Abstract
Mesenchymal stem cells (MSCs) are of great interest in cell therapies due to the immunomodulatory and other effects they have after autologous or allogeneic transplantation. In most clinical applications, a high number of MSCs is required; therefore, the isolated MSC population must be expanded in the cell culture until the desired number is reached. Analysing freshly isolated MSCs is challenging due to their rareness and heterogeneity, which is noticeable among donors, tissues, and cell subpopulations. Although the phenotype of MSCs in tissue can differ from those of cultured cells, phenotyping and counting are usually performed only after MSC proliferation. As MSC applicability is a developing and growing field, there is a need to implement phenotyping and counting methods for freshly isolated MSCs, especially in new one-step procedures where isolated cells are implanted immediately without cell culturing. Only by analysing harvested cells can we correctly evaluate such studies. This review describes multilevel heterogeneity and concentrations of MSCs and different strategies for phenotype determination and enumeration of freshly isolated MSCs.
1. Introduction
Multiple studies have been conducted since 1990 to investigate the regenerative and immune modulatory properties of mesenchymal stem cells (MSCs) [1,2,3,4,5,6]. These studies paved the way for the use of MSCs in preclinical and clinical trials. Over time, the number of applications using MSCs has increased. By 2023, registered clinical trials involving MSCs for treatment at clinicaltrials.gov (accessed on 1 December 2023) numbered over 1500. The most frequently listed studies included cardiology, traumatology, pneumology, neurology, haematology, ophthalmology, and plastic surgery. Studies have shown that MSC therapy is feasible and safe and that MSCs can effectively treat diseases or reduce the side effects of other treatments in some cases and under certain conditions. Studies have demonstrated that the main therapeutic effects of MSCs came from their production and secretion of different bioactive molecules. They can produce immunosuppressive factors such as IDO (indoleamine-2,3-dioxygenase), NO (nitric oxide), IL-10 (interleukin 10), TGF-β (transforming growth factor β), CCL2 (chemokine ligand 2), PGE2 (prostaglandin E2), IL-1Ra (IL-1 receptor antagonist), M-CSF (macrophage colony-stimulating factor), TSG-6 (tumour necrosis growth factor-induced protein 6), SOD-3 (superoxide dismutase 3), and HLA-G5 (human leukocyte antigen-G5) [7,8,9,10,11]. MSCs also secrete many trophic factors with mitogenic, antiapoptotic, neurodegenerative, angiogenetic, and other properties that accelerate the repair of damaged tissues. These factors include EGF (epidermal growth factor), VEGF (vascular endothelial growth factor), hGF (hepatocyte growth factor), PDGF (platelet growth factor), Ang (angiopoietin), and IGF-1 (insulin-like growth factor-1) [12]. The release of trophic factors, which stimulate endogenous mechanisms for regeneration, is essential for their effectiveness. MSCs can also exert antimicrobial activity by secreting IL-37 (human cathelicidin) and lipocalin [13].
Because of their immunomodulatory abilities, MSCs are increasingly gaining recognition not only in human medicine but also in veterinary medicine, where they represent a potential therapeutic option for various diseases, including orthopaedic, orodental, and digestive tract diseases. Human MSCs are under investigation as a treatment for more than twenty clinical conditions. Even though the majority of MSCs die soon after application and engraftment has been found to be limited [14,15,16], their therapeutic usefulness has been shown in many cases. As there are still numerous trials in progress, many researchers believe their results will open up new and more extensive possibilities for their use in future treatments. Commercial products with allogeneic MSCs have already been approved for routine use in the last few years, and this shows us that MSCs are also commercially attractive therapeutic tools.
However, there is still a lack of standardisation in the MSC manufacturing and quality control process. Cell preparation starts with MSC harvesting from a tissue sample. A review of currently registered clinical trials showed that therapeutic MSCs are most frequently obtained from the iliac crest, placenta, or adipose tissue. MSC dosages vary between different therapeutic targets, patients’ conditions, delivery routes, and the types of MSCs used [17]. An analysis of clinical trials with intravenous administrated MSCs between 2004 and 2018, which reported positive outcomes, indicated minimal effective doses ranging from 70 to 190 million MSCs [18]. The MSC count in the management of knee osteoarthritis is slightly lower; in the meta-analysis of results of clinical trials, the group of patients with 50–100 million MSCs delivered to the target site showed significant improvement in pain and functional outcomes, while a higher dose was less effective [19]. Multiple administrations of MSCs with up to several hundred million cells are needed for certain patients and diseases to achieve the desired therapeutic effect [20,21]. To reach such high numbers, MSCs must be expanded in the cell culture. Isolated cells are usually placed in a cell culture without prior determination of their number. MSCs are counted only later, during their proliferation in cell culture, or at the end of the manufacturing process when the final cell product is prepared for application.
Although it is still not routinely performed, MSC counting immediately after isolation from harvested tissue has value. Determining the starting cell number allows us to better plan the cell production process and reduce costs. In Europe and the USA, expanded MSCs are classified as advanced therapy medicinal products (ATMPs). While this regulation ensures quality products, it is also associated with higher costs. For some clinical conditions, using MSCs without multiplying them is becoming increasingly attractive because such minimal manipulation of cells allows MSCs to fall outside the ATMP classification, making the procedure more widely applicable. However, in these newer procedures, freshly isolated cell counting becomes essential because the cell application is performed on the same day within a hospital setting. An example of such one-step procedures involves using autologous bone marrow cells, which take approximately two hours from bone marrow aspiration to cell implantation [12].
Regardless of whether MSCs are used after proliferation or immediately after being obtained from tissue, determination of the initial MSC count is not routinely performed. The most probable reason MSC counting is not carried out before cell culturing or before direct re-implantation is that the MSC population in tissue is sparse. For example, MSCs present only 0.001–0.01% of the mononuclear cells in bone marrow [2]. The second reason is their phenotypic complexity (Figure 1). MSCs cannot be detected based on single marker protein expression; their identification is based on the presence of some specific surface markers and, simultaneously, on the absence of others. Besides this, MSCs show considerable phenotypic differences depending on their source [17]. The heterogeneity of MSCs is also seen in the tissue itself and is reflected in various MSC subpopulations. Furthermore, changes in MSC phenotype can be detected during cell expansion in vitro [22].
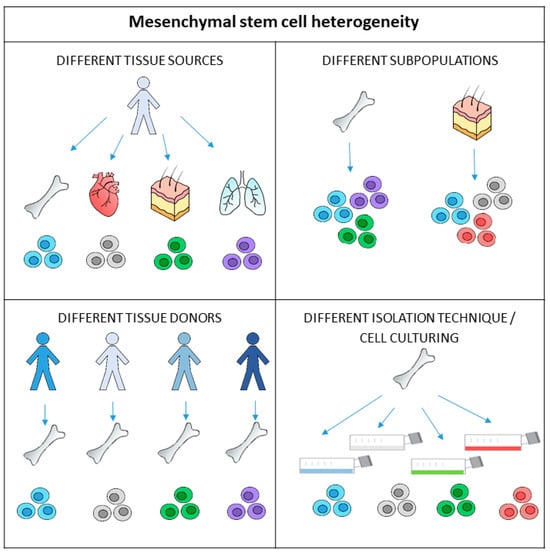
Figure 1.
Mesenchymal stem cells (MSCs) exhibit heterogeneity on multiple levels. The different biological properties and functions of MSCs are due to the anatomical tissue sources they come from as the environment affects the genetically identical cells. Differences can also be observed in MSCs taken from the same tissue sources in different individuals. There are also different subpopulations of MSCs present in tissues, and their proliferation, differentiation, or immunomodulation functions may differ. Furthermore, isolation and cell culturing techniques may affect the phenotype and functional properties of cells before transplantation.
In this review, we discuss MSC multilevel phenotypic diversity. As a result of their heterogenicity coupled with their low numbers, MSC phenotypisation and further number determination is not routinely performed before cell expansion. We also summarise all currently available phenotypisation and quantification methodologies for freshly isolated MSCs, although these procedures are not standardised and used regularly, as in the case of multiplied cells.
2. Mesenchymal Stem Cell Concentrations in the Source Tissues
Preparing cells for transplantation starts with cell isolation from the tissue source. Because of the small number of MSCs, isolated cells usually do not qualify for transplantation and have to be expanded in vitro over several population doublings to obtain enough cells for implantation. MSCs were first isolated from bone marrow [23], and later, they were obtained from almost every tissue of the body, i.e., bone marrow, adipose tissue, umbilical cord blood, Wharton’s jelly, amniotic fluid, neurospheres, placenta, dental pulp, peripheral blood, synovium and synovial fluid, endometrium, compact bone, dermis, pancreatic islets, adult brain tissues, skeletal muscle tissues, the lungs, heart, and hair follicles. Bone marrow aspiration is an invasive procedure, but it is still a frequently used method of obtaining MSCs for cell therapy. Bone marrow is the most studied source of MSCs and represents the most common source of MSCs for clinical applications in orthopaedics [24,25]. Bone marrow-derived MSCs are also used for acute myocardial infarction and ischaemic heart failure [26,27,28]. The separation of MSCs from peripheral blood after their mobilisation from bone marrow represents an alternative method of cell collection [29]. From 25 mL of bone marrow placed in an adherent cell culture, 100–150 million MSCs can be produced in a few weeks [30]. MSC expansion from the umbilical cord and peripheral blood is performed similarly [31].
Methods of isolating adipose-derived MSCs rely on the digestion of the adipose tissue using enzymes, such as collagenase, which destroys the extracellular matrix [32,33]. Whereas cutting, harvesting, and centrifuging are considered to be minimal manipulations of the cells, enzymatic digestion is a substantial manipulation. Enzymes can cause changes in cell characteristics, and MSCs obtained this way are classified as advanced therapy medicinal products (ATMPs). Some try to achieve cell isolation by optimizing various procedures to avoid enzymatic digestion by concentrating the lipoaspirate or isolating the stem cells with the use of mechanical procedures [34]. In general, these isolation procedures are still not as effective as those with the help of enzymes. One such method for isolating MSCs is the explant culture method, where tissue from Wharton’s jelly, bone, or cornea is cut into smaller pieces and placed in culture dishes [35]. Other methods are immuno-magnetic cell separation and the cell sorting methodology; however, these methods require MSC identification before cell isolation and are primarily used for research purposes. Identification or phenotypisation is also needed whenever we want to count isolated cells. MSCs derived from different anatomical tissues may have different developmental origins and different properties and functions, which can complicate their identification.
Cell amounts vary between tissues, but relatively low MSC concentrations are a feature of all MSC deposits in the body. The number of MSCs in tissue may depend on many factors. The concentration of MSCs in bone marrow depends on the patient’s age, medicaments used, condition of health, and bone remodelling diseases [36]. Studies examining the relationship between age and cell concentration in tissues have produced varying results [12,37,38,39,40]. Still, the majority suggest that the number of MSCs decreases with age. This decline in MSC numbers has been observed in bone marrow MSCs and adipose tissue-derived MSCs [12,39,40]. Cell growth was also more vigorous when cells were isolated from younger rather than older donors [41]. This is consistent with the observation that the ability of MSCs to regenerate tissue is probably impaired by the loss of stem cell numbers and function with age [42].
In the case of bone marrow, it has been proven that the initial number of stem cells also depends on the aspiration technique used to collect MSCs [12,43]. Stem cells are located in different niches in the medullary cavity [44], and different aspiration techniques may be more or less effective in preferentially extracting cells from various niches. Moreover, it has been demonstrated that the number of harvested cells significantly decreases during repeated aspirations. Table 1 shows data about native MSC numbers, which have been determined from the tissues most commonly used as a source of cells for therapies.

Table 1.
Amount of mesenchymal stem cells (MSCs) in different tissue sources.
3. Heterogeneity of Mesenchymal Stem Cells
MSCs exhibit heterogeneity on multiple levels (Figure 1). This heterogeneity is noticeable among donors, tissues, and cell populations. Studies have shown that age, sex, and physiological status could result in functional differences in MSCs derived from the same tissue of origin of different donors [63,64]. Studies have also compared bone marrow MSCs from various donors and found significant differences in cell growth rates and alkaline phosphatase enzyme activity [65]. The variability in the chondrogenic, osteogenic, or endothelial differentiation from different donors was shown as well [64,66,67].
Another level of heterogeneity among MSCs is an outcome of variability between different tissues. Since the microenvironment has a clear and substantial impact on the cells, heterogeneity is inevitable even when the cells are genetically identical. It has already been shown that the proliferation or differentiation of MSCs depends on the cell source. Studies also report different immunomodulatory properties of MSCs harvested from different tissues [68]. MSC heterogeneity is also present within the same tissue. Heterogeneity in a particular tissue is manifested in different subpopulations with distinct expression profiles and functional properties [69]. MSC plating density, different components in growth media (serum, growth factor combinations), and oxygen tension may affect the mesenchymal population’s gene profile, epigenomic state, and phenotype. Clones derived from even single proliferating MSCs can be heterogenous in vitro [70]. Additionally, clonal analysis of expanded MSCs has shown that the diversity is dramatically lowered to just a few clones after multiple passages [71]. As a result, the MSC subpopulations in cell culture do not represent the most abundant clones present at the beginning [71,72,73,74]. Therefore, it must be considered that the expression profile of MSCs in tissue probably differs from that of proliferating MSCs in culture. The phenotype during culturing can be monitored after each cell passage. With a larger number of passages, more MSCs are obtained for clinical application. However, since the number of cell passages can affect the MSCs’ properties, the most commonly used passages for treating include passages 3 to 7.
A consequence of MSC heterogeneity is the complexity of their identification, especially in the tissue. This is further complicated by the fact that MSC subpopulations are not well defined and are difficult to distinguish from each other. Examples of different combinations of phenotype markers used for MSC identification are collected in Table 2. Various marker combinations were used to confirm the identity of MSCs from different sources, i.e., bone marrow, adipose tissue, umbilical cord, and placenta. The most commonly used markers were CD29, CD44, CD73, CD90, and CD105, which were positively expressed, while among negatively expressed markers, CD34, CD45, and HLA-DR were most common.

Table 2.
An overview of different combinations of phenotype markers used for MSC identification.
4. Counting Mesenchymal Stem Cells Harvested from Tissue
4.1. The Mesenchymal Stem Cell Counts per Total Nucleated Cells
MSC estimation per total nucleated cells is the most simple method for determining native MSC numbers, but it is inaccurate. Hernigou et al. reported an equation for the prediction of the number of nucleated cells in bone marrow, as follows: N(108/kg) = (V × NP) − (V − 100) × NS/P, where V is the total volume of aspirate, NP is the nuclear cell count per ml of bone marrow aspirate, NS is the nuclear cell count per ml of peripheral blood, and P is the patient’s weight [112]. The above calculation was applied to MSCs isolated from bone marrow. Stem and progenitor cells in the uncultured stromal vascular fraction (SVF) from adipose tissue usually amount to up to 3% of the whole number of cells, which is 2500-fold greater than the frequency of stem cells in bone marrow [113].
4.2. Estimation of Mesenchymal Stem Cell Count with the CFU-F Test
MSCs were initially referred to as fibroblast-like cells that can generate colony-forming unit fibroblasts or CFU-F. Determining the colony-forming units present is the oldest way of estimating the actual stem cells in the suspension of different cells [114]. A suspension of mixed cells is plated on plastic culture dishes at a low density. Culturing under specific conditions allows stem cells to adhere, proliferate, and form colonies. After the growth of visible colonies, they are counted using light microscopy. The number of colonies indicates the number of cells that were able to proliferate, as each colony is presumed to originate from a single stem cell.
The CFU-F assay has some shortcomings. Cell culturing takes several days before the identification of colonies; the method is time-consuming and inappropriate for routine use, the counting of colonies is subjective, not standardised; and the technique lacks accuracy [115]. Even though this procedure gives us the number of functional, proliferating MSCs in a tissue, there is a need for further investigation to explore alternative approaches to assessing the quantity of MSCs.
5. Mesenchymal Stem Cell Quantification with Immunophenotyping
For accurate counting of MSCs, their identity has to be ascertained first. Identification is based on the characteristic protein expression profile of MSCs. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) defined an MSC phenotype that is used irrespective of tissue source. Expanded cells are determined to be MSCs when expressing CD105, CD90, and CD73 and not expressing CD45, CD34, CD14 (CD11b), CD79 (CD19), or human lymphocyte antigen-DR (HLA-DR) [75]. This method is usually not used in the case of freshly isolated MSCs. Besides this, in recent years, new markers with positive or negative expression and new marker combinations have been recognised, among them CD49a, CD106 (VCAM-1), CD140b, CD146, CD271 (LNGFR), MSCA-1, GD2, and STRO-1 [12,79,116,117,118]. Many of these markers were used to identify MSC subpopulations expressing different types of regulatory proteins that function in hematopoiesis, angiogenesis, neural activities, and immunity processes [63].
In order to quantify MSCs before culturing, it is necessary to identify the MSC (sub)population from the harvested cell suspension where MSCs are still suspended with other cells. MSCs from the same tissue are heterogeneous and may not share the same phenotypical markers. Additionally, there is a lack of consensus on the markers identifying or distinguishing MSCs derived from different tissues. The chosen phenotype markers may influence the count; for example, the CD45–/CD271+ MSCs correlate more with CFU-F numbers than the CD45–/CD73+/CD90+/CD105+ MSCs [12]. Therefore, the identification of native MSCs is much more complicated than expanded MSCs. The biological capacity of MSCs (i.e., immunomodulatory capacity, differentiation potential to a specific cell type, and endogenous stem cell mobilising capacity) of one tissue source may differ from others. Isolation or production of a specific subpopulation of MSCs for a particular medical indication should improve outcomes.
