The Dynamic Role of Curcumin in Mitigating Human Illnesses: Recent Advances in Therapeutic Applications
Abstract
1. Introduction
2. Preclinical and Clinical Evidence for Therapeutic Applications of Curcumin
2.1. Cancer
2.2. Inflammatory Bowel Disease
2.3. Osteoarthritis
2.4. Atherosclerosis
2.5. Peptic Ulcer
2.6. COVID-19
2.7. Psoriasis
2.8. Vitiligo
2.9. Depression
2.10. Diabetes Mellitus
2.11. Malaria
2.12. Antimicrobial Properties
2.13. Neuroprotective Properties
3. Curcumin’s Bioavailability: Challenges and Innovative Formulations
4. Safety Profile of Curcumin
5. Probable Mechanisms of Action of Curcumin
| S. No. | Pathway/Methods | Study Type | Main Findings | References |
|---|---|---|---|---|
| 1. | DPPH scavenging method | In vitro | Curcumin and nanosuspension exhibited more potency in scavenging of superoxide free radicals followed by demethoxycurcumin and bisdemethoxycurcumin. | [211,212,213] |
| 2. | Radiation-induced lipid peroxidation DFT studies | In vitro, in vivo, and in silico | Curcumin inhibited lipid peroxidation by 82% and dimethoxy curcumin by 24%. In curcumin, the hydrogen of -OH is more labile for separation than the hydrogen of -CH(2). | [214] |
| 3. | ABTS and DMPD radical scavenging assay | In vitro | Curcumin exhibited free radical scavenging activity against DPPH, ABTS, DMPD, superoxide anion free radical, and H2O2, as well as for ferrous (Fe2+) ion chelation and ferric ion (Fe3+) reduction. | [62] |
| 4. | Styrene oxidation assay | In vitro | Curcumin produced phenolic chain-breaking antioxidant activity. | [203] |
| 5. | Phosphomolybdenum peroxidation and linoleic acid peroxidation assay | In vitro | Curcumin exhibited maximum antioxidant activity followed by demethoxycurcumin and then bisdemethoxycurcumin. | [215] |
| 6. | Keap1/Nrf2/ARE signaling pathway | In vitro | Curcumin demonstrated resistance to oxidizing agents by activating the Nrf2-Keap1 pathway and boosting the activity of antioxidant enzymes. | [216] |
| In vivo | Curcumin demonstrated Nrf2 activation by inhibiting the upregulation of Keap1 induced by inflammatory signals, thereby contributing to its antioxidant benefits and alleviating insulin resistance in high-fat-diet obese mice. | [217] | ||
| 7. | PI3K/Akt-1/mTOR signaling pathway | In vitro | Curcumin stimulates autophagy by inhibiting the PI3K/AKT/mTOR signaling pathway, which is achieved by at least partially inhibiting the phosphorylation of both Akt and mTOR. | [218,219] |
| 8. | Modulation of inflammatory cytokines | In vivo | A curcuminoid preparation (180 mg/day) produces a significant reduction in TNFα, IL-6, substance P, hs-CRP, CGRP, and TGF-β as compared to a placebo control. | [220,221] |
| In vivo | In patients with metabolic syndrome, the administration of curcumin (1 g/day) once daily results in a substantial decrease in serum levels of inflammatory cytokines. | [222] | ||
| In vitro | Curcumin inhibited the release of inflammatory cytokines, including IL-8, monocyte inflammatory protein-1 (MIP-1α), monocyte chemotactic protein-1 (MCP-1), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α), from lipopolysaccharide-induced monocytes and macrophages extracted from the peripheral blood and alveoli of humans, respectively. | [223] | ||
| In vitro | Curcumin reduces LPS-induced NO and pro-inflammatory cytokine production in microglial cells. | [224] | ||
| In vitro | Curcumin suppresses PHA-induced T-cell growth, production of IL-2, NO generation, and LPS-induced NF-κB, while increasing NK cell cytotoxicity. | [225] | ||
| 9. | Modulation of growth factors | In vitro | Curcumin suppressed the EGF/EGFR signaling pathway, which in turn prevented hyperglycemia-driven EGF-induced pancreatic cancer from migrating and invading by downregulating the ERK and Akt signaling molecules. | [226] |
| In vitro | Curcumin suppresses angiogenesis by inhibiting FGF-induced neovascularization in cultured corneal cells. | [227] | ||
| In vivo and in vitro | Both in vitro and in vivo models exhibited the angiogenesis-inhibiting effects of curcumin through its inhibitory action on VEGF and VEGFR2. | [228,229] | ||
| 10. | Modulation of growth factