Novel 2,5-Diketopiperazines with In Vitro Activities against Protozoan Parasites of Tropical Diseases
Abstract
1. Introduction
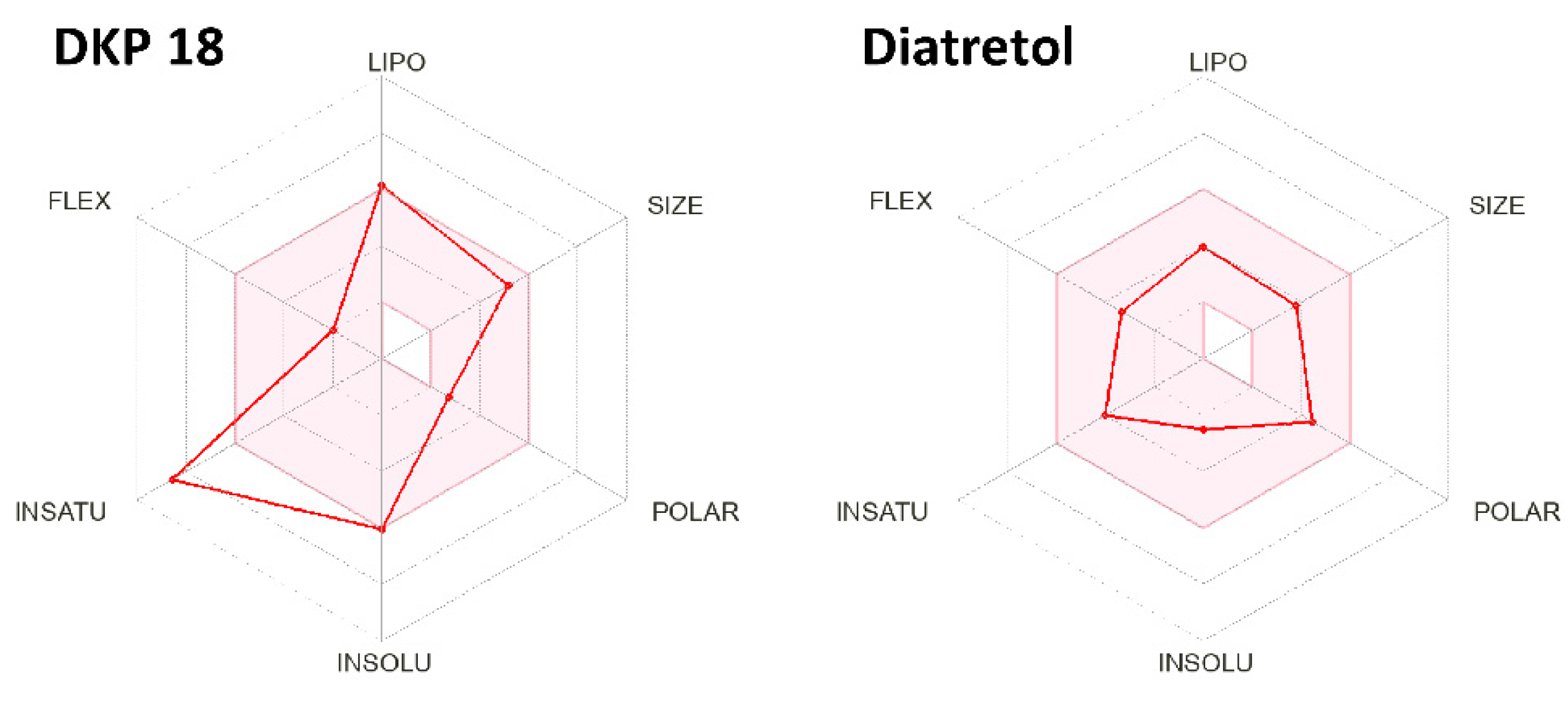
2. Results and Discussion
3. Materials and Methods
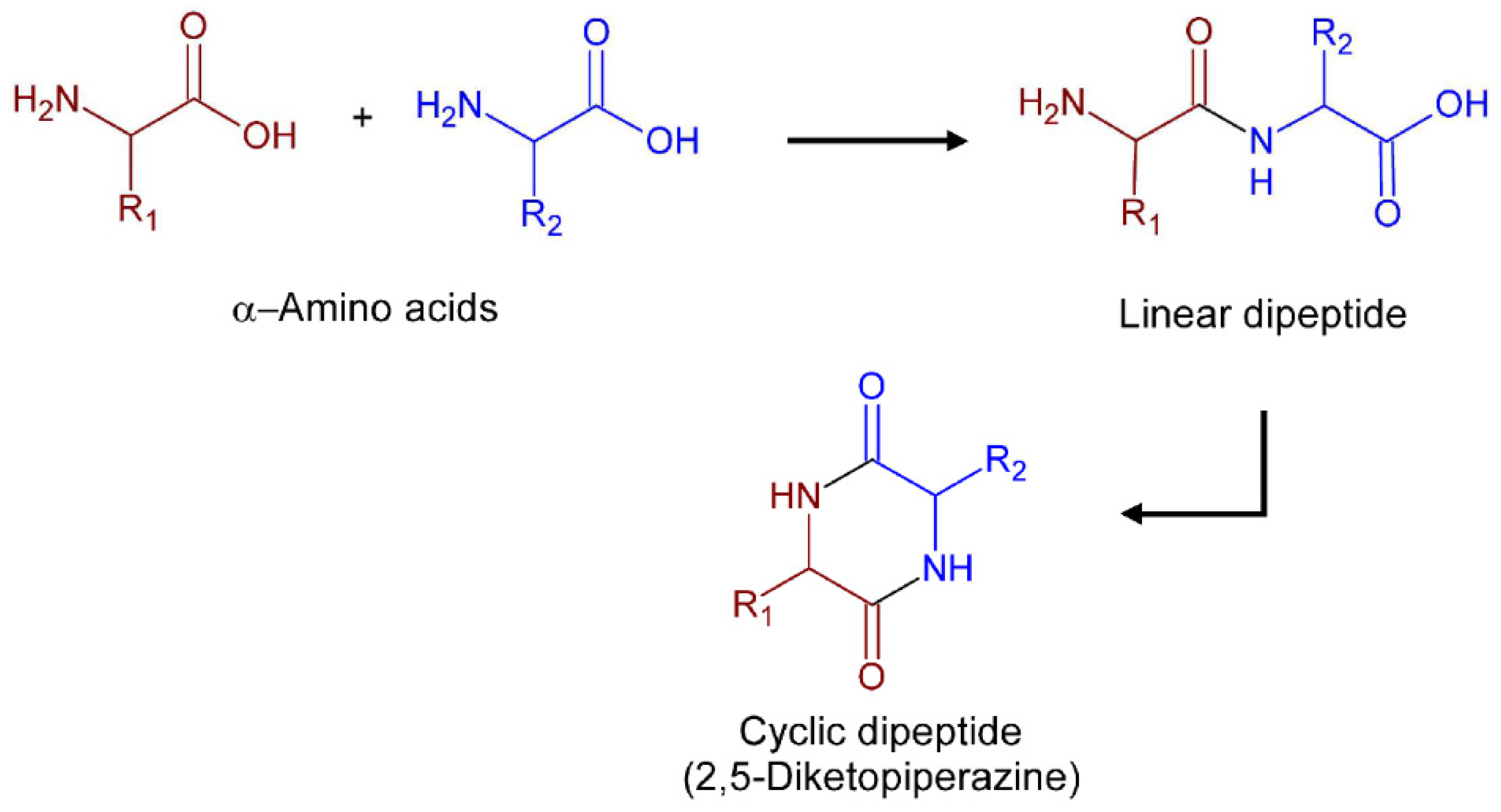
3.1. 2,5-Diketopiperazines
3.2. UHPLC-HRMS
3.3. Anti-Plasmodium falciparum Activity Assay
3.4. Anti-Trypanosoma cruzi Activity Assay
3.5. Anti-Leishmania Infantum Activity Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). World Malaria Report 2022. Available online: Https://www.who.int/Publications/i/Item/9789240064898 (accessed on 19 July 2023).
