1. Introduction
Cancer is a leading cause of death around the world, where lung cancer is the most common cause of cancer-related deaths. Approximately 85% of all lung cancers are non-small cell lung carcinoma (NSCLC) [
1]. Unfortunately, existing therapies do not cure most NSCLC patients. Even chemotherapy (e.g., platinum-based drugs such as cisplatin (CisPt)), the preferred and most effective treatment for lung cancer, only shows a complete response in 30% of individuals with NSCLC [
2]. Additionally, chemoresistance, which results in more aggressive cancer cells, limits the efficacy of chemotherapy. Approximately 10–15% of patients receiving CisPt will relapse and invariably develop chemoresistance, with an expected survival time of no longer than a year [
3]. In the last decade, the approvals for novel targeted and immunogenic anticancer agents have increased exponentially. From 2020 to 2023, sixteen of these new drugs were approved for the treatment of lung cancer, e.g., nivolumab (Opdivo
®) and ipilimumab (Yervoy
®) combined with a platinum-based drug is the first-line therapy for PDL1+ NSCLC [
4]. For these reasons, new and adjuvant therapies are still needed. Phytochemicals such as triterpenes have demonstrated great potential against different cancers [
5] and could work to overcome drug resistance mechanisms [
6].
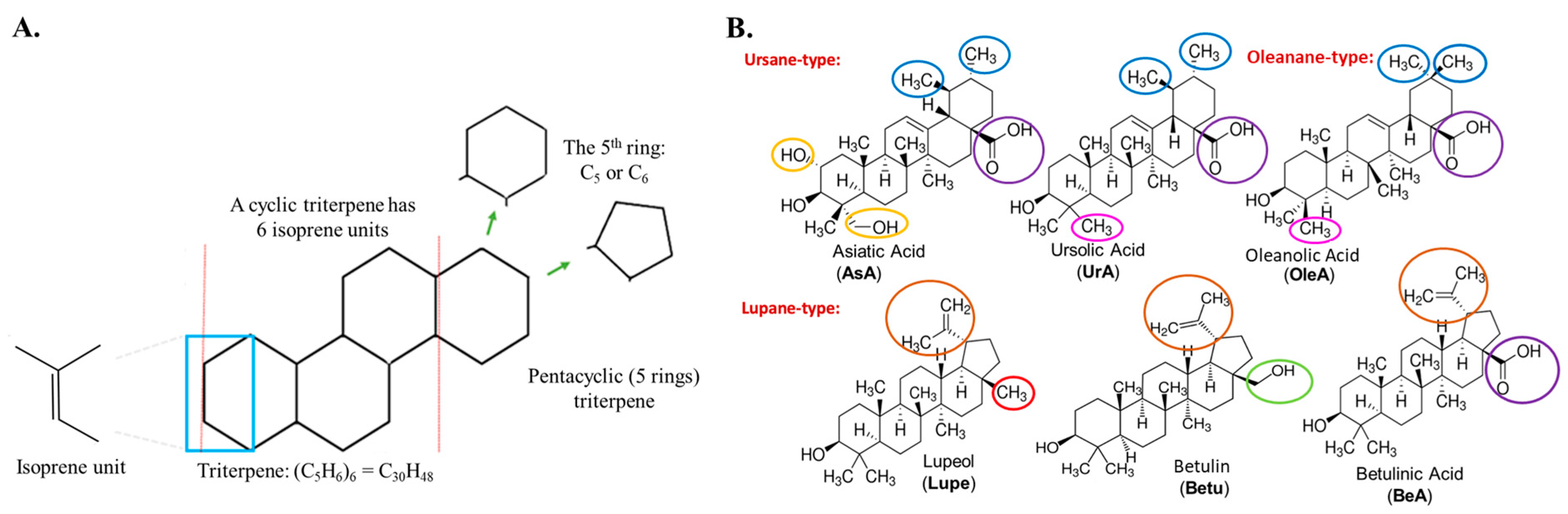
Triterpenes are secondary metabolites abundantly found in numerous plant species and composed of six isoprene units, resulting in a total of 30 carbon atoms in their molecular structure (see
Figure 1A). Among the various triterpenes, pentacyclic triterpenoids exhibit approximately 200 different skeletons, showcasing remarkable structural diversity and potent biomedical activity [
7]. Pentacyclic triterpenes that possess either five six-carbon (C
6) rings (C 6-6-6-6-6) or four six-carbon (C
6) rings and one five-carbon (C
5) ring (C 6-6-6-6-5) are considered the most bioactive triterpenoids [
8].
The ursane, lupane, and oleanane types are widely distributed within higher plants, including edible species. Within the ursane and oleanane types, asiatic acid (AsA), ursolic acid (UrA), and oleanolic acid (OleA) present a C 6-6-6-6-6 structural arrangement. These compounds exhibit very similar structures, with only slight variations in the substituent groups of the fifth C
6 ring. For instance, AsA possesses an additional hydroxyl group at position 2 and a hydroxymethyl substituent at position 4. On the other hand, OleA differs from UrA in that a methyl substituent has been moved from position 19 to position 20. Interestingly, the lupane-type pentacyclic triterpenes, namely lupeol (Lupe), betulin (Betu), and betulinic acid (BeA), exhibit a C 6-6-6-6-5 structural arrangement. The primary distinction among these three compounds lies in the substituent group at position 17. Specifically, Lupe possesses a methyl group, Betu contains a hydroxymethyl group, and BeA features a carboxylic acid group at this position. These subtle but specific variations among these six pentacyclic triterpenoids may play a crucial role in their activity across metabolic pathways, especially in cancer [
9].
Figure 1B provides a visual representation highlighting the differences between these pentacyclic triterpenes.
Herein, we investigated a library of six structurally similar pentacyclic triterpenes, AsA, UrA, OleA, Lupe, Betu, and BeA, against NSCLC. To study their cellular mechanistic effects, we determined the impact on the cell cycle, DNA, production of reactive oxygen (ROS), and activation of apoptosis and autophagy. In addition, we determined the protein expression of the mitogen-activated protein kinase/phosphatidylinositol 3-kinase (MAPK/PI3K) and the gene expression of ribonucleotide reductase (RR), programmed death-ligand 1 (PDL1), and signal transducer and activator of transcription 3 (STAT3), key markers of chemoresistance in NSCLC. This study opens up promising perspectives for further research on the role of triterpenoids as therapeutics, which ultimately will contribute to their more rational application in lung cancer therapy.