Despite the numerous markers for MSC identification at our disposal, no agreement exists over the correct composite marker panel for defining MSCs in freshly isolated and non-cultured populations. For this reason, a precise characterisation of MSCs derived from different tissues and their proper ties relating to their therapeutic potential represents an essential requirement for the exploitation and development of optimal MSC-based therapies. Additional studies should be conducted to test the correlation between phenotype markers and CFU-F and other biological effects. Together with multicentric study data, the ISCT could recommend criteria for native MSCs analysis.
6. Towards the Standardisation of Mesenchymal Stem Cell-Based Therapies
Knowing the number of MSCs before transplantation would help clarify the efficacy and limitations of cell therapy. When planning for autologous transplantations, it is important to have information on the initial number of cells in order to anticipate cell proliferation and determine the date of application (freezing, thawing, and additional culturing can be avoided). In cases where one-step procedures without cell expansion are used, it would be beneficial to determine the count and subpopulations of MSCs present for further evaluation of the treatment. By detecting MSC properties and sorting selected subpopulations, it may be possible to utilise the most efficient cells for a given clinical condition.
There are many groups that are currently working on standardising therapies that involve the use of MSCs. Their focus is on standardising MSC isolation techniques, enzymatic digestion, explant culture, or enrichment techniques. They are also discussing nomenclature and trying to set identifying criteria to distinguish MSCs from different tissue cells and other fibroblastic cells. The ISCT has recently published ISO standardisation documents that are focused on the biobanking of MSCs from two tissue sources, Wharton’s Jelly and bone marrow [119,120,121,122]. There is an awareness that these documents are evolving documents with iterations amending various sections with revisions as the science progresses and we gain deeper understanding of MSC biology and functionality.
7. Conclusions
There is still a lack of standardisation in the MSC manufacturing and quality control process; however, there are many groups that are currently working on standardising therapies that involve the use of MSCs with a recognition that premature standards or inappropriate scope may distort or inhibit the adoption of MSC-based therapies. Currently, there is some information available on cultured cells, but more research is needed on native MSCs, especially those used for one-step procedures. Uncultured MSCs offer an alternative in some clinical applications. The main reason that initial MSCs are not counted is their low concentration in native tissue and their multilevel heterogeneity. Consequently, the identification of native MSCs is more complicated than that of cultured cells. Since the identification of MSCs with cell surface markers is necessary for their counting, there is a need to develop a consensus regarding standards for more accurately identifying native MSCs isolated from different sources and different MSC subpopulations.
Author Contributions
E.M. and K.J. designed and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was supported by the Slovenian Research Agency (project number L3-3176).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lazarus, H.M.; Haynesworth, S.E.; Gerson, S.L.; Rosenthal, N.S.; Caplan, A.I. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): Implications for therapeutic use. Bone Marrow. Transplant. 1995, 16, 557–564. [Google Scholar]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Gotherstrom, C.; Hassan, M.; Uzunel, M.; Ringden, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Galderisi, U.; Marino, I.R. From the laboratory bench to the patient’s bedside: An update on clinical trials with mesenchymal stem cells. J. Cell. Physiol. 2007, 211, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef]
- De Miguel, M.P.; Fuentes-Julian, S.; Blazquez-Martinez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr. Mol. Med. 2012, 12, 574–591. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Ryu, S.; Kim, D.S.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Strategies to improve the immunosuppressive properties of human mesenchymal stem cells. Stem Cell Res. Ther. 2015, 6, 179. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Rebolj, K.; Veber, M.; Drobnic, M.; Malicev, E. Hematopoietic stem cell and mesenchymal stem cell population size in bone marrow samples depends on patient’s age and harvesting technique. Cytotechnology 2018, 70, 1575–1583. [Google Scholar] [CrossRef]
- Boomsma, R.A.; Geenen, D.L. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS ONE 2012, 7, e35685. [Google Scholar] [CrossRef]
- Toma, C.; Wagner, W.R.; Bowry, S.; Schwartz, A.; Villanueva, F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: In vivo observations of cell kinetics. Circ. Res. 2009, 104, 398–402. [Google Scholar] [CrossRef]
- von Bahr, L.; Batsis, I.; Moll, G.; Hagg, M.; Szakos, A.; Sundberg, B.; Uzunel, M.; Ringden, O.; Le Blanc, K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 2012, 30, 1575–1578. [Google Scholar] [CrossRef]
- Leibacher, J.; Henschler, R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res. Ther. 2016, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fuentes, D.E.; Fernandez-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldana, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in mesenchymal stem cell clinical trials 2004–2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl. Med. 2020, 9, 17–27. [Google Scholar] [CrossRef]
- Muthu, S.; Mir, A.A.; Kumar, R.; Yadav, V.; Jeyaraman, M.; Khanna, M. What is the clinically significant ideal mesenchymal stromal cell count in the management of osteoarthritis of the knee?—Meta-analysis of randomized controlled trials. J. Clin. Orthop. Trauma 2022, 25, 101744. [Google Scholar] [CrossRef]
- El-Kheir, W.A.; Gabr, H.; Awad, M.R.; Ghannam, O.; Barakat, Y.; Farghali, H.A.; El Maadawi, Z.M.; Ewes, I.; Sabaawy, H.E. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014, 23, 729–745. [Google Scholar] [CrossRef]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Aguayo, C.; Bonilla, C.; Marin, E.; Tapiador, N.; Sevilla, M.; Vazquez, D.; Carballido, J.; et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy 2018, 20, 806–819. [Google Scholar] [CrossRef]
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef]
- Friedenstein, A.J. Precursor cells of mechanocytes. Int. Rev. Cytol. 1976, 47, 327–359. [Google Scholar] [CrossRef]
- Narbona-Carceles, J.; Vaquero, J.; Suarez-Sancho, S.; Forriol, F.; Fernandez-Santos, M.E. Bone marrow mesenchymal stem cell aspirates from alternative sources: Is the knee as good as the iliac crest? Injury 2014, 45 (Suppl. S4), S42–S47. [Google Scholar] [CrossRef]
- Hyer, C.F.; Berlet, G.C.; Bussewitz, B.W.; Hankins, T.; Ziegler, H.L.; Philbin, T.M. Quantitative assessment of the yield of osteoblastic connective tissue progenitors in bone marrow aspirate from the iliac crest, tibia, and calcaneus. J. Bone Jt. Surg. Am. 2013, 95, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.F.; Tirouvanziam, A.M.; Perigaud, C.; Fernandes, S.; Fusellier, M.S.; Desfontis, J.C.; Toquet, C.S.; Heymann, M.F.; Crochet, D.P.; Lemarchand, P.F. Cell distribution after intracoronary bone marrow stem cell delivery in damaged and undamaged myocardium: Implications for clinical trials. Stem Cell Res. Ther. 2010, 1, 4. [Google Scholar] [CrossRef]
- Schaefer, A.; Zwadlo, C.; Fuchs, M.; Meyer, G.P.; Lippolt, P.; Wollert, K.C.; Drexler, H. Long-term effects of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: 5-year results from the randomized-controlled BOOST trial—An echocardiographic study. Eur. J. Echocardiogr. 2010, 11, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yu, J.; Zhang, N.; Li, W.; Wang, J.; Cai, G.; Chen, Y.; Yang, Y.; Liu, Z. Bone marrow mesenchymal stem cells transfer in patients with ST-segment elevation myocardial infarction: Single-blind, multicenter, randomized controlled trial. Stem Cell Res. Ther. 2021, 12, 33. [Google Scholar] [CrossRef]
- Kassis, I.; Zangi, L.; Rivkin, R.; Levdansky, L.; Samuel, S.; Marx, G.; Gorodetsky, R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006, 37, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F. Mesenchymal stem cells from adult bone marrow. Methods Mol. Biol. 2008, 449, 27–44. [Google Scholar] [CrossRef]
- Salehinejad, P.; Moshrefi, M.; Eslaminejad, T. An Overview on Mesenchymal Stem Cells Derived from Extraembryonic Tissues: Supplement Sources and Isolation Methods. Stem Cells Cloning 2020, 13, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Bertheuil, N.; Chaput, B.; Menard, C.; Varin, A.; Garrido, I.; Grolleau, J.L.; Sensebe, L.; Watier, E.; Tarte, K. Adipose-derived stromal cells: History, isolation, immunomodulatory properties and clinical perspectives. Ann. Chir. Plast. Esthet. 2015, 60, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I.; et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015, 4, 713. [Google Scholar] [CrossRef] [PubMed]
- Hendijani, F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017, 50, e12334. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Guan, J.; Zhang, C. Mesenchymal stem cells: Mechanisms and role in bone regeneration. Postgrad. Med. J. 2014, 90, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Stenderup, K.; Justesen, J.; Eriksen, E.F.; Rattan, S.I.; Kassem, M. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J. Bone Miner. Res. 2001, 16, 1120–1129. [Google Scholar] [CrossRef]
- Kassem, M.; Marie, P.J. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell 2011, 10, 191–197. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schafer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, K.J.; Kim, M.K.; Lee, S.J.; Ryu, Y.H.; Seo, B.F.; Oh, D.Y.; Ahn, S.T.; Lee, H.Y.; Rhie, J.W. The stem cell potential and multipotency of human adipose tissue-derived stem cells vary by cell donor and are different from those of other types of stem cells. Cells Tissues Organs 2014, 199, 373–383. [Google Scholar] [CrossRef]
- Gharibi, B.; Farzadi, S.; Ghuman, M.; Hughes, F.J. Inhibition of Akt/mTOR attenuates age-related changes in mesenchymal stem cells. Stem Cells 2014, 32, 2256–2266. [Google Scholar] [CrossRef]
- McLain, R.F.; Fleming, J.E.; Boehm, C.A.; Muschler, G.F. Aspiration of osteoprogenitor cells for augmenting spinal fusion: Comparison of progenitor cell concentrations from the vertebral body and iliac crest. J. Bone Jt. Surg. Am. 2005, 87, 2655–2661. [Google Scholar] [CrossRef]
- Yoshioka, S.; Miura, Y.; Iwasa, M.; Fujishiro, A.; Yao, H.; Miura, M.; Fukuoka, M.; Nakagawa, Y.; Yokota, A.; Hirai, H.; et al. Isolation of mesenchymal stromal/stem cells from small-volume umbilical cord blood units that do not qualify for the banking system. Int. J. Hematol. 2015, 102, 218–229. [Google Scholar] [CrossRef]
- Castro-Malaspina, H.; Gay, R.E.; Resnick, G.; Kapoor, N.; Meyers, P.; Chiarieri, D.; McKenzie, S.; Broxmeyer, H.E.; Moore, M.A. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood 1980, 56, 289–301. [Google Scholar] [CrossRef]
- Caplan, A.I. Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef]
- Brozovich, A.; Sinicrope, B.J.; Bauza, G.; Niclot, F.B.; Lintner, D.; Taraballi, F.; McCulloch, P.C. High Variability of Mesenchymal Stem Cells Obtained via Bone Marrow Aspirate Concentrate Compared with Traditional Bone Marrow Aspiration Technique. Orthop. J. Sports Med. 2021, 9, 23259671211058459. [Google Scholar] [CrossRef]
- Cuthbert, R.; Boxall, S.A.; Tan, H.B.; Giannoudis, P.V.; McGonagle, D.; Jones, E. Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy 2012, 14, 431–440. [Google Scholar] [CrossRef]
- Consentius, C.; Mirenska, A.; Jurisch, A.; Reinke, S.; Scharm, M.; Zenclussen, A.C.; Hennig, C.; Volk, H.D. In situ detection of CD73+ CD90+ CD105+ lineage: Mesenchymal stromal cells in human placenta and bone marrow specimens by chipcytometry. Cytometry Part A 2018, 93, 889–893. [Google Scholar] [CrossRef]
- Rickard, D.J.; Kassem, M.; Hefferan, T.E.; Sarkar, G.; Spelsberg, T.C.; Riggs, B.L. Isolation and characterization of osteoblast precursor cells from human bone marrow. J. Bone Miner. Res. 1996, 11, 312–324. [Google Scholar] [CrossRef]
- Bruder, S.P.; Jaiswal, N.; Haynesworth, S.E. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J. Cell. Biochem. 1997, 64, 278–294. [Google Scholar] [CrossRef]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef]
- Oedayrajsingh-Varma, M.J.; van Ham, S.M.; Knippenberg, M.; Helder, M.N.; Klein-Nulend, J.; Schouten, T.E.; Ritt, M.J.; van Milligen, F.J. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 2006, 8, 166–177. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, T.; Song, K.; Fan, X.; Ma, X.; Cui, Z. Adipose-derived stem cell: A better stem cell than BMSC. Cell Biochem. Funct. 2008, 26, 664–675. [Google Scholar] [CrossRef]
- Baer, P.C.; Geiger, H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012, 2012, 812693. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Bieback, K.; Kern, S.; Kluter, H.; Eichler, H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004, 22, 625–634. [Google Scholar] [CrossRef]
- Rogers, I.; Casper, R.F. Umbilical cord blood stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 893–908. [Google Scholar] [CrossRef]
- Karahuseyinoglu, S.; Cinar, O.; Kilic, E.; Kara, F.; Akay, G.G.; Demiralp, D.O.; Tukun, A.; Uckan, D.; Can, A. Biology of stem cells in human umbilical cord stroma: In situ and in vitro surveys. Stem Cells 2007, 25, 319–331. [Google Scholar] [CrossRef]
- Weiss, M.L.; Medicetty, S.; Bledsoe, A.R.; Rachakatla, R.S.; Choi, M.; Merchav, S.; Luo, Y.; Rao, M.S.; Velagaleti, G.; Troyer, D. Human umbilical cord matrix stem cells: Preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 2006, 24, 781–792. [Google Scholar] [CrossRef]
- Raynaud, C.M.; Maleki, M.; Lis, R.; Ahmed, B.; Al-Azwani, I.; Malek, J.; Safadi, F.F.; Rafii, A. Comprehensive characterization of mesenchymal stem cells from human placenta and fetal membrane and their response to osteoactivin stimulation. Stem Cells Int. 2012, 2012, 658356. [Google Scholar] [CrossRef]
- Brooke, G.; Rossetti, T.; Pelekanos, R.; Ilic, N.; Murray, P.; Hancock, S.; Antonenas, V.; Huang, G.; Gottlieb, D.; Bradstock, K.; et al. Manufacturing of human placenta-derived mesenchymal stem cells for clinical trials. Br. J. Haematol. 2009, 144, 571–579. [Google Scholar] [CrossRef]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: From characterization to application. NPJ Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef]
- Siddappa, R.; Licht, R.; van Blitterswijk, C.; de Boer, J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J. Orthop. Res. 2007, 25, 1029–1041. [Google Scholar] [CrossRef]
- Phinney, D.G.; Kopen, G.; Righter, W.; Webster, S.; Tremain, N.; Prockop, D.J. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J. Cell. Biochem. 1999, 75, 424–436. [Google Scholar] [CrossRef]
- Payne, K.A.; Didiano, D.M.; Chu, C.R. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthr. Cartil. 2010, 18, 705–713. [Google Scholar] [CrossRef]
- Portalska, K.J.; Groen, N.; Krenning, G.; Georgi, N.; Mentink, A.; Harmsen, M.C.; van Blitterswijk, C.; de Boer, J. The effect of donor variation and senescence on endothelial differentiation of human mesenchymal stromal cells. Tissue Eng. Part A 2013, 19, 2318–2329. [Google Scholar] [CrossRef]
- Wegmeyer, H.; Broske, A.M.; Leddin, M.; Kuentzer, K.; Nisslbeck, A.K.; Hupfeld, J.; Wiechmann, K.; Kuhlen, J.; von Schwerin, C.; Stein, C.; et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013, 22, 2606–2618. [Google Scholar] [CrossRef]
- Phinney, D.G.; Hill, K.; Michelson, C.; DuTreil, M.; Hughes, C.; Humphries, S.; Wilkinson, R.; Baddoo, M.; Bayly, E. Biological activities encoded by the murine mesenchymal stem cell transcriptome provide a basis for their developmental potential and broad therapeutic efficacy. Stem Cells 2006, 24, 186–198. [Google Scholar] [CrossRef]
- Rennerfeldt, D.A.; Van Vliet, K.J. Concise Review: When Colonies Are Not Clones: Evidence and Implications of Intracolony Heterogeneity in Mesenchymal Stem Cells. Stem Cells 2016, 34, 1135–1141. [Google Scholar] [CrossRef]
- Selich, A.; Daudert, J.; Hass, R.; Philipp, F.; von Kaisenberg, C.; Paul, G.; Cornils, K.; Fehse, B.; Rittinghausen, S.; Schambach, A.; et al. Massive Clonal Selection and Transiently Contributing Clones during Expansion of Mesenchymal Stem Cell Cultures Revealed by Lentiviral RGB-Barcode Technology. Stem Cells Transl. Med. 2016, 5, 591–601. [Google Scholar] [CrossRef]
- Roobrouck, V.D.; Vanuytsel, K.; Verfaillie, C.M. Concise review: Culture mediated changes in fate and/or potency of stem cells. Stem Cells 2011, 29, 583–589. [Google Scholar] [CrossRef]
