Effects of Chicory (Cichorium intybus L.) Extract on Male Rat Reproductive System, Pregnancy and Offspring Development
Abstract
1. Introduction
2. Results
2.1. The Effect of CE on the Reproductive Function of Male Rats
2.2. The Effect of CE on Morpho-Functional Parameters of Epididymal Spermatozoa of Male Rats
2.3. Characteristics of the Functional State of Leydig Cells
2.4. Effects of CE on Male Rat Fertility
2.5. The Effect of CE Male Rat Treatment on Offspring Development
3. Discussion
4. Materials and Methods
4.1. Phytodrug
4.2. Experimental Animals
4.3. CE Treatment
4.4. Analysis of CE Effects on the Male Reproductive System
4.5. Analysis of CE Effects on Male Fertility
4.6. Investigation of PADE Effects on Fetal and Offspring Development
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mann, U.; Shiff, B.; Patel, P. Reasons for worldwide decline in male fertility. Curr. Opin. Urol. 2020, 30, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Methorst, C.; Perrin, J.; Faix, A.; Huyghe, E. Infertilité masculine, environnement et mode de vie. Progrès En Urol. 2023, 33, 613–623. [Google Scholar] [CrossRef]
- Perheentupa, A.; Toppari, J. Male fertility and semen quality are decreasing—Do we have the expertise to deal with this challenge? Acta Obstet. Et Gynecol. Scand. 2023, 102, 1606–1607. [Google Scholar] [CrossRef] [PubMed]
- Ameratunga, D.; Gebeh, A.; Amoako, A. Obesity and male infertility. Best. Pract. Res. Clin. Obstet. Gynaecol. 2023, 90, 102393. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Minhas, S.; Dhillo, W.S.; Jayasena, C.N. Male infertility due to testicular disorders. J. Clin. Endocrinol. Metab. 2021, 106, e442–e459. [Google Scholar] [CrossRef] [PubMed]
- Karomatov, I.D.; Abduvokhidov, A.T. Medicinal herbs possessing spermatoprotective properties. Biol. Integr. Med. 2018, 6, 96–116. [Google Scholar]
