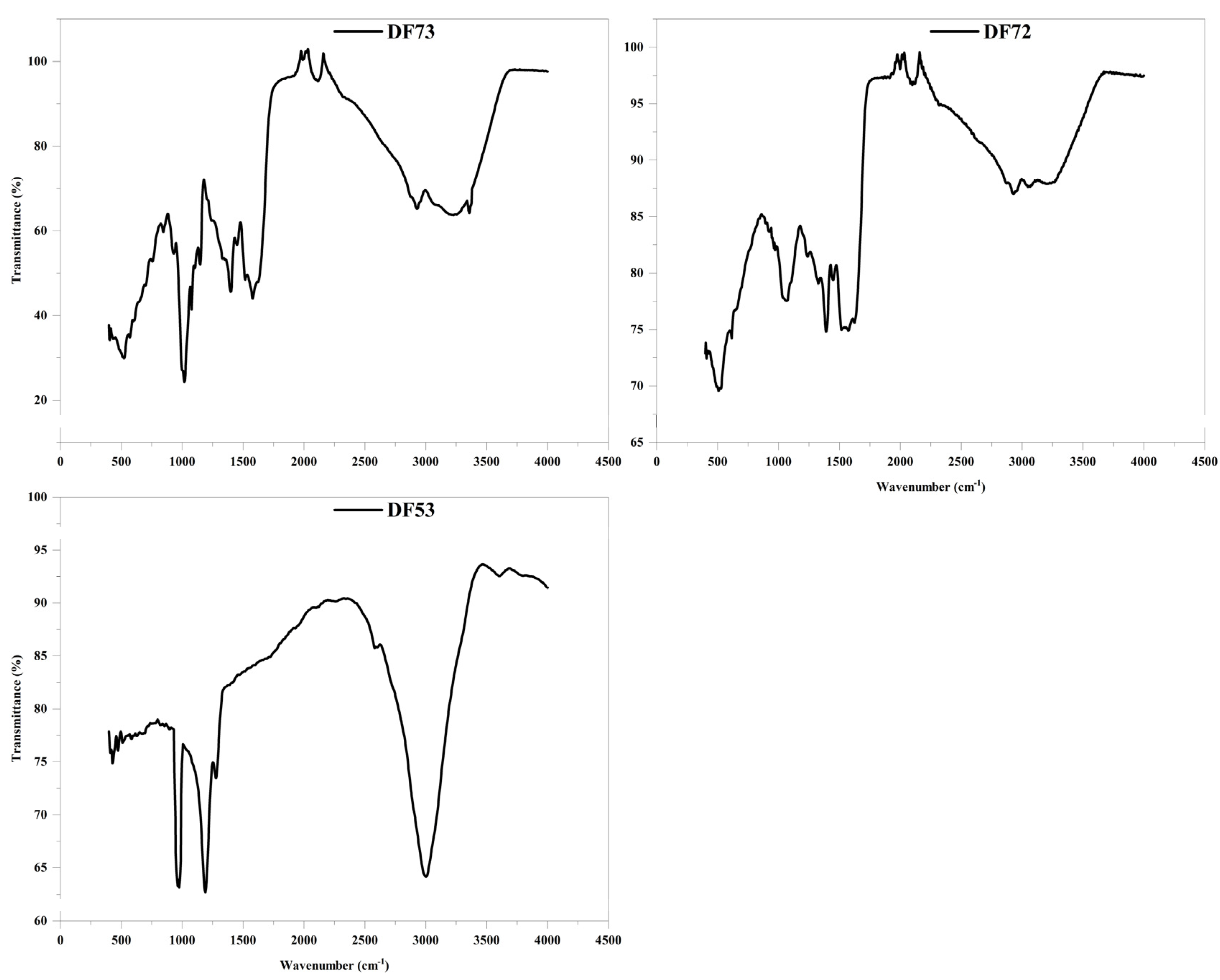
2.4. IR Spectra of Oligosaccharide Fractions
2.5. In Vitro Alpha-Amylase Inhibition Activity of the Fractions
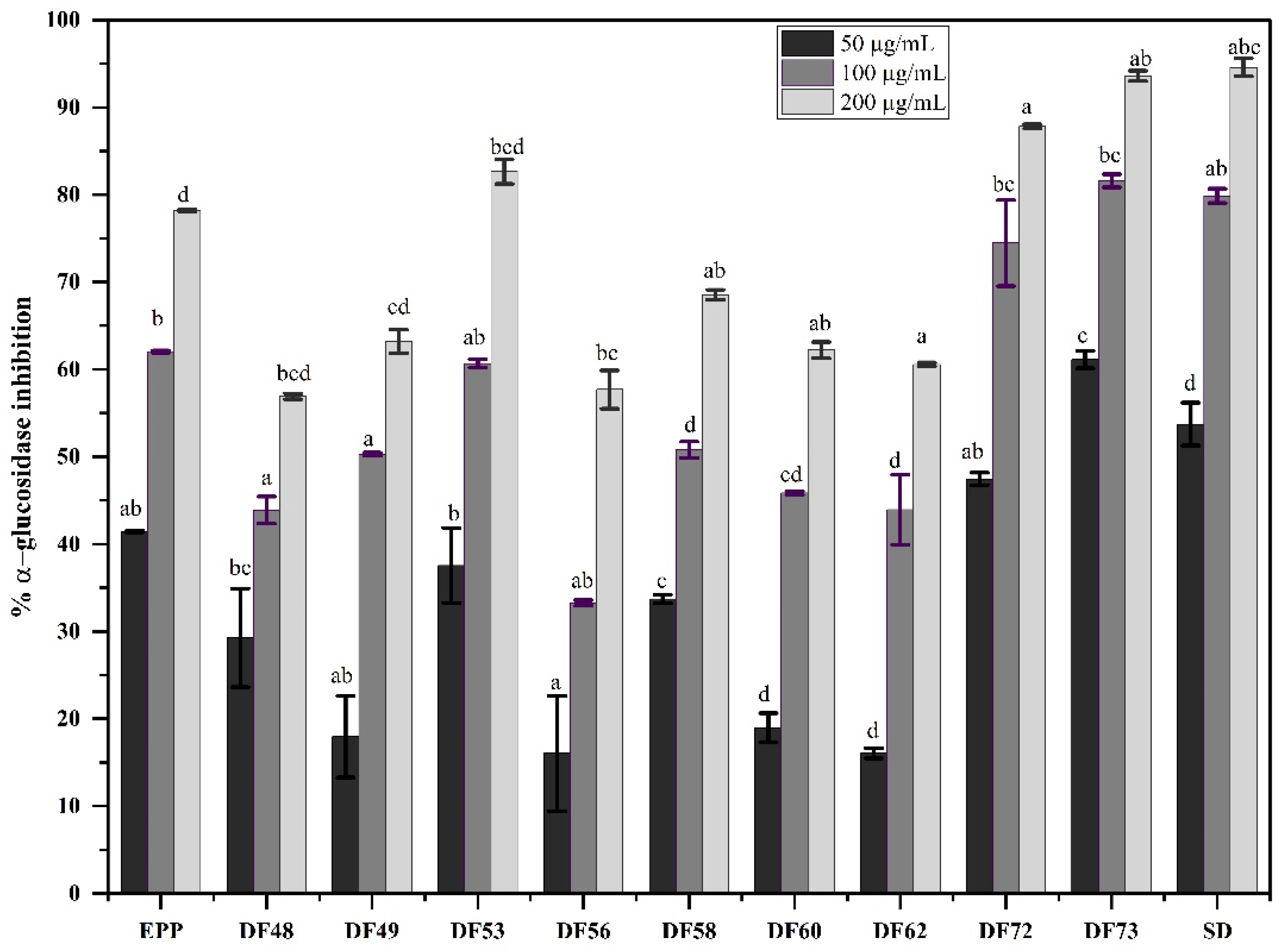
2.6. In Vitro Alpha-Glucosidase Inhibition Potential of Oligosaccharide Fractions
2.7. Hypoglycemic Effect of Oligosaccharide Fractions
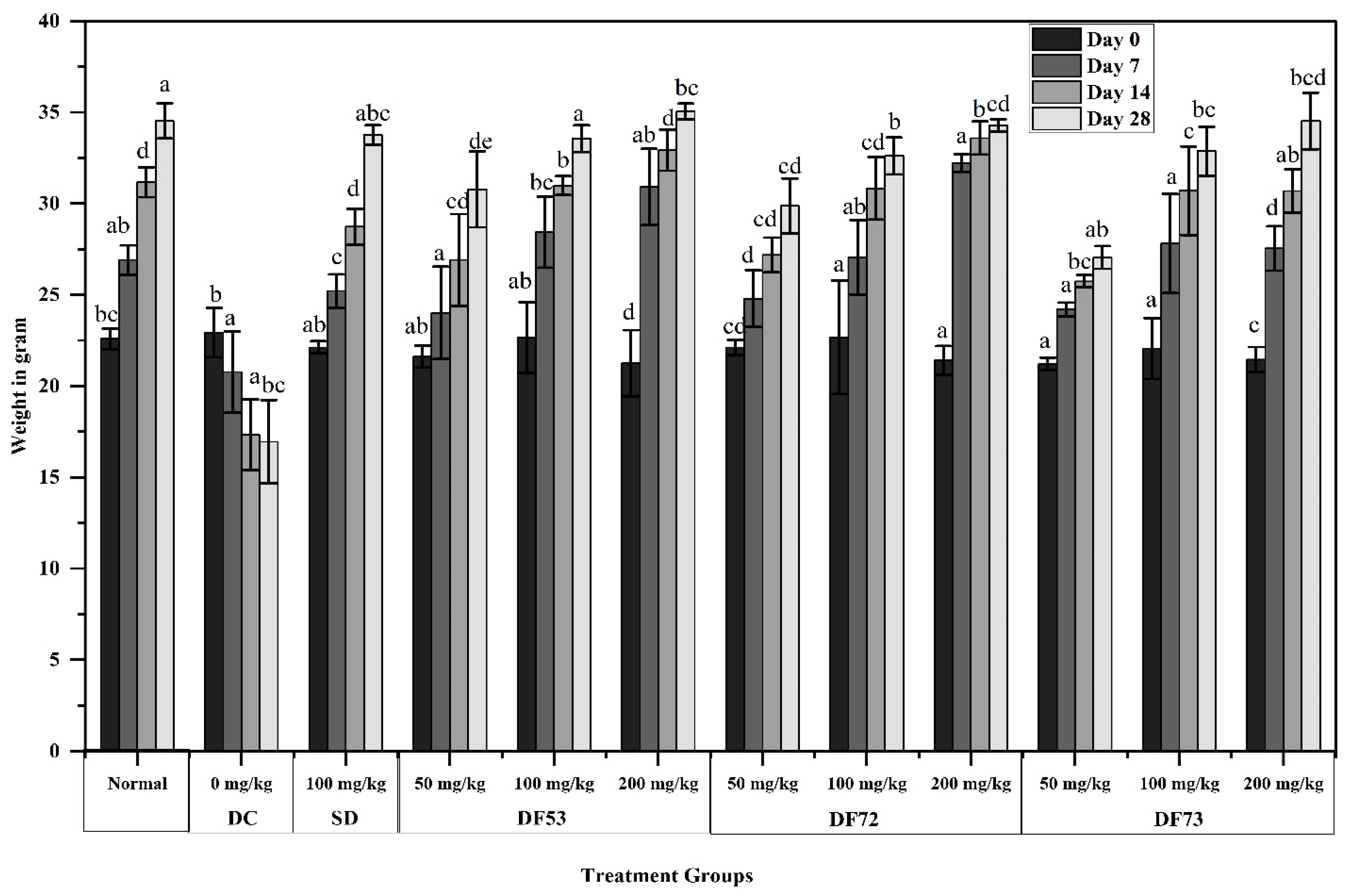
2.8. Effect of Oligosaccharide Treatment on the Weight of STZ-Induced Diabetic Mice
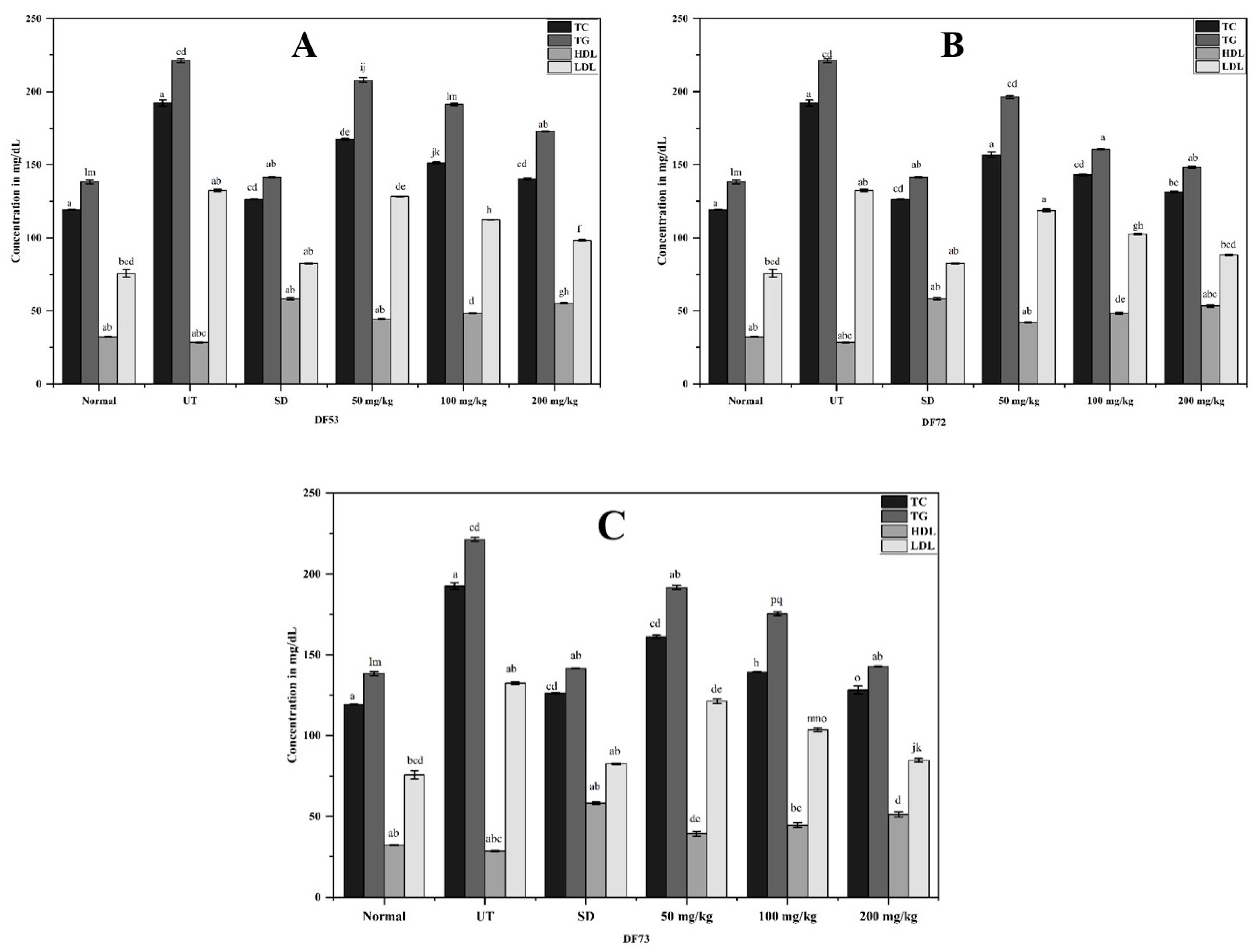
2.9. Effect of Oligosaccharides Fraction on Blood Lipid Profile
2.10. Effect of Oligosaccharide Fractions on Blood Serum Profiles
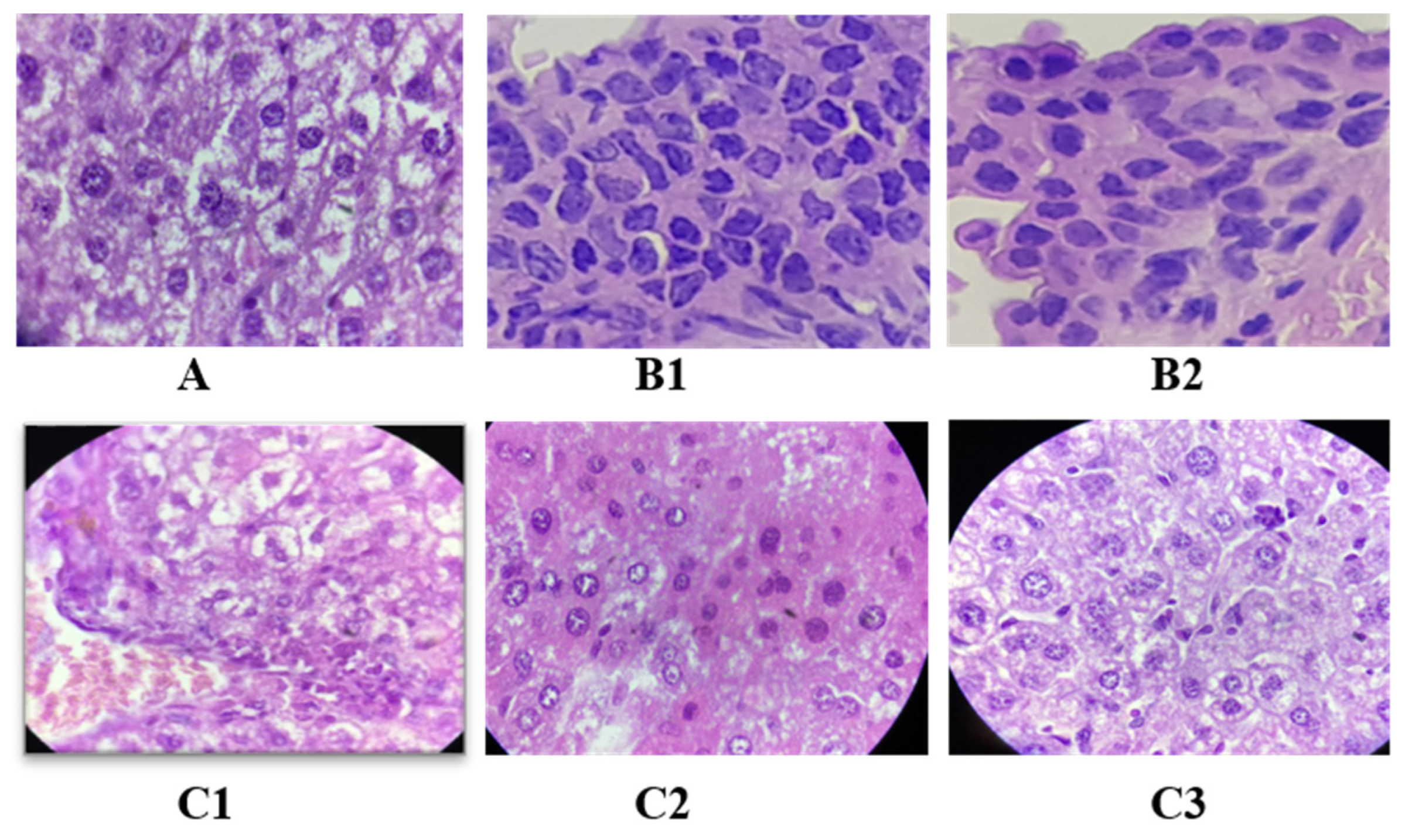
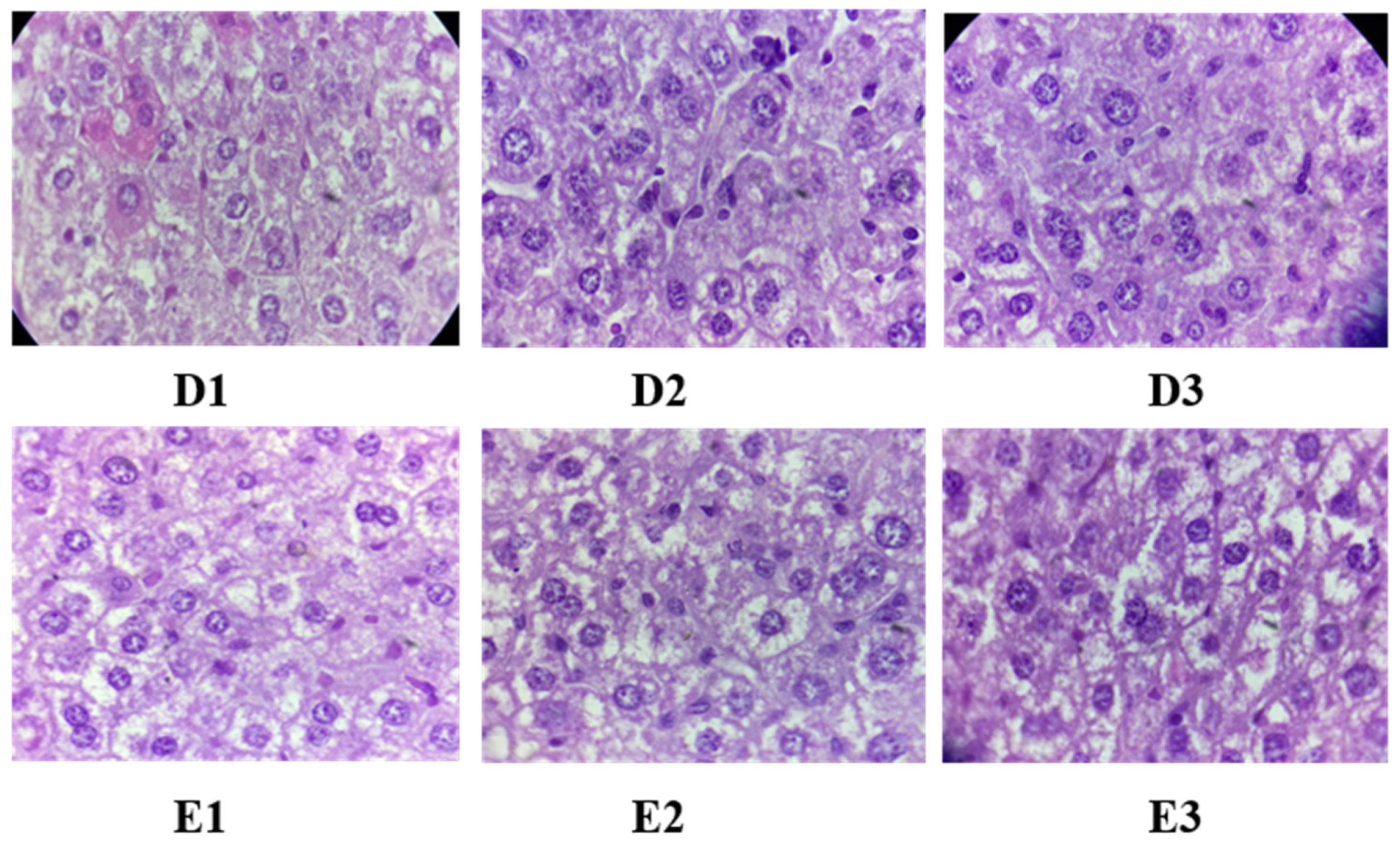
2.11. Effect of Oligosaccharide Fractions on Liver Histology
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Polysaccharide Extraction
3.3. In Vitro Fermentation Using Yeast Strains
3.4. Gel Permeation Chromatography (GPC)
3.5. Biochemical Analysis
3.6. Fourier Transformed Infrared (FT-IR) Spectroscopy
3.7. Gas Chromatography-Mass Spectrometry (GC-MS)
3.8. In Vitro α-Amylase Inhibition Assay
3.9. In Vitro α-Glucosidase Inhibition Assay
3.10. In Vivo Antidiabetic Assay in Streptozotocin-Induced Diabetic Mice
3.10.1. Induction of Diabetes
3.10.2. Experimental Design
3.11. Blood Biochemical Analyses
3.12. Histopathology of Mouse Livers
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| BGL | Blood glucose levels |
| DC | Diabetic control |
| DNS | dinitrosalicylic acid |
| EPP | Ethanol precipitated polysaccharide |
| FBGL | Fasting blood glucose level |
| FT-IR | Fourier transform infrared |
| GC-MS | Gas chromatography-mass spectrometry |
| GLP | Good Laboratory Practices |
| GPC | Gel permeation chromatography |
| H&E | Hematoxylin and eosin |
| HDL | High-density lipoproteins |
| HPLC | High-Performance Liquid Chromatography |
| IC50 | Half-maximal inhibitor concentration |
| IR | Infrared spectroscopy |
| LDL | Low-density lipoproteins |
| NH4Cl | Ammonium chloride |
| PSA | Phenol–sulfuric acid |
| RBPS | Rice brain polysaccharides |
| SGOT | Serum glutamic-oxaloacetic transaminase |
| SGPT | Serum glutamic-pyruvic transaminase |
| STZ | Streptozotocin |
| T2DM | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TCC | Total carbohydrates content |
| TG | Total glycerides |
| UV | Ultraviolet |
References
- Association, A.D. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S7–S14. [Google Scholar] [CrossRef]
- Aschner, P.; Karuranga, S.; James, S.; Simmons, D.; Basit, A.; Shaw, J.E.; Wild, S.H.; Ogurtsova, K.; Saeedi, P. The International Diabetes Federation’s guide for diabetes epidemiological studies. Diabetes Res. Clin. Pract. 2021, 172, 108630. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5,6 & 6,6)-oxa-oxa annulated sugars as glycosidase inhibitors from 2-formyl galactal using iodocyclization as a key step. Arkivoc 2022, 2022, 5–23. [Google Scholar]