- World Health Organization (WHO). Chagas Disease. Available online: https://www.who.int/News-Room/Fact-Sheets/Detail/Chagas-Disease-(American-Trypanosomiasis) (accessed on 19 July 2023).
- World Health Organization (WHO). Leishmaniasis. Available online: Https://www.who.int/News-Room/Fact-Sheets/Detail/Leishmaniasis (accessed on 19 July 2023).
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current Trends in the Pharmacological Management of Chagas Disease. Int. J. Parasitol. Drugs Drug Resist. 2020, 12, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Majumder, N.; Banerjee, A.; Saha, S. A Review on New Natural and Synthetic Anti-Leishmanial Chemotherapeutic Agents and Current Perspective of Treatment Approaches. Acta Trop. 2023, 240, 106846. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, M.P.; Abraham, W.-R. Antimicrobial and Biofilm Inhibiting Diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Hou, Y.; Yang, Q.; Li, X.; Wu, S.; Wang, C.-Y.; Wang, B.-G. Marine Drugs Structures and Biological Activities of Diketopiperazines from Marine Organisms: A Review. Mar. Drugs 2021, 19, 403. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Yao, J.; Kong, J.; Yu, A.; Wei, J.; Dong, Y.; Song, R.; Shan, D.; Zhong, X.; Lv, F.; et al. 2,5-Diketopiperazines: A Review of Source, Synthesis, Bioactivity, Structure, and MS Fragmentation. Curr. Med. Chem. 2023, 30, 1060–1085. [Google Scholar] [CrossRef] [PubMed]
- Bojarska, J.; Mieczkowski, A.; Ziora, Z.M.; Skwarczynski, M.; Toth, I.; Shalash, A.O.; Parang, K.; El-Mowafi, S.A.; Mohammed, E.H.M.; Elnagdy, S.; et al. Cyclic Dipeptides: The Biological and Structural Landscape with Special Focus on the Anti-Cancer Proline-Based Scaffold. Biomolecules 2021, 11, 1515. [Google Scholar] [CrossRef]
- Maity, A.; Hazra, A.; Palit, P.; Mondal, S.; Sanchaita, L.; Mondal, N.B. The Cytotoxic Effects of Diketopiperaizes against Leishmania donovani Promastigotes and Amastigotes. Med. Chem. Res. 2013, 22, 3425–3458. [Google Scholar] [CrossRef]
- Guimarães Tunes, L.; Nicolau Gonçalves, V.; Nabak Bueno, D.; Leomar Zani, C.; Henrique Rosa, L.; Barros Cota, B.; Cruz, O.; Horizonte, B. Diketopiperazine Alkaloids Produced by the Endophytic Fungus Penicillium Citrinum and Evaluation of Their Antileishmanial Activity. Afr. J. Microbiol. Res. 2019, 13, 562–567. [Google Scholar]
- Martins-Teixeira, M.B.; Campo, V.L.; Biondo, M.; Sesti-Costa, R.; Carneiro, Z.A.; Silva, J.S.; Carvalho, I. α-Selective Glycosylation Affords Mucin-Related GalNAc Amino Acids and Diketopiperazines Active on Trypanosoma cruzi. Bioorg Med. Chem. 2013, 21, 1978–1987. [Google Scholar] [CrossRef]