3. Discussion
Triterpenes are known for their wide range of benefits, including their anti-inflammatory, antimicrobial, antiviral, analgesic, anti-tumorigenic, and immunomodulatory characteristics [
5,
6,
7,
8,
11]. For this project, we aimed to study a library of six pentacyclic triterpenes with similar structures to determine which one was the most potent against lung carcinoma, one of the deadliest cancers. While previous research has demonstrated the potency of these triterpenes, our study aimed to determine which one had the most effective structural arrangement for potency, selectivity, and the targeting of key pathways against chemoresistance, the major problem contributing to chemotherapy failure. We selected pentacyclic triterpenes because, in our previous study, we found that the fifth ring of the structure is essential for the cytotoxic activity in NSCLC A549 cells. For instance, even at the highest concentration of 100 μM, β-sitosterol and cholesterol (tetracyclic triterpenes) did not kill A549 cells [
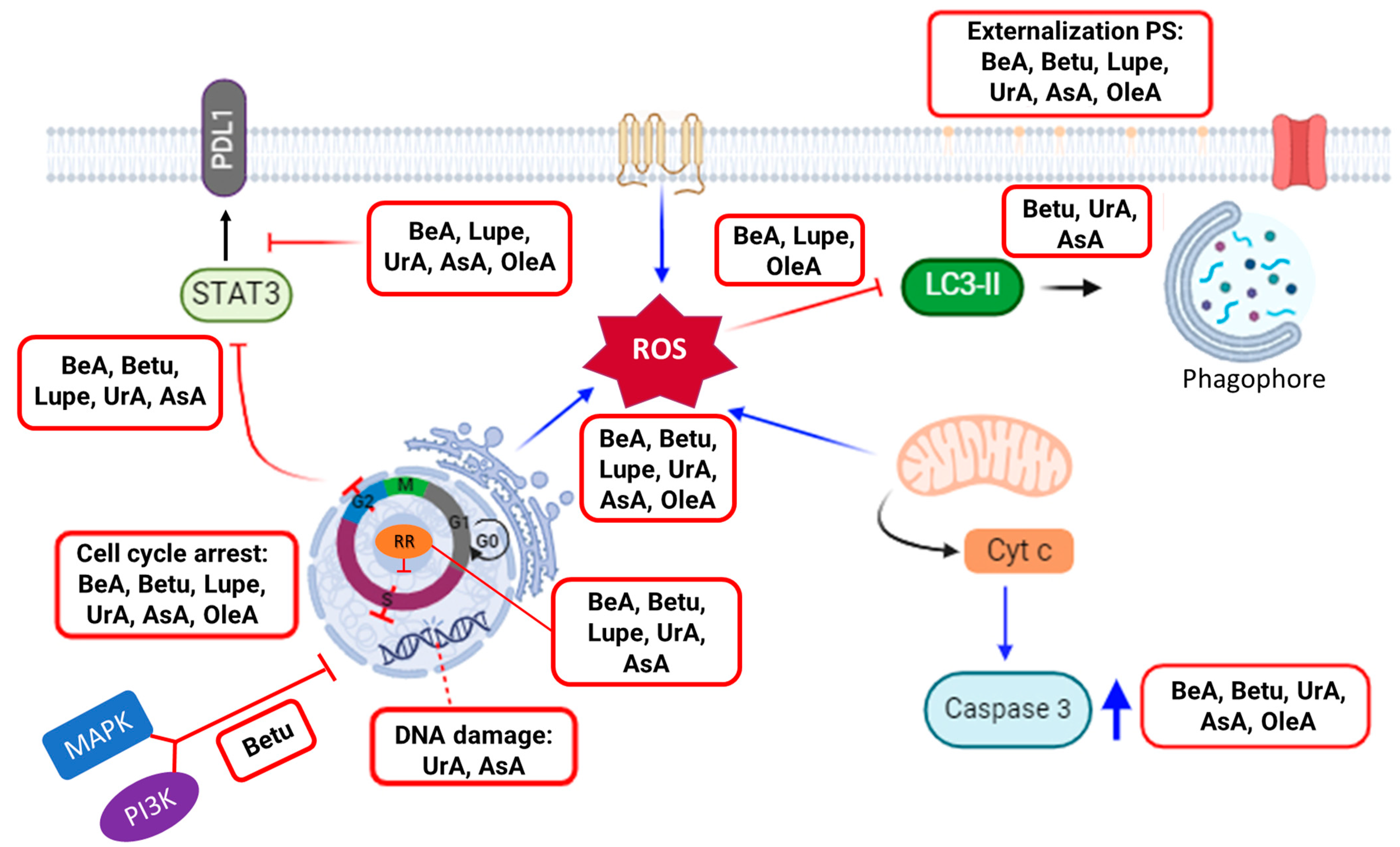
10]. Thus, this study explored the underlying mechanistic principles (
Figure 7) with a particular emphasis on key pathways to induce cell death (i.e., cell cycle arrest, expression of RR, DNA damage, activation of caspase 3, and oxidative stress) but also in chemoresistance-related processes (i.e., inhibition of autophagy; MAPK, PI3K, STAT3, and PDL1 expression).
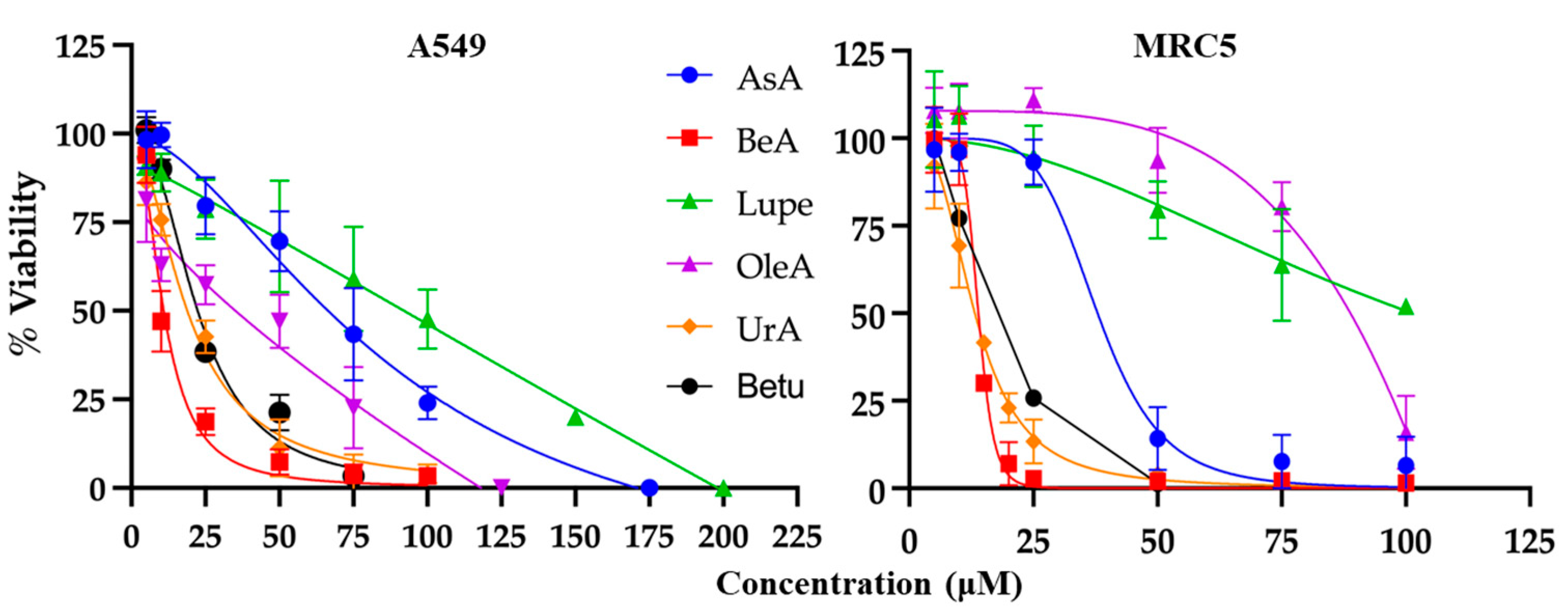
Our experimental results revealed distinct patterns in the response of NSCLC cells to the six pentacyclic triterpenes under scrutiny. The viability assays demonstrated that Lupe had the highest IC
50, OleA had the best TI, and BeA had the lowest IC
50 with a TI >1. Normal lung cells such as MRC5 have been considered as cancer-associated fibroblasts (CAF), even when they are non-cancerous cells [
12]. This means that an effective therapy must kill both cell lines (NSCLC and CAF) but behave aggressively towards cancerous cells. In this context, BeA showed the lowest IC
50 to kill both cell lines (NSCLC and CAF) but behaved more aggressively towards the NSCLC A549 cells among the six triterpenes. These results suggest that a methyl substituent in lupane-type triterpenes, instead of carboxylic acid or hydroxyl, can drastically diminish their cytotoxicity. On the other hand, changes in the methyl substituents in OleA (vs. UrA) diminished their cytotoxicity in cells but increased their selectivity. Previous studies have found similar IC
50 values in the μM range in a 24 h treatment period for the six selected pentacyclic triterpenes exposed to different cancer cell types [
13,
14,
15,
16,
17,
18]. This highlights the versatility of using triterpenes against cancer in different organs and tissues.
