An Assessment of Different Decision Support Software from the Perspective of Potential Drug–Drug Interactions in Patients with Chronic Kidney Diseases
Abstract
1. Introduction
2. Results
Potential Drug–Drug Interactions
3. Discussion
3.1. Frequency and Severity of Potential Drug–Drug Interactions
3.2. Comparison of Potential Drug–Drug Interaction Information from Different Sources
3.3. Limitations
4. Materials and Methods
4.1. Setting and Patient Characteristics
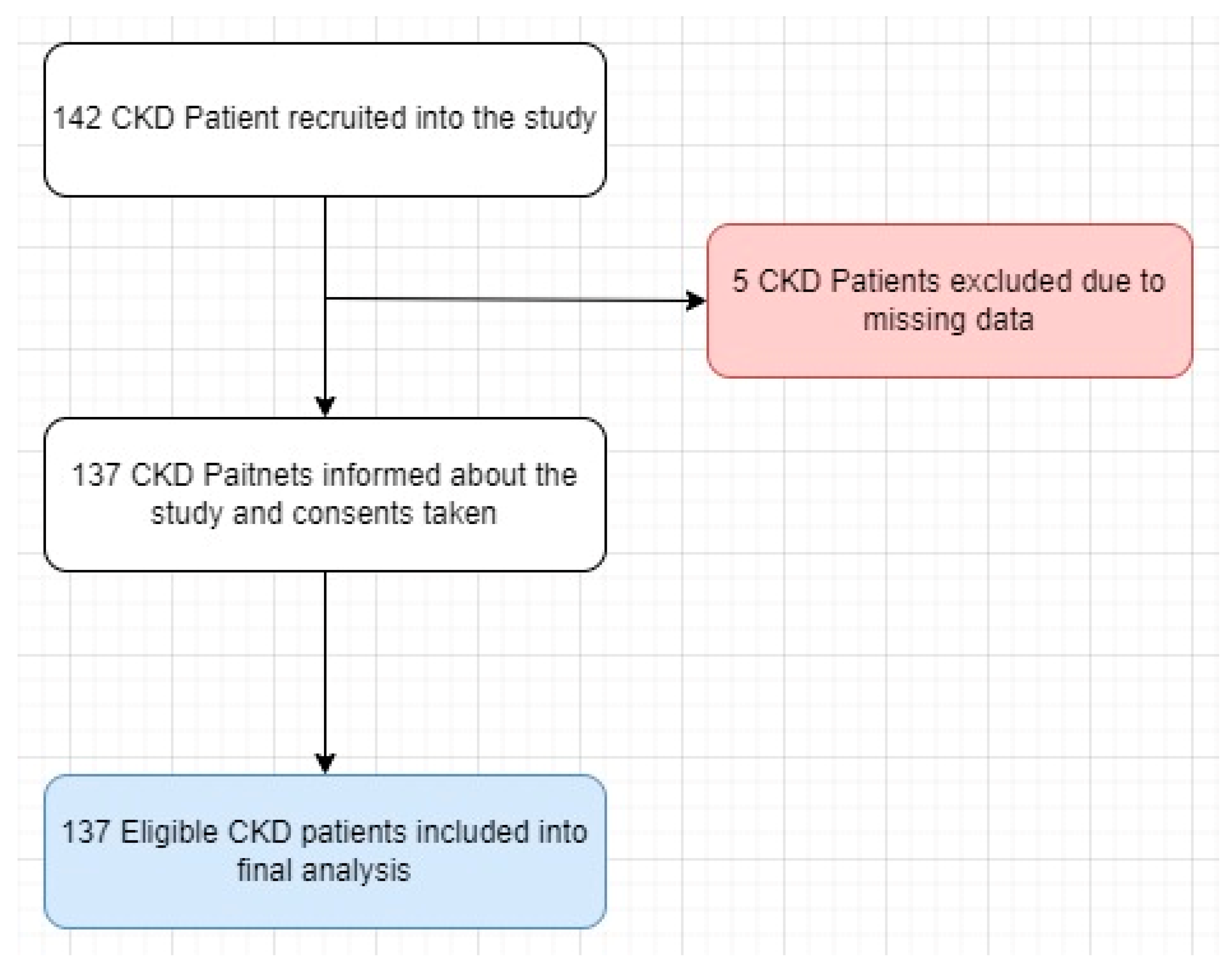
4.2. Sample Size
4.3. Data Acquisition and Evaluating the pDDIs
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magro, L.; Moretti, U.; Leone, R. Epidemiology and characteristics of adverse drug reactions caused by drug–drug interactions. Expert Opin. Drug Saf. 2012, 11, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Juurlink, D.N.; Mamdani, M.; Kopp, A.; Laupacis, A.; Redelmeier, D.A. Drug-Drug Interactions among Elderly Patients Hospitalized for Drug Toxicity. JAMA 2003, 289, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Teich, J.M.; Osheroff, J.A.; Pifer, E.A.; Sittig, D.F. Clinical decision support in electronic prescribing: Recommendations and an action plan: Report of the joint clinical decision support workgroup. J. Am. Med. Inform. Assoc. 2005, 12, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, G.J.; Bobb, A.; Payne, T.H.; Avery, A.J.; Gandhi, T.K.; Burns, G.; Classen, D.C.; Bates, D.W. Medication-related Clinical Decision Support in Computerized Provider Order Entry Systems: A Review. J. Am. Med. Inform. Assoc. 2007, 14, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Glassman, P.A.; Simon, B.; Belperio, P.; Lanto, A. Improving recognition of drug interactions. Med. Care 2002, 40, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.M.; Lee, T.H.; Bae, T.; Yamamoto, R.; Horsky, J.; Bates, D.W. Survey of physicians’ experience using a handheld drug reference Guide. In Proceedings of the AMIA Symposium 2000, Los Angeles, CA, USA, 4–8 November 2000; p. 1125. [Google Scholar]
- Smithburger, P.L.; Kane-Gill, S.L.; Seybert, A.L. Drug-drug interactions in cardiac and cardiothoracic intensive care units. Drug Saf. 2010, 33, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Abarca, J.; Malone, D.C.; Armstrong, E.P.; Grizzle, A.J.; Hansten, P.D.; Van Bergen, R.C.; Lipton, R.B. Concordance of severity ratings provided in four drug interaction compendia. J. Am. Pharm. Assoc. 2004, 44, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Gyebi, L.; Soltani, Z.; Reisin, E. Lipid nephrotoxicity: New concept for an old disease. Curr. Hypertens. Rep. 2012, 14, 177–181. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sánchez-Lozada, L.G.; Kang, D.-H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transplant. 2013, 28, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Hostetter, T.H.; Olson, J.L.; Rennke, H.G.; Venkatachalam, M.A.; Brenner, B.M.; Geraci, S.; Chacon-Caldera, J.; Cullen-McEwen, L.; Schad, L.R.; Sticht, C.; et al. Hyperfiltration in remnant nephrons: A potentially adverse response to renal ablation. Am. J. Physiol. Physiol. 1981, 241, F85–F93. [Google Scholar] [CrossRef] [PubMed]
