From Eye Care to Hair Growth: Bimatoprost
Abstract
1. Introduction
2. Methods
3. Results
4. Selected Comparison Studies on Bimatoprost
4.1. Bimatoprost vs. Bimatoprost
4.2. Bimatoprost vs. Beta Blockers and α2 Agonists
4.3. Bimatoprost vs. Prostaglandin Analogues
4.4. Bimatoprost vs. Multi-Class IOP Lowering Medications
4.5. Bimatoprost vs. Fixed Combination Therapies
5. Complications of Bimatoprost
6. Bimatoprost in Other Fields
7. Bimatoprost and Hair Growth
8. Evolution of Bimatoprost Therapies
9. Novel Delivery Technologies for Bimatoprost
10. Future Perspective for Bimatoprost
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subbulakshmi, S.; Kavitha, S.; Venkatesh, R. Prostaglandin analogs in ophthalmology. Indian. J. Ophthalmol. 2023, 71, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Mantravadi, A.V.; Myers, J.S. Patient considerations in ocular hypertension: Role of Bimatoprost ophthalmic solution. Clin. Ophthalmol. 2017, 11, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, A.; Riva, I.; Bozkurt, B.; Hollo, G.; Quaranta, L.; Oddone, F.; Irkec, M.; Dutton, G.N.; Konstas, A.G. A new look at the safety and tolerability of prostaglandin analogue eyedrops in glaucoma and ocular hypertension. Expert. Opin. Drug Saf. 2022, 21, 525–539. [Google Scholar] [CrossRef]
- Meymandi, S.S.; Safari, A.; Meymandi, M.S.; Aflatoonian, M. The role of fractional laser-assisted drug delivery in enhancing the efficacy of topical bimatoprost solution in the treatment of alopecia areata: An intra-patient comparative randomized clinical trial. J. Cosmet. Dermatol. 2024, 23, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Klimko, P.G.; Sharif, N.A. Discovery, characterization and clinical utility of prostaglandin agonists for the treatment of glaucoma. Br. J. Pharmacol. 2019, 176, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Jiang, M.; Ye, B.; Zhang, G.T.; Li, W.; Tang, P.; Huang, Z.; Chen, F. A unified strategy to prostaglandins: Chemoenzymatic total synthesis of cloprostenol, Bimatoprost, PGF(2alpha), fluprostenol, and travoprost guided by biocatalytic retrosynthesis. Chem. Sci. 2021, 12, 10362–10370. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.S.; Meyer, J.J. Bimatoprost Ophthalmic Solution; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Doucette, L.P.; Walter, M.A. Prostaglandins in the eye: Function, expression, and roles in glaucoma. Ophthalmic Genet. 2017, 38, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Kobelt, G.; Texier-Richard, B.; Buchholz, P.; Bron, A.; Renard, J.P.; Rouland, J.F.; Nordmann, J.P. Treatment of glaucoma in clinical practice: Four-year results from a patient registry in France. J. Glaucoma 2010, 19, 199–206. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5311027, Bimatoprost. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bimatoprost (accessed on 13 April 2024).
- Yamagishi, R.; Aihara, M.; Araie, M. Neuroprotective effects of prostaglandin analogues on retinal ganglion cell death independent of intraocular pressure reduction. Exp. Eye Res. 2011, 93, 265–270. [Google Scholar] [CrossRef]
- Faulkner, R.; Sharif, N.A.; Orr, S.; Sall, K.; Dubiner, H.; Whitson, J.T.; Moster, M.; Craven, E.R.; Curtis, M.; Pailliotet, C.; et al. Aqueous humor concentrations of Bimatoprost free acid, Bimatoprost and travoprost free acid in cataract surgical patients administered multiple topical ocular doses of LUMIGAN or TRAVATAN. J. Ocul. Pharmacol. Ther. 2010, 26, 147–156. [Google Scholar] [CrossRef]
- Brubaker, R.F. Mechanism of action of bimatoprost (Lumigan). Surv. Ophthalmol. 2001, 45, S347–S351. [Google Scholar] [CrossRef]
- Woodward, D.F.; Krauss, A.H.; Nilsson, S.F. Bimatoprost effects on aqueous humor dynamics in monkeys. J. Ophthalmol. 2010, 2010, 926192. [Google Scholar] [CrossRef][Green Version]
- Vyas, P.; Naik, U.; Gangaiah, J.B. Efficacy of Bimatoprost 0.03% in reducing intraocular pressure in patients with 360 degrees synechial angle-closure glaucoma: A preliminary study. Indian. J. Ophthalmol. 2011, 59, 13–16. [Google Scholar]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Shen, J.; Goodkin, M.L.; Tong, W.; Attar, M. Ocular pharmacokinetics and tolerability of Bimatoprost ophthalmic solutions administered once or twice daily in rabbits, and clinical dosing implications. Clin. Ophthalmol. 2017, 11, 1761–1767. [Google Scholar] [CrossRef]
- Heo, J.Y.; Ooi, Y.H.; Rhee, D.J. Effect of prostaglandin analogs: Latanoprost, Bimatoprost, and unoprostone on matrix metalloproteinases and their inhibitors in human trabecular meshwork endothelial cells. Exp. Eye Res. 2020, 194, 108019. [Google Scholar] [CrossRef]
- Hwang, H.B.; Kim, S.Y. The Effect of Prostaglandin Analogues on the Ciliary Zonular Fibers of the Rabbit Crystalline Lens. Curr. Eye Res. 2018, 43, 1357–1361. [Google Scholar] [CrossRef]
- Stamer, W.D.; Piwnica, D.; Jolas, T.; Carling, R.W.; Cornell, C.L.; Fliri, H.; Martos, J.; Pettit, S.N.; Wang, J.W.; Woodward, D.F. Cellular basis for Bimatoprost effects on human conventional outflow. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5176–5181. [Google Scholar] [CrossRef]
- Angeli, A.; Supuran, C.T. Prostaglandin receptor agonists as antiglaucoma agents (a patent review 2013–2018). Expert. Opin. Ther. Pat. 2019, 29, 793–803. [Google Scholar] [CrossRef]
- Li, X.; Liu, G.; Wang, Y.; Yu, W.; Xiang, H.; Liu, X. A case hypersensitive to Bimatoprost and dexamethasone. J. Ocul. Pharmacol. Ther. 2011, 27, 519–523. [Google Scholar] [CrossRef]
- Comparison of Bimatoprost SR to Selective Laser Trabeculoplasty in Patients with Open-Angle Glaucoma or Ocular Hypertension; Identifier NCT02507687; U.S. National Library of Medicine: Bethesda, MD, USA, 2023. Available online: https://clinicaltrials.gov/ct2/show/study/NCT02507687 (accessed on 14 April 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Loftus, J.; Christopoulou, D.; Blak, B.T.; Hards, M. Topical prostaglandin fixed combinations in UK primary care: Observational study using data from the health improvement network. Eur. J. Ophthalmol. 2012, 22, 376–387. [Google Scholar] [CrossRef]
- Rahman, M.Q.; Abeysinghe, S.S.; Kelly, S.; Roskell, N.S.; Shannon, P.R.; Abdlseaed, A.A.; Montgomery, D.M. Persistence of glaucoma medical therapy in the Glasgow Glaucoma Database. Br. J. Ophthalmol. 2011, 95, 966–970. [Google Scholar] [CrossRef]
- Heo, J.H.; Rascati, K.L.; Wilson, J.P.; Lawson, K.A.; Richards, K.M.; Nair, R. Comparison of Prostaglandin Analog Treatment Patterns in Glaucoma and Ocular Hypertension. J. Manag. Care Spec. Pharm. 2019, 25, 1001–1010. [Google Scholar] [CrossRef]
- Dams, I.; Wasyluk, J.; Prost, M.; Kutner, A. Therapeutic uses of prostaglandin F(2alpha) analogues in ocular disease and novel synthetic strategies. Prostaglandins Other Lipid Mediat. 2013, 104, 109–121. [Google Scholar] [CrossRef]
- Woodward, D.F.; Phelps, R.L.; Krauss, A.H.; Weber, A.; Short, B.; Chen, J.; Liang, Y.; Wheeler, L.A. Bimatoprost: A novel antiglaucoma agent. Cardiovasc. Drug Rev. 2004, 22, 103–120. [Google Scholar] [CrossRef]
- Blondeau, P.; Hamid, M.; Ghalie, Z. Prospective randomized clinical trial on the effects of Latanoprost, travoprost and Bimatoprost on Latanoprost non-responders. J. Fr. Ophtalmol. 2019, 42, 894–899. [Google Scholar] [CrossRef]
- Crichton, A.C.; Nixon, D.R.; Simonyi, S.; Bhogal, M.; Sigouin, C.S.; Discepola, M.J.; Hutnik, C.M.; Baptiste, D.C.; Yan, D.B. An observational study of Bimatoprost 0.01% in patients on prior intraocular pressure-lowering therapy: The Canadian Lumigan((R)) RC Early Analysis Review (CLEAR) trial. Clin. Ophthalmol. 2014, 8, 1031–1038. [Google Scholar]
- Campbell, J.H.; Schwartz, G.; Labounty, B.; Kowalski, J.; Patel, V.D. Comparison of adherence and persistence with Bimatoprost 0.01% versus Bimatoprost 0.03% topical ophthalmic solutions. Curr. Med. Res. Opin. 2013, 29, 1201–1209. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Wierzbowska, J.; Siemiatkowska, A.; Fuksinska, B.; Robaszkiewicz, J.; Zegadlo, A.; Ehrlich, R.; Siesky, B.; Harris, A. The additive effect of dorzolamide hydrochloride (Trusopt) and a morning dose of Bimatoprost (Lumigan) on intraocular pressure and retrobulbar blood flow in patients with primary open-angle glaucoma. Br. J. Ophthalmol. 2010, 94, 1307–1311. [Google Scholar] [CrossRef]
- Inoue, K.; Shiokawa, M.; Fujimoto, T.; Tomita, G. Effects of treatment with Bimatoprost 0.03% for 3 years in patients with normal-tension glaucoma. Clin. Ophthalmol. 2014, 8, 1179–1183. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Sonty, S.; Ahmad, A. Evaluating intraocular pressure-lowering solutions for the treatment of open-angle glaucoma: Comparison between Bimatoprost 0.03% and Bimatoprost 0.01%—An observational switch study. Clin. Ophthalmol. 2017, 11, 1371–1376. [Google Scholar] [CrossRef]
- Asendrych-Wicik, K.; Zarczuk, J.; Walaszek, K.; Ciach, T.; Markowicz-Piasecka, M. Trends in development and quality assessment of pharmaceutical formulations—F2alpha analogues in the glaucoma treatment. Eur. J. Pharm. Sci. 2023, 180, 106315. [Google Scholar] [CrossRef] [PubMed]
- Diaconita, V.; Quinn, M.; Jamal, D.; Dishan, B.; Malvankar-Mehta, M.S.; Hutnik, C. Washout Duration of Prostaglandin Analogues: A Systematic Review and Meta-analysis. J. Ophthalmol. 2018, 2018, 3190684. [Google Scholar] [CrossRef]
- Lim, C.W.; Diaconita, V.; Liu, E.; Ault, N.; Lizotte, D.; Nguyen, M.; Hutnik, C.M.L. Effect of 6-week washout period on intraocular pressure following chronic prostaglandin analogue treatment: A randomized controlled trial. Can. J. Ophthalmol. 2020, 55, 143–151. [Google Scholar] [CrossRef]
- Bimatoprost. Drugs and Lactation Database (LactMed(R)); Bimatoprost: Bethesda, MD, USA, 2006. [Google Scholar]
- Shazly, T.A.; Latina, M.A. Comparison of intraocular pressure-lowering effect of every night versus every other night dosing of Bimatoprost 0.03%. J. Ocul. Pharmacol. Ther. 2011, 27, 369–371. [Google Scholar] [CrossRef]
- Nixon, D.R.; Simonyi, S.; Bhogal, M.; Sigouin, C.S.; Crichton, A.C.; Discepola, M.; Hutnik, C.M.; Yan, D.B. An observational study of Bimatoprost 0.01% in treatment-naive patients with primary open angle glaucoma or ocular hypertension: The CLEAR trial. Clin. Ophthalmol. 2012, 6, 2097–2103. [Google Scholar]
- Wang, K.; Xu, L.; Yuan, Z.; Yao, K.; Zhao, J.; Xu, L.; Fang, A.; Zhang, M.; Wu, L.; Ji, J.; et al. Intraocular pressure-lowering efficacy and safety of Bimatoprost 0.03% therapy for primary open-angle glaucoma and ocular hypertension patients in China. BMC Ophthalmol. 2014, 14, 21. [Google Scholar] [CrossRef][Green Version]
- Tsumura, T.; Yoshikawa, K.; Suzumura, H.; Kimura, T.; Sasaki, S.; Kimura, I.; Takeda, R. Bimatoprost ophthalmic solution 0.03% lowered intraocular pressure of normal-tension glaucoma with minimal adverse events. Clin. Ophthalmol. 2012, 6, 1547–1552. [Google Scholar] [CrossRef]
- Konstas, A.G.; Hollo, G.; Mikropoulos, D.; Tsironi, S.; Haidich, A.B.; Embeslidis, T.; Georgiadou, I.; Irkec, M.; Melamed, S. Twenty-four-hour intraocular pressure control with Bimatoprost and the Bimatoprost/timolol fixed combination administered in the morning, or evening in exfoliative glaucoma. Br. J. Ophthalmol. 2010, 94, 209–213. [Google Scholar] [CrossRef]
- Day, D.G.; Walters, T.R.; Schwartz, G.F.; Mundorf, T.K.; Liu, C.; Schiffman, R.M.; Bejanian, M. Bimatoprost 0.03% preservative-free ophthalmic solution versus Bimatoprost 0.03% ophthalmic solution (Lumigan) for glaucoma or ocular hypertension: A 12-week, randomized, double-masked trial. Br. J. Ophthalmol. 2013, 97, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.; Gil Pina, R.; Lanzagorta-Aresti, A.; Schiffman, R.M.; Liu, C.; Bejanian, M. Bimatoprost 0.03%/timolol 0.5% preservative-free ophthalmic solution versus Bimatoprost 0.03%/timolol 0.5% ophthalmic solution (Ganfort) for glaucoma or ocular hypertension: A 12-week randomized controlled trial. Br. J. Ophthalmol. 2014, 98, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Ogundele, A.B.; Jasek, M.C. Aqueous humor penetration of topical Bimatoprost 0.01% and Bimatoprost 0.03% in rabbits. Clin. Ophthalmol. 2010, 4, 1447–1450. [Google Scholar] [CrossRef] [PubMed]
- Chang-Lin, J.E.; Batoosingh, A.L.; Hollander, D.A.; Schiffman, R.M.; Tang-Liu, D.D. Aqueous humor penetration of topical Bimatoprost 0.01% and Bimatoprost 0.03% in rabbits: Response to authors. Clin. Ophthalmol. 2011, 5, 1119–1120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pfennigsdorf, S.; Ramez, O.; von Kistowski, G.; Mader, B.; Eschstruth, P.; Frobose, M.; Thelen, U.; Spraul, C.; Schnober, D.; Cooper, H.; et al. Multicenter, prospective, open-label, observational study of Bimatoprost 0.01% in patients with primary open-angle glaucoma or ocular hypertension. Clin. Ophthalmol. 2012, 6, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, M.; Campagna, G.; Ciampa, N.; Fioretto, G.; Giannini, R.; Marino, P.F.; dell’Omo, R.; Costagliola, C. Ocular Tolerability of Bimatoprost 0.1 mg/mL Preservative-Free versus Bimatoprost 0.1 mg/mL with Benzalkonium Chloride or Bimatoprost 0.3 mg/mL Preservative-Free in Patients with Primary Open-Angle Glaucoma. J. Clin. Med. 2022, 11, 3518. [Google Scholar] [CrossRef]
- Petricca, S.; Celenza, G.; Costagliola, C.; Tranfa, F.; Iorio, R. Cytotoxicity, Mitochondrial Functionality, and Redox Status of Human Conjunctival Cells after Short and Chronic Exposure to Preservative-Free Bimatoprost 0.03% and 0.01%: An In Vitro Comparative Study. Int. J. Mol. Sci. 2022, 23, 14113. [Google Scholar] [CrossRef] [PubMed]
- Nixon, D.R. A randomized, prospective study of Bimatoprost 0.01% or travoprost/timolol in patients previously treated with Latanoprost and timolol to reduce intraocular pressure. J. Ocul. Pharmacol. Ther. 2013, 29, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.J.; Cohen, J.S.; Batoosingh, A.L.; Felix, C.; Shu, V.; Schiffman, R.M. Twelve-month, randomized, con-trolled trial of Bimatoprost 0.01%, 0.0125%, and 0.03% in patients with glaucoma or ocular hypertension. Am. J. Ophthalmol. 2010, 149, 661–671 e1. [Google Scholar] [CrossRef]
- Figus, M.; Nardi, M.; Piaggi, P.; Sartini, M.; Guidi, G.; Martini, L.; Lazzeri, S. Bimatoprost 0.01% vs. Bimatoprost 0.03%: A 12-month prospective trial of clinical and in vivo confocal microscopy in glaucoma patients. Eye 2014, 28, 422–429. [Google Scholar] [CrossRef]
- Xu, K.M.; Cho, R.; Chan, T.Y.B. Retrospective Analysis of Switching Bimatoprost 0.01% to Bimatoprost 0.03% in Pa-tients with Various Types of Glaucoma and Ocular Hypertension. Clin. Ophthalmol. 2022, 16, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.D.; Tafreshi, A.; Weinreb, R.N.; Slight, J.R.; Medeiros, F.A.; Liu, J.H. Twenty-four-hour effects of Bima-toprost 0.01% monotherapy on intraocular pressure and ocular perfusion pressure. BMJ Open 2012, 2, e001106. [Google Scholar] [CrossRef] [PubMed]