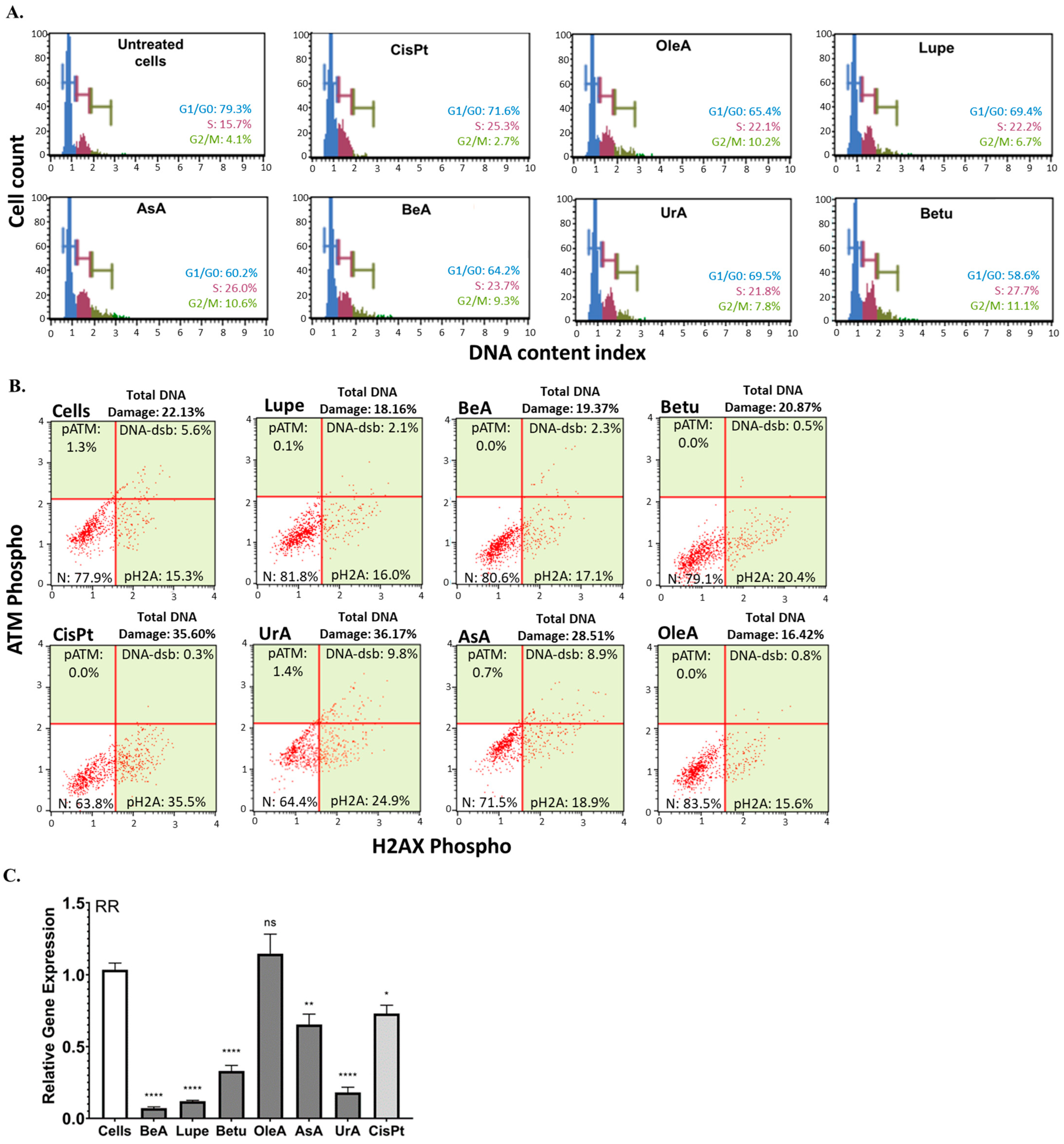
Nuclear processes such as the cell cycle are important pathways that promote cancer progression. Cell cycle arrest in response to DNA damage is a critical mechanism that helps to prevent the propagation of cancer cells and to induce apoptosis [
19]. In this study, all triterpenes produced cell cycle arrest in the S phase and G2/M phase. There are several studies that have shown similar results, where these triterpenes exhibited S-phase and G2/M-phase arrest in myeloma, gastric, breast, and lung cancer cells [
20,
21,
22,
23]. These results suggest that, regardless of their structural variations, these six pentacyclic triterpenes may trigger cell cycle arrest in these two critical divisions in NSCLC, implying that they all may be able to prevent the proliferation of these cancer cells. In addition, only the ursane-type UrA and AsA caused DNA damage, implying their other potential targets to kill cancer cells compared to the other four triterpenes. These results revealed that, unlike lupane-type triterpenes, ursane-type triterpenes might employ DNA damage machinery to induce apoptosis. These results support previous studies that highlight UrA’s ability to increase DNA damage and chemosensitize A549 cells [
24]. In contrast, there are several studies that have demonstrated that lupane-type triterpenes are able to cause DNA damage [
25,
26]. RR is an enzyme complex composed of two subunits: a catalytic alpha subunit (RRM1) and a radical-generating beta subunit (RRM2). These subunits are responsible for catalyzing the rate-limiting step for DNA synthesis and are known to be elevated in a variety of cancers because of their influence on invasion, including NSCLC [
27]. This study found that the lupane- and ursane-type triterpenes downregulated the RRM1 subunit gene. No previous studies have demonstrated that these pentacyclic triterpenes affect RR. However, the literature indicates that another pentacyclic (C6-6-6-6-6) triterpene, celastrol, downregulates RRM2 [
28].