- Santos, H.O.; Howell, S.; Teixeira, F.J. Beyond tribulus (Tribulus terrestris L.): The effects of phytotherapics on testosterone, sperm and prostate parameters. J. Ethnopharmacol. 2019, 235, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Ramgir, S.S.; Renu, K.; Vellingiri, B.; George, A.; Tirupapuliyur, D.; Thiagarajan, P.; Valsala Gopalakrishnan, A. Phytomedicinal therapeutics for male infertility: Critical insights and scientific updates. J. Nat. Med. 2022, 76, 546–573. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.F. Ethnobiology and Ethnopharmacology of Lepidium meyenii (Maca), a Plant from the Peruvian Highlands. Evid. Based Complement. Altern. Med. 2012, 2012, 193496. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, G.K.; Mahajan, A.Y.; Mahajan, R.T. Efficacy of Aphrodisiac Plants towards Improvement in Semen Quality and Motility in Infertile Males. J. Complement. Integr. Med. 2012, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tahvilzadeh, M.; Hajimahmoodi, M.; Toliyat, T.; Karimi, M.; Rahimi, R. An evidence-based approach to medicinal plants for the treatment of sperm abnormalities in traditional Persian medicine. Andrologia 2016, 48, 860–879. [Google Scholar] [CrossRef] [PubMed]
- Boroujeni, S.N.; Malamiri, F.A.; Bossaghzadeh, F.; Esmaeili, A.; Moudi, E. The most important medicinal plants affecting sperm and testosterone production: A systematic review. JBRA Assist. Reprod. 2022, 26, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Krepkova, L.V.; Babenko, A.N.; Lemyaseva, S.V.; Saybel, O.L.; Sherwin, C.M.; Enioutina, E.Y. Modulation of Hepatic Functions by Chicory (Cichorium intybus L.) Extract: Preclinical Study in Rats. Pharmaceuticals 2023, 16, 1471. [Google Scholar] [CrossRef] [PubMed]
- Mehran, D.; Seyyed Mansour, S.; Marzieh Noroozi Tabrizi, N. Beneficial effects of Cichorium intybus L. extract on oxidative status and reproductive parameters in male Wistar rats: An experimental study. Int. J. Reprod. Biomed. 2019, 17, 425–434. [Google Scholar] [CrossRef]
