Arnicolide C Suppresses Tumor Progression by Targeting 14-3-3θ in Breast Cancer
Abstract
1. Introduction
2. Results
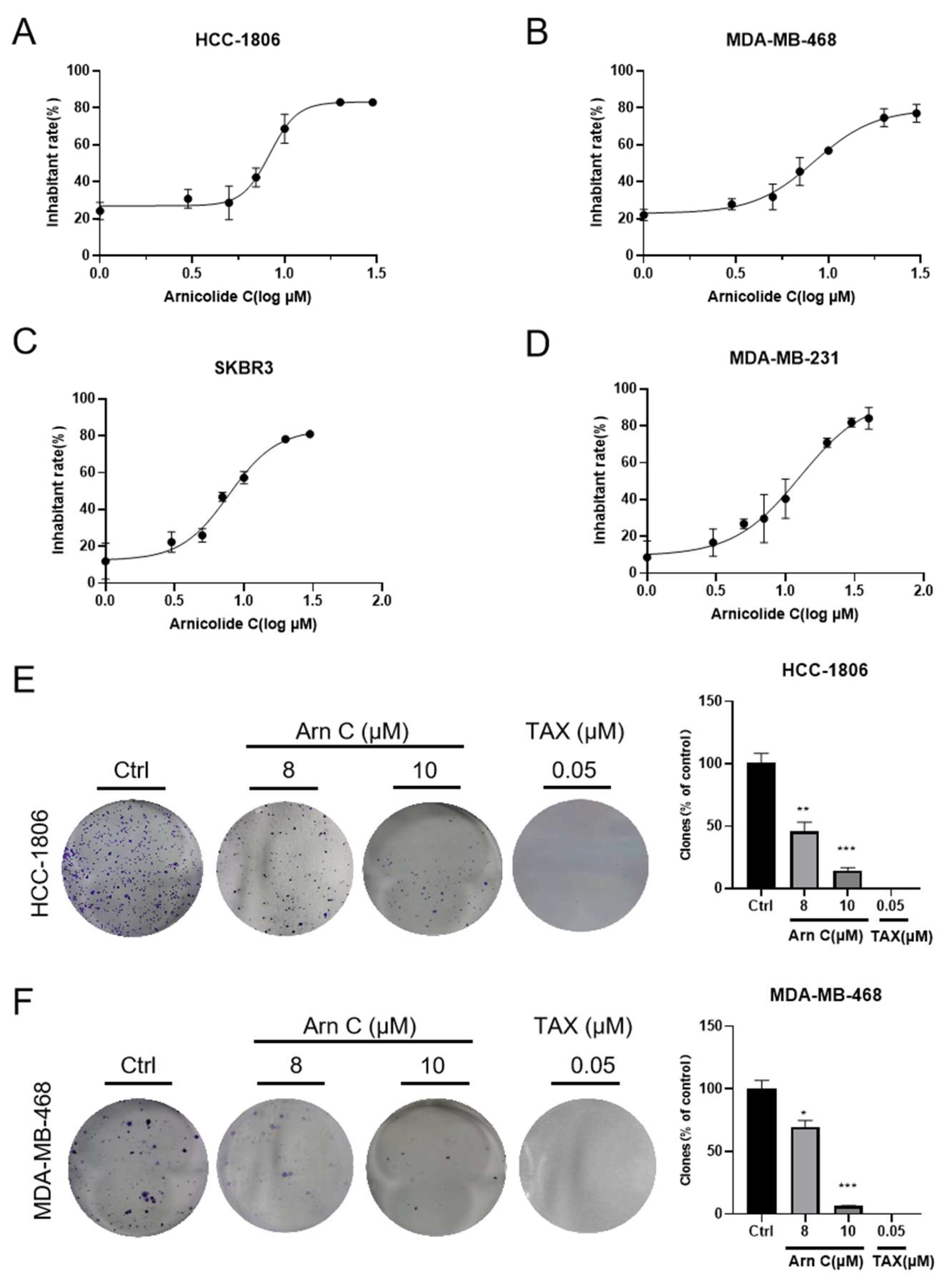
2.1. Arnicolide C Inhibited the Growth of Breast Cancer Cell Lines
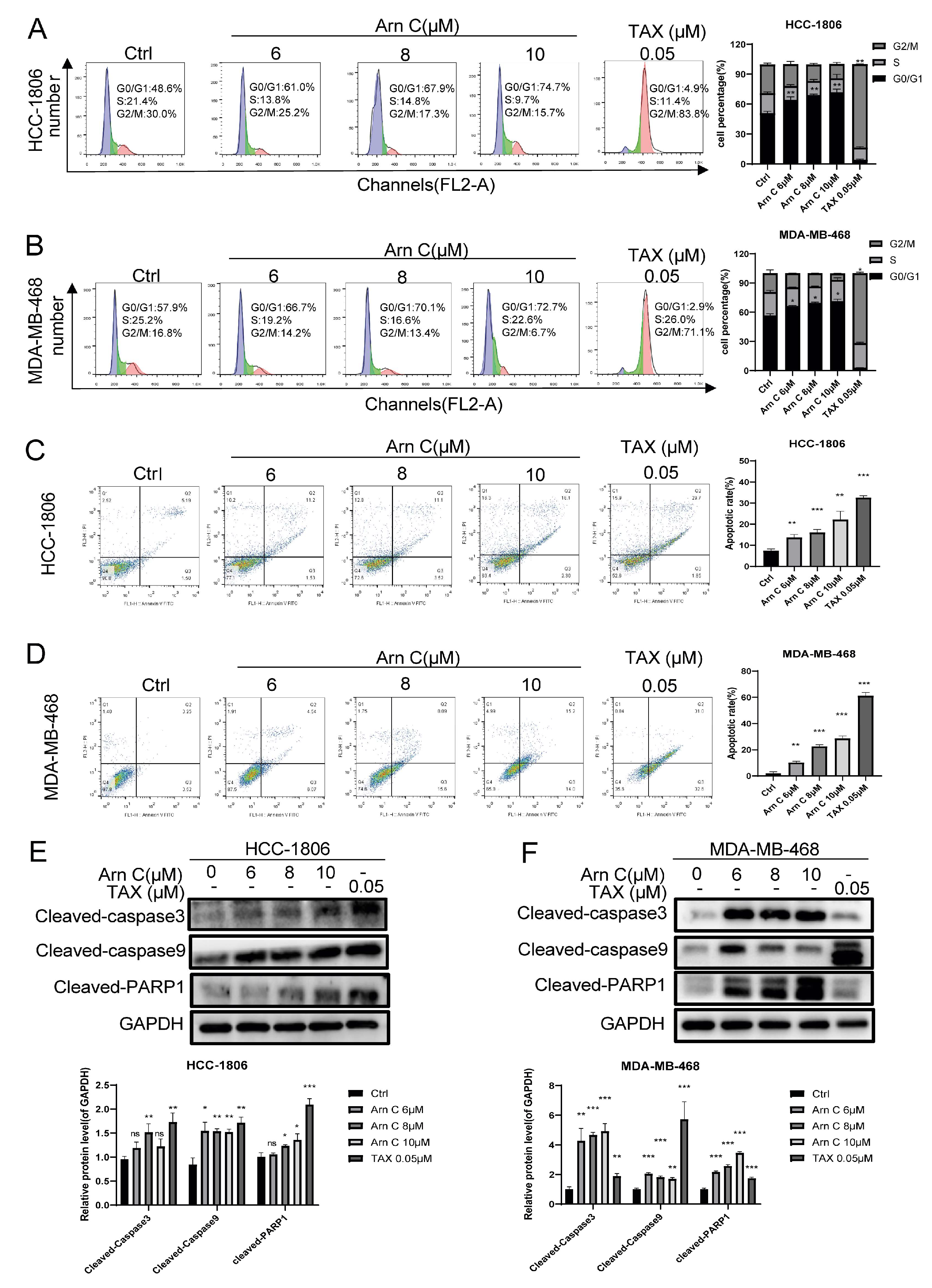
2.2. Arnicolide C-Induced Apoptosis and Cell Cycle Arrest in Breast Cancer Cells
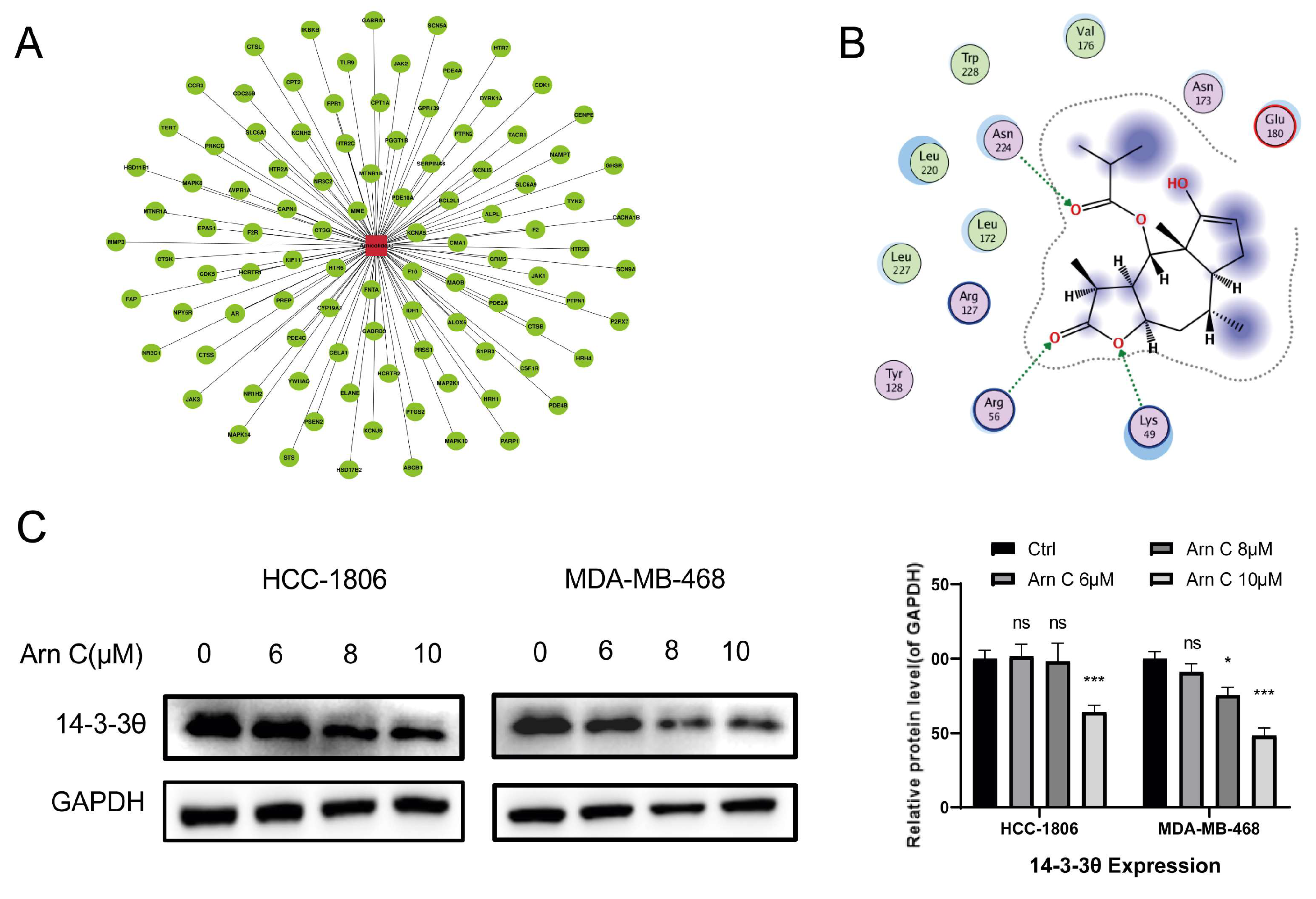
2.3. Effects of Arnicolide C on 14-3-3θ
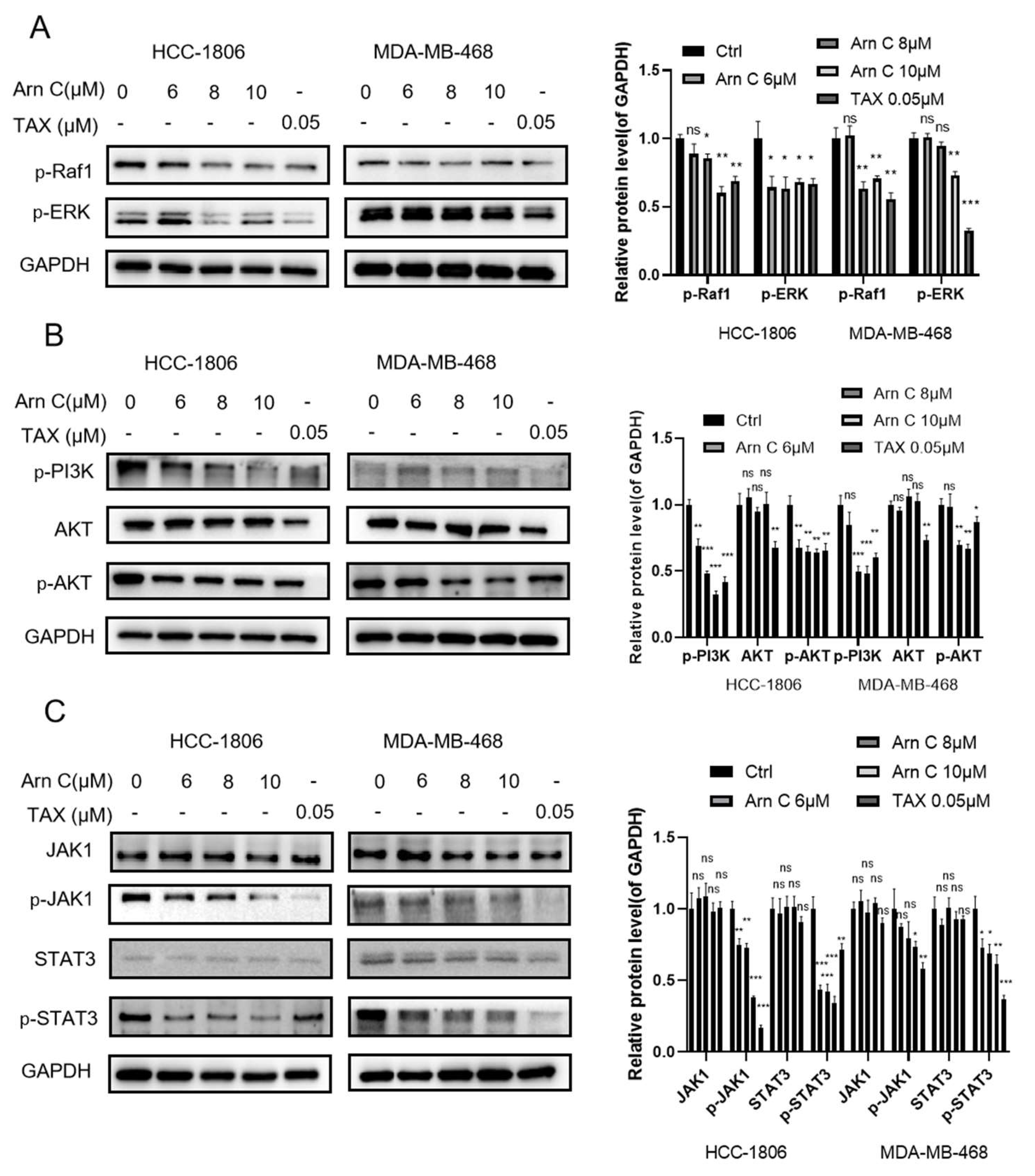
2.4. Arnicolide C Inhibits Proliferation-Related Signaling Pathways in Breast Cancer Cells
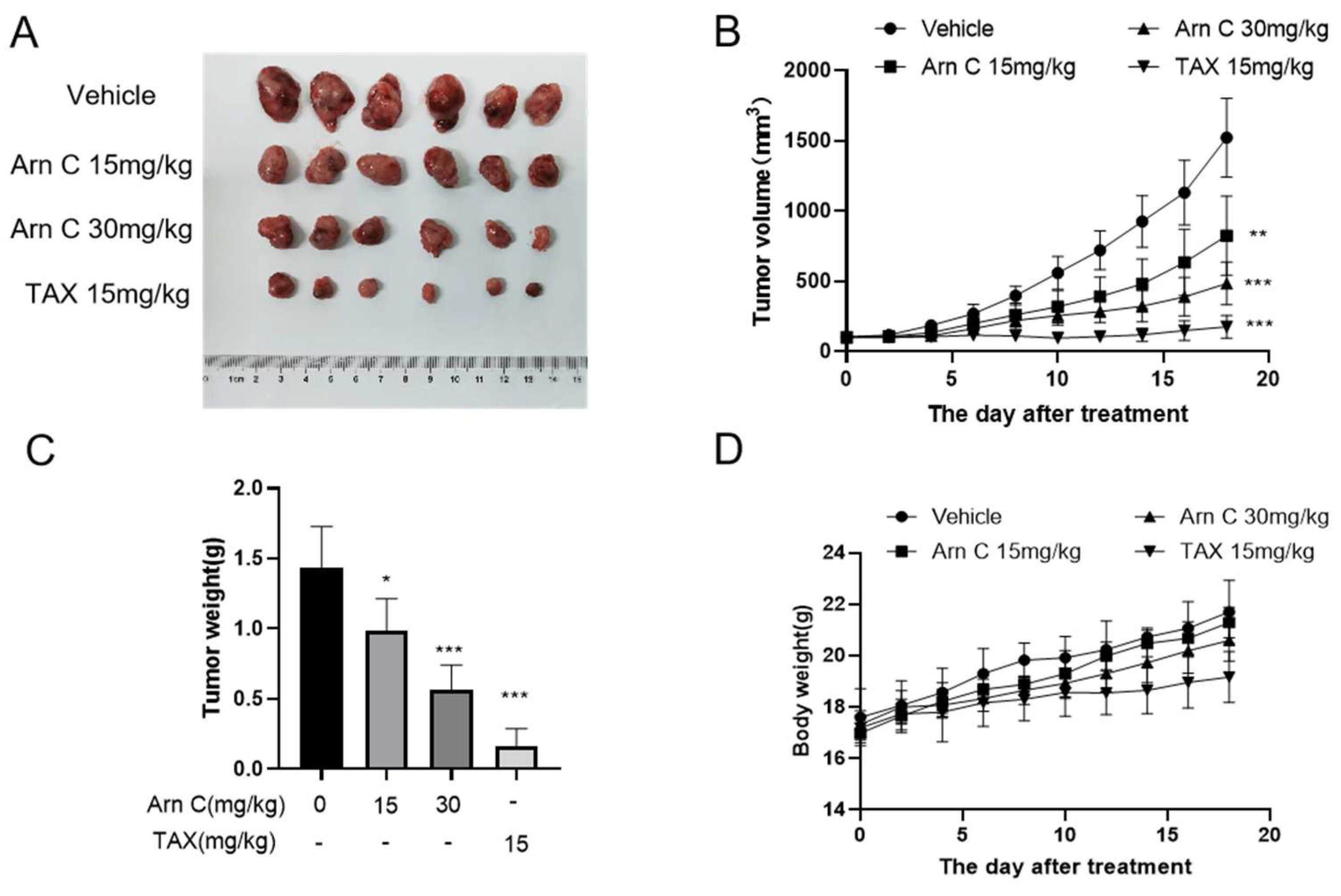
2.5. Arnicolide C Exerted Antitumor Effects on Xenograft Tumors
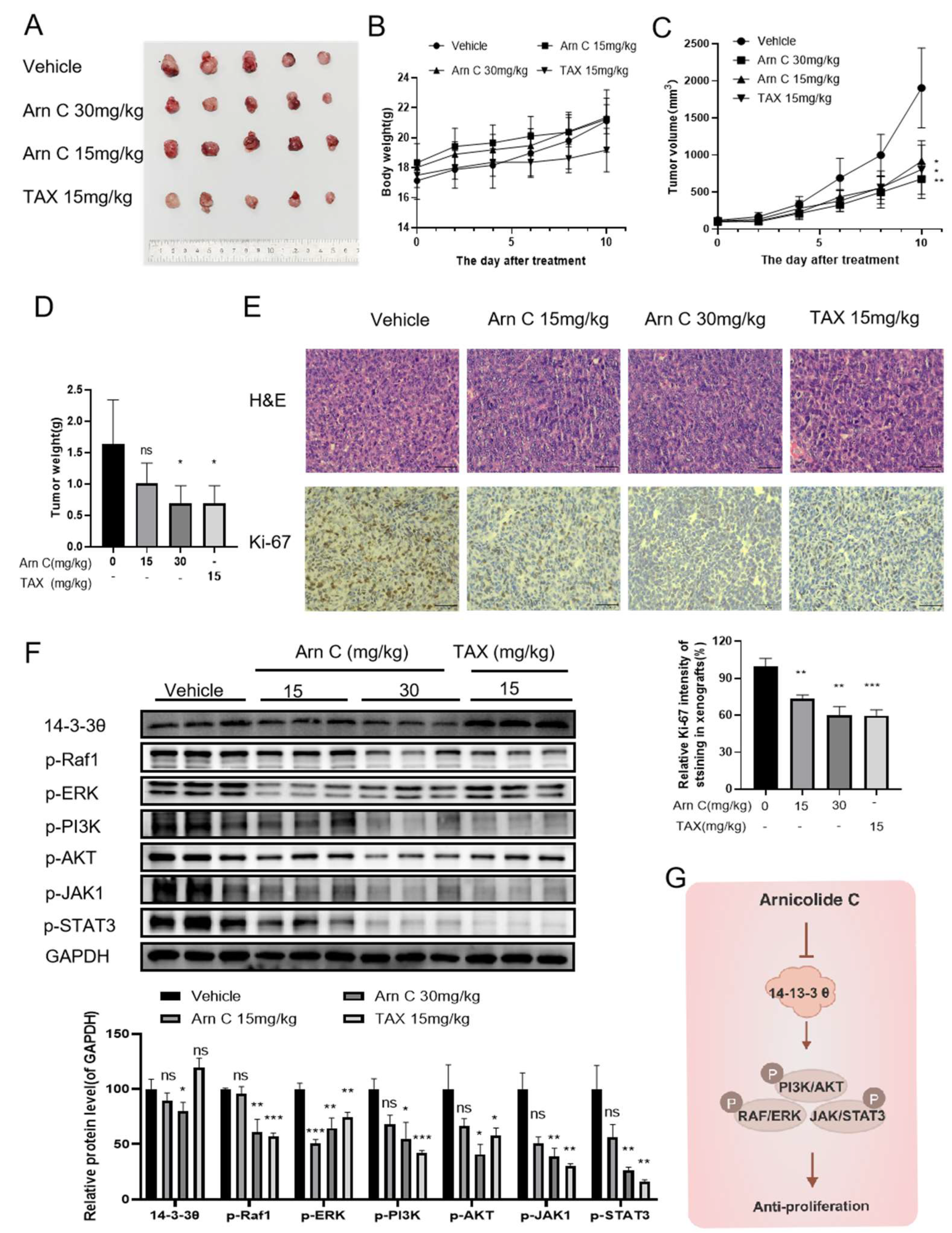
2.6. Arnicolide C Exerted Antitumor Effects via 14-3-3θ in the Patient-Derived Tumor Xenograft (PDX) Model
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Colony Formation Assay
4.5. Apoptosis Detection and Cell Cycle Analysis
4.6. Western Blotting
4.7. Molecular Docking
4.8. Nude Mouse Xenograft Study
4.9. Patient-Derived Tumor Xenograft Study
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Thi Thuy, L.; Nguyen Thi Thu, H.; Nguyen Thanh, T.; Le Thi Tu, A.; Nguyen Van, T.; Ninh The, S. Medicinal Plant Centipeda Minima: A Resource of Bioactive Compounds. Mini-Rev. Med. Chem. 2021, 21, 273–287. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Zhou, G.B. Promising anticancer activities and mechanisms of action of active compounds from the medicinal herb Centipeda minima (L.) A. Braun & Asch. Phytomedicine 2022, 106, 154397. [Google Scholar] [CrossRef] [PubMed]
- Jincheng, T.; Zhiping, Q.; Mingjing, M.; Fan, Z.; Hiu Yee, K.; Keying, Z.; Chunfang, Y.; Yechun, W.; Mi, Z.; Zhongqiu, L.; et al. Centipeda minima: An update on its phytochemistry, pharmacology and safety. J. Ethnopharmacol. 2022, 292, 115027. [Google Scholar] [CrossRef]
- Chan, B.D.; Wong, W.Y.; Lee, M.M.; Leung, T.W.; Shum, T.Y.; Cho, W.C.; Chen, S.; Tai, W.C. Centipeda minima Extract Attenuates Dextran Sodium Sulfate-Induced Acute Colitis in Mice by Inhibiting Macrophage Activation and Monocyte Chemotaxis. Front. Pharmacol. 2021, 12, 738139. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Chiu, C.S.; Lin, T.H.; Lee, M.M.; Lee, C.Y.; Chang, S.J.; Hou, W.C.; Huang, G.J.; Deng, J.S. Antioxidant and anti-inflammatory activities of aqueous extract of Centipeda minima. J. Ethnopharmacol. 2013, 147, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Bao, F.; Dong, X.; Tan, R.; Zhang, C.; Lu, Q.; Cheng, Y. Antibacterial thymol derivatives isolated from Centipeda minima. Molecules 2007, 12, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.X.; Bao, F.K.; Dong, X.P.; Zhu, H.J.; Lu, X.J.; Shi, M.; Lu, Q.; Cheng, Y.X. Two new antibacterial sesquiterpenoids from Centipeda minima. Chem. Biodivers. 2007, 4, 2810–2816. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Sun, H.Y.; Chan, C.O.; Liu, B.B.; Wu, J.H.; Chan, S.W.; Mok, D.K.; Tse, A.K.; Yu, Z.L.; Chen, S.B. Centipeda minima (Ebushicao) extract inhibits PI3K-Akt-mTOR signaling in nasopharyngeal carcinoma CNE-1 cells. Chin. Med. 2015, 10, 26. [Google Scholar] [CrossRef]
- Lee, D.; Kwak, H.J.; Kim, B.H.; Kim, D.W.; Kim, H.Y.; Kim, S.H.; Kang, K.S. Brevilin A Isolated from Centipeda minima Induces Apoptosis in Human Gastric Cancer Cells via an Extrinsic Apoptotic Signaling Pathway. Plants 2022, 11, 1658. [Google Scholar] [CrossRef]
- You, P.; Wu, H.; Deng, M.; Peng, J.; Li, F.; Yang, Y. Brevilin A induces apoptosis and autophagy of colon adenocarcinoma cell CT26 via mitochondrial pathway and PI3K/AKT/mTOR inactivation. Biomed. Pharmacother. 2018, 98, 619–625. [Google Scholar] [CrossRef]
