Population Pharmacokinetics of Dasatinib in Healthy Subjects
Abstract
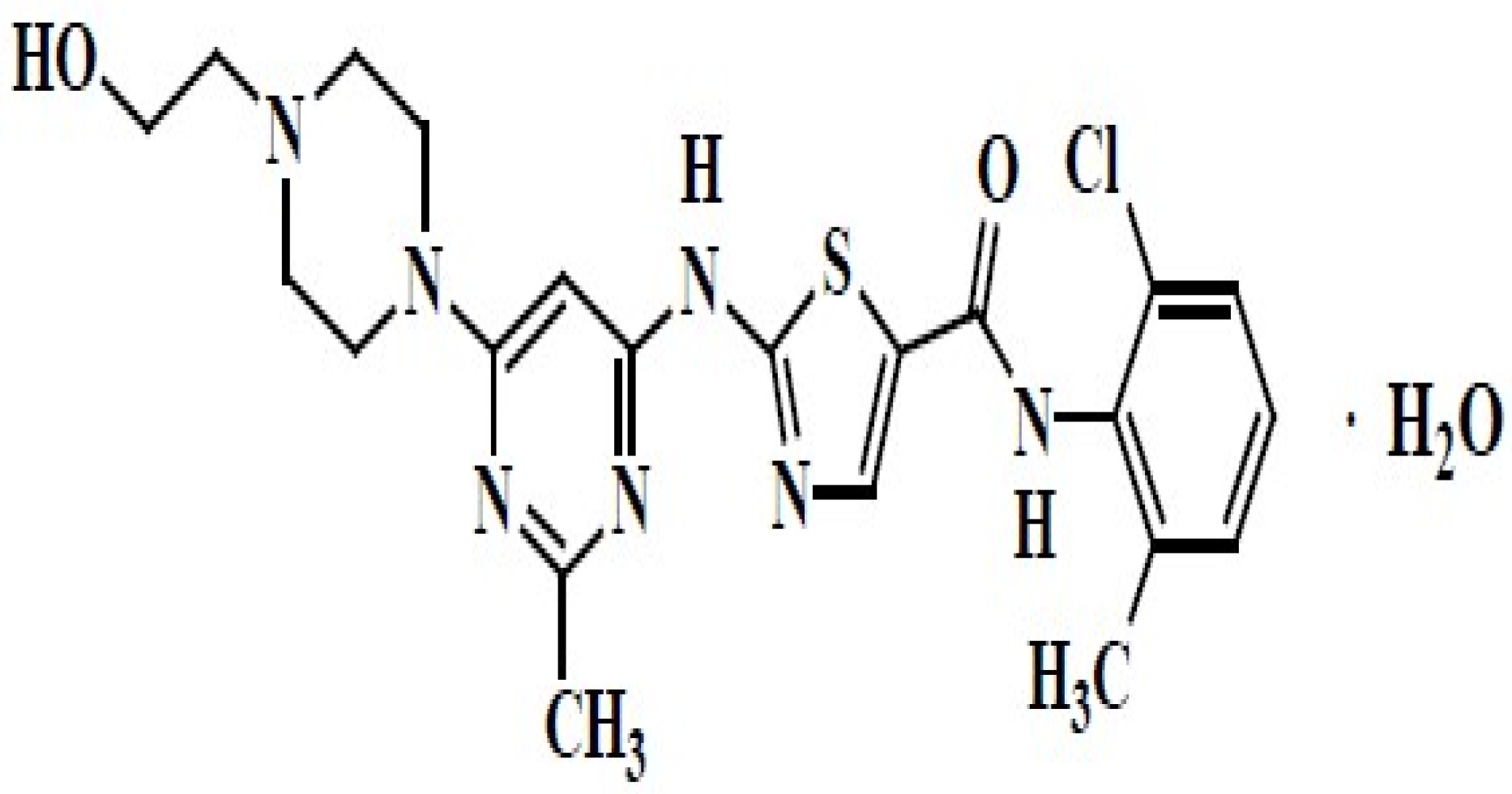
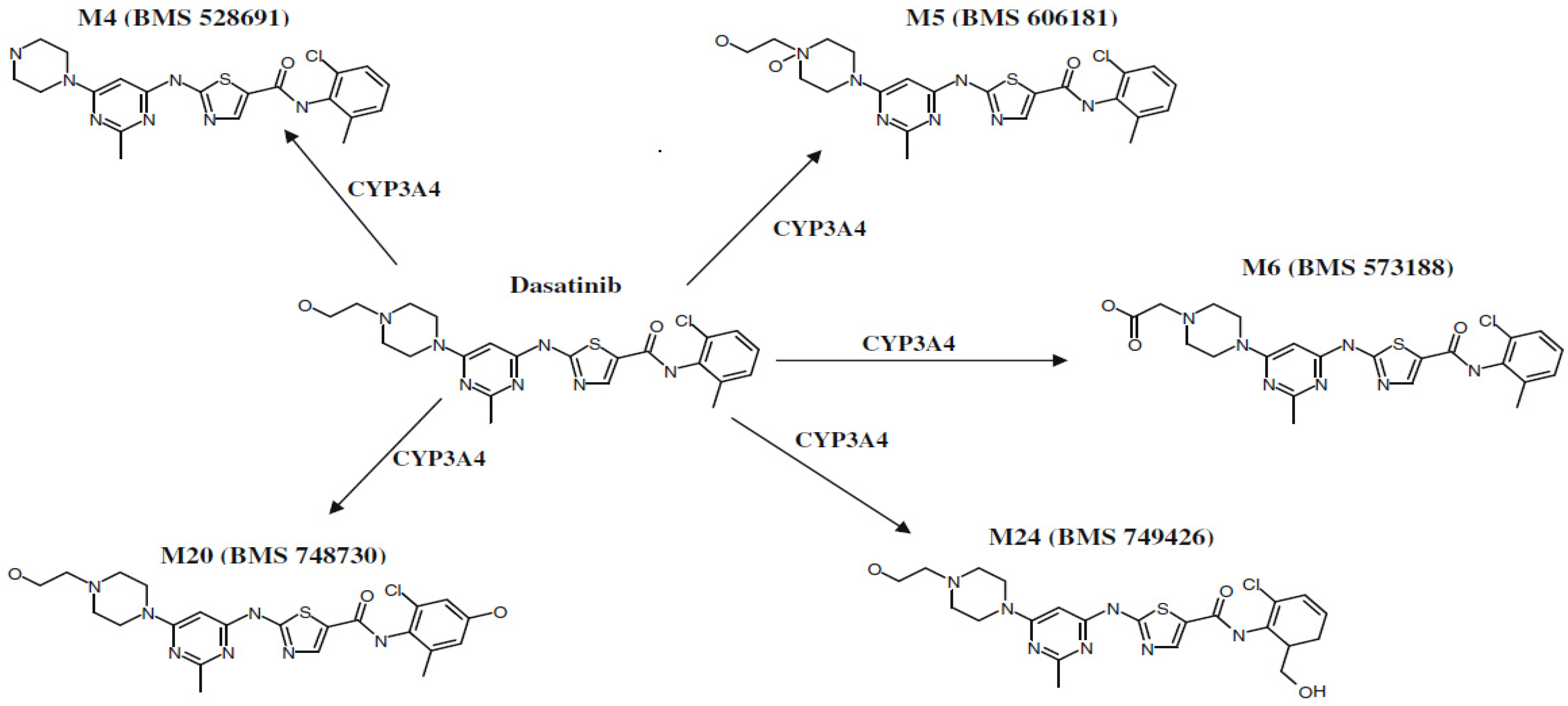
1. Introduction
2. Results
