Hydrogen Sulfide Delivery to Enhance Bone Tissue Engineering Cell Survival
Abstract
1. Introduction
2. Results and Discussion
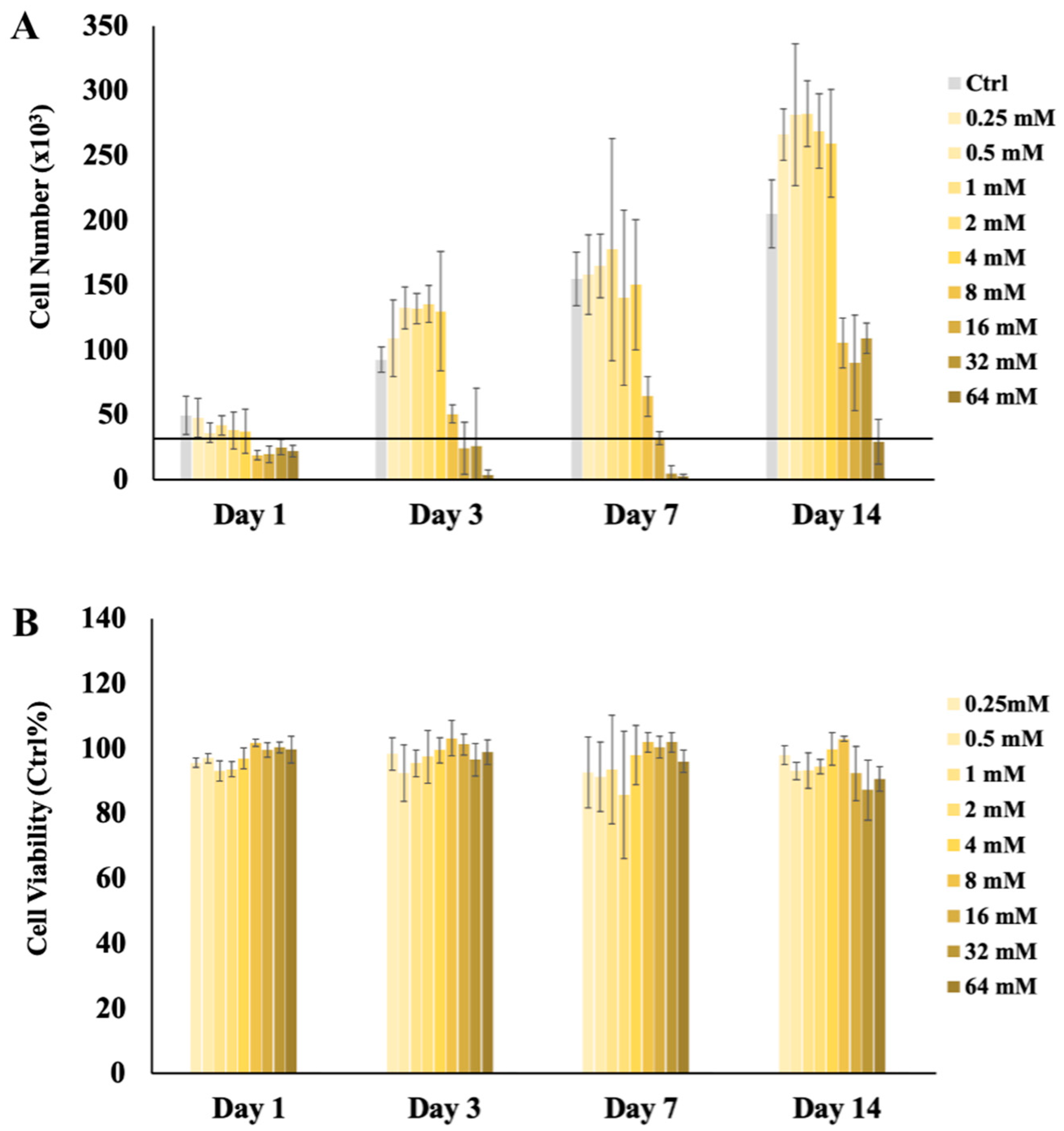
2.1. H2S Cytotoxicity
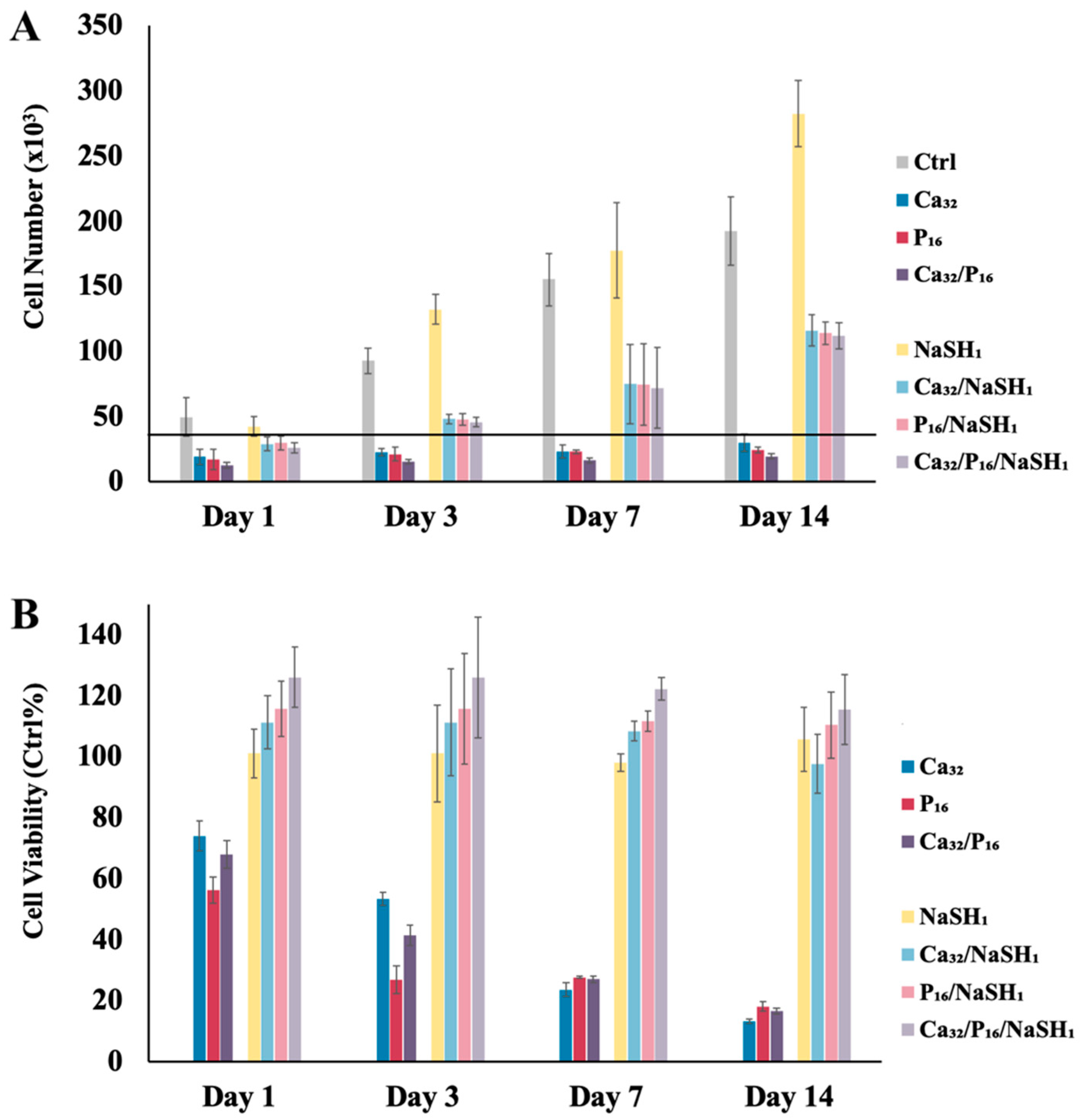
Cytoprotective Effect of H2S at High Ca2+ and/or Pi Concentrations
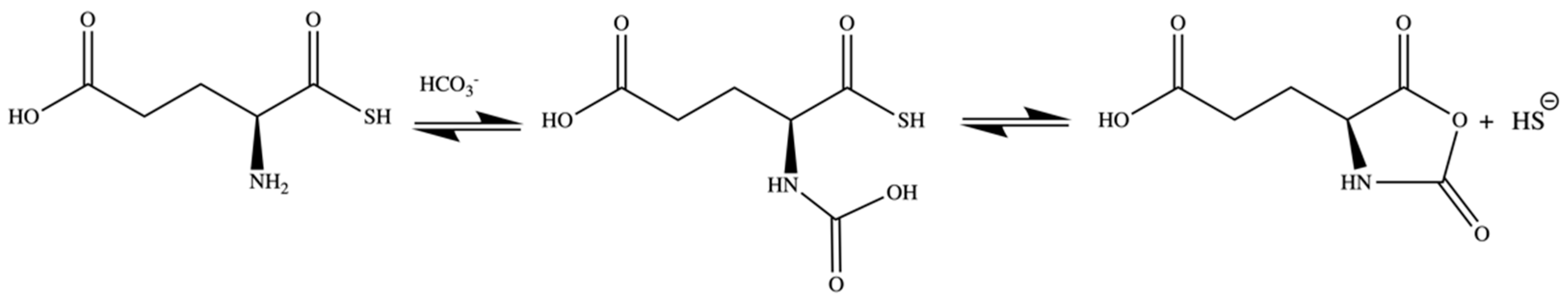
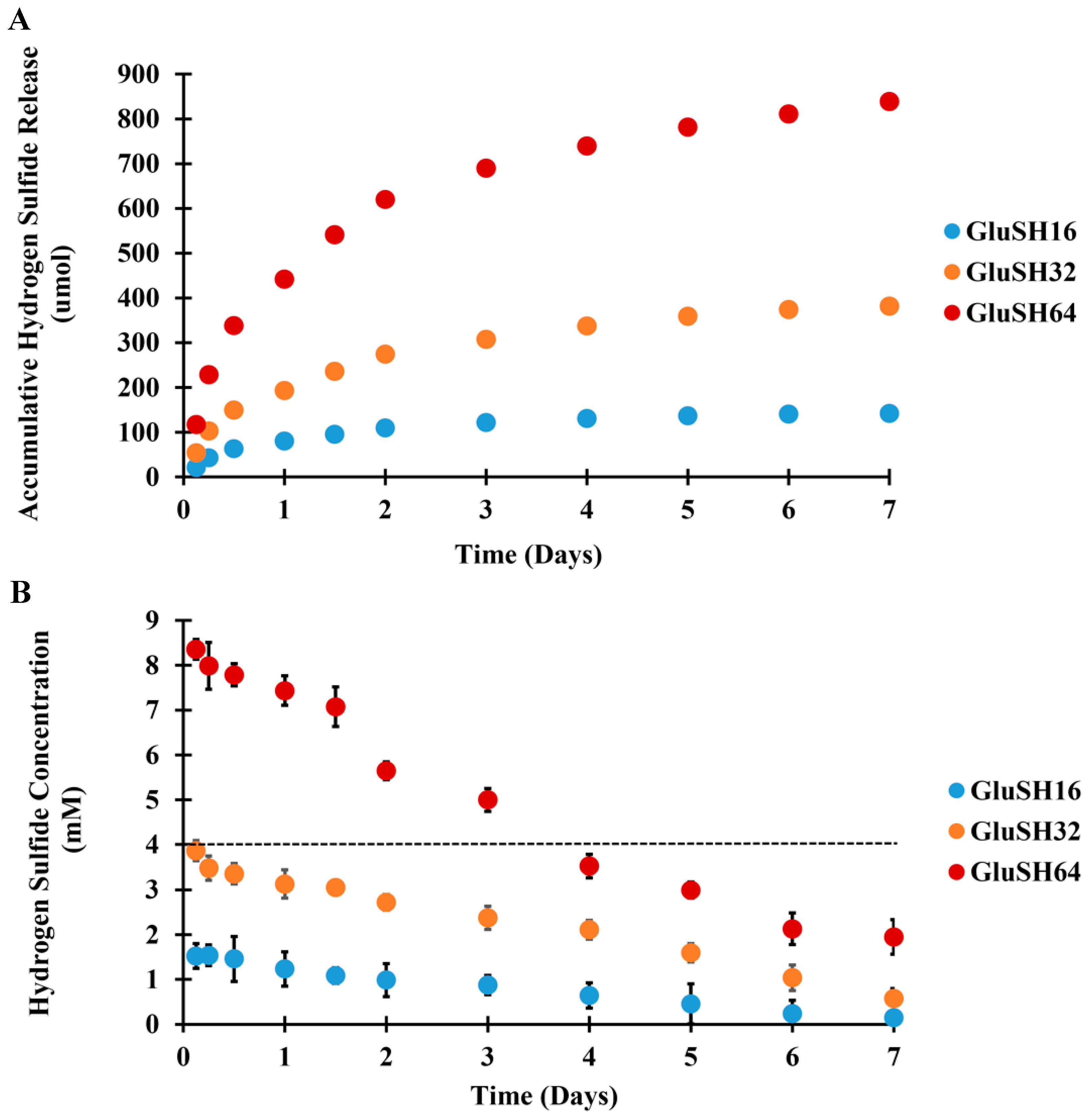
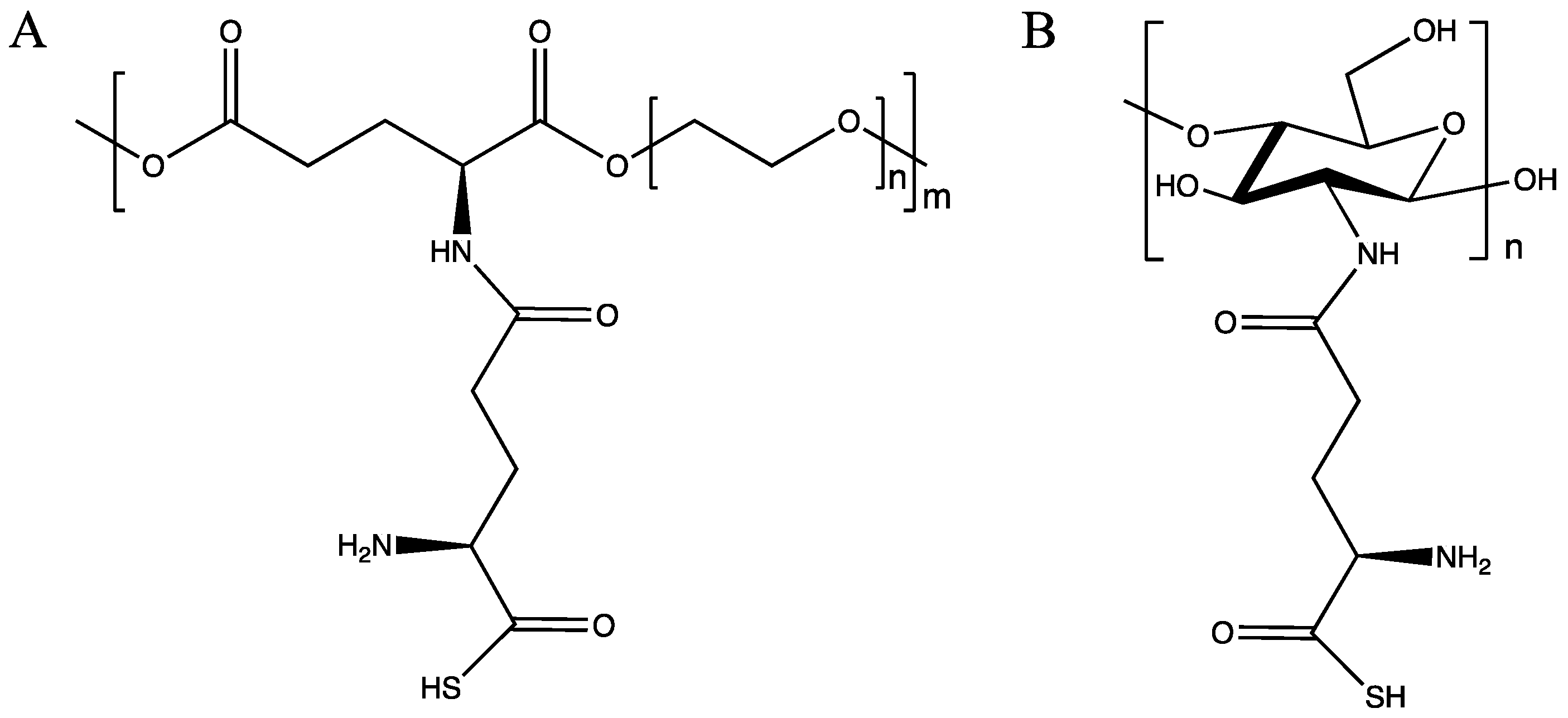
2.2. H2S Release from GluSH
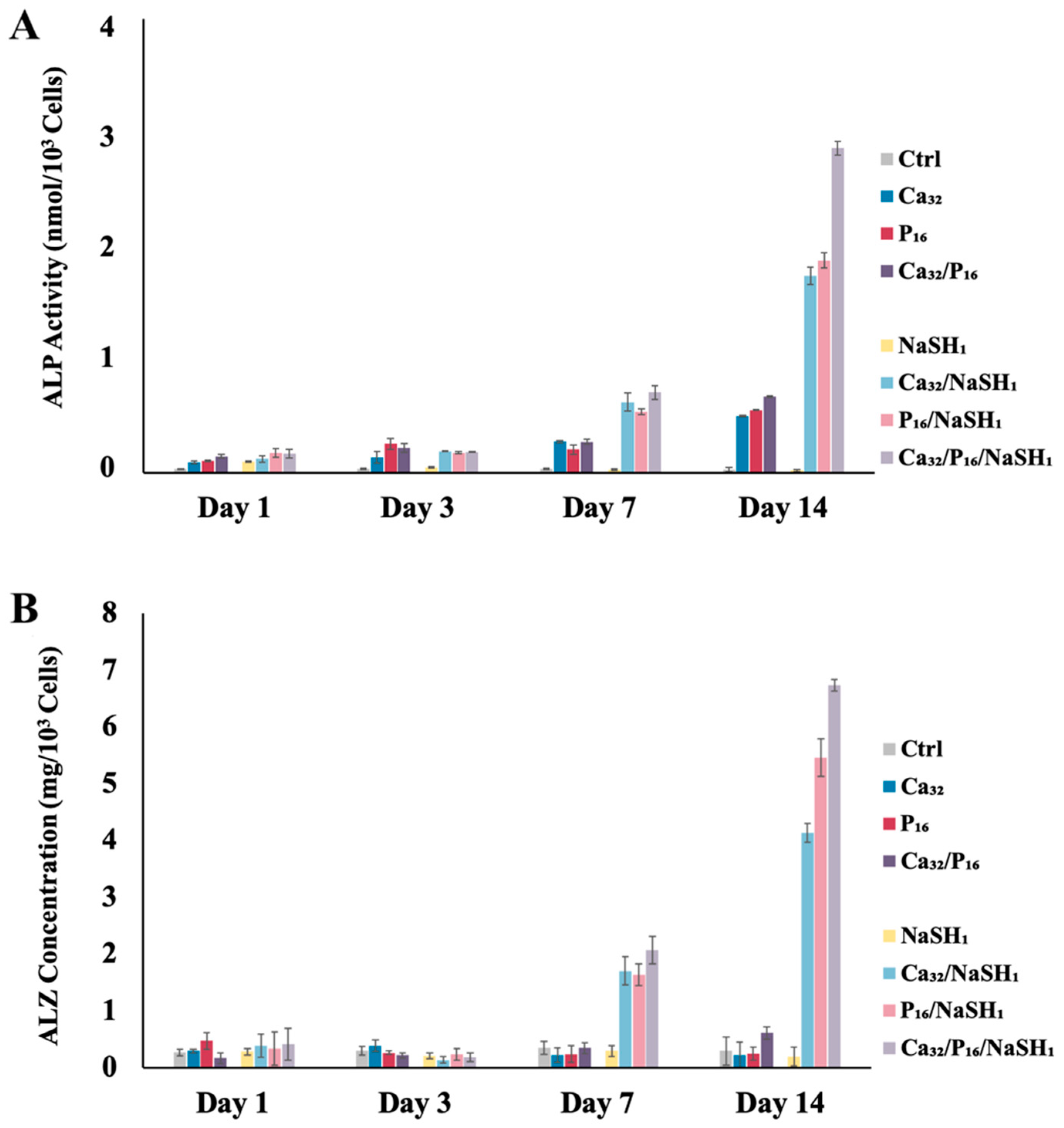
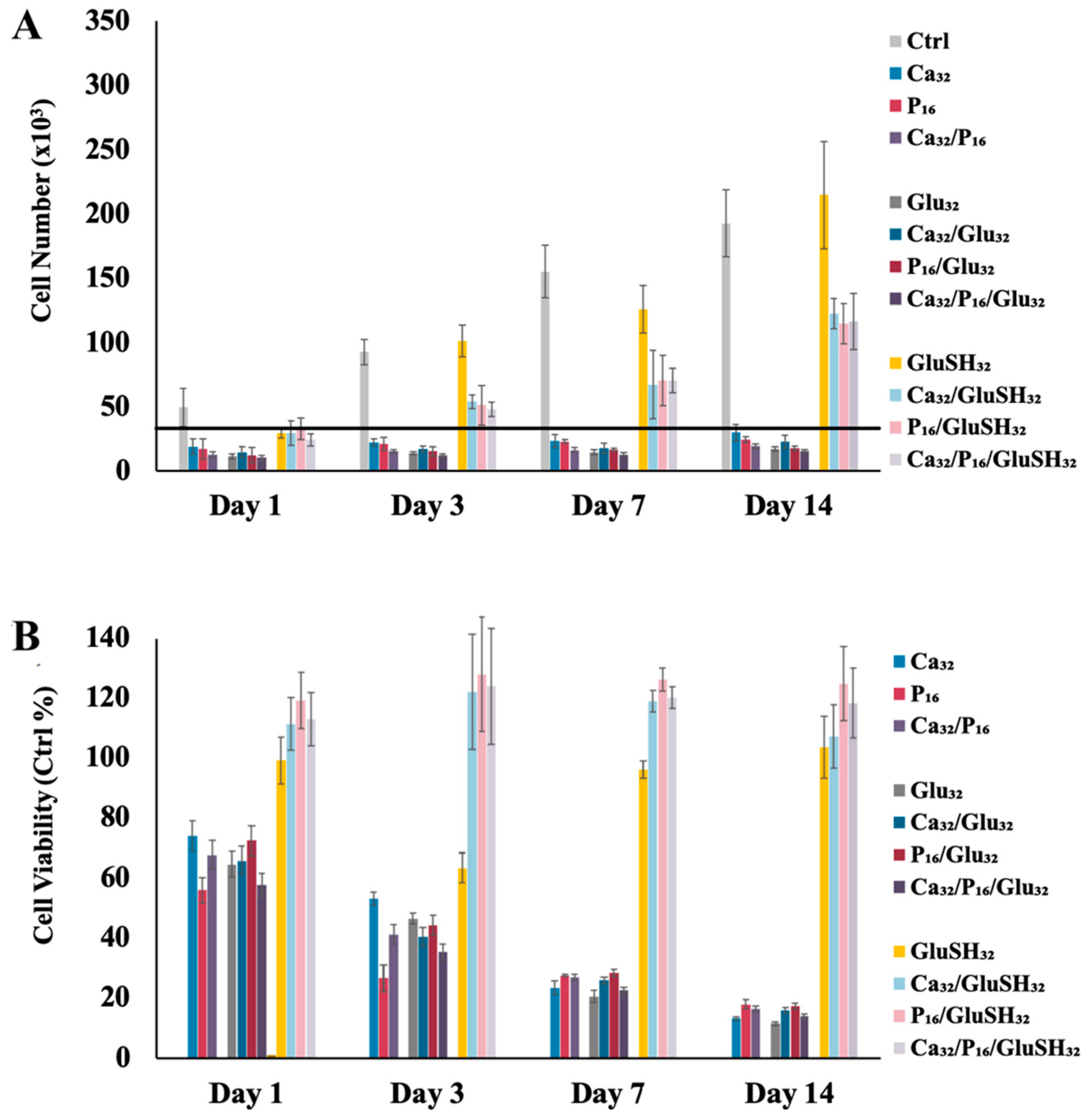
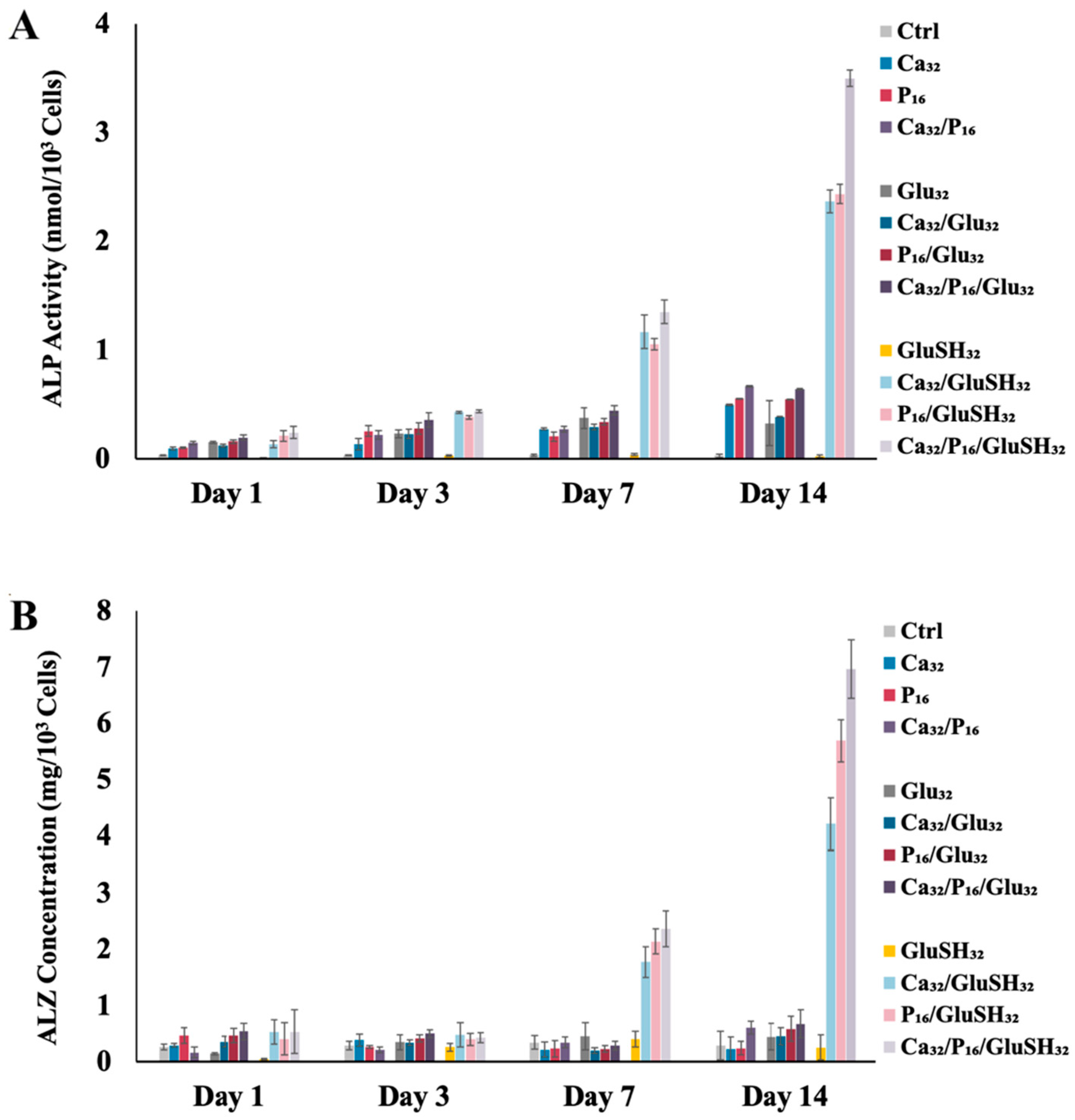
2.3. GluSH-Mediated Stem Cell Proliferation and Differentiation
3. Material and Methods
3.1. Preparation of H2S, Ca2+, and Pi Containing Media
3.2. Cell Culture and Seeding
3.3. Proliferation Assay
3.4. Viability Assay
3.5. Alkaline Phosphatase Activity Assay
3.6. Mineralization Assay
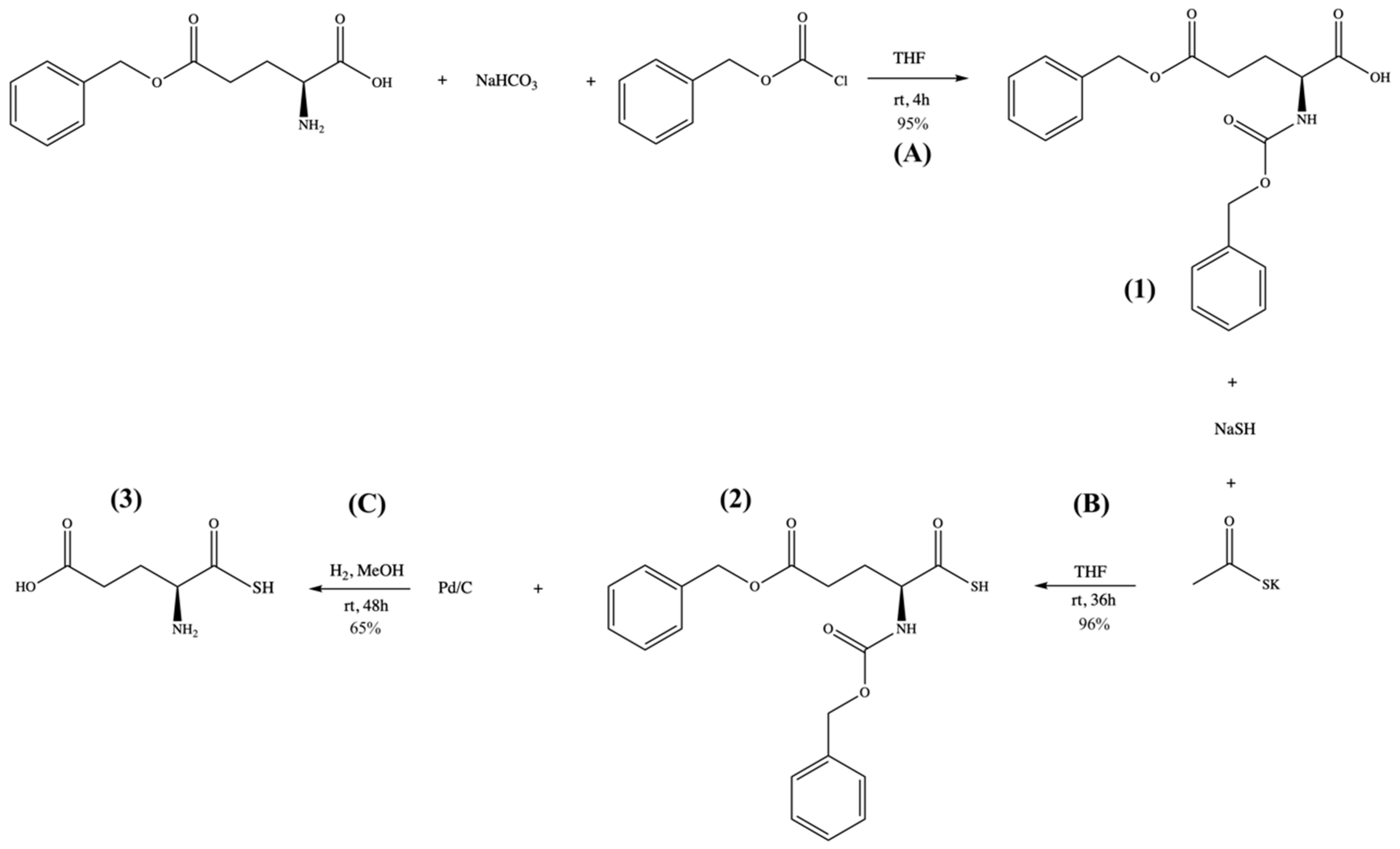
3.7. GluSH Synthesis
3.8. H2S Release Study
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, Regional, and National Life Expectancy, All-Cause Mortality, and Cause-Specific Mortality for 249 Causes of Death, 1980–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; Khoshhal, K.I. Calcium Metabolism and Oxidative Stress in Bone Fractures: Role of Antioxidants. Curr. Drug Metab. 2007, 8, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Marenzana, M.; Arnett, T.R. The Key Role of the Blood Supply to Bone. Bone Res. 2013, 1, 203–215. [Google Scholar] [CrossRef]