- Pehlivanli, A.; Eren-Sadioglu, R.; Aktar, M.; Eyupoglu, S.; Sengul, S.; Keven, K.; Erturk, S.; Basgut, B.; Ozcelikay, A.T. Potential drug-drug interactions of immunosuppressants in kidney transplant recipients: Comparison of drug interaction resources. Pharm. Weekbl. 2022, 44, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Tecen-Yucel, K.; Bayraktar-Ekincioglu, A.; Yildirim, T.; Yilmaz, S.R.; Demirkan, K.; Erdem, Y. Assessment of Clinically Relevant Drug Interactions by Online Programs in Renal Transplant Recipients. J. Manag. Care Spéc. Pharm. 2020, 26, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Alkan, A.; Yaşar, A.; Karcı, E.; Köksoy, E.B.; Ürün, M.; Şenler, F.; Ürün, Y.; Tuncay, G.; Ergün, H.; Akbulut, H. Severe drug interactions and potentially inappropriate medication usage in elderly cancer patients. Support. Care Cancer 2016, 25, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, C.-F.; Feng, Y.-F.; Chen, H. Potential drug-drug interactions in drug therapy for older adults with chronic coronary syndrome at hospital discharge: A real-world study. Front. Pharmacol. 2022, 13, 946415. [Google Scholar] [CrossRef] [PubMed]
- Santos-Díaz, G.; Pérez-Pico, A.M.; Suárez-Santisteban, M.; García-Bernalt, V.; Mayordomo, R.; Dorado, P. Prevalence of Potential Drug–Drug Interaction Risk among Chronic Kidney Disease Patients in a Spanish Hospital. Pharmaceutics 2020, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Okoro, R.N.; Farate, V.T. Evaluation of potential drug–drug interactions among patients with chronic kidney disease in northeastern Nigeria. Afr. J. Nephrol. 2019, 22, 77–81. [Google Scholar] [CrossRef]
- Adibe, M.O.; Ewelum, P.C.; Amorha, K.C. Evaluation of drug-drug interactions among patients with chronic kidney disease in a South-Eastern Nigeria tertiary hospital: A retrospective study. Pan Afr. Med. J. 2017, 28, 199. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.; Seeling, A.; Rupprecht, H. Adverse Drug Events in Patients with Chronic Kidney Disease Associated with Multiple Drug Interactions and Polypharmacy. Drugs Aging 2020, 37, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Masood, I.; Khan, T.M. Clinical relevancy and determinants of potential drug–drug interactions in chronic kidney disease patients: Results from a retrospective analysis. Integr. Pharm. Res. Pract. 2017, 6, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hedge, S.; Udaykumar, P.; Manjuprasad, M.S. Potential drug interactions in chronic kidney disease patients-A cross sectional study. Int. J. Recent Trends Sci. Technol. 2015, 16, 56–60. [Google Scholar]
- Bektay, M.Y.; Seker, Z.; Eke, H.K.; Turk, H.M.; Izzettin, F.V. Comparison of different decision support software programs in perspective of potential drug–drug interactions in the oncology clinic. J. Oncol. Pharm. Pract. 2022, 29, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Matzke, G.R.; Aronoff, G.R.; Atkinson, A.J.; Bennett, W.M.; Decker, B.S.; Eckardt, K.-U.; Golper, T.; Grabe, D.W.; Kasiske, B.; Keller, F.; et al. Drug dosing consideration in patients with acute and chronic kidney disease—A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011, 80, 1122–1137. [Google Scholar] [CrossRef] [PubMed]
