Pharmacovigilance Practices by Healthcare Providers in Oncology: A Cross-Sectional Study
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
2.2. PV Knowledge and Reporting Practices
2.3. PV-Reporting Process
2.4. ADRs Reported in Specific Oncology Medications
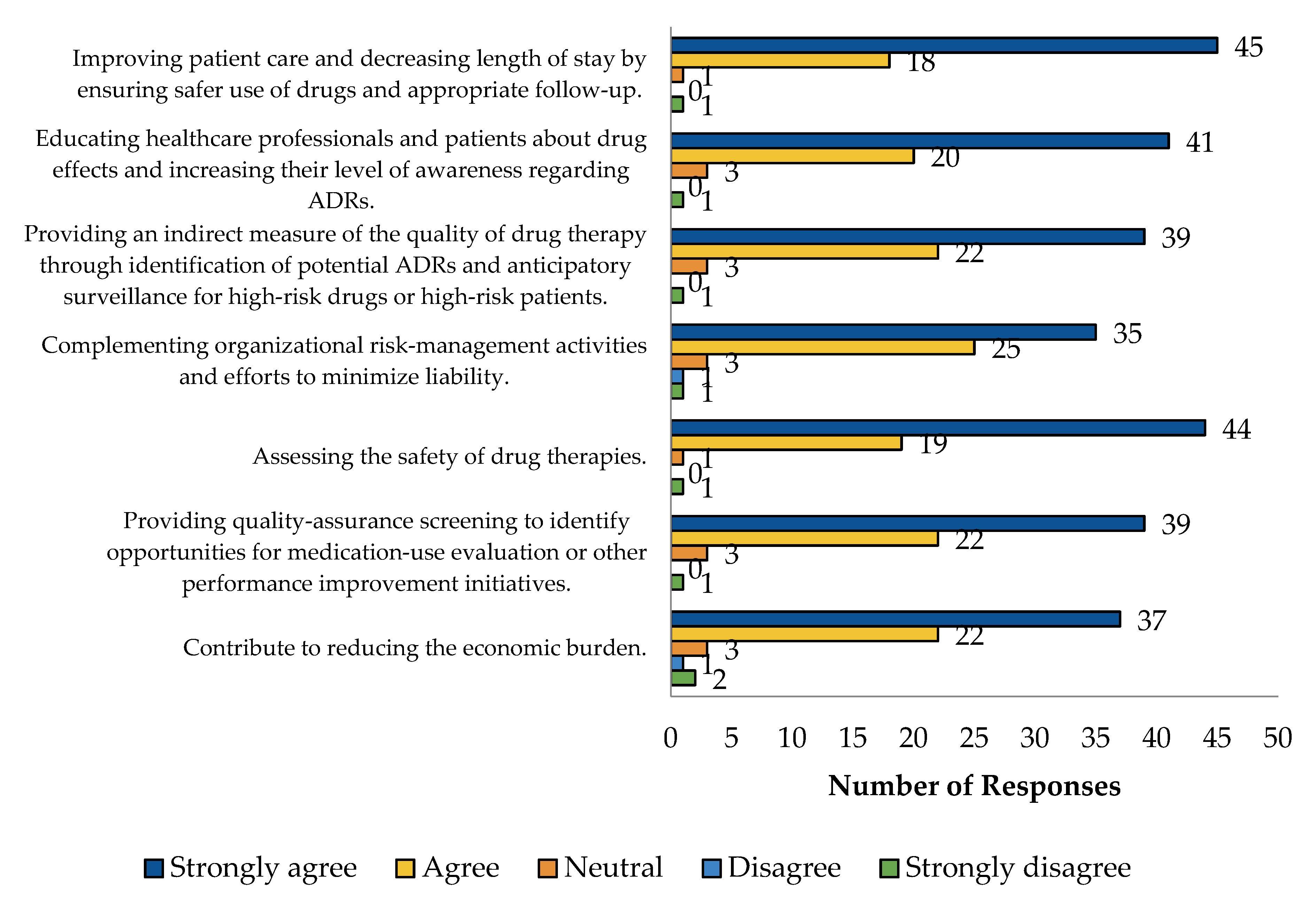
2.5. HCP Perspective Regarding PV
3. Discussion
4. Materials and Methods
4.1. Study Design, Settings, and Population
4.2. Study Questionnaire
4.3. Data Collection and Source
4.4. Study Variables
4.5. Data Presentation and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fornasier, G.; Francescon, S.; Leone, R.; Baldo, P. An historical overview over Pharmacovigilance. Int. J. Clin. Pharm. 2018, 40, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Curtis, K.; Van, C.; Tabish Razi Zaidi, S.; Yeo, C.Y.; Arun Kali, C.; Zaheen, M.; Therese Moujalli, G.; Castelino, R. Why hospital-based healthcare professionals do not report adverse drug reactions: A mixed methods study using the Theoretical Domains Framework. Eur. J. Clin. Pharmacol. 2022, 78, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Alharf, A.; Alqahtani, N.; Saeed, G.; Alshahrani, A.; Alshahrani, M.; Aljasser, N.; Alquwaizani, M.; Bawazir, S. Saudi Vigilance Program: Challenges and lessons learned. Saudi Pharm. J. 2018, 26, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, T.M.; Alshakka, M.; Aljadhey, H. Pharmacovigilance system in Saudi Arabia. Saudi Pharm. J. 2017, 25, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Aljadhey, H.; Mahmoud, M.A.; Hassali, M.A.; Alrasheedy, A.; Alahmad, A.; Saleem, F.; Sheikh, A.; Murray, M.; Bates, D.W. Challenges to and the future of medication safety in Saudi Arabia: A qualitative study. Saudi Pharm. J. 2014, 22, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Karatas, Y.; Hamid, S.M. Evaluation of health care professionals’ knowledge, attitudes, practices and barriers to pharmacovigilance and adverse drug reaction reporting: A cross-sectional multicentral study. PLoS ONE 2023, 18, e0285811. [Google Scholar] [CrossRef] [PubMed]
- Baldo, P.; Fornasier, G.; Ciolfi, L.; Sartor, I.; Francescon, S. Pharmacovigilance in oncology. Int. J. Clin. Pharm. 2018, 40, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Tuccori, M.; Montagnani, S.; Capogrosso-Sansone, A.; Mantarro, S.; Antonioli, L.; Fornai, M.; Blandizzi, C. Adverse reactions to oncologic drugs: Spontaneous reporting and signal detection. Expert Rev. Clin. Pharmacol. 2015, 8, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Bearz, A.; Cecco, S.; Francescon, S.; Re, F.L.; Corona, G.; Baldo, P. Safety Profiles and Pharmacovigilance Considerations for Recently Patented Anticancer Drugs: Lung Cancer. Recent Pat. Anticancer Drug Discov. 2019, 14, 242–257. [Google Scholar] [CrossRef]
- Lavan, A.H.; O’Mahony, D.; Buckley, M.; O’Mahony, D.; Gallagher, P. Adverse Drug Reactions in an Oncological Population: Prevalence, Predictability, and Preventability. Oncologist 2019, 24, e968–e977. [Google Scholar] [CrossRef]