- Alany, R.G. Adherence, persistence and cost-consequence comparison of Bimatoprost topical ocular formulations. Curr. Med. Res. Opin. 2013, 29, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Hollo, G.; Thelen, U.; Teus, M.A.; Quaranta, L.; Ferkova, S.; Babic, N.; Misiuk-Hojlo, M.; Mikropoulos, D.G.; Kaluzny, B.J.; Kozobolis, V.; et al. Long-term outcomes of prostaglandin analog versus timolol maleate in ocular hypertensive or primary open-angle glaucoma patients in Europe. J. Ocul. Pharmacol. Ther. 2011, 27, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Giannico, A.T.; Lima, L.; Shaw, G.C.; Russ, H.H.; Froes, T.R.; Montiani-Ferreira, F. Effects of prostaglandin analogs on blood flow velocity and resistance in the ophthalmic artery of rabbits. Arq. Bras. Oftalmol. 2016, 79, 33–36. [Google Scholar] [CrossRef][Green Version]
- Natt, N.K.; Gupta, A.; Singh, G.; Singh, T. A pharmacoeconomic analysis to determine the relative cost-effectiveness of Bimatoprost 0.03% eye drops and brimonidine 0.2% eye drops in patients of primary open-angle glaucoma/ocular hypertension. Indian J. Ophthalmol. 2014, 62, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Artunay, O.; Yuzbasioglu, E.; Unal, M.; Rasier, R.; Sengul, A.; Bahcecioglu, H. Bimatoprost 0.03% versus brimonidine 0.2% in the prevention of intraocular pressure spike following neodymium:yttrium-aluminum-garnet la-ser posterior capsulotomy. J. Ocul. Pharmacol. Ther. 2010, 26, 513–517. [Google Scholar] [CrossRef]
- Stalmans, I.; Oddone, F.; Cordeiro, M.F.; Hommer, A.; Montesano, G.; Ribeiro, L.; Sunaric-Megevand, G.; Rossetti, L. Comparison of preservative-free Latanoprost and preservative-free Bimatoprost in a multicenter, random-ized, investigator-masked cross-over clinical trial, the SPORT trial. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Orme, M.; Collins, S.; Dakin, H.; Kelly, S.; Loftus, J. Mixed treatment comparison and meta-regression of the effi-cacy and safety of prostaglandin analogues and comparators for primary open-angle glaucoma and ocular hypertension. Curr. Med. Res. Opin. 2010, 26, 511–528. [Google Scholar] [CrossRef]
- Gimenez-Gomez, R.; Garcia-Catalan, M.R.; Gallardo-Galera, J.M. Tear clearance and ocular symptoms in patients treated with preservative-free prostaglandins. Arch. Soc. Esp. Oftalmol. 2013, 88, 88–91. [Google Scholar] [CrossRef]
- Hommer, A.; Kimmich, F. Switching patients from preserved prostaglandin-analog monotherapy to preservative-free tafluprost. Clin. Ophthalmol. 2011, 5, 623–631. [Google Scholar] [PubMed]
- Ranno, S.; Sacchi, M.; Brancato, C.; Gilardi, D.; Lembo, A.; Nucci, P. A prospective study evaluating IOP changes after switching from a therapy with prostaglandin eye drops containing preservatives to non-preserved tafluprost in glaucoma patients. Sci. World J. 2012, 2012, 804730. [Google Scholar] [CrossRef] [PubMed]
- Kara, C.; Sen, E.M.; Elgin, K.U.; Serdar, K.; Yilmazbas, P. Does the intraocular pressure-lowering effect of prostaglandin analogues continue over the long term? Int. Ophthalmol. 2017, 37, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.H.; Schwartz, G.F.; LaBounty, B.; Kowalski, J.W.; Patel, V.D. Patient adherence and persistence with topical ocular hypotensive therapy in real-world practice: A comparison of Bimatoprost 0.01% and travoprost Z 0.004% ophthalmic solutions. Clin. Ophthalmol. 2014, 8, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.S.; Vold, S.; Zaman, F.; Williams, J.M.; Hollander, D.A. Bimatoprost 0.01% or 0.03% in patients with glaucoma or ocular hypertension previously treated with Latanoprost: Two randomized 12-week trials. Clin. Ophthalmol. 2014, 8, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, F.; Liu, K.; Duan, X. Efficacy and safety of prostaglandin analogues in primary open-angle glaucoma or ocular hypertension patients: A meta-analysis. Medicine 2019, 98, e16597. [Google Scholar] [CrossRef]
- Turan-Vural, E.; Torun-Acar, B.; Acar, S. Effect of ketorolac add-on treatment on intraocular pressure in glaucoma patients receiving prostaglandin analogues. Ophthalmologica 2012, 227, 205–209. [Google Scholar] [CrossRef]
- Ozyol, P.; Ozyol, E.; Erdogan, B.D. The Interaction of Nepafenac and Prostaglandin Analogs in Primary Open-angle Glaucoma Patients. J. Glaucoma 2016, 25, e145-9. [Google Scholar] [CrossRef] [PubMed]
- Faridi, U.A.; Saleh, T.A.; Ewings, P.; Venkateswaran, M.; Cadman, D.H.; Samarasinghe, R.A.; Vodden, J.; Claridge, K.G. Comparative study of three prostaglandin analogues in the treatment of newly diagnosed cases of ocular hypertension, open-angle and normal tension glaucoma. Clin. Exp. Ophthalmol. 2010, 38, 678–682. [Google Scholar] [CrossRef]
- Brennan, N.; Dehabadi, M.H.; Nair, S.; Quartilho, A.; Bunce, C.; Reekie, I.; Obikpo, R. Efficacy and safety of Bimatoprost in glaucoma and ocular hypertension in non-responder patients. Int. J. Ophthalmol. 2017, 10, 1251–1254. [Google Scholar]
- Zhou, L.; Zhan, W.; Wei, X. Clinical pharmacology and pharmacogenetics of prostaglandin analogues in glaucoma. Front. Pharmacol. 2022, 13, 1015338. [Google Scholar] [CrossRef] [PubMed]
- Priluck, A.Z.; Havens, S.J. Variation in Prostaglandin Analog Prices Paid for Through Medicare Part D. J. Glaucoma 2019, 28, e17–e20. [Google Scholar] [CrossRef] [PubMed]
- Lazcano-Gomez, G.; Hernandez-Oteyza, A.; Iriarte-Barbosa, M.J.; Hernandez-Garciadiego, C. Topical glaucoma therapy cost in Mexico. Int. Ophthalmol. 2014, 34, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, V.L.; Liu, P.; Dhruva, S.S.; Shah, N.D.; Bollinger, K.E.; Ross, J.S. Prostaglandin Coverage and Costs to Medicare and Medicare Beneficiaries, 2009–2017. J. Manag. Care Spec. Pharm. 2020, 26, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Kammer, J.A.; Katzman, B.; Ackerman, S.L.; Hollander, D.A. Efficacy and tolerability of Bimatoprost versus travoprost in patients previously on Latanoprost: A 3-month, randomised, masked-evaluator, multicentre study. Br. J. Ophthalmol. 2010, 94, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Kausar, N.; Thapa, K. Comparative study of Latanoprost (0.005%) and Bimatoprost (0.03%) in primary open angle glaucoma. Nepal. J. Ophthalmol. 2022, 14, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhao, Y.J.; Chew, P.T.; Sng, C.C.; Wong, H.T.; Yip, L.W.; Wu, T.S.; Bautista, D.; Teng, M.; Khoo, A.L.; et al. Comparative efficacy and tolerability of topical prostaglandin analogues for primary open-angle glaucoma and ocular hypertension. Ann. Pharmacother. 2014, 48, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Brown, M.M. Patient Preference-Based Comparative Effectiveness and Cost-Utility Analysis of the Prostamides for Open-Angle Glaucoma. J. Ocul. Pharmacol. Ther. 2019, 35, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Calugaru, D.; Calugaru, M. Monotherapy with lipid structural derivatives in glaucoma. Oftalmologia 2010, 54, 30–43. [Google Scholar]
- Mishra, D.; Sinha, B.P.; Kumar, M.S. Comparing the efficacy of Latanoprost (0.005%), Bimatoprost (0.03%), travoprost (0.004%), and timolol (0.5%) in the treatment of primary open angle glaucoma. Korean J. Ophthalmol. 2014, 28, 399–407. [Google Scholar] [CrossRef]
- Kook, M.S.; Simonyi, S.; Sohn, Y.H.; Kim, C.Y.; Park, K.H. Bimatoprost 0.01% for previously treated patients with open-angle glaucoma or ocular hypertension in the Korean clinical setting. Jpn. J. Ophthalmol. 2015, 59, 325–334. [Google Scholar] [CrossRef]
- Solish, A.M.; James, F.; Walt, J.G.; Chiang, T.H. Paired-eye comparison of medical therapies for glaucoma. Clin. Ophthalmol. 2010, 4, 1131–1135. [Google Scholar] [CrossRef]
- Macky, T.A. Bimatoprost versus travoprost in an Egyptian population: A hospital-based prospective, randomized study. J. Ocul. Pharmacol. Ther. 2010, 26, 605–610. [Google Scholar] [CrossRef]
- Inoue, K.; Setogawa, A.; Tomita, G. Nonresponders to Prostaglandin Analogs Among Normal-Tension Glaucoma Patients. J. Ocul. Pharmacol. Ther. 2016, 32, 90–96. [Google Scholar] [CrossRef]
- Sato, S.; Hirooka, K.; Baba, T.; Mizote, M.; Fujimura, T.; Tenkumo, K.; Ueda, H.; Shiraga, F. Efficacy and safety of switching from topical Latanoprost to Bimatoprost in patients with normal-tension glaucoma. J. Ocul. Pharmacol. Ther. 2011, 27, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Sawada, A.; Yamamoto, T. Switching efficacy on intraocular pressure from Latanoprost to Bimatoprost in eyes with open angle glaucoma: Implication to the changes of central corneal thickness. Jpn. J. Ophthalmol. 2014, 58, 423–428. [Google Scholar] [CrossRef]
- Germano, R.A.; Susanna, R., Jr.; De Moraes, C.G.; Susanna, B.N.; Susanna, C.N.; Chibana, M.N. Effect of Switching from Latanoprost to Bimatoprost in Primary Open-Angle Glaucoma Patients Who Experienced Intraocular Pressure Elevation During Treatment. J. Glaucoma 2016, 25, e359–e366. [Google Scholar] [CrossRef] [PubMed]
- El Hajj Moussa, W.G.; Farhat, R.G.; Nehme, J.C.; Sahyoun, M.A.; Schakal, A.R.; Jalkh, A.E.; Abi Karam, M.P.; Azar, G.G. Comparison of Efficacy and Ocular Surface Disease Index Score between Bimatoprost, Latanoprost, Travoprost, and Tafluprost in Glaucoma Patients. J. Ophthalmol. 2018, 2018, 1319628. [Google Scholar] [CrossRef] [PubMed]
- Whitson, J.T.; Trattler, W.B.; Matossian, C.; Williams, J.; Hollander, D.A. Ocular surface tolerability of prosta-glandin analogs in patients with glaucoma or ocular hypertension. J. Ocul. Pharmacol. Ther. 2010, 26, 287–292. [Google Scholar] [CrossRef]
- Craven, E.R.; Liu, C.C.; Batoosingh, A.; Schiffman, R.M.; Whitcup, S.M. A randomized, controlled comparison of macroscopic conjunctival hyperemia in patients treated with Bimatoprost 0.01% or vehicle who were previously con-trolled on Latanoprost. Clin. Ophthalmol. 2010, 4, 1433–1440. [Google Scholar] [CrossRef]
- Demirel, S.; Doganay, S.; Gurses, I.; Iraz, M. Toxic-inflammatory effects of prostaglandin analogs on the ocular surface. Ocul. Immunol. Inflamm. 2013, 21, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Crichton, A.C.; Vold, S.; Williams, J.M.; Hollander, D.A. Ocular surface tolerability of prostaglandin analogs and prostamides in patients with glaucoma or ocular hypertension. Adv. Ther. 2013, 30, 260–270. [Google Scholar] [CrossRef]
- Pellinen, P.; Huhtala, A.; Tolonen, A.; Lokkila, J.; Maenpaa, J.; Uusitalo, H. The cytotoxic effects of preserved and preservative-free prostaglandin analogs on human corneal and conjunctival epithelium in vitro and the distribution of benzalkonium chloride homologs in ocular surface tissues in vivo. Curr. Eye Res. 2012, 37, 145–154. [Google Scholar] [CrossRef]
- Aihara, M.; Shirato, S.; Sakata, R. Incidence of deepening of the upper eyelid sulcus after switching from Latanoprost to Bimatoprost. Jpn. J. Ophthalmol. 2011, 55, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Shiokawa, M.; Wakakura, M.; Tomita, G. Deepening of the upper eyelid sulcus caused by 5 types of prostaglandin analogs. J. Glaucoma 2013, 22, 626–631. [Google Scholar] [CrossRef]
- Sakata, R.; Shirato, S.; Miyata, K.; Aihara, M. Recovery from deepening of the upper eyelid sulcus after switching from Bimatoprost to Latanoprost. Jpn. J. Ophthalmol. 2013, 57, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; McCluskey, P. Clinical utility and differential effects of prostaglandin analogs in the management of raised intraocular pressure and ocular hypertension. Clin. Ophthalmol. 2010, 4, 741–764. [Google Scholar]
- Yanagi, M.; Kiuchi, Y.; Yuasa, Y.; Yoneda, T.; Sumi, T.; Hoshikawa, Y.; Kobayashi, M.; Fukushima, A. Association between glaucoma eye drops and hyperemia. Jpn. J. Ophthalmol. 2016, 60, 72–77. [Google Scholar] [CrossRef]
- Guedes, R.A.; Guedes, V.M.; Freitas, S.M.; Chaoubah, A. Quality of life of glaucoma patients under medical therapy with different prostaglandins. Clin. Ophthalmol. 2012, 6, 1749–1753. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Bernabei, F.; Senni, C.; Aloi, M.; Scalzo, G.C.; Ceravolo, D.; Iovino, C.; Scorcia, V. Comparative analysis of ocular redness score evaluated automatically in glaucoma patients under different topical medications. Eur. J. Ophthalmol. 2021, 31, 2405–2411. [Google Scholar] [CrossRef]
- Schmier, J.K.; Lau, E.C.; Covert, D.W. Two-year treatment patterns and costs in glaucoma patients initiating treat-ment with prostaglandin analogs. Clin. Ophthalmol. 2010, 4, 1137–1143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schmier, J.K.; Covert, D.W.; Robin, A.L. First-year treatment costs among new initiators of topical prostaglandin analog identified from November 2007 through April 2008. Curr. Med. Res. Opin. 2010, 26, 2769–2777. [Google Scholar] [CrossRef] [PubMed]
- Alm, A. Latanoprost in the treatment of glaucoma. Clin. Ophthalmol. 2014, 8, 1967–1985. [Google Scholar]
- Arias, A.; Schargel, K.; Ussa, F.; Canut, M.I.; Robles, A.Y.; Sanchez, B.M. Patient persistence with first-line an-tiglaucomatous monotherapy. Clin. Ophthalmol. 2010, 4, 261–267. [Google Scholar]
- Hahn, S.R.; Kotak, S.; Tan, J.; Kim, E. Physicians’ treatment decisions, patient persistence, and interruptions in the continuous use of prostaglandin therapy in glaucoma. Curr. Med. Res. Opin. 2010, 26, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Schmier, J.K.; Covert, D.W. First-year treatment costs among new initiators of topical prostaglandin analogs: Pooled results. Clin. Ophthalmol. 2010, 4, 437–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berenson, K.L.; Kymes, S.; Hollander, D.A.; Fiscella, R.; Burk, C.; Patel, V.D. Cost-offset analysis: Bimatoprost versus other prostaglandin analogues in open-angle glaucoma. Am. J. Manag. Care 2011, 17, e365-74. [Google Scholar] [PubMed]
- Islam, S.; Spry, C. Prostaglandin Analogues for Ophthalmic Use: A Review of Comparative Clinical Effectiveness, Cost-Effectiveness, and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020. [Google Scholar]
- Birt, C.M.; Buys, Y.M.; Kiss, A.; Trope, G.E.; Toronto Area Glaucoma, S. The influence of central corneal thickness on response to topical prostaglandin analogue therapy. Can. J. Ophthalmol. 2012, 47, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Ichhpujani, P.; Katz, L.J.; Hollo, G.; Shields, C.L.; Shields, J.A.; Marr, B.; Eagle, R.; Alvim, H.; Wizov, S.S.; Acheampong, A.; et al. Comparison of human ocular distribution of Bimatoprost and Latanoprost. J. Ocul. Pharmacol. Ther. 2012, 28, 134–145. [Google Scholar] [CrossRef]
- Stevens, A.; Iliev, M.E.; de Jong, L.; Grobeiu, I.; Hommer, A. A combined analysis of four observational studies evaluating the intraocular pressure-lowering ability and tolerability of Bimatoprost 0.01% in patients with primary open-angle glaucoma or ocular hypertension. Clin. Ophthalmol. 2016, 10, 635–641. [Google Scholar] [CrossRef]