- Maison, O.; Tardy, C.; Cabelguenne, D.; Parat, S.; Ducastelle, S.; Piriou, V.; Lepape, A.; Lalande, L. Drug incompatibilities in intravenous therapy: Evaluation and proposition of preventive tools in intensive care and hematology units. Eur. J. Clin. Pharmacol. 2018, 75, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ademola, O.A.; Ibiene, E.O.; Olumuyiwa, J.F.; Akinwumi, A.A.; Oluwole, B.A. Prevalence and pattern of potential drug-drug interactions among chronic kidney disease patients in south-western Nigeria. Niger. Postgrad. Med. J. 2017, 24, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Marquito, A.B.; Fernandes, N.M.d.S.; Colugnati, F.A.B.; de Paula, R.B. Identifying potential drug interactions in chronic kidney disease patients. Braz. J. Nephrol. 2014, 36, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Al-Ramahi, R.; Raddad, A.R.; Rashed, A.O.; Bsharat, A.; Abu-Ghazaleh, D.; Yasin, E.; Shehab, O. Evaluation of potential drug- drug interactions among Palestinian hemodialysis patients. BMC Nephrol. 2016, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Perazella, M.A. NSAIDs in CKD: Are They Safe? Am. J. Kidney Dis. 2020, 76, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Fasipe, O.J.; Akhideno, P.E.; Nwaiwu, O.; Adelosoye, A.A. Assessment of prescribed medications and pattern of distribution for potential drug–drug interactions among chronic kidney disease patients attending the Nephrology Clinic of Lagos University Teaching Hospital in Sub-Saharan West Africa. Clin. Pharmacol. Adv. Appl. 2017, 9, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Monteith, S.; Glenn, T. A comparison of potential psychiatric drug interactions from six drug interaction database programs. Psychiatry Res. 2019, 275, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Bykov, K.; Gagne, J.J. Generating Evidence of Clinical Outcomes of Drug–Drug Interactions. Drug Saf. 2017, 40, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.J.; Daly, T.M.; Liu, X.; Goldstein, K.; Johnston, J.A.; Ryan, T.P. Co-prescription trends in a large cohort of subjects predict substantial drug-drug interactions. PLoS ONE 2015, 10, e0118991. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-M.; Ko, Y.; Chan, A. Clinically significant drug–drug interactions between oral anticancer agents and nonanticancer agents: Profiling and comparison of two drug compendia. Ann. Pharmacother. 2008, 42, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Warholak, T.L.; Hines, L.E.; Song, M.C.; Gessay, A.; Menke, J.M.; Sherrill, D.; Reel, S.; Murphy, J.E.; Malone, D.C. Medical, nursing, and pharmacy students’ ability to recognize potential drug-drug interactions: A comparison of healthcare professional students. J. Am. Acad. Nurse Pr. 2011, 23, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Kongsholm, G.G.; Nielsen, A.K.T.; Damkier, P. Drug interaction databases in medical literature: Transparency of ownership, funding, classification