- Sharma, P.K.; Misra, A.K.; Gupta, A.; Singh, S.; Dhamija, P.; Pareek, P. A retrospective analysis of reporting of adverse drug reactions to oncology drugs: An experience from a national center of clinical excellence. Indian J. Pharmacol. 2018, 50, 273–278. [Google Scholar] [PubMed]
- Manohar, H.D.; Adiga, S.; Thomas, J.; Sharma, A. Adverse drug reaction profile of microtubule-damaging antineoplastic drugs: A focused pharmacovigilance study in India. Indian J. Pharmacol. 2016, 48, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulos, S.; Brown, C.A.; Felix, T.; Grampp, G.; Getz, K.A. A Survey of Adverse Event Reporting Practices Among US Healthcare Professionals. Drug Saf. 2016, 39, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, W.S.; Almufareh, N.A.; Domiaty, D.M.; Albasher, G.; Alduwish, M.A.; Alkhalaf, H.; Almuzzaini, B.; Al-Marshidy, S.S.; Alfraihi, R.; Elasbali, A.M.; et al. Epidemiology of cancer in Saudi Arabia thru 2010–2019: A systematic review with constrained meta-analysis. AIMS Public Health 2020, 7, 679–696. [Google Scholar] [PubMed]
- Chaudhri, E.; Fathi, W.; Hussain, F.; Hashmi, S.K. The Increasing Trends in Cases of the Most Common Cancers in Saudi Arabia. J. Epidemiol. Glob. Health 2020, 10, 258–262. [Google Scholar] [CrossRef]
- Baldo, P.; De Paoli, P. Pharmacovigilance in oncology: Evaluation of current practice and future perspectives. J. Eval. Clin. Pract. 2014, 20, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Fornasier, G.; Taborelli, M.; Francescon, S.; Polesel, J.; Aliberti, M.; De Paoli, P.; Baldo, P. Targeted therapies and adverse drug reactions in oncology: The role of clinical pharmacist in pharmacovigilance. Int. J. Clin. Pharm. 2018, 40, 795–802. [Google Scholar] [CrossRef]
- Crestan, D.; Trojniak, M.P.; Francescon, S.; Fornasier, G.; Baldo, P. Pharmacovigilance of anti-cancer medicines: Opportunities and challenges. Expert Opin. Drug Saf. 2020, 19, 849–860. [Google Scholar] [CrossRef]
- Curtain, C.M.; Chang, J.Y.; Cousins, J.; Parameswaran Nair, N.; Bereznicki, B.; Bereznicki, L. Medication Regimen Complexity Index Prediction of Adverse Drug Reaction-Related Hospital Admissions. Ann. Pharmacother. 2020, 54, 996–1000. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, S.; Khanal, S. Polypharmacy in elderly cancer patients: Challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019, 2, 42–49. [Google Scholar] [CrossRef]
- Parzianello, A.; Fornasier, G.; Kiren, V.; Pigato, F.; Orzetti, S.; Zamagni, G.; Arbo, A.; Baldo, P.; Rossi, P.; Rabusin, M.; et al. Improving Drug Safety in Pediatric and Young Adult Patients with Hemato-Oncological Diseases: A Prospective Study of Active Pharmacovigilance. Pharmaceuticals 2024, 17, 106. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.; Jesus, M.; Pereira, L.; Monteiro, C.; Duarte, A.P.; Morgado, M. Hematological Events Potentially Associated with CDK4/6 Inhibitors: An Analysis from the European Spontaneous Adverse Event Reporting System. Pharmaceuticals 2023, 16, 1340. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R., Sr.; Jindal, A., Sr.; Garg, P.; Kaur, A.; Kumar, S.; Tilak Raj, R.; Singh, S. Pharmacovigilance Study of Anticancer Drugs in a Tertiary Care Teaching Hospital in North India: A Retrospective Study. Cureus 2023, 15, e44984. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumari, K.M.; Manohar, H.D.; Bairy, K.L.; Thomas, J. Pattern of adverse drug reactions due to cancer chemotherapy in a tertiary care hospital in South India. Perspect. Clin. Res. 2015, 6, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.; Rehan, H.S.; Sharma, V.; Mishra, R. Chemotherapy-induced adverse drug reactions in oncology patients: A prospective observational survey. Indian J. Med. Paediatr. Oncol. 2016, 37, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Seruga, B.; Sterling, L.; Wang, L.; Tannock, I.F. Reporting of serious adverse drug reactions of targeted anticancer agents in pivotal phase III clinical trials. J. Clin. Oncol. 2011, 29, 174–185. [Google Scholar] [CrossRef]
- Vera-Badillo, F.E.; Napoleone, M.; Krzyzanowska, M.K.; Alibhai, S.M.; Chan, A.W.; Ocana, A.; Seruga, B.; Templeton, A.J.; Amir, E.; Tannock, I.F. Bias in reporting of randomised clinical trials in oncology. Eur. J. Cancer 2016, 61, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Biagi, C.; Montanaro, N.; Buccellato, E.; Roberto, G.; Vaccheri, A.; Motola, D. Underreporting in pharmacovigilance: An intervention for Italian GPs (Emilia-Romagna region). Eur. J. Clin. Pharmacol. 2013, 69, 237–244. [Google Scholar] [CrossRef]
- Herdeiro, M.T.; Figueiras, A.; Polónia, J.; Gestal-Otero, J.J. Physicians’ attitudes and adverse drug reaction reporting: A case-control study in Portugal. Drug Saf. 2005, 28, 825–833. [Google Scholar] [CrossRef]
- Reumerman, M.; Tichelaar, J.; van Eekeren, R.; van Puijenbroek, E.P.; Richir, M.C.; van Agtmael, M.A. The potential of training specialist oncology nurses in real-life reporting of adverse drug reactions. Eur. J. Clin. Pharmacol. 2021, 77, 1531–1542. [Google Scholar] [CrossRef]
- Brabete, A.C.; Greaves, L.; Maximos, M.; Huber, E.; Li, A.; Lê, M.L. A Sex- and Gender-Based Analysis of Adverse Drug Reactions: A Scoping Review of Pharmacovigilance Databases. Pharmaceuticals 2022, 15, 298. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, P.E. Gender differences in risk perception: Theoretical and methodological perspectives. Risk Anal. 1998, 18, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Alshabi, A.M.; Shaikh, M.A.K.; Shaikh, I.A.; Alkahtani, S.A.; Aljadaan, A. Knowledge, attitude and practice of hospital pharmacists towards pharmacovigilance and adverse drug reaction reporting in Najran, Saudi Arabia. Saudi Pharm. J. 2022, 30, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Alshayban, D.; Mahmoud, M.A.; Islam, M.A.; Alshammari, S.; Alsulaiman, D. Pharmacovigilance Perception and Knowledge Among Pharmacists and Interns in Saudi Arabia. Risk Manag. Healthc. Policy 2020, 13, 55–61. [Google Scholar] [CrossRef]
