Abstract
The importance of natural plant materials in modern medicine is considerable, and raw materials with antiviral, antibacterial, antifungal, and anticancer properties are still sought because of microbe resistance and difficulties in anticancer therapy. This review focuses on the lemongrass Cymbopogon citratus (DC.) Stapf. and on the lemongrass oil properties and applications. Multiple applications of this plant were described in different latitudes and cultures, including cases of digestive disorders and anti-inflammatory, antipyretic, diaphoretic, stimulating, and antispasmodic conditions. Data from the literature on the composition of essential oil and extracts from C. citratus were analyzed, and the results of research on the antifungal, antibacterial, and antiviral effects were quoted. Essential oil inhibits the growth of fungi (Aspergillus niger, A. fumigatus, Candida spp.) and has an antibacterial effect (Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa). It also shows antiviral activity and deters insects. Lemongrass contains active substances with potential anticancer effects. This plant has apoptosis-stimulating properties, mainly through the activity of apigenin, which is the main active flavonoid in this plant. This active substance helps inhibit cell proliferation by stopping the cell cycle and directing cancer cells toward apoptosis.
1. Introduction
According to WHO data from the 2007 report, 4 billion people in the world, representing 80% of the human population, use herbal preparations to treat common health conditions [1]. Medicines and supplements of plant origin are a major element in various types of traditional medicine such as Ayurveda, homeopathy, traditional oriental medicine, and Indian medicine. It is also estimated that among plant-derived medical devices, approximately 74% are used in modern medicine in a way that correlates directly with their traditional use in folk medicine [2,3,4,5,6]. About one-fourth of annually prescribed drugs and about 7000 different medical devices contain ingredients of plant origin or their derivatives, with a market value of about USD 40 billion. Nearly 33% of medicines produced in highly developed countries are preparations obtained from plants [3,4,5,6]. One such commonly used medicinal plant is lemongrass (Cymbopogon citratus (DC.) Stapf) (Figure 1).

Figure 1.
Depiction of Cymbopogon citratus cultivated on experimental plots of the Garden of Cosmetic Plants and Raw Materials (Research and Science Innovation Centre): (A) Collection of whole plants; (B) Cleaned shoots; (C) Plants before harvesting.
Cymbopogon citratus (DC.) Stapf, previously described as Andropogon citratus by De Candolle and reclassified by Otto Stapf, belongs to the Poaceae family, which includes approximately 500 genera and 8000 species of plants, commonly called grasses. The Cymbopogon genus includes 30 species of grasses native to Old World countries. The name “Cymbopogon” comes from a combination of the Greek words “kymbe”—boat and “pogon”—beard and refers to the arrangement of flower spikes. The part “citratus” refers to the Latin term meaning lemon-scented leaves [7]. Shah et al. [8] provide common names for Cymbopogon citratus, which is used in various regions of the world—Brazil: capim-cidrao, capim-santo, Egypt: lemon grass, Great Britain: lemongrass, citronella, squinant, Ethiopia: tej-sar, India: sera, verveine, Indonesia: sereh, Italy: cimbopogone, Malaysia: sakumau, Mexico: zacate limon, Germany: zitronengras, Sweden: citrongräss, Thailand: ta-khrai, Turkey: limon out, and the USA: citronella [7,8,9].
C. citratus comes from the southwest part of Asia and now grows in all tropical regions of the world and on savannahs. It is a perennial plant that grows in tall, 2-m clumps with short roots. It has long leaves up to 1 m long and 5–15 mm wide. It has an inflorescence composed of spikes arranged on the top of the blade [10]. It is also possible to grow this species in a one-year system in temperate climate conditions [11].
Although numerous literature data present Cymbopogon citratus as a plant source of biologically active ingredients with a broad spectrum of action, this review aims to critically evaluate the pharmacological activity not only of the essential oil as the most popular product obtained from this plant but also of other therapeutic products. A new approach in this review is to combine and summarize information regarding the origin of Cymbopogon citratus, its use, the chemical composition of products obtained from it, and the antibacterial, antiviral, antifungal, and anticancer effects of this plant’s raw material. Although there are many varieties and species of the genus Cymbopogon, this work focuses on the one selected species and takes into account its chemical composition, the presence of biologically active substances, and their health effects based on clinical evidence. The work will present the mechanisms of anticancer effects of various products obtained from C. citratus and active ingredients obtained from this plant. Finally, we will indicate future research directions that should be undertaken to continue the search for active compounds with anticancer properties and to prioritize further progress in the use of C. citratus as a raw material for medicinal use. To achieve the above objectives, a review of the literature from the last 20 years was performed, with particular emphasis on the latest research.
2. Cymbopogon citratus Application
Infusions and decoctions prepared from fresh or dried lemongrass leaves are popular and often used almost all over the world in order to relieve various ailments. Chemical compounds extracted from C. citratus have an equally wide range of applications, and essential oil obtained from this plant is particularly common. In India, lemongrass is used for gastrointestinal ailments, while in China, it is used as a component of antidepressant mixtures. In the Malay Peninsula, it is commonly used to treat flu, fever, pneumonia, and gastrointestinal problems and as a diaphoretic drug. In Nigeria, it is used as an antipyretic and for its stimulating and antispasmodic properties, while in Indonesia, the plant is recommended as an ingredient that aids in digestion, promotes diuresis and sweating, and regulates the menstrual cycle. In Africa and Asia, its antitussive, antiseptic, diaphoretic, and antirheumatic properties are known, and it is used to treat lower back pain, sprains, and hemoptysis (Figure 2) [12,13,14,15,16,17].
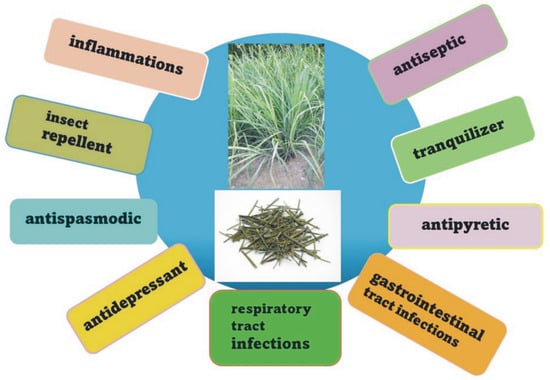
Figure 2.
Traditional applications of the Cymbopogon citratus.
Lemongrass is a commonly used medicine in traditional folk medicine in Cuba and many other countries in the Caribbean region. In addition to the above-mentioned properties, its analgesic and anti-inflammatory effects are also widely known and important. In the Trinidad and Tobago region, it is a popular herb used to fight diabetes. In traditional Suriname medicine, it is used against cough, wounds, asthma, and bladder diseases and as a diaphoretic and headache reliever. It is also applied as a repellent and carminative. In Brazil, it is a very common herbal medicine recommended for many ailments, including as a tonic, digestive aid, antitussive, anti-cold, analgesic, treatment of heart disease, antipyretic, anti-inflammatory in urinary tract diseases, diuretic, antispasmodic, diaphoretic, and antiallergic. There is also evidence of lemongrass being used as a mild sedative [16,18,19,20]. In addition to medicinal uses, lemongrass—most often as an essential oil obtained from it—is widely used in the food, perfume, and cosmetics industries. The oil is part of some oils applied for massage and aromatherapy. Lemongrass is also an important ingredient in oriental cuisine. Very diluted lemongrass oils are used in the food industry to flavor food products and drinks. However, in undiluted form, they may be toxic or even fatal if a large dose is taken orally. Due to the high content of citronellal, the oil is used as a repellent [11,21,22,23,24,25,26,27,28,29,30,31].