algorithms, level of documentation, and staff qualifications. A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Scheife, R.T.; Hines, L.E.; Boyce, R.D.; Chung, S.P.; Momper, J.D.; Sommer, C.D.; Abernethy, D.R.; Horn, J.R.; Sklar, S.J.; Wong, S.K.; et al. Consensus recommendations for systematic evaluation of drug–drug interaction evidence for clinical decision support. Drug Saf. 2015, 38, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Tilson, H.; Hines, L.E.; McEvoy, G.; Weinstein, D.M.; Hansten, P.D.; Matuszewski, K.; le Comte, M.; Higby-Baker, S.; Hanlon, J.T.; Pezzullo, L.; et al. Recommendations for selecting drug–drug interactions for clinical decision support. Am. J. Heal. Pharm. 2016, 73, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Vitry, A.I. Comparative assessment of four drug interaction compendia. Br. J. Clin. Pharmacol. 2006, 63, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, K.M.; Nelson, S.D.; Hines, L.; Empey, P.; Boyce, R.D.; Hochheiser, H. Information needs for making clinical recommendations about potential drug-drug interactions: A synthesis of literature review and interviews. BMC Med. Inform. Decis. Mak. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hines, L.E.; Malone, D.C.; Murphy, J.E. Recommendations for generating, evaluating, and implementing drug-drug interaction evidence. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2012, 32, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Wong, M.; Lightwood, J.M.; Cheng, C.M. Black box warning contraindicated comedications: Concordance among three major drug interaction screening programs. Ann. Pharmacother. 2010, 44, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Acharya, L.; Attur, R.; Rama, M.; Viswanathan, G.; Reddy, P.; Raghavan, S. Assessment of Drug-Drug Interactions among Renal Failure Patients of Nephrology Ward in a South Indian Tertiary Care Hospital. Indian J. Pharm. Sci. 2012, 74, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J.; van der Sijs, H.; E Haefeli, W.; Slight, S.P.; E McDowell, S.; Seidling, H.M.; Eiermann, B.; Aarts, J.; Ammenwerth, E.; E Ferner, R.; et al. On the alert: Future priorities for alerts in clinical decision support for computerized physician order entry identified from a European workshop. BMC Med. Inform. Decis. Mak. 2013, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Castro, V.; Centurion, I.G.; Espinosa, J.; Keller, G.A.; Gonzalez, C.D.; Riera, M.C.S.; Saubidet, C.L.; Di Girolamo, G.; Pujol, G.S.; et al. A Systematic Approach to Assess the Burden of Drug Interactions in Adult Kidney Transplant Patients. Curr. Drug Saf. 2016, 11, 156–163. [Google Scholar] [CrossRef] [PubMed]
| pDDI Software Program | Lexicomp Drug Interactions® | Medscape® |
|---|---|---|
| Language | English | English |
| Clinical effect | Yes | Yes |
| Online/offline * | Online | Online |
| Access/payment | Required | Not Required |
| Risk rating | Yes | Yes |
| Risk rating categories | X, D, C, B, A | Contraindicated, Serious-Use Alternative, Monitor Closely, Minor, None |
| Mechanism of interaction | Yes | Yes |