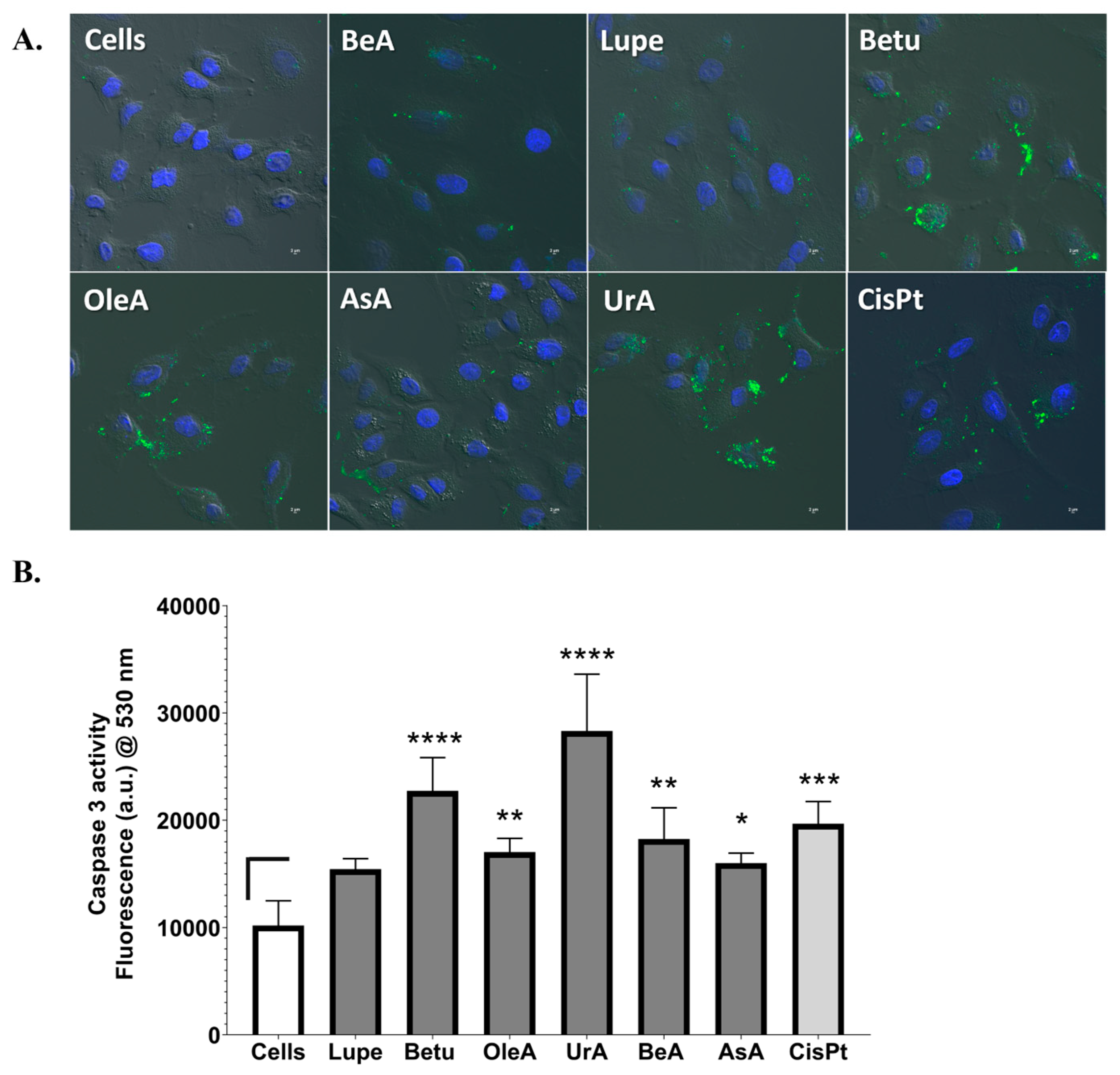
Another important pathway is the activation of apoptosis through the mitochondrial response and the externalization of PS on the cell membrane. The disruption of the mitochondrial membrane potential can lead to the release of cytochrome c, which activates caspase 3, initiating intrinsic apoptosis and, then, cell death [
29]. Another explanation for this mechanism is that the proapoptotic protein Bid can directly activate caspase 8 or translocate to the mitochondrion, leading to the release of cytochrome c to activate caspases 3/7, regardless of the mitochondrial potential [
30]. Most studies in the literature show that pentacyclic triterpenes induce both intrinsic and extrinsic apoptosis by caspase 3 and caspase 8, respectively [
31]. In the current study, all triterpenes except Lupe activated caspase 3. In addition, all triterpenes activated the early apoptotic cellular process on the A549 cells, where Lupe and BeA induced the lowest level of activation. Interestingly, BeA demonstrated the low activation of early apoptosis and caspase 3 and no DNA damage, while UrA showed the activation of the DNA damage machinery, as well as the strongest activation of caspase 3 and the externalization of PS. Structurally, BeA and UrA were demonstrated to more aggressively attack different pathways in NSCLC.