3. Chemical Composition of Cymbopogon citratus Essential Oil and Extracts
Among the many species and varieties of the genus Cymbopogon, the species C. citratus, in particular, is cultivated for its essential oil, which is used in the cosmetics, perfumery, and food industries. Its wide range of applications results from the high citral content (70–80%) [32,33,34,35,36,37,38,39]. The total amount of essential oil obtained from the leaves ranges from 0.28 to 1.4%. The maximum reported isolated amount of essential oil is 3.0% and was obtained by hydrodistillation of dry leaves. The yield of essential oil depends on the growing conditions and the condition of the plants; from dry material, the average amount is 3.5–12.8 mL/kg (or 0.35–1.28%). The highest yield of lemongrass essential oil produced (2.63%) was obtained from dried lemongrass stems that were heated. Lemongrass can be distilled fresh or after wilting. Withering the herbs before the distillation process reduces the moisture content and increases the oil yield. Available data on the yield of essential oil obtained by this method vary significantly and range from 0.24% to 0.71% [13,30,33,34,37,38,40,41,42,43,44,45,46,47,48,49]. The oil has a scent described as lemon, even though the main ingredients of both oils—lemon and lemongrass—are different. Citral predominates in lemongrass oil (approximately 26.5% in essential oil from leaves water distillation), while limonene predominates in lemon pericarp oil (Citrus limon (L.) Burm.), and there is little citral [50]. However, the chemical composition of the oil (Table 1) varies depending on factors such as the geographical origin of the plant; the main ingredients are terpene hydrocarbons, alcohols, ketones, esters, and aldehydes. Among the substances isolated from lemongrass leaves and roots, the most common are alkaloids, saponins, sterols, terpenes, alcohols, ketones, flavonoids, acids, i.e., chlorogenic, caffeic, p-coumaric acids, and sugars [51]. The chemical composition of the oil may vary slightly, depending on the plant’s growth conditions, cultivation region, agrotechnics, plant age, and harvest date [13,30,33,34,37,38,40,41,42,43,44,45,46,47,48,49].

Table 1.
Main ingredients and their content in lemongrass leaf essential oil [10,11,37,38,46,48,49,52,53,54,55].
In the group of mono-, di- and sesquiterpenes isolated from lemongrass, the most common is myrcene, which occurs in various amounts—from 2 to 25.3%, and limonene isolated in an amount of 0.2 to 13.8% [10,11,37,38,52,53,54,55]. α-pinene, α-caryophyllene, phelandrene, and α-oxobisabolene are present in smaller amounts [54]. During research on the chemical composition of wax obtained from the leaves of C. citratus, two chemical compounds belonging to the group of triterpenes—cymbopogon and cymbopogonol, were identified, and further research indicated that cymbopogon is probably not a natural product but an artifact produced during isolation of cimbopogonol, which in turn is classified as an alcohol. Among the ketones, ionones and methylheptenone have been isolated, constituting over 25% of the total composition of the essential oil. The main component of the oil is an aldehyde-citral (3,7-dimethyl-2,6-octadienal), which is a mixture of neral and geranial, which, depending on the place of origin of the plant, constitutes from 30 to even 93.74% of the composition [55]. This is the compound that determines the lemon scent of the plant. Research conducted in the Philippines has shown that oil obtained from plants during the dry season (March to June) contains a larger amount of citral. Hexane extraction also provides a larger amount of citral (up to 86.83%) than other solvents [55]. Isocitral, decinal and citronellal, cinnamic, salicylic, and anise aldehyde were isolated by steam distillation. The phenolic components found in lemongrass oil are primarily hydroquinone, catechol, elemycin, myrcene, quercetin, kaempferol, and apigenin [56]. The most common compound from the group of alcohols and esters is geraniol, the amount of which depends on the origin of the plant. The remaining compounds from the alcohol group are linalool, citronellol, metaheptanol, 1,8-cineol, menthol, neomenthol, terpineol, nerol, and farnesol. They are also from the group of esters: geranyl formate, citronellol acetate, terpinyl acetate, linalyl formate, linalyl acetate, and geranyl acetate. Other compounds present in the essential oil are acids-chlorogenic, caffeic, coumaric, tannins, and sitosterol (Figure 3) [46,48,57].
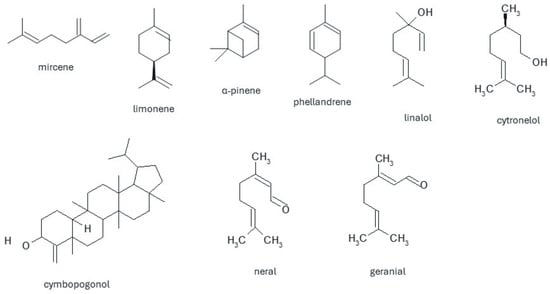
Figure 3.
Chemical structures of selected compounds present in Cymbopogon citratus species extracts and essential oil.
Extracts prepared from leaves and roots using alcohol, hexane, and chloroform differ in chemical composition. Their composition was analyzed in terms of the content of tannins, flavonoids, phenols, hydrocarbons, and essential oils, and the results obtained are presented in Table 2. Substances, such as tannins and phenolic compounds, are responsible for the antibacterial and antifungal properties of the plant. Particularly important is the content of tannins, which in herbal medicine play an important role in stopping hemorrhages because by causing precipitation of proteins, they make them resistant to the action of proteolytic enzymes. They form a membrane of coagulated protein, thus protecting the gastrointestinal mucosa [58,59,60,61,62].

Table 2.
Chemical composition of C. citratus leaf and root extracts [58].
Both the extracts and the essential oil have antiviral, antibacterial, antifungal, and anticancer properties (Figure 4).
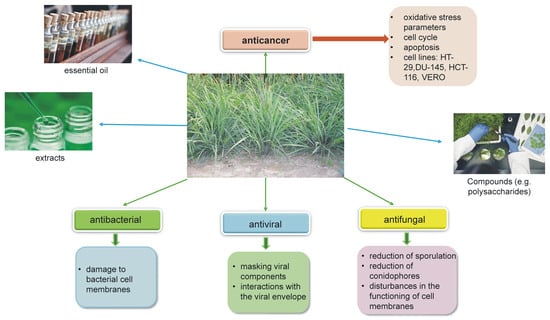
Figure 4.
Mechanisms of biological action of essential oil and extracts from Cymbopogon citratus.
4. Antifungal Activity of Cymbopogon citratus
Recently, a significant increase in the number of available antifungal preparations based on plant raw materials has been observed due to the growing resistance among many species of fungi to traditionally used drugs obtained by chemical synthesis. C. citratus essential oil has antifungal activity against Candida albicans, Candida pseudotropicalis, Mycosporum gypseum, Botritis cinerea, Aspergillus niger, Beauveria bassiana. It is also effective against fungi from the dermatophyte group such as the following: Trichophyton rubrum, Microsporum gypseum, Aspergillus fumigatus, Cladosporium trichoides, Trichophyton mentagrophytes, Epidermophyton floccosum, Botrytis cinerea and Aspergillus nidulans [51,63,64].
The essential oil shows statistically significantly high activity against Aspergillus niger and A. fumigatus at a concentration of 5 µL/0.4 L of air. Results obtained by Sulaiman [52] also confirm the strong activity of volatile substances obtained from essential oil against the maturation of spores of both above-mentioned fungal species (Table 3). The maturation of A. fumigatus and A. niger spores was completely inhibited by volatile substances at a concentration of 10 µL/0.4 L of air, while A. flavus spores lost their ability to survive at a concentration of 15 µL/0.4 L of air (Table 3). Studies carried out using a light microscope showed morphological changes in the species A. niger after exposure to 5 µL of oil and 0.4 L of air. In control samples, a regular structure with visible hyphae containing conidia was visible. However, after exposure to C. citratus essential oil, the mycelium presented morphological changes, including reduced sporulation, reduced pigmentation, and the reduction and distortion of conidiophores. In addition to inhibition of hyphal growth and spore formation, disturbances in the structure and functioning of cell membranes and the disorganization of mitochondrial structures were also observed at the cellular level. Moreover, it has been shown that essential oil causes the loss of cytoplasm within the fungal hyphae, which become significantly thinner [65,66]. According to literature data, aromatic volatile compounds isolated from plants have stronger antifungal and antibacterial effects than non-aromatic compounds [67]. Essential oils are also more effective than individual ingredients isolated from them. Like essential oil, its main ingredient—citral—has antifungal activity. The action of the oil depends on the concentration of citral in it [68,69,70,71].