- Tseng, P.S.; Ande, C.; Moremen, K.W.; Crich, D. Influence of side chain conformation on the activity of glycosidase inhibitors. Angew. Chem. 2023, 135, e202217809. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Q.; Cao, J.; Xu, Y.; Pei, Z.; Fan, H.; Yuan, Y.; Shen, X.; Li, C. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in Goto-Kakizaki rats. Food Chem. Toxicol. 2020, 135, 110886. [Google Scholar] [CrossRef]
- Rajput, R.; Kumar, K. Protective effect of ethanolic extract of guava leaves (Psidium guajava L.) in alloxan-induced diabetic mice. Mater. Today Proc. 2021, 47, 437–439. [Google Scholar] [CrossRef]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic phytochemicals from medicinal plants: Prospective candidates for new drug discovery and development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.J.B.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Song, J.; Chen, M.; Gong, P.; Jia, W.; Li, G. Hypoglycemic effects of Auricularia auricula polysaccharides on high fat diet and streptozotocin-induced diabetic mice using metabolomics analysis. Food Funct. 2021, 12, 9994–10007. [Google Scholar] [CrossRef]
- Han, R.; Pang, D.; Wen, L.; You, L.; Huang, R.; Kulikouskaya, V. In vitro digestibility and prebiotic activities of a sulfated polysaccharide from Gracilaria Lemaneiformis. J. Funct. Foods 2020, 64, 103652. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Aweya, J.J.; Li, N.; Deng, R.-Y.; Chen, W.-Y.; Tang, J.; Cheong, K.-L. Microbial catabolism of Porphyra haitanensis polysaccharides by human gut microbiota. Food Chem. 2019, 289, 177–186. [Google Scholar] [CrossRef]
- Kučka, M.; Ražná, K.; Harenčár, Ľ.; Kolarovičová, T. Plant seed mucilage—Great potential for sticky matter. Nutraceuticals 2022, 2, 253–269. [Google Scholar] [CrossRef]
- Barzegar, H.; Alizadeh Behbahani, B.; Mehrnia, M.A. Quality retention and shelf life extension of fresh beef using Lepidium sativum seed mucilage-based edible coating containing Heracleum lasiopetalum essential oil: An experimental and modeling study. Food Sci. Biotechnol. 2020, 29, 717–728. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Oraby, H.F. Lepidium sativum seeds: Therapeutic significance and health-promoting potential. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–289. [Google Scholar]
- Al-Snafi, A.E. Chemical constituents and pharmacological effects of lepidium sativum. Int. J. Curr. Pharm. Res. 2019, 11, 1–10. [Google Scholar] [CrossRef]
