Serious Safety Signals and Prediction Features Following COVID-19 mRNA Vaccines Using the Vaccine Adverse Event Reporting System
Abstract
1. Introduction
2. Results
2.1. ICSRs by Demographic Characteristics
2.2. Serious AEFIs
2.3. Disproportionality Analysis for Signal Detection of Serious AEFIs
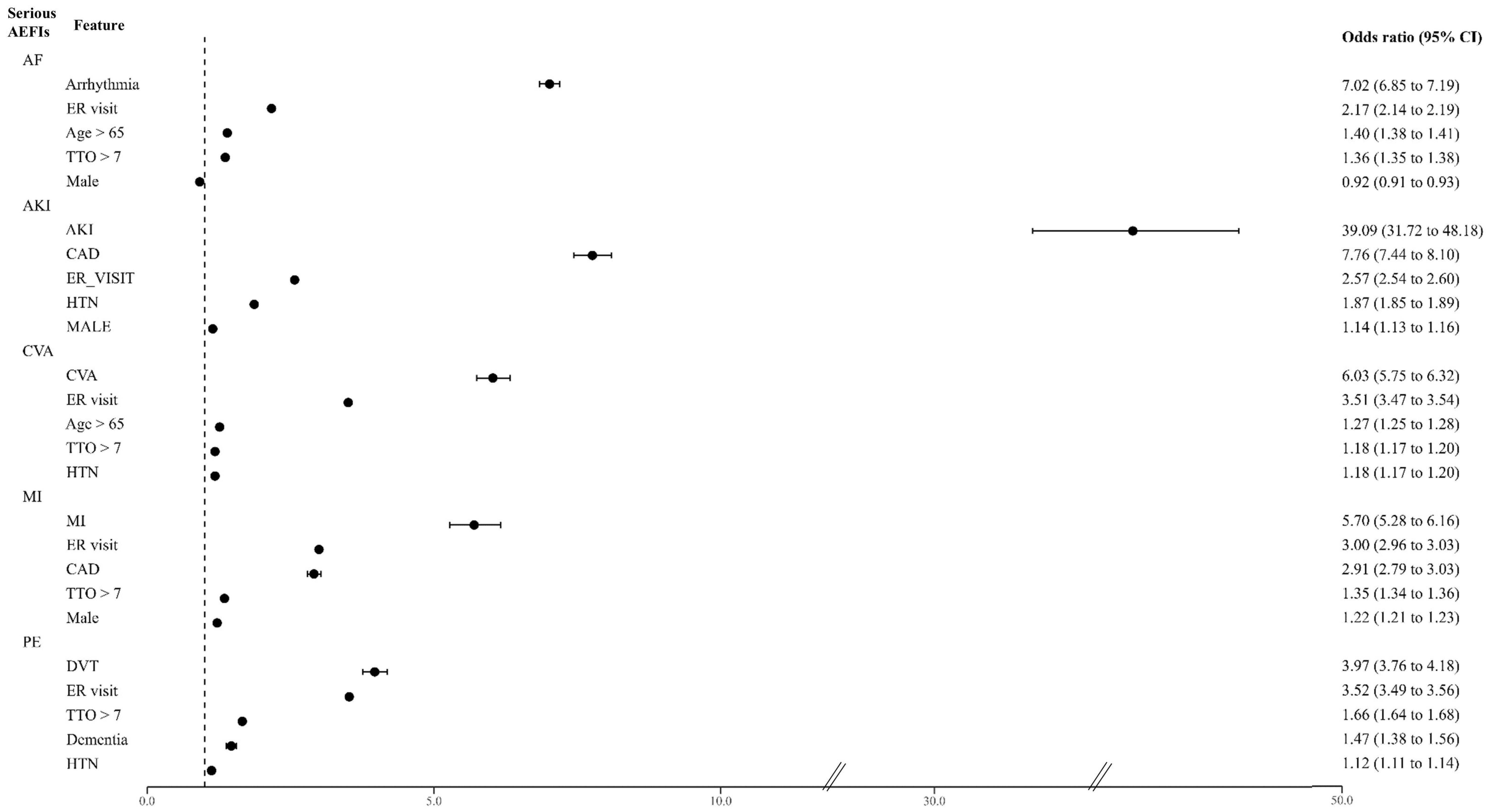
2.4. Predicting the Incidence of the Serious AEFIs and the Associated Features
3. Discussion
4. Materials and Methods
4.1. Data Source
4.2. Patient Medical History Coding
4.3. Study Design
4.3.1. Incidences of ICSR and Serious AEFI
4.3.2. Signal Detection for Serious AEFI
4.3.3. Prediction for Serious AEFIs
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Machhi, J.; Shahjin, F.; Das, S.; Patel, M.; Abdelmoaty, M.M.; Cohen, J.D.; Singh, P.A.; Baldi, A.; Bajwa, N.; Kumar, R.; et al. Nanocarrier vaccines for SARS-CoV-2. Adv. Drug Deliv. Rev. 2021, 171, 215–239. [Google Scholar] [CrossRef] [PubMed]
- WHO. Serious AEFI. 2022. Available online: https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/aefi/serious-aefi (accessed on 9 April 2023).
- Amanat, F.; Krammer, F. SARS-CoV-2 vaccines: Status report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef]
- Lorini, C.; Collini, F.; Gasparini, F.; Paolini, D.; Grazzini, M.; Ierardi, F.; Galletti, G.; Zanobini, P.; Gemmi, F.; Bonaccorsi, G.; et al. Health literacy, vaccine confidence and influenza vaccination uptake among nursing home staff: A cross-sectional study conducted in Tuscany. Vaccines 2020, 8, 154. [Google Scholar] [CrossRef]
- Yadete, T.; Batra, K.; Netski, D.M.; Antonio, S.; Patros, M.J.; Bester, J.C. Assessing acceptability of COVID-19 vaccine booster dose among adult Americans: A cross-sectional study. Vaccines 2021, 9, 1424. [Google Scholar] [CrossRef]
- Phillips, N. The coronavirus is here to stay-here’s what that means. Nature 2021, 590, 382–384. [Google Scholar] [CrossRef]
- Convertino, I.; Tuccori, M.; Ferraro, S.; Valdiserra, G.; Cappello, E.; Focosi, D.; Blandizzi, C. Exploring pharmacological approaches for managing cytokine storm associated with pneumonia and acute respiratory distress syndrome in COVID-19 patients. Crit. Care 2020, 24, 331. [Google Scholar] [CrossRef]
- Varricchio, F.; Iskander, J.; Destefano, F.; Ball, R.; Pless, R.; Braun, M.M.; Chen, R.T. Understanding vaccine safety information from the vaccine adverse event reporting system. Pediatr. Infect. Dis. J. 2004, 23, 287–294. [Google Scholar] [CrossRef]
