Natural Compounds and Breast Cancer: Chemo-Preventive and Therapeutic Capabilities of Chlorogenic Acid and Cinnamaldehyde
Abstract
1. Introduction
2. Conventional Treatment Methods for Breast Cancer and Their Challenges
3. The Need for Safer/More Effective Alternative Breast Cancer Treatment Approaches
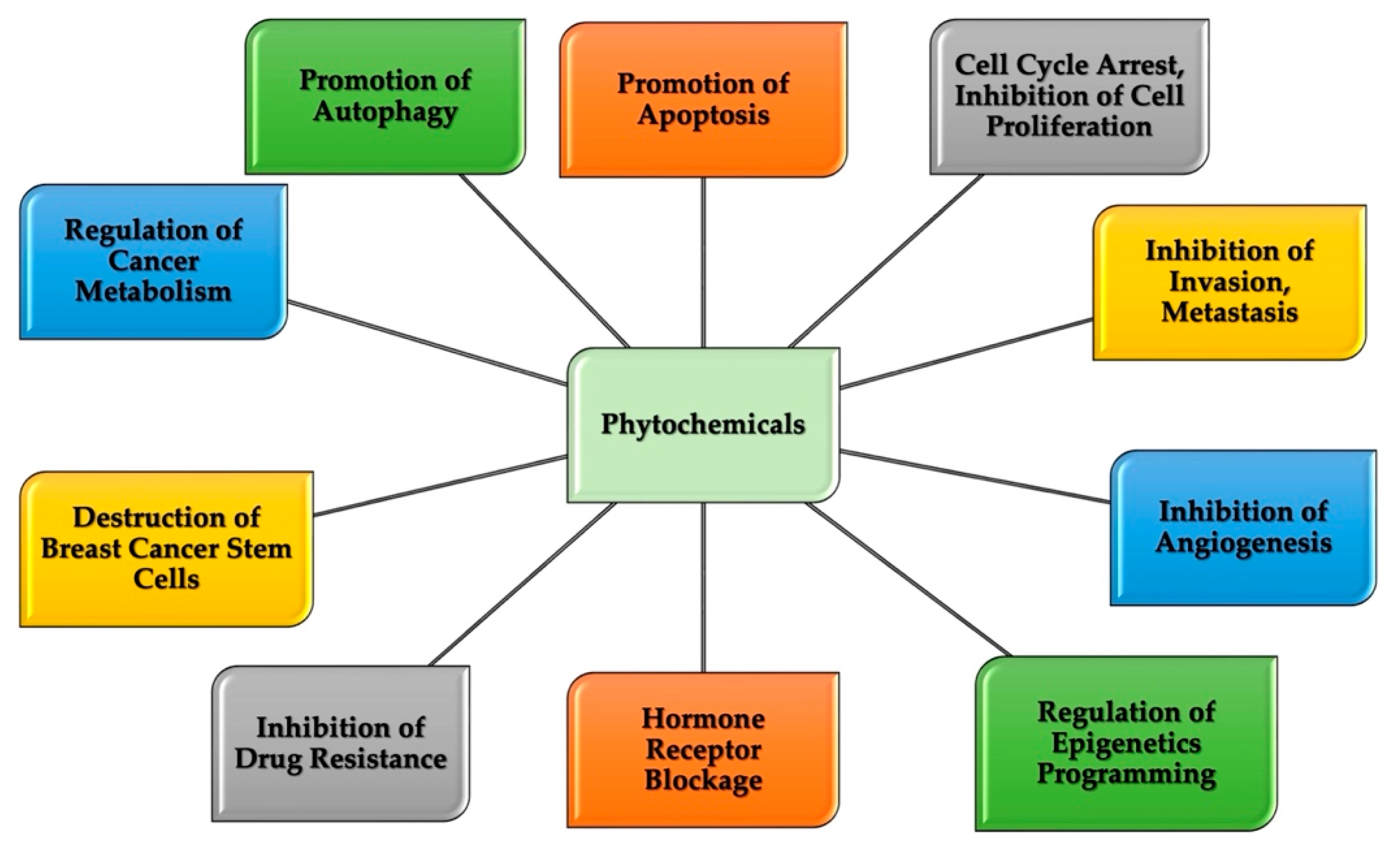
4. Breast Cancer and Chemoprevention Characteristics of Natural Compounds
5. Therapeutic Potential of Natural Compounds in Breast Cancer
6. Chlorogenic Acid and Cinnamaldehyde
6.1. Chlorogenic Acid and Cinnamaldehyde as Effective Chemo-Preventive and Therapeutic Agents in Breast Cancer
6.2. Multidrug Resistance in Breast Cancer Cells: The Potential Intervention of Chlorogenic Acid and Cinnamaldehyde
6.3. The Protective Abilities of Chlorogenic Acid and Cinnamaldehyde in Epigenetic Reprogramming-Driven Breast Cancer Cells
6.4. Synergism of CGA and CA in Breast Cancer
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac. J. Cancer Prev. 2019, 20, 2015–2020. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 36, 1551–1566. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative Approaches for Cancer Treatment: Current Perspectives and New Challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Kumar, A.P.; Ghosh, R. Food-Based Natural Products for Cancer Management: Is the Whole Greater than the Sum of the Parts? Semin. Cancer Biol. 2016, 40, 233–246. [Google Scholar] [CrossRef]
- Orlikova, B.; Dicato, M.; Diederich, M. 1000 Ways to Die: Natural Compounds Modulate Non-Canonical Cell Death Pathways in Cancer Cells. Phytochem. Rev. 2014, 13, 277–293. [Google Scholar] [CrossRef]
- Lee, J.; Gollahon, L. Nek2-Targeted ASO or SiRNA Pretreatment Enhances Anticancer Drug Sensitivity in Triple-Negative Breast Cancer Cells. Int. J. Oncol. 2013, 42, 839–847. [Google Scholar] [CrossRef]
- Gollahon, L.S.; Lee, K.; Finckbone, V.; Jeong, Y. The Natural Product NI-07 Demonstrates Effective Anti-Cancer Properties against Numerous Cancer Cell Types. J. Solid. Tumors 2013, 3, 30. [Google Scholar] [CrossRef]
- Diederich, M.; Cerella, C. Non-Canonical Programmed Cell Death Mechanisms Triggered by Natural Compounds. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2016; pp. 4–34. [Google Scholar] [CrossRef]
- Greco, G.; Catanzaro, E.; Fimognari, C. Natural Products as Inducers of Non-Canonical Cell Death: A Weapon against Cancer. Cancers 2021, 13, 304. [Google Scholar] [CrossRef]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3- Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef]
- Lukitasari, M.; Nugroho, D.A.; Widodo, N. Chlorogenic Acid: The Conceivable Chemosensitizer Leading to Cancer Growth Suppression. J. Evid.-Based Integr. Med. 2018, 23, 2515690X18789628. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.L.; Xue, N.N.; Li, C.; Guo, H.H.; Ren, T.K.; Zhan, Y.; Li, W.B.; Zhang, J.; Chen, X.G.; et al. Chlorogenic Acid Effectively Treats Cancers through Induction of Cancer Cell Differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef]
- Li, Y.; Pu, R.; Zhou, L.; Wang, D.; Li, X. Effects of a Chlorogenic Acid-Containing Herbal Medicine (LASNB) on Colon Cancer. Evid.-Based Complement. Altern. Med. 2021, 2021, 9923467. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Li, Y.; Hu, Y.; Zhang, Q.; Huang, Y.; Shi, K.; Ran, C.; Hou, J.; Zhou, G.; et al. Chlorogenic Acid Decreases Malignant Characteristics of Hepatocellular Carcinoma Cells by Inhibiting DNMT1 Expression. Front. Pharmacol. 2020, 11, 867. [Google Scholar] [CrossRef]
- Hsu, P.H.; Chen, W.H.; Juanlu, C.; Hsieh, S.C.; Lin, S.C.; Mai, R.T.; Chen, S.Y. Hesperidin and Chlorogenic Acid Synergistically Inhibit the Growth of Breast Cancer Cells via Estrogen Receptor/Mitochondrial Pathway. Life 2021, 11, 950. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Villeneuve, N.F.; Lamore, S.D.; Bause, A.S.; Jiang, T.; Zhang, D.D. The Cinnamon-Derived Dietary Factor Cinnamic Aldehyde Activates the Nrf2-Dependent Antioxidant Response in Human Epithelial Colon Cells. Molecules 2010, 15, 3338–3355. [Google Scholar] [CrossRef]
- Liu, Y.; An, T.; Wan, D.; Yu, B.; Fan, Y.; Pei, X. Targets and Mechanism Used by Cinnamaldehyde, the Main Active Ingredient in Cinnamon, in the Treatment of Breast Cancer. Front. Pharmacol. 2020, 11, 582719. [Google Scholar] [CrossRef]
- Nagle, A.A.; Gan, F.F.; Jones, G.; So, C.L.; Wells, G.; Chew, E.H. Induction of Tumor Cell Death through Targeting Tubulin and Evoking Dysregulation of Cell Cycle Regulatory Proteins by Multifunctional Cinnamaldehydes. PLoS ONE 2012, 7, e50125. [Google Scholar] [CrossRef]
- Zhang, J.H.; Liu, L.Q.; He, Y.L.; Kong, W.J.; Huang, S.A. Cytotoxic Effect of Trans-Cinnamaldehyde on Human Leukemia K562 Cells. Acta Pharmacol. Sin. 2010, 31, 861–866. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wang, L.; Chen, B.; Zhu, M.; Ma, C.; Mu, C.; Tao, A.; Li, S.; Luo, L.; et al. Cinnamaldehyde Suppressed EGF-Induced EMT Process and Inhibits Ovarian Cancer Progression Through PI3K/AKT Pathway. Front. Pharmacol. 2022, 13, 779608. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 2017, 15, 81–94. [Google Scholar] [CrossRef]
- Ishii, G.; Ochiai, A.; Neri, S. Phenotypic and Functional Heterogeneity of Cancer-Associated Fibroblast within the Tumor Microenvironment. Adv. Drug Deliv. Rev. 2016, 99, 186–196. [Google Scholar] [CrossRef]