- Watts, K.R.; Ratnam, J.; Ang, K.H.; Tenney, K.; Compton, J.E.; McKerrow, J.; Crews, P. Assessing the Trypanocidal Potential of Natural and Semi-Synthetic Diketopiperazines from Two Deep Water Marine-Derived Fungi. Bioorg Med. Chem. 2010, 18, 2566–2574. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, A.; Hokari, R.; Nonaka, K.; Chiba, T.; Miura, H.; Otoguro, K.; Iwatsuki, M. Diatretol, an α, A′-Dioxo-Diketopiperazine, Is a Potent In Vitro and In Vivo Antimalarial. J. Antibiot. 2021, 74, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Picaso, L.; Olivo, H.F.; Argotte-Ramos, R.; Rodríguez-Gutiérrez, M.; Rios, M.Y. Linear and Cyclic Dipeptides with Antimalarial Activity. Bioorg Med. Chem. Lett. 2012, 22, 7048–7051. [Google Scholar] [CrossRef] [PubMed]
- Ugi, I. The α-Addition of Immonium Ions and Anions to Isonitriles Accompanied by Secondary Reactions. Angew. Chem. Int. Ed. 1962, 1, 8–21. [Google Scholar] [CrossRef]
- Mendes, L.L.; Varejão, J.O.S.; De Souza, J.A.; Carneiro, J.W.D.M.; Valdo, A.K.S.M.; Martins, F.T.; Ferreira, B.W.; Barreto, R.W.; Da Silva, T.I.; Kohlhoff, M.; et al. 2,5-Diketopiperazines via Intramolecular N-Alkylation of Ugi Adducts: A Contribution to the Synthesis, Density Functional Theory Study, X-ray Characterization, and Potential Herbicide Application. J. Agric. Food Chem. 2022, 70, 1799–1809. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of In Vitro Bioassay Methods: Application in Herbal Drug Research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [CrossRef]
- Zin, N.M.; Baba, M.S.; Zainal-Abidin, A.H.; Latip, J.; Mazlan, N.W.; Edrada-Ebel, R.A. Gancidin W, a Potential Low-Toxicity Antimalarial Agent Isolated from an Endophytic Streptomyces SUK10. Drug Des. Devel. Ther. 2017, 11, 351–363. [Google Scholar] [CrossRef]
- Buedenbender, L.; Grkovic, T.; Duffy, S.; Kurtböke, D.I.; Avery, V.M.; Carroll, A.R. Naseseazine C, a New Anti-Plasmodial Dimeric Diketopiperazine from a Marine Sediment Derived Streptomyces Sp. Tetrahedron Lett. 2016, 57, 5893–5895. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Lu, M.; Totokotsopoulos, S.; Heretsch, P.; Gigue, D.; Sun, Y.-P.; Sarlah, D.; Nguyen, T.H.; Wolf, I.C.; Smee, D.F.; et al. Synthesis and Biological Evaluation of Epidithio-, Epitetrathio-, and Bis-(Methylthio)Diketopiperazines: Synthetic Methodology, Enantioselective Total Synthesis of Epicoccin G, 8,8′-Epi-Ent-Rostratin B, Gliotoxin, Gliotoxin G, Emethallicin E, and Haematocin and Discovery of New Antiviral and Antimalarial Agents. J. Am. Chem. Soc. 2012, 134, 17320–17332. [Google Scholar] [CrossRef]
- Nilanonta, C.; Isaka, M.; Kittakoop, P.; Saenboonr, J.; Rukachaisirikul, V.; Kongsaereec, P.; Thebtaranonthb, Y. New Diketopiperazines from the Entomopathogenic Fungus Verticillium Hemipterigenum BCC 1449. J. Antibiot. 2003, 56, 647–651. [Google Scholar] [CrossRef][Green Version]