- Trifirò, G.; Pariente, A.; Coloma, P.M.; Kors, J.A.; Polimeni, G.; Miremont-Salamé, G.; Catania, M.A.; Salvo, F.; David, A.; Moore, N.; et al. Data mining on electronic health record databases for signal detection in pharmacovigilance: Which events to monitor? Pharmacoepidemiol. Drug Saf. 2009, 18, 1176–1184. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Sun, M.; Zhou, Y.; Yin, M.; Zhao, B.; Li, X. COVID-19 mRNA vaccines are generally safe in the short term: A vaccine vigilance real-world study says. Front. Immunol. 2021, 12, 1843. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 125. [Google Scholar] [CrossRef]
- Persad, G.; Emanuel, E.J.; Sangenito, S.; Glickman, A.; Phillips, S.; Largent, E.A. Public perspectives on COVID-19 vaccine prioritization. JAMA Netw. Open 2021, 4, e217943. [Google Scholar] [CrossRef]
- Meyboom, R.H.; Egberts, A.C.; Edwards, I.R.; Hekster, Y.A.; de Koning, F.H.; Gribnau, F.W. Principles of signal detection in pharmacovigilance. Drug Saf. 1997, 16, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Green, M.S.; Peer, V.; Magid, A.; Hagani, N.; Anis, E.; Nitzan, D. Gender differences in adverse events following the Pfizer-BioNTech COVID-19 vaccine. Vaccines 2022, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A. Gender-based differences in the toxicity of pharmaceuticals—The Food and Drug Administration’s perspective. Int. J. Toxicol. 2001, 20, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Lim, J.; Choe, K.-W.; Lee, K.-D.; Jo, D.H.; Kim, M.J.; Kim, J.M.; Kim, K.N. Reactogenicity after the first and second doses of BNT162b2 mRNA coronavirus disease vaccine: A single-center study. Clin. Exp. Vaccine Res. 2021, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yuan, J.; Li, M.; Jiang, B.; Lu, Z.K. Age and gender disparities in adverse events following COVID-19 vaccination: Real-world evidence based on big data for risk management. Front. Med. 2021, 8, 700014. [Google Scholar] [CrossRef] [PubMed]
- Montano, D. Frequency and associations of adverse reactions of COVID-19 vaccines reported to pharmacovigilance systems in the European Union and the United States. Front. Public Health 2022, 2237, 756633. [Google Scholar] [CrossRef] [PubMed]
- Pfizer. COMIRNATY Full Prescribing Information. 2022. Available online: https://www.comirnaty.com/prescribing-information (accessed on 9 April 2023).
- Moderna. SPIKEVAX Full Prescribing Information. 2022. Available online: https://www.fda.gov/media/155675/download?attachment (accessed on 4 March 2024).