- Taylor, R.; Towers, G. Antibacterial constituents of the Nepalese medicinal herb, Centipeda minima. Phytochemistry 1998, 47, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, H.; Zhang, Q.; Mou, X.; Zhao, Y.; Fan, H.; Xu, H.; Chen, D.; Qiu, F.; Zhao, F. Centipeda minima Two sesquiterpene lactones, arnicolide B and arnicolide C, isolated from, exert anti-inflammatory effects in LPS stimulated RAW 264.7 macrophages via inactivation of the MAPK pathway. Nat. Prod. Res. 2023, 37, 2969–2972. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Hao, X.; Yan, Y.; Hong, M.; Wei, S.; Zhou, Y.; Wang, Q.; Cheng, Y.; Liu, Y. Centipeda minima Ethanol Extract of Exerts Antioxidant and Neuroprotective Effects via Activation of the Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 9421037. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pang, Y.; Wang, H.; Wu, P.; Chen, Z.; Huang, D.; Peng, D.; Yan, Y.; Liu, C.; Wu, L.; et al. Identification of arnicolide C as a novel chemosensitizer to suppress mTOR/E2F1/FANCD2 axis in non-small cell lung cancer. Br. J. Pharmacol. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.; Perez, V.; Gehring, M. Assay and regional distribution of a soluble protein characteristic of the nervous system. J. Neurochem. 1968, 15, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, M.; Rittinger, K.; Volinia, S.; Caron, P.; Aitken, A.; Leffers, H.; Gamblin, S.; Smerdon, S.; Cantley, L. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 1997, 91, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lee, W.; Sobott, F.; Papagrigoriou, E.; Robinson, C.; Grossmann, J.; Sundström, M.; Doyle, D.; Elkins, J. Structural basis for protein-protein interactions in the 14-3-3 protein family. Proc. Natl. Acad. Sci. USA 2006, 103, 17237–17242. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Subramanian, R.; Masters, S. 14-3-3 proteins: Structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 617–647. [Google Scholar] [CrossRef]