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer Heterogeneity: Implications for Targeted Therapeutics. Br. J. Cancer 2013, 108, 479. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA—J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, QLD, Australia, 2022; Chapter 3. Available online: https://www.ncbi.nlm.nih.gov/books/NBK583808/ (accessed on 23 February 2024).
- Cummings, M.C.; Chambers, R.; Simpson, P.T.; Lakhani, S.R. Molecular Classification of Breast Cancer: Is It Time to Pack up Our Microscopes? Pathology 2011, 43, 1–8. [Google Scholar] [CrossRef]
- Eliyatkın, N.; Yalçın, E.; Zengel, B.; Aktaş, S.; Vardar, E. Molecular Classification of Breast Carcinoma: From Traditional, Old-Fashioned Way to A New Age, and A New Way. J. Breast Health 2015, 11, 59. [Google Scholar] [CrossRef]
- Mubtasim, N.; Moustaid-Moussa, N.; Gollahon, L. The Complex Biology of the Obesity-Induced, Metastasis-Promoting Tumor Microenvironment in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 2480. [Google Scholar] [CrossRef]
- Zagami, P.; Carey, L.A. Triple Negative Breast Cancer: Pitfalls and Progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef]
- Smolarz, B.; Zadrożna Nowak, A.; Romanowicz, H. Breast Cancer—Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Nakamura, H.; Maeda, H. Cancer Chemotherapy. In Fundamentals of Pharmaceutical Nanoscience; Springer: Berlin/Heidelberg, Germany, 2023; pp. 401–427. [Google Scholar] [CrossRef]
- Chemotherapy Side Effects|American Cancer Society. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/chemotherapy-side-effects.html (accessed on 1 May 2023).
- Possible Side Effects|SEER Training. Available online: https://training.seer.cancer.gov/treatment/chemotherapy/ (accessed on 1 May 2023).
- Liu, S.; Kurzrock, R. Toxicity of Targeted Therapy: Implications for Response and Impact of Genetic Polymorphisms. Cancer Treat. Rev. 2014, 40, 883–891. [Google Scholar] [CrossRef]
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef]
- Sledge, G.W.; Mamounas, E.P.; Hortobagyi, G.N.; Burstein, H.J.; Goodwin, P.J.; Wolff, A.C. Past, Present, and Future Challenges in Breast Cancer Treatment. J. Clin. Oncol. 2014, 32, 1979. [Google Scholar] [CrossRef]
- Moulder, S.; Hortobagyi, G.N. Advances in the Treatment of Breast Cancer. Clin. Pharmacol. Ther. 2008, 83, 26–36. [Google Scholar] [CrossRef]
- Galmarini, C.M. Why We Do What We Do. A Brief Analysis of Cancer Therapies. EXCLI J. 2020, 19, 1401. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Tillotson, G.S.; Zinner, S.H. Expert Review of Anti-Infective Therapy Paul Ehrlich, a Prescient Pioneer in the Field of Antimicrobial Chemotherapy: What Did He Foresee a Century Ago? Expert. Rev. Anti-Infect. Ther. 2013, 11, 113–115. [Google Scholar] [CrossRef][Green Version]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Schuster, C.; Wolpert, N.; Moustaid-Moussa, N.; Gollahon, L.S. Combinatorial Effects of the Natural Products Arctigenin, Chlorogenic Acid, and Cinnamaldehyde Commit Oxidation Assassination on Breast Cancer Cells. Antioxidants 2022, 11, 591. [Google Scholar] [CrossRef]
- Gollahon, L.S.; Jeong, Y.; Finckbone, V.; Lee, K.; Park, J.S. The Natural Product NI-07 Is Effective Against Breast Cancer Cells While Showing No Cytotoxicity to Normal Cells. Open Breast Cancer J. 2011, 3, 31–44. [Google Scholar] [CrossRef]
- Steward, W.P.; Brown, K. Cancer Chemoprevention: A Rapidly Evolving Field. Br. J. Cancer 2013, 109, 1. [Google Scholar] [CrossRef]
- Mohan Shankar, G.; Swetha, M.; Keerthana, C.K.; Rayginia, T.P.; Anto, R.J. Cancer Chemoprevention: A Strategic Approach Using Phytochemicals. Front. Pharmacol. 2022, 12, 9308. [Google Scholar] [CrossRef]
- Ganesan, K.; Du, B.; Chen, J. Effects and Mechanisms of Dietary Bioactive Compounds on Breast Cancer Prevention. Pharmacol. Res. 2022, 178, 105974. [Google Scholar] [CrossRef]
- Noel, B.; Singh, S.K.; Lillard, J.W.; Singh, R. Role of Natural Compounds in Preventing and Treating Breast Cancer. Front. Biosci. 2020, 12, 137. [Google Scholar] [CrossRef]
- Chen, C.; Ah-Ng, T.K. Dietary Cancer-Chemopreventive Compounds: From Signaling and Gene Expression to Pharmacological Effects. Trends Pharmacol. Sci. 2005, 26, 318–326. [Google Scholar] [CrossRef]
- Espiritu, M.J.; Chen, J.; Yadav, J.; Larkin, M.; Pelletier, R.D.; Chan, J.M.; Gc, J.B.; Natesan, S.; Harrelson, J.P. Mechanisms of Herb-Drug Interactions Involving Cinnamon and Cyp2a6: Focus on Time-Dependent Inhibition by Cinnamaldehyde and 2-Methoxycinnamaldehyde. Drug Metab. Dispos. 2020, 48, 1028–1043. [Google Scholar] [CrossRef]
- Mocanu, M.M.; Nagy, P.; Szöllosi, J.; Mayence, A. Chemoprevention of Breast Cancer by Dietary Polyphenols. Molecules 2015, 20, 22578–22620. [Google Scholar] [CrossRef]
- Belkaid, A.; Currie, J.C.; Desgagnés, J.; Annabi, B. The Chemopreventive Properties of Chlorogenic Acid Reveal a Potential New Role for the Microsomal Glucose-6-Phosphate Translocase in Brain Tumor Progression. Cancer Cell Int. 2006, 6, 7. [Google Scholar] [CrossRef]
- Israel, B.B.; Tilghman, S.L.; Parker-Lemieux, K.; Payton-Stewart, F. Phytochemicals: Current Strategies for Treating Breast Cancer. Oncol. Lett. 2018, 15, 7471. [Google Scholar] [CrossRef]