- See, E.J.; Toussaint, N.D.; Bailey, M.; Johnson, D.W.; Polkinghorne, K.R.; Robbins, R.; Bellomo, R. Risk factors for major adverse kidney events in the first year after acute kidney injury. Clin. Kidney J. 2021, 14, 556–563. [Google Scholar] [CrossRef] [PubMed]
- VanWagner, L.B.; Serper, M.; Kang, R.; Levitsky, J.; Hohmann, S.; Abecassis, M.; Skaro, A.; Lloyd-Jones, D.M. Factors associated with major adverse cardiovascular events after liver transplantation among a national sample. Am. J. Transplant. 2016, 16, 2684–2694. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Causality Assessment of an Adverse Event Following Immunization (AEFI): User Manual for the Revised WHO Classification. 2019. Available online: https://www.who.int/publications/i/item/9789241516990 (accessed on 4 March 2024).
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Jaffe, E.; Levi, R. Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave. Sci. Rep. 2022, 12, 6978. [Google Scholar] [CrossRef] [PubMed]
- Terentes-Printzios, D.; Gardikioti, V.; Solomou, E.; Emmanouil, E.; Gourgouli, I.; Xydis, P.; Christopoulou, G.; Georgakopoulos, C.; Dima, I.; Miliou, A.; et al. The effect of an mRNA vaccine against COVID-19 on endothelial function and arterial stiffness. Hypertens. Res. 2022, 45, 846–855. [Google Scholar] [CrossRef]
- Hause, A.M.; Baggs, J.; Marquez, P.; Myers, T.R.; Su, J.R.; Blanc, P.G.; Baumblatt, J.A.G.; Woo, E.J.; Gee, J.; Shimabukuro, T.T. Safety monitoring of COVID-19 vaccine booster doses among adults—United States, September 22, 2021–February 6, 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 249. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Dao, T.L.; Truong, T.M.D.; Nguyen, T.H.; Phan, T.N.; Nguyen, H.M.; Pham, T.D.; Nguyen, X.B.; Nguyen, T.B.; Hoang, V.T.; et al. Short-Term Adverse Effects Immediately after the Start of COVID-19 Booster Vaccination in Vietnam. Vaccines 2022, 10, 1325. [Google Scholar] [CrossRef]
- Singleton, J.A.; Lloyd, J.C.; Mootrey, G.T.; Salive, M.E.; Chen, R.T.; Ellenberg, S.; Rastogi, S.; Krueger, C.; Braun, M.; Wise, R.; et al. An overview of the vaccine adverse event reporting system (VAERS) as a surveillance system. Vaccine 1999, 17, 2908–2917. [Google Scholar] [CrossRef]
- VAERS. VAERS Data Use Guide. 2021. Available online: https://vaers.hhs.gov/docs/VAERSDataUseGuide_en_September2021.pdf (accessed on 9 April 2023).