2.1. Base Model Selection and Full Multi-Covariate Model
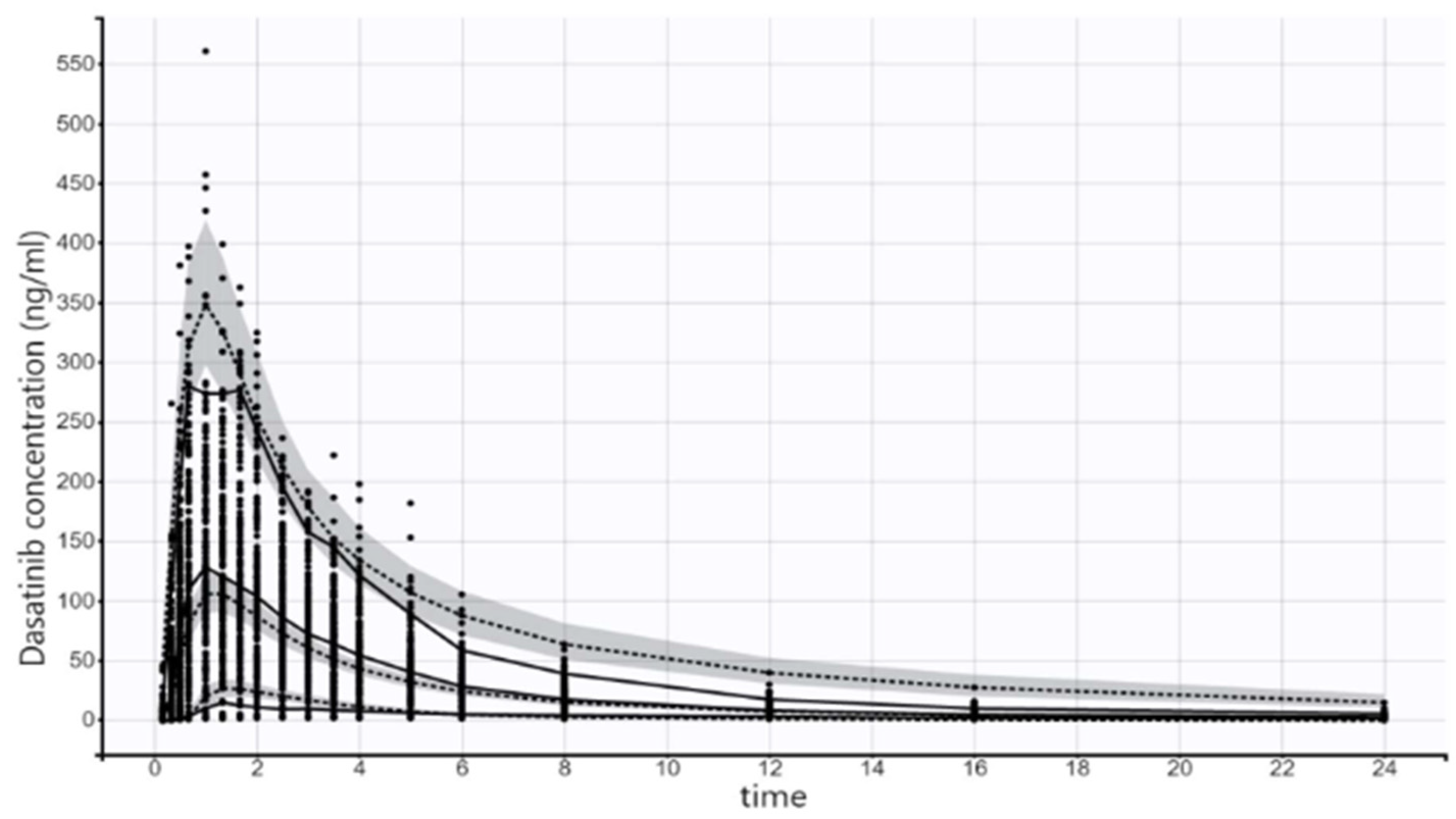
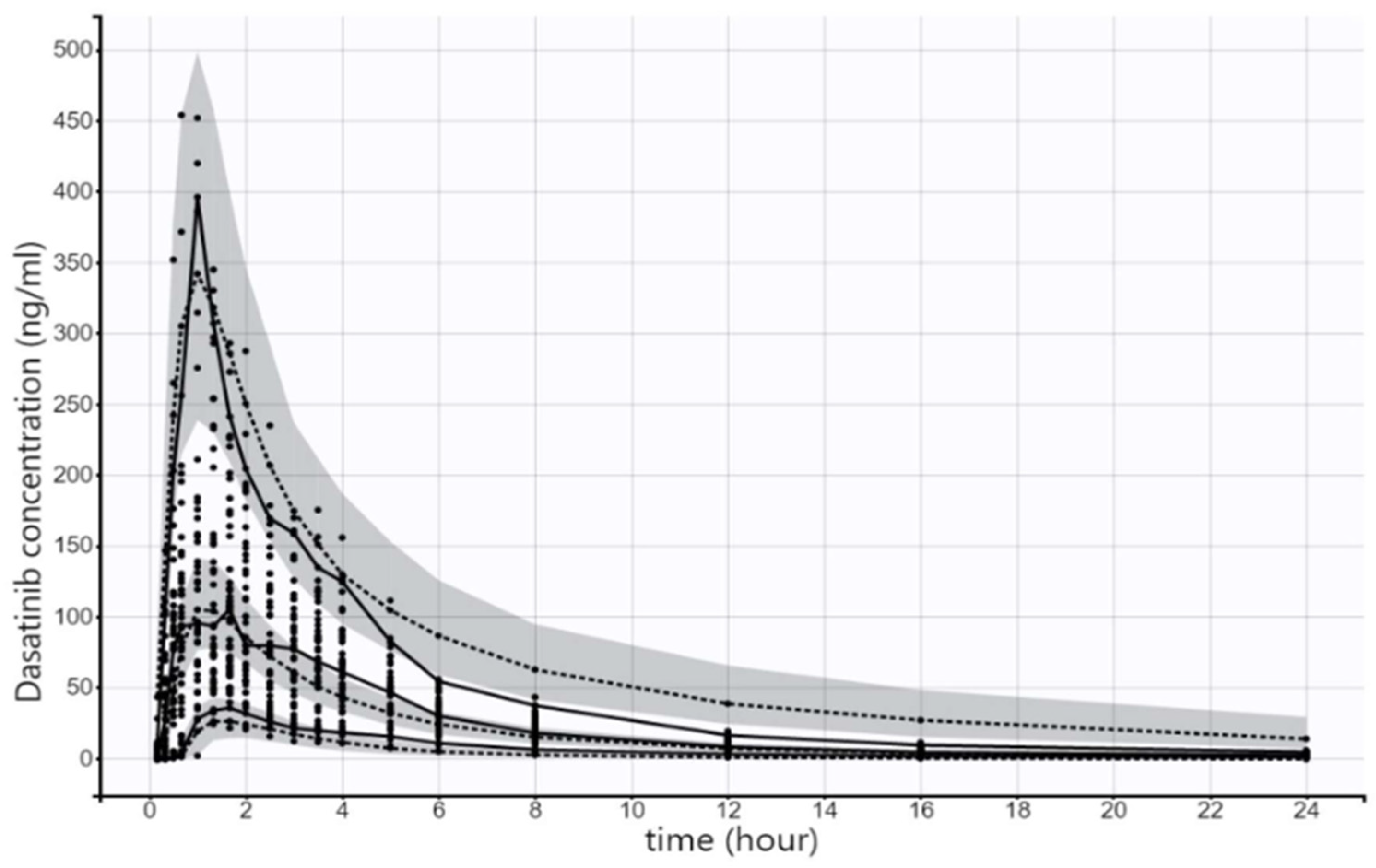
2.2. Final Model
3. Discussion
- Underweight—BMI < 18.5 kg/m2;
- Normal weight—BMI ≥ to 18.5 to 24.9 kg/m2;
- Overweight—BMI ≥ to 25 to 29.9 kg/m2;
- Obesity—BMI ≥ to 30 kg/m2.
- Tmax is the time in which the absorption rate equals the elimination rate and it can be calculated as per the equation below:
- Tmax = ln(Ka/K) Ka − K;
- Tmax: time to reach peak concentrations;
- Ka: absorption rate constant;
- K: elimination rate constant.