- Gardino, A.; Yaffe, M. 14-3-3 proteins as signaling integration points for cell cycle control and apoptosis. Semin. Cell Dev. Biol. 2011, 22, 688–695. [Google Scholar] [CrossRef]
- Muslin, A.; Tanner, J.; Allen, P.; Shaw, A. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 1996, 84, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Hausser, A.; Storz, P.; Link, G.; Stoll, H.; Liu, Y.; Altman, A.; Pfizenmaier, K.; Johannes, F. Protein kinase C mu is negatively regulated by 14-3-3 signal transduction proteins. J. Biol. Chem. 1999, 274, 9258–9264. [Google Scholar] [CrossRef] [PubMed]
- Tzivion, G.; Avruch, J. 14-3-3 proteins: Active cofactors in cellular regulation by serine/threonine phosphorylation. J. Biol. Chem. 2002, 277, 3061–3064. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, E.; Mimae, T.; Ito, M.; Kadoya, T.; Miyata, Y.; Ito, A.; Okada, M. Breast cancer cell motility is promoted by 14-3-3γ. Breast Cancer 2019, 26, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Holmes, T.; Al Matouq, J.; Holmes, M.; Sioda, N.; Rudd, J.; Bloom, C.; Nicola, L.; Palermo, N.; Madson, J.; Lovas, S.; et al. Targeting 14-3-3ε activates apoptotic signaling to prevent cutaneous squamous cell carcinoma. Carcinogenesis 2021, 42, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wu, L.; Chen, S.; Cai, R.; Dai, Y.; Yang, H.; Tang, L.; Li, Y. Arsenic trioxide enhances the chemotherapeutic efficiency of cisplatin in cholangiocarcinoma cells via inhibiting the 14-3-3ε-mediated survival mechanism. Cell Death Discov. 