- Brown, E.G.; Wood, L.; Wood, S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999, 20, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J.; Waller, P.C.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Lanes, S.; Sacks, S.T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 2004, 13, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Bate, A.; Lindquist, M.; Edwards, I.R.; Olsson, S.; Orre, R.; Lansner, A.; De Freitas, R.M. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 1998, 54, 315–321. [Google Scholar] [CrossRef]
- DuMouchel, W.; Pregibon, D. Empirical bayes screening for multi-item associations. In Proceedings of the Seventh ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 26–29 August 2001; pp. 67–76. [Google Scholar]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Guyon, I.; Weston, J.; Barnhill, S.; Vapnik, V. Gene selection for cancer classification using support vector machines. Mach. Learn. 2002, 46, 389–422. [Google Scholar] [CrossRef]
| Characteristics | Pfizer–BioNTech Vaccine (BNT162b2) (n = 266,369) | Moderna Vaccine (mRNA-1273) (n = 288,664) | |||
|---|---|---|---|---|---|
| Serious ICSR (n = 28,267) | Non-Serious ICSR (n = 238,102) | Serious ICSR (n = 23,231) | Non-Serious ICSR (n = 265,433) | ||
| n (%) | n (%) | n (%) | n (%) | ||
| Sex | Female | 15,368 (54.4) | 172,592 (72.5) | 12,132 (52.2) | 196,952 (74.2) |
| Male | 12,899 (45.6) | 65,510 (27.5) | 11,099 (47.8) | 68,481 (25.8) | |
| Age (years) | Average ± SD | 60.9 ± 19.0 | 48.4 ± 16.7 | 63.0 ± 18.5 | 52.5 ± 17.5 |
| 18–64 | 14,784 (52.3) | 192,886 (81.0) | 10,907 (47.0) | 187,333 (70.6) | |
| over 65 | 13,483 (47.7) | 45,216 (19.0) | 12,324 (53.1) | 78,100 (29.4) | |
| Time to onset of AEFIs (days) | 0–7 | 12,004 (42.5) | 194,233 (81.6) | 10,612 (45.7) | 199,417 (75.1) |
| >7 | 15,398 (54.5) | 32,432 (13.6) | 11,934 (51.4) | 51,702 (19.5) | |
| Unknown | 865 (3.1) | 11,437 (4.8) | 685 (3.0) | 14,314 (5.4) | |
| Emergency room or urgent care visit | Visit | 12,024 (42.5) | 29,810 (12.5) | 9041 (38.9) | 22,932 (8.6) |
| No visit | 16,243 (57.5) | 208,292 (87.5) | 14,190 (61.1) | 242,501 (91.4) | |
| Doctor or other healthcare provider office/clinic visit | Visit | 8005 (28.3) | 55,531 (23.3) | 5915 (25.5) | 50,206 (18.9) |
| No visit | 20,262 (71.7) | 182,571 (76.7) | 17,316 (74.5) | 215,227 (81.1) | |
| Prognosis after the AEFIs | Recovered | 4734 (16.8) | 79,115 (33.2) | 4639 (20.0) | 97,522 (36.7) |
| Not recovered | 14,290 (50.6) | 94,345 (39.6) | 11,226 (48.3) | 90,091 (33.9) | |