Table 3.
Inhibitory effect of different concentrations in the air of volatile substances of lemongrass essential oil (Cymbopogon citratus (DC.) Stapf) on the maturation of Aspergillus spores.
5. Antibacterial Activity of Cymbopogon citratus
The plant extract and essential oil have antibacterial properties. Especially the essential oil, due to the presence of citral, has a particularly strong antibacterial effect against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Straptococcus pneumoniae, S. pyogenes, Neisseria gonorhoeae, Clostridium perfrigens, Pseudomonas fluorescens, Acinetobacter baumannii, Aeromonas veronii biogroup sober, Enterobacter faecalis, Klebsiella pneumoniae, Salmonella enterica subsp. Enetrica sorotipo typhimurium, Serratia marcescens, Proteus mirabilis, Shigella flexneri, and Salmonella typhi [49,72,73,74,75].
The mechanism of antibacterial action of essential oil is based on the structure and properties of its bioactive substances. Hydrophobic substances such as hydrocarbons can affect chemical interactions and thus determine the structural stability of the bacterial cell and its macromolecular systems and disturb its vital functions [76]. According to Moreira et al. [77], the lipophilic components of essential oil bind to the lipid bilayer of the cell membrane, increasing its permeability and causing the cell contents to flow into the environment and damaging the cell’s enzymatic system. Souza et al. [78] found that even small changes in the structure of the cytoplasmic membrane can affect the metabolism of the bacterial cell, including the synthesis of macromolecules.
Extracts from the root and leaves of the plant prepared using various solvents (methanol, hexane, and chloroform) have various bactericidal effects (Table 4). Extracts obtained from the plant root have a stronger effect. Water and alcohol extracts from C. citratus have a much weaker antibacterial effect than essential oil. The water extract has no antibacterial effect at all, while the alcoholic extract has little activity. The antibacterial activity of two types of extracts and the essential oil is summarized in Table 5 and Table 6. The high antimicrobial activity of Cymbopogon citratus oil and extracts makes them willingly used as a replacement or supplement to synthetic preservatives used in the production of food and cosmetics [39,49,79,80,81].

Table 4.
Antibacterial activity of the chloroform extract of C. citratus [58].

Table 5.
Antibacterial activity of C. citratus essential oil against selected bacterial strains [82].

Table 6.
Antibacterial activity of alcoholic and water extracts of C. citratus against selected bacterial strains [83].
7. Anticancer Activity of Cymbopogon citratus
Literature data indicate the anticancer effect of the Cymbopogon citratus plant, especially the essential oil and extracts obtained from this plant. According to literature data, lemongrass has anticancer effects by reducing cell viability due to increasing oxidative stress parameters in SiHa renal cancer cells. At the same time, it turns out that lemongrass has an antioxidant effect on healthy VERO kidney cells, increasing their viability and proliferation and reducing oxidative stress [102]. Lemongrass has also emerged as a promising medicinal plant that can be used as an adjunct to chemotherapy to enhance the antitumor response and reduce the resistance of prostate cancer. The studies were carried out in vitro using the DU-145 cell line. In addition to reducing the viability and proliferation of cancer cells, lemongrass also inhibits the formation of colonies. Also, in the tested cell line, it stimulates oxidative stress and causes cell cycle arrest in the G0/G1 phase. It is worth emphasizing that the extract had a selective effect on cancer cells while not causing cytotoxicity in healthy cells [103]. Ethanolic lemongrass extract has the ability to stimulate the production of reactive oxygen species (ROS) and induce apoptosis in lymphoma and leukemia cell models-MV-4-11 Chronic myelomonocytic leukemia cell line, U-937 non-Hodgkin’s histiocytic lymphoma cell line, L-540 Hodgkin lymphoma, HD-MYZ Hodgkin lymphoma, KM-H2 Hodgkin lymphoma [104]. Lemongrass extract has proven to be effective in both in vitro and in vivo studies. It induced apoptosis in colon cancer cells-colon cancer cell line HT-29 and colon cancer cell line HCT-116, depending on time and dose, while not damaging healthy cells represented in the study by normal colon mucosa cell line and normal colon mucosa cell line NCM-460 in vitro. In vivo studies showed that oral administration of lemongrass extract was well tolerated and effective in inhibiting colon cancer xenograft growth in mice. The tested extract also increased the anticancer effectiveness of drugs traditionally used in therapy and reduced their side effects, such as weight loss. Hence, it is concluded that this extract has potential as an adjunct treatment for colorectal cancer [105]. The antioxidant effect, potential anti-inflammatory effect (by inhibiting lipoxygenase), and cytotoxic effect of the essential oil of Cymbopogon citratus (DC.) Stapf. were also tested on various cell lines, including the prostate cancer and glioblastoma multiforme cell lines (LNCaP, PC-3, SF-767, and SF-763) [106]. Inhibition of the proliferation of oral epidermoid carcinoma cell lines under the influence of C. citratus essential oil has been demonstrated in mice and humans [107].
Several previous studies have demonstrated the anticancer effects of C. citratus leaf extracts, especially their bioactive components. For example, α-myrcene, α-limonene, and geraniol have been shown to have antitumor activity against breast cancer, liver cancer, and intestinal mucosa cancer cells in mouse models [108]. It is believed that C. citratus leaf extracts contain inhibitors of the developmental phase of skin cancer. Additionally, experiments conducted on mice confirmed that C. citratus leaf extract inhibits the development of colon cancer [109]. The inhibitory effect of extracts obtained from C. citratus on the process of hepatocarcinogenesis in rats, which was initiated by treatment with diethylnitrosamine, was also reported [110]. C. citratus extracts were isolated by solvent maceration, and then their antiproliferative activity was tested on five different cancer cell lines—human colon cancer (HCT-116), breast cancer (MCF-7 and MDA-MB 231), and ovarian cancer (SKOV -3 and COAV)—and a normal liver cell line (WRL 68). The obtained results suggest the antiproliferative effectiveness of the ethanol extract of C. citratus against selected human cancer cell lines [111]. Essential oil, which was extracted from the culm and leaf of Cymbopogon citratus collected from different regions of Vietnam, caused apoptosis induction and cell cycle arrest in A549 cells, as concluded based on the results obtained from flow cytometry and fluorescent nuclear staining tests. Western blot analysis showed that the apoptotic effect was caused by changes in the activity of proteins regulating the apoptosis process, such as caspase-3, Bcl-2, and Bax (Figure 5) [112].
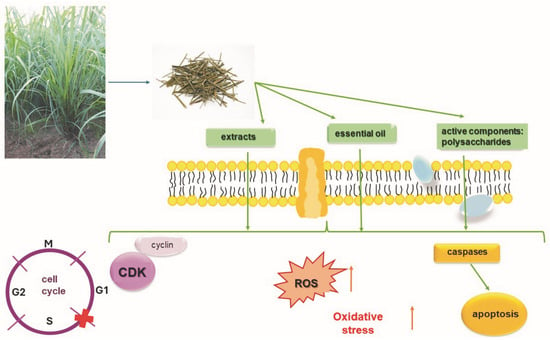
Figure 5.
Molecular mechanisms of action of bioactive ingredients, essential oil, and C. citratus extracts in cancer cells. Possibility of penetration of active substances of C. citratus through cell membranes and induction of apoptosis in cancer cells through their influence on the activation of caspases. Increasing the level of oxidative stress in cancer cells and the effect of C. citratus extracts, essential oil, and selected active components on cell cycle arrest.