- Lee, J.; Gollahon, L. Mitotic Perturbations Induced by Nek2 Overexpression Require Interaction with TRF1 in Breast Cancer Cells. Cell Cycle 2013, 12, 3599–3614. [Google Scholar] [CrossRef][Green Version]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women with Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J. Clin. Oncol. 2016, 34, 1689–1701. [Google Scholar] [CrossRef]
- Liu, R.; Yu, X.; Chen, X.; Zhong, H.; Liang, C.; Xu, X.; Xu, W.; Cheng, Y.; Wang, W.; Yu, L.; et al. Individual Factors Define the Overall Effects of Dietary Genistein Exposure on Breast Cancer Patients. Nutr. Res. 2019, 67, 1–16. [Google Scholar] [CrossRef]
- Gu, J.W.; Makey, K.L.; Tucker, K.B.; Chinchar, E.; Mao, X.; Pei, I.; Thomas, E.Y.; Miele, L. EGCG, a Major Green Tea Catechin Suppresses Breast Tumor Angiogenesis and Growth via Inhibiting the Activation of HIF-1α and NFκB, and VEGF Expression. Vasc. Cell 2013, 5, 9. [Google Scholar] [CrossRef]
- Sen, T.; Chatterjee, A. Epigallocatechin-3-Gallate (EGCG) Downregulates EGF-Induced MMP-9 in Breast Cancer Cells: Involvement of Integrin Receptor A5β1 in the Process. Eur. J. Nutr. 2011, 50, 465–478. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Sulaiman, G.M.; Anwar, S.S.; Tawfeeq, A.T.; Khan, R.A.; Mohammed, S.A.A.; Al-Omar, M.S.; Alsharidah, M.; Rugaie, O.A.; Al-Amiery, A.A. Quercetin against MCF7 and CAL51 Breast Cancer Cell Lines: Apoptosis, Gene Expression and Cytotoxicity of Nano-Quercetin. Nanomedicine 2021, 16, 1937–1961. [Google Scholar] [CrossRef]
- Ham, S.L.; Nasrollahi, S.; Shah, K.N.; Soltisz, A.; Paruchuri, S.; Yun, Y.H.; Luker, G.D.; Bishayee, A.; Tavana, H. Phytochemicals Potently Inhibit Migration of Metastatic Breast Cancer Cells. Integr. Biol. 2015, 7, 792–800. [Google Scholar] [CrossRef]
- Wu, D.; Jia, H.; Zhang, Z.; Li, S. Capsaicin Suppresses Breast Cancer Cell Viability by Regulating the CDK8/PI3K/Akt/Wnt/Β-catenin Signaling Pathway. Mol. Med. Rep. 2020, 22, 4868–4876. [Google Scholar] [CrossRef]
- Johnson, K.P.; Yearby, L.A.; Stoute, D.; Burow, M.E.; Rhodes, L.V.; Gray, M.; Carriere, P.; Tilghman, S.L.; McLachlan, J.A.; Ochieng, J. In Vitro and In Vivo Evaluation of Novel Anticancer Agents in Triple Negative Breast Cancer Models. J. Health Care Poor Underserved 2013, 24, 104. [Google Scholar] [CrossRef]
- Adams, L.S.; Phung, S.; Yee, N.; Seeram, N.P.; Li, L.; Chen, S. Blueberry Phytochemicals Inhibit Growth and Metastatic Potential of MDA-MB-231 Breast Cancer Cells Through Modulation of the Phosphatidylinositol 3-Kinase Pathway. Cancer Res. 2010, 70, 3594. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Wang, X.; Li, J.; Cui, H.; Niu, M. Ganoderic Acids Suppress Growth and Angiogenesis by Modulating the NF-ΚB Signaling Pathway in Breast Cancer Cells. Int. J. Clin. Pharmacol. Ther. 2012, 50, 712–721. [Google Scholar] [CrossRef]
- Khan, M.A.; Siddiqui, S.; Ahmad, I.; Singh, R.; Mishra, D.P.; Srivastava, A.N.; Ahmad, R. Phytochemicals from Ajwa Dates Pulp Extract Induce Apoptosis in Human Triple-Negative Breast Cancer by Inhibiting AKT/MTOR Pathway and Modulating Bcl-2 Family Proteins. Sci. Rep. 2021, 11, 10322. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting Cancer Stem Cells and Signaling Pathways by Phytochemicals: Novel Approach for Breast Cancer Therapy. Semin. Cancer Biol. 2016, 40–41, 192–208. [Google Scholar] [CrossRef]
- Paplomata, E.; O’regan, R. The PI3K/AKT/MTOR Pathway in Breast Cancer: Targets, Trials and Biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Asúnsolo, Á.; Buján, J.; García-Honduvilla, N.; Coca, S. Signal Transduction Pathways in Breast Cancer: The Important Role of PI3K/Akt/MTOR. J. Oncol. 2020, 2020, 9258396. [Google Scholar] [CrossRef]
- Prajapati, K.S.; Gupta, S.; Kumar, S. Targeting Breast Cancer-Derived Stem Cells by Dietary Phytochemicals: A Strategy for Cancer Prevention and Treatment. Cancers 2022, 14, 2864. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mazumdar, M.; Chakraborty, S.; Manna, A.; Saha, S.; Khan, P.; Bhattacharjee, P.; Guha, D.; Adhikary, A.; Mukhjerjee, S.; et al. Curcumin Inhibits Breast Cancer Stem Cell Migration by Amplifying the E-Cadherin/β-Catenin Negative Feedback Loop. Stem Cell Res. Ther. 2014, 5, 116. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Xie, C.; Zhu, J.; Meng, Y.; Chen, Y.; Li, Y.; Jiang, Y.; Yang, X.; Wang, S.; et al. Sonic Hedgehog and Wnt/β-Catenin Pathways Mediate Curcumin Inhibition of Breast Cancer Stem Cells. Anticancer Drugs 2018, 29, 208–215. [Google Scholar] [CrossRef]
- Fu, Y.; Chang, H.; Peng, X.; Bai, Q.; Yi, L.; Zhou, Y.; Zhu, J.; Mi, M. Resveratrol Inhibits Breast Cancer Stem-Like Cells and Induces Autophagy via Suppressing Wnt/β-Catenin Signaling Pathway. PLoS ONE 2014, 9, e102535. [Google Scholar] [CrossRef]
- Ock, C.W.; Kim, G.D. Dioscin Decreases Breast Cancer Stem-like Cell Proliferation via Cell Cycle Arrest by Modulating P38 Mitogen-Activated Protein Kinase and AKT/MTOR Signaling Pathways. J. Cancer Prev. 2021, 26, 183. [Google Scholar] [CrossRef]