- Seta, K.A.; Yuan, Y.; Spicer, Z.; Lu, G.; Bedard, J.; Ferguson, T.K.; Pathrose, P.; Cole-Strauss, A.; Kaufhold, A.; Millhorn, D.E. The Role of Calcium in Hypoxia-Induced Signal Transduction and Gene Expression. Cell Calcium 2004, 36, 331–340. [Google Scholar] [CrossRef]
- Eisenhauer, A.; Müller, M.; Heuser, A.; Kolevica, A.; Glüer, C.-C.; Both, M.; Laue, C.; Hehn, U.V.; Kloth, S.; Shroff, R.; et al. Calcium Isotope Ratios in Blood and Urine: A New Biomarker for the Diagnosis of Osteoporosis. Bone Rep. 2019, 10, 100200. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The Biology of Fracture Healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Nicotera, P. The Calcium Ion and Cell Death. J. Neural Transm. Suppl. 1994, 43, 1–11. [Google Scholar]
- Zeng, J.-H.; Liu, S.-W.; Xiong, L.; Qiu, P.; Ding, L.-H.; Xiong, S.-L.; Li, J.-T.; Liao, X.-G.; Tang, Z.-M. Scaffolds for the Repair of Bone Defects in Clinical Studies: A Systematic Review. J. Orthop. Surg. Res. 2018, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Properties of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Fernandes, G.; Abhyankar, V.; O’Dell, J.M. Calcium Sulfate as a Scaffold for Bone Tissue Engineering: A Descriptive Review. J. Dent. Oral Disord. Ther. 2021, 9, 1–22. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [PubMed]