- Kawaguchi, I.; Higashide, T.; Ohkubo, S.; Kawaguchi, C.; Sugiyama, K. Comparison of efficacy of four prosta-glandin analogues by bilateral treatment in healthy subjects. Jpn. J. Ophthalmol. 2012, 56, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, S.; Rossetti, L.; Oddone, F.; Sunaric-Megevand, G.; Hommer, A.; Vandewalle, E.; Francesca Cor-deiro, M.; McNaught, A.; Montesano, G.; Stalmans, I. Comparison of preserved Bimatoprost 0.01% with preserva-tive-free tafluprost: A randomised, investigator-masked, 3-month crossover, multicentre trial, SPORT II. Eur. J. Ophthalmol. 2021, 32, 11206721211006573. [Google Scholar]
- Suzuki, K.; Teranishi, S.; Sagara, T.; Yoshino, H.; Nakayama, M.; Enoki, M.; Nuno, Y.; Hirano, S.; Wakuta, M.; Takahashi, N.; et al. Safety and Efficacy of Benzalkonium Chloride-optimized Tafluprost in Japanese Glaucoma Patients with Existing Superficial Punctate Keratitis. J. Glaucoma 2015, 24, e145-50. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.V.; Gupta, P.K.; Vudathala, D.K.; Blair, I.A.; Tanna, A.P. Thermal stability of Bimatoprost, Latanoprost, and travoprost under simulated daily use. J. Ocul. Pharmacol. Ther. 2011, 27, 51–59. [Google Scholar] [CrossRef] [PubMed]
- DuBiner, H.B.; Hubatsch, D.A. Late-day intraocular pressure-lowering efficacy and tolerability of travoprost 0.004% versus Bimatoprost 0.01% in patients with open-angle glaucoma or ocular hypertension: A randomized trial. BMC Ophthalmol. 2014, 14, 151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Birt, C.M.; Buys, Y.M.; Ahmed, I.I.; Trope, G.E.; The Toronto Area Glaucoma Society. Prostaglandin efficacy and safety study undertaken by race (the PRESSURE study). J. Glaucoma 2010, 19, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Shiokawa, M.; Higa, R.; Sugahara, M.; Soga, T.; Wakakura, M.; Tomita, G. Adverse periocular reac-tions to five types of prostaglandin analogs. Eye 2012, 26, 1465–1472. [Google Scholar] [CrossRef]
- Kucukevcilioglu, M.; Bayer, A.; Uysal, Y.; Altinsoy, H.I. Prostaglandin associated periorbitopathy in patients using Bimatoprost, Latanoprost and travoprost. Clin. Exp. Ophthalmol. 2014, 42, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Hikage, F.; Ida, Y.; Ohguro, H. Prostaglandin F2alpha Agonists Negatively Modulate the Size of 3D Organ-oids from Primary Human Orbital Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef]
- Patil, A.J.; Vajaranant, T.S.; Edward, D.P. Bimatoprost—A review. Expert Opin. Pharmacother. 2009, 10, 2759–2768. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, J.E.; Lee, J.W.; Park, H.J.; Lee, J.E.; Jung, J.H. In vitro study of antiadipogenic profile of Lat-anoprost, travoprost, Bimatoprost, and tafluprost in human orbital preadiopocytes. J. Ocul. Pharmacol. Ther. 2012, 28, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Jamison, A.; Okafor, L.; Ullrich, K.; Schiedler, V.; Malhotra, R. Do Prostaglandin Analogue Lash Lengtheners Cause Eyelid Fat and Volume Loss? Aesthet. Surg. J. 2022, 42, 1241–1249. [Google Scholar] [CrossRef]
- Nakakura, S.; Terao, E.; Fujisawa, Y.; Tabuchi, H.; Kiuchi, Y. Changes in Prostaglandin-associated Periorbital Syndrome After Switch from Conventional Prostaglandin F2alpha Treatment to Omidenepag Isopropyl in 11 Consecutive Patients. J. Glaucoma 2020, 29, 326–328. [Google Scholar] [CrossRef] [PubMed]
- El-Khamery, A.A.; Mohamed, A.I.; Swify, H.E.; Mohamed, A.I. Cost-effectiveness of glaucoma management with monotherapy medications in Egypt. J. Adv. Pharm. Technol. Res. 2017, 8, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lindsley, K.; Rouse, B.; Hong, H.; Shi, Q.; Friedman, D.S.; Wormald, R.; Dickersin, K. Comparative Effectiveness of First-Line Medications for Primary Open-Angle Glaucoma: A Systematic Review and Network Meta-analysis. Ophthalmology 2016, 123, 129–140. [Google Scholar] [CrossRef]
- Inoue, K.; Ishida, K.; Tomita, G.; Noma, H. A scoping review and network meta-analysis for efficacy and safety of glaucoma medication in Japanese patients. Jpn. J. Ophthalmol. 2020, 64, 103–113. [Google Scholar] [CrossRef]
- Priluck, A.Z.; Dietze, J. Ophthalmologist and Optometrist Glaucoma Prescribing Patterns Based on 2015 Medicare Part D Data. Ophthalmol. Glaucoma 2019, 2, 63–66. [Google Scholar] [CrossRef]
- Lazcano-Gomez, G.; Alvarez-Ascencio, D.; Haro-Zuno, C.; Turati-Acosta, M.; Garcia-Huerta, M.; Jimenez-Arroyo, J.; Castaneda-Diez, R.; Castillejos-Chevez, A.; Gonzalez-Salinas, R.; Dominguez-Duenas, F.; et al. Glaucoma Medication Preferences among Glaucoma Specialists in Mexico. J. Curr. Glaucoma Pract. 2017, 11, 97–100. [Google Scholar]
- Rennie, G.; Wilkinson, A.; White, A.; Ruospo, M.; Teixeira-Pinto, A.; Strippoli, G. Topical medical therapy and ocular perfusion pressure in open angle glaucoma: A systematic review and meta-analysis. Curr. Med. Res. Opin. 2019, 35, 1421–1431. [Google Scholar] [CrossRef]
- Stewart, W.C.; Konstas, A.G.; Kruft, B.; Mathis, H.M.; Stewart, J.A. Meta-analysis of 24-h intraocular pressure fluctuation studies and the efficacy of glaucoma medicines. J. Ocul. Pharmacol. Ther. 2010, 26, 175–180. [Google Scholar] [CrossRef]
- Huang, H.L.; Sun, X.H.; Xiao, M. Comparison of intraocular pressure reducing effects of three prostaglandin eye drops in open-angle glaucoma. Zhonghua Yan Ke Za Zhi 2011, 47, 109–113. [Google Scholar] [PubMed]
- Tamcelik, N.; Izgi, B.; Temel, A.; Yildirim, N.; Okka, M.; Ozcan, A.; Yuksel, N.; Elgin, U.; Altan, C.; Ozer, B. Prospective, non-interventional, multicenter study of the intraocular pressure-lowering effects of prostaglandin ana-log/prostamide-containing therapies in previously treated patients with open-angle glaucoma or ocular hypertension. Clin. Ophthalmol. 2017, 11, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, X.; Yu, M. Meta-analysis of randomized controlled trials comparing Latanoprost with other glaucoma medications in chronic angle-closure glaucoma. Eur. J. Ophthalmol. 2015, 25, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.P. Bimatoprost: A review of its use in open-angle glaucoma and ocular hypertension. Drugs Aging 2009, 26, 1049–1071. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lopez, A.; Paczka, J.A.; Jimenez-Roman, J.; Hartleben, C. Efficacy and tolerability of fixed-combination Bimatoprost/timolol versus fixed-combination dorzolamide/brimonidine/timolol in patients with primary open-angle glaucoma or ocular hypertension: A multicenter, prospective, crossover study. BMC Ophthalmol. 2014, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Brief, G.; Lammich, T.; Nagel, E.; Pfennigsdorf, S.; Spraul, C.W.; Ho, S. Fixed combination of Bimatoprost and timolol in patients with primary open-angle glaucoma or ocular hypertension with inadequate IOP adjustment. Clin. Ophthalmol. 2010, 4, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, A.; Misiuk-Hojlo, M.; Grabska-Liberek, I.; Romanowska-Dixon, B.; Wierzbowska, J.; Wasyluk, J.; Mulak, M.; Szuscik, I.; Sierdzinski, J.; Ehrlich, R.; et al. Intraocular pressure and ocular hemody-namics in patients with primary open-angle glaucoma treated with the combination of morning dosing of Bimatoprost and dorzolamide hydrochloride. Acta Ophthalmol. 2011, 89, e57–e63. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Wang, T.H.; Liu, C.; Wu, K.Y.; Chiu, S.L.; Simonyi, S.; Lu, D.W. Tolerability and efficacy of Bima-toprost 0.01% in patients with open-angle glaucoma or ocular hypertension evaluated in the Taiwanese clinical setting: The Asia Pacific Patterns from Early Access of Lumigan 0.01% (APPEAL Taiwan) study. BMC Ophthalmol. 2016, 16, 162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Selen, F.; Tekeli, O.; Yanik, O. Assessment of the Anterior Chamber Flare and Macular Thickness in Patients Treated with Topical Antiglaucomatous Drugs. J. Ocul. Pharmacol. Ther. 2017, 33, 170–175. [Google Scholar] [CrossRef]
- Xing, Y.; Zhu, L.; Zhang, K.; Huang, S. The efficacy of the fixed combination of Latanoprost and timolol versus other fixed combinations for primary open-angle glaucoma and ocular hypertension: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0229682. [Google Scholar] [CrossRef]
- Guven Yilmaz, S.; Degirmenci, C.; Karakoyun, Y.E.; Yusifov, E.; Ates, H. The efficacy and safety of Bimato-prost/timolol maleate, Latanoprost/timolol maleate, and travoprost/timolol maleate fixed combinations on 24-h IOP. Int. Ophthalmol. 2018, 38, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, L.; Sacchi, M.; Karabatsas, C.H.; Topouzis, F.; Vetrugno, M.; Centofanti, M.; Boehm, A.; Vorwerk, C.; Goldblum, D.; Fogagnolo, P. Comparison of the effects of Bimatoprost and a fixed combination of Latanoprost and timolol on 24-hour blood and ocular perfusion pressures: The results of a randomized trial. BMC Ophthalmol. 2015, 15, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, A.W.; Gan, L.Y.; Yao, X.; Zhou, J. Long-term assessment of prostaglandin analogs and timolol fixed combinations vs. prostaglandin analogs monotherapy. Int. J. Ophthalmol. 2016, 9, 750–756. [Google Scholar] [PubMed]
- Rotsos, T.G.; Kliafa, V.G.; Asher, K.J.; Papaconstantinou, D. Bimatoprost/timolol fixed combination (BTFC) in pa-tients with primary open angle glaucoma or ocular hypertension in Greece. Int. J. Ophthalmol. 2016, 9, 69–75. [Google Scholar] [PubMed]
- Choi, E.Y.; Johnson, N.A.; Stinnett, S.; Rosdahl, J.; Moya, F.; Herndon, L.W. The Effect of Bimatoprost Implant on Glaucoma Patients: An Observational Study. J. Glaucoma 2024. [Google Scholar] [CrossRef] [PubMed]
- Aptel, F.; Cucherat, M.; Denis, P. Efficacy and tolerability of prostaglandin-timolol fixed combinations: A meta-analysis of randomized clinical trials. Eur. J. Ophthalmol. 2012, 22, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, A.; Dastiridou, A.I.; Fanariotis, M.; Kotoula, M.; Tsironi, E.E. Bimatoprost and Bimatoprost/timolol fixed combination in patients with open-angle glaucoma and ocular hypertension. J. Ocul. Pharmacol. Ther. 2011, 27, 67–71. [Google Scholar] [CrossRef]
- Shim, S.H.; Kim, J.M.; Choi, C.Y.; Kim, C.Y. Diurnal intraocular pressure with Bimatoprost/timolol fixed combi-nation versus Latanoprost/timolol fixed combination in healthy subjects. Korean J. Ophthalmol. 2014, 28, 39–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, Y.; Ling, Z.; Sun, X. Fixed-combination treatments for intraocular hypertension in Chinese patients—Focus on Bimatoprost-timolol. Drug Des. Devel. Ther. 2015, 9, 2617–2625. [Google Scholar]
- Shin, D.H.; Feldman, R.M.; Sheu, W.P.; Fixed Combination Latanoprost/Timolol Study Group. Efficacy and safety of the fixed combinations Latanoprost/timolol versus dorzolamide/timolol in patients with elevated intraocular pressure. Ophthalmology 2004, 111, 276–282. [Google Scholar] [CrossRef]
- Calugaru, M.; Calugaru, D. The latest developments in glaucoma therapy using fixed combination products. Oftalmologia 2011, 55, 53–69. [Google Scholar] [PubMed]
- Ling, Z.; Zhang, M.; Hu, Y.; Yin, Z.; Xing, Y.; Fang, A.; Ye, J.; Chen, X.; Liu, D.; Wang, Y.; et al. Safety and efficacy of Bimatoprost/timolol fixed combination in Chinese patients with open-angle glaucoma or ocular hypertension. Chin. Med. J. 2014, 127, 905–910. [Google Scholar] [CrossRef]
- Mrukwa-Kominek, E.; Misiuk-Hojlo, M.; Csutak, A.; Stalmans, I.; Garhofer, G. A randomized clinical trial comparing three fixed combinations of Bimatoprost with timolol in patients with open-angle glaucoma or ocular hypertension. Curr. Med. Res. Opin. 2023, 39, 775–783. [Google Scholar] [CrossRef]
- Lequeu, I.; Theuwis, K.; Abegao Pinto, L.; Vandewalle, E.; Stalmans, I. Long term IOP lowering efficacy of Bimatoprost/timolol fixed combination: A 12 month prospective study. Bull. Soc. Belge. Ophtalmol. 2013, 322, 105–110. [Google Scholar]
- Konstas, A.G.; Haidich, A.B.; Rossetti, L.; Webers, C. Prostaglandin-timolol fixed combinations efficacy: Myth or reality? Eur. J. Ophthalmol. 2012, 22, 1–4. [Google Scholar] [CrossRef]
- Belfort, R., Jr.; Paula, J.S.; Lopes Silva, M.J.; Della Paolera, M.; Kim, T.; Chen, M.Y.; Goodkin, M.L. Fixed-combination Bimatoprost/Brimonidine/Timolol in Glaucoma: A Randomized, Masked, Controlled, Phase III Study Conducted in Brazil(☆). Clin. Ther. 2020, 42, 263–275. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Kaarniranta, K.; Lorenz, K.; Traverso, C.E.; Vuorinen, J.; Ropo, A. Changes in ocular signs and symptoms in patients switching from Bimatoprost-timolol to tafluprost-timolol eye drops: An open-label phase IV study. BMJ Open 2019, 9, e024129. [Google Scholar] [CrossRef]
- Nucci, C.; Varesi, C.; Martucci, A.; Cesareo, M.; Cedrone, C.; Mancino, R.; Cerulli, L. Efficacy of Timolol 0.1% Gel and a Prostaglandin Analog in an Unfixed Combination Compared to the Corresponding Fixed Combinations. Eur. J. Ophthalmol. 2013, 23, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, L.; Biagioli, E.; Riva, I.; Rulli, E.; Poli, D.; Katsanos, A.; Floriani, I. Prostaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: A systematic review and meta-analysis. J. Ocul. Pharmacol. Ther. 2013, 29, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Wang, H.; Zong, Y.; Cheng, J.W.; Wei, R.L. Efficacy and tolerability of prostaglandin-timolol fixed combinations: An updated systematic review and meta-analysis. Curr. Med. Res. Opin. 2015, 31, 1139–1147. [Google Scholar] [CrossRef]
- Maruyama, Y.; Ikeda, Y.; Mori, K.; Ueno, M.; Yoshikawa, H.; Kinoshita, S. Comparison between Bimatoprost and Latanoprost-timolol fixed combination for efficacy and safety after switching patients from Latanoprost. Clin. Ophthalmol. 2015, 9, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lin, M.; Duan, X.; Zhang, C.; Ming, J. Patient satisfaction with fixed-combination Bimatoprost/timolol ophthalmic solution: A survey study in patients with glaucoma in China. Patient Prefer. Adherence 2017, 11, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Mesci, C.; Aydin, N.; Erbil, H.H. Twenty-four-hour intraocular pressure control with Latanoprost-timolol-fixed combination versus Bimatoprost in patients who switched from timolol. J. Glaucoma 2011, 20, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Konstas, A.G.; Hollo, G.; Mikropoulos, D.G.; Haidich, A.B.; Dimopoulos, A.T.; Empeslidis, T.; Teus, M.A.; Ritch, R. 24-hour efficacy of the Bimatoprost-timolol fixed combination versus Latanoprost as first choice therapy in subjects with high-pressure exfoliation syndrome and glaucoma. Br. J. Ophthalmol. 2013, 97, 857–861. [Google Scholar] [CrossRef]
- Gutierrez-Diaz, E.; Silva Cotta, J.; Munoz-Negrete, F.J.; Gutierrez-Ortiz, C.; Morgan-Warren, R.J.; Maltman, J. Bimatoprost/timolol fixed combination versus Latanoprost in treatment-naive glaucoma patients at high risk of progression: A pilot study. Clin. Ophthalmol. 2014, 8, 725–732. [Google Scholar] [CrossRef]