| Unknown | 9243 (32.7) | 64,642 (27.2) | 7366 (31.7) | 77,820 (29.3) | |
| Top 5 comorbidity * | Hypertension | 6247 (22.1) | 23,523 (9.9) | 5215 (22.5) | 24,228 (9.1) |
| Type 2 diabetes mellitus | 3470 (12.3) | 10,763 (4.5) | 2992 (12.9) | 11,357 (4.3) | |
| Hyperlipidemia | 3028 (10.7) | 8912 (3.7) | 2543 (11.0) | 9485 (3.6) | |
| Asthma | 1428 (5.1) | 14,640 (6.2) | 1068 (4.6) | 13,541 (5.1) | |
| Hypothyroid disease | 1339 (4.7) | 8756 (3.7) | 1206 (5.2) | 8682 (3.3) | |
| Unknown or vague description | 12,751 (45.1) | 130,241 (54.7) | 10,907 (47.0) | 168,965 (63.7) | |
| SOC | PT | Serious AEFI (Total n = 271,444) | Pfizer–BioNTech Vaccine (BNT162b2) (n = 150,575) | Moderna Vaccine (mRNA-1273) (n = 120,869) | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| General disorders and administration site conditions | Death | 7694 | 2.8 | 4021 | 2.7 | 3673 | 3.0 |
| Pyrexia | 6536 | 2.4 | 3455 | 2.3 | 3081 | 2.6 | |
| Fatigue | 5668 | 2.1 | 3160 | 2.1 | 2508 | 2.1 | |
| Asthenia | 5183 | 1.9 | 2765 | 1.8 | 2418 | 2.0 | |
| Pain | 4336 | 1.6 | 2420 | 1.6 | 1916 | 1.6 | |
| Chest pain | 4333 | 1.6 | 2439 | 1.6 | 1894 | 1.6 | |
| Condition aggravated | 3927 | 1.5 | 2200 | 1.5 | 1727 | 1.4 | |
| Chills | 3168 | 1.2 | 1659 | 1.1 | 1509 | 1.3 | |
| Malaise | 2648 | 1.0 | 1375 | 0.9 | 1273 | 1.1 | |
| Others | 17,656 | 6.5 | 9205 | 6.1 | 8451 | 7.0 | |
| Total | 61,149 | 22.5 | 32,699 | 21.7 | 28,450 | 23.5 | |
| Nervous system disorders | Headache | 4517 | 1.7 | 2463 | 1.6 | 2054 | 1.7 |
| Dizziness | 3503 | 1.3 | 1943 | 1.3 | 1560 | 1.3 | |
| Cerebrovascular accident | 2230 | 0.8 | 1139 | 0.8 | 1091 | 0.9 | |
| Hypoaesthesia | 2156 | 0.8 | 1264 | 0.8 | 892 | 0.7 | |
| Others | 27,345 | 10.1 | 14,981 | 10.0 | 12,364 | 10.2 | |
| Total | 39,751 | 14.6 | 21,790 | 14.5 | 17,961 | 14.9 | |
| Respiratory, thoracic, and mediastinal disorders | Dyspnoea | 10,752 | 4.0 | 6042 | 4.0 | 4710 | 3.9 |
| Cough | 5039 | 1.9 | 3093 | 2.1 | 1946 | 1.6 | |
| Pulmonary embolism | 2396 | 0.9 | 1237 | 0.8 | 1159 | 1.0 | |
| Hypoxia | 2259 | 0.8 | 1280 | 0.9 | 979 | 0.8 | |
| Others | 18,195 | 6.7 | 10,457 | 6.9 | 7738 | 6.4 | |
| Total | 38,641 | 14.2 | 22,109 | 14.7 | 16,532 | 13.7 | |
| Infections and infestations | COVID-19 | 14,143 | 5.2 | 8628 | 5.7 | 5515 | 4.6 |
| COVID-19 pneumonia | 2907 | 1.1 | 1831 | 1.2 | 1076 | 0.9 | |
| Pneumonia | 2065 | 0.8 | 1115 | 0.7 | 950 | 0.8 | |
| Others | 9189 | 3.4 | 5324 | 3.5 | 3865 | 3.2 | |
| Total | 28,304 | 10.4 | 16,898 | 11.2 | 11,406 | 9.4 | |
| Gastrointestinal disorders | Nausea | 3960 | 1.5 | 2155 | 1.4 | 1805 | 1.5 |