As mentioned above, literature data indicate that essential oils obtained from Cymbopogon citratus have wide medical and pharmaceutical applications resulting from their numerous antibacterial, antiviral, antifungal, and anticancer properties. However, recently, there have been data indicating the biological activity of polysaccharides found in the plant and their potential anticancer effect. The anti-inflammatory and anticancer effects of C. citratus polysaccharides on cancer cells were analyzed in vitro. Literature data indicate that the mechanism of action of polysaccharides in inducing apoptosis in cancer cells involves inducing the internal pathway. Polysaccharide fractions had cytotoxic and pro-apoptotic effects on Siha and LNCap cancer cells. They induced apoptosis in these cells by increasing the level of caspase 3 and downregulating the bcl-2 family genes, followed by the release of cytochrome c [113]. The newly identified high molecular weight polysaccharide isolated from C. citratus also inhibited the proliferation of MDA-MB-231 cells, reduced the expression of cyclin D1 and CDK4, and induced cell arrest in the G0/G1 phase. This compound induced apoptosis of MDA-MB-231 cells by triggering the Fas/FasL-mediated death receptor pathway. Chen et al. indicate that the results provide a theoretical basis for the use of C. citratus polysaccharide as a potential anti-breast cancer agent in functional foods and medicine [114].
8. Limitations and Side Effects of Using C. citratus Products
The pharmacological use of agents derived from C. citratus should include restrictions on their use. Citral, which is the main component of essential oil, is considered potentially allergenic, so information about it should be clearly provided on the packaging of the dermatological preparation [115]. Machraoui et al. [9] and Ekpenyong et al. [35] also indicate possible potential toxic properties of C. citratus extracts at high doses. This information applies to highly concentrated preparations. However, Negrelle and Gomes [7] report that drinking a standard infusion of dried C. citratus does not cause any side or toxic effects. Some literature data indicate that the high content of lemongrass oil in food products may have an adverse effect on the human body, in particular on the organs of taste and smell [116]. On the other hand, lemongrass essential oil used in low concentrations is considered safe for human consumption [117]. In vitro studies have shown that the antimicrobial activity of lemongrass oil mainly ranges from 0.2 to 10 L/mL. In food products, the concentration of essential oil is much higher (25–100 times) to achieve comparable antimicrobial activity. In many countries, the use of essential oils is not regulated in any way. Moreover, improper and accidental uses of lemongrass essential oil may cause health problems due to genetic damage, carcinogenicity, and mutations [118]. Therefore, further research on the toxicity of lemongrass oil and a comprehensive safety assessment are necessary.
9. Conclusions
Plant medicinal preparations are becoming more and more popular in modern societies as an alternative to compounds obtained by chemical synthesis. This is due to, among other reasons, the fewer side effects they cause, the increasing availability, and the wide range of these preparations. One such raw material is lemongrass, which is very often used as a medicinal plant and in the food, cosmetic, and perfume industries. Raw materials from it are still being analyzed, and its antibacterial, antiviral, antifungal, and anticancer properties are now known. It is likely that subsequent research results will expand the possibilities of its applications in medicine. The potential anticancer properties of products obtained from lemongrass, such as essential oil and extract, as well as its chemical components, prompt extensive research on the molecular mechanisms of their action. This can be seen in the large number of emerging research results on the effects of lemongrass on cancer cells. The cytotoxic effect of this raw material on cancer cells and the cytoprotective effect on healthy cells indicate the need to continue research in this direction.
Author Contributions
Conceptualization, A.J.-T. and A.K.-D.; methodology, A.J.-T. and A.K.-D.; software, A.J.-T., A.K.-D. and J.E.; writing—original draft preparation, A.K.-D., A.J.-T. and J.E.; writing—review and editing, A.K.-D., A.J.-T. and J.E.; visualization, A.K.-D., A.J.-T. and J.E.; project administration, A.J.-T.; funding acquisition, A.J.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Ministry of Education and Science, Poland, under the research project number WZ/WB-IIŚ/6/2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues. 5 June 2007. Available online: https://www.who.int/publications/i/item/9789241594448 (accessed on 15 November 2023).
- Saggar, S.; Mir, P.A.; Kumar, N.; Chawla, A.; Uppal, J.; Kaur, A. Traditional and Herbal Medicines: Opportunities and Challenges. Pharmacogn. Res. 2022, 14, 107–114. Available online: https://www.phcogres.com/article/2022/14/2/105530pres14215 (accessed on 15 November 2023). [CrossRef]
- Barkat, M.A.; Goyal, A.; Barkat, H.A.; Salauddin, M.; Pottoo, F.H.; Anwer, E.T. Herbal medicine: Clinical perspective and regulatory status. Comb. Chem. High Throughput Screen. 2021, 24, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Parveen, B.; Parveen, A.; Parveen, R.; Ahmad, S.; Ahmad, M.; Iqbal, M. Challenges and opportunities for traditional herbal medicine today, with special reference to its status in India. Ann. Phytomed. 2020, 9, 97–112. [Google Scholar] [CrossRef]
- Choudhury, A.; Singh, P.A.; Bajwa, N.; Dash, S.; Bisht, P. Pharmacovigilance of herbal medicines: Concerns and future prospects. J. Ethnopharmacol. 2023, 309, 116383. [Google Scholar] [CrossRef] [PubMed]
- Al-Worafi, Y.M. Herbal medicines safety issues. In Drug Safety in Developing Countries; Academic Press: Cambridge, MA, USA, 2020; pp. 163–178. [Google Scholar] [CrossRef]
- Negrelle, R.R.B.; Gomes, E.C. Cymbopogon citratus (DC) Stapf: Chemical Composition and Biological Activities. Rev. Bras. Plantas Med. 2007, 9, 80–92. Available online: https://api.semanticscholar.org/CorpusID:56066104 (accessed on 15 November 2023).
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A.S. Scientific basis for the therapeutic use of Cymbopogon citratus, Stapf (Lemon grass). J. Adv. Pharm. Tech. Res. 2011, 2, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Machraoui, M.; Kthiri, Z.; Ben Jabeur, M.; Hamada, W. Ethnobotanical and phytopharmacological notes on Cymbopogon citratus (DC.) Stapf. J. New Sci. Agric. Biotechnol. 2018, 55, 3642–3652. [Google Scholar]
- Srivastava, V.; Dubey, S.; Mishra, A. A review on lemon grass: Agricultural and medicinal aspect. Int. Res. J. Pharm. 2013, 4, 42–44. [Google Scholar] [CrossRef]
- Kiełtyka-Dadasiewicz, A.; Ludwiczuk, A.; Tarasevičienė, Ž.; Michalak, M.; Głowacka, A.; Baj, T.; Kręcisz, B.; Krochmal-Marczak, B. Chemical and nutritional compounds of different parts of lemongrass (Cymbopogon citratus (DC) Stapf.) cultivated in temperate climate of Poland. J. Oleo Sci. 2021, 70, 125–133. [Google Scholar] [CrossRef]
- Kassahun, T.; Girma, B.; Joshi, R.K.; Sisay, B.; Tesfaye, K.; Taye, S.; Tesema, S.; Abera, T.; Teka, F. Ethnobotany, traditional use, phytochemistry and pharmacology of Cymbopogon citratus. Int. J. Herb. Med. 2020, 8, 80–87. [Google Scholar]
- Zahra, A.A.; Hartati, R.; Fidrianny, I. Review of the chemical properties, pharmacological properties, and development studies of Cymbopogon sp. Biointerface Res. Appl. Chem. 2020, 11, 10341–10350. [Google Scholar] [CrossRef]
- Magotra, S.; Singh, A.P.; Singh, A.P. A review on pharmacological activities of Cymbopogon citratus. Int. J. Pharm. Drug Anal. 2021, 9, 151–157. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Wardhani, D.H.; Retnowati, D.S.; Haryani, K. A brief review on the characteristics, extraction and potential industrial applications of citronella grass (Cymbopogon nardus) and lemongrass (Cymbopogon citratus) essential oils. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012118. [Google Scholar] [CrossRef]
- Tibenda, J.J.; Yi, Q.; Wang, X.; Zhao, Q. Review of phytomedicine, phytochemistry, ethnopharmacology, toxicology, and pharmacological activities of Cymbopogon genus. Front. Pharmacol. 2022, 13, 997918. [Google Scholar] [CrossRef] [PubMed]
- Syarif, L.I.; Junita, A.R.; Hatta, M.; Dwiyanti, R.; Kaelan, C.; Sabir, M.; Noviyanthi, R.A.; Primaguna, M.R.; Purnamasari, N.I. A mini review: Medicinal plants for typhoid fever in Indonesia. Syst. Rev. Pharm. 2020, 11, 1171–1180. [Google Scholar]
- Ortiz, R.S.; Marrero, G.V.; Navarro, A.L.T. Instructivo tecnico del cultivo de Cymbopogon citratus (DC) Stapf (cana santa). Rev. Plantas Med. 2002, 7, 2. [Google Scholar]
- Simões, D.M.; Malheiros, J.; Antunes, P.E.; Figueirinha, A.; Cotrim, M.D.; Fonseca, D.A. Vascular activity of infusion and fractions of Cymbopogon citratus (DC) Stapf. in human arteries. J. Ethnopharmacol. 2020, 258, 112947. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, M.; Wang, S.; Li, J.; Zhang, J.; Liu, D. Ethnopharmacology, chemical composition and functions of Cymbopogon citratus. Chin. Herb. Med. 2023; in press. [Google Scholar] [CrossRef]
- Olorunsanya, A.O.; Olorunsanya, E.O.; Bolu, S.A.O.; Adejumobi, C.T.; Kayode, R.M.O. Effect of graded levels of lemongrass (Cymbopogon citratus) on oxidative stability of raw or cooked pork patties. Pak. J. Nutr. 2010, 9, 467–470. [Google Scholar] [CrossRef]
- Thakur, C.; Bhardwaj, M.; Verma, A.K.; Bhatia, A. A review on post harvest management of lemongrass. Just Agric. 2020, 1, 029. [Google Scholar]
- Salvador, M.J.; Lopes, G.N.; Nascimento Filho, V.F.; Zucchi, O.L.A.D. Quality control of commercial tea by x-ray fluorescence. X-ray Spectr. 2002, 31, 141–144. [Google Scholar] [CrossRef]
- Newerli-Guz, J.; Śmiechowska, M.; Piotrzkowska, J. Aroma substances as ingredients of herbal-fruit teas. Zesz. Nauk. Akad. Morsk. Gdyn. 2009, 61, 19–32. [Google Scholar]
- Steinka, I. Biostatic properties of multicomponent tea. Bromat. Chem. Toksykol. 2012, 45, 538–542. [Google Scholar]
- Michalak-Majewska, M. Analysing quality and the consumer desirable of selected red leaf teas [in Polish: Analiza jakości i pożądalności konsumenckiej wybranych czerwonych herbat liściastych]. Towarozn. Probl. Jak. 2013, 3, 92–102. [Google Scholar]
- Adamczak, A.; Forycka, A.; Buchwald, W. The composition of fruit teas available on the Polish market of foodstuffs. Post. Fitoter. 2015, 16, 216–222. [Google Scholar]
- Tajidin, N.E.; Ahmad, S.H.; Rosenani, A.B.; Munirah, M. Growth performance and nutrient concentration of ‘Hijau’ Lemongrass (Cymbopogon citratus) as affected by maturity stages at harvest. Trans. Malays. Soc. Plant Physiol. 2011, 19, 35–38. [Google Scholar]
- Hagvall, L.; Bråred Christensson, J. Cross-reactivity between citral and geraniol—Can it be attributed to oxidized geraniol. Contact Dermat. 2014, 71, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Uraku, A.J.; Okaka, A.N.C.; Ogbanshi, M.E.; Onuoha, S.C. Nutritive and anti-nutritive potentials of Cymbopopgon citratus leaves. Am. J. Food Nutr. 2016, 6, 14–22. [Google Scholar]
- de Andrade, S.F.; Rocha, C.; Pinheiro, E.J.; Pereira-Leite, C.; Costa, M.D.C.; Monteiro Rodrigues, L. Revealing the protective effect of topically applied Cymbopogon citratus essential oil in human skin through a contact model. Cosmetics 2023, 10, 29. [Google Scholar] [CrossRef]
- Saleem, M.; Afza, N.; Anwar, M.A.; Hai, S.M.A.; Ali, M.S. A comparative study of essential oils of Cymbopogon citratus and some members of the genus Citrus. Nat. Prod. Res. 2003, 17, 369–373. [Google Scholar] [CrossRef]
- Linares, S.; Gonzalez, N.; Gomez, E. Effect of the fertilization, plant density and time of cutting on yield and quality of the essential oil of Cymbopogon citratus Stapf. Rev. Fac. Agron. (LUZ) 2005, 22, 247–260. [Google Scholar]
- Kassahun, B.M.; Mekonnen, S.A.; Abedena, Z.T.; Kidanemariam, H.G.; Yalemtesfa, B.; Atnafu, G.; Melka, B.; Mengesha, W.K.; da Silva, J.A. Performance of lemongrass (Cymbopogon citratus L. (DC) Stapf) agronomic and chemical traits in different agro-ecologies of ethiopia. Med. Arom. Plant Sci. Biotechnol. 2011, 5, 133–138. [Google Scholar]
- Ekpenyong, C.E.; Akpan, E.E.; Nyebuk, D.E. Phytochemical constituents, therapeutic applications and toxicological profile of Cymbopogon citratus Stapf (DC) leaf extract. J. Pharmacog. Phytochem. 2014, 3, 133–141. [Google Scholar]