- Lee, M.Y.; Teh, N.C.; Nur Zulekha, M.; Thayanithi, S.; Jelinar, M.N.; Rizal, A.M.; Arfah, W. The Effects of Fixed Combination of Bimatoprost-Timolol and Travoprost-Timolol on Intraocular Pressure in Patients with Primary Open-Angle Glaucoma or Ocular Hypertension, Previously on Non-fixed Combination of Latanoprost and Timolol. Asia Pac. J. Ophthalmol. 2012, 1, 208–212. [Google Scholar] [CrossRef]
- Hommer, A. Combination therapy in the medical treatment of glaucoma. Klin. Monbl. Augenheilkd. 2013, 230, 133–140. [Google Scholar] [PubMed]
- Lipatov, D.V.; Chistiakov, T.A.; Kuz’min, A.G.; Tolkacheva, A.A. Evaluation of the effectiveness of Ganfort treatment in patients with secondary neovascular glaucoma associated with diabetes mellitus. Vestn. Oftalmol. 2014, 130, 45–48. [Google Scholar]
- Cordeiro, M.F.; Goldberg, I.; Schiffman, R.; Bernstein, P.; Bejanian, M. Efficacy of a preservative-free formulation of fixed-combination Bimatoprost and timolol (Ganfort PF) in treatment-naive patients vs. previously treated patients. Clin. Ophthalmol. 2015, 9, 1605–1611. [Google Scholar] [CrossRef][Green Version]
- Takagi, Y.; Santo, K.; Hashimoto, M.; Fukuchi, T. Ocular hypotensive effects of prostaglandin analogs in Japanese patients with normal-tension glaucoma: A literature review. Clin. Ophthalmol. 2018, 12, 1837–1844. [Google Scholar] [CrossRef]
- Centofanti, M.; Oddone, F.; Gandolfi, S.; Hommer, A.; Boehm, A.; Tanga, L.; Sangermani, C.; Sportelli, V.; Haustein, M.; Manni, G.; et al. Comparison of Travoprost and Bimatoprost plus timolol fixed combinations in open-angle glaucoma patients previously treated with Latanoprost plus timolol fixed combination. Am. J. Ophthalmol. 2010, 150, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Macky, T.A. Bimatoprost/timolol versus travoprost/timolol fixed combinations in an Egyptian population: A hospi-tal-based prospective randomized study. J. Glaucoma 2014, 23, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Hartleben, C.; Parra, J.C.; Batoosingh, A.; Bernstein, P.; Goodkin, M. A Masked, Randomized, Phase 3 Comparison of Triple Fixed-Combination Bimatoprost/Brimonidine/Timolol versus Fixed-Combination Brimonidine/Timolol for Lowering Intraocular Pressure. J. Ophthalmol. 2017, 2017, 4586763. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.G.; Goodkin, M.L. Triple Fixed-Combination Bimatoprost/Brimonidine/Timolol in Glaucoma and Ocular Hypertension in India: A Multicenter, Open-Label, Phase 3 Study. Clin. Ophthalmol. 2022, 16, 3559–3569. [Google Scholar] [CrossRef] [PubMed]
- Susanna, B.N.; Susanna, C.N.; Susanna, F.N.; Mota, R.T.; Barbosa, G.C.S.; Lima, V.L.; Susanna, R., Jr. Intraocular Peak Pressure in Patients Under Treatment with Fixed Combination of Bimatoprost/Timolol/Brimonidine Once Daily Versus Twice Daily. J. Glaucoma 2022, 31, e96–e100. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.W.; Cheng, S.W.; Gao, L.D.; Lu, G.C.; Wei, R.L. Intraocular pressure-lowering effects of commonly used fixed-combination drugs with timolol: A systematic review and meta-analysis. PLoS ONE 2012, 7, e45079. [Google Scholar] [CrossRef]
- Shen, J.; Bejanian, M. Effect of preservative removal from fixed-combination Bimatoprost/timolol on intraocular pres-sure lowering: A potential timolol dose-response phenomenon. Clin. Ophthalmol. 2016, 10, 373–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, E.J.; Kim, Y.H.; Kang, S.H.; Lee, K.W.; Park, Y.J. In vitro effects of preservative-free and preserved prosta-glandin analogs on primary cultured human conjunctival fibroblast cells. Korean J. Ophthalmol. 2013, 27, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Schnober, D.; Hubatsch, D.A.; Scherzer, M.L. Efficacy and safety of fixed-combination travoprost 0.004%/timolol 0.5% in patients transitioning from Bimatoprost 0.03%/timolol 0.5% combination therapy. Clin. Ophthalmol. 2015, 9, 825–832. [Google Scholar] [CrossRef]
- Imasawa, M.; Tanabe, J.; Kashiwagi, F.; Kashiwagi, K. Efficacy and Safety of Switching Latanoprost Monotherapy to Bimatoprost Monotherapy or Combination of Brinzolamide and Latanoprost. Open Ophthalmol. J. 2016, 10, 94–102. [Google Scholar] [CrossRef][Green Version]
- Paranhos, A.; Mendonca, M.; Silva, M.J.; Giampani, J.; Torres, R.J.; Della Paolera, M.; Russ, H.; Lottenberg, C.L. Hyperemia reduction after administration of a fixed combination of Bimatoprost and timolol maleate to patients on prostaglandin or prostamide monotherapy. J. Ocul. Pharmacol. Ther. 2010, 26, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Scherzer, M.L.; Liehneova, I.; Negrete, F.J.; Schnober, D. Travoprost 0.004%/timolol 0.5% fixed combination in patients transitioning from fixed or unfixed Bimatoprost 0.03%/timolol 0.5%. Adv. Ther. 2011, 28, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Jothi, R.; Ismail, A.M.; Senthamarai, R.; Pal, S. A comparative study on the efficacy, safety, and cost-effectiveness of Bimatoprost/timolol and dorzolamide/timolol combinations in glaucoma patients. Indian J. Pharmacol. 2010, 42, 362–365. [Google Scholar] [PubMed]
- Soto, J.; Diaz, S. Does the fixed combination of Bimatoprost/timolol really produce a better benefit/risk balance than the fixed combination of Latanoprost/timolol? Eur. J. Ophthalmol. 2010, 20, 246–247, author reply 247-8. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, M.; Specchia, C.; Williams, S.E.; Villani, E.; Nucci, P. Efficacy of Bimatoprost Plus Timolol Fixed Combina tion in Open Angle Glaucoma Patients Previously Treated with Dorzolamide Plus Timolol Fixed Combination. Curr. Eye Res. 2016, 41, 1433–1437. [Google Scholar] [CrossRef]
- Pfennigsdorf, S.; de Jong, L.; Makk, S.; Fournichot, Y.; Bron, A.; Morgan-Warren, R.J.; Maltman, J. A combined analysis of five observational studies evaluating the efficacy and tolerability of Bimatoprost/timolol fixed combination in patients with primary open-angle glaucoma or ocular hypertension. Clin. Ophthalmol. 2013, 7, 1219–1225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aptel, F.; Denis, P. Balancing efficacy and tolerability of prostaglandin analogues and prostaglandin-timolol fixed combinations in primary open-angle glaucoma. Curr. Med. Res. Opin. 2011, 27, 1949–1958. [Google Scholar] [CrossRef]
- Russ, H.H.; Nogueira-Filho, P.A.; Barros, J.N.; Faria, N.V.; Montiani-Ferreira, F.; Gomes, J.A.; Mello, P.A. Ocular surface evaluation in patients treated with a fixed combination of prostaglandin analogues with 0.5% timolol maleate topical monotherapy: A randomized clinical trial. Clinics 2013, 68, 1318–1324. [Google Scholar] [CrossRef]
- Day, D.G.; Sharpe, E.D.; Beischel, C.J.; Jenkins, J.N.; Stewart, J.A.; Stewart, W.C. Safety and efficacy of Bimato-prost 0.03% versus timolol maleate 0.5%/dorzolamide 2% fixed combination. Eur. J. Ophthalmol. 2005, 15, 336–342. [Google Scholar] [CrossRef]
- Lafuma, A.; Salmon, J.F.; Robert, J.; Berdeaux, G. Treatment persistence and cost-effectiveness of Latano-prost/Latanoprost-timolol, Bimatoprost/Bimatoprost-timolol, and travoprost/travoprost-timolol in glaucoma: An analysis based on the United Kingdom general practitioner research database. Clin. Ophthalmol. 2011, 5, 361–367. [Google Scholar]
- Xu, C.; Guo, R.; Huang, D.; Ji, J.; Liu, W. Daily Costs and Cost Effectiveness of Glaucoma Fixed Combinations in China. J. Ophthalmol. 2020, 2020, 2406783. [Google Scholar] [CrossRef] [PubMed]
- Kosakyan, S.M.; Robustova, O.V.; Bessmertny, A.M.; Kalinina, O.M.; Vasilenkova, L.V. Effectiveness and safety of drug combination therapy in patients with advanced primary open-angle glaucoma. Vestn. Oftalmol. 2020, 136, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Sumi, T.; Yoneda, T.; Fukuda, K.; Hoshikawa, Y.; Kobayashi, M.; Yanagi, M.; Kiuchi, Y.; Yasumitsu-Lovell, K.; Fukushima, A. Development of automated conjunctival hyperemia analysis software. Cornea 2013, 32 (Suppl. S1), S52-9. [Google Scholar] [CrossRef] [PubMed]
- Kymes, S.M.; Burk, C.; Feinman, T.; Williams, J.M.; Hollander, D.A. Demonstration of an online tool to assist managed care formulary evidence-based decision making: Meta-analysis of topical prostaglandin analog efficacy. Ther. Clin. Risk Manag. 2011, 7, 283–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, J.; Vu, J.T.; Hong, B.; Gottlieb, C. Uveitis and cystoid macular oedema secondary to topical prostaglandin analogue use in ocular hypertension and open angle glaucoma. Br. J. Ophthalmol. 2020, 104, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Holmstrom, S.; Buchholz, P.; Walt, J.; Wickstrom, J.; Aagren, M. Analytic review of bimatoprost, latanoprost and travoprost in primary open angle glaucoma. Curr. Med. Res. Opin. 2005, 21, 1875–1883. [Google Scholar] [CrossRef]
- Denis, P.; Lafuma, A.; Khoshnood, B.; Mimaud, V.; Berdeaux, G. A meta-analysis of topical prostaglandin analogues intra-ocular pressure lowering in glaucoma therapy. Curr. Med. Res. Opin. 2007, 23, 601–608. [Google Scholar] [CrossRef]
- Noecker, R.J.; Earl, M.L.; Mundorf, T.; Peace, J.; Williams, R.D. Bimatoprost 0.03% versus travoprost 0.004% in black Americans with glaucoma or ocular hypertension. Adv. Ther. 2003, 20, 121–128. [Google Scholar] [CrossRef]
- Noecker, R.S.; Dirks, M.S.; Choplin, N.T.; Bernstein, P.; Batoosingh, A.L.; Whitcup, S.M.; Bimatoprost/Latanoprost Study, G. A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am. J. Ophthalmol. 2003, 135, 55–63. [Google Scholar] [CrossRef]
- Parrish, R.K.; Palmberg, P.; Sheu, W.P.; Group, X.L.T.S. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: A 12-week, randomized, masked-evaluator multicenter study. Am. J. Ophthalmol. 2003, 135, 688–703. [Google Scholar] [CrossRef]
- Cantor, L.B.; WuDunn, D.; Cortes, A.; Hoop, J.; Knotts, S. Ocular hypotensive efficacy of bimatoprost 0.03% and travoprost 0.004% in patients with glaucoma or ocular hypertension. Surv. Ophthalmol. 2004, 49 (Suppl. S1), S12–S18. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Mori, C.; Koshiba, H.; Yuminaga, R.; Tanabe, K.; Ohtsu, F. Pregnancy Loss Signal from Prostaglandin Eye Drop Use in Pregnancy: A Disproportionality Analysis Using Japanese and US Spontaneous Reporting Databases. Drugs-Real World Outcomes 2022, 9, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Ishida, A.; Ichioka, S.; Takayanagi, Y.; Tsutsui, A.; Manabe, K.; Shirakami, T.; Sugihara, K.; Matsuo, M. Proposal of a simple grading system integrating cosmetic and tonometric aspects of prostaglandin-associated periorbitopathy. Medicine 2021, 100, e26874. [Google Scholar] [CrossRef]
- Hikage, F.; Ida, Y.; Ouchi, Y.; Watanabe, M.; Ohguro, H. Omidenepag, a Selective, Prostanoid EP2 Agonist, Does Not Suppress Adipogenesis in 3D Organoids of Human Orbital Fibroblasts. Transl. Vis. Sci. Technol. 2021, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Patradul, C.; Tantisevi, V.; Manassakorn, A. Factors Related to Prostaglandin-Associated Periorbitopathy in Glaucoma Patients. Asia Pac. J. Ophthalmol. 2017, 6, 238–242. [Google Scholar]
- Sarnoff, D.S.; Gotkin, R.H. Bimatoprost-induced chemical blepharoplasty. J. Drugs Dermatol. 2015, 14, 472–477. [Google Scholar]
- Ida, Y.; Watanabe, M.; Umetsu, A.; Ohguro, H.; Hikage, F. Addition of EP2 agonists to an FP agonist additively and synergistically modulates adipogenesis and the physical properties of 3D 3T3-L1 sphenoids. Prostaglandins Leukot. Essent. Fatty Acids 2021, 171, 102315. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Cao, M.; Liu, K.; Duan, X. Analysis of the Responsiveness of Latanoprost, Travoprost, Bimatoprost, and Tafluprost in the Treatment of OAG/OHT Patients. J. Ophthalmol. 2021, 2021, 5586719. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Robinson, M.R.; Weinreb, R.N. Episcleral Venous Pressure and the Ocular Hypotensive Effects of Topical and Intracameral Prostaglandin Analogs. J. Glaucoma 2019, 28, 846–857. [Google Scholar] [CrossRef]
- Kaliaperumal, S.; Govindaraj, I.; Kopparapu, P.K.; Ahuja, S. Hirsutism following the use of Bimatoprost eyedrops for glaucoma. J. Pharmacol. Pharmacother. 2014, 5, 208–210. [Google Scholar] [CrossRef]
- Sano, I.; Takahashi, H.; Inoda, S.; Sakamoto, S.; Arai, Y.; Takahashi, Y.; Ohkubo, A.; Kawashima, H.; Mayama, C. Shortening of Interpupillary Distance after Instillation of Topical Prostaglandin Analog Eye Drops. Am. J. Ophthalmol. 2019, 206, 11–16. [Google Scholar] [CrossRef]
- Hutchison, D.M.; Duffens, A.; Yale, K.; Park, A.; Cardenas, K.; Mesinkovska, N.A. Eyelash trichomegaly: A systematic review of acquired and congenital aetiologies of lengthened lashes. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 536–546. [Google Scholar] [CrossRef]
- Skorin, L., Jr.; Dailey, K.H. Clicking Eyelids: A New Finding of Prostaglandin-Associated Periorbitopathy. Optom. Vis. Sci. 2016, 93, 779–781. [Google Scholar] [CrossRef]
- Karslioglu, M.Z.; Hosal, M.B.; Tekeli, O. Periocular changes in topical Bimatoprost and Latanoprost use. Turk. J. Med. Sci. 2015, 45, 925–930. [Google Scholar] [CrossRef]
- Deveau, A.P.; da Silva, F.N.; Ly, T.Y.; Hussain, A. Periocular invasive melanoma manifestation in a patient using Bimatoprost: Case report and literature review. Orbit 2023, 42, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kent, T.L.; Custer, P.L. Structural and Histologic Eyelid Changes Associated with 6 Months of Topical Bimatoprost in the Rabbit. J. Glaucoma 2017, 26, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Noma, K.; Kakizaki, H. Bilateral upper eyelid retraction caused by topical Bimatoprost therapy. Ophthalmic Plast. Reconstr. Surg. 2012, 28, e33-5. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Choi, Y.J.; Lee, K.W.; Lee, M.J. Periorbital changes associated with prostaglandin analogs in Korean patients. BMC Ophthalmol. 2017, 17, 126. [Google Scholar] [CrossRef]
- Jbara, D.; Eiger-Moscovich, M.; Didkovsky, E.; Keshet, Y.; Avisar, I. In Vivo Effects of Prostaglandin Analogues Application by Topical Drops or Retrobulbar Injections on the Orbital Fat of a Rat Model. Ocul. Immunol. Inflamm. 2023, 31, 298–303. [Google Scholar] [CrossRef]
- Rabinowitz, M.P.; Katz, L.J.; Moster, M.R.; Myers, J.S.; Pro, M.J.; Spaeth, G.L.; Sharma, P.; Stefanyszyn, M.A. Unilateral Prostaglandin-Associated Periorbitopathy: A Syndrome Involving Upper Eyelid Retraction Distinguishable from the Aging Sunken Eyelid. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 373–378. [Google Scholar] [CrossRef]
- Higashiyama, T.; Minamikawa, T.; Kakinoki, M.; Sawada, O.; Ohji, M. Decreased orbital fat and enophthalmos due to Bimatoprost: Quantitative analysis using magnetic resonance imaging. PLoS ONE 2019, 14, e0214065. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, K.; Vagefi, M.R.; Lee, V.; Hui, J.Z.; Zhu, M.; Dine, K.; Anderson, R.L.; Koeberlein, B.; Sulaimankutty, R.; Shindler, K.S. In Vivo Effects of Retrobulbar Bimatoprost Injection on Orbital Fat. Ophthalmic Plast. Reconstr. Surg. 2018, 34, 201–204. [Google Scholar] [CrossRef]