- Wang, R.; Yang, L.; Li, S.; Ye, D.; Yang, L.; Liu, Q.; Zhao, Z.; Cai, Q.; Tan, J.; Li, X. Quercetin Inhibits Breast Cancer Stem Cells via Downregulation of Aldehyde Dehydrogenase 1A1 (ALDH1A1), Chemokine Receptor Type 4 (CXCR4), Mucin 1 (MUC1), and Epithelial Cell Adhesion Molecule (EpCAM). Med. Sci. Monit. 2018, 24, 412. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Sun, S.; Chen, Z.; Xiang, S.; Ding, Z.; Huang, Z.; Zhang, B. Understanding the Versatile Roles and Applications of EpCAM in Cancers: From Bench to Bedside. Exp. Hematol. Oncol. 2022, 11, 97. [Google Scholar] [CrossRef]
- Binienda, A.; Ziolkowska, S.; Pluciennik, E. The Anticancer Properties of Silibinin: Its Molecular Mechanism and Therapeutic Effect in Breast Cancer. Anticancer Agents Med. Chem. 2020, 20, 1787–1796. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Chattopadhyay, S.; Siddiqui, F.A.; Ur Rehman, A.; Siddiqui, S.; Prakasam, G.; Khan, A.; Sultana, S.; Bamezai, R.N.K. Silibinin Induces Metabolic Crisis in Triple-Negative Breast Cancer Cells by Modulating EGFR-MYC-TXNIP Axis: Potential Therapeutic Implications. FEBS J. 2021, 288, 471–485. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharmacol. 2018, 9, 409609. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Kaur, J.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Kulkarni, S.; Kaur, G.; Sak, K.; Kumar, M.; et al. Garcinol Exhibits Anti-Neoplastic Effects by Targeting Diverse Oncogenic Factors in Tumor Cells. Biomedicines 2020, 8, 103. [Google Scholar] [CrossRef]
- Castro, N.P.; Rangel, M.C.; Merchant, A.S.; MacKinnon, G.; Cuttitta, F.; Salomon, D.S.; Kim, Y.S. Sulforaphane Suppresses the Growth of Triple-Negative Breast Cancer Stem-like Cells in Vitro and in Vivo. Cancer Prev. Res. 2019, 12, 147. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic Acid: A Comprehensive Review of the Dietary Sources, Processing Effects, Bioavailability, Beneficial Properties, Mechanisms of Action, and Future Directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 1396. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The Potential Effects of Chlorogenic Acid, the Main Phenolic Components in Coffee, on Health: A Comprehensive Review of the Literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Kapil, A.; Koul, I.B.; Suri, P. Antihepatotoxic Effects of Chlorogenic Acid from Anthocephalus Cadamba. Phytother. Res. 1995, 9, 189–193. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic Acid and Mental Diseases: From Chemistry to Medicine. Curr. Neuropharmacol. 2016, 15, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Yang, K.; Chen, L.; Liu, M.; Geng, Q.; He, X.; Li, Y.; Liu, Y.; Tian, J. Cinnamaldehyde, a Promising Natural Preservative Against Aspergillus Flavus. Front. Microbiol. 2019, 10, 2895. [Google Scholar] [CrossRef]
- Kim, T.W. Cinnamaldehyde Induces Autophagy-Mediated Cell Death through ER Stress and Epigenetic Modification in Gastric Cancer Cells. Acta Pharmacol. Sin. 2022, 43, 712–723. [Google Scholar] [CrossRef]
- Lu, L.; Xiong, Y.; Zhou, J.; Wang, G.; Mi, B.; Liu, G. The Therapeutic Roles of Cinnamaldehyde against Cardiovascular Diseases. Oxid. Med. Cell Longev. 2022, 2022, 9177108. [Google Scholar] [CrossRef]
- Pineda, C.T.; Ramanathan, S.; Fon Tacer, K.; Weon, J.L.; Potts, M.B.; Ou, Y.H.; White, M.A.; Potts, P.R. Degradation of AMPK by a Cancer-Specific Ubiquitin Ligase. Cell 2015, 160, 715–728. [Google Scholar] [CrossRef]
- Moral, R.; Escrich, E. Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms. Molecules 2022, 27, 477. [Google Scholar] [CrossRef]
- Gullett, N.P.; Ruhul Amin, A.R.M.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Aggarwal, B.B.; Surh, Y.J.; Kucuk, O. Cancer Prevention with Natural Compounds. Semin. Oncol. 2010, 37, 258–281. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rahman, T. The difficulties in cancer treatment. Ecancermedicalscience 2012, 6, ed16. [Google Scholar] [CrossRef] [PubMed]
- ShiDu Yan, S. Disrupting Cancer Cell Function by Targeting Mitochondria. Integr. Cancer Sci. Ther. 2014, 1, 17–25. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.; Goldkorn, A. Telomere and Telomerase Therapeutics in Cancer. Genes 2016, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Ma, J.; Xu, Y.; Wang, Y.; Hu, M.; Ma, F.; Qin, Z.; Xue, R.; Tao, N. Cinnamaldehyde Treatment of Prostate Cancer-Associated Fibroblasts Prevents Their Inhibitory Effect on T Cells through Toll-like Receptor 4. Drug Des. Devel Ther. 2020, 14, 3363–3372. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Hao, J.; Zhang, Q.; Jin, R.; Luo, Z.; Yang, X.; Liu, Y.; Lu, Q.; Ouyang, Y.; Guo, H. Chlorogenic Acid Inhibits Epithelial-Mesenchymal Transition and Invasion of Breast Cancer by Down-Regulating LRP6S. J. Pharmacol. Exp. Ther. 2023, 384, 254–264. [Google Scholar] [CrossRef]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic Acid for Cancer Prevention and Therapy: Current Status on Efficacy and Mechanisms of Action. Pharmacol. Res. 2022, 186, 106505. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic Acid Induces Apoptosis, Inhibits Metastasis and Improves Antitumor Immunity in Breast Cancer via the NF-ΚB Signaling Pathway. Oncol. Rep. 2021, 45, 717–727. [Google Scholar] [CrossRef]
- Padmanaban, V.; Krol, I.; Suhail, Y.; Szczerba, B.M.; Aceto, N.; Bader, J.S.; Ewald, A.J. E-Cadherin Is Required for Metastasis in Multiple Models of Breast Cancer. Nature 2019, 573, 439–444. [Google Scholar] [CrossRef]