4. Methodology and Study Design
4.1. Overall Study Design Description
- -
- Inclusion criteria
- Age 18 to 55 years, inclusive.
- Body mass index (BMI) range is within 18.5–30.0 Kg/m2.
- Subject does not have a known allergy to the drug under investigation or any of its ingredients or any other related drugs.
- Medical history and physical examination within medically acceptable criteria.
- Standard 12-lead ECG assessment is normal.
- Males and their female partners need to practice adequate contraception for at least one week after the dasatinib dose.
- Laboratory investigation tests within laboratory reference ranges (ALP and creatinine are accepted if below the reference range). Hematology tests within 5% of reference limits.
- -
- Exclusion criteria
- Medical demographics performed not longer than two weeks before the initiation of the clinical study with significant deviations from the normal ranges.
- Presence of any clinically significant results from laboratory tests; however, ALP and creatinine will be accepted if below reference range. Hematology tests with deviation of more than 5% of the reference limits. Laboratory tests are performed not longer than two weeks before the initiation of the clinical study.
- History of drug or alcohol abuse.
- Subject is a heavy smoker (more than 10 cigarettes per day).
- Positive drug abuse test screening and/or positive alcohol test at screening.
- Subject does not agree not to take any prescription or non-prescription drugs with systemic absorption within at least two weeks preceding the first study drug administration until donating the last sample of the study.
- Subject does not agree not to take any vitamins for nutritional purposes within at least two days before first study drug administration until donating the last sample of the study.
- Subject is on a special diet (for example, subject is a vegetarian).
- Subject consumes large quantities of alcohol or beverages containing methylxanthines, e.g., caffeine (coffee, tea, cola, energy drinks, chocolate, etc).
- Subject does not agree not to consume any beverages or food containing alcohol at least 14 days prior to first study drug administration until donating the last sample of the study.
- Subject does not agree not to consume any beverages or food containing methylxanthines, e.g., caffeine (coffee, tea, cola, chocolate, etc.) at least 24 h prior to the study drug administration of each study period until the end of confinement period.
- Subject does not agree not to consume any beverages or food containing grapefruit at least two weeks prior to first study drug administration until donating the last sample in the study.
- Subject has a history of severe diseases which have direct impact on the study.
- Participation in a bioequivalence study or in a clinical study within the last 80 days before first study drug administration.
- Subject intends to be hospitalized within 3 months after first study drugs’ administration.
- Subject donated blood or its derivatives in the past 3 months or who through completion of this study, would have donated more than 1250 mL in 120 days, 1500 mL in 180 days, 2000 mL in 270 days, 2500 mL of blood in 1 year.
- A positive pregnancy test or subject is lactating during screening or study period if the subject is female.
- Subject has a history of significant asthma, peptic or gastric ulcer, sinusitis, pharyngitis, renal disorder (impaired renal function), hepatic disorder (impaired hepatic function), cardiovascular disorder, neurological disease such as epilepsy, hematological disorders or diabetes, psychiatric, cardiopulmonary disease, congenital long QT syndrome, fluid retention, dermatologic or immunological disorders.
- Subject does not agree not to engage in strenuous exercise at least one day prior to study drug administration until donating the last sample in each respective period.
- Subject having at screening examination a pulse outside the normal range of (60–100 beats per minute) or a body temperature outside the normal range of (35.0–37.2 °C) or a respiratory rate outside the normal range of (14–20 breaths per minute) or a sitting blood pressure less than 100/60 mm Hg or more than or equal to 140/90 mm Hg.
- Subject has history of difficulties in swallowing or any gastrointestinal disease which could affect the drug absorption.
- Subject does not agree not to consume any medication or food which may affect CYP3A4 enzyme at least two weeks prior to first study drug administration until donating the last sample of the study.
- Subject has history of malabsorption within the past year or presence of clinically significant gastrointestinal disease.
- Subject has galactose or fructose intolerance, sucrase-isomaltase insufficiency, Lapp lactase insufficiency, galactosemia, or glucose–galactose malabsorption syndrome.