- Taketani, Y.; Yamagishi, R.; Fujishiro, T.; Igarashi, M.; Sakata, R.; Aihara, M. Activation of the prostanoid FP receptor inhibits adipogenesis leading to deepening of the upper eyelid sulcus in prostaglandin-associated periorbitopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1269–1276. [Google Scholar] [CrossRef]
- Ida, Y.; Hikage, F.; Itoh, K.; Ida, H.; Ohguro, H. Prostaglandin F2alpha agonist-induced suppression of 3T3-L1 cell adipogenesis affects spatial formation of extra-cellular matrix. Sci. Rep. 2020, 10, 7958. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Berke, S. Latanoprost-induced prostaglandin-associated periorbitopathy. Optom. Vis. Sci. 2013, 90, e245–e247. [Google Scholar] [CrossRef]
- Wang, P.X.; Koh, V.T.; Cheng, J.F. Periorbital muscle atrophy associated with topical Bimatoprost therapy. Clin. Ophthalmol. 2014, 8, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Priluck, J.C.; Fu, S. Latisse-induced periocular skin hyperpigmentation. Arch. Ophthalmol. 2010, 128, 792–793. [Google Scholar] [CrossRef][Green Version]
- Lewis, R.A.; Gross, R.L.; Sall, K.N.; Schiffman, R.M.; Liu, C.C.; Batoosingh, A.L.; Ganfort Investigators, G., II. The safety and efficacy of Bimatoprost/timolol fixed combination: A 1-year double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J. Glaucoma 2010, 19, 424–426. [Google Scholar] [CrossRef]
- Park, K.H.; Simonyi, S.; Kim, C.Y.; Sohn, Y.H.; Kook, M.S. Bimatoprost 0.01% in treatment-naive patients with open-angle glaucoma or ocular hypertension: An observational study in the Korean clinical setting. BMC Ophthalmol. 2014, 14, 160. [Google Scholar] [CrossRef]
- Sun, X.; Yao, K.; Liu, Q.; Zhang, H.; Xing, X.; Fang, A.; Duan, X.; Yu, M.; Chen, M.Y.; Yang, J.; et al. Safety of Fixed-Combination Bimatoprost 0.03%/Timolol 0.5% Ophthalmic Solution at 6 Months in Chinese Patients with Open-Angle Glaucoma or Ocular Hypertension. Ophthalmol. Ther. 2023, 12, 341–353. [Google Scholar] [CrossRef]
- Aydin, S.; Isikligil, I.; Teksen, Y.A.; Kir, E. Recovery of orbital fat pad prolapsus and deepening of the lid sulcus from topical Bimatoprost therapy: 2 case reports and review of the literature. Cutan. Ocul. Toxicol. 2010, 29, 212–216. [Google Scholar] [CrossRef]
- Jayaprakasam, A.; Ghazi-Nouri, S. Periorbital fat atrophy—An unfamiliar side effect of prostaglandin analogues. Orbit 2010, 29, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, H.K.; Moon, J.I. Changes to upper eyelid orbital fat from use of topical Bimatoprost, travoprost, and Latanoprost. Jpn. J. Ophthalmol. 2011, 55, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Sahay, P.; Padhy, D.; Sarangi, S.; Suar, M.; Modak, R.; Rao, A. Tear biomarkers in Latanoprost and Bimatoprost treated eyes. PLoS ONE 2018, 13, e0201740. [Google Scholar] [CrossRef] [PubMed]
- Ulas, F.; Balbaba, M.; Celebi, S. Effect of prophylactic intraocular pressure-lowering medication on pain during cataract surgery. J. Ocul. Pharmacol. Ther. 2013, 29, 658–662. [Google Scholar] [CrossRef]
- Bafa, M.; Georgopoulos, G.; Mihas, C.; Stavrakas, P.; Papaconstantinou, D.; Vergados, I. The effect of prostaglandin analogues on central corneal thickness of patients with chronic open-angle glaucoma: A 2-year study on 129 eyes. Acta Ophthalmol. 2011, 89, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shen, X.; Yu, J.; Tan, H.; Cheng, Y. The comparison of the effects of Latanoprost, travoprost, and Bimatoprost on central corneal thickness. Cornea 2011, 30, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, N.; Celikay, O. Effects of topical prostaglandin therapy on corneal layers thickness in primary open-angle glaucoma patients using anterior segment optical coherence tomography. Int. Ophthalmol. 2023, 43, 3175–3184. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yim, H.W. Effect of Topical Prostaglandin Analogue Therapy on Central Corneal Thickness: A Systematic Review. J. Clin. Med. 2022, 12, 44. [Google Scholar] [CrossRef]
- Wu, J.H.; Wang, T.H.; Huang, J.Y.; Su, C.C. Ocular Surface Disease in Glaucoma Patients Randomized to Benzalkonium Chloride-Containing Latanoprost and Preservative-Free Bimatoprost. J. Ocul. Pharmacol. Ther. 2021, 37, 556–564. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Yu, A.; Wu, J.; Zhu, M.; Jiang, M.; Li, X.; Zhu, D.; Zhang, P.; Zheng, X.; et al. Effect of travoprost, Latanoprost and Bimatoprost PGF2alpha treatments on the biomechanical properties of in-vivo rabbit cornea. Exp. Eye Res. 2022, 215, 108920. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, N.; Ekici, E.; Celikay, O. The effect of topical Bimatoprost on corneal clarity in primary open-angle glaucoma: A longitudinal prospective assessment. Int. Ophthalmol. 2022, 42, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Matsuura, E.; Takahashi, M.; Nagano, T.; Kawazu, K. Effects of Anti-Glaucoma Prostaglandin Ophthalmic Solutions on Cultured Human Corneal Epithelial Cells. Curr. Eye Res. 2019, 44, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Pozarowska, D.; Pozarowski, P.; Darzynkiewicz, Z. Cytometric assessment of cytostatic and cytotoxic effects of topical glaucoma medications on human epithelial corneal line cells. Cytometry B Clin. Cytom. 2010, 78, 130–137. [Google Scholar] [CrossRef]
- Padhy, D.; Rao, A. Bimatoprost (0.03%)-induced accommodative spasm and pseudomyopia. BMJ Case Rep. 2015, 2015, bcr2015211820. [Google Scholar]
- Agange, N.; Mosaed, S. Prostaglandin-induced cystoid macular edema following routine cataract extraction. J. Ophthalmol. 2010, 2010, 690707. [Google Scholar] [CrossRef] [PubMed]
- Wendel, C.; Zakrzewski, H.; Carleton, B.; Etminan, M.; Mikelberg, F.S. Association of Postoperative Topical Prostaglandin Analog or Beta-Blocker Use and Incidence of Pseudophakic Cystoid Macular Edema. J. Glaucoma 2018, 27, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.H.; Badar, A.; Bakhsh, S.; Jusufbegovic, D. Bilateral Cystoid Macular Edema Following Bimatoprost Implants. Retin. Cases Brief. Rep. 2022, 18, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Nitoda, E.; Chatziralli, I.P.; Panos, G.D.; Demopoulos, C.A. Impact of prostaglandin glaucoma drops on platelet-activating factor action: An in vitro study. Drug Des. Devel Ther. 2016, 10, 3977–3981. [Google Scholar] [CrossRef] [PubMed]
- Addison, P.K.; Papadopoulos, M.; Nischal, K.K.; Hykin, P.G. Serous retinal detachment induced by topical Bimatoprost in a patient with Sturge-Weber syndrome. Eye 2011, 25, 124–125. [Google Scholar] [CrossRef][Green Version]
- Ogundele, A.B.; Li, G.; Ellis, J.J. Impact of topical Bimatoprost 0.01% and Bimatoprost 0.03% on conjunctival irritation in rabbits. Clin. Ophthalmol. 2010, 4, 77–80. [Google Scholar] [CrossRef][Green Version]
- Nakakura, S.; Noguchi, A.; Tabuchi, H.; Kiuchi, Y. Bimatoprost-induced late-onset choroidal detachment after trabeculectomy: A case report and review of the literature. Medicine 2017, 96, e5927. [Google Scholar] [CrossRef]
- Akyol, N.; Kalkisim, A.; Turk, A.; Kola, M.; Imamoglu, H.I. Evaluation of the effects on choroidal thickness of Bimatoprost 0.03% versus a brinzolamide 1.0%/timolol maleate 0.5% fixed combination. Cutan. Ocul. Toxicol. 2017, 36, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.A.; Kim, N.W.; Kang, S.S.; Kim, M.L.; Sung, K.R. In vitro Effects of Prostaglandin Analogs on Cultured Astrocytes Obtained from the Lamina Cribrosa. Curr. Eye Res. 2016, 41, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Ogundele, A.B.; Earnest, D.; McLaughlin, M.A. In vivo comparative study of ocular vasodilation, a relative indicator of hyperemia, in guinea pigs following treatment with Bimatoprost ophthalmic solutions 0.01% and 0.03%. Clin. Ophthalmol. 2010, 4, 649–652. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, H.; Pauly, A.; Riancho, L.; Baudouin, C.; Brignole-Baudouin, F. Toxicological evaluation of preservative-containing and preservative-free topical prostaglandin analogues on a three-dimensional-reconstituted corneal epithelium system. Br. J. Ophthalmol. 2011, 95, 869–875. [Google Scholar] [CrossRef]
- Barabino, S.; Antonelli, S.; Cimbolini, N.; Mauro, V.; Bouzin, M. The effect of preservatives and antiglaucoma treatments on the ocular surface of mice with dry eye. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6499–6504. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, E.J.; Kim, Y.H.; Kim, Y.I.; Lee, S.H.; Jung, J.C.; Lee, K.W.; Park, Y.J. In Vivo Effects of Preservative-free and Preserved Prostaglandin Analogs: Mouse Ocular Surface Study. Korean J. Ophthalmol. 2015, 29, 270–279. [Google Scholar] [CrossRef]
- Liang, H.; Baudouin, C.; Daull, P.; Garrigue, J.S.; Brignole-Baudouin, F. In Vitro Corneal and Conjunctival Wound-Healing Assays as a Tool for Antiglaucoma Prostaglandin Formulation Characterization. Front. Biosci. (Landmark Ed) 2022, 27, 147. [Google Scholar] [CrossRef] [PubMed]
- Nakakura, S.; Tabuchi, H.; Kiuchi, Y. Latanoprost therapy after sunken eyes caused by travoprost or Bimatoprost. Optom. Vis. Sci. 2011, 88, 1140–1144. [Google Scholar] [CrossRef]
- Altieri, M.; Ferrari, E. Do prostaglandin analogs affect eyelid position and motility? J. Ocul. Pharmacol. Ther. 2011, 27, 511–517. [Google Scholar] [CrossRef]
- Radcliffe, N.M. The impact of timolol maleate on the ocular tolerability of fixed-combination glaucoma therapies. Clin. Ophthalmol. 2014, 8, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Hikage, F.; Ichioka, H.; Watanabe, M.; Umetsu, A.; Ohguro, H.; Ida, Y. Addition of ROCK inhibitors to prostaglandin derivative (PG) synergistically affects adipogenesis of the 3D spheroids of human orbital fibroblasts (HOFs). Hum. Cell 2022, 35, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ida, Y.; Sato, T.; Umetsu, A.; Watanabe, M.; Furuhashi, M.; Hikage, F.; Ohguro, H. Addition of ROCK Inhibitors Alleviates Prostaglandin-Induced Inhibition of Adipogenesis in 3T3L-1 Spheroids. Bioengineering 2022, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Agnifili, L.; Mastropasqua, R.; Fasanella, V.; Brescia, L.; Scatena, B.; Oddone, F.; Mastropasqua, L. Meibomian Gland Features and Conjunctival Goblet Cell Density in Glaucomatous Patients Controlled with Prostaglandin/Timolol Fixed Combinations: A Case Control, Cross-sectional Study. J. Glaucoma 2018, 27, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Pfennigsdorf, S.; Eschstruth, P.; Hasemeyer, S.; Feuerhake, C.; Brief, G.; Grobeiu, I.; Shirlaw, A. Preservative-free Bimatoprost 0.03%/timolol 0.5% fixed combination in patients with glaucoma in clinical practice. Clin. Ophthalmol. 2016, 10, 1837–1846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pillunat, L.E.; Eschstruth, P.; Hasemeyer, S.; Thelen, U.; Foja, C.; Leaback, R.; Pfennigsdorf, S. Preservative-free Bimatoprost 0.03% in patients with primary open-angle glaucoma or ocular hypertension in clinical practice. Clin. Ophthalmol. 2016, 10, 1759–1765. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inoue, K.; Shiokawa, M.; Sugahara, M.; Higa, R.; Wakakura, M.; Tomita, G. Iris and periocular adverse reactions to Bimatoprost in Japanese patients with glaucoma or ocular hypertension. Clin. Ophthalmol. 2012, 6, 111–116. [Google Scholar] [CrossRef]
- Wirta, D.; Vandenburgh, A.M.; Weng, E.; Whitcup, S.M.; Kurstjens, S.; Beddingfield, F.C., 3rd. Long-term safety evaluation of Bimatoprost ophthalmic solution 0.03%: A pooled analysis of six double-masked, randomized, active-controlled clinical trials. Clin. Ophthalmol. 2011, 5, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, E.J.; Schuman, J.S.; Goldberg, I.; Gross, R.L.; VanDenburgh, A.M.; Chen, K.; Whitcup, S.M. One-year, randomized study comparing Bimatoprost and timolol in glaucoma and ocular hypertension. Arch. Ophthalmol. 2002, 120, 1286–1293. [Google Scholar] [CrossRef]
- de Faria, N.V.; Russ, H.H.; Rose, P.; Noronha, L.; Mello, P.A.; Montiani-Ferreira, F.; Sobrinho, S.C. Conjunctival changes and inflammatory aspects in rabbits’ conjunctivas induced by fixed combinations of prostaglandin analogues and timolol maleate. J. Ophthalmic Inflamm. Infect. 2013, 3, 22. [Google Scholar] [CrossRef][Green Version]
- Trzeciecka, A.; Paterno, J.J.; Toropainen, E.; Koskela, A.; Podracka, L.; Korhonen, E.; Kauppinen, A.; Kaarniranta, K.; Smedowski, A. Long-term topical application of preservative-free prostaglandin analogues evokes macrophage infiltration in the ocular adnexa. Eur. J. Pharmacol. 2016, 788, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Shibata, S.; Shibata, N.; Hagihara, K.; Yaguchi, H.; Osada, H.; Takahashi, N.; Kubo, E.; Sasaki, H. Safety comparison of additives in antiglaucoma prostaglandin (PG) analog ophthalmic formulations. Clin. Ophthalmol. 2013, 7, 515–520. [Google Scholar] [CrossRef][Green Version]
- Whitson, J.T.; Petroll, W.M. Corneal epithelial cell viability following exposure to ophthalmic solutions containing preservatives and/or antihypertensive agents. Adv. Ther. 2012, 29, 874–888. [Google Scholar]
- Liang, H.; Baudouin, C.; Labbe, A.; Riancho, L.; Brignole-Baudouin, F. Conjunctiva-associated lymphoid tissue (CALT) reactions to antiglaucoma prostaglandins with or without BAK-preservative in rabbit acute toxicity study. PLoS ONE 2012, 7, e33913. [Google Scholar] [CrossRef] [PubMed]
- Ayaki, M.; Iwasawa, A.; Niwano, Y. Cell viability score as an integrated indicator for cytotoxicity of benzalkonium chloride-containing antiglaucoma eyedrops. Biocontrol Sci. 2012, 17, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Seibold, L.K.; Ammar, D.A.; Kahook, M.Y. Acute effects of glaucoma medications and benzalkonium chloride on pre-adipocyte proliferation and adipocyte cytotoxicity in vitro. Curr. Eye Res. 2013, 38, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Yunoki, T.; Tojo, N.; Hayashi, A. Effectiveness of Blepharoptosis Surgery in Patients with Deepening of the Upper Eyelid Sulcus. J. Craniofac Surg. 2020, 31, 1284–1286. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Yoneda, M.; Gosho, M.; Kato, T.; Zako, M. Bimatoprost, Latanoprost, and tafluprost induce differential expression of matrix metalloproteinases and tissue inhibitor of metalloproteinases. BMC Ophthalmol. 2016, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Cheggour, M.; Gambrelle, J. Massive choroidal detachment secondary to topical use of Bimatoprost: Report of two cases. J. Fr. Ophtalmol. 2012, 35, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Turnbull, C.; Akhtar, M.M.; Fleet, J. Ganfort, a blinding drug to the physician. BMJ Case Rep. 2012, 2012, bcr0120125648. [Google Scholar] [CrossRef]
- Miki, T.; Naito, T.; Fujiwara, M.; Araki, R.; Kiyoi, R.; Shiode, Y.; Fujiwara, A.; Morizane, Y.; Shiraga, F. Effects of pre-surgical administration of prostaglandin analogs on the outcome of trabeculectomy. PLoS ONE 2017, 12, e0181550. [Google Scholar] [CrossRef]