- Kondakova, L.I.; Kalashnikova, S.A.; Polyakova, L.V. Morphofunctional status of interstitial endocrinocytes (Leydig cells) with premature aging caused by dark deprivation. J. Volgogr. State Med. Univ. 2023, 20, 70–73. [Google Scholar] [CrossRef]
- Kiss, P.; Tamas, A.; Lubics, A.; Szalai, M.; Szalontay, L.; Lengvari, I.; Reglodi, D. Development of neurological reflexes and motor coordination in rats neonatally treated with monosodium glutamate. Neurotox. Res. 2005, 8, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Abramova, O.; Ushakova, V.; Zorkina, Y.; Zubkov, E.; Storozheva, Z.; Morozova, A.; Chekhonin, V. The Behavior and Postnatal Development in Infant and Juvenile Rats after Ultrasound-Induced Chronic Prenatal Stress. Front. Physiol. 2021, 12, 659366. [Google Scholar] [CrossRef] [PubMed]
- Dorostghoal, M.; Seyyednejad, S.M.; Nejad, M.N.T. Cichorium intybus L. extract ameliorates testicular oxidative stress induced by lead acetate in male rats. Clin. Exp. Reprod. Med. 2020, 47, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Eltamany, E.E.; Mosalam, E.M.; Mehanna, E.T.; Awad, B.M.; Mosaad, S.M.; Abdel-Kader, M.S.; Ibrahim, A.K.; Badr, J.M.; Goda, M.S. Potential Gonado-Protective Effect of Cichorium endivia and Its Major Phenolic Acids against Methotrexate-Induced Testicular Injury in Mice. Biomedicines 2022, 10, 1986. [Google Scholar] [CrossRef] [PubMed]
- Koloko, B.L.; Bushra, I.; Wankeu-Nya, M.; Ngaha Njila, M.I.; Kenmogne, H.; Nyonseu Nzeubang, D.C.; Nzangueu, C.B.; Dimo, T.; Dongmo, A.B.; Massoma Lembe, D. In vivo effects of Rauvolfia vomitoria (Apocynaceae) ethanolic extract on sexual performance and reproductive activity in male rats. Andrologia 2020, 52, e13414. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhang, Z.; Liu, Y. Difference analysis of different parts of chicory based on HPLC fingerprint and multi-component content determination. Chin. Herb. Med. 2022, 14, 317–323. [Google Scholar] [CrossRef]