- Arnone, A.; Nasini, G.; De Pava, O.V.; Capelli, S.; Meille, S.V. Structure Elucidation of Diatretol—A New Diketopiperazine Metabolite from the Fungus Clitocybe diatreta. Liebigs Ann. Chem 1996, 1996, 1875–1877. [Google Scholar] [CrossRef]
- Takahashi, S.; Kimishima, A.; Hirose, T.; Yamada, T.; Sugawara, A.; Shirahata, T.; Noguchi, Y.; Iwatsuki, M.; Hokari, R.; Ishiyama, A.; et al. Unified Enantioselective Total Synthesis of 3,6-Dioxygenated Diketopiperazine Natural Products, Diatretol and Lepistamides A, B and C. Tetrahedron Lett. 2021, 67, 15289. [Google Scholar] [CrossRef]
- Leesombun, A.; Iijima, M.; Pagmadulam, B.; Orkhon, B.; Doi, H.; Issiki, K.; Sawa, R.; Nihei, C.; Nishikawa, Y. Metacytofilin Has Potent Anti-Malarial Activity. Parasitol. Int. 2021, 81, 102267. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Vitorino, I.; de la Cruz, M.; Díaz, C.; Cautain, B.; Annang, F.; Pérez-Moreno, G.; Gonzalez, I.; Tormo, J.R.; Martin, J.; et al. Diketopiperazines and Other Bioactive Compounds from Bacterial Symbionts of Marine Sponges. Antonie Leeuwenhoek 2020, 113, 875–887. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Teague, S.J.; Davis, A.M.; Leeson, P.D.; Oprea, T. The Design of Leadlike Combinatorial Libraries. Angew. Chem. Int. Ed. 1999, 38, 3743–3748. [Google Scholar] [CrossRef]
- Siqueira, E.; Ceravolo, I.; Kohlhoff, M.; Krettli, A.; Zani, C. Synthesis and Antiplasmodial Activity of 2-Methyl-3-Carboxyl-Naphtho [2,3-b] Furan Quinone Derivatives. J. Med. Chem. Drug Des. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Oduola, A.M.J.; Milhous, W.K.; Weatherly, N.F.; Bowdre, J.H.; Desjardins, R.E. Plasmodium Falciparum: Induction of Resistance to Mefloquine in Cloned Strains by Continuous Drug Exposure In Vitro. Exp. Parasitol. 1988, 67, 354–360. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and Inexpensive Fluorescence-Based Technique for High-Throughput Antimalarial Drug Screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Ceravolo, I.P.; Zani, C.L.; Figueiredo, F.J.B.; Kohlhoff, M.; Santana, A.E.G.; Krettli, A.U. Aspidosperma Pyrifolium, a Medicinal Plant from the Brazilian Caatinga, Displays a High Antiplasmodial Activity and Low Cytotoxicity. Malar. J. 2018, 17, 436. [Google Scholar] [CrossRef] [PubMed]
- Condo, C.; Ninoska, F.; Salamanca, E.A.; Ticona, J.; Natalio, M.; Enrique, U.; Benigno, C.; Ivan, L.; Giménez, A. Actividad Antiparasitaria In Vitro de Plantas de La Medicina Tra-Dicional Tacana Sobre Plas-modium Faciparum a Través Del Método Fluorométrico-SYBR GreenI. Con-Cienc. 2020, 8, 21–28. [Google Scholar]