- Rodriguez-Agramonte, F.; Jimenez, J.C.; Montes, J.R. Periorbital Changes associated with Topical Prostaglandins Analogues in a Hispanic Population. P. R. Health Sci. J. 2017, 36, 218–222. [Google Scholar] [PubMed]
- Subedi, L.; Pandey, P.; Shim, J.H.; Kim, K.T.; Cho, S.S.; Koo, K.T.; Kim, B.J.; Park, J.W. Preparation of topical Bimatoprost with enhanced skin infiltration and in vivo hair regrowth efficacy in androgenic alopecia. Drug Deliv. 2022, 29, 328–341. [Google Scholar] [CrossRef]
- Manabe, M.; Tsuboi, R.; Itami, S.; Osada, S.I.; Amoh, Y.; Ito, T.; Inui, S.; Ueki, R.; Ohyama, M.; Kurata, S.; et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version. J. Dermatol. 2018, 45, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Boubertakh, B.; Courtemanche, O.; Marsolais, D.; Di Marzo, V.; Silvestri, C. New role for the anandamide metabolite prostaglandin F(2alpha) ethanolamide: Rolling preadipocyte proliferation. J. Lipid Res. 2023, 64, 100444. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Evans, K.; North, R.V.; Jones, L.; Purslow, C. Impact of Eye Cosmetics on the Eye, Adnexa, and Ocular Surface. Eye Contact Lens 2016, 42, 211–220. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, A.; Pedrotti, E.; Stevan, G.; Scala, A.; Lambiase, A.; Morselli, S. Floppy eyelid syndrome and ectropion improvement after 1 month of 0.03% Bimatoprost topical therapy. Am. J. Ophthalmol. Case Rep. 2020, 20, 100938. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hao, Z.; Qi, W.; Wang, Z.; Zhou, M.; Guo, N. The efficacy of topical prostaglandin analogs for hair loss: A systematic review and meta-analysis. Front. Med. 2023, 10, 1130623. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-Garza, J.; Griggs, J.; Tosti, A. New drugs under investigation for the treatment of alopecias. Expert. Opin. Investig. Drugs 2019, 28, 275–284. [Google Scholar] [CrossRef]
- Barron-Hernandez, Y.L.; Tosti, A. Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia. Expert. Opin. Investig. Drugs 2017, 26, 515–522. [Google Scholar] [CrossRef]
- Alshahrani, Y.F.; Alghamdi, S.; Alkhathami, A.; Alshahrani, A.; Alshahrani, S.M.; Korkoman, A. Attitudes and Practices of Female University Students in Saudi Arabia Regarding the Cosmetic Use of Careprost (Bimatoprost) Eye Drops. Cureus 2024, 16, e56233. [Google Scholar] [CrossRef]
- Vergilis-Kalner, I.J. Application of Bimatoprost ophthalmic solution 0.03% for the treatment of eyebrow hypotrichosis: Series of ten cases. Dermatol. Online J. 2014, 20, 8. [Google Scholar] [CrossRef]
- Sarsik, S.M.; El-Amawy, H.S. Uses of eye drops in dermatology, literature review. J. Dermatolog. Treat. 2022, 33, 2758–2770. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, S.; Kamata, M.; Kasanuki, T.; Yokobori, M.; Takeoka, S.; Hayashi, K.; Tanaka, T.; Fukuyasu, A.; Ishikawa, T.; Ohnishi, T.; et al. Open-label pilot study to evaluate the effectiveness of topical Bimatoprost on rhododendrol-induced refractory leukoderma. J. Dermatol. 2018, 45, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.L.; Rivkin, A.; Dayan, S.; Shamban, A.; Werschler, W.P.; Teller, C.F.; Kaminer, M.S.; Sykes, J.M.; Weinkle, S.H.; Garcia, J.K. Multimodal Facial Aesthetic Treatment on the Appearance of Aging, Social Confidence, and Psychological Well-being: HARMONY Study. Aesthet. Surg. J. 2022, 42, NP115–NP124. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Chanasumon, N.; Sriphojanart, T. Efficacy and Safety of Bimatoprost 0.01% for the Treatment of Eyebrow Hypotrichosis: A Randomized, Double-Blind, Vehicle-Controlled Study. Dermatol. Surg. 2019, 45, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Steinsapir, K.D.; Steinsapir, S.M.G. Revisiting the Safety of Prostaglandin Analog Eyelash Growth Products. Dermatol. Surg. 2021, 47, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Zaher, H.; Gawdat, H.I.; Hegazy, R.A.; Hassan, M. Bimatoprost versus Mometasone Furoate in the Treatment of Scalp Alopecia Areata: A Pilot Study. Dermatology 2015, 230, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Levy, L.L.; Emer, J.J. Female pattern alopecia: Current perspectives. Int. J. Womens Health 2013, 5, 541–556. [Google Scholar]
- Wikramanayake, T.C.; Haberland, N.I.; Akhundlu, A.; Laboy Nieves, A.; Miteva, M. Prevention and Treatment of Chemotherapy-Induced Alopecia: What Is Available and What Is Coming? Curr. Oncol. 2023, 30, 3609–3626. [Google Scholar] [CrossRef]
- Rubio-Gonzalez, B.; Juhasz, M.; Fortman, J.; Mesinkovska, N.A. Pathogenesis and treatment options for chemotherapy-induced alopecia: A systematic review. Int. J. Dermatol. 2018, 57, 1417–1424. [Google Scholar] [CrossRef]
- Bhusal, M.; Bhattarai, S.; Thapa, B.; Shrestha, P.R.; Sagar, G.C. Bimatoprost versus Clobetasol Propionate in Scalp Alopecia Areata: A Prospective Non-Randomized Open-Label Clinical Trial. Indian. Dermatol. Online J. 2023, 14, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, T.; Fukumoto, R.; Magno, E.; Oka, M.; Nishigori, C.; Horita, N. Treatments for alopecia areata: A systematic review and network meta-analysis. Dermatol. Ther. 2021, 34, e14916. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Sarkar, R.; Udayan, U.K.; Roy, P.K.; Jha, A.K.; Chaudhary, R.K.P. Bimatoprost in Dermatology. Indian Dermatol. Online J. 2018, 9, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K. An effect of Bimatoprost: Adverse for some, therapeutic for others. Int. J. Trichol. 2011, 3, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.O.; Camacho Martinez, F.M. Bimatoprost in the treatment of eyelash universalis alopecia areata. Int. J. Trichol. 2010, 2, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Li, A.W.; Antaya, R.J. Successful Treatment of Pediatric Alopecia Areata of the Scalp Using Topical Bimatoprost. Pediatr. Dermatol. 2016, 33, e282–e283. [Google Scholar] [CrossRef] [PubMed]
- Anbar, T.S.; El-Ammawi, T.S.; Barakat, M.; Fawzy, A. Skin pigmentation after NB-UVB and three analogues of prostaglandin F(2alpha) in guinea pigs: A comparative study. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Yoelin, S.; Walt, J.G.; Earl, M. Safety, effectiveness, and subjective experience with topical Bimatoprost 0.03% for eyelash growth. Dermatol. Surg. 2010, 36, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Harii, K.; Arase, S.; Tsuboi, R.; Weng, E.; Daniels, S.; VanDenburgh, A. Bimatoprost for eyelash growth in Japanese subjects: Two multicenter controlled studies. Aesthetic Plast. Surg. 2014, 38, 451–460. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S. Eyebrow growth pattern analysis in patients with eyebrow hypotrichosis after receiving topical treatment: A retrospective study. J. Cosmet. Dermatol. 2020, 19, 1404–1408. [Google Scholar] [CrossRef]
- Banaszek, A. Company profits from side effects of glaucoma treatment. Can. Med. Assoc. J. 2011, 183, E1058. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.V.; Friedman, P.M.; Agron, S.; Konda, A.; Parker, C.; Geronemus, R.G. Enhanced Uptake and Retention of 0.03% Bimatoprost, 0.5% 5-Fluorouracil, and 5% Minoxidil After 1,550-nm or 1,927-nm Nonablative Laser Pretreatment. Dermatol. Surg. 2022, 48, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Waibel, J.S.; Rudnick, A.; Arheart, K.L.; Nagrani, N.; Gonzalez, A.; Gianatasio, C. Re-pigmentation of Hypopigmentation: Fractional Lasers vs. Laser-Assisted Delivery of Bimatoprost vs. Epidermal Melanocyte Harvesting System. J. Drugs Dermatol. 2019, 18, 1090–1096. [Google Scholar] [PubMed]
- Nguyen, N.; Helms, J.; Conza, A.; Petty, A.; Akanji, J.; Imahiyerobo-Ip, J. Microneedling with Bimatoprost to Treat Hypopigmented Skin Caused by Burn Scars. Cutis 2023, 111, E31–E33. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R. Laser-assisted drug delivery for the treatment of androgenetic alopecia: Ablative laser fractional photothermolysis to enhance cutaneous topical delivery of platelet-rich plasma—With or without concurrent Bimatoprost and/or minoxidil. Dermatol. Online J. 2019, 25, 3. [Google Scholar] [CrossRef]
- Massaki, A.B.; Fabi, S.G.; Fitzpatrick, R. Repigmentation of hypopigmented scars using an erbium-doped 1,550-nm fractionated laser and topical Bimatoprost. Dermatol. Surg. 2012, 38 Pt 1, 995–1001. [Google Scholar] [CrossRef]
- Regis, A.; MacGregor, J.; Chapas, A. Fractional Resurfacing and Topical Bimatoprost for the Treatment of Laser Induced Postinflammatory Hypopigmentation on the Lower Extremities. Dermatol. Surg. 2018, 44, 883–886. [Google Scholar] [CrossRef]
- Wilson, B.N.; Aleisa, A.; Menzer, C.; Rossi, A.M. Bimatoprost drug delivery with fractional laser and microneedling for the management of COVID-19 prone positioning-induced facial atrophy and hypopigmentation. JAAD Case Rep. 2021, 15, 26–29. [Google Scholar] [CrossRef]
- Zubair, R.; Lyons, A.B.; Vellaichamy, G.; Peacock, A.; Hamzavi, I. What’s New in Pigmentary Disorders. Dermatol. Clin. 2019, 37, 175–181. [Google Scholar] [CrossRef]
- Kanokrungsee, S.; Pruettivorawongse, D.; Rajatanavin, N. Clinical outcomes of topical Bimatoprost for nonsegmental facial vitiligo: A preliminary study. J. Cosmet. Dermatol. 2021, 20, 812–818. [Google Scholar] [CrossRef]
- Barbulescu, C.C.; Goldstein, N.B.; Roop, D.R.; Norris, D.A.; Birlea, S.A. Harnessing the Power of Regenerative Therapy for Vitiligo and Alopecia Areata. J. Investig. Dermatol. 2020, 140, 29–37. [Google Scholar] [CrossRef]
- Silpa-Archa, N.; Likittanasombat, S.; Apinuntham, C.; Pruksaeakanan, C.; Charoenpipatsin, N.; Chaiyabutr, C.; Wongpraparut, C. The efficacy of Bimatoprost ophthalmic solution combined with NB-UVB phototherapy in non-segmental and segmental vitiligo: A single-blind randomized controlled study. Sci. Rep. 2023, 13, 6438. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Prasad, S.; Sinha, R. Bimatoprost ophthalmic solution in facial vitiligo. J. Cosmet. Dermatol. 2018, 17, 437–440. [Google Scholar] [CrossRef]
- Kanokrungsee, S.; Khunkhet, S.; Rojhirunsakool, S.; Thadvibun, K.; Sahaspot, T. Triple combination therapy of narrowband ultraviolet B, fractional carbon dioxide laser and topical Bimatoprost 0.01% for non-segmental vitiligo on non-facial areas: A randomized half-body, double-blind, placebo-controlled, comparative study. Dermatol. Ther. 2022, 35, e15198. [Google Scholar] [CrossRef] [PubMed]
- Kreeshan, F.C.; Madan, V. Idiopathic Guttate Hypomelanosis Treated with 308-nm Excimer Light and Topical Bimatoprost. J. Cutan. Aesthet. Surg. 2021, 14, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Peabody, T.; Reitz, S.; Smith, J.; Teti, B. Clinical management of trichotillomania with Bimatoprost. Optom. Vis. Sci. 2013, 90, e167-71. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, E.S.; Pinchover, L.; Bernstein, R.M. Topical Bimatoprost for the treatment of eyebrow hypotrichosis. J. Drugs Dermatol. 2012, 11, 106–108. [Google Scholar]
- Smith, S.; Fagien, S.; Whitcup, S.M.; Ledon, F.; Somogyi, C.; Weng, E.; Beddingfield, F.C., 3rd. Eyelash growth in subjects treated with Bimatoprost: A multicenter, randomized, double-masked, vehicle-controlled, parallel-group study. J. Am. Acad. Dermatol. 2012, 66, 801–806. [Google Scholar] [CrossRef]
- Jones, D. Enhanced eyelashes: Prescription and over-the-counter options. Aesthetic Plast. Surg. 2011, 35, 116–121. [Google Scholar] [CrossRef]
- Rahman, M.S.; Yoshida, N.; Hanafusa, M.; Matsuo, A.; Zhu, S.; Stub, Y.; Takahashi, C.; Tsuboi, H.; Matsushita, R.; Maekawa, K.; et al. Screening and quantification of undeclared PGF(2alpha) analogs in eyelash-enhancing cosmetic serums using LC-MS/MS. J. Pharm. Biomed. Anal. 2022, 219, 114940. [Google Scholar] [CrossRef]
- Fagien, S. Management of hypotrichosis of the eyelashes: Focus on Bimatoprost. Clin. Cosmet. Investig. Dermatol. 2010, 3, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Woodward, D.F.; Tang, E.S.; Attar, M.; Wang, J.W. The biodisposition and hypertrichotic effects of Bimatoprost in mouse skin. Exp. Dermatol. 2013, 22, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Wirta, D.; Baumann, L.; Bruce, S.; Ahluwalia, G.; Weng, E.; Daniels, S. Safety and Efficacy of Bimatoprost for Eyelash Growth in Postchemotherapy Subjects. J. Clin. Aesthet. Dermatol. 2015, 8, 11–20. [Google Scholar] [PubMed]
- Ahluwalia, G.S. Safety and efficacy of Bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J. Investig. Dermatol. Symp. Proc. 2013, 16, S73–S76. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.L.; Stinnett, S.; Woodward, J. The role of Bimatoprost eyelash gel in chemotherapy-induced madarosis: An analysis of efficacy and safety. Int. J. Trichol. 2011, 3, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Caro, G.; Fortuna, M.C.; Pigliacelli, F.; D’Arino, A.; Carlesimo, M. Prevention and Treatment of Chemotherapy-Induced Alopecia. Dermatol. Pract. Concept. 2020, 10, e2020074. [Google Scholar] [CrossRef] [PubMed]
- Bitton, E.; Courey, C.; Giancola, P.; Diaconu, V.; Wise, J.; Wittich, W. Effects of LATISSE (Bimatoprost 0.03 per cent topical solution) on the ocular surface. Clin. Exp. Optom. 2017, 100, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Giannico, A.T.; Lima, L.; Russ, H.H.; Montiani-Ferreira, F. Eyelash growth induced by topical prostaglandin analogues, Bimatoprost, tafluprost, travoprost, and Latanoprost in rabbits. J. Ocul. Pharmacol. Ther. 2013, 29, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Emer, J.J.; Stevenson, M.L.; Markowitz, O. Novel treatment of female-pattern androgenetic alopecia with injected Bimatoprost 0.03% solution. J. Drugs Dermatol. 2011, 10, 795–798. [Google Scholar]
- Gupta, A.K.; Bamimore, M.A.; Foley, K.A. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: A systematic review with network meta-analyses, and an assessment of evidence quality. J. Dermatol. Treat. 2022, 33, 62–72. [Google Scholar] [CrossRef]
- McElwee, K.J.; Shapiro, J.S. Promising therapies for treating and/or preventing androgenic alopecia. Skin. Therapy Lett. 2012, 17, 1–4. [Google Scholar] [PubMed]
- Khidhir, K.G.; Woodward, D.F.; Farjo, N.P.; Farjo, B.K.; Tang, E.S.; Wang, J.W.; Picksley, S.M.; Randall, V.A. The prostamide-related glaucoma therapy, Bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 2013, 27, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Law, S.K. Bimatoprost in the treatment of eyelash hypotrichosis. Clin. Ophthalmol. 2010, 4, 349–358. [Google Scholar] [CrossRef]
- Ricar, J.; Cetkovska, P.; Hordinsky, M.; Ricarova, R. Topical Bimatoprost in the treatment of eyelash loss in alopecia totalis and universalis: A prospective, open-label study. Dermatol. Ther. 2022, 35, e15438. [Google Scholar] [CrossRef]