- Ciudad-Mulero, M.; Morales, P.; Fernández-Ruiz, V.; Murai, T.; Matsuda, S. The Chemopreventive Effects of Chlorogenic Acids, Phenolic Compounds in Coffee, against Inflammation, Cancer, and Neurological Diseases. Molecules 2023, 28, 2381. [Google Scholar] [CrossRef]
- Yi, X.; Pei, T.; Li, S.; Huang, L. Cinnamic Aldehyde Induces Apoptosis of Breast Cancer Cells via STAT3/CMyc Pathway. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Jeong, H.W.; Han, D.C.; Son, K.H.; Han, M.Y.; Lim, J.S.; Ha, J.H.; Lee, C.W.; Kim, H.M.; Kim, H.C.; Kwon, B.M. Antitumor Effect of the Cinnamaldehyde Derivative CB403 through the Arrest of Cell Cycle Progression in the G(2)/M Phase. Biochem. Pharmacol. 2003, 65, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Changizi, Z.; Moslehi, A.; Rohani, A.H.; Eidi, A. Chlorogenic Acid Inhibits Growth of 4T1 Breast Cancer Cells through Involvement in Bax/Bcl2 Pathway. J. Cancer Res. Ther. 2020, 16, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Hwang, S.J.; Park, J.H.; Lee, H.J. Chlorogenic Acid Inhibits Hypoxia-Induced Angiogenesis via down-Regulation of the HIF-1α/AKT Pathway. Cell. Oncol. 2015, 38, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hu, J.; Zhou, X.; Cheung, P.C.K. Inhibition of Vascular Endothelial Growth Factor-Induced Angiogenesis by Chlorogenic Acid via Targeting the Vascular Endothelial Growth Factor Receptor 2-Mediated Signaling Pathway. J. Funct. Food 2017, 32, 285–295. [Google Scholar] [CrossRef]
- Bae, W.Y.; Choi, J.S.; Kim, J.E.; Jeong, J.W. Cinnamic Aldehyde Suppresses Hypoxia-Induced Angiogenesis via Inhibition of Hypoxia-Inducible Factor-1α Expression during Tumor Progression. Biochem. Pharmacol. 2015, 98, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Muntean, D.M.; Sturza, A.; Pavel, I.Z.; Duicu, O.M. Modulation of Cancer Metabolism by Phytochemicals—A Brief Overview. Anticancer Agents Med. Chem. 2018, 18, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, H.J.; Sim, D.Y.; Park, J.E.; Ahn, C.H.; Park, S.Y.; Jang, E.; Kim, B.; Kim, S.H. The Antitumor Effect of Cinnamaldehyde Derivative CB-PIC in Hepatocellular Carcinoma Cells via Inhibition of Pyruvate and STAT3 Signaling. Int. J. Mol. Sci. 2022, 23, 6461. [Google Scholar] [CrossRef] [PubMed]
- Skupień, K.; Kostrzewa-Nowak, D.; Oszmiański, J.; Tarasiuk, J. In Vitro Antileukaemic Activity of Extracts from Chokeberry (Aronia Melanocarpa [Michx] Elliott) and Mulberry (Morus alba L.) Leaves against Sensitive and Multidrug Resistant HL60 Cells. Phytother. Res. 2008, 22, 689–694. [Google Scholar] [CrossRef]
- Ganesan, M.; Kanimozhi, G.; Pradhapsingh, B.; Khan, H.A.; Alhomida, A.S.; Ekhzaimy, A.; Brindha, G.R.; Prasad, N.R. Phytochemicals Reverse P-Glycoprotein Mediated Multidrug Resistance via Signal Transduction Pathways. Biomed. Pharmacother. 2021, 139, 111632. [Google Scholar] [CrossRef]
- Yun, M.; Lee, D.; Park, M.N.; Kim, E.O.; Sohn, E.J.; Kwon, B.M.; Kim, S.H. Cinnamaldehyde Derivative (CB-PIC) Sensitizes Chemo-Resistant Cancer Cells to Drug-Induced Apoptosis via Suppression of MDR1 and Its Upstream STAT3 and AKT Signalling. Cell Physiol. Biochem. 2015, 35, 1821–1830. [Google Scholar] [CrossRef]
- Lee, W.J.; Zhu, B.T. Inhibition of DNA Methylation by Caffeic Acid and Chlorogenic Acid, Two Common Catechol-Containing Coffee Polyphenols. Carcinogenesis 2006, 27, 269–277. [Google Scholar] [CrossRef]
- Bartolomeu, A.R.; Romualdo, G.R.; Lisón, C.G.; Besharat, Z.M.; Corrales, J.A.M.; Chaves, M.Á.G.; Barbisan, L.F. Caffeine and Chlorogenic Acid Combination Attenuate Early-Stage Chemically Induced Colon Carcinogenesis in Mice: Involvement of OncomiR MiR-21a-5p. Int. J. Mol. Sci. 2022, 23, 6292. [Google Scholar] [CrossRef]
- Nakayama, T.; Funakoshi-Tago, M.; Tamura, H. Coffee Reduces KRAS Expression in Caco-2 Human Colon Carcinoma Cells via Regulation of MiRNAs. Oncol. Lett. 2017, 14, 1109–1114. [Google Scholar] [CrossRef]
- Wang, R.P.; Wang, G.; Sun, Q.M.; Wu, J.; Zou, X. Inhibitory effect of cinnamaldehyde on invasion capacities of human breast cancer cell line MDA-MB-435S and its relation with regulating the expression of miR-27a. Chin. J. Integr. Tradit. West. Med. 2014, 34, 964–969. [Google Scholar]
- Chen, R.; Wu, J.; Lu, C.; Yan, T.; Qian, Y.; Shen, H.; Zhao, Y.; Wang, J.; Kong, P.; Zhang, X. Systematic Transcriptome Analysis Reveals the Inhibitory Function of Cinnamaldehyde in Non-Small Cell Lung Cancer. Front. Pharmacol. 2021, 11, 2479. [Google Scholar] [CrossRef]
- Tian, F.; Yu, C.T.; Ye, W.D.; Wang, Q. Cinnamaldehyde Induces Cell Apoptosis Mediated by a Novel Circular RNA Hsa_circ_0043256 in Non-Small Cell Lung Cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1260–1266. [Google Scholar] [CrossRef]
- Banerjee, S.; Banerjee, S. Anticancer Potential and Molecular Mechanisms of Cinnamaldehyde and Its Congeners Present in the Cinnamon Plant. Physiologia 2023, 3, 173–207. [Google Scholar] [CrossRef]
- Chu, S.C.; Hsieh, Y.S.; Hsu, L.S.; Lin, C.Y.; Lai, Y.A.; Chen, P.N. Cinnamaldehyde Decreases the Invasion and U-PA Expression of Osteosarcoma by down-Regulating the FAK Signalling Pathway. Food Funct. 2022, 13, 6574–6582. [Google Scholar] [CrossRef]