- Subject has a history of hypokalemia or hypomagnesemia.
4.2. Bioanalysis
4.3. Population Pharmacokinetic Modeling
4.3.1. Base Model
4.3.2. Building the Full Multi-Covariate Model
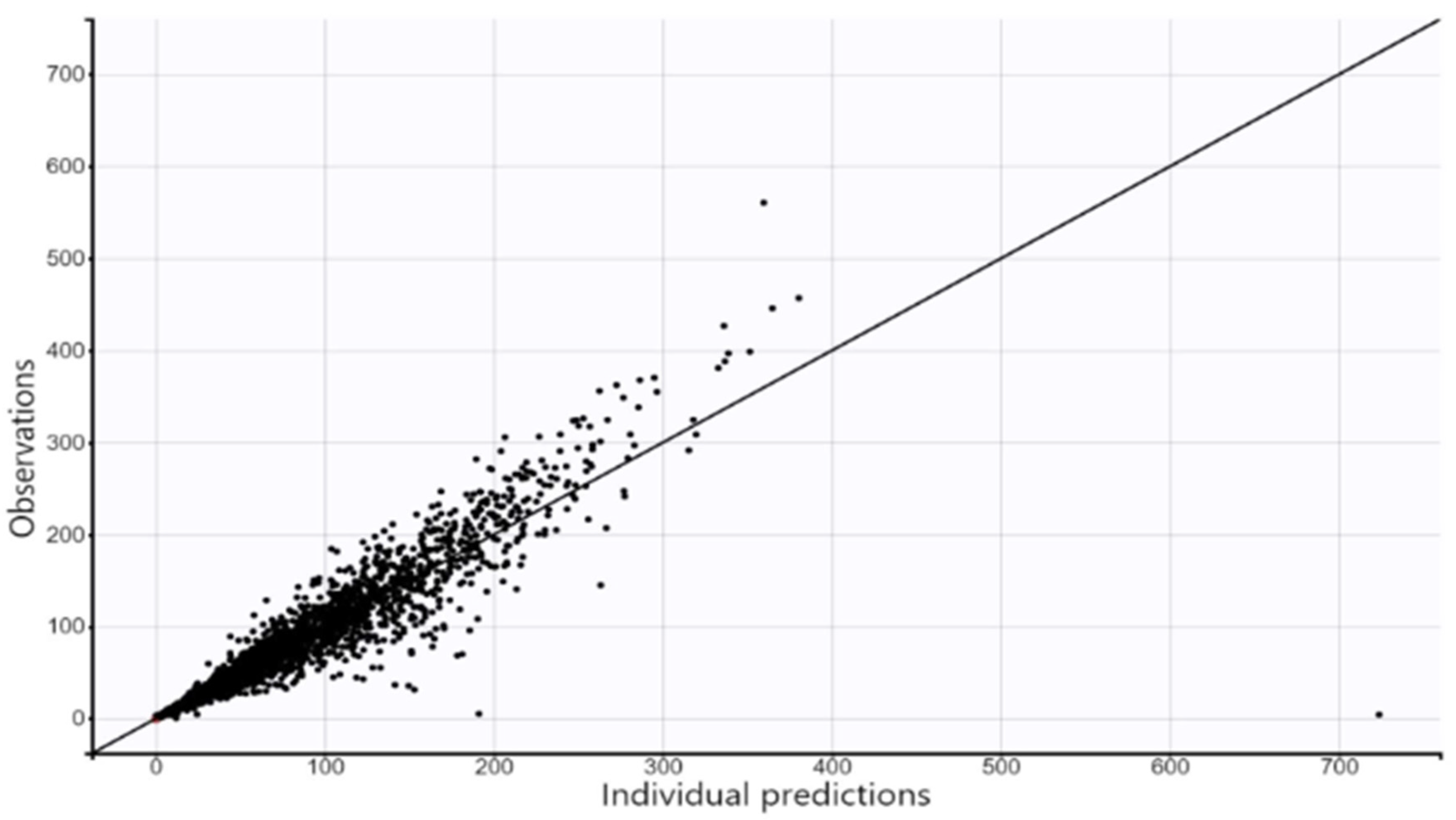
4.3.3. Model Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.-W.; Goh, Y.-T.; Hsiao, H.-H.; Caguioa, P.B.; Kim, D.; Kim, W.-S.; Saikia, T.; Agrawal, S.; Roy, A.; Dai, D.; et al. Clinical profile of dasatinib in Asian and non-Asian patients with chronic myeloid leukemia. Int. J. Hematol. 2009, 89, 664–672. [Google Scholar] [CrossRef] [PubMed]
- van Erp, N.P.; Gelderblom, H.; Guchelaar, H.-J. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat. Rev. 2009, 35, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Levêque, D.; Becker, G.; Bilger, K.; Natarajan-Amé, S. Clinical Pharmacokinetics and Pharmacodynamics of Dasatinib. Clin. Pharmacokinet. 2020, 59, 849–856. [Google Scholar] [CrossRef] [PubMed]
- U.S. FDA. Highlights of Prescribing Information of Sprycel (Dasatinib) Tablets, for Oral Use; U.S. FDA: Washington, DC, USA, 2006.