- Roy-Choudhury, A.; Venkatakrishna-Bhatt, H. Spermatogenic inhibition by Cichorium intybus L. aqueous root suspension in mice. Naturwissenschaften 1983, 70, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Pizent, A.; Tariba, B.; Živković, T. Reproductive toxicity of metals in men. Arh. Hig. Rada Toksikol. 2012, 63 (Suppl. S1), 35–46. [Google Scholar] [CrossRef]
- Türk, E.; Ozan Tekeli, I.; Özkan, H.; Uyar, A.; Cellat, M.; Kuzu, M.; Yavas, I.; Alizadeh Yegani, A.; Yaman, T.; Güvenç, M. The protective effect of esculetin against aluminium chloride-induced reproductive toxicity in rats. Andrologia 2021, 53, e13930. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Azarnia, M.; Hajebrahimi, Z.; Nejad Ebrahimi, S. The effect of hydroalcoholic extract of Cichorium intybus leaf on aryl hydrocarbon receptor expression in the testis of Wistar rats exposed to cigarette smoke. Avicenna J. Phytomed. 2023, 13, 58–69. [Google Scholar] [CrossRef]
- Liu, Z.J.; Liu, Y.H.; Huang, S.Y.; Zang, Z.J. Insights into the Regulation on Proliferation and Differentiation of Stem Leydig Cells. Stem Cell Rev. Rep. 2021, 17, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Chen, G.; Wang, H. Epigenetic programming of TBX2/CX43 mediates lower sperm quality in male offspring induced by prenatal dexamethasone exposure. Toxicol. Sci. 2023, 192, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Tuimah Alabedi, G.S.; Al-Baghdady, H.F.; Alahmer, M.A.; Bustani, G.S.; Al-Dhalimy, A.M.B. Effects of Ocimum tenuiflorum on Induced Testicular Degeneration by Filgrastim in Wistar Rats. Arch. Razi Inst. 2021, 76, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A. Oxidative Stress and Male Infertility: The Protective Role of Antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Onuah, C.L.; Singh, S.K. Plants in the management of male infertility. Andrologia 2020, 52, e13509. [Google Scholar] [CrossRef] [PubMed]
- Safarnavadeh, T.; Rastegarpanah, M. Antioxidants and infertility treatment, the role of Satureja Khuzestanica: A mini-systematic review. Iran. J. Reprod. Med. 2011, 9, 61–70. [Google Scholar]