- Borenfreund, E.; Babich, H.; Martin-Alguacil, N. Comparisons of Two in Vitro Cytotoxicity Assays—The Neutral Red (NR) and Tetrazolium MTT Tests. Toxicol. Vitr. 1988, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- do Céu de Madureira, M.; Paula Martins, A.; Gomes, M.; Paiva, J.; Proença da Cunha, A.; do Rosário, V. Antimalarial Activity of Medicinal Plants Used in Traditional Medicine in S. Tomé and Príncipe Islands. J. Ethnopharmacol. 2002, 81, 23–29. [Google Scholar] [CrossRef]
- Bézivin, C.; Tomasi, S.; Lohézic-Le Dévéhat, F.; Boustie, J. Cytotoxic Activity of Some Lichen Extracts on Murine and Human Cancer Cell Lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef]
- Romanha, A.J.; Lisboa De Castro, S.; De Nazaré, M.; Soeiro, C.; Lannes-Vieira, J.; Ribeiro, I.; Talvani, A.; Bourdin, B.; Blum, B.; Olivieri, B.; et al. In Vitro and In Vivo Experimental Models for Drug Screening and Development for Chagas Disease. Mem. Inst. Oswaldo Cruz 2010, 105, 233–238. [Google Scholar] [CrossRef]
- Buckner, F.S.; Verlinde, C.L.M.J.; La Flamme, A.C.; Van Voorhis, W.C. Efficient Technique for Screening Drugs for Activity against Trypanosoma cruzi Using Parasites Expressing-Galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef]
- De Moura Lodi Cruz, M.G.F.; Murta Santi, A.M.; De Morais-Teixeira, E.; Portes Caldeira, A.S.; Pessoa de Sequeira, E.; Oliveira, E.; De Almeida Alves, T.M.; Fonseca Murta, S.M. Anti-Leishmania Compounds Can Be Screened Using Leishmania Spp. Expressing Red Fluorescence (tdTomato). Antimicrob. Agents Chemother. 2023, e00509-23, in press. [Google Scholar] [CrossRef]

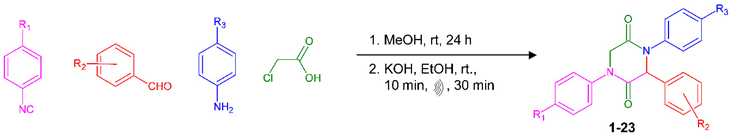 | |||
|---|---|---|---|
| Compound | R1 | R2 | R3 |
| 1 | Cl | H | H |
| 2 | Cl | 4-Et | H |
| 3 | Cl | 4-NMe2 | H |
| 4 | Cl | 4-Cl | H |
| 5 | Cl | 2-F | H |
| 6 | Cl | 4-OMe | H |
| 7 | Cl | 4-NHCOMe | H |
| 8 | Cl | 3-Br, 4-OMe | H |
| 9 | Cl | 2,5-OMe | H |
| 10 | Cl | 3-Cl | H |
| 11 | Cl | 4-F | H |
| 12 | OMe | 4-OMe | OMe |
| 13 | OMe | H | OMe |
| 14 | Cl | 4-OH | H |
| 15 | Cl | 3-Br, 4-OH | H |
| 16 | Cl | 2-F | OMe |
| 17 | Cl | 2-F | Cl |
| 18 | Cl | 4-Cl | OMe |
| 19 | Cl | 2-F | Br |
| 20 | Cl | 2-F | F |
| 21 | Cl | 4-Cl | F |
| 22 | Cl | 2-Cl, 6-F | H |
| 23 | Cl | 2,4,6-OMe | H |
| Parasite | Compound | CC50 (µg/mL) a | IC50 (µg/mL) b | SI c | Status |
|---|---|---|---|---|---|
| P. falciparum | 5 | ≤3.1 | 15.6 ± 2.6 | <1 | toxic |
| 10 | NT | 28.1 ± 0.3 | NA | inactive | |
| 15 | 18.5 ± 3.4 | 14.9 ± 0.7 | 1 | toxic | |
| 17 | 138.6 ± 18.7 | 9.5 ± 2.1 | 15 | ACTIVE | |
| 19 | 110.0 ± 11.3 | 5.4 ± 1.7 | 20 | ACTIVE | |
| 20 | 9.4 ± 5.1 | 17.6 ± 1.3 | <1 | toxic | |
| 21 | NT | ≥50 | NA | inactive | |
| Chloroquine | 221.8 ± 28.8 | 0.174 ± 0.055 | 1257 | Control | |
| T. cruzi | 5 | 29.2 | 9.3 ± 0.1 | 3 | toxic |
| 8 | >400 | 7.3 ± 1.5 | >55 | ACTIVE | |