- Lu, Y.; He, Y.; Wang, X.; Wang, H.; Qiu, Q.; Wu, B.; Wu, X. Screening, characterization, and determination of suspected additives Bimatoprost and Latanoprost in cosmetics using NMR and LC-MS methods. Anal. Bioanal. Chem. 2023, 415, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, G.; Park, H.N.; Kim, J.; Kim, N.S.; Park, S.; Park, S.K.; Baek, S.Y.; Kang, H. Determination of illegal adulteration of dietary supplements with synthetic hair-growth compounds by UPLC and LC-Q-TOF/MS. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 191–199. [Google Scholar] [CrossRef]
- Johansson, M.; Fransson, D.; Rundlof, T.; Huynh, N.H.; Arvidsson, T. A general analytical platform and strategy in search for illegal drugs. J. Pharm. Biomed. Anal. 2014, 100, 215–229. [Google Scholar] [CrossRef]
- Yazdanian, N.; Mozafarpoor, S.; Goodarzi, A. Phosphodiesterase inhibitors and prostaglandin analogues in dermatology: A comprehensive review. Dermatol. Ther. 2021, 34, e14669. [Google Scholar] [CrossRef]
- Nasir, W.; Aguilera, S.B.; Weiss, E. Non-Surgical Eyebrow Rejuvenation Techniques: A Review. J. Drugs Dermatol. 2021, 20, 970–978. [Google Scholar] [CrossRef]
- Riahi, R.R.; Cohen, P.R. Topical Treatment of Eyebrow Hypotrichosis with Bimatoprost 0.03% Solution: Case Report and Literature Review. Cureus 2018, 10, e2666. [Google Scholar] [CrossRef]
- Borchert, M.; Bruce, S.; Wirta, D.; Yoelin, S.G.; Lee, S.; Mao, C.; VanDenburgh, A. An evaluation of the safety and efficacy of Bimatoprost for eyelash growth in pediatric subjects. Clin. Ophthalmol. 2016, 10, 419–429. [Google Scholar] [PubMed]
- Zaky, M.S.; Hashem, O.A.; Mahfouz, S.M.; Elsaie, M.L. Comparative study of the efficacy and safety of topical minoxidil 2% versus topical Bimatoprost 0.01% versus topical Bimatoprost 0.03% in treatment of eyebrow hypotrichosis: A randomized controlled trial. Arch. Dermatol. Res. 2023, 315, 2635–2641. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Lei, T.C.; Xing, F. Bimatoprost promotes hair growth of reconstructed hair follicles in mice through activation of the Wnt/beta-catenin signaling pathway. Zhonghua Yi Xue Za Zhi 2021, 101, 1529–1534. [Google Scholar] [PubMed]
- Gupta, A.K.; Carviel, J.; Abramovits, W. Treating Alopecia Areata: Current Practices Versus New Directions. Am. J. Clin. Dermatol. 2017, 18, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Fagien, S.; Walt, J.G.; Carruthers, J.; Cox, S.E.; Wirta, D.; Weng, E.; Beddingfield, F.C., 3rd. Patient-reported outcomes of Bimatoprost for eyelash growth: Results from a randomized, double-masked, vehicle-controlled, parallel-group study. Aesthet. Surg. J. 2013, 33, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Kim, J.Y.; Paik, S.H.; Jeon, H.C.; Jung, Y.J.; Lee, Y.; Baek, J.H.; Chun, J.H.; Lee, W.S.; Lee, J.Y.; et al. Long-term utility and durability of the therapeutic effects of Bimatoprost 0.03% for eyelash augmentation in healthy Asian subjects. Dermatology 2014, 229, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Beer, K.R.; Julius, H.; Dunn, M.; Wilson, F. Treatment of eyebrow hypotrichosis using Bimatoprost: A randomized, double-blind, vehicle-controlled pilot study. Dermatol. Surg. 2013, 39, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.A.; Haggerty, C.J.; Stinnett, S.S.; Williams, Z.Y. Bimatoprost 0.03% gel for cosmetic eyelash growth and enhancement. J. Cosmet. Dermatol. 2010, 9, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Wester, S.T.; Lee, W.W.; Shi, W. Eyelash growth from application of Bimatoprost in gel suspension to the base of the eyelashes. Ophthalmology 2010, 117, 1024–1031. [Google Scholar] [CrossRef]
- Zaleski-Larsen, L.A.; Ruth, N.H.; Fabi, S.G. Retrospective Evaluation of Topical Bimatoprost and Iris Pigmentation Change. Dermatol. Surg. 2017, 43, 1431–1433. [Google Scholar] [CrossRef]
- Carruthers, J.; Beer, K.; Carruthers, A.; Coleman, W.P., 3rd; Draelos, Z.D.; Jones, D.; Goldman, M.P.; Pucci, M.L.; VanDenburgh, A.; Weng, E.; et al. Bimatoprost 0.03% for the Treatment of Eyebrow Hypotrichosis. Dermatol. Surg. 2016, 42, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Harnchoowong, S.; Chanasumon, N.; Sriphojanart, T. Comparison of the efficacy and safety of using 0.01% versus 0.03% Bimatoprost for the treatment of eyebrow hypotrichosis: A randomized, double-blind, split-face, comparative study. J. Cosmet. Dermatol. 2020, 19, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Yoelin, S.G.; Fagien, S.; Cox, S.E.; Davis, P.G.; Campo, A.; Caulkins, C.A.; Gallagher, C.J. A retrospective review and observational study of outcomes and safety of Bimatoprost ophthalmic solution 0.03% for treating eyelash hypotrichosis. Dermatol. Surg. 2014, 40, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Diehl, J.; Levins, P.C. Promising alternative clinical uses of prostaglandin F2alpha analogs: Beyond the eyelashes. J. Am. Acad. Dermatol. 2015, 72, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Glaser, D.A.; Hossain, P.; Perkins, W.; Griffiths, T.; Ahluwalia, G.; Weng, E.; Beddingfield, F.C. Long-term safety and efficacy of Bimatoprost solution 0.03% application to the eyelid margin for the treatment of idiopathic and chemotherapy-induced eyelash hypotrichosis: A randomized controlled trial. Br. J. Dermatol. 2015, 172, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Sayed, K.M.; Mostafa, E.M.; Mounir, A.; Abd Elhaliem, N.G.; Alsmman, A.H. Analysis of Bimatoprost-Induced changes on Rabbits eyelash Follicle: Clinical and Electron microscopic study. Clin. Ophthalmol. 2019, 13, 2421–2426. [Google Scholar] [CrossRef]
- Shin, D.W. The physiological and pharmacological roles of prostaglandins in hair growth. Korean J. Physiol. Pharmacol. 2022, 26, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Chu, H.; Ahn, Y.; Kim, J.; Kim, D.Y. HMGB1 promotes hair growth via the modulation of prostaglandin metabolism. Sci. Rep. 2019, 9, 6660. [Google Scholar] [CrossRef] [PubMed]
- Chanasumon, N.; Sriphojanart, T.; Suchonwanit, P. Therapeutic potential of Bimatoprost for the treatment of eyebrow hypotrichosis. Drug Des. Devel Ther. 2018, 12, 365–372. [Google Scholar] [CrossRef]
- Wirta, D.; Pariser, D.M.; Yoelin, S.G.; Arase, S.; McMichael, A.; Weng, E.; Mao, C.; Demos, G.; Vandenburgh, A. Bimatoprost 0.03% for the Treatment of Eyelash Hypotrichosis: A Pooled Safety Analysis of Six Randomized, Double-masked Clinical Trials. J. Clin. Aesthet. Dermatol. 2015, 8, 17–29. [Google Scholar]
- Ali, M.S.; Hafiz, H.S.A.; Ahmed, N.A.; Galal, S.A. Combined microneedling with topical vitamin D3 or Bimatoprost versus microneedling alone in the treatment of alopecia areata: A comparative randomized trial. J. Cosmet. Dermatol. 2023, 22, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, X.; Wu, M. Topical medication instillation techniques for glaucoma. Cochrane Database Syst. Rev. 2017, 2, CD010520. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.J.; Scheman, A.; Tanna, A.P.; Bueno, A. The effect of cutaneous prostaglandin application on nail growth, nail brittleness, and intraocular pressure. J. Cosmet. Dermatol. 2018, 17, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Reardon, G.; Schwartz, G.F.; Kotak, S. Persistence on prostaglandin ocular hypotensive therapy: An assessment using medication possession and days covered on therapy. BMC Ophthalmol. 2010, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Aref, A.A. Sustained drug delivery for glaucoma: Current data and future trends. Curr. Opin. Ophthalmol. 2017, 28, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kesav, N.P.; Young, C.E.C.; Ertel, M.K.; Seibold, L.K.; Kahook, M.Y. Sustained-release drug delivery systems for the treatment of glaucoma. Int. J. Ophthalmol. 2021, 14, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Gillmann, K.; Mansouri, K. Minimally Invasive Surgery, Implantable Sensors, and Personalized Therapies. J. Ophthalmic Vis. Res. 2020, 15, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A.; Sheybani, A.; Shah, M.M.; Rivas, M.; Bai, Z.; Werts, E.; Ahmed, I.I.K.; Craven, E.R. Single Administration of Intracameral Bimatoprost Implant 10 microg in Patients with Open-Angle Glaucoma or Ocular Hypertension. Ophthalmol. Ther. 2022, 11, 1517–1537. [Google Scholar] [CrossRef]
- Gautam, M.; Gupta, R.; Singh, P.; Verma, V.; Verma, S.; Mittal, P.; Karkhur, S.; Sampath, A.; Mohan, R.R.; Sharma, B. Intracameral Drug Delivery: A Review of Agents, Indications, and Outcomes. J. Ocul. Pharmacol. Ther. 2023, 39, 102–116. [Google Scholar] [CrossRef]
- Kompella, R.; Patil, M. Pharmacokinetic-pharmacodynamic model to predict drug concentrations and intraocular pressure lowering effect for a Bimatoprost six-month slow-release system. Eur. J. Ophthalmol. 2022, 33, 11206721221135910. [Google Scholar] [CrossRef]
- Wong, M.K.; Bowers, M.E.; Ventimiglia, J.; Niknam, R.M.; Moster, M.R.; Pro, M.J.; Dale, E.; Kolomeyer, N.N.; Lee, D.; Zheng, C.X. Short-Term Outcomes of Bimatoprost Sustained-Release Intracameral Implant in Glaucoma. J. Glaucoma 2023, 32, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Almazan, A.; Decker, S.; Zhong, Y.; Ghebremeskel, A.N.; Hughes, P.; Robinson, M.R.; Burke, J.A.; Weinreb, R.N. Intraocular Pressure Effects and Mechanism of Action of Topical Versus Sustained-Release Bimatoprost. Transl. Vis. Sci. Technol. 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.A.; Christie, W.C.; Day, D.G.; Craven, E.R.; Walters, T.; Bejanian, M.; Lee, S.S.; Goodkin, M.L.; Zhang, J.; Whitcup, S.M.; et al. Bimatoprost Sustained-Release Implants for Glaucoma Therapy: 6-Month Results from a Phase I/II Clinical Trial. Am. J. Ophthalmol. 2017, 175, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Christie, W.C.; Medeiros, F.A.; Craven, E.R.; Kim, K.; Nguyen, A.; Bejanian, M.; Wirta, D.L. Single Administration of Bimatoprost Implant: Effects on 24-Hour Intraocular Pressure and 1-Year Outcomes. Ophthalmol. Glaucoma 2023, 6, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Craven, E.R.; Walters, T.; Christie, W.C.; Day, D.G.; Lewis, R.A.; Goodkin, M.L.; Chen, M.; Wangsadipura, V.; Robinson, M.R.; Bejanian, M.; et al. 24-Month Phase I/II Clinical Trial of Bimatoprost Sustained-Release Implant (Bimatoprost SR) in Glaucoma Patients. Drugs 2020, 80, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Robinson, M.R.; Struble, C.; Attar, M. Nonclinical Pharmacokinetic and Pharmacodynamic Assessment of Bimatoprost Following a Single Intracameral Injection of Sustained-Release Implants. Transl. Vis. Sci. Technol. 2020, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Di Trani, N.; Jain, P.; Chua, C.Y.X.; Ho, J.S.; Bruno, G.; Susnjar, A.; Pons-Faudoa, F.P.; Sizovs, A.; Hood, R.L.; Smith, Z.W.; et al. Nanofluidic microsystem for sustained intraocular delivery of therapeutics. Nanomedicine 2019, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Belamkar, A.; Harris, A.; Zukerman, R.; Siesky, B.; Oddone, F.; Verticchio Vercellin, A.; Ciulla, T.A. Sustained release glaucoma therapies: Novel modalities for overcoming key treatment barriers associated with topical medications. Ann. Med. 2022, 54, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, P.; Kansara, N.D.; Frankfort, B.J.; Blieden, L.S.; Chang, P.T. Intraocular Pressure and Eyedrop Usage Reduction with Intracameral Bimatoprost Implant. J. Ocul. Pharmacol. Ther. 2023, 39, 398–403. [Google Scholar] [CrossRef]
- Sirinek, P.E.; Lin, M.M. Intracameral sustained release bimatoprost implants (Durysta). Semin. Ophthalmol. 2022, 37, 385–390. [Google Scholar] [CrossRef]
- Satyanarayana, S.D.; Abu Lila, A.S.; Moin, A.; Moglad, E.H.; Khafagy, E.S.; Alotaibi, H.F.; Obaidullah, A.J.; Charyulu, R.N. Ocular Delivery of Bimatoprost-Loaded Solid Lipid Nanoparticles for Effective Management of Glaucoma. Pharmaceuticals 2023, 16, 1001. [Google Scholar] [CrossRef] [PubMed]
- Seal, J.R.; Robinson, M.R.; Burke, J.; Bejanian, M.; Coote, M.; Attar, M. Intracameral Sustained-Release Bimatoprost Implant Delivers Bimatoprost to Target Tissues with Reduced Drug Exposure to Off-Target Tissues. J. Ocul. Pharmacol. Ther. 2019, 35, 50–57. [Google Scholar] [CrossRef]
- Lee, S.S.; Burke, J.; Shen, J.; Almazan, A.; Orilla, W.; Hughes, P.; Zhang, J.; Li, H.; Struble, C.; Miller, P.E.; et al. Bimatoprost sustained-release intracameral implant reduces episcleral venous pressure in dogs. Vet. Ophthalmol. 2018, 21, 376–381. [Google Scholar] [CrossRef]
- Yadav, M.; Guzman-Aranguez, A.; Perez de Lara, M.J.; Singh, M.; Singh, J.; Kaur, I.P. Bimatoprost loaded nanovesicular long-acting sub-conjunctival in-situ gelling implant: In vitro and in vivo evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109730. [Google Scholar] [CrossRef]
- Yadav, M.; Guzman-Aranguez, A.; Perez de Lara, M.J.; Singh, M.; Singh, J.; Kaur, I.P. Safety data on in situ gelling Bimatoprost loaded nanovesicular formulations. Data Brief. 2019, 25, 104361. [Google Scholar] [CrossRef] [PubMed]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Rodrigues, L.B.; Bravo, R.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.; et al. Bimatoprost-loaded ocular inserts as sustained release drug delivery systems for glaucoma treatment: In vitro and in vivo evaluation. PLoS ONE 2014, 9, e95461. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A.; Walters, T.R.; Kolko, M.; Coote, M.; Bejanian, M.; Goodkin, M.L.; Guo, Q.; Zhang, J.; Robinson, M.R.; Weinreb, R.N.; et al. Phase 3, Randomized, 20-Month Study of Bimatoprost Implant in Open-Angle Glaucoma and Ocular Hypertension (ARTEMIS 1). Ophthalmology 2020, 127, 1627–1641. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, C.; Rajagopalan, L.; Ugarte, S.; Mistry, S.; Orilla, W.; Goodkin, M.L.; Robinson, M.R.; Engles, M.; Dibas, M. Intraocular Pressure-Lowering Efficacy of a Sustained-Release Bimatoprost Implant in Dog Eyes Pretreated with Selective Laser Trabeculoplasty. J. Ocul. Pharmacol. Ther. 2022, 38, 311–318. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Bacharach, J.; Brubaker, J.W.; Medeiros, F.A.; Bejanian, M.; Bernstein, P.; Robinson, M.R. Bimatoprost Implant Biodegradation in the Phase 3, Randomized, 20-Month ARTEMIS Studies. J. Ocul. Pharmacol. Ther. 2023, 39, 55–62. [Google Scholar] [CrossRef]
- Desai, A.R.; Maulvi, F.A.; Desai, D.M.; Shukla, M.R.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Sandeman, S.; Shah, D.O. Multiple drug delivery from the drug-implants-laden silicone contact lens: Addressing the issue of burst drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110885. [Google Scholar] [CrossRef]
- Miller, P.E.; Eaton, J.S. Medical anti-glaucoma therapy: Beyond the drop. Vet. Ophthalmol. 2021, 24 (Suppl. S1), 2–15. [Google Scholar] [CrossRef]
- Kenley, R.A.; Filippone, H.; Giske, M.; Beidler, D.; Vehige, J.; Fleitman, J. Equilibrium binding interactions between lotrafilcon a soft contact lenses and the two prostaglandin antiglaucoma drugs Bimatoprost and tafluprost. Eye Contact Lens 2013, 39, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Soni, P.D.; Patel, P.J.; Desai, A.R.; Desai, D.T.; Shukla, M.R.; Shah, S.A.; Shah, D.O.; Willcox, M.D.P. Controlled Bimatoprost release from graphene oxide laden contact lenses: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2021, 208, 112096. [Google Scholar] [CrossRef]