- Yimam, M.; Lee, Y.-C.; Hyun, E.-J.; Jia, Q. Reproductive and Developmental Toxicity of Orally Administered Botanical Composition, UP446-Part III: Effects on Fertility and Early Embryonic Development to Implantation in Sprague Dawley Rats. Birth Defects Res. Part. B Dev. Reprod. Toxicol. 2015, 104, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, M.S.; Kim, T.H.; Alraek, T.; Zaslawski, C.; Kim, J.W.; Moon, D.G. Ginseng for erectile dysfunction. Cochrane Database Syst. Rev. 2021, 4, Cd012654. [Google Scholar] [CrossRef]
- Salgado, R.M.; Marques-Silva, M.H.; Gonçalves, E.; Mathias, A.C.; Aguiar, J.G.; Wolff, P. Effect of oral administration of Tribulus terrestris extract on semen quality and body fat index of infertile men. Andrologia 2017, 49. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-H.; Tsai, H.-C.; Hsu, G.-L.; Chen, C.-C.; Hsu, C.-Y. Herb formula enhances treatment of impotent patients after penile venous stripping: A randomised clinical trials. Andrologia 2016, 48, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Saad, B. A Review of the Anti-Obesity Effects of Wild Edible Plants in the Mediterranean Diet and Their Active Compounds: From Traditional Uses to Action Mechanisms and Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 12641. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.N. Guidelines for Preclinical Studies of Drugs. Part 1; Grif and K: Moscow, Russia, 2012. [Google Scholar]
- European Convention for the Protection of Vertebrate Animals Used for Experimental and other Scientific Purposes (ETS 123). Strasbourg, 1986. Available online: https://rm.coe.int/168007a67b (accessed on 13 February 2024).
- Bortnikova, V.V.; Krepkova, L.V.; Babenko, A.N.; Saybel, O.L.; Borovkova, M.V.; Kuzina, O.S.; Mizina, P.G.; Job, K.M.; Sherwin, C.M.; Enioutina, E.Y. A perspective botanical drug: Hepatoprotective activity of dry extract prepared from the aerial part of chicory plant (Cichorium intybus L.), ACCP annual conference abstract #119. Clin. Pharmacol. Drug Dev. 2021, 10 (Suppl. S1), 100. [Google Scholar]