- Moniri, H.; Farahmandfar, R.; Motamedzadegan, A. Cress seed (Lepidium sativum) gum dried by vacuum, freeze, and microwave drying methods: Structural, rheological, emulsifying, and foaming properties. J. Food Process Eng. 2020, 43, e13408. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Huo, D.; Cao, C.; Li, Y.; Liang, Y.; Li, B.; Li, L. Preliminary characterization, antioxidant and α-glucosidase inhibitory activities of polysaccharides from Mallotus furetianus. Carbohydr. Polym. 2019, 215, 307–315. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, B.; Alonso, J.L.; Labidi, J.; Gullón, P. Vine shoots as new source for the manufacture of prebiotic oligosaccharides. Carbohydr. Polym. 2019, 207, 34–43. [Google Scholar] [CrossRef]
- Bhatia, L.; Sharma, A.; Bachheti, R.K.; Chandel, A.K. Lignocellulose derived functional oligosaccharides: Production, properties, and health benefits. Prep. Biochem. Biotechnol. 2019, 49, 744–758. [Google Scholar] [CrossRef]
- Rebnegger, C.; Vos, T.; Graf, A.B.; Valli, M.; Pronk, J.T.; Daran-Lapujade, P.; Mattanovich, D. Pichia pastoris exhibits high viability and a low maintenance energy requirement at near-zero specific growth rates. Appl. Environ. Microbiol. 2016, 82, 4570–4583. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, X.; Zhuang, X.; Han, W.; Guo, W.; Xiong, J.; Zhang, X. Rice bran polysaccharides and oligosaccharides modified by Grifola frondosa fermentation: Antioxidant activities and effects on the production of NO. Food Chem. 2017, 223, 49–53. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Wu, J.; Weng, L.; Zhang, H.; Zhang, J.; Wu, A. An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydr. Polym. 2020, 227, 115332. [Google Scholar] [CrossRef]
- Oh, S.; Kim, D.-Y. Characterization, antioxidant activities, and functional properties of mucilage extracted from corchorus olitorius L. Polymers 2022, 14, 2488. [Google Scholar] [CrossRef]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; Lo Curto, R.B.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, chemical, and biochemical characterization of cladodes from prickly pear [Opuntia ficus-indica (L.) Mill.]. J. Agric. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef]
- Koocheki, A.; Hesarinejad, M.A.; Mozafari, M.R. Lepidium perfoliatum seed gum: Investigation of monosaccharide composition, antioxidant activity and rheological behavior in presence of salts. Chem. Biol. Technol. Agric. 2022, 9, 61. [Google Scholar] [CrossRef]
- Duran, H.; Yavuz, E.; Sismanoglu, T.; Senkal, B.F. Functionalization of gum arabic including glycoprotein and polysaccharides for the removal of boron. Carbohydr. Polym. 2019, 225, 115139. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.-X.; Cao, Y.-J.; Lin, Y.-C.; Guo, W.-L.; Liu, J.-Y.; Bai, W.-D.; Zhang, Y.-Y.; Ni, L.; Liu, B.; et al. Preparation of Ganoderma lucidum polysaccharide-chromium(III) complex and its hypoglycemic and hypolipidemic activities in high-fat and high-fructose diet-induced pre-diabetic mice. Int. J. Biol. Macromol. 2019, 140, 782–793. [Google Scholar] [CrossRef]