- Humeau, J.; Bravo-San Pedro, J.M.; Vitale, I.; Nuñez, L.; Villalobos, C.; Kroemer, G.; Senovilla, L. Calcium Signaling and Cell Cycle: Progression or Death. Cell Calcium 2018, 70, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.C.; Carlier, A.; Bolander, J.; Roberts, S.J.; Geris, L.; Schrooten, J.; Van Oosterwyck, H.; Luyten, F.P. Current Views on Calcium Phosphate Osteogenicity and the Translation into Effective Bone Regeneration Strategies. Acta Biomater. 2012, 8, 3876–3887. [Google Scholar] [CrossRef] [PubMed]
- Ali Akbari Ghavimi, S.; Allen, B.N.; Stromsdorfer, J.L.; Kramer, J.S.; Li, X.; Ulery, B.D. Calcium and Phosphate Ions as Simple Signaling Molecules with Versatile Osteoinductivity. Biomed. Mater. 2018, 13, 055005. [Google Scholar] [CrossRef] [PubMed]
- Ghavimi, S.A.A.; Lungren, E.S.; Faulkner, T.J.; Josselet, M.A.; Wu, Y.; Sun, Y.; Pfeiffer, F.M.; Goldstein, C.L.; Wan, C.; Ulery, B.D. Inductive Co-Crosslinking of Cellulose Nanocrystal/Chitosan Hydrogels for the Treatment of Vertebral Compression Fractures. Int. J. Biol. Macromol. 2019, 130, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ghavimi, S.A.A.; Lungren, E.S.; Stromsdorfer, J.L.; Darkow, B.T.; Nguyen, J.A.; Sun, Y.; Pfieffer, F.M.; Goldstein, C.L.; Wan, C.; Ulery, B.D. Effect of Dibasic Calcium Phosphate Incorporation on Cellulose