- Wu, C.; Zhuang, Y.; Jiang, S.; Tian, F.; Teng, Y.; Chen, X.; Zheng, P.; Liu, S.; Zhou, J.; Wu, J.; et al. Cinnamaldehyde Induces Apoptosis and Reverses Epithelial-Mesenchymal Transition through Inhibition of Wnt/β-Catenin Pathway in Non-Small Cell Lung Cancer. Int. J. Biochem. Cell Biol. 2017, 84, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Nakagawara, A. Role of P53 in Cell Death and Human Cancers. Cancers 2011, 3, 994. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of P53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. P21 in Cancer: Intricate Networks and Multiple Activities. Nat. Rev. Cancer 2009, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Engeland, K. Cell Cycle Regulation: P53-P21-RB Signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95. [Google Scholar] [CrossRef]
- Ma, J.H.; Qin, L.; Li, X. Role of STAT3 Signaling Pathway in Breast Cancer. Cell Commun. Signal. 2020, 18, 33. [Google Scholar] [CrossRef]
- Yang, H.; Said, A.M.; Huang, H.; Papa, A.P.D.; Jin, G.; Wu, S.; Ma, N.; Lan, L.; Shangguan, F.; Zhang, Q. Chlorogenic Acid Depresses Cellular Bioenergetics to Suppress Pancreatic Carcinoma through Modulating C-Myc-TFR1 Axis. Phytother. Res. 2021, 35, 2200–2210. [Google Scholar] [CrossRef]
- Vélez-Vargas, L.C.; Santa-González, G.A.; Uribe, D.; Henao-Castañeda, I.C.; Pedroza-Díaz, J. In Vitro and In Silico Study on the Impact of Chlorogenic Acid in Colorectal Cancer Cells: Proliferation, Apoptosis, and Interaction with β-Catenin and LRP6. Pharmaceuticals 2023, 16, 276. [Google Scholar] [CrossRef]
- Lin, B.; Ma, R.; Wu, J.; Du, S.; Lv, Y.; Yu, H.; Zhang, W.; Mao, S.; Liu, G.; Bu, Y.; et al. Cinnamaldehyde Alleviates Bone Loss by Targeting Oxidative Stress and Mitochondrial Damage via the Nrf2/HO-1 Pathway in BMSCs and Ovariectomized Mice. J. Agric. Food Chem. 2023, 71, 17362–17378. [Google Scholar] [CrossRef] [PubMed]
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018, 8, 248. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in Cancer. Vasc. Health Risk Manag. 2006, 2, 213. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, K.; Nam, S.; Anderson, R.A.; Jove, R.; Wen, W. Novel Angiogenesis Inhibitory Activity in Cinnamon Extract Blocks VEGFR2 Kinase and Downstream Signaling. Carcinogenesis 2010, 31, 481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Powell, K.; Li, L. Breast Cancer Stem Cells: Biomarkers, Identification and Isolation Methods, Regulating Mechanisms, Cellular Origin, and Beyond. Cancers 2020, 12, 3765. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Farzaneh, M. Signaling Pathways Governing Breast Cancer Stem Cells Behavior. Stem Cell Res. Ther. 2021, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Wang, K.; Wang, Y.; Yin, W.; Li, L. P38/NF-ΚB/Snail Pathway Is Involved in Caffeic Acid-Induced Inhibition of Cancer Stem Cells-Like Properties and Migratory Capacity in Malignant Human Keratinocyte. PLoS ONE 2013, 8, e58915. [Google Scholar] [CrossRef]
- Chen, J.C.; Hsieh, P.S.; Chen, S.M.; Hwang, J.H. Effects of Cinnamaldehyde on the Viability and Expression of Chemokine Receptor Genes in Temozolomide-Treated Glioma Cells. Vivo 2020, 34, 595. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Izawa, Y.; Onodera, D.; Tagami, M. Chlorogenic Acid Regulates Apoptosis and Stem Cell Marker-Related Gene Expression in A549 Human Lung Cancer Cells. Mol. Cell Biochem. 2018, 441, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Läsche, M.; Emons, G.; Gründker, C. Shedding New Light on Cancer Metabolism: A Metabolic Tightrope Between Life and Death. Front. Oncol. 2020, 10, 409. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 2581. [Google Scholar] [CrossRef]
- Li, Y.J.; Lei, Y.H.; Yao, N.; Wang, C.R.; Hu, N.; Ye, W.C.; Zhang, D.M.; Chen, Z.S. Autophagy and Multidrug Resistance in Cancer. Chin. J. Cancer 2017, 36, 52. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, F.U.; Sufiyan Chhipa, A.; Mishra, V.; Gupta, V.K.; Rawat, S.G.; Kumar, A.; Pathak, C. Molecular and Cellular Paradigms of Multidrug Resistance in Cancer. Cancer Rep. 2022, 5, e1291. [Google Scholar] [CrossRef]
- Liu, C.-M.; Su, M.; An, L.; Shao, Z.; Wu, Z.; Han, N.; Ma, Y.; Chen, C.-Y. Anticancer and Overcoming Multidrug Resistance Activities of Potential Phytochemicals. Chem. Pharm. Res. 2022, 4, 1–11. [Google Scholar] [CrossRef]
- Hamed, A.R.; Abdel-Azim, N.S.; Shams, K.A.; Hammouda, F.M. Targeting Multidrug Resistance in Cancer by Natural Chemosensitizers. Bull. Natl. Res. Cent. 2019, 43, 8. [Google Scholar] [CrossRef]
- Tinoush, B.; Shirdel, I.; Wink, M. Phytochemicals: Potential Lead Molecules for MDR Reversal. Front. Pharmacol. 2020, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- İsaoğlu, M.; Güllüce, M.; Karadayı, M. Plant-Derived Natural Products as Multidrug Resistance Modulators in Cancer Therapy. Anatol. J. Biol. 2020, 2, 1–51. [Google Scholar]
- Ahmad, B.; Rizwan, M.; Rauf, A.; Raza, M.; Bashir, S.; Molnar, J.; Csonka, A.; Szabo, D.; Mubarak, M.S.; Noor, M.; et al. Isolation of Chlorogenic Acid from Soil Borne Fungi Screlotium Rolfsii, Their Reversal of Multidrug Resistance and Anti-Proliferative in Mouse Lymphoma Cells. Med. Chem. 2017, 13, 721–726. [Google Scholar] [CrossRef] [PubMed]
- US Patent for Use of Chlorogenic Acid in Preparation of Drug for Treating Chordoma Patent (Patent # 11,547,715 Issued January 10, 2023)—Justia Patents Search. Available online: https://patents.justia.com/patent/11547715 (accessed on 18 May 2023).