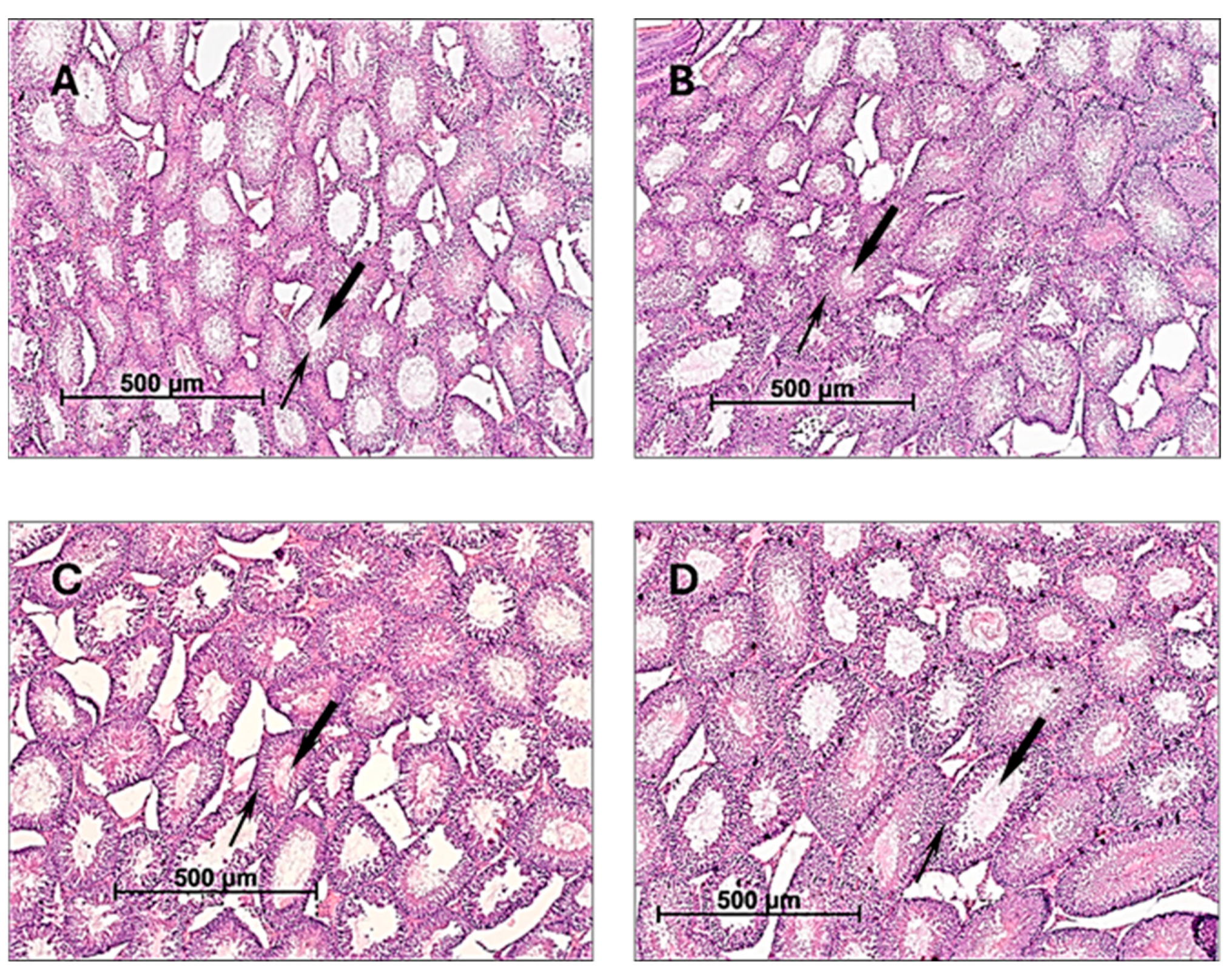

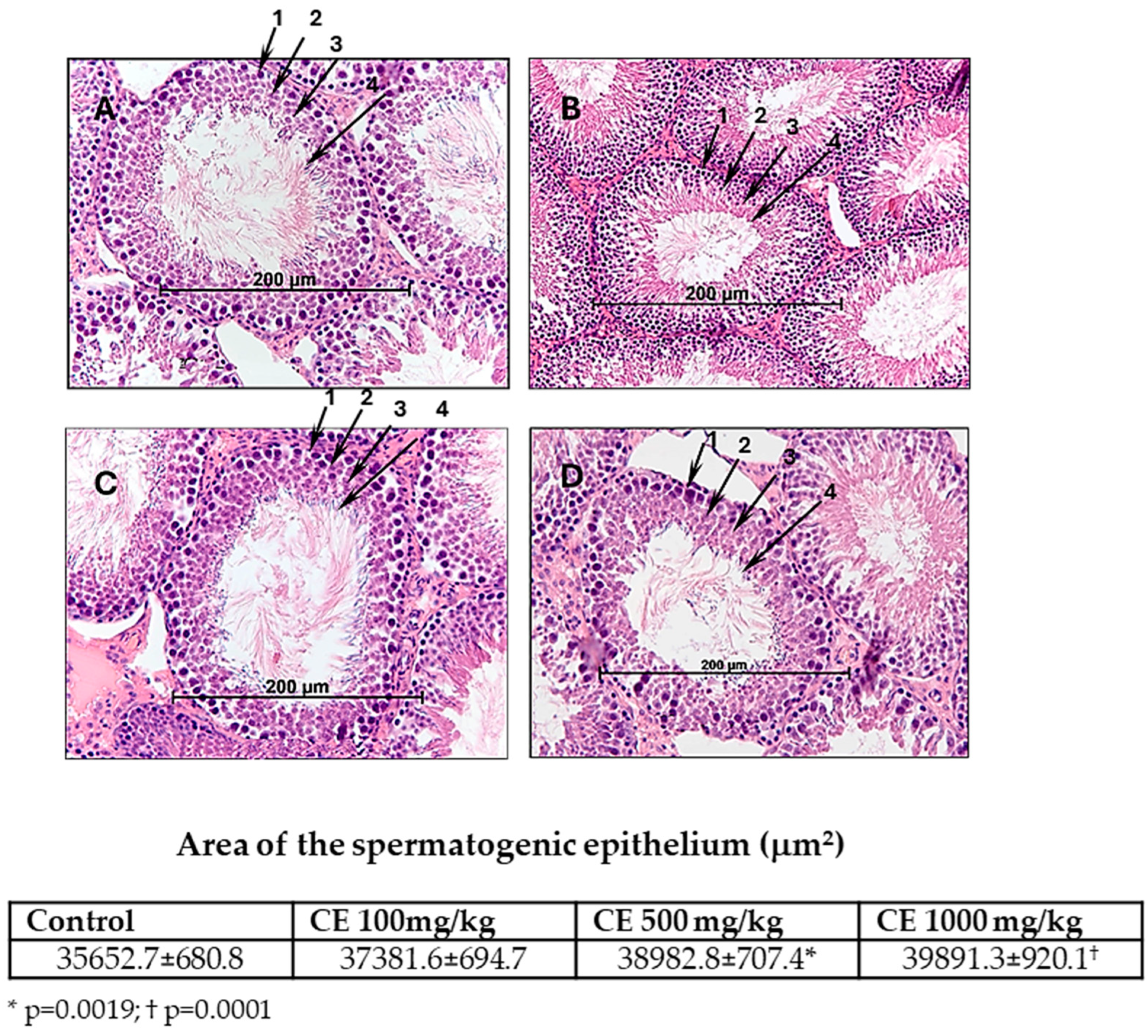
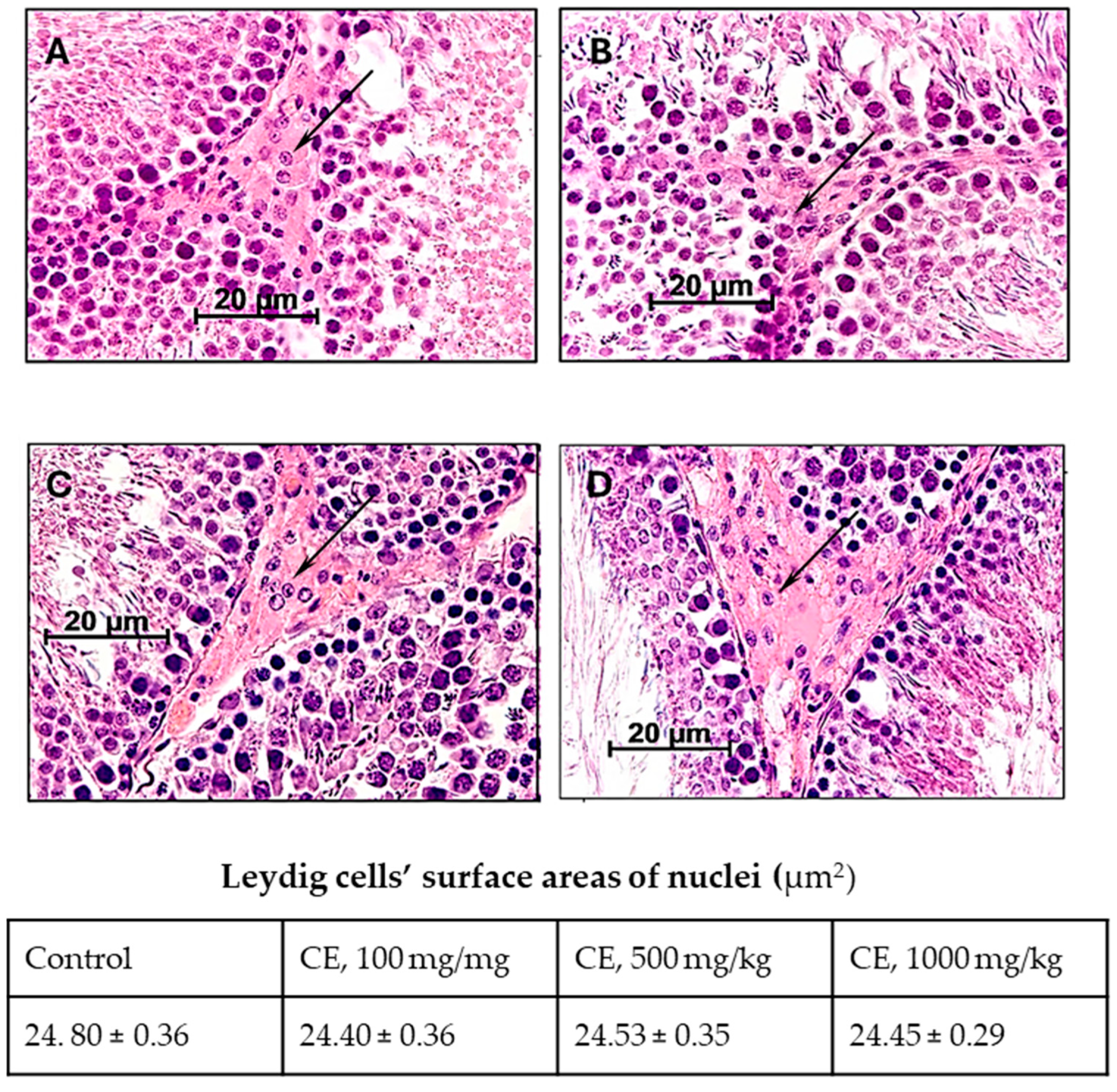

| Parameters | Experimental Groups a | |||
|---|---|---|---|---|
| Control (H2O) | CE 100 mg/kg bw/day | CE 500 mg/kg bw/day | CE 1000 mg/kg bw/day | |
| Number of animals evaluated | 10 | 10 | 10 | 10 |
| Weight of testes (g) b | 3.21 ± 0.20 | 3.20 ± 0.21 ns | 3.20 ± 0.07 ns | 3.20 ± 0.14 ns |
| Relative weight (%) b | 0.96 ± 0.04 | 0.96 ± 0.04 ns | 0.94 ± 0.09 ns | 0.98 ± 0.08 ns |
| Parameters | Experimental Groups a | |||
|---|---|---|---|---|
| Control (H2O) | CE 100 mg/kg bw/day | CE 500 mg/kg bw/day | CE 1000 mg/kg bw/day | |
| Number of animals evaluated | 10 | 10 | 10 | 10 |
| Spermatogenesis index b | 3.47 ± 0.02 | 3.49 ± 0.02 ns | 3.71 ± 0.02 * | 3.76 ± 0.02 ** |
| Parameters | Groups a | |||
|---|---|---|---|---|
| Control (H2O) | CE 100 mg/kg bw/day | CE 500 mg/kg bw/day | CE 1000 mg/kg bw/day | |
| The number of animals evaluated | 10 | 10 | 10 | 10 |
| Total number of spermatozoa (×106) b | 23.5 ± 2.4 | 36.3 ± 3.3 * | 37.4 ± 3.6 * | 40.0 ± 4.6 * |
| Motile spermatozoa (×106) b | 17.2 ± 1.7 | 23.7 ± 2.4 * | 23.8 ± 2.2 * | 32.9 ± 2.6 * |
| Duration of spermatozoon movement (min.) b | 360.0 ± 23.5 | 362.2 ± 26.0 ns | 345.3 ± 25.6 ns | 352.7 ± 23.5 ns |
| Pathologic forms of spermatozoa (%) | 1.5 ± 0.2 | 1.7 ± 0.2 ns | 1.6 ± 0.1 ns | 1.7 ± 0.2 ns |
| Parameters | Experimental Groups a | |||
|---|---|---|---|---|
| ♂ × ♀ | ♂ × ♀ | ♂ × ♀ | ♂ × ♀ | |
| H2O × Intact | CE, 100 mg/kg × Intact | CE, 500 mg/kg × Intact | CE, 1000 mg/kg × Intact | |
| Number of mated females | 15 | 15 | 15 | 15 |
| Number of fertile females | 14 | 14 | 14 | 14 |
| Number of pregnant females | 14 | 14 | 14 | 14 |
| Fertility index (%) | 93.3 | 93.3 | 93.3 | 93.3 |
| Pregnancy index (%) | 100 | 100 | 100 | 100 |