- Sekar, P.; Chauhan, A. Effect of vitamin-E integration on delivery of prostaglandin analogs from therapeutic lenses. J. Colloid Interface Sci. 2019, 539, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ma, C.; Ma, Y.; Ma, Y.; Mao, Y.; Meng, Z. Sustained Bimatoprost release using gold nanoparticles laden contact lenses. J. Biomater. Sci. Polym. Ed. 2021, 32, 1618–1634. [Google Scholar] [CrossRef]
- Shirley, M. Bimatoprost Implant: First Approval. Drugs Aging 2020, 37, 549, Erratum in Drugs Aging 2020, 37, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Lambert, W.S.; Carlson, B.J.; van der Ende, A.E.; Shih, G.; Dobish, J.N.; Calkins, D.J.; Harth, E. Nanosponge-Mediated Drug Delivery Lowers Intraocular Pressure. Transl. Vis. Sci. Technol. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Dams, I.; Chodynski, M.; Krupa, M.; Pietraszek, A.; Zezula, M.; Cmoch, P.; Kosinska, M.; Kutner, A. A novel convergent synthesis of the antiglaucoma PGF2alpha analogue Bimatoprost. Chirality 2013, 25, 170–179. [Google Scholar] [CrossRef]
- Yasmeen, A.; Syed, M.H.; Alqahtani, S.S.; Kashan Syed, N.; Meraya, A.M.; Wazaify, M.; Van Hout, M.C. Suspected inappropriate use of prescription and non-prescription drugs among requesting customers: A Saudi community pharmacists’ perspective. Saudi Pharm. J. 2023, 31, 1254–1264. [Google Scholar] [CrossRef]
- Ichioka, H.; Ida, Y.; Watanabe, M.; Ohguro, H.; Hikage, F. Prostaglandin F2alpha and EP2 agonists, and a ROCK inhibitor modulate the formation of 3D organoids of Grave’s orbitopathy related human orbital fibroblasts. Exp. Eye Res. 2021, 205, 108489. [Google Scholar] [CrossRef]
- Draman, M.S.; Morris, D.S.; Evans, S.; Haridas, A.; Pell, J.; Greenwood, R.; Foy, C.; Taylor, P.; Pooprasert, P.; Muller, I.; et al. Prostaglandin F2-Alpha Eye Drops (Bimatoprost) in Graves’ Orbitopathy: A Randomized Controlled Double-Masked Crossover Trial (BIMA Trial). Thyroid 2019, 29, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Melovatskiy, P.D.; Krylova, E.I.; Grusha, Y.O. Prospects of the use of prostaglandin analogues in the treatment of patients with thyroid eye disease. Vestn. Oftalmol. 2022, 138, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Eiger-Moscovich, M.; Stiebel-Kalish, H.; Yassur, I.; Barash, D.; Gaton, D.; Avisar, I. Prostaglandin analogue drops for the treatment of soft tissue expansion and exophthalmos in patients with inactive thyroid eye disease. Can. J. Ophthalmol. 2019, 54, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, T.; Ohji, M. Treatment with Bimatoprost for exophthalmos in patients with inactive thyroid-associated ophthalmopathy. Clin. Ophthalmol. 2018, 12, 2415–2421. [Google Scholar] [CrossRef] [PubMed]
- Weber, U. Central corneal thickness and corneal endothelial morphology with and without therapy using commercially available antiglaucomatous drugs. Klin. Monbl Augenheilkd. 2012, 229, 716–723. [Google Scholar] [PubMed]
- Salvetat, M.L.; Zeppieri, M.; Tosoni, C.; Parisi, L.; Brusini, P. Non-conventional perimetric methods in the detection of early glaucomatous functional damage. Eye 2010, 24, 835–842. [Google Scholar] [CrossRef][Green Version]
- Brusini, P.; Salvetat, M.L.; Parisi, L.; Zeppieri, M.; Tosoni, C. Discrimination between normal and early glaucomatous eyes with scanning laser polarimeter with fixed and variable corneal compensator settings. Eur. J. Ophthalmol. 2005, 15, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Fogagnolo, P.; Figus, M.; Frezzotti, P.; Iester, M.; Oddone, F.; Zeppieri, M.; Ferreras, A.; Brusini, P.; Rossetti, L.; Orzalesi, N. Test-retest variability of intraocular pressure and ocular pulse amplitude for dynamic contour tonometry: A multicentre study. Br. J. Ophthalmol. 2010, 94, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Salvetat, M.L.; Zeppieri, M.; Tosoni, C.; Brusini, P. Baseline factors predicting the risk of conversion from ocular hypertension to primary open-angle glaucoma during a 10-year follow-up. Eye 2016, 30, 784–795. [Google Scholar] [CrossRef]
- Yoneda, T.; Sumi, T.; Takahashi, A.; Hoshikawa, Y.; Kobayashi, M.; Fukushima, A. Automated hyperemia analysis software: Reliability and reproducibility in healthy subjects. Jpn. J. Ophthalmol. 2012, 56, 1–7. [Google Scholar] [CrossRef]
- Bastia, E.; Sgambellone, S.; Lucarini, L.; Provensi, G.; Brambilla, S.; Galli, C.; Almirante, N.; Impagnatiello, F. NCX 470 Restores Ocular Hemodynamics and Retinal Cell Physiology After ET-1-Induced Ischemia/Reperfusion Injury of Optic Nerve and Retina in Rabbits. J. Ocul. Pharmacol. Ther. 2022, 38, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Chakrabarti, A.; Nazm, N.; Mehta, R.; Edward, D.P. Newer advances in medical management of glaucoma. Indian J. Ophthalmol. 2022, 70, 1920–1930. [Google Scholar] [PubMed]
- Walters, T.R.; Kothe, A.C.; Boyer, J.L.; Usner, D.W.; Lopez, K.; Duquesroix, B.; Fechtner, R.D.; Navratil, T. A Randomized, Controlled Comparison of NCX 470 (0.021%, 0.042%, and 0.065%) and Latanoprost 0.005% in Patients with Open-angle Glaucoma or Ocular Hypertension: The Dolomites Study. J. Glaucoma 2022, 31, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Impagnatiello, F.; Bastia, E.; Almirante, N.; Brambilla, S.; Duquesroix, B.; Kothe, A.C.; Bergamini, M.V.W. Prostaglandin analogues and nitric oxide contribution in the treatment of ocular hypertension and glaucoma. Br. J. Pharmacol. 2019, 176, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Impagnatiello, F.; Toris, C.B.; Batugo, M.; Prasanna, G.; Borghi, V.; Bastia, E.; Ongini, E.; Krauss, A.H. Intraocular Pressure-Lowering Activity of NCX 470, a Novel Nitric Oxide-Donating Bimatoprost in Preclinical Models. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6558–6564. [Google Scholar] [CrossRef][Green Version]
- Woodward, D.F.; Coleman, R.A.; Woodrooffe, A.J.; Spada, C.S.; Wang, J.W. Effect of the Antiglaucoma Agent JV-GL1 and Related Compounds in the Canine Eye. J. Ocul. Pharmacol. Ther. 2020, 36, 636–648. [Google Scholar] [CrossRef]
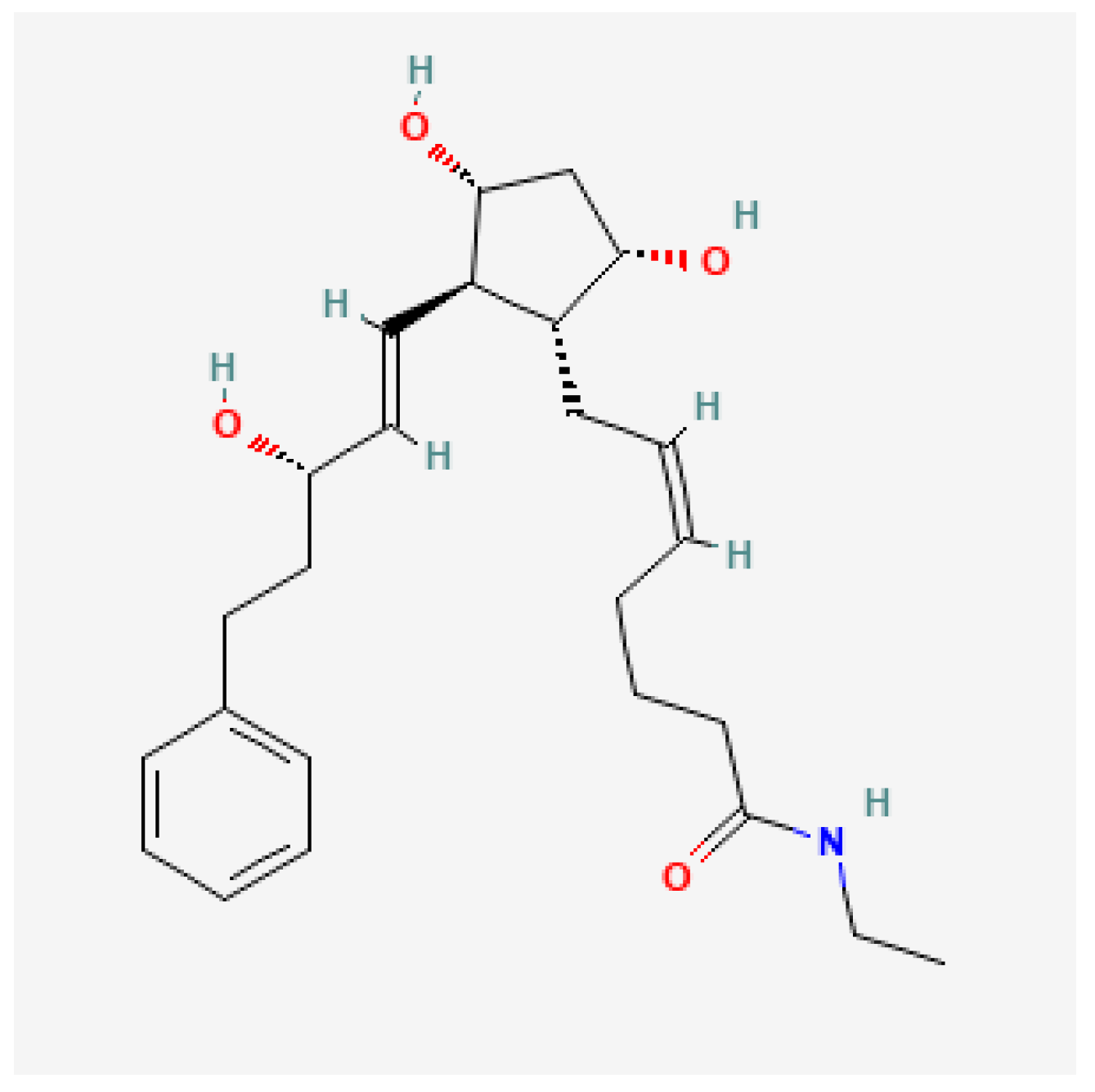
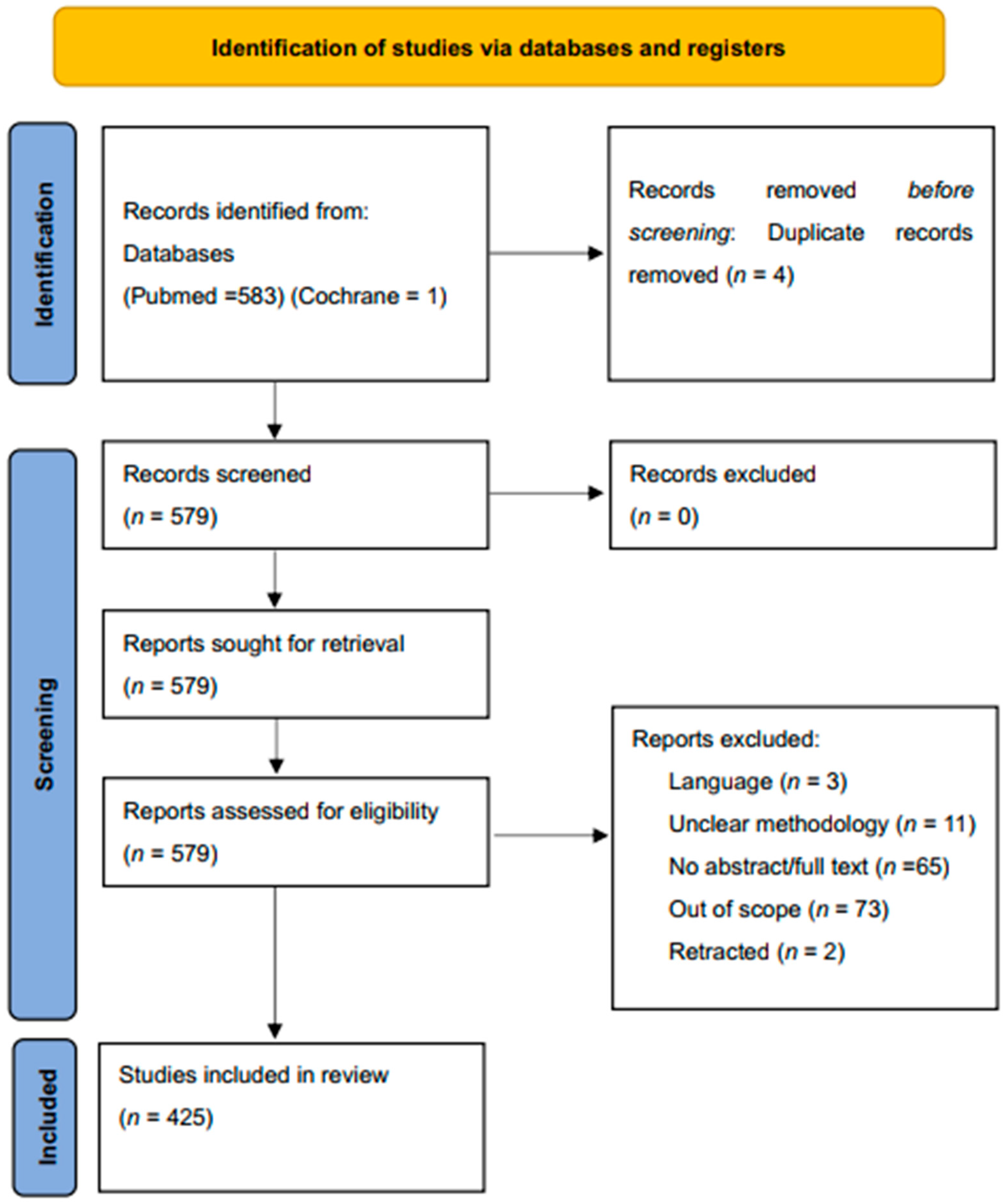
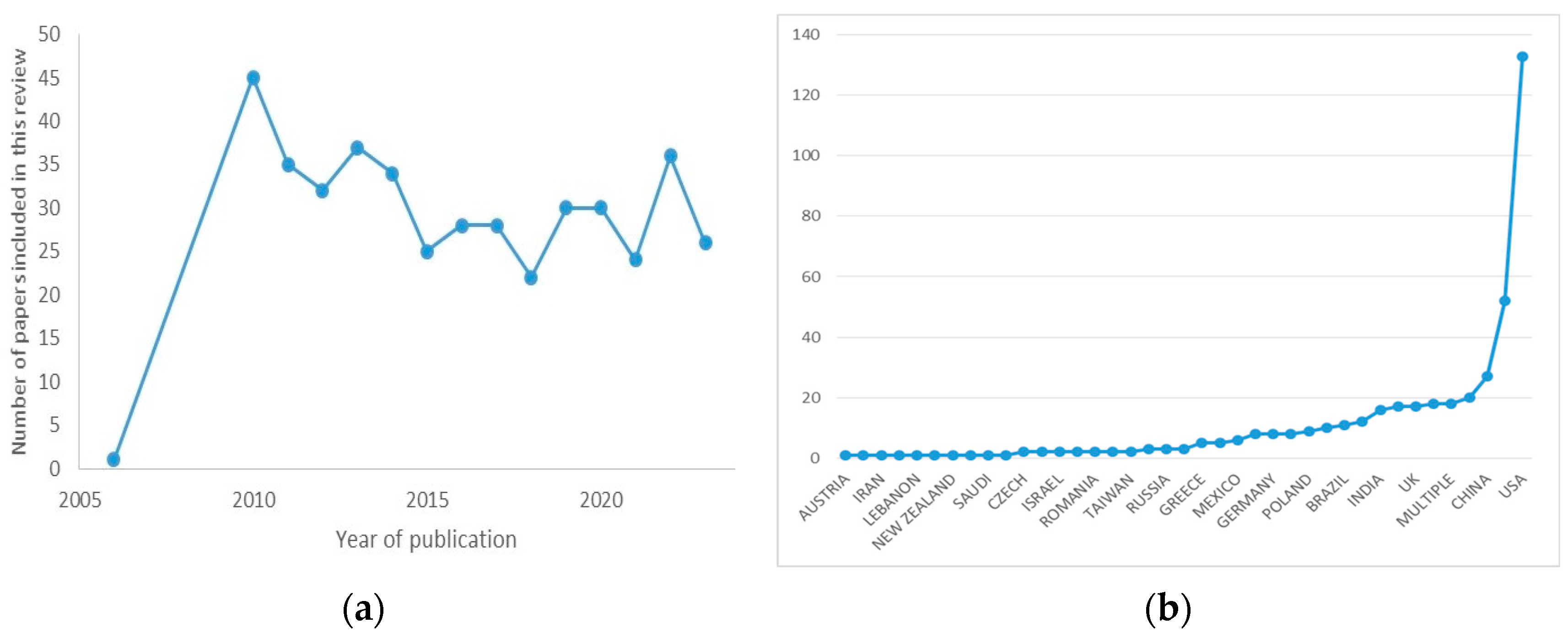

| Study and Year | Design | Study Population | Main Outcomes with p Values |
|---|---|---|---|
| Holstrom et al., 2005 [201] | Meta-analysis | 9295 (42 studies) | 30.3% reduction in IOP from baseline with bimatoprost compared to 28.7% with travoprost and 26.7% with latanoprost (p = 0.001) |
| Denis et al., 2007 [202] | Meta-analysis | 1318 (6 studies) | bimatoprost produced the largest reduction in IOP (−1.04 mmHg, 95% CI: −2.11; 0.04; p = 0.06) compared to bimatoprost (−0.98 mmHg, 95% CI: −2.08; 0.13; p = 0.08) and a reference latanoprost treated population |
| Noecker et al., 2003 [203] | Clinical Trial | 31 | bimatoprost produced a mean IOP reduction of 8.4 mmHg (33.7%) compared to 7.9 mmHg (30%) with travoprost (p = 0.671) |
| Noecker et al., 2003 [204] | Clinical Trial | 269 | From baselines before treatments of 24.0 ± 0.27 mm Hg (bimatoprost) and 23.3 ± 0.27 mm Hg (latanoprost), IOP reduced statistically more in the bimatoprost group as compared to the latanoprost group (16.5–17.4 mm Hg; 17.6–18.9 mm Hg, p ≤ 0.008) |
| Parrish et al., 2003 [205] | Clinical Trial | 393 | Three separate groups of participants achieved IOP lowering of 8.6 ± 0.3 mmHg, 8.7 ± 0.3 mmHg, and 8.0 ± 0.3 mmHg when treated with latanoprost, bimatoprost or travporost respectively (p = 0.128 for difference among groups). |
| Cantor et al., 2004 [206] | Clinical Trial | 26 | IOP reduced by 7.4–8.8 mmHg with bimatoprost as compared to 4.6–7.2 mmHg with travoprost (p = 0.057) |
| Summary of Findings | Formulation | Location of Complication | Authors |
|---|---|---|---|
| Bimatoprost use was implicated in the sudden expression of non-vellus hairs in a female patient | Lumigan 0.03%, (Allergan, Dublin, ON, Canada) | Face | Kaliaperumal et al. [215] |
| The bimatoprost regimen was associated with a reduction in IPD | Lumigan 0.03%, (Senju Pharmaceutical Co., Osaka, Japan) | Interpupillary distance | Sano et al. [216] |
| Bimatoprost is implicated in eyelash trichomegaly | Bimatoprost * | Eyelash | Hutchinson et al. [217] |
| The doctor noticed audible clicking of eyelashes secondary to eyelash elongation | Bimatoprost * | Eyelash | Skorin and Dailey [218] |
| Bimatoprost produced more upper eyelid changes as compared to latanoprost | Bimatoprost 0.03% * | Eyelid | Karslioglu et al. [219] |
| Hypopigmentation progressing to Lentigo Maligna Melanoma | Bimatoprost 0.03% * | Eyelid tissue | Deveau et al. [220] |
| Bimatoprost use resulted in eyelid tightening and shortening in the mouse model | Bimatoprost 0.03% * | Eyelid tissue | Kent and Custer [221] |
| Bilateral upper lid retraction was observed with bimatoprost use | Bimatoprost 0.03% * | Eyelids (upper) | Noma and Kakizaki [222] |
| DUES was the most common complication seen in an Asian study | Bimatoprost * | Orbit | Kim et al. [223] |
| Topical and retrobulbar injection of bimatoprost resulted in a drop in adipocyte cell count | Lumigan 0.03%, Allergan | Orbital fat | Jbara et al. [224] |
| Orbital fat reduced as evidenced by adnexal photographs | Bimatoprost * | Orbital fat | Rabinowitz et al. [225] |
| MRI and computer image algorithms respectively showed reduced orbital fat after bimatoprost use | bimatoprost (Allergan, Inc.), | Orbital fat | Higoshima et al. [226], and Eftekhari et al. [227] |
| Prostaglandins inhibit adipogenesis resulting in DUES | Bimaopost and Bimatoprost acid (Cayman Chemical Co., Ann Arbor, MI, USA) | Orbital tissue | Taketani et al. [228] and Ida et al. [229] |
| Bimatoprost implicated in peri-orbitopathy | Bimatoprost * | Periorbital | Tan and Berke [230] |
| Atrophy of the eyelid levator muscle was observed after the use of bimatoprost monocularly | Lumigan 0.03%, Allergan | Periorbital muscle | Wang et al. [231] |
| Bimatoprost (Latisse) induced skin discoloration | Bimatoprost solution, 0.3% (Latisse; Allergan Inc., Dublin, ON, Canada) | Periocular discoloration | Priluck and Fu [232] |
| Adverse effects include conjunctival hyperemia, burning sensation, and growth of eyelashes | Bimatoprost 0.03% *, Bimatoprost 0.01% * | External eye | Lewis et al. [233] and Park et al. [234] |
| Hyperemia and eyelash elongation were the most common side effects reported in a large Asian study | Lumigan 0.03%, Allergan | external eye | Sun et al. [235] |
| Bimatoprost use resulted in orbital fat prolapse and iris pigmentation | Lumigan 0.03%, Allergan | Eyeball tissue and iris | Aydin et al. [236], Jayaprakasam and Ghazi-Nouri [237], and Park et al. [238] |
| The tear profile of patients on bimatoprost revealed elevated cytokines mediating allergenic pathway | Bimaotoprost 0.01% * | Tears | Reddy et al. [239] |
| Bimatoprost scored highest on the pain scale in cataract surgery patients | Bimaotoprost * | Anterior eye | Ulas et al. [240] |
| A slight but significant increase in thickness observed | Bimatoprost 0.03% * | Central corneal thickness | Bafa et al. [241] |
| A slight but significant reduction in thickness observed | Bimatoprost 0.03% * | Central corneal thickness | Zhong et al. [242] and Eraslan and Celikay [243] |
| A systematic review showed that prostaglandin analogs result in thinning of the CCT | Bimatoprost * | Central corneal thickness (CCT) | Kim and Yim [244] |
| Bimatoprost was associated with conjunctival hyperemia | Lumigan PF 0.03% | Conjunctiva/cornea | Wu et al. [245] |
| PGF2α including bimatoprost-induced reduction in tissue stiffness and cornea microstructure changes | Bimatoprost * | Cornea | Wang et al. [246] |
| Scheimflug-aided analysis showed a longitudinal reduction in CCD after bimatoprost use | Bimatoprost * | Cornea cell density (CCD) | Eraslan et al. [247] |
| Bimatoprost demonstrated cytotoxicity to tested cells | Lumigan 0.01%, and Lumigan 0.03% | Corneal endothelial cells | Tong et al. [248] |
| Bimatoprost was found to cause cell necrosis and stifle proliferation | Bimatoprost * | Corneal epithelium | Pozarowska et al. [249] |
| Bimatoprost 0.03% triggered accommodative spasm and the patient manifested pseudomyopia | bimatoprost 0.03% * | Accommodative system | Padhy and Rao [250] |
| Cystoid macula edema resulted after bimatoprost therapy | Lumigan 0.03% | Macula | Agange and Mosaed [251] |
| Prostaglandin use postoperatively associated with cystoid macular edema | Bimatoprost * | Macula | Wendel et al. [252] |
| A geriatric patient was found to have developed bilateral macula edema after receiving bimatoprost implants | Bimatoprost implant (Durysta) | Macula | Patel et al. [253] |
| Bimatoprost-induced inhibition of platelet activation action. | Lumigan UD® (bimatoprost 0.3 mg/mL | Platelets | Moschos et al. [254] |
| Serous retinal detachment occurred after starting the bimatoprost regimen | Bimatoprost * | Retina | Addison et al. [255] |
| Bimatoprost 0.03 and 0.01 both resulted in conjunctival congestion after topical administration | Bimatoprost 0.01% | Scleral blood vessels | Ogundele et al. [256] |
| A case report of bimatoprost inducing late onset choroidal detachment in a patient after trabeculectomy | Bimatoprost 0.03% | Choroid | Nakakura et al. [257] |
| Bimatoprost 0.03% was associated with the thickening of the choroid. | Bimatoprost 0.03% * | Choroidal layer | Akyol et al. [258] |
| In-vitro analysis of the effect of bimatoprost on lamina cribosa astrocytes revealed apoptosis | Bimatoprost Acid * | Optic nerve head astrocytes | Shin et al. [259] |
| Bimatoprost resulted in prolonged vasodilation of ocular vessels, an indication of hyperemia | Bimatoprost 0.01% or Bimatoprost 0.03% * | Pig eyes in-vivo | Ogundele et al. [260] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeppieri, M.; Gagliano, C.; Spadea, L.; Salati, C.; Chukwuyem, E.C.; Enaholo, E.S.; D’Esposito, F.; Musa, M. From Eye Care to Hair Growth: Bimatoprost. Pharmaceuticals 2024, 17, 561. https://doi.org/10.3390/ph17050561
Zeppieri M, Gagliano C, Spadea L, Salati C, Chukwuyem EC, Enaholo ES, D’Esposito F, Musa M. From Eye Care to Hair Growth: Bimatoprost. Pharmaceuticals. 2024; 17(5):561. https://doi.org/10.3390/ph17050561
Chicago/Turabian StyleZeppieri, Marco, Caterina Gagliano, Leopoldo Spadea, Carlo Salati, Ekele Caleb Chukwuyem, Ehimare Samuel Enaholo, Fabiana D’Esposito, and Mutali Musa. 2024. "From Eye Care to Hair Growth: Bimatoprost" Pharmaceuticals 17, no. 5: 561. https://doi.org/10.3390/ph17050561
APA StyleZeppieri, M., Gagliano, C., Spadea, L., Salati, C., Chukwuyem, E. C., Enaholo, E. S., D’Esposito, F., & Musa, M. (2024). From Eye Care to Hair Growth: Bimatoprost. Pharmaceuticals, 17(5), 561. https://doi.org/10.3390/ph17050561