- Wang, L.; Zhang, Y.; Liu, Y.; Xu, M.; Yao, Z.; Zhang, X.; Sun, Y.; Zhou, T.; Shen, M. Effects of Chlorogenic Acid on Antimicrobial, Antivirulence, and Anti-Quorum Sensing of Carbapenem-Resistant Klebsiella Pneumoniae. Front. Microbiol. 2022, 13, 5051. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhang, Y.; Fu, S.; Li, Z.; Zhang, J.; Xu, Y.; Han, X.; Miao, J. Application of Chlorogenic Acid as a Substitute for Antibiotics in Multidrug-Resistant Escherichia Coli-Induced Mastitis. Int. Immunopharmacol. 2023, 114, 109536. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Cvijanović, O.; Šušnić, V.; Katalinić, N. Renoprotective Mechanisms of Chlorogenic Acid in Cisplatin-Induced Kidney Injury. Toxicology 2014, 324, 98–107. [Google Scholar] [CrossRef]
- Toumia, I.B.; Sobeh, M.; Ponassi, M.; Banelli, B.; Dameriha, A.; Wink, M.; Ghedira, L.C.; Rosano, C. A Methanol Extract of Scabiosa Atropurpurea Enhances Doxorubicin Cytotoxicity against Resistant Colorectal Cancer Cells In Vitro. Molecules 2020, 25, 5265. [Google Scholar] [CrossRef]
- Tan, S.; Dong, X.; Liu, D.; Hao, S.; He, F. Anti-Tumor Activity of Chlorogenic Acid by Regulating the MTORC2 Signaling Pathway and Disrupting F-Actin Organization. Int. J. Clin. Exp. Med. 2019, 12, 4818–4828. [Google Scholar]
- Yan, Y.; Li, J.; Han, J.; Hou, N.; Song, Y.; Dong, L. Chlorogenic Acid Enhances the Effects of 5-Fluorouracil in Human Hepatocellular Carcinoma Cells through the Inhibition of Extracellular Signal-Regulated Kinases. Anticancer Drugs 2015, 26, 540–546. [Google Scholar] [CrossRef]
- Ekbatan, S.S.; Li, X.Q.; Ghorbani, M.; Azadi, B.; Kubow, S. Chlorogenic Acid and Its Microbial Metabolites Exert Anti-Proliferative Effects, S-Phase Cell-Cycle Arrest and Apoptosis in Human Colon Cancer Caco-2 Cells. Int. J. Mol. Sci. 2018, 19, 723. [Google Scholar] [CrossRef]
- Aliyah, A.N.; Lintangsari, G.; Maran, G.G.; Hermawan, A.; Meiyanto, E. Cinnamon Oil as a Co-Chemotherapy Agent through Inhibition of Cell Migration and MMP-9 Expression on 4T1 Cells. J. Complement. Integr. Med. 2021, 19, 921–928. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Fu, J.; Liu, R.; Zhou, X. Cinnamaldehyde Downregulation of Sept9 Inhibits Glioma Progression through Suppressing Hif-1 α via the Pi3k/Akt Signaling Pathway. Dis. Markers 2022, 2022, 6530934. [Google Scholar] [CrossRef]
- Li, J.; Teng, Y.; Liu, S.; Wang, Z.; Chen, Y.; Zhang, Y.; Xi, S.; Xu, S.; Wang, R.; Zou, X. Cinnamaldehyde Affects the Biological Behavior of Human Colorectal Cancer Cells and Induces Apoptosis via Inhibition of the PI3K/Akt Signaling Pathway. Oncol. Rep. 2016, 35, 1501–1510. [Google Scholar] [CrossRef]
- Vo, A.T.; Millis, R.M. Epigenetics and Breast Cancers. Obs. Gynecol. Int. 2012, 2012, 602720. [Google Scholar] [CrossRef]
- Schröder, R.; Illert, A.L.; Erbes, T.; Flotho, C.; Lübbert, M.; Duque-Afonso, J. The Epigenetics of Breast Cancer—Opportunities for Diagnostics, Risk Stratification and Therapy. Epigenetics 2021, 17, 612–624. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Daniels, K.D.; Grunwald, J.T.; Ramos, S.A.; Propper, C.R.; Selmin, O.I. Epigenetics of Breast Cancer: Modifying Role of Environmental and Bioactive Food Compounds. Mol. Nutr. Food Res. 2016, 60, 1310. [Google Scholar] [CrossRef]
- Huang, Y.; Nayak, S.; Jankowitz, R.; Davidson, N.E.; Oesterreich, S. Epigenetics in Breast Cancer: What’s New? Breast Cancer Res. 2011, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Arora, I.; Tollefsbol, T.O. Impact of Stilbenes as Epigenetic Modulators of Breast Cancer Risk and Associated Biomarkers. Int. J. Mol. Sci. 2021, 22, 10033. [Google Scholar] [CrossRef] [PubMed]
- Carlos-Reyes, Á.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.A.; Astudillo-De La Vega, H.; Hernández De La Cruz, O.N.; López-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, L.C.; Machado, A.R.T.; Tuttis, K.; Ribeiro, D.L.; Aissa, A.F.; Dévoz, P.P.; Antunes, L.M.G. Caffeic Acid and Chlorogenic Acid Cytotoxicity, Genotoxicity, and impact on Global DNA Methylation in Human Leukemic Cell Lines. Genet. Mol. Biol. 2020, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Xu, Y.M.; Lau, A.T.Y. The Epigenetic Effects of Coffee. Molecules 2023, 28, 1770. [Google Scholar] [CrossRef] [PubMed]
- Corrie, K.; Hardman, J.G. Mechanisms of Drug Interactions: Pharmacodynamics and Pharmacokinetics. Anaesth. Intensive Care Med. 2017, 18, 331–334. [Google Scholar] [CrossRef]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The Synergistic and Antagonistic Antioxidant Interactions of Dietary Phytochemical Combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.A.T.; Paterson, J.; Bucknall, M.; Arcot, J. Interactions between Phytochemicals from Fruits and Vegetables: Effects on Bioactivities and Bioavailability. Crit. Rev. Food Sci. Nutr. 2018, 58, 1310–1329. [Google Scholar] [CrossRef]