- Zha, S.; Zhao, Q.; Chen, J.; Wang, L.; Zhang, G.; Zhang, H.; Zhao, B. Extraction, purification and antioxidant activities of the polysaccharides from maca (Lepidium meyenii). Carbohydr. Polym. 2014, 111, 584–587. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.-Y.; Nie, S.-P.; Li, C.; Wang, Y.-X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Ren, G.; Xu, L.; Lu, T.; Yin, J. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int. J. Biol. Macromol. 2018, 115, 1202–1210. [Google Scholar] [CrossRef]
- Telagari, M.; Hullatti, K. In-vitro α-amylase and α-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar]
- Rafique, R.; Khan, K.M.; Arshia; Kanwal; Chigurupati, S.; Wadood, A.; Rehman, A.U.; Karunanidhi, A.; Hameed, S.; Taha, M.; et al. Synthesis of new indazole based dual inhibitors of α-glucosidase and α-amylase enzymes, their in vitro, in silico and kinetics studies. Bioorg. Chem. 2020, 94, 103195. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Li, C.; Huang, Q.; You, L.-J.; Chen, C.; Fu, X.; Liu, R.H. Comparative study on the physicochemical properties and bioactivities of polysaccharide fractions extracted from Fructus Mori at different temperatures. Food Funct. 2019, 10, 410–421. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Liu, L. Novel polysaccharide from Chaenomeles speciosa seeds: Structural characterization, α-amylase and α-glucosidase inhibitory activity evaluation. Int. J. Biol. Macromol. 2020, 153, 755–766. [Google Scholar] [CrossRef]
- Amamou, S.; Lazreg, H.; Hafsa, J.; Majdoub, H.; Rihouey, C.; Le Cerf, D.; Achour, L. Effect of extraction condition on the antioxidant, antiglycation and α-amylase inhibitory activities of Opuntia macrorhiza fruit peels polysaccharides. LWT 2020, 127, 109411. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, Y.; Zhang, Z.; Yao, W.; Gao, X. Hypoglycemic, hypolipidemic and antioxidant effects of Sarcandra glabra polysaccharide in type 2 diabetic mice. Food Funct. 2014, 5, 2850–2860. [Google Scholar] [CrossRef]
- Jia, X.; Hu, J.; He, M.; Zhang, Q.; Li, P.; Wan, J.; He, C. α-Glucosidase inhibitory activity and structural characterization of polysaccharide fraction from Rhynchosia minima root. J. Funct. Foods 2017, 28, 76–82. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Wu, J.; Zhang, H.; Zhu, L.; Zhan, X. Potential application of a low-viscosity and high-transparency xanthan gum produced from Xanthomonas campestris CCTCC M2015714 in foods. Prep. Biochem. Biotechnol. 2018, 48, 402–407. [Google Scholar] [CrossRef]
- Hsu, W.-K.; Hsu, T.-H.; Lin, F.-Y.; Cheng, Y.-K.; Yang, J.P.-W. Separation, purification, and α-glucosidase inhibition of polysaccharides from Coriolus versicolor LH1 mycelia. Carbohydr. Polym. 2013, 92, 297–306. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Liu, X.; Lu, W.; Jia, S.; Hong, T.; Li, R.; Zhang, H.; Peng, L.; Zhan, X. Anti-diabetic activity evaluation of a polysaccharide extracted from Gynostemma pentaphyllum. Int. J. Biol. Macromol. 2019, 126, 209–214. [Google Scholar] [CrossRef]