- Ghazanfari, R.; Zacharaki, D.; Li, H.; Ching Lim, H.; Soneji, S.; Scheding, S. Human Primary Bone Marrow Mesenchymal Stromal Cells and Their In Vitro Progenies Display Distinct Transcriptional Profile Signatures. Sci. Rep. 2017, 7, 10338. [Google Scholar] [CrossRef]
- Krinner, A.; Zscharnack, M.; Bader, A.; Drasdo, D.; Galle, J. Impact of oxygen environment on mesenchymal stem cell expansion and chondrogenic differentiation. Cell Prolif. 2009, 42, 471–484. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Khatun, M.; Sorjamaa, A.; Kangasniemi, M.; Sutinen, M.; Salo, T.; Liakka, A.; Lehenkari, P.; Tapanainen, J.S.; Vuolteenaho, O.; Chen, J.C.; et al. Niche matters: The comparison between bone marrow stem cells and endometrial stem cells and stromal fibroblasts reveal distinct migration and cytokine profiles in response to inflammatory stimulus. PLoS ONE 2017, 12, e0175986. [Google Scholar] [CrossRef]
- Simmons, P.J.; Torok-Storb, B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 1991, 78, 55–62. [Google Scholar] [CrossRef]
- Gronthos, S.; Zannettino, A.C.; Hay, S.J.; Shi, S.; Graves, S.E.; Kortesidis, A.; Simmons, P.J. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J. Cell Sci. 2003, 116, 1827–1835. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P.G.; Riminucci, M.; et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007, 131, 324–336. [Google Scholar] [CrossRef]
- Kuci, S.; Kuci, Z.; Kreyenberg, H.; Deak, E.; Putsch, K.; Huenecke, S.; Amara, C.; Koller, S.; Rettinger, E.; Grez, M.; et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica 2010, 95, 651–659. [Google Scholar] [CrossRef]
- Quirici, N.; Soligo, D.; Bossolasco, P.; Servida, F.; Lumini, C.; Deliliers, G.L. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp. Hematol. 2002, 30, 783–791. [Google Scholar] [CrossRef]
- Jones, E.A.; Kinsey, S.E.; English, A.; Jones, R.A.; Straszynski, L.; Meredith, D.M.; Markham, A.F.; Jack, A.; Emery, P.; McGonagle, D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002, 46, 3349–3360. [Google Scholar] [CrossRef]
- Rasini, V.; Dominici, M.; Kluba, T.; Siegel, G.; Lusenti, G.; Northoff, H.; Horwitz, E.M.; Schafer, R. Mesenchymal stromal/stem cells markers in the human bone marrow. Cytotherapy 2013, 15, 292–306. [Google Scholar] [CrossRef]
- Bhat, S.; Viswanathan, P.; Chandanala, S.; Prasanna, S.J.; Seetharam, R.N. Expansion and characterization of bone marrow derived human mesenchymal stromal cells in serum-free conditions. Sci. Rep. 2021, 11, 3403. [Google Scholar] [CrossRef]
- Campioni, D.; Lanza, F.; Moretti, S.; Ferrari, L.; Cuneo, A. Loss of Thy-1 (CD90) antigen expression on mesenchymal stromal cells from hematologic malignancies is induced by in vitro angiogenic stimuli and is associated with peculiar functional and phenotypic characteristics. Cytotherapy 2008, 10, 69–82. [Google Scholar] [CrossRef]
- Aslan, H.; Zilberman, Y.; Kandel, L.; Liebergall, M.; Oskouian, R.J.; Gazit, D.; Gazit, Z. Osteogenic differentiation of noncultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells 2006, 24, 1728–1737. [Google Scholar] [CrossRef]
- Battula, V.L.; Bareiss, P.M.; Treml, S.; Conrad, S.; Albert, I.; Hojak, S.; Abele, H.; Schewe, B.; Just, L.; Skutella, T.; et al. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation 2007, 75, 279–291. [Google Scholar] [CrossRef]
- Pinho, S.; Lacombe, J.; Hanoun, M.; Mizoguchi, T.; Bruns, I.; Kunisaki, Y.; Frenette, P.S. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 2013, 210, 1351–1367. [Google Scholar] [CrossRef]
- Buhring, H.J.; Battula, V.L.; Treml, S.; Schewe, B.; Kanz, L.; Vogel, W. Novel markers for the prospective isolation of human MSC. Ann. N. Y. Acad. Sci. 2007, 1106, 262–271. [Google Scholar] [CrossRef]
- Buhring, H.J.; Treml, S.; Cerabona, F.; de Zwart, P.; Kanz, L.; Sobiesiak, M. Phenotypic characterization of distinct human bone marrow-derived MSC subsets. Ann. N. Y. Acad. Sci. 2009, 1176, 124–134. [Google Scholar] [CrossRef]
- Mohanty, S.T.; Cairney, C.J.; Chantry, A.D.; Madan, S.; Fernandes, J.A.; Howe, S.J.; Moore, H.D.; Thompson, M.J.; Chen, B.; Thrasher, A.; et al. A small molecule modulator of prion protein increases human mesenchymal stem cell lifespan, ex vivo expansion, and engraftment to bone marrow in NOD/SCID mice. Stem Cells 2012, 30, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yu, W.; Ye, G.; Li, J.; Zheng, G.; Liu, W.; Lin, J.; Su, Z.; Che, Y.; Ye, F.; et al. Single-cell RNA sequencing analysis of human bone-marrow-derived mesenchymal stem cells and functional subpopulation identification. Exp. Mol. Med. 2022, 54, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Han, Z.B.; Ji, Y.R.; Wang, Y.W.; Liang, L.; Chi, Y.; Yang, S.G.; Li, L.N.; Luo, W.F.; Li, J.P.; et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS ONE 2013, 8, e59354. [Google Scholar] [CrossRef]
- Harkness, L.; Zaher, W.; Ditzel, N.; Isa, A.; Kassem, M. CD146/MCAM defines functionality of human bone marrow stromal stem cell populations. Stem Cell Res. Ther. 2016, 7, 4. [Google Scholar] [CrossRef]
- Busser, H.; Najar, M.; Raicevic, G.; Pieters, K.; Velez Pombo, R.; Philippart, P.; Meuleman, N.; Bron, D.; Lagneaux, L. Isolation and Characterization of Human Mesenchymal Stromal Cell Subpopulations: Comparison of Bone Marrow and Adipose Tissue. Stem Cells Dev. 2015, 24, 2142–2157. [Google Scholar] [CrossRef]
- Widowati, W.; Noverina, R.; Ayuningtyas, W.; Kurniawan, D.; Kusuma, H.S.W.; Arumwardana, S.; Artie, D.S.; Sholihah, I.A.; Handayani, R.A.S.; Laksmitawati, D.R.; et al. Proliferation, Characterization and Differentiation Potency of Adipose Tissue-Derived Mesenchymal Stem Cells (AT-MSCs) Cultured in Fresh Frozen and non-Fresh Frozen Plasma. Int. J. Mol. Cell. Med. 2019, 8, 283–294. [Google Scholar] [CrossRef]