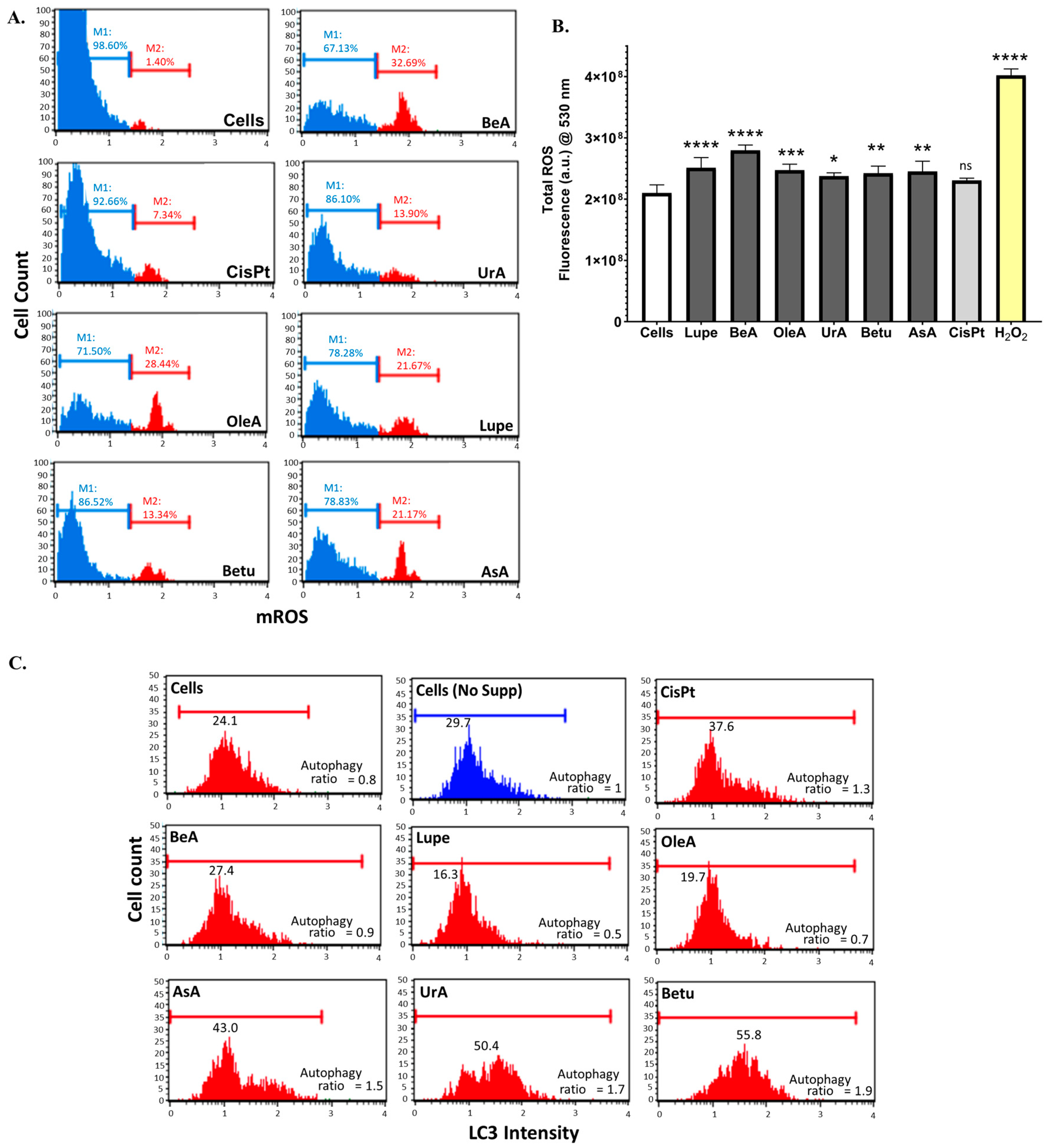
Oxidative stress, such as ROS levels, can modulate autophagy. Mild amounts of ROS promote the autophagic response, while high concentrations inhibit autophagy and promote cell death [
32]. To determine the mROS, we used the DHE dye, which reacts mainly with superoxide species [
33]. Although all triterpenes produced mROS, compared to the untreated cells, the amounts of mROS from BeA, OleA, and Lupe were, on average, 26.2%, while Betu, AsA, and UrA showed an average of 10.1%. Thus, BeA, OleA, and Lupe did not activate autophagy because of the high concentration of ROS. On the other hand, Betu, AsA, and UrA activated the autophagic response via the lower levels of ROS. Although mitochondria are the main suppliers of ROS, other organelles, such as the endoplasmic reticulum, also play important roles in ROS production [
34]. Thus, we decided to test the total amount of ROS using the DCF dye, which labels mostly the peroxide species as the end products of free radicals during oxidation reactions. Again, BeA showed the highest increase in total ROS, in agreement with the increase in mROS. Numerous studies agree with our results showing that triterpenes can induce cell death via the production of ROS, as summarized in the review in [
31]. It is important to understand that autophagy can suppress tumor development in the early stages, but, once the tumor is established, it promotes chemotherapy resistance [
35]. Based on the structural aspects of these triterpenes, our results suggest that the skeletal carbon ring structure of the pentacyclic triterpenes is essential in triggering the formation of mROS. Moreover, the results revealed that most of the differences in the six pentacyclic triterpenes’ structures and their substituents changed the ROS production levels, but this was not sufficient to inhibit this process. A possible explanation is that these triterpenes inactivate iron complex I and iron complex III in the electron transport chain. Thus, under increased ROS levels, iron is released from these complexes and apoptosis is switched to a process called ferroptosis, which depends on the presence of free iron and high oxidant conditions [
36]. In this way, BeA can activate apoptosis but mostly ferroptosis by high ROS and no autophagy. In contrast, UrA can activate mostly apoptosis and autophagy by DNA damage and moderate levels of ROS.
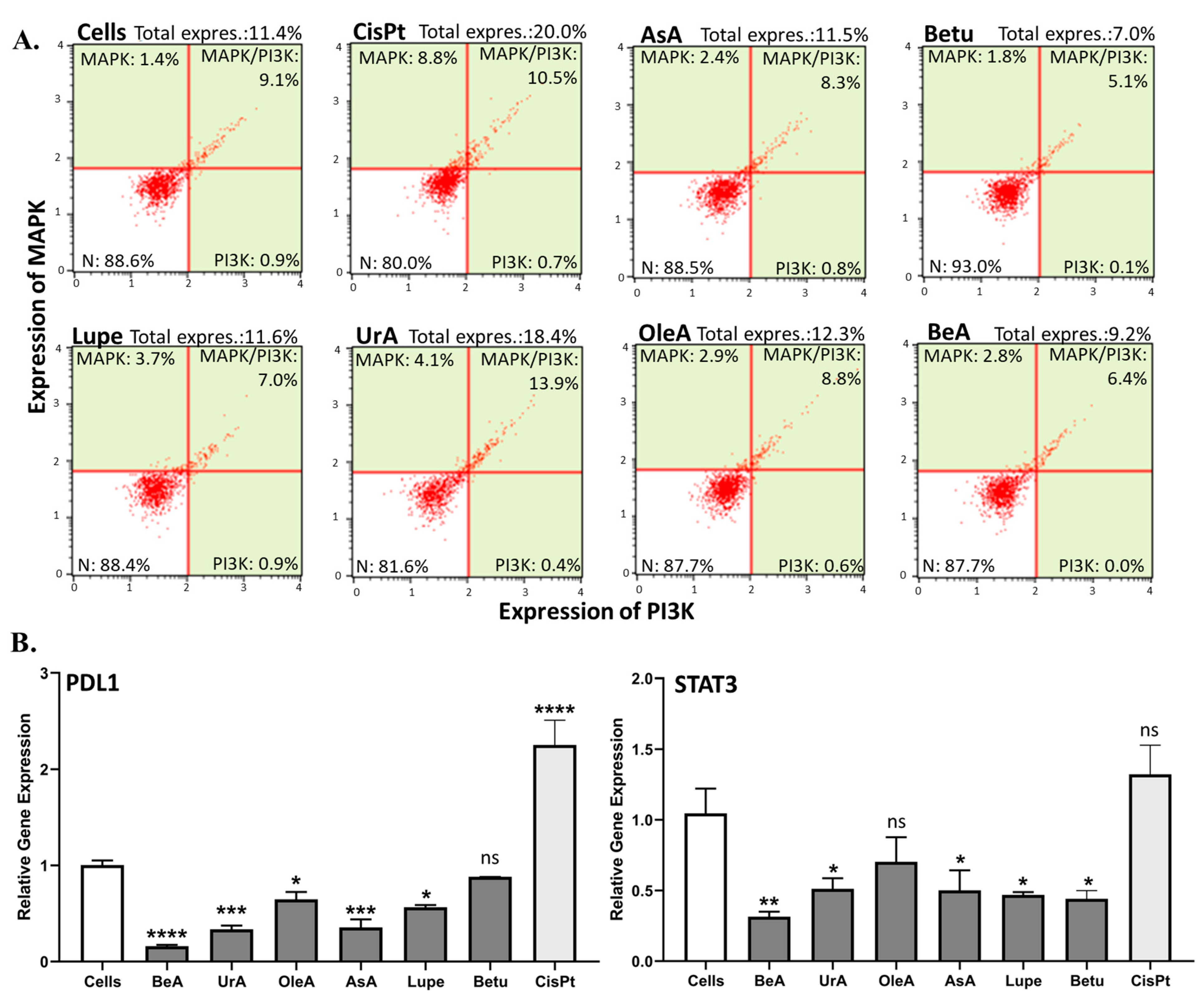
MAPK/PI3K has been linked to the upregulation of PDL1, which leads to immune suppression and chemoresistance [
9,
37]. Meanwhile, the STAT3-mediated upregulation of PDL1 expression in cancer cells has been observed [
37]. In our experiments, only UrA slightly increased the expression of MAPK/PI3K when compared to the negative (untreated cells) and positive (CisPt) controls. These results suggest that lupane-type triterpenes with a 6-6-6-6-5 structural arrangement (where Betu < BeA < Lupe) are superior in limiting cell progression and survival by MAPK/PI3K. Regarding the chemoresistance markers STAT3 and PDL1, our results revealed that most of the pentacyclic triterpenes suppressed these two genes, where the chemotherapy CisPt clearly upregulated PDL1. However, BeA was the greater inhibitor of PDL1 and STAT3 gene expression, while it did not increase MAPK/PI3K expression. This outcome is also in agreement with our hypothesis of the activation of ferroptosis, which may sensitize chemoresistant cancer cells [
36]. In the literature, we found similar results for MAPK, PI3K, PDL1, and STAT3 after BeA treatment [
38,
39]. UrA was also shown to downregulate STAT3 and PDL1 [
40].
Computational approaches provide detailed information to predict the pharmacokinetic properties, biological activity, and molecular mechanisms of small molecules, including their interactions with specific protein targets with associated signaling pathways [
41]. For example, from a database of 339 proteins, ChEMBL predicted interactions with approximately 25 targets with an activity threshold of 5–7.5. In evaluating the data, we selected targets with stronger predictive properties: lower activity threshold values and confidence intervals of 70, 80, and 90%. These protein targets were of the following types: (a) membrane-associated proteins, receptors, and ion channels; (b) nuclear proteins; (c) cytoplasmic enzymes; and (d) cytokines and signaling molecules (shown in