| Parameters | Experimental Groups a | ||||
|---|---|---|---|---|---|
| ♂ × ♀ | ♂ × ♀ | ♂ × ♀ | ♂ × ♀ | ||
| H2O × Intact | CE, 100 mg/kg × Intact | CE, 500 mg/kg × Intact | CE, 1000 mg/kg × Intact | ||
| Number of pregnant female rats investigated | 7 | 7 | 7 | 7 | |
| Embryonic Death (%) b | Pre-implantation | 7.7 ± 0.8 | 7.6 ± 0.7 ns | 7.2 ± 0.3 ns | 6.4 ± 0.6 ns |
| Post-implantation | 4.7 ± 0.5 | 4.5 ± 0.3 ns | 4.2 ± 0.3 ns | 3.8 ± 0.4 ns | |
| Embryonic weight on day 20 (g) b | 2.4 ± 0.1 | 2.3 ± 0.1 ns | 2.4 ± 0.03 ns | 2.3 ± 0.1 ns | |
| Parameters | Experimental Groups a | |||||
|---|---|---|---|---|---|---|
| ♂ × ♀ | ♂ × ♀ | ♂ × ♀ | ♂ × ♀ | |||
| H2O × Intact | CE, 100 mg/kg × Intact | CE, 500 mg/kg × Intact | CE, 1000 mg/kg × Intact | |||
| Number of litters evaluated | 7 | 7 | 7 | 7 | ||
| Number of newborn pups in the litter b | 8.4 ± 0.4 | 8.1 ± 0.7 ns | 8.5± 0.3 ns | 9.3 ± 0.8 ns | ||
| Number of surviving pups per female rat post-delivery | Day 7 | Number b | 8.1 ± 0.6 | 8.1 ± 0.7 ns | 8.5± 0.3 ns | 9.3 ± 0.8 ns |
| % | 96.4 | 100 | 100 | 100 | ||
| Day 14 | Number b | 8.1 ± 0.6 | 7.9 ± 0.6 ns | 8.5± 0.3 ns | 9.3 ± 0.8 ns | |
| % | 96.4 | 97.5 | 100 | 100 | ||
| Day 21 | Number b | 7.9 ± 0.6 | 7.7 ± 0.6 ns | 8.3 ± 0.4 ns | 9.3 ± 0.8 ns | |
| % | 94.0 | 95.1 | 98.0 | 100 | ||
| Pups weight (g) b | Day 1 | 6.2 ± 0.2 | 6.3 ± 0.3 ns | 6.1± 0.4 ns | 6.0 ± 0.2 ns | |
| Day 4 | 9.1 ± 0.5 | 9.0 ± 0.5 ns | 8.9 ± 0.7 ns | 8.9 ± 0.3 ns | ||
| Day 7 | 12.3 ± 0.8 | 12.7 ± 1.1 ns | 11.9 ± 0.5 ns | 11.8 ± 0.6 ns | ||
| Day 14 | 22.6 ± 0.9 | 22.9 ± 0.9 ns | 20.9 ±1.5 ns | 20.9 ± 1.3 ns | ||
| Day 21 | 32.4 ± 2.2 | 33.2± 2.0 ns | 32.2 ± 3.4 ns | 31.2 ± 3.0 ns | ||
| Parameters | Groups a | ||||||
|---|---|---|---|---|---|---|---|
| Control (H2O) | CE 100 mg/kg | CE 500 mg/kg | CE 1000 mg/kg | ||||
| Number of pups evaluated | 51 | 52 | 50 | 54 | |||
| Time of cliff avoidance (sec.) b | 5.0 ± 0.6 | 4.7 ± 0.4 ns | 6.2 ± 0.5 ns | 5.3 ± 0.5 ns | |||