Nanocrystal/Chitosan Hydrogel Properties for the Treatment of Vertebral Compression Fractures. AAPS J. 2019, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, M.M.; Snyder, S.H. Hydrogen Sulfide as a Gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Goto, Y.-I.; Kimura, H. Hydrogen Sulfide Increases Glutathione Production and Suppresses Oxidative Stress in Mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.-Z.; Liu, Y.; Bian, J.-S. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxidative Medicine and Cellular Longevity. Oxidative Med. Cell. Longev. 2016, 2016, 6043038. [Google Scholar] [CrossRef]
- Yi, J.; Yuan, Y.; Zheng, J.; Zhao, T. Hydrogen Sulfide Alleviates Uranium-Induced Rat Hepatocyte Cytotoxicity via Inhibiting Nox4/ROS/P38 MAPK Pathway. J. Biochem. Mol. Toxicol. 2018, 33, e22255. [Google Scholar] [CrossRef]
- Wu, B.; Teng, H.; Zhang, L.; Li, H.; Li, J.; Wang, L.; Li, H. Interaction of Hydrogen Sulfide with Oxygen Sensing under Hypoxia. Oxidative Med. Cell. Longev. 2015, 2015, 758678. [Google Scholar] [CrossRef] [PubMed]
- Gambari, L.; Grigolo, B.; Grassi, F. Hydrogen Sulfide in Bone Tissue Regeneration and Repair: State of the Art and New Perspectives. Int. J. Mol. Sci. 2019, 20, 5231. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, G.; Cirino, G.; Santagada, V.; Wallace, J.L. Synthesis and Biological Effects of Hydrogen Sulfide (H2S): Development of H2S-Releasing Drugs as Pharmaceuticals. J. Med. Chem. 2010, 53, 6275–6286. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.-H.; Moore, P.K. Characterization of a Novel, Water-Soluble Hydrogen Sulfide–Releasing Molecule (GYY4137). Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Hu, X.; Zhuang, X.; Liao, L.; Li, W. GYY4137, a Novel Hydrogen Sulfide-Releasing Molecule, Likely Protects against High Glucose-Induced Cytotoxicity by Activation of the AMPK/MTOR Signal Pathway in H9c2 Cells. Mol. Cell. Biochem. 2013, 389, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M.; Papapetropoulos, A.; Vellecco, V.; Zhou, Z.; Pyriochou, A.; Roussos, C.; Roviezzo, F.; Brancaleone, V.; Cirino, G. Hydrogen Sulfide Is an Endogenous Inhibitor of Phosphodiesterase Activity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Oshita, A.; Rothstein, G.; Lonngi, G. CGMP Stimulation of Stem Cell Proliferation. Blood 1977, 49, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Caro, A.A.; Thompson, S.; Tackett, J. Increased Oxidative Stress and Cytotoxicity by Hydrogen Sulfide in HepG2 Cells Overexpressing Cytochrome P450 2E1. Cell Biol. Toxicol. 2011, 27, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Wagner, E.D.; Gaskins, H.R.; Plewa, M.J. Hydrogen Sulfide Induces Direct Radical-Associated DNA Damage. Mol. Cancer Res. 2007, 5, 455–459. [Google Scholar] [CrossRef]