Table S1). Thus,
Table 2 shows only the cancer-related targets (~five for each triterpene). Most of these triterpenes have very similar targets. Interleukin-2 is a common target of all six triterpenes. Dihydrofolate reductase and WD repeat-containing protein 5 are targeted by all except AsA. TNF-alpha is targeted by all except Lupe and Betu. The LSD1/CoREST complex is targeted by all except OleA and UrA. Adenosine deaminase is only targeted by lupane-type triterpenes. On the other hand, mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1) and gamma-secretase are only targeted by AsA, and carbonic anhydrase IX is only targeted by Lupe. Knowing that ChEMBL calculates the probability of each triterpene interacting with the protein targets in the database (not their specific activation or inhibition), we can make several important connections based on the molecules predicted to be targeted. The most important findings from these predictions are that these pentacyclic triterpenes have the ability to trigger an inflammatory response (IL-2 and TNF-alpha) by the activation of adenosine deaminase. This process may induce immunostimulation to attack tumor cells [
42]. In addition, only AsA could target MALT1, which acts as a scaffolding protein to drive immune activation [
43]. This prediction confirmed our experimental results on the downregulation of PDL1 and STAT3. Furthermore, these triterpenes can interact with proteins such as the LSD1/CoREST complex, WD repeat-containing protein 5, and dihydrofolate reductase, which regulate the cell cycle, DNA synthesis, and genomic instability [
19]. This prediction could explain our experimental results regarding cell cycle arrest in the S phase and G2/M. Similarly, targeting carbonic anhydrase IX could disrupt the stability of the hypoxic tumor environment. Targeting carbonic anhydrase IX is a potential therapeutic strategy to starve tumors and limit their spread [
44]. In particular, AsA is predicted to interact with gamma-secretase, which is a novel therapeutic strategy in cancer that involves blocking Notch cleavage [
45].
In summary, BeA emerges as the triterpene exerting the most widespread influence on several key pathways related to ROS-mediated cell death, with the lowest IC
50 and a TI > 1, and inhibits the chemoresistance-related genes STAT3 and PDL1. Theoretically, BeA can also modulate the immune response by interacting with IL-2, TNF-alpha, and adenosine deaminase. Based on the structure, the C 6-6-6-6-5 arrangement substituted with a carboxylic acid and a HC=C-CH
2 group is the most potent against NSCLC. These findings collectively indicate that triterpenes impact different pathways depending on the substituents of their rings. While BeA has the lower IC
50 of 11 μM, it is important to note that its TI is narrow but above 1. However, OleA showed the best TI (= 2), making it a promising candidate for derivatization. A low IC
50 reflects the potency of a drug in inhibiting cancer cell growth, while a high TI is crucial in ensuring the safety and tolerability of the drug. Both parameters are important considerations in cancer therapeutics, with the ideal drug having both a low IC
50 and a high TI. However, MEK inhibitors, clinically approved chemotherapeutics, have a TI ~ 1 [
46]. Based on these findings, future research can utilize the most effective patterns of parent molecules BeA and OleA for derivatization. Specifically, the alternate methyl groups of the fifth C
6 ring of OleA could be the key to higher selectivity. A possibility to consider is a BeA derivative that includes the original substituent of the HC=C-CH
2 group in the fifth C
5 ring to maintain the potency, as well as the alternate methyl groups of OleA to increase the selectivity. To validate our arguments about OleA, two synthetic derivatives of OleA, CDDO-Me and CDDO-Im, were studied in phase I clinical trials [
47,
48]. Furthermore, OleA in combination with curcumin was examined in a phase I clinical trial [
49]. On the other hand, selectivity of BeA could be increased by its encapsulation into a targeted drug delivery system (DDS). Thus, we previously loaded BeA in a protein-based DDS, demonstrating that BeA was synergized with doxorubicin chemotherapy in A549 cells [
50].