- Kpoviessi, S.; Bero, J.; Agbani, P.; Gbaguidi, F.; Kpadonou-Kpoviessi, B.; Sinsin, B.; Accrombessi, G.; Frédérich, M.; Moudachirou, M.; Quetin-Leclercq, J. Chemical composition, cytotoxicity and in vitro antitrypanosomal and antiplasmodial activity of the essential oils of four Cymbopogon species from Benin. J. Ethnopharmacol. 2014, 151, 652–659. [Google Scholar] [CrossRef]
- Rajeswara Rao, B.R.; Adinarayana, G.; Kumar, A.N.; Rajput, D.K.; Syamasundar, K.V. Chemical-profile variations in essential oils isolated from lemongrass (Cymbopogon flexuosus) biomass and condensate wastewater by re-distillation and solvent extraction techniques. J. Essent. Oil Res. 2016, 28, 557–564. [Google Scholar] [CrossRef]
- Hamed, E.S.; Toaima, W.I.M.; El-Shazly, M. Effect of planting density and biofertilization on growth and productivity of Cymbopogon citratus (DC.) Stapf. (Lemongrass) plant under Siwa Oasis conditions. J. Med. Plants Stud. 2017, 5, 195–203. [Google Scholar]
- Majewska, E.; Kozłowska, M.; Gruczyńska-Sękowska, E.; Kowalska, D.; Tarnowska, K. Lemongrass (Cymbopogon citratus) essential oil: Extraction, composition, bioactivity and uses for food preservation—A review. Pol. J. Food Nutr. Sci. 2019, 69, 327–341. [Google Scholar] [CrossRef]
- Soraes, M.O.; Alves, R.C.; Pires, P.C.; Oliveira, M.B.; Vinha, A.F. Angolan Cymbopogon citratus used for therapeutic benefits: Nutritional composition and influence of solvents in phytochemicals content and antioxidant activity of leaf extracts. Food Chem. Toxicol. 2013, 60, 413–418. [Google Scholar] [CrossRef]
- Anal, J.M.H. Trace and essential elements analysis in Cymbopogon citratus (DC.) Stapf samples by graphite furnace-atomic absorption spectroscopy and its health concern. J. Toxicol. 2014, 2014, 690758. [Google Scholar] [CrossRef]
- d’Ávila, J.V.; Martinazzo, A.P.; dos Santos, F.S.; Teodoro, C.E.d.S.; Portz, A. Essential oil production of lemongrass (Cymbopogon citratus) under organic compost containing sewage sludge. Rev. Bras. Eng. Agríc. Ambient. 2016, 20, 811–816. [Google Scholar] [CrossRef]
- El-Mahrouk, E.M.; Abido, A.I.; Radwan, F.I.; Hamed, E.S.; El-Nagar, E.E. Vegetative growth and essential oil productivity of lemongrass (Cymbopogon citratus) as affected by NPK and some growth stimulators. Int. J. Bot. Stud. 2018, 3, 48–55. [Google Scholar]
- AL-Joburi, M.A. The effect of spraying a mixture of micronutrients and plant growth regulators on a vegetative growth the chemical contents and some physical characters for volatile oils of lemon grass plant (Cymbopogon citratus L.). Tikrit. J. Pure Sci. 2018, 23, 49–59. [Google Scholar] [CrossRef]
- Zigene, Z.D.; Kassahun, B.M.; Degu, B. Agronomic characterstics and essential oil yield of Java Citronella (Cymbopogon Winterianus Jowitt) as affected by harvesting age and plant population density. Acad. Res. J. Agric. Sci. Res. 2018, 6, 70–76. [Google Scholar]
- De Silva, G.B.V.U.; Dharmadasa, R.M.; Senanayake, R.A.S.P.; Lintha, A.; Sewwandi, S.K.U. Comparison of essential oil content and composition of different parts of Cymbopogon citratus (DC.) Stapf (Poaceae) grown in Sri Lanka. World J. Agric. Res. 2020, 8, 1–5. [Google Scholar]
- Guleria, K.; Sehgal, A. Appraisal of antioxidant effect of fresh and dried leaves of lemongrass (Cymbopogon citratus). Plant Arch. 2020, 20 (Suppl. S2), 2554–2557. [Google Scholar]
- Piasecki, B.; Biernasiuk, A.; Skiba, A.; Skalicka-Woźniak, K.; Ludwiczuk, A. Composition, anti-MRSA activity and toxicity of essential oils from Cymbopogon species. Molecules 2021, 26, 7542. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Galovičová, L.; Borotová, P.; Vukovic, N.L.; Vukic, M.; Kačániová, M. Cymbopogon citratus essential oil: Its application as an antimicrobial agent in food preservation. Agronomy 2022, 12, 155. [Google Scholar] [CrossRef]
- Mbili, N.C.; Opara, U.L.; Lennox, C.L.; Vries, F.A. Citrus and lemongrass essential oils inhibit Botris cinerea on ‘Golden Delicious’, ‘Pink Lady’ and ‘Granny Smith’ apples. J. Plant Dis. Protect. 2017, 124, 499–511. [Google Scholar] [CrossRef]
- He, L.; Zhao, Y.; Ye, M.; Zhan, J.; Tao, L.; Yang, Y.; Fan, L.; Su, F.; Chen, Q. Antifungal Activity of Cymbopogon citratus Essential Oils from Different Habitats against Botrytis cinerea. 2022. Available online: https://ssrn.com/abstract=4074553 (accessed on 25 November 2023). [CrossRef]
- Sulaiman, A.A.Y. Antifungal activity of volatiles from Lemongrass (Cymbopogon ctratus) and Peppermint (Mentha piperita) oils against some respiratory pathogenic species of Aspergillus. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 261–272. [Google Scholar]
- Cimanga, K.; Apers, S.; de Bruyne, T.; Van Miert, S.; Hermans, N.; Totte, J.; Vlietinck, A.J.; Kambu, K.; Tona, L. Chemical composition and antifungal activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Essent. Oil Res. 2002, 14, 382–387. [Google Scholar] [CrossRef]
- Rozzi, N.L.; Phippen, W.; Simon, J.E.; Singh, R.K. Supercritical fluid extraction of essential oil components from lemon-scented botanicals. LWT—Food Sci. Technol. 2002, 35, 319–324. [Google Scholar] [CrossRef]
- Schaneberg, B.T.; Khan, I.A. Comparison of extraction methods for marker compounds in the essential oil of lemon grass by cg. J. Agric. Food Chem. 2002, 50, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Miean, K.H.; Mohamed, S. Flavonoid (Myricitin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Nakiyingi, C. Determination of Quality and Quantity of Essential Oils from Lemon Grass (Cymbopogon citratus) of Different Maturity Stages and from Different Environments. Bachelor’s Thesis, School of Physical Sciences (Phys-Sciences) Collection, Makerere University, Kampala, Uganda, 2021. Available online: https://hdl.handle.net/20.500.12281/10085 (accessed on 12 January 2022).
- Ewansiha, J.U.; Garba, S.A.; Mawak, J.D.; Oyewole, O.A. Antimicrobial activity of Cymbopogon citratus (Lemon Grass) and it’s phytochemical properties. Front. Sci. 2012, 2, 214–220. [Google Scholar]
- Gao, X.; Hu, Y.; Tao, Y.; Liu, S.; Chen, H.; Li, J.; Zhao, Y.; Sheng, J.; Tian, Y.; Fan, Y. Cymbopogon citratus (DC.) Stapf aqueous extract ameliorates loperamide-induced constipation in mice by promoting gastrointestinal motility and regulating the gut microbiota. Front. Microbiol. 2022, 13, 1017804. [Google Scholar] [CrossRef] [PubMed]
- Hasan-Al-Sharif, M.; Hossain, M.M.; Asha, A.S.; Al-Mamun, M. Lemongrass (Cymbopogon Citratus) Essential Oil Improves Gut Health and Production Performance in Broiler: A Review. Int. J. Curr. Sci. 2023, 13, 788–801. Available online: https://rjpn.org/ijcspub/papers/IJCSP23C1205.pdf (accessed on 15 January 2024).