- Benders, K.E.M.; Weeren, P.R.v.; Badylak, S.F.; Saris, D.B.F.; Dhert, W.J.A.; Malda, J. Extracellular Matrix Scaffolds for Cartilage and Bone Regeneration. Trends Biotechnol. 2013, 31, 169–176. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Zheng, Q.; Wan, X.; Ouyang, S.; Yin, Y.; Sui, X.; Liu, J.; Yang, X. Hydrogen Sulfide Prevents Hypoxia-Induced Apoptosis via Inhibition of an H2O2-Activated Calcium Signaling Pathway in Mouse Hippocampal Neurons. Biochem. Biophys. Res. Commun. 2012, 425, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Varanyuwatana, P.; Halestrap, A.P. The Roles of Phosphate and the Phosphate Carrier in the Mitochondrial Permeability Transition Pore. Mitochondrion 2012, 12, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.M.; Olson, M.S. The Regulation of Pyruvate Dehydrogenase in Isolated Beef Heart Mitochondria: The role of calcium, magnesium, and permeant anions. J. Biol. Chem. 1974, 249, 7159–7165. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.W.; Coetzee, W.A.; Lefer, D.J. Novel Insights into Hydrogen Sulfide–Mediated Cytoprotection. Antioxid. Redox Signal. 2010, 12, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, V.; Artiukhov, A.; Oppermann, H.; Kazantsev, A.; Lukashev, N.; Bunik, V. Mitochondrial Impairment May Increase Cellular NAD(P)H: Resazurin Oxidoreductase Activity, Perturbing the NAD(P)H-Based Viability Assays. Cells 2015, 4, 427–451. [Google Scholar] [CrossRef]