- Mildmay-White, A.; Khan, W. Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2017, 12, 484–492. [Google Scholar] [CrossRef]
- Ferraro, G.A.; De Francesco, F.; Nicoletti, G.; Paino, F.; Desiderio, V.; Tirino, V.; D’Andrea, F. Human adipose CD34+ CD90+ stem cells and collagen scaffold constructs grafted in vivo fabricate loose connective and adipose tissues. J. Cell. Biochem. 2013, 114, 1039–1049. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Kohli, N.; Al-Delfi, I.R.T.; Snow, M.; Sakamoto, T.; Miyazaki, T.; Nakajima, H.; Uchida, K.; Johnson, W.E.B. CD271-selected mesenchymal stem cells from adipose tissue enhance cartilage repair and are less angiogenic than plastic adherent mesenchymal stem cells. Sci. Rep. 2019, 9, 3194. [Google Scholar] [CrossRef]
- Smith, R.J.P.; Faroni, A.; Barrow, J.R.; Soul, J.; Reid, A.J. The angiogenic potential of CD271+ human adipose tissue-derived mesenchymal stem cells. Stem Cell Res. Ther. 2021, 12, 160. [Google Scholar] [CrossRef]
- Sarugaser, R.; Lickorish, D.; Baksh, D.; Hosseini, M.M.; Davies, J.E. Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells 2005, 23, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Guan, H.; Zhou, F. Biological Characteristics of Umbilical Cord Mesenchymal Stem Cells and Its Therapeutic Potential for Hematological Disorders. Front. Cell Dev. Biol. 2021, 9, 570179. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Ghanbarvand, F.; Reza Behvarz, M.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int. J. Stem Cells 2014, 7, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Gatta, V.; D’Aurora, M.; Lanuti, P.; Pierdomenico, L.; Sperduti, S.; Palka, G.; Gesi, M.; Marchisio, M.; Miscia, S.; Stuppia, L. Gene expression modifications in Wharton’s Jelly mesenchymal stem cells promoted by prolonged in vitro culturing. BMC Genom. 2013, 14, 635. [Google Scholar] [CrossRef] [PubMed]
- Nagamura-Inoue, T.; He, H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells 2014, 6, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Y.; Wang, L.; Zheng, Y.; Yu, H.; Dong, X.; He, W.; Yin, Z.; Wang, Z. Study of the biological characteristics of human umbilical cord mesenchymal stem cells after long-time cryopreservation. Cell Tissue Bank. 2022, 23, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.Y.; Subramanian, A.; Biswas, A.; Gauthaman, K.; Srikanth, P.; Hande, M.P.; Bongso, A. Derivation efficiency, cell proliferation, freeze-thaw survival, stem-cell properties and differentiation of human Wharton’s jelly stem cells. Reprod. Biomed. Online 2010, 21, 391–401. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, R.; Zou, Q.; Chen, Y.; Zhou, M.; Li, X.; Ran, R.; Chen, Q. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci. Rep. 2018, 8, 5014. [Google Scholar] [CrossRef]
- Fukuchi, Y.; Nakajima, H.; Sugiyama, D.; Hirose, I.; Kitamura, T.; Tsuji, K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells 2004, 22, 649–658. [Google Scholar] [CrossRef]
- Papait, A.; Vertua, E.; Magatti, M.; Ceccariglia, S.; De Munari, S.; Silini, A.R.; Sheleg, M.; Ofir, R.; Parolini, O. Mesenchymal Stromal Cells from Fetal and Maternal Placenta Possess Key Similarities and Differences: Potential Implications for Their Applications in Regenerative Medicine. Cells 2020, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Poignard, A.; Manicom, O.; Mathieu, G.; Rouard, H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J. Bone Jt. Surg. Br. 2005, 87, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Zhu, M.; Wulur, I.; Alfonso, Z. Adipose-derived stem cells. Methods Mol. Biol. 2008, 449, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974, 17, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Cercone, M.; Greenfield, M.R.; Fortier, L.A. Bone Marrow Concentrate Mesenchymal Stromal Cells Do not Correlate With Nucleated Cell Count or Colony Forming Units. J. Cartil. Jt. Preserv. 2021, 1, 100017. [Google Scholar] [CrossRef]
- Shi, S.; Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef]
- Battula, V.L.; Treml, S.; Bareiss, P.M.; Gieseke, F.; Roelofs, H.; de Zwart, P.; Muller, I.; Schewe, B.; Skutella, T.; Fibbe, W.E.; et al. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica 2009, 94, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Wilson, A.J.; Brown, N.; Rand, E.; Genever, P.G. Attitudes towards Standardization of Mesenchymal Stromal Cells—A Qualitative Exploration of Expert Views. Stem Cells Transl. Med. 2023, 12, 745–757. [Google Scholar] [CrossRef]
- Wright, A.; Arthaud-Day, M.L.; Weiss, M.L. Therapeutic Use of Mesenchymal Stromal Cells: The Need for Inclusive Characterization Guidelines to Accommodate All Tissue Sources and Species. Front. Cell Dev. Biol. 2021, 9, 632717. [Google Scholar] [CrossRef]
- Garcia-Munoz, E.; Vives, J. Towards the standardization of methods of tissue processing for the isolation of mesenchymal stromal cells for clinical use. Cytotechnology 2021, 73, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Blanc, K.L.; Ciccocioppo, R.; Dagher, G.; Filiano, A.J.; Galipeau, J.; Krampera, M.; Krieger, L.; Lalu, M.M.; Nolta, J.; et al. An International Society for Cell and Gene Therapy Mesenchymal Stromal Cells (MSC) Committee perspectives on International Standards Organization/Technical Committee 276 Biobanking Standards for bone marrow-MSCs and umbilical cord tissue-derived MSCs for research purposes. Cytotherapy 2023, 25, 803–807. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).