4. Materials and Methods
4.1. Chemicals and Reagents
Aqueous solutions were prepared with sterile (autoclave conditions: 121 °C and 18 PSI), high-quality nanopure water (18.2 MΩ cm resistivity, Thermo Scientific™ Barnstead™ Easypure™ II system (Thermo Fisher Scientific, Waltham, MA, USA)).
Asiatic acid, ursolic acid, oleanolic acid, betulin, lupeol, betulinic acid, 2′,7′-dichlorodihydrofluorescein diacetate (DCF) and cis-diamminedichloroplatinum (II) (cisplatin, CisPt) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The cell lines A549 (human lung adenocarcinoma; ATCC CCL-185) and MRC5 (human fibroblast-like cells; ATCC CCL-171) were obtained from the American Type Culture Collection (Manassas, VA, USA). The CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent) was obtained from the Promega Corporation (Madison, WI, USA). The cell cycle, MAPK/PI3K expression, DNA damage, and mitochondrial oxidative stress assays were obtained from the Luminex Corporation (Austin, TX, USA). Reagents for Taqman gene expression assays were purchased from Applied Biosystems (Waltham, MA, USA). All other chemicals were purchased from various suppliers, of analytical grade, and used without further purification.
4.2. Cell Culture Conditions
The human NSCLC A549 and normal lung MRC5 cell lines were maintained following the ATCC protocols. These cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM-high glucose) containing 1% L-glutamine, 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin. Cells were kept in a humidified incubator under 5% CO2 and 95% air at 37 °C. We conducted all of the experiments before the cells reached 25 passages. For all experiments, the triterpenes were initially dissolved in pure dimethylformamide (DMF) and subsequently diluted in phosphate-buffered saline (PBS), ensuring a final DMF concentration in the cells of less than 1%. CisPt (25 μM) was included as a control to compare the effects of the triterpenes with the most used chemotherapy in NSCLC.
4.3. Cell Viability
The 50% inhibitory concentration (IC
50) of some of these triterpenes in A549 NSCLC cells were previously determined by us [
10]. A similar procedure was performed to determine the IC
50 of the triterpenes in a normal lung fibroblast cell line (MRC5). In brief, the A549 and MRC5 cells were seeded into 96-well plates at a density of 1 × 10
5 cells/mL in supplemented DMEM medium. The cells were treated with specific concentrations (A549: 5, 10, 25, 50, 75, 100, 125,150, 175, 200 μM; MRC5: 5, 10, 25, 50, 75, 100 μM) of the triterpenes and incubated for 24 h. Then, 10 uL of the MTS reagent from the CellTiter 96
® Aqueous Non-Radioactive Cell Proliferation Assay (Promega G5421) was added to each well. Then, we incubated the plates at 37 °C and in a 5% CO
2 atmosphere for 1 h. After incubation, the absorbance was measured at 492 nm (
n = 8) in a microplate reader spectrophotometer (Thermoscientific Multiskan FC). The cell viability was calculated as follows:
The cell viability data were presented by plotting the values with an average of eight measurements for each treatment (mean ± SD) in at least three independent experiments. The IC50 values were calculated with the GraphPad Prism 9 software (Prism 9; GraphPad by Dotmatics, San Diego, CA, USA) using the dose–response curve. The data were normalized by the non-linear fit of the log (drug inhibition) vs. normalized response variable slope to obtain the best fit IC50 and R-squared values.
4.4. Apoptosis Externalization of Phospatidylserine (PS) Annexin V Assay
Early apoptotic activity was evaluated by the externalization of PS using the Alexa Fluor® 488 Annexin V Apoptosis Reagent (Molecular Probes™) and NucBlue ReadyProbes Reagent™ (Thermo Fisher Scientific). For this assay, A549 cells were seeded in 8-well cover slip plates with a density of 1 × 105 cells/mL for 24 h in supplemented DMEM. Then, the A549 cells were incubated with the triterpenes (IC25) and controls for 24 h. Afterward, the cells were washed with 1× PBS and incubated with NucBlue (DAPI analog) and Annexin V for 15 min in the dark. The cells were fixed with 3.7% formaldehyde solution for 15 min, washed three times with 1× PBS, and covered with glycerol before visualization. The plates were observed by confocal microscopy using a Nikon Eclipse Ti microscope (Nikon Instruments Inc., El Segundo, CA, USA), utilizing a 20× oil objective with filter sets compatible with DAPI (Ex/Em = 405/460 nm) and Annexin V Alexa Fluor (Ex/Em = 488/525 nm). The fluorescence intensity was analyzed using the NIS-Elements Viewer program (version 5.21 64-bit) (Nikon Instruments Inc.). Each sample was analyzed in duplicate in two independent experiments. The fluorescence intensity was adjusted using untreated cells for each fluorescence channel (green and blue). Subsequently, this calibrated intensity was applied for the analysis of all treated cells.
4.5. Caspase 3 Activity Assay
The intrinsic apoptosis activity was evaluated by the quantification of caspase 3 enzymatic activity using the Apo-ONE® Homogeneous Caspase-3/7 Assay (Promega G7792). A549 cells were seeded in a concentration of 1 × 105 cells/mL into black 96-well plates with a clear bottom. After 24 h, the cells were incubated with the triterpenes (IC50) and controls for 24 h. After the completion of the incubation, 50 μL of Apo-ONE® Caspase-3/7 Reagent (1:100 rhodamine 110 bis-(N-CBZ-l-aspartyl-l-glutamyl-l-valyl-aspartic acid amide) (Z-DEVD-R110) substrate diluted in lysis buffer) was added to each well. The plate was gently mixed using a plate shaker at 150 rpm for 2 h at room temperature. Then, the fluorescence of each well was measured (Ex/Em = 485/530 nm) in the Tecan® M200 plate reader.
The caspase 3 activity experiments were performed in triplicate. All data were expressed by plotting the values with an average of three measurements for each treatment condition as the mean ± SD and analyzed with the software GraphPad Prism 9 (San Diego, CA, USA). Statistical analysis was performed using one-way analysis of variance (ANOVA) to compare the mean value of each type of treated cells versus the control (untreated cells). The statistical significance defined by the software for p values was **** < 0.0001, *** from 0.001 to 0.0001, ** from 0.001 to 0.01, * from 0.01 to 0.05, and non-significant (ns) ≥ 0.05.
4.6. Flow Cytometry Analysis
We used flow cytometry to analyze several cellular mechanistic pathways in A549 cells after incubation with the triterpenes. In general, the cells were seeded in a 6-well plate at a density of 1 × 106 cells/mL and incubated for 24 h in supplemented DMEM. After 24 h, the A549 cells were treated with the triterpenes, following each manufacturer’s protocol. All of the following experiments were performed in a Muse® Cell Analyzer (EMD Millipore Corporation, Temecula, CA). The instrument was calibrated before each assay using the Muse® System Check Kit (Luminex MCH100101, Luminex Corporation). Each assay was run at least in two independent experiments. For each experiment, all samples were analyzed in triplicate in the instrument and the capillary line was washed after each sample run to avoid artifacts. In addition, to address the issue of cell doublets, we used the Muse® Cell Dispersal Reagent (Luminex Corporation) in all experiments. The concentration of the triterpenes in each assay was selected depending on the cellular concentration necessary to generate the gain signal in the instrument. This means that we attempted to select concentrations avoiding excessive cytotoxicity to accurately determine the respective signal from each assay.