| Gives advice for clinical management | Yes | Yes |
| Reliability rating | Yes | No |
| Reliability rating categories | Good, Fair, Poor | No |
| Reference list | Yes | No |
| Date of last update | 2 February 2024 | Not Available |
| Source | Wolters Kluwer Clinical Drug Information | Medscape Publishers’ Circle |
| CKD Classification | G3a 45–59 mL/min/1.73 m2 | G3b 30–44 mL/min/1.73 m2 | G4 15–29 mL/min/1.73 m2 | G5 <15 mL/min/1.73 m2 | Total | p |
|---|---|---|---|---|---|---|
| Number of Patients (n, %) | 20, 14.60 | 40, 29.20 | 55, 40.15 | 22, 16.06 | 137, 100 | 0.379 |
| Gender (n, %) | 0.043 | |||||
| Women | 9, 6.6 | 20, 14.6 | 33, 24.1 | 18, 13.1 | 80, 58.4 | |
| Men | 11, 8 | 20, 14.6 | 22, 16.1 | 4, 2.9 | 57, 41.6 | |
| Working Status (n, %) | 0.236 | |||||
| Employed | 8, 5.8 | 18, 13.1 | 17, 12.4 | 12, 8.8 | 55, 40.1 | |
| Unemployed | 12, 8.8 | 22, 16.1 | 38, 27.7 | 10, 7.3 | 82, 59.9 | |
| Smoking (n, %) | 0.375 | |||||
| Yes | 4, 2.9 | 10, 7.3 | 8, 5.8 | 2, 1.5 | 24, 17.5 | |
| No | 16, 11.7 | 30, 21.9 | 47, 34.3 | 20, 14.6 | 113, 82.5 | |
| Alcohol (n, %) | 0.808 | |||||
| Yes | - | 1, 0.7 | 1, 0.7 | - | 2, 1.5 | |
| No | 20, 14.6 | 39, 28.5 | 54, 39.4 | 22, 16.1 | 135, 98.5 | |
| Age (years) (Mean ± SD) | 62.35 ± 12.67 | 61.75 ± 15.85 | 68.36 ± 13.56 | 63.68 ± 15.38 | 64.8 ± 14.59 | 0.13 |
| BMI (Mean ± SD) | 30.52 ± 6.28 | 28.97 ± 5.46 | 28.05 ± 4.65 | 27.78 ± 5.85 | 28.63 ± 5.36 | 0.28 |
| Weight (Kg) | 88.8 ± 23.71 | 77.95 ± 14.28 | 74.85 ± 13.67 | 73.91 ± 16.04 | 77.64 ± 16.57 | 0.01 |
| Height (Cm) | 169.75 ± 10.18 | 164.25 ± 8.87 | 163.4 ± 10.57 | 163.23 ± 8.68 | 164.55 ± 9.9 | 0.008 |
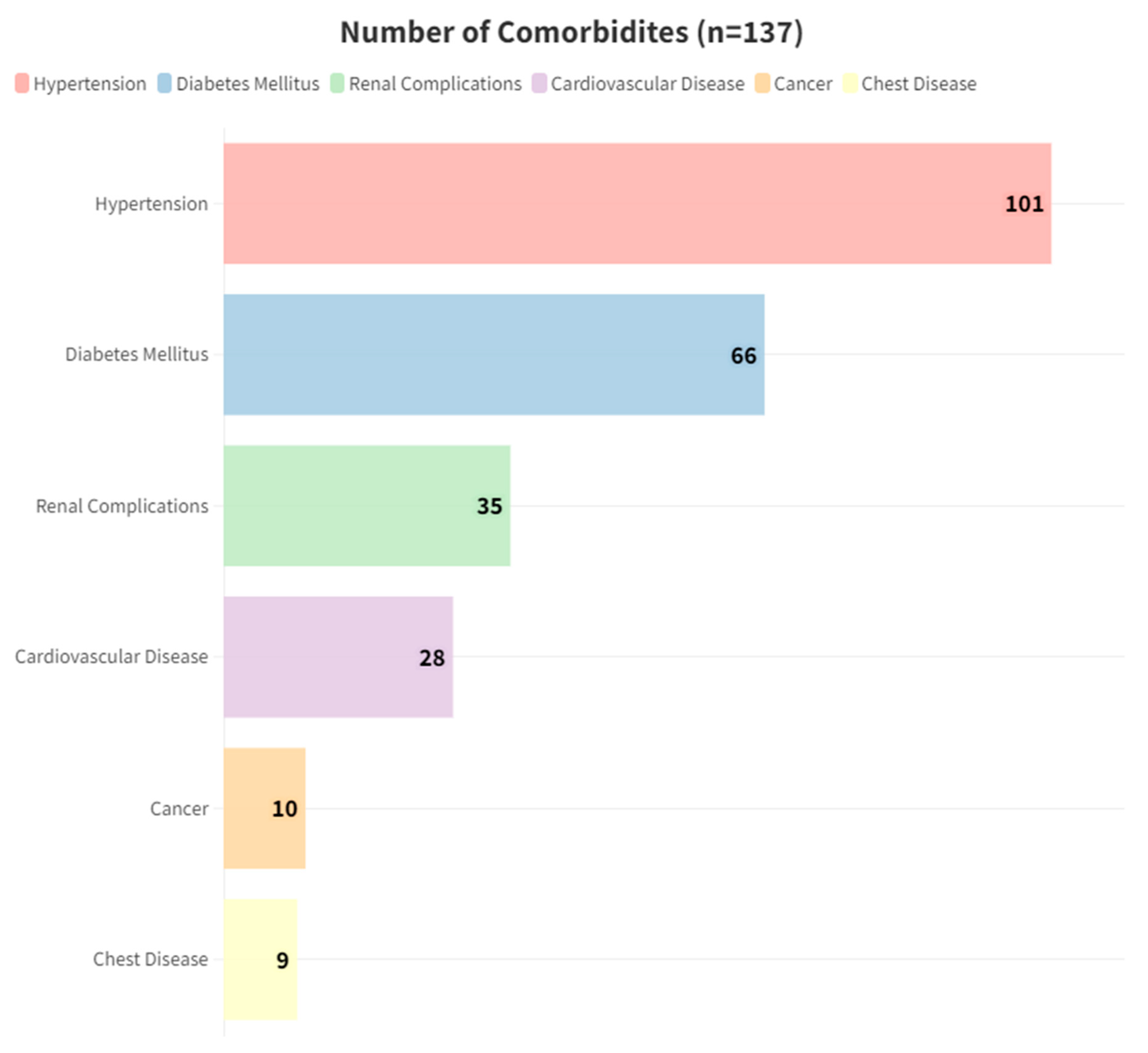
| No. of Comorbidities (Mean ± SD) | 2.8 ± 0.89 | 2.23 ± 1.19 | 2.22 ± 1.13 | 2.05 ± 1.21 | 2.28 ± 1.14 | 0.15 |
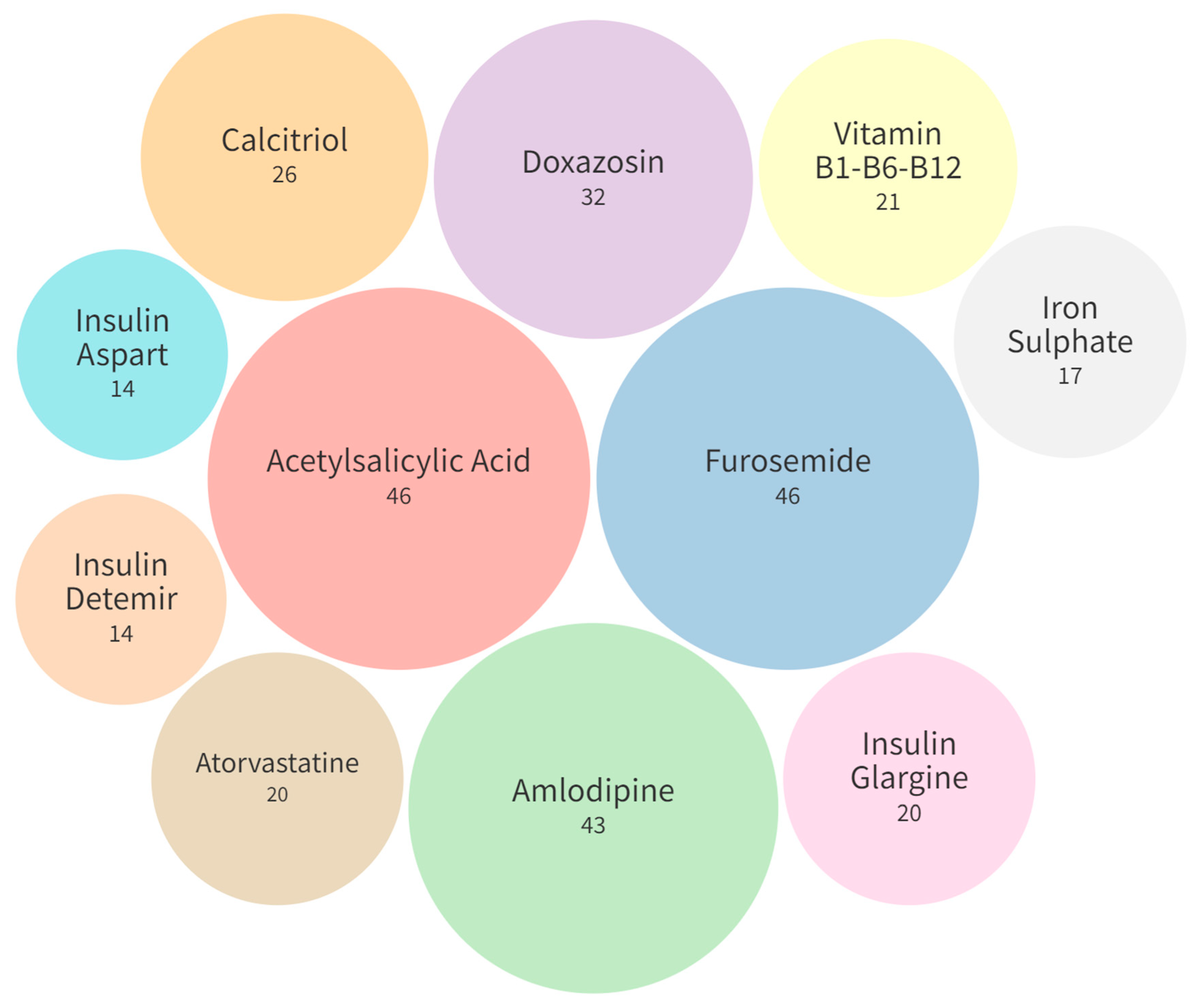
| No. of Drugs Used per Patient (Mean ± SD) | 8.6 ± 3.84 | 7.25 ± 3.7 | 8.4 ± 3.95 | 8.95 ± 3.79 | 8.18 ± 3.84 | 0.30 |
| No. of Comorbidities (Median, [IQR]) | 3, [2–3.75] | 2, [1.25–3] | 2, [1–3] | 2, [1–3] | 2, [1–3] | 0.099 |
| 0 (n, %) | 0, 0 | 3, 2.2 | 1, 0.7 | 1, 0.7 | 5, 3.65 | |
| 1 (n, %) | 1, 0.7 | 7, 5.1 | 18, 13.1 | 7, 5.1 | 33, 24.08 | |
| 2 (n, %) | 7, 5.1 | 15, 10.9 | 12, 8.8 | 8, 5.8 | 42, 30.65 | |
| 3 (n, %) | 7, 5.1 | 9, 6.6 | 17, 12.4 | 3, 2.2 | 36, 26.27 | |
| 4 (n, %) | 5, 3.6 | 5, 3.6 | 6, 4.4 | 2, 1.5 | 18, 13.13 | |
| 5 (n, %) | - | 1, 0.7 | 1, 0.7 | 1, 0.7 | 3, 2.18 | |
| No. of pDDIs (Mean ± SD) * | 0.15 ± 0.37 | 0.15 ± 0.36 | 0.18 ± 0.47 | 0.45 ± 0.74 | 0.21 ± 0.49 | 0.088 |
| No. of pDDIs (Mean ± SD) ** | 0.15 ± 0.49 | 0.75 ± 1.03 | 0.47 ± 0.77 | 0.45 ± 0.74 | 0.5 ± 0.83 | 0.059 |
| Years with CKD (Mean ± SD) | 5.95 ± 4.65 | 7.27 ± 7.15 | 5.98 ± 4.66 | 6.8 ± 5.91 | 6.48 ± 5.66 | 0.70 |
| Capability of Self- Care (n, %) | 0.333 | |||||
| Yes | 16, 11.7 | 24, 17.5 | 36, 26.3 | 12, 8.8 | 88, 64.1 | |
| No | 4, 2.9 | 16, 11.7 | 19, 13.9 | 10, 7.3 | 49, 35.8 | |
| Systolic Blood Pressure (mmHg) | 135 ± 23.95 | 137 ± 21.51 | 93 ± 22.53 | 55 ± 24.59 | 136.69 ± 22.58 | 0.91 |
| Diastolic Blood Pressure (mmHg) | 70.5 ± 19.59 | 78.25 ± 12.79 | 75.55 ± 8.85 | 80 ± 15.12 | 76.31 ± 13.25 | 0.076 |