- Di Gion, P.; Kanefendt, F.; Lindauer, A.; Scheffler, M.; Doroshyenko, O.; Fuhr, U.; Wolf, J.; Jaehde, U. Clinical Pharmacokinetics of Tyrosine Kinase Inhibitors. Clin. Pharmacokinet. 2011, 50, 551–603. [Google Scholar] [CrossRef] [PubMed]
- Christopher, L.J.; Cui, D.; Wu, C.; Luo, R.; Manning, J.A.; Bonacorsi, S.J.; Lago, M.; Allentoff, A.; Lee, F.Y.; McCann, B.; et al. Metabolism and disposition of dasatinib after oral administration to humans. Drug Metab. Dispos. 2008, 36, 1357–1364. [Google Scholar] [CrossRef]
- Yoshitsugu, H.; Imai, Y.; Seriu, T.; Hiraoka, M. Markov Chain Monte Carlo Bayesian Analysis for Population Pharmacokinetics of Dasatinib in Japanese Adult Subjects with Chronic Myeloid Leukemia and Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia. Jpn. J. Clin. Pharmacol. Ther. 2012, 43, 29–41. [Google Scholar] [CrossRef]
- Demetri, G.D.; Lo Russo, P.; MacPherson, I.R.J.; Wang, D.; Morgan, J.A.; Brunton, V.G.; Paliwal, P.; Agrawal, S.; Voi, M.; Jeffry Evans, T.R. Phase I Dose-Escalation and Pharmacokinetic Study of Dasatinib in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2009, 15, 6232–6240. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Wang Roy, A.; Kantarjian, H.; Chen, T.T.; Shah, N.P. Differential effects of dosing regimen on the safety and efficacy of dasatinib: Retrospective exposure–response analysis of a Phase III study. In Clinical Pharmacology: Advances and Applications; Taylor & Francis: London, UK, 2013; p. 85. [Google Scholar]
- Smit, C.; De Hoogd, S.; Brüggemann, R.J.M.; Knibbe, C.A.J. Obesity and drug pharmacology: A review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hanley, M.J.; Abernethy, D.R.; Greenblatt, D.J. Effect of Obesity on the Pharmacokinetics of Drugs in Humans. Clin. Pharmacokinet 2010, 49, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P.J. Basic Pharmacokine, 2nd ed.; Pharmaceutical Press: London, UK, 2012. [Google Scholar]
- Erstad, L.B. Dosing of medications in morbidly obese patients in the intensive care unit setting. Intensive Care Med. 2004, 30, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Barras, M.; Legg, A. Drug dosing in obese adults. Aust. Prescr. 2017, 40, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Neath, A.A.; Cavanaugh, J.E. The Bayesian information criterion: Background, derivation, and applications. Wiley Interdiscip. Rev. Comput. Stat. 2012, 4, 199–203. [Google Scholar] [CrossRef]
- Ayral, G.; Si Abdallah, J.F.; Magnard, C.; Chauvin, J. A novel method based on unbiased correlations tests for covariate selection in nonlinear mixed effects models: The COSSAC approach. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 318–329. [Google Scholar] [CrossRef] [PubMed]


| System/Organ | Adverse Events |
|---|---|
| Blood and vascular system | Thrombocytopenia a, neutropenia a, bleedings b,c |