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Fan, T.; Chen, L.; Yang, M.; Wai Wong, V.K.; Chen, D.; Liu, Z.; Zhou, Y.; Wu, W.; Qiu, Z.; et al. Design, synthesis and antitumor evaluation of novel 1H-indole-2-carboxylic acid derivatives targeting 14-3-3η protein. Eur. J. Med. Chem. 2022, 238, 114402. [Google Scholar] [CrossRef]
- Jung, J.; Koh, S.; Lee, K.; Kim, J. via14-3-3 Sigma Protein Contributes to Hepatocyte Growth Factor-mediated Cell Proliferation and Invasion Matrix Metalloproteinase-1 Regulation in Human Gastric Cancer. Anticancer Res. 2022, 42, 519–530. [Google Scholar] [CrossRef]
- Huang, Y.; Li, S.; Liu, Q.; Wang, Z.; Li, S.; Liu, L.; Zhao, W.; Wang, K.; Zhang, R.; Wang, L.; et al. The LCK-14-3-3ζ-TRPM8 axis regulates TRPM8 function/assembly and promotes pancreatic cancer malignancy. Cell Death Dis. 2022, 13, 524. [Google Scholar] [CrossRef]
- Hermeking, H. The 14-3-3 cancer connection. Nat. Rev. Cancer 2003, 3, 931–943. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, J.; Liu, F.; Liu, J.; Li, M. Downregulation of 14-3-3β inhibits proliferation and migration in osteosarcoma cells. Mol. Med. Rep. 2018, 17, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Wang, G.; Ye, J.; Li, T.; Bai, H.; Wang, W. 14-3-3β regulates the proliferation of glioma cells through the GSK3β/β-catenin signaling pathway. Oncol. Rep. 2013, 30, 2976–2982. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, Y.; Yang, Z.; Ke, Z.; Yao, Y.; Hu, X.; Sun, Y.; Li, H.; Yin, J.; Zeng, C. Expression of 14-3-3γ in patients with breast cancer: Correlation with clinicopathological features and prognosis. Cancer Epidemiol. 2012, 36, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Raungrut, P.; Wongkotsila, A.; Lirdprapamongkol, K.; Svasti, J.; Geater, S.L.; Phukaoloun, M.; Suwiwat, S.; Thongsuksai, P. Prognostic significance of 14-3-3γ overexpression in advanced non-small cell lung cancer. Asian Pac. J. Cancer Prev. 2014, 15, 3513–3518. [Google Scholar] [CrossRef]