- Olalekan, O.; Olalekan, O.; Adebanjo, F.; Onasanya, A.; Elumalero, G.; Apenah, M.; Akinbile, O. Cymbopogon citratus reaction on aluminium nitrate induced stomach damage in adult female Wistar rat. Niger. J. Sci. Environ. 2021, 19, 152–162. [Google Scholar]
- Villalobos, M.C.; Nicolas, M.G.; Trinidad, T.P. Antihyperglycemic and cholesterol-lowering potential of dietary fibre from lemongrass (Cymbopogon citratus Stapf.). Mediterr. J. Nutr. Metab. 2021, 14, 453–467. [Google Scholar] [CrossRef]
- Lima, E.O.; Gompertz, O.F.; Giesbrecht, A.M.; Paulo, M.Q. In vitro antifungal activity of essential oil from officinal plants against dermatophyte. Mycoses 1993, 36, 333–363. [Google Scholar] [CrossRef]
- Sahal, G.; Woerdenbag, H.J.; Hinrichs, W.L.; Visser, A.; Tepper, P.G.; Quax, W.J.; van der Mei, H.C.; Bilkay, I.S. Antifungal and biofilm inhibitory effect of Cymbopogon citratus (lemongrass) essential oil on biofilm forming by Candida tropicalis isolates; an in vitro study. J. Ethnopharmacol. 2020, 246, 112188. [Google Scholar] [CrossRef]
- Pawar, V.C.; Thaker, V.S. In vitro efficacy of 75 essential oils against Aspergillus Niger. Mycoses 2006, 49, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tripathi, A. Effects of Citrus sinesis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus Niger (L.) Van Tieghem. Microbiol. Res. 2008, 163, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Chen, P.F.; Chang, S.-T. Antifungal activities of essential oils and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi. Bioresour. Technol. 2005, 96, 813–818. [Google Scholar] [CrossRef]
- Silva, C.B.; Guterres, S.S.; Weisheimer, V.; Schapoval, E.E.S. Antifungal activity of the lemongrass oil citral against Candida spp. Braz. J. Infect. Dis. 2008, 12, 63–66. [Google Scholar] [CrossRef]
- Hamza, I.S.; Ahmed, S.H.; Aoda, H. Study on the antimicrobial activity of Lemongrass leaf extracts. Iraq. J. Mark. Res. Consum. Protec. 2009, 1, 198–212. [Google Scholar]
- Chaves-Quirós, C.; Usuga-Usuga, J.S.; Morales-Uchima, S.M.; Tofiño-Rivera, A.P.; Tobón-Arroyave, S.I.; Martínez-Pabón, M.C. Assessment of cytotoxic and antimicrobial activities of two components of Cymbopogon citratus essential oil. J. Clin. Exp. Dent. 2020, 12, e749–e754. [Google Scholar] [CrossRef]
- Moustafa, M.A.; Hassan, N.N.; Alfuhaid, N.A.; Amer, A.; Awad, M. Insights into the toxicity, biochemical activity, and molecular docking of Cymbopogon citratus essential oils and citral on Spodoptera littoralis (Lepidoptera: Noctuidae). J. Econ. Entomol. 2023, 116, 1185–1195. [Google Scholar] [CrossRef]
- Hamer, K.A.; Carson, C.F.; Riley, T.V. In vitro activity of essential oils, in particular Melaleuca alterniflolia (tea tree) oil and tea tree oil products, against Candida spp. J. Antimicrob. Chemother. 1998, 42, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Onawunmi, G.O.; Yisak, W.A.; Ogunlana, G.O. Antibacterial constituents in the essential oil of Cymbopogon citratus (D.C.) Stapf. J. Ethnopharmacol. 1984, 12, 279–286. [Google Scholar] [CrossRef]
- Premathilake, U.G.A.T.; Wathugala, D.L.; Dharmadasa, R.M. Evaluation of chemical composition and assessment of antimicrobial activities of essential oil of lemongrass (Cymbopogon citratus (DC.) Stapf). Int. J. Minor Fruits Med. Arom. Plants 2018, 4, 13–19. [Google Scholar]
- Selim, S.A. Chemical composition, antioxidant and antimicrobial activity of the essential oil and methanol extract of the Egyptian lemongrass Cymbopogon proximus Stapf. Gras. Aceites 2011, 62, 55–61. [Google Scholar] [CrossRef]
- Sylvestre, M.; Pichette, A.; Longtin, A.; Nagau, F.; Legault, J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L.; from Guadeloupe. J. Ethnopharmacol. 2006, 103, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.R.; Ponce, A.G.; del Valle, C.E.; Roura, S.I. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT—Food Sci. Technol. 2005, 38, 565–570. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Barros, J.C.; de Oliveira, C.E.; da Conceição, M.L. Influence of Origanum vulgare L. essential oil on enterotoxin production, membrane permeability and surface characteristics of Staphylococcus aureus. Int. J. Food Microbiol. 2010, 137, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Boeira, C.P.; Piovesan, N.; Flores, D.C.; Soquetta, M.B.; Lucas, B.N.; Heck, R.T.; dos Santos, A.J.; Campagnol, B.C.; dos Santos, D.; Flores, E.M.; et al. Phytochemical characterization and antimicrobial activity of Cymbopogon citratus extract for application as natural antioxidant in fresh sausage. Food Chem. 2020, 319, 126553. [Google Scholar] [CrossRef] [PubMed]
- Nguefack, J.; Dongmo, J.B.; Dakole, C.D.; Leth, V.; Vismer, H.F.; Torp, J.; Guemdjom, E.F.; Mbeffo, M.; Tamgue, O.; Fotio, D.; et al. Food preservative potential of essential oils and fractions from Cymbopogon citratus, Ocimum gratissimum and Thymus vulgaris against mycotoxigenic fungi. Int. J. Food Microbiol. 2009, 131, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.I.; Fomda, B.A.; Jaykumar, E.; Bhat, J.A. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selscted pathogenic bacterias. Asian Pac. J. Trop. Med. 2010, 3, 535–538. [Google Scholar] [CrossRef]
- Nyarko, H.D.; Barku, V.Y.; Batama, J. Antimicrobial Examinations of Cymbopogon citratus and Adiatum capillus-Veneris Used in Ghanaian Folkloric Medicine. Int. J. Life Sci. Pharm. Res. 2012, 2, 115–121. Available online: http://hdl.handle.net/123456789/5966 (accessed on 21 December 2023).
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential Oils of Aromatic Plants with Antibacterial, Antifungal, Antiviral, and Cytotoxic Properties—An Overview. Forsch. Komplementärmedizin/Res. Complement. Med. 2009, 16, 79–90. [Google Scholar] [CrossRef]
- Salih, A.H.; Salih, R.H.; Ahmed, Y.H. Bioactivity of Cymbopogon citratus aqueous extract against measles virus and some bacterial isolates. Casp. J. Environ. Sci. 2022, 20, 585–592. [Google Scholar] [CrossRef]
- Sharma, A.D.; Kaur, I. Targeting H3N2 Influenza Virus RNA-dependent RNA Polymerase by Using Bioactives from Essential Oils from Eucalyptus polybrachtea, Cymbopogon citratus and Cymbopogon khasianus. Biol. Med. Nat. Prod. Chem. 2023, 12, 515–524. [Google Scholar] [CrossRef]
- Thuy, B.T.P.; Nhan, V.D.; Quang, N.M.; Duoc, N.T.; Van Tat, P. Evaluation of SARS-CoV-2 inhibition of some compounds in Cymbopogon citratus oil combining docking and molecular dynamics simulations. Vietnam J. Chem. 2021, 59, 790–799. [Google Scholar] [CrossRef]
- Kaur, S.; Thakur, B.; Kaur, G.; Kaur, R. Phytochemicals and Phyto-Essential Oils as Potent Antiviral Agents. In Promising Antiviral Herbal and Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2024; pp. 62–78. [Google Scholar] [CrossRef]
- Jadhav, A.K.; Karuppayil, S.M. Essential Oil Components: Anti-viral Properties. In Antimicrobials in Pharmaceutical and Medicinal Research; CRC Press: Boca Raton, FL, USA, 2023; pp. 109–124. ISBN 9781003268932. [Google Scholar]