4.6.1. Cell Cycle Arrest
A549 cells were incubated with the triterpenes (IC25) for 24 h. Then, the cells were scraped, centrifuged, and fixed using cold 70% ethanol. Afterward, the fixed cell pellet was incubated with the propidium iodide (PI)-based cell cycle reagent (Luminex MCH100106 kit, Luminex Corporation) for 30 min for immediate measurement.
4.6.2. DNA Damage Induction
A549 cells were incubated with the triterpenes (IC50) for 24 h. Then, the A549 cells were scraped and centrifuged. The pellet was suspended in the assay buffer from the Luminex MCH200107 kit (Luminex Corporation). Later, the cell pellet was fixed and permeabilized. Afterward, the antibodies anti-pH2AX (histone) and anti-pATM (ataxia-telangiectasia mutated) were added and incubated for 30 min. Next, the cell pellet was washed and suspended in fresh assay buffer for measurement.
4.6.3. Mitochondrial Oxidative Stress
The production of reactive oxygen species (ROS) by mitochondria was determined using the Oxidative Stress Kit (Luminex MCH100111, Luminex Corporation). After the A549 cells were treated with the triterpenes (IC25) for 24 h, the cells were scraped and centrifuged. For ROS, the cell pellet was incubated with dihydroethidium (DHE) dye for 1 h at 37 °C.
4.6.4. Autophagy Activation
The Muse® Autophagy LC3 Antibody-Based Kit (Luminex MCH200109, Luminex Corporation) was used to determine autophagy activation by monitoring lipidated LC3-II. After the A549 cells were treated with the triterpenes (IC25) for 24 h, autophagy reagent A was added for 4 h to prevent the lysosomal degradation of LC3-II. Then, the cells were detached and centrifuged. The pellet was suspended and incubated for 30 min in the autophagy reagent B containing anti-LC3-II mouse monoclonal antibody. Finally, the cell pellet was washed and suspended in fresh assay buffer for measurement. The autophagy response was calculated using the following formula:
Autophagy ratio = mean autophagy value of treated or untreated cells/mean autophagy value of starving cells.
The starving cells were labeled ‘No supp’ as non-supplemented cells. These served as the positive signal for autophagy and were designated a ratio of 1 since they were compared to themselves. A ratio ≥1 indicates the activation of the autophagic response.
4.6.5. MAPK/PI3K Expression
The Muse® P13K/MAPK Dual Pathway Activation Kit (Luminex MCH200108, Luminex Corporation) was used to determine MAPK/PI3K expression. After the incubation treatment with the triterpenes (IC25) for 24 h, the A549 cells were scraped, centrifuged, and washed once with 1× PBS. Next, the fixation buffer was incubated for 10 min and then the permeabilization buffer for another 10 min. Both incubations were performed on ice. Later, the antibody cocktail containing anti-phospho-Akt and anti-phospho-ERK1/2 was incubated for 30 min. Afterward, the cell pellet was washed and suspended in fresh assay buffer for measurement.
4.7. Total Oxidative Stress
The production of total ROS was determined using the 2′,7′-dichlorodihydrofluorescein diacetate (DCF) fluorescent dye. A549 cells were seeded in a concentration of 1 × 105 cells/mL into black 96-well plates with a clear bottom. After 24 h, the cells were incubated with the triterpenes (IC25) and controls for 24 h. Then, 50 μL of DCF (10 μM) was added to each well and they were incubated for 1 h at room temperature. Then, the fluorescence of each well was measured (Ex/Em = 485/530 nm) in the Tecan® M200 plate reader.
This experiment was performed in triplicate. All data were expressed by plotting the values as an average of three measurements for each treatment condition as the mean ± SD and analyzed with the software GraphPad Prism 9. Statistical analysis was performed using one-way analysis of variance (ANOVA) to compare the mean value of each treated type of cells versus the control (untreated cells). The statistical significance defined by the software for p values was **** < 0.0001, *** from 0.001 to 0.0001, ** from 0.001 to 0.01, * from 0.01 to 0.05, and non-significant (ns) ≥ 0.05.
4.8. RNA Extraction and Real-Time (RT) Quantitative PCR
A549 cells were seeded into 6-well plates at a density of 1 × 106 cells/mL in supplemented DMEM. The cells were incubated with the triterpenes (IC25) for 24 h. Total RNA was extracted from the cells using the TRIzol reagent (Invitrogen Corporation, Carlsbad, CA, USA) according to the manufacturer’s instructions. The purity and concentration of RNA were assessed using a NanoDrop spectrophotometer (Thermo Fisher Scientific). cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer’s instructions.
RT-qPCR was performed on a StepOnePlus® Real-Time PCR System (Thermo Fisher Scientific) using TaqMan Gene Expression Assays (Applied Biosystems) for PDL1 (CD274; Assay ID: Hs01125297_m1), ribonucleotide reductase (RR) M1 (Assay ID: Hs01040697_m1), and STAT3 (Assay ID: Hs01047586_g1). Each reaction consisted of 10 ng of cDNA, TaqMan Gene Expression Master Mix (Applied Biosystems), and TaqMan probes. The PCR cycling conditions were as follows: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. The relative gene expression levels were calculated using the ΔΔCt method. GAPDH (Assay ID: Hs02758991_g1) was used as the endogenous control for normalization.
All RT-qPCR experiments were performed at least in triplicate. All data were expressed by plotting values with an average of four measurements for each treatment condition as the mean ± SD and analyzed with the software GraphPad Prism 9. Statistical analysis was performed using one-way analysis of variance (ANOVA) to compare the mean value of each treated type of cells versus the control (untreated cells). The statistical significance defined by the software for p values was **** < 0.0001, *** from 0.001 to 0.0001, ** from 0.001 to 0.01, * from 0.01 to 0.05, and non-significant (ns) ≥ 0.05.
4.9. Theoretical Target Predictive Analysis
Computational analysis is an approach that helps to identify novel targets from extensive databases. We selected the curated database of bioactive molecules ChEMBL (from EMBL’s European Bioinformatics Institute) for our analysis. ChemBL was accessed through the online platform (
https://www.ebi.ac.uk/chembl/) (accessed on 1 May 2024), where we searched the chemical structures and ChemIDs of the six pentacyclic triterpenes of interest. By applying filters such as human species specificity, active/inactive, confidence levels, and activity thresholds (logarithmic potency values), we retrieved the predictive information on the targets, including the protein names and triterpene compound IDs. The target prediction returned a result of ‘active’ or ‘inactive’ depending on whether the compound was predicted to interact or not with the target, ‘empty’ if the model was unable to make a prediction, or ‘both’ if it could not make a conclusion.