- Yang, Y.F.; Lee, Y.C.; Wang, Y.Y.; Wang, C.H.; Hou, M.F.; Yuan, S.F. YWHAE promotes proliferation, metastasis, and chemoresistance in breast cancer cells. Kaohsiung J. Med. Sci. 2019, 35, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Yan, L.; Gu, H.; Mu, Y.; Tong, G.; Zhang, G. 14-3-3ε functions as an oncogene in SGC7901 gastric cancer cells through involvement of cyclin E and p27kip1. Mol. Med. Rep. 2014, 10, 3145–3150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, T.A.; Jan, Y.J.; Ko, B.S.; Liang, S.M.; Chen, S.C.; Wang, J.; Hsu, C.; Wu, Y.M.; Liou, J.Y. 14-3-3ε overexpression contributes to epithelial-mesenchymal transition of hepatocellular carcinoma. PLoS ONE 2013, 8, e57968. [Google Scholar] [CrossRef]
- Zhen, J.; Jiao, K.; Yang, K.; Wu, M.; Zhou, Q.; Yang, B.; Xiao, W.; Hu, C.; Zhou, M.; Li, Z. The 14-3-3η/GSK-3β/β-catenin complex regulates EndMT induced by 27-hydroxycholesterol in HUVECs and promotes the migration of breast cancer cells. Cell Biol. Toxicol. 2021, 37, 515–529. [Google Scholar] [CrossRef]
- Shen, J.; Jiang, F.; Yang, Y.; Huang, G.; Pu, F.; Liu, Q.; Chen, L.; Ju, L.; Lu, M.; Zhou, F.; et al. 14-3-3η is a novel growth-promoting and angiogenic factor in hepatocellular carcinoma. J. Hepatol. 2016, 65, 953–962. [Google Scholar] [CrossRef]
- Wu, Q.; Fan, H.; Lang, R.; Li, X.; Zhang, X.; Lv, S.; He, Q. Overexpression of 14-3-3 σ Modulates Cholangiocarcinoma Cell Survival by PI3K/Akt Signaling. BioMed Res. Int. 2020, 2020, 3740418. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, L.; Chen, Y.; Liu, X.; Kawakami, M.; Mustachio, L.M.; Roszik, J.; Ferry-Galow, K.V.; Parchment, R.E.; Liu, X.; et al. Loss of ubiquitin-specific peptidase 18 destabilizes 14-3-3ζ protein and represses lung cancer metastasis. Cancer Biol. Ther. 2022, 23, 265–280. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Sheng, Y.; Wu, B.; Song, Y.; Ye, G.; Qi, Y.; Zhao, S. PRDM5 suppresses oesophageal squamous carcinoma cells and modulates 14-3-3zeta/Akt signalling pathway. Clin. Exp. Pharmacol. Physiol. 2022, 49, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Scheibner, K.; Teaboldt, B.; Hauer, M.; Chen, X.; Cherukuri, S.; Guo, Y.; Kelley, S.; Liu, Z.; Baer, M.; Heimfeld, S.; et al. MiR-27a functions as a tumor suppressor in acute leukemia by regulating 14-3-3θ. PLoS ONE 2012, 7, e50895. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Wang, H.; Wang, Y.; Chen, Y.; Zhao, Z.; Ma, J.; Ji, Y.; Huang, Q.; Chen, J.; Chen, H.; et al. Shuttling SLC2A4RG is regulated by 14-3-3θ to modulate cell survival via caspase-3 and caspase-6 in human glioma. EBioMedicine 2019, 40, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, H.; Fan, J.; Tong, C.; Yang, J.; Wei, H.; Yi, J.; Ling, R. Overexpression of 14-3-3θ promotes tumor metastasis and indicates poor prognosis in breast carcinoma. Oncotarget 2014, 5, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, X.; Guan, Y.; Gong, M.; He, K.; Huang, B. 1,3-Dicaffeoylquinic acid targeting 14-3-3 tau suppresses human breast cancer cell proliferation and metastasis through IL6/JAK2/PI3K pathway. Biochem. Pharmacol. 2020, 172, 113752. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, K.; Lin, H.; Bellam, N.; Ling, S.; Lin, W. 14-3-3Tau regulates ubiquitin-independent proteasomal degradation of p21, a novel mechanism of p21 downregulation in breast cancer. Mol. Cell. Biol. 2010, 30, 1508–1527. [Google Scholar] [CrossRef] [PubMed]