| Vomiting | 3096 | 1.1 | 1652 | 1.1 | 1444 | 1.2 | |
| Diarrhea | 2567 | 1.0 | 1493 | 1.0 | 1074 | 0.9 | |
| Others | 9278 | 3.4 | 5155 | 3.4 | 4123 | 3.4 | |
| Total | 18,901 | 7.0 | 10,455 | 6.9 | 8446 | 7.0 | |
| Musculoskeletal and connective tissue disorders | Pain in extremity | 3047 | 1.1 | 1668 | 1.1 | 1379 | 1.1 |
| Arthralgia | 2209 | 0.8 | 1239 | 0.8 | 970 | 0.8 | |
| Others | 11,886 | 4.4 | 6556 | 4.4 | 5330 | 4.4 | |
| Total | 17,142 | 6.3 | 9463 | 6.3 | 7679 | 6.4 | |
| SOC | Pfizer–BioNTech Vaccine (BNT162b2) ROR [95% CI] | Moderna Vaccine (mRNA-1273) ROR [95% CI] |
|---|---|---|
| Cardiac disorders | 3.12 [2.91–3.34] | 3.24 [3.02–3.48] |
| Infections and infestations | 2.62 [2.50–2.75] | 2.16 [2.06–2.27] |
| Renal and urinary disorders | 2.18 [1.95–2.44] | 2.04 [1.82–2.28] |
| PT | Pfizer–BioNTech Vaccine (BNT162b2) | Moderna Vaccine (mRNA-1273) | ||
|---|---|---|---|---|
| n | ROR [95% CI] | n | ROR [95% CI] | |
| Acute myocardial infarction | 467 | 10.75 [6.20–18.66] | 358 | 10.27 [5.90–17.86] |
| Pulmonary embolism | 1237 | 8.27 [6.14–11.13] | 1159 | 9.66 [7.17–13.02] |
| Acute kidney injury | 1001 | 7.51 [5.48–10.31] | 620 | 5.79 [4.20–7.97] |
| Myocardial infarction | 526 | 3.84 [2.79–5.28] | 500 | 4.55 [3.31–6.26] |
| Atrial fibrillation | 814 | 3.54 [2.76–4.52] | 703 | 3.81 [2.97–4.87] |
| Cerebrovascular accident | 1139 | 3.49 [2.84–4.29] | 1091 | 4.17 [3.39–5.13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.Y.; Lee, Y.; Park, N.G.; Kim, M.S.; Rhie, S.J. Serious Safety Signals and Prediction Features Following COVID-19 mRNA Vaccines Using the Vaccine Adverse Event Reporting System. Pharmaceuticals 2024, 17, 356. https://doi.org/10.3390/ph17030356
Choi JY, Lee Y, Park NG, Kim MS, Rhie SJ. Serious Safety Signals and Prediction Features Following COVID-19 mRNA Vaccines Using the Vaccine Adverse Event Reporting System. Pharmaceuticals. 2024; 17(3):356. https://doi.org/10.3390/ph17030356
Chicago/Turabian StyleChoi, Jung Yoon, Yongjoon Lee, Nam Gi Park, Mi Sung Kim, and Sandy Jeong Rhie. 2024. "Serious Safety Signals and Prediction Features Following COVID-19 mRNA Vaccines Using the Vaccine Adverse Event Reporting System" Pharmaceuticals 17, no. 3: 356. https://doi.org/10.3390/ph17030356
APA StyleChoi, J. Y., Lee, Y., Park, N. G., Kim, M. S., & Rhie, S. J. (2024). Serious Safety Signals and Prediction Features Following COVID-19 mRNA Vaccines Using the Vaccine Adverse Event Reporting System. Pharmaceuticals, 17(3), 356. https://doi.org/10.3390/ph17030356