| Albumin (g/dL) (Mean ± SD) | 4.15 ± 0.26 | 4.1 ± 0.29 | 4.71 ± 4.72 | 3.99 ± 0.28 | 4.34 ± 3.02 | 0.71 |
| Potassium (mmol/L) (Mean ± SD) | 4.39 ± 1.12 | 4.68 ± 0.55 | 4.67 ± 0.58 | 4.78 ± 0.51 | 4.65 ± 0.67 | 0.26 |
| Calcium (mg/dL) (Mean ± SD) | 9.46 ± 0.48 | 9.08 ± 1.5 | 9.08 ± 0.51 | 8.77 ± 0.73 | 9.09 ± 0.95 | 0.13 |
| Creatinine (mg/dL) (Mean ± SD) | 1.24 ± 0.21 | 6.13 ± 27.71 | 2.5 ± 0.47 | 4.22 ± 1.1 | 3.65 ± 14.96 | 0.58 |
| Ferritin (ng/mL) (Mean ± SD) | 108.7 ± 101.11 | 122.89 ± 97.81 | 162.28 ± 203.14 | 202.22 ± 107.06 | 149.37 ± 152.63 | 0.13 |
| MEDSCAPE® | LEXICOMP® | |||||
|---|---|---|---|---|---|---|
| Contraindicated | Serious-Use Alternative | Monitor Closely | X | D | C | |
| G3a 45–59 mL/min/1.73 m2 | 0.05 ± 0.22 | 0.15 ± 0.37 | 3.55 ± 3.14 | 0.05 ± 0.22 | 0.30 ± 0.47 | 2.70 ± 3.25 |
| G3b 30–44 mL/min/1.73 m2 | - | 0.15 ± 0.43 | 4.59 ± 4.27 | 0.03 ± 0.16 | 0.36 ± 0.74 | 3.49 ± 4.30 |
| G4 15–29 mL/min/1.73 m2 | - | 0.24 ± 0.54 | 4.36 ± 3.94 | 0.07 ± 0.26 | 0.55 ± 0.83 | 3.87 ± 3.61 |
| G5 <15 mL/min/1.73 m2 | - | 0.27 ± 0.46 | 7.23 ± 6.55 | 0.14 ± 0.35 | 0.45 ± 0.67 | 6.18 ± 4.93 |
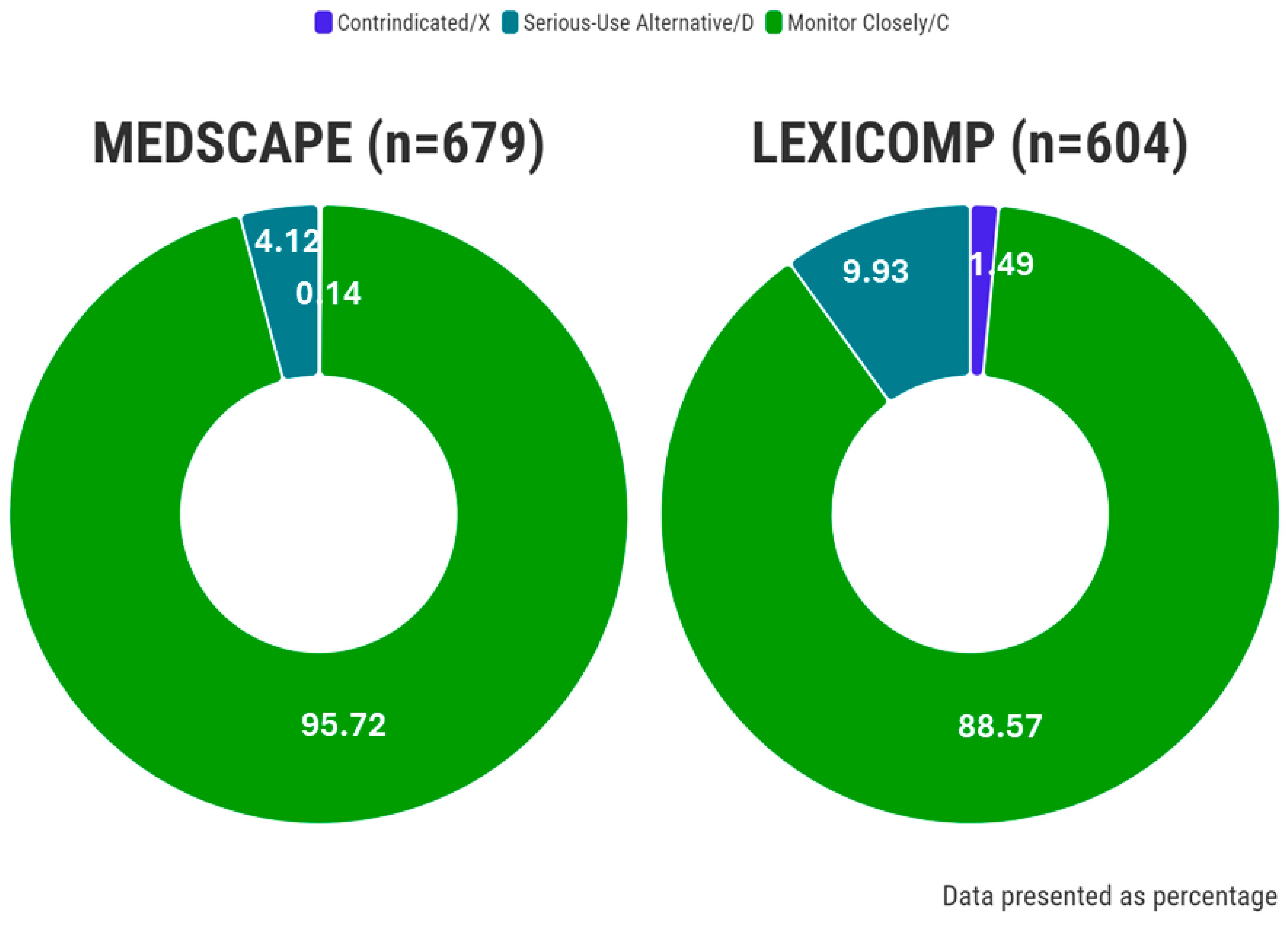
| Total (Mean ± SD) 1 | 0.01 ± 0.09 | 0.21 ± 0.47 | 4.77 ± 4.54 | 0.07 ± 0.25 | 0.44 ± 0.74 | 3.96 ± 4.10 |
| Total No. of pDDIs (n, %) | 1, 0.15 | 28, 4.12 | 650, 95.73 | 9, 1.49 | 60, 9.93 | 535, 88.58 |
| Inter-Item Correlation Matrix | Medscape | p-value | |||
| Lexicomp | 0.187 | <0.001 | |||
| Intraclass Correlation Coefficient | |||||
| Intraclass Correlation Coefficient | 95% Confidence Interval | ||||
| Lower Bound | Upper Bound | p-value | |||
| Cronbach’s α | 0.315 | 0.287 | 0.369 | 0.028 | |
| Kendall Coefficient of Concordance of Lexicomp and Medscape Software | |||||
| Kendall W | Chi-Square | Strength of agreement | p-value | n | |
| Overall | 0.073 | 9.981 | Poor | <0.001 | 137 |
| G3a 45–59 mL/min/1.73 m2 | 0.173 | 0.200 | Poor | 0.655 | 20 |
| G3b 30–44 mL/min/1.73 m2 | 0.268 | 10.714 | Slight | 0.001 | 40 |
| G4 15–29 mL/min/1.73 m2 | 0.091 | 5.000 | Poor | 0.025 | 55 |
| G5 <15 mL/min/1.73 m2 | 0.006 | 0.143 | Poor | 0.705 | 22 |
| Drug-Drug Interactions | LEXICOMP | MEDSCAPE | Explanation | Severity/Reliability Rating | ||
|---|---|---|---|---|---|---|
| Severity | n | Severity | n | |||
| Acetylsalicylic Acid–Furosemide | C | 25 | Monitor closely | 25 | Acetylsalicylic acid may reduce diuretic effect of furosemide. May increase serum concentration. | Moderate/Good |
| Acetylsalicylic Acid–Metoprolol | No interactions | Monitor closely | 17 | Acetylsalicylic acid reduces PD antagonism effect of metoprolol. Both increase serum potassium levels. | NA | |
| Allopurinol–Furosemide | C | 19 | No interactions | Furosemide may increase toxic effect of allopurinol. Increases serum concentration. | Moderate/Fair | |
| Sodium bicarbonate–Iron Sulfate | D | 14 | Monitor closely | 14 | Sodium bicarbonate reduces absorption of iron sulfate. | Minor/Fair |
| Acetylsalicylic Acid–Carvedilol | No interactions | Monitor closely | 13 | Acetylsalicylic acid decreases effects of carvedilol by PD antagonism. | NA | |