- Tian, Z.H.; Li, Z.F.; Zhou, S.B.; Liang, Y.Y.; He, D.C.; Wang, D.S. Differentially Expressed Proteins of MCF-7 Human Breast Cancer Cells Affected by Zilongjin, a Complementary Chinese Herbal Medicine. Proteomics Clin Appl 2010, 4, 550–559. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Yao, R.; Li, J.; Yan, Y.; La Regina, M.; Lemon, W.L.; Grubbs, C.J.; Lubet, R.A.; You, M. Cancer Chemopreventive Activity of a Mixture of Chinese Herbs (Antitumor B) in Mouse Lung Tumor Models. Oncogene 2004 2004, 23, 3841–3850. [Google Scholar] [CrossRef]
- Pressete, C.G.; Viegas, F.P.D.; Campos, T.G.; Caixeta, E.S.; Hanemann, J.A.C.; Ferreira-Silva, G.Á.; Zavan, B.; Aissa, A.F.; Miyazawa, M.; Viegas, C., Jr.; et al. Piperine–Chlorogenic Acid Hybrid Inhibits the Proliferation of the SK-MEL-147 Melanoma Cells by Modulating Mitotic Kinases. Pharmaceuticals 2023, 16, 145. [Google Scholar] [CrossRef]
- Meng, M.; Geng, S.; Du, Z.; Yao, J.; Zheng, Y.; Li, Z.; Zhang, Z.; Li, J.; Duan, Y.; Du, G. Berberine and Cinnamaldehyde Together Prevent Lung Carcinogenesis. Oncotarget 2017, 8, 76385. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.T.; Rashidi, S.; Mahmoudi, R.; Douna, B.K. Cytotoxic Activity of Epigallocatechin and Trans-Cinnamaldehyde in Gastric Cancer Cell Line. Asian Pac. J. Cancer Biol. 2019, 4, 71–74. [Google Scholar] [CrossRef]
- Olayiwola, Y.; Gollahon, S.L. Combinatorial chlorogenic acid and cinnamaldehyde demonstrate anti-metastasis and apoptosis induction in MCF7 and MDA-MB-231 breast cancer cells. Obesity Research Institute, Texas Tech University, Lubbock, TX, USA. 2024; Manuscript in preparation. [Google Scholar]

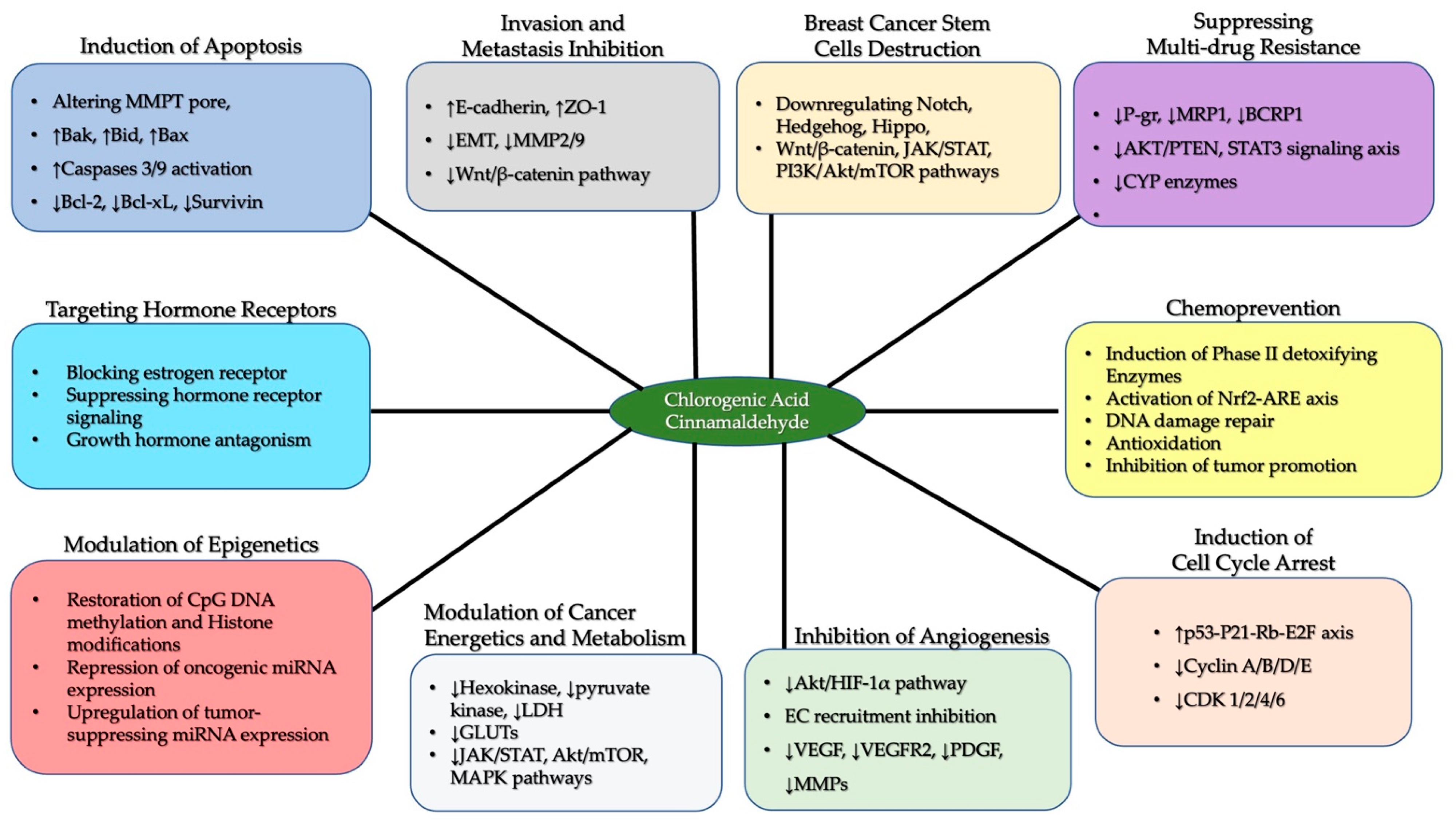
| Anticancer Effect on Breast Cancer Cells | Mechanisms of Action | ||||
|---|---|---|---|---|---|
| Chlorogenic Acid | Ref | Cinnamaldehyde | Ref | ||
| 1. | Anti-invasion and anti- metastasis | 1. Inhibition of EMT 2. Downregulation of LRP6, the part of canonical wnt/β-catenin pathway receptor complex | [96,97,98] | Promotion of E-cadherin expression | [99] |
| 2. | Halting cell cycle progress | 1. Upregulation of the expression and activity of tumor suppressor proteins p53 and p21 2. Downregulation of the MAPK pathway | [13,15,21,97,100] | Inhibition of CDK1, CDC25, CDC20 and survivin expression resulting in: 1. Arrest at G2/M transition 2. Disruption of spindle assembly formation | [19,101,102] |
| 3. | Promoting apoptosis | Promoting mitochondrial-mediated cell death via: 1. Downregulation of Bcl-2 and upregulation of Bax 2. Stabilization of p53 and caspase 3 activity | [98,103] | Driving the intrinsic apoptotic pathway via: The downregulation of Bcl-2 through the downregulation of the JAK2/STAT3/cMyc pathway | [101] |
| 4. | Anti- angiogenesis | 1. Blockage of the Akt/HIF-1α pathway 2. Downregulation of VEGF and VEGF receptor-2 mediated signaling. | [104,105] | Preventing the stabilization of HIF-1α | [106] |
| 5. | Modulation of rewired cancer metabolism | Regulation of the PI3K/Akt/mTOR pathway and HIF-1α expression | [43,107] | 1. Inhibition of the activities of hexokinase and pyruvate kinase, key glycolytic enzymes. 2. Suppression of STAT3 signaling | [108] |
| 6. | Suppression of multi-drug resistance | 1. Downregulation of the expression of P-glycoprotein 2.Modulation of the PI3K/AKT/mTOR/PTEN signaling axis to shut down ABC transporter expression | [100,109] | Suppression of the P-gp ABC pump via the regulation of Akt/STAT3 signal transduction | [110,111] |
| 7. | Regulation of epigenetic programming | 1. Prevention of DNA hypermethylation via inhibition of the DNMT enzyme 2. Downregulation of oncogenic microRNA (oncomiR) such as miR-21a-5p. 3. Promotion of the expression of tumor-suppressing microRNAs (such as miR-30c and miR-96) to target oncogenic transcripts such as KRAS | [112,113,114] | 1. Downregulation of onco-miR such as miR-27a 2. Promotion of tumor-suppressing microRNA and long non-coding RNA to target oncogenes 3. Induction of endogenous circular RNA expression to promote apoptosis and inhibit the wnt/β-catenin pathway. | [115,116,117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olayiwola, Y.; Gollahon, L. Natural Compounds and Breast Cancer: Chemo-Preventive and Therapeutic Capabilities of Chlorogenic Acid and Cinnamaldehyde. Pharmaceuticals 2024, 17, 361. https://doi.org/10.3390/ph17030361
Olayiwola Y, Gollahon L. Natural Compounds and Breast Cancer: Chemo-Preventive and Therapeutic Capabilities of Chlorogenic Acid and Cinnamaldehyde. Pharmaceuticals. 2024; 17(3):361. https://doi.org/10.3390/ph17030361
Chicago/Turabian StyleOlayiwola, Yusuff, and Lauren Gollahon. 2024. "Natural Compounds and Breast Cancer: Chemo-Preventive and Therapeutic Capabilities of Chlorogenic Acid and Cinnamaldehyde" Pharmaceuticals 17, no. 3: 361. https://doi.org/10.3390/ph17030361
APA StyleOlayiwola, Y., & Gollahon, L. (2024). Natural Compounds and Breast Cancer: Chemo-Preventive and Therapeutic Capabilities of Chlorogenic Acid and Cinnamaldehyde. Pharmaceuticals, 17(3), 361. https://doi.org/10.3390/ph17030361