- Golson, M.L.; Maulis, M.F.; Dunn, J.C.; Poffenberger, G.; Schug, J.; Kaestner, K.H.; Gannon, M.A. Activated FoxM1 attenuates streptozotocin-mediated β-cell death. Mol. Endocrinol. 2014, 28, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Uddin Zim, A.I.; Khatun, J.; Khan, M.F.; Hossain, M.A.; Haque, M.M. Evaluation of in vitro antioxidant activity of okra mucilage and its antidiabetic and antihyperlipidemic effect in alloxan-induced diabetic mice. Food Sci. Nutr. 2021, 9, 6854–6865. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Prabhu, G.K. Image analysis tools for evaluation of microscopic views of immunohistochemically stained specimen in medical research–a review. J. Med. Syst. 2012, 36, 2621–2631. [Google Scholar] [CrossRef]
- Seedevi, P.; Ramu Ganesan, A.; Moovendhan, M.; Mohan, K.; Sivasankar, P.; Loganathan, S.; Vairamani, S.; Shanmugam, A. Anti-diabetic activity of crude polysaccharide and rhamnose-enriched polysaccharide from G. lithophila on Streptozotocin (STZ)-induced in Wistar rats. Sci. Rep. 2020, 10, 556. [Google Scholar] [CrossRef]
- Zhu, D.; Yan, Q.; Liu, J.; Wu, X.; Jiang, Z. Can functional oligosaccharides reduce the risk of diabetes mellitus? FASEB J. 2019, 33, 11655. [Google Scholar] [CrossRef]
- Chung, I.-M.; Kim, E.-H.; Yeo, M.-A.; Kim, S.-J.; Seo, M.C.; Moon, H.-I. Antidiabetic effects of three Korean sorghum phenolic extracts in normal and streptozotocin-induced diabetic rats. Food Res. Int. 2011, 44, 127–132. [Google Scholar] [CrossRef]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef]
- Bari, M.W.; Islam, M.M.; Khatun, M.; Sultana, M.J.; Ahmed, R.; Islam, A.; Hossain, M.I.; Rahman, M.M.; Islam, M.A. Antidiabetic effect of Wedelia chinensis leaf extract in alloxan induced Swiss albino diabetic mice. Clin. Phytosci. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Wen, J.; Huang, Y.; Lu, Y.; Yuan, H. Associations of non-high-density lipoprotein cholesterol, triglycerides and the total cholesterol/HDL-c ratio with arterial stiffness independent of low-density lipoprotein cholesterol in a Chinese population. Hypertens. Res. 2019, 42, 1223–1230. [Google Scholar] [CrossRef]
- Imtiaz, F.; Islam, M.; Saeed, H.; Ahmed, A.; Rathore, H.A. Assessment of the antidiabetic potential of extract and novel phytoniosomes formulation of Tradescantia pallida leaves in the alloxan-induced diabetic mouse model. FASEB J. 2023, 37, e22818. [Google Scholar] [CrossRef]