| Furosemide–Doxazosin | C | 11 | No interactions | Acetylsalicylic acid may increase hypotensive effect of doxazosin. | Moderate/Fair | |
| Furosemide–Hydrochlorothiazide | C | 11 | Monitor closely | 11 | Furosemide may enhance hypotensive effect of antihypertensive agents. | Moderate/Fair |
| Metoprolol–Furosemide | C | 11 | Monitor closely | 11 | Metoprolol increases serum potassium levels, decreases furosemide. | Moderate/Fair |
| Metoprolol–Doxazosin | C | 10 | Monitor closely | 10 | Metoprolol may enhance orthostatic hypotensive effect of doxazosin. | Moderate/Fair |
| Acetylsalicylic Acid–Doxazosin | No interactions | Monitor closely | 11 | Acetylsalicylic acid reduces effect of doxazosin by PD antagonism. | NA | |
| Metformin–Hydrochlorothiazide | C | 9 | Minor | 9 | Hydrochlorothiazide may reduce therapeutic effect of metformin. | Moderate/Fair |
| Acetylsalicylic Acid–Hydrochlorothiazide | No interactions | Monitor closely | 11 | Acetylsalicylic acid increases serum potassium levels, decreases hydrochlorothiazide. | NA | |
| Acetylsalicylic Acid–Valsartan | No interactions | Monitor closely | 10 | PD synergism/both increase serum potassium levels. | NA | |
| Carvedilol–Valsartan | No interactions | Monitor closely | 10 | Pharmacodynamic synergism. | NA | |
| Acetylsalicylic Acid–Clopidogrel | C | 9 | Monitor closely | 8 | Both enhance antiplatelet effects of each other. | Moderate/Fair |
| Metoprolol–Doxazosin | B | 9 | Monitor closely | 9 | Metoprolol may enhance hypotensive effect of doxazosin. | Minor/Fair |
| Insulin Aspart–Furosemide | C | 8 | No interactions | Furosemide reduces therapeutic effect of insulin. | Moderate/Fair | |
| Acetylsalicylic Acid–Insulin Glargine | C | 8 | Monitor closely | 8 | Acetylsalicylic acid may increase effect of insulin glargine. | Moderate/Fair |
| Sodium bicarbonate–Allopurinol | No interactions | Monitor closely | 9 | Sodium bicarbonate reduces allopurinol levels by inhibition of gastrointestinal absorption. | NA | |
| Metoprolol–Amlodipine | No interactions | Monitor closely | 8 | Doxazosin and amlodipine both increase anti-hypertensive channel blocking. | NA | |
| Acetylsalicylic Acid–Clopidogrel | C | 8 | Monitor closely | 8 | Agents with antiplatelet properties may enhance antiplatelet effect of other agents with antiplatelet properties. | Moderate/Fair |
| Doxazosin–Amlodipine | B | 8 | Monitor closely | 8 | Antihypertensive agents may enhance hypotensive effect of doxazosin. | Minor/Fair |
| Doxazosin–Carvedilol | B | 8 | Monitor closely | 8 | Antihypertensive agents may enhance hypotensive effect of doxazosin. | Minor/Fair |
| Nebivolol–Acetylsalicylic Acid | No interactions | Monitor closely | 7 | Acetylsalicylic acid decreases effects of nebivolol by PD antagonism. | NA | |
| Nebivolol–Hydrochlorothiazide | No interactions | Monitor closely | 7 | Nebivolol increases and hydrochlorothiazide decreases serum potassium. | NA | |
| Furosemide–Carvedilol | C | 7 | Monitor closely | Furosemide may enhance hypotensive effect of antihypertensive agents. | Moderate/Fair | |