- Garan, L.; Xiao, Y.; Lin, W. 14-3-3τ drives estrogen receptor loss via ERα36 induction and GATA3 inhibition in breast cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2209211119. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.; Goel, N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Adv. Protein Chem. Struct. Biol. 2021, 125, 73–120. [Google Scholar] [CrossRef]
- D’Arcy, M. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Zhao, T.; Li, Z.; He, J.; Zhang, B.; Du, H.; Jiang, J.; Yuan, S.; Sun, L. Topotecan induces apoptosis via ASCT2 mediated oxidative stress in gastric cancer. Phytomed. Int. J. Phytother. Phytopharm. 2019, 57, 117–128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Lyu, X.; Chen, J.; Zhang, B.; Xie, S.; Yuan, Y.; Sun, L.; Yuan, S.; Yu, H.; Ding, J.; et al. Arnicolide C Suppresses Tumor Progression by Targeting 14-3-3θ in Breast Cancer. Pharmaceuticals 2024, 17, 224. https://doi.org/10.3390/ph17020224
Liu Z, Lyu X, Chen J, Zhang B, Xie S, Yuan Y, Sun L, Yuan S, Yu H, Ding J, et al. Arnicolide C Suppresses Tumor Progression by Targeting 14-3-3θ in Breast Cancer. Pharmaceuticals. 2024; 17(2):224. https://doi.org/10.3390/ph17020224
Chicago/Turabian StyleLiu, Zhengrui, Xiaodan Lyu, Jiaxu Chen, Benteng Zhang, Siman Xie, Yan Yuan, Li Sun, Shengtao Yuan, Hong Yu, Jian Ding, and et al. 2024. "Arnicolide C Suppresses Tumor Progression by Targeting 14-3-3θ in Breast Cancer" Pharmaceuticals 17, no. 2: 224. https://doi.org/10.3390/ph17020224
APA StyleLiu, Z., Lyu, X., Chen, J., Zhang, B., Xie, S., Yuan, Y., Sun, L., Yuan, S., Yu, H., Ding, J., & Yang, M. (2024). Arnicolide C Suppresses Tumor Progression by Targeting 14-3-3θ in Breast Cancer. Pharmaceuticals, 17(2), 224. https://doi.org/10.3390/ph17020224