- Yin, P.; Wang, Y.; Yang, L.; Sui, J.; Liu, Y. Hypoglycemic effects in alloxan-induced diabetic rats of the phenolic extract from mongolian oak cups enriched in ellagic acid, kaempferol and their derivatives. Molecules 2018, 23, 1046. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, J.; Zheng, Y.; Xu, Z.; Li, Y.; Zhou, S.; Zhang, C. Beneficial effects of polysaccharide-rich extracts from Apocynum venetum leaves on hypoglycemic and gut microbiota in type 2 diabetic mice. Biomed. Pharmacother. 2020, 127, 110182. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-M.; Dai, R.; Luo, Y.; Xiao, J.-H. Glucose-lowering and hypolipidemic activities of polysaccharides from Cordyceps taii in streptozotocin-induced diabetic mice. BMC Complement. Altern. Med. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Rehman, H.u.; Ullah, K.; Rasool, A.; Manzoor, R.; Yuan, Y.; Tareen, A.M.; Kaleem, I.; Riaz, N.; Hameed, S.; Bashir, S. Comparative impact of streptozotocin on altering normal glucose homeostasis in diabetic rats compared to normoglycemic rats. Sci. Rep. 2023, 13, 7921. [Google Scholar] [CrossRef]
- Mandal, A.; Bhattarai, B.; Kafle, P.; Khalid, M.; Jonnadula, S.K.; Lamicchane, J.; Kanth, R.; Gayam, V. Elevated liver enzymes in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Cureus 2018, 10, e3626. [Google Scholar] [CrossRef]
- Aleissa, M.S.; Mohammed, A.-Z.; Alneghery, L.M.; Hasnain, M.S.; Almutairi, B.; Ali, D.; Alarifi, S.; Alkahtani, S. Comparative study of the anti-diabetic effect of mucilage and seed extract of Abelmoschus esculentus against streptozotocin-induced diabetes in rat model. J. King Saud Univ.-Sci. 2022, 34, 102297. [Google Scholar] [CrossRef]
- Kumar Bhateja, P.; Singh, R. Antidiabetic activity of Acacia tortilis (Forsk.) Hayne ssp. raddiana polysaccharide on streptozotocin-nicotinamide induced diabetic rats. BioMed Res. Int. 2014, 2014, 572013. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. IJBS 2008, 4, 89. [Google Scholar] [CrossRef]
- Mahfoz, A.M.; Gawish, A.Y.J.D.; Syndrome, M. Insight into the hepatoprotective, hypolipidemic, and antidiabetic impacts of aliskiren in streptozotocin-induced diabetic liver disease in mice. Diabetol. Metab. Syndr. 2022, 14, 163. [Google Scholar] [CrossRef]
- Karazhiyan, H.; Razavi, S.M.; Phillips, G.O. Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocoll. 2011, 25, 915–920. [Google Scholar] [CrossRef]
- Azzouz, Z.; Bettache, A.; Boucherba, N.; Prieto, A.; Martinez, M.J.; Benallaoua, S.; de Eugenio, L.I. Optimization of β-1, 4-endoxylanase production by an Aspergillus niger strain growing on wheat straw and application in xylooligosaccharides production. Molecules 2021, 26, 2527. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Molecular weight distribution of polysaccharides from edible seaweeds by high-performance size-exclusion chromatography (HPSEC). Talanta 2012, 93, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, C.; Sempéré, R. Analytical methods for the determination of sugars in marine samples: A historical perspective and future directions. Methods 2005, 3, 419–454. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, C.; Chen, Z.; Li, W.; Yuan, G.; Chen, H. Physicochemical properties and antidiabetic effects of a polysaccharide from corn silk in high-fat diet and streptozotocin-induced diabetic mice. Carbohydr. Polym. 2017, 164, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Pettolino, F.A.; Walsh, C.; Fincher, G.B.; Bacic, A. Determining the polysaccharide composition of plant cell walls. Nat. Protoc. 2012, 7, 1590–1607. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-B.; Son, S.-U.; Lee, J.-E.; Lee, S.-H.; Kang, C.-H.; Kim, Y.-S.; Shin, K.-S.; Park, H.-Y. Characterization, prebiotic and immune-enhancing activities of rhamnogalacturonan-I-rich polysaccharide fraction from molokhia leaves. Int. J. Biol. Macromol. 2021, 175, 443–450. [Google Scholar] [CrossRef]
- Sabitha, V.; Panneerselvam, K.; Ramachandran, S. In vitro α–glucosidase and α–amylase enzyme inhibitory effects in aqueous extracts of Abelmoscus esculentus (L.) Moench. Asian Pac. J. Trop. Biomed. 2012, 2, S162–S164. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-Safi, I.; Louba, A.; Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alharbi, M.S.; Alqahtani, A.; Bari, A.; et al. In vitro alpha-amylase and alpha-glucosidase inhibitory activity and in vivo antidiabetic activity of Withania frutescens L. Foliar extract. Molecules 2021, 26, 293. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, M.P.; Kimuni, N.; Ngeranwa, N.; Orinda, O.; Njagi, M.; Maina, D.; Agyirifo, S.; Gathumbi, K. Antidiabetic and safety of Lantana rhodesiensis in alloxan induced diabetic rats. J. Dev. Drugs 2015, 2015, 1–10. [Google Scholar]
- Li, D.; Liu, Q.; Lu, X.; Li, Z.; Wang, C.; Leung, C.-H.; Wang, Y.; Peng, C.; Lin, L. α-Mangostin remodels visceral adipose tissue inflammation to ameliorate age-related metabolic disorders in mice. Aging 2019, 11, 11084. [Google Scholar] [CrossRef]



| Types of Monosaccharaides | DF53 | DF72 | DF73 | |
|---|---|---|---|---|
| Neutral Monosaccharides | Arabinose | 11.67 | 4.53 | 3.12 |
| Fucose | 4.56 | 2.09 | 1.96 | |
| Xylose | 8.73 | 2.13 | Traces | |
| Galactose | 13.27 | 5.21 | 2.06 | |
| Glucose | 21.21 | 11.67 | 8.61 | |
| Rhamnose | - | 4.03 | 5.61 | |
| Sucrose | 9.28 | 7.32 | 3.16 | |
| Maltose | 0.7 | 1.31 | 2.67 | |
| Acidic Monosaccharaides | Galacturonic Acid | 26.34 | 54.58 | 67.38 |
| Glucuronic Acid | 4.24 | 7.13 | 5.43 |
| α-Amylase (IC50 in µg/mL) | α-Glucosidase (IC50 in µg/mL) | |
|---|---|---|
| EPP | 84.48 ± 2.86 | 67.19 ± 3.18 |
| DF48 | 95.04 ± 1.98 | 139.48 ± 2.09 |
| DF49 | 108.92 ± 4.21 | 120.88 ± 1.73 |
| DF53 | 89.12 ± 3.09 | 72.87 ± 1.66 |
| DF56 | 133.55 ± 2.17 | 161.13 ± 5.61 |