- Al-Abdulkarim, D.A.; Aljadhey, H.S.; Mahmoud, M.A.; Poff, G.A.; Hassali, M.A.; Ali, S. Knowledge and Barriers Among Physicians Toward Adverse Drug Reaction Reporting at a Tertiary Care Hospital in Saudi Arabia. Hosp. Pharm. 2021, 56, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Danekhu, K.; Shrestha, S.; Aryal, S.; Shankar, P.R. Health-care Professionals’ Knowledge and Perception of Adverse Drug Reaction Reporting and Pharmacovigilance in a Tertiary Care Teaching Hospital of Nepal. Hosp. Pharm. 2021, 56, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Valinciute, A.; Gerbutaviciene, R.J.; Paukstaitiene, R.; Kubiliene, L. Pharmacovigilance and Adverse Drug Reaction Reporting among the General Public in Lithuania: A Cross-Sectional Study. Healthcare 2023, 11, 1133. [Google Scholar] [CrossRef] [PubMed]
- García-Abeijon, P.; Costa, C.; Taracido, M.; Herdeiro, M.T.; Torre, C.; Figueiras, A. Factors Associated with Underreporting of Adverse Drug Reactions by Health Care Professionals: A Systematic Review Update. Drug Saf. 2023, 46, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Hariraj, V.; Aziz, Z. Patient Reporting of Adverse Drug Reactions (ADRs): Survey of Public Awareness and Predictors of Confidence to Report. Ther. Innov. Regul. Sci. 2018, 52, 757–763. [Google Scholar] [CrossRef]
- Staniszewska, A.; Dąbrowska-Bender, M.; Olejniczak, D.; Duda-Zalewska, A.; Bujalska-Zadrożny, M. Patient knowledge on reporting adverse drug reactions in Poland. Patient Prefer. Adherence 2017, 11, 47–53. [Google Scholar] [CrossRef]
- Mutair, A.A.; Alhumaid, S.; Shamsan, A.; Zaidi, A.R.Z.; Mohaini, M.A.; Al Mutairi, A.; Rabaan, A.A.; Awad, M.; Al-Omari, A. The Effective Strategies to Avoid Medication Errors and Improving Reporting Systems. Medicines 2021, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Clarke, J.; Ashrafian, H.; Darzi, A.; Neves, A.L. The Impact of Electronic Health Record Interoperability on Safety and Quality of Care in High-Income Countries: Systematic Review. J. Med. Internet Res. 2022, 24, e38144. [Google Scholar] [CrossRef] [PubMed]
- Bossaer, J.B.; Eskens, D.; Gardner, A. Sensitivity and specificity of drug interaction databases to detect interactions with recently approved oral antineoplastics. J. Oncol. Pharm. Pract. 2022, 28, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tao, W.; Li, Z.; Sun, Z.; Li, F.; Fenton, S.; Xu, H.; Tao, C. Artificial intelligence-powered pharmacovigilance: A review of machine and deep learning in clinical text-based adverse drug event detection for benchmark datasets. J. Biomed. Inform. 2024, 152, 104621. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Overall Sample (n = 65) | Female (n = 32) | Male (n = 33) | p-Value * |
|---|---|---|---|---|
| Age in years: | 0.772 | |||
| 25–35 | 37 (56.9%) | 17 (53.1) | 20 (60.6) | |
| 36–45 | 17 (26.2%) | 9 (28.1) | 8 (24.2) | |
| 46–55 | 8 (12.3%) | 5 (15.6) | 3 (9.1) | |
| 56–65 | 3 (4.6%) | 1 (3.1) | 2 (6.1) | |
| 66+ | 0 (0%) | 0 (0%) | 0 (0%) | |
| Years in practice: | 0.934 | |||
| Less than 1 year | 4 (6.2%) | 2 (6.2) | 2 (6.1) | |