| 13 | NT | 22.3 ± 1.1 | NA | inactive | |
| 15 | 81.8 | 11.2 ± 6.0 | 7 | toxic | |
| 20 | 32.1 | 15.4 ± 3.0 | 2 | toxic | |
| 21 | 16.8 | 16.1 ± 2.0 | 1 | toxic | |
| 22 | >400 | 7.2 ± 0.9 | >56 | ACTIVE | |
| Benznidazole | 540 | 0.5 ± 0.1 | 1080 | Control | |
| L. infantum | 2 | NT | >100 | NA | inactive |
| 3 | NT | 49.1 ± 1.6 | NA | inactive | |
| 4 | NT | 35.7 ± 0.8 | NA | inactive | |
| 7 | 679 ± 1 | 16.0 ± 1.0 | 42 | part. ACTIVE | |
| 9 | NT | 50.8 ± 1.1 | NA | inactive | |
| 11 | NT | 38.2 ± 1.8 | NA | inactive | |
| 14 | 611 ± 1 | 8.3 ± 0.2 | 74 | ACTIVE | |
| Amphotericin B | 367 ± 1 | 0.228 ± 0.025 | 1610 | Control |
| Compound | MW (Da) | HBA | HBD | Log P(o/w) | NRB | TPSA (Å2) | Water Solubility | GI | Drug-Likeness * | Lead-Likeness * |
|---|---|---|---|---|---|---|---|---|---|---|
| Rules | ≤500 | ≤10 | ≤5 | ≤4.15 | ≤9 | ≤130 | ||||
| 7 | 433.89 | 3 | 1 | 3.01 | 5 | 69.72 | o | + | 0 | 2 |
| 8 | 485.76 | 3 | 0 | 3.86 | 4 | 49.85 | − | + | 0 | 2 |
| 14 | 392.83 | 3 | 1 | 3.07 | 3 | 60.85 | o | + | 0 | 2 |
| 17 | 429.27 | 3 | 0 | 4.48 | 3 | 40.62 | − | + | 1 | 2 |
| 19 | 473.72 | 3 | 0 | 4.58 | 3 | 40.62 | − | + | 1 | 2 |
| 22 | 429.27 | 3 | 0 | 4.48 | 3 | 40.62 | − | + | 1 | 2 |
| Diatretol | 306.36 | 4 | 3 | 0.86 | 5 | 87.66 | + | + | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceravolo, I.P.; Leoni, L.F.; Krettli, A.U.; Murta, S.M.F.; Resende, D.d.M.; Cruz, M.G.F.d.M.L.; Varejão, J.O.S.; Mendes, L.L.; Varejão, E.V.V.; Kohlhoff, M. Novel 2,5-Diketopiperazines with In Vitro Activities against Protozoan Parasites of Tropical Diseases. Pharmaceuticals 2024, 17, 223. https://doi.org/10.3390/ph17020223
Ceravolo IP, Leoni LF, Krettli AU, Murta SMF, Resende DdM, Cruz MGFdML, Varejão JOS, Mendes LL, Varejão EVV, Kohlhoff M. Novel 2,5-Diketopiperazines with In Vitro Activities against Protozoan Parasites of Tropical Diseases. Pharmaceuticals. 2024; 17(2):223. https://doi.org/10.3390/ph17020223
Chicago/Turabian StyleCeravolo, Isabela P., Letícia F. Leoni, Antoniana U. Krettli, Silvane M. F. Murta, Daniela de M. Resende, Mariza G. F. de M. L. Cruz, Jodieh O. S. Varejão, Lorena L. Mendes, Eduardo V. V. Varejão, and Markus Kohlhoff. 2024. "Novel 2,5-Diketopiperazines with In Vitro Activities against Protozoan Parasites of Tropical Diseases" Pharmaceuticals 17, no. 2: 223. https://doi.org/10.3390/ph17020223
APA StyleCeravolo, I. P., Leoni, L. F., Krettli, A. U., Murta, S. M. F., Resende, D. d. M., Cruz, M. G. F. d. M. L., Varejão, J. O. S., Mendes, L. L., Varejão, E. V. V., & Kohlhoff, M. (2024). Novel 2,5-Diketopiperazines with In Vitro Activities against Protozoan Parasites of Tropical Diseases. Pharmaceuticals, 17(2), 223. https://doi.org/10.3390/ph17020223