- Minami, M.; Kita, M.; Nakaya, T.; Yamamoto, T.; Kuriyama, H.; Imanishi, J. The inhibitory effect of essential oils on herpes simplex virus type 1 replication in vitro. Microbiol. Immunol. 2003, 47, 681–684. [Google Scholar] [CrossRef]
- Bacon, T.H.; Levin, M.J.; Leary, J.J.; Sarisky, R.T.; Sutton, D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 2003, 16, 114–128. [Google Scholar] [CrossRef]
- Morfin, F.; Thouvenot, D. Herpes simplex virus resistance to antiviral drugs. J. Clin. Virol. 2003, 26, 29–37. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Prado, G.M.; Prado, J.C.S.; Pinheiro, C.V.F.; Daza-Cardona, E.A.; Barbosa, F.C.B.; de Souza, E.B.; dos Santos Fontenelle, R.O. Cymbopogon sp. from ethnobotany to antimicrobial: An integrative review. Res. Soc. Dev. 2022, 11, e19211931587. [Google Scholar] [CrossRef]
- Tshibangu, D.S.; Matondo, A.; Lengbiye, E.M.; Inkoto, C.L.; Ngoyi, E.M.; Kabengele, C.N.; Bongo, G.N.; Gbolo, B.Z.; Kilembe, J.T.; Mwanangombo, D.T.; et al. Possible effect of aromatic plants and essential oils against COVID-19: Review of their antiviral activity. J. Complement. Altern. Med. Res. 2020, 11, 10–22. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L. Antiviral effects of plant-derived essential oils and their components: An updated review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Tang, L.I.C.; Ling, A.P.; Koh, R.Y.; Chye, S.M.; Voon, K.G. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement. Altern. Med. 2012, 12, 3. [Google Scholar] [CrossRef]
- Juan-Pablo, B.P.; David, P.E.; Mónica, S.R.; Ashutosh, S.; Daniel, N.A.; Dealmy, D.G.; Rubén, G.-G.; Sergio-Everardo, V.-G.; Agustina, R.-M.; María-Del-Carmen, V.-M.; et al. Medicinal Plants with Anti-dengue and Immunomodulatory Activity. Curr. Pharm. Biotechnol. 2023, 24, 486–494. [Google Scholar] [CrossRef]
- Pal, D.; Lal, P. Herbal Drugs and Medicinal Plants in Controlling and Treatment of Diseases Caused by Dengue Virus (DEN-1 & 2): Ethnopharmacology, Chemistry, and Clinical and Preclinical Studies. In Anti-Viral Metabolites from Medicinal Plants; Springer International Publishing: Cham, Switzerland, 2023; pp. 683–746. [Google Scholar] [CrossRef]
- Lim, S.Y.M.; Chieng, J.Y.; Pan, Y. Recent insights on anti-dengue virus (DENV) medicinal plants: Review on in vitro, in vivo and in silico discoveries. All Life 2021, 14, 1–33. [Google Scholar] [CrossRef]
- Azad, M.M.; Ahammed, M.M.; Islam, M.; Mannan, M.A. Role of Medicinal Plants against Dengue Virus: A Review. J. Hamdard Univ. Bangladesh 2021, 7, 1–2. [Google Scholar]
- Pan, D.; Machado, L.; Bica, C.G.; Machado, A.K.; Steffani, J.A.; Cadoná, F.C. In Vitro Evaluation of Antioxidant and Anticancer Activity of Lemongrass (Cymbopogon citratus (D.C.) Stapf). Nutr. Cancer 2022, 74, 1474–1488. [Google Scholar] [CrossRef]
- Gomes, L.F.; Longhi, P.J.H.; Machado, L.; da Cruz, I.B.M.; Montano, M.A.E.; Martins, M.; Machado, S.A.; Steffani, J.A.; Cadoná, F.C. Lemongrass (Cymbopogon citratus (D.C.) Stapf) Presents Antitumoral Effect and Improves Chemotherapy Activity in Prostate Cancer Cells. Anticancer Agents Med. Chem. 2021, 21, 2337–2350. [Google Scholar] [CrossRef]
- Philion, C.; Ma, D.; Ruvinov, I.; Mansour, F.; Pignanelli, C.; Noel, M.; Saleem, A.; Arnason, J.; Rodrigues, M.; Singh, I.; et al. Cymbopogon citratus and Camellia sinensis extracts selectively induce apoptosis in cancer cells and reduce growth of lymphoma xenografts in vivo. Oncotarget 2017, 8, 110756–110773. [Google Scholar] [CrossRef]
- Ruvinov, I.; Nguyen, C.; Scaria, B.; Vegh, C.; Zaitoon, O.; Baskaran, K.; Mehaidli, A.; Nunes, M.; Pandey, S. Lemongrass Extract Possesses Potent Anticancer Activity against Human Colon Cancers, Inhibits Tumorigenesis, Enhances Efficacy of FOLFOX, and Reduces Its Adverse Effects. Integr. Cancer Ther. 2019, 18, 1. [Google Scholar] [CrossRef]
- Bayala, B.; Bassole, I.H.N.; Maqdasy, S.; Baron, S.; Simpore, J.; Lobaccaro, J.A. Cymbopogon citratus and Cymbopogon giganteus essential oils have cytotoxic effects on tumor cell cultures. Identification of citral as a new putative anti-proliferative molecule. Biochimie 2018, 153, 162–170. [Google Scholar] [CrossRef]
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef]
- Zheng, G.Q.; Kenney, P.M.; Lam, L.T. Potential anticarcinogenic natural products isolated from lemongrass oil and galanga root oil. J. Agric. Food Chem. 1993, 41, 153–156. [Google Scholar] [CrossRef]
- Suaeyun, R.; Kinouchi, T.; Arimochi, H.; Vinitketkumnuen, U.; Ohnishi, Y. Inhibitory effects of lemon grass (Cymbopogon citratus Stapf) on formation of azoxymethane-induced DNA adducts and abberant crypt foci in the rat colon. Carcinogenesis 1997, 18, 949–955. [Google Scholar] [CrossRef]
- Puatanachokchai, R.; Kishida, H.; Denda, A.; Murata, N.; Konishi, Y.; Vinitketkumnuen, U.; Nakae, D. Inhibitory effects of lemon grass (Cymbopogon citratus Stapf) extract on the early phase of hepatocarcinogenesis after initiation with diehtylnitrosamine in male Fischer 344 rats. Cancer Lett. 2002, 183, 9–15. [Google Scholar] [CrossRef]
- Halabi, M.F.; Sheikh, B.Y. Anti-proliferative effect and phytochemical analysis of Cymbopogon citratus extract. BioMed Res. Int. 2014, 2014, 906239. [Google Scholar] [CrossRef]
- Trang, D.T.; Hoang, T.K.V.; Nguyen, T.T.M.; Van Cuong, P.; Dang, N.H.; Dang, H.D.; Nguyen Quang, T.; Dat, N.T. Essential Oils of Lemongrass (Cymbopogon citratus Stapf) Induces Apoptosis and Cell Cycle Arrest in A549 Lung Cancer Cells. BioMed Res. Int. 2020, 2020, 5924856. [Google Scholar] [CrossRef]
- Thangam, R.; Sathuvan, M.; Poongodi, A.; Suresh, V.; Pazhanichamy, K.; Sivasubramanian, S.; Kanipandian, N.; Ganesan, N.; Rengasamy, R.; Thirumurugan, R.; et al. Activation of intrinsic apoptotic signaling pathway in cancer cells by Cymbopogon citratus polysaccharide fractions. Carbohydr. Polym. 2014, 17, 138–150. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, S.; Liu, H.; Xing, H.; Chen, P. Structural Characterization and Anti-breast Cancer Activity in vitro of a Novel Polysaccharide from Cymbopogon citratus. Front. Nutr. 2022, 11, 911838. [Google Scholar] [CrossRef]
- IFRA Standards. Available online: https://ifrafragrance.org/docs/default-source/ifra-code-of-practice-and-standards/ifra-standards---48th-amendment/ifra-standards-in-full---booklet.pdf (accessed on 22 May 2024).
- Smith, R.L.; Cohen, S.M.; Doull, J. GRAS flavouring substances 22. Food Technol. 2005, 59, 24–62. [Google Scholar]
- Sinha, S.; Jothiramajayam, M.; Ghosh, M.; Mukherjee, A. Evaluation of toxicity of essential oils palmarosa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem. Toxicol. 2014, 68, 71–77. [Google Scholar] [CrossRef]
- Sousa, S.M.; Silva, P.S.; Viccini, L.F. Cytogenotoxicity of Cymbopogon citratus (DC) Stapf (lemon grass) aqueous extracts in vegetal test systems. An. Acad. Bras. Cienc. 2010, 82, 305–311. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).