| 1–4 years | 16 (24.6%) | 9 (28.1) | 7 (21.2) | |
| 5–9 years | 15 (23.1%) | 6 (18.8) | 9 (27.3) | |
| 10–19 years | 24 (36.9%) | 12 (37.5) | 12 (36.4) | |
| 20 years or more | 6 (9.2%) | 3 (9.4) | 3 (9.1) | |
| Practice Setting: | 0.037 | |||
| Ministry of Health Facilities | 14 (21.6%) | 10 (31.2) | 4 (12.1) | |
| Military Hospitals | 11 (16.9%) | 4 (12.5) | 7 (21.2) | |
| Ministry of the Interior Hospitals | 1 (1.6%) | 0 (0.0) | 1 (3.0) | |
| Referral Hospitals | 6 (9.2%) | 0 (0.0) | 6 (18.2) | |
| University Hospitals | 25 (38.4%) | 15 (46.9) | 10 (30.3) | |
| Private Facilities | 8 (12.3%) | 3 (9.4) | 5 (15.2) | |
| Role: | 0.070 | |||
| Physician | 7 (10.8%) | 2 (6.2) | 5 (15.2) | |
| Nurse | 17 (26.2%) | 13 (40.6) | 4 (12.1) | |
| Pharmacist | 33 (50.8%) | 13 (40.6) | 20 (60.6) | |
| Pharmacy technician | 7 (10.8%) | 3 (9.4) | 4 (12.1) | |
| Other | 1 (1.5%) | 1 (3.1) | 0 (0.0) |
| Characteristics | Overall Sample (n = 65) | Female (n = 32) | Male (n = 33) | p-Value * |
|---|---|---|---|---|
| To which organization do HCPs report ADRs? | 0.844 | |||
| Internally (the healthcare organization where HCPs work) | 38 (58.5) | 18 (56.2) | 20 (60.6) | |
| Ministry of Health | 6 (9.2) | 3 (9.4) | 3 (9.1) | |
| Drug manufacturer | 4 (6.1) | 2 (6.2) | 2 (6.0) | |
| SFDA PV Program | 8 (12.3) | 5 (15.6) | 3 (9.1) | |
| Never reported | 9 (13.8) | 4 (12.5) | 5 (15.2) | |
| Did HCPs receive ADR-related education? | 0.602 | |||
| Yes | 50 (76.9) | 26 (81.2) | 24 (72.7) | |
| No | 15 (23.1%) | 6 (18.8) | 9 (27.3) | |
| Are HCPs familiar with a formal procedure for reviewing reports submitted to the incident-reporting system? | 0.561 | |||
| Yes | 25 (38.5%) | 13 (40.6) | 12 (36.4) | |
| No | 4 (6.2%) | 1 (3.1) | 3 (9.1) | |
| Unsure | 35 (53.8%) | 18 (56.2) | 17 (51.5) | |
| Not applicable | 1 (1.5%) | 0 (0.0) | 1 (3.0) | |
| How do HCPs identify ADRs? | 0.148 | |||
| Temporal relationship between the onset of a drug therapy and the adverse reaction | 21 (31.7%) | 6 (18.8) | 15 (45.5) | |
| There was a dechallenge or rechallenge | 18 (27.0%) | 12 (37.5) | 6 (18.2) | |
| Signs and symptoms of ADRs | 8 (12.7%) | 4 (12.5) | 4 (12.1) | |
| Laboratory tests | 3 (4.8%) | 1 (3.1) | 2 (6.1) | |
| Patient complaint | 15 (23.8%) | 9 (28.1) | 6 (18.2) |
| Electronic Systems’ Implementation | n (%) |
|---|---|
| Electronic Health Records (EHR)/Electronic Medical Records (EMR) | |
| Fully Implemented | 51 (77.8%) |
| Not Implemented/Paper-based Methods Only | 1 (1.6%) |
| Partially Implemented | 12 (19.0%) |
| Unsure | 1 (1.6%) |
| Barcode-enabled Medication Administration (BCMA) | |
| Fully Implemented | 32 (49.2%) |
| Not Implemented/Paper-based Methods Only | 6 (9.5%) |
| Partially Implemented | 24 (36.5%) |
| Unsure | 3 (4.8%) |
| Computerized Physician Order Entry (CPOE) System | |
| Fully Implemented | 52 (79.4%) |
| Not Implemented/Paper-based Methods Only | 1 (1.6%) |
| Partially Implemented | 10 (15.9%) |
| Unsure | 2 (3.2%) |
| BarCode Medication Preparation Technologies (BCMP) | |