| Flip/roll over onto all four paws | Pups with positive reaction | No. | 51 | 52 | 50 | 54 | |
| % | 100 | 100 | 100 | 100 | |||
| Negative geotaxis (sec) b | 11.3 ± 1.2 | 10.9 ± 1.1 ns | 11.5 ±1.3 ns | 10.9 ± 1.0 ns | |||
| Open-field test | Total squares crossed b | 32.9 ± 3.2 | 37.6 ± 3.9 ns | 37.8 ± 4.0 ns | 38.0 ± 3.7 ns | ||
| Head dipping b | 3.17 ± 0.35 | 3.20 ± 0.33 ns | 3.50 ± 0.35 ns | 3.60 ± 0.35 ns | |||
| Grooming frequency b | 1.20 ± 0.13 | 1.20 ± 0.12 ns | 1.10 ± 0.11 ns | 1.10 ± 0.13 ns | |||
| Defecation b | 2.90 ± 0.22 | 3.10 ± 0.30 ns | 3.40 ± 0.34 ns | 3.60 ± 0.41 ns | |||
| Rearing frequency b | 0.10 ± 0.10 | 0.20 ± 0.13 ns | 0.20 ± 0.13 ns | 0.20 ± 0.13 ns | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babenko, A.N.; Krepkova, L.V.; Borovkova, M.V.; Kuzina, O.S.; Mkhitarov, V.A.; Job, K.M.; Enioutina, E.Y. Effects of Chicory (Cichorium intybus L.) Extract on Male Rat Reproductive System, Pregnancy and Offspring Development. Pharmaceuticals 2024, 17, 700. https://doi.org/10.3390/ph17060700
Babenko AN, Krepkova LV, Borovkova MV, Kuzina OS, Mkhitarov VA, Job KM, Enioutina EY. Effects of Chicory (Cichorium intybus L.) Extract on Male Rat Reproductive System, Pregnancy and Offspring Development. Pharmaceuticals. 2024; 17(6):700. https://doi.org/10.3390/ph17060700
Chicago/Turabian StyleBabenko, Alexandra N., Lubov V. Krepkova, Marina V. Borovkova, Olga S. Kuzina, Vladimir A. Mkhitarov, Kathleen M. Job, and Elena Y. Enioutina. 2024. "Effects of Chicory (Cichorium intybus L.) Extract on Male Rat Reproductive System, Pregnancy and Offspring Development" Pharmaceuticals 17, no. 6: 700. https://doi.org/10.3390/ph17060700
APA StyleBabenko, A. N., Krepkova, L. V., Borovkova, M. V., Kuzina, O. S., Mkhitarov, V. A., Job, K. M., & Enioutina, E. Y. (2024). Effects of Chicory (Cichorium intybus L.) Extract on Male Rat Reproductive System, Pregnancy and Offspring Development. Pharmaceuticals, 17(6), 700. https://doi.org/10.3390/ph17060700