| DF58 | 84.85 ± 1.88 | 96.04 ± 2.69 |
| DF60 | 100.81 ± 0.9 | 127.81 ± 2.74 |
| DF62 | 97.88 ± 2.12 | 135.76 ± 11.25 |
| DF72 | 86.1 ± 2.64 | 50.47 ± 5.18 |
| DF73 | 38.2 ± 1.12 | 29.26 ± 2.68 |
| SD | 29.18 ± 1.76 | 41.29 ± 3.12 |
| Treatments | Doses (mg/kg) | SGOT (U/L) | SGPT (U/L) | ALP (U/L) | Creatinine (mg/dL) |
|---|---|---|---|---|---|
| NC | 0.3 mL | 65.63 ± 1.23 ab | 47.85 ± 1.52 bc | 165.45 ± 0.38 cd | 1.23 ± 0.12 a |
| DC | 0.3 mL | 178.38 ± 0.92 a | 156.29 ± 2.56 cd | 361.28 ± 0.19 cd | 3.60 ± 0.17 ab |
| SD | 100 | 74.66 ± 0.57 abc | 53.73 ± 1.83 a | 160.57 ± 1.56 b | 1.25 ± 0.09 c |
| DF53 | 50 | 158.75 ± 1.53 bc | 147.26 ± 0.87 a | 284.33 ± 0.93 a | 3.12 ± 0.51 d |
| 100 | 119.27 ± 2.68 ab | 119.41 ± 0.36 abc | 221.46 ± 0.07 abc | 2.73 ± 0.08 a | |
| 200 | 78.28 ± 3.71 ab | 73.54 ± 1.24 cd | 182.55 ± 1.09 ab | 2.08 ± 1.01 ab | |
| DF72 | 50 | 165.23 ± 0.93 a | 136.48 ± 3.21 ab | 294.26 ± 1.28 bc | 3.03 ± 0.19 bc |
| 100 | 112.52 ± 3.51 b | 103.33 ± 1.27 a | 272.58 ± 0.76 cd | 2.03 ± 0.07 c | |
| 200 | 71.89 ± 0.83 d | 70.58 ± 0.97 c | 202.39 ± 0.82 ab | 1.55 ± 0.16 bcd | |
| DF73 | 50 | 159.43 ± 1.27 cd | 129.27 ± 0.58 bc | 254.18 ± 1.08 a | 2.43 ± 0.09 b |
| 100 | 96.28 ± 0.57 ab | 94.56 ± 0.92 ab | 199.92 ± 0.99 a | 1.85 ± 0.15 cd | |
| 200 | 54.67 ± 0.57 ab | 50.12 ± 1.35 c | 167.33 ± 1.23 a | 1.29 ± 0.24 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.U.; Jamil, Y.; Khan, A.; Ahmad, J.; Iqbal, A.; Ali, S.; Hamayun, M.; Hussain, A.; Alrefaei, A.F.; Almutairi, M.H.; et al. Pichia pastoris Mediated Digestion of Water-Soluble Polysaccharides from Cress Seed Mucilage Produces Potent Antidiabetic Oligosaccharides. Pharmaceuticals 2024, 17, 704. https://doi.org/10.3390/ph17060704
Khan IU, Jamil Y, Khan A, Ahmad J, Iqbal A, Ali S, Hamayun M, Hussain A, Alrefaei AF, Almutairi MH, et al. Pichia pastoris Mediated Digestion of Water-Soluble Polysaccharides from Cress Seed Mucilage Produces Potent Antidiabetic Oligosaccharides. Pharmaceuticals. 2024; 17(6):704. https://doi.org/10.3390/ph17060704
Chicago/Turabian StyleKhan, Imdad Ullah, Yusra Jamil, Aiman Khan, Jalwa Ahmad, Amjad Iqbal, Sajid Ali, Muhammad Hamayun, Anwar Hussain, Abdulwahed Fahad Alrefaei, Mikhlid H. Almutairi, and et al. 2024. "Pichia pastoris Mediated Digestion of Water-Soluble Polysaccharides from Cress Seed Mucilage Produces Potent Antidiabetic Oligosaccharides" Pharmaceuticals 17, no. 6: 704. https://doi.org/10.3390/ph17060704
APA StyleKhan, I. U., Jamil, Y., Khan, A., Ahmad, J., Iqbal, A., Ali, S., Hamayun, M., Hussain, A., Alrefaei, A. F., Almutairi, M. H., & Ahmad, A. (2024). Pichia pastoris Mediated Digestion of Water-Soluble Polysaccharides from Cress Seed Mucilage Produces Potent Antidiabetic Oligosaccharides. Pharmaceuticals, 17(6), 704. https://doi.org/10.3390/ph17060704