| Fully Implemented | 32 (49.2%) |
| Not Implemented/Paper-based Methods Only | 6 (9.5%) |
| Partially Implemented | 22 (33.3%) |
| Unsure | 5 (7.9%) |
| Electronic Incident-Reporting (IR) System | |
| Fully Implemented | 45 (69.8%) |
| Not Implemented/Paper-based Methods Only | 1 (1.6%) |
| Partially Implemented | 14 (22.2%) |
| Unsure | 4 (6.3%) |
| Electronic Medication Administration Record (eMAR) | |
| Fully Implemented | 45 (71.4%) |
| Partially Implemented | 17 (25.4%) |
| Unsure | 2 (3.2%) |
| Reporting Processes | n (%) |
|---|---|
| Most encountered type of ADRs | |
| Nausea and vomiting | 9 (14.5%) |
| Dermatological toxicities | 8 (11.4%) |
| Febrile neutropenia | 7 (10.4%) |
| Cardiovascular toxicity | 6 (9.3%) |
| Diarrhea or constipation | 5 (7.8%) |
| Fatigue | 4 (6.2%) |
| Mucositis | 4 (6.2%) |
| Thrombosis | 3 (5.2%) |
| Infusion reactions | 3 (5.2%) |
| Neuropathic pain | 3 (5.2%) |
| Central venous catheters-related complications | 2 (3.6%) |
| Infections | 2 (3.6%) |
| Most observed reaction type * | |
| Unprovoked/Unexpected reaction | 36 (56.1%) |
| Exaggerated pharmacological action | 29 (43.9%) |
| Most observed level of ADR severity ** | |
| Category 1 | 20 (30%) |
| Category 2 | 15 (23.3%) |
| Category 3 | 14 (21.1%) |
| Category 4 | 12 (18.9%) |
| Category 5 | 3 (4.4%) |
| Category 6 | 1 (2.2%) |
| Action taken most often once an ADR is detected | |
| Discontinue suspect medication(s) | 17 (26.9%) |
| Treatment with medications | 13 (20.7%) |
| Adjust dose, route, frequency | 13 (20.7%) |
| Therapy held | 11 (16.6%) |
| Switch to alternative agent | 8 (11.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkofide, H.; Almalag, H.M.; Alromaih, M.; Alotaibi, L.; Altuwaijri, N.; Al Aloola, N.; Alsabhan, J.F.; Bawazeer, G.A.; Al Juffali, L.; Alfaraj, R.; et al. Pharmacovigilance Practices by Healthcare Providers in Oncology: A Cross-Sectional Study. Pharmaceuticals 2024, 17, 683. https://doi.org/10.3390/ph17060683
Alkofide H, Almalag HM, Alromaih M, Alotaibi L, Altuwaijri N, Al Aloola N, Alsabhan JF, Bawazeer GA, Al Juffali L, Alfaraj R, et al. Pharmacovigilance Practices by Healthcare Providers in Oncology: A Cross-Sectional Study. Pharmaceuticals. 2024; 17(6):683. https://doi.org/10.3390/ph17060683
Chicago/Turabian StyleAlkofide, Hadeel, Haya M. Almalag, Mashael Alromaih, Lama Alotaibi, Njoud Altuwaijri, Noha Al Aloola, Jawza F. Alsabhan, Ghada A. Bawazeer, Lobna Al Juffali, Rihaf Alfaraj, and et al. 2024. "Pharmacovigilance Practices by Healthcare Providers in Oncology: A Cross-Sectional Study" Pharmaceuticals 17, no. 6: 683. https://doi.org/10.3390/ph17060683
APA StyleAlkofide, H., Almalag, H. M., Alromaih, M., Alotaibi, L., Altuwaijri, N., Al Aloola, N., Alsabhan, J. F., Bawazeer, G. A., Al Juffali, L., Alfaraj, R., Alkhudair, N., Aljadeed, R., Aljadeed, R., & Alnaim, L. S. (2024). Pharmacovigilance Practices by Healthcare Providers in Oncology: A Cross-Sectional Study. Pharmaceuticals, 17(6), 683. https://doi.org/10.3390/ph17060683