| Respiratory system | Pleural effusions, dyspnea, dry cough, chest pain, pulmonary arterial hypertension a, pulmonary edema |
| Cardiovascular system | Ischemia, palpitations, arrhythmias, QT Prolongation d, congestive heart failure and cardiac dysfunction |
| Skin | Rash, Stevens–Johnson syndrome (SJS) and erythema multiforme, pruritus |
| Nervous system | headache |
| Gastrointestinal system | Diarrhea, abdominal pain, nausea, vomiting, constipation and mucositis |
| Musculoskeletal system | Myalgia, arthralgia, muscle spasms |
| Infections | Bacterial, viral and fungal infections |
| General | Decreased appetite, fluid retention and pain |
| Characteristic | (Mean ± Standard Deviation (SD)) | Median (Range) |
|---|---|---|
| Gender | Males | NA |
| Count | 116 | NA |
| Age (Years) | 32 ± 8.36 | 33 (18–49) |
| Body weight (Kg) | 72 ± 12.71 | 70 (51–100) |
| Height (cm) | 175 ± 5.86 | 175 (160–192) |
| BMI (Kg/m2) | 23.6 ± 3.50 | 22.9 (18.6–29.8) |
| Smoking history * | 14.66% were non-smokers, 85.34% were smokers | NA |
| Race | Middle Eastern | NA |
| Parameter | Description | Population Parameter (%RSE) | Between-Subject Variability (Standard Deviation (%RSE)) | Inter-Occasion Variability (Standard Deviation) |
|---|---|---|---|---|
| Ktr | Transit time rate constant | 18.8 (9.34) | 0.49 (26) | 0.84 (9.54) |
| Mtt | Meant transit time | 0.48 (4.63) | 0.3 (16.1) | 0.4 (8.04) |
| Ka | Absorption rate constant | 0.37 (4.8) | 0.36 (12.7) | 0.31 (10.1) |
| βBMI | Regression coefficient for the effect of body mass index (BMI) on Absorption rate constant | −0.85 (36.9) | ||
| Cl | Clearance | 273.14 (7.37) | 0.62 (9.82) | 0.42 (7.94) |
| V1 | Volume of the central compartment (L) | 18.98 (8.94) | ||
| Q | Intercompartmental clearance (L/h) | 64.62 (3.92) | ||
| V2 | Volume of the peripheral compartment (L) | 487.9 (3.18) | ||
| a (constant) | Residual variability | 0.78 (5) | ||
| b (proportional) | Residual variability | 0.22 (1.72) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassouneh, W.B.; Al-Ghazawi, M.A.; Saleh, M.I.; Najib, N. Population Pharmacokinetics of Dasatinib in Healthy Subjects. Pharmaceuticals 2024, 17, 671. https://doi.org/10.3390/ph17060671
Hassouneh WB, Al-Ghazawi MA, Saleh MI, Najib N. Population Pharmacokinetics of Dasatinib in Healthy Subjects. Pharmaceuticals. 2024; 17(6):671. https://doi.org/10.3390/ph17060671
Chicago/Turabian StyleHassouneh, Walaa B., Mutasim A. Al-Ghazawi, Mohammad I. Saleh, and Naji Najib. 2024. "Population Pharmacokinetics of Dasatinib in Healthy Subjects" Pharmaceuticals 17, no. 6: 671. https://doi.org/10.3390/ph17060671
APA StyleHassouneh, W. B., Al-Ghazawi, M. A., Saleh, M. I., & Najib, N. (2024). Population Pharmacokinetics of Dasatinib in Healthy Subjects. Pharmaceuticals, 17(6), 671. https://doi.org/10.3390/ph17060671