- Zhan, F.; Watanabe, Y.; Shimoda, A.; Hamada, E.; Kobayashi, Y.; Maekawa, M. Evaluation of Serum Bone Alkaline Phosphatase Activity in Patients with Liver Disease: Comparison between Electrophoresis and Chemiluminescent Enzyme Immunoassay. Clin. Chim. Acta 2016, 460, 40–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vimalraj, S. Alkaline Phosphatase: Structure, Expression and Its Function in Bone Mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Gambari, L.; Amore, E.; Raggio, R.; Bonani, W.; Barone, M.; Lisignoli, G.; Grigolo, B.; Motta, A.; Grassi, F. Hydrogen Sulfide-Releasing Silk Fibroin Scaffold for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 102, 471–482. [Google Scholar] [CrossRef]
- Chen, W.; Melamed, M.L.; Abramowitz, M.K. Serum Bicarbonate and Bone Mineral Density in US Adults. Am. J. Kidney Dis. 2015, 65, 240–248. [Google Scholar] [CrossRef]
- Ali Akbari Ghavimi, S.; Tata, R.R.; Greenwald, A.J.; Allen, B.N.; Grant, D.A.; Grant, S.A.; Lee, M.W.; Ulery, B.D. Controlled Ion Release from Novel Polyester/Ceramic Composites Enhances Osteoinductivity. AAPS J. 2017, 19, 1029–1044. [Google Scholar] [CrossRef]