| Carvedilol–Hydrochlorothiazide | No interactions | Monitor closely | 7 | Carvedilol increases serum potassium levels, decreases hydrochlorothiazide. | NA | |
| Sodium bicarbonate–Nebivolol | No interactions | Monitor closely | 7 | Sodium bicarbonate reduces nebivolol levels by inhibition of gastrointestinal absorption. | NA | |
| Pantoprazole–Iron Sulfate | B | 7 | Monitor closely | 7 | Inhibitors of proton pump may decrease absorption of iron preparations. | Minor/Fair |
| Insulin Glargine–Furosemide | C | 7 | No interactions | Furosemide reduces therapeutic effect of allopurinol. | Moderate/Fair | |
| Iron sulfate–Levothyroxine | D | 6 | Monitor closely | 6 | Iron II glycine sulfate may decrease serum concentration of levothyroxine. | Moderate/Good |
| Metformin–Furosemide | C | 6 | Minor | 6 | Furosemide may reduce therapeutic effect of metformin. | Moderate/Fair |
| Insulin Glargine–Insulin Aspart | C | 6 | No interactions | Insulin glargine increases hypoglycemic effect of insulin aspartate. | Moderate/Fair | |
| Pantoprazole–Clopidogrel | C | 6 | Monitor closely | 6 | Pantoprazole reduces serum concentration of clopidogrel. | Major/Fair |
| Insulin Glargine–Metformin | C | 5 | Monitor closely | 5 | Metformin increases hypoglycemic effect of insulin glargine. | Moderate/Fair |
| Insulin Aspart–Linagliptin | D | 4 | No interactions | Linagliptin may increase hypoglycemic effect of insulin aspart. | Moderate/Fair | |
| Acetylsalicylic Acid–Escitalopram | C | 4 | Monitor closely | 4 | Escitalopram increases antiplatelet effect of acetylsalicylic acid. | Moderate/Fair |
| Amlodipine–Clopidogrel | C | 4 | Monitor closely | 4 | Amlodipine reduces therapeutic effect of clopidogrel. | Moderate/Fair |
| Gliclazide–Furosemide | C | 4 | No interactions | Gliclazide may reduce therapeutic effect of furosemide. | Moderate/Fair | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bektay, M.Y.; Buker Cakir, A.; Gursu, M.; Kazancioglu, R.; Izzettin, F.V. An Assessment of Different Decision Support Software from the Perspective of Potential Drug–Drug Interactions in Patients with Chronic Kidney Diseases. Pharmaceuticals 2024, 17, 562. https://doi.org/10.3390/ph17050562
Bektay MY, Buker Cakir A, Gursu M, Kazancioglu R, Izzettin FV. An Assessment of Different Decision Support Software from the Perspective of Potential Drug–Drug Interactions in Patients with Chronic Kidney Diseases. Pharmaceuticals. 2024; 17(5):562. https://doi.org/10.3390/ph17050562
Chicago/Turabian StyleBektay, Muhammed Yunus, Aysun Buker Cakir, Meltem Gursu, Rumeyza Kazancioglu, and Fikret Vehbi Izzettin. 2024. "An Assessment of Different Decision Support Software from the Perspective of Potential Drug–Drug Interactions in Patients with Chronic Kidney Diseases" Pharmaceuticals 17, no. 5: 562. https://doi.org/10.3390/ph17050562
APA StyleBektay, M. Y., Buker Cakir, A., Gursu, M., Kazancioglu, R., & Izzettin, F. V. (2024). An Assessment of Different Decision Support Software from the Perspective of Potential Drug–Drug Interactions in Patients with Chronic Kidney Diseases. Pharmaceuticals, 17(5), 562. https://doi.org/10.3390/ph17050562