- Zhou, Z.; von Wantoch Rekowski, M.; Coletta, C.; Szabo, C.; Bucci, M.; Cirino, G.; Topouzis, S.; Papapetropoulos, A.; Giannis, A. Thioglycine and L-Thiovaline: Biologically Active H2S-Donors. Bioorg. Med. Chem. 2012, 20, 2675–2678. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali Akbari Ghavimi, S.; Faulkner, T.J.; Tata, R.R.; Hemmerla, A.J.; Huddleston, S.E.; Rezaei, F.; Lungren, E.S.; Zhang, R.; Bumann, E.E.; Ulery, B.D. Hydrogen Sulfide Delivery to Enhance Bone Tissue Engineering Cell Survival. Pharmaceuticals 2024, 17, 585. https://doi.org/10.3390/ph17050585
Ali Akbari Ghavimi S, Faulkner TJ, Tata RR, Hemmerla AJ, Huddleston SE, Rezaei F, Lungren ES, Zhang R, Bumann EE, Ulery BD. Hydrogen Sulfide Delivery to Enhance Bone Tissue Engineering Cell Survival. Pharmaceuticals. 2024; 17(5):585. https://doi.org/10.3390/ph17050585
Chicago/Turabian StyleAli Akbari Ghavimi, Soheila, Trent J. Faulkner, Rama Rao Tata, August J. Hemmerla, Samantha E. Huddleston, Farnoushsadat Rezaei, Ethan S. Lungren, Rui Zhang, Erin E. Bumann, and Bret D. Ulery. 2024. "Hydrogen Sulfide Delivery to Enhance Bone Tissue Engineering Cell Survival" Pharmaceuticals 17, no. 5: 585. https://doi.org/10.3390/ph17050585
APA StyleAli Akbari Ghavimi, S., Faulkner, T. J., Tata, R. R., Hemmerla, A. J., Huddleston, S. E., Rezaei, F., Lungren, E. S., Zhang, R., Bumann, E. E., & Ulery, B. D. (2024). Hydrogen Sulfide Delivery to Enhance Bone Tissue Engineering Cell Survival. Pharmaceuticals, 17(5), 585. https://doi.org/10.3390/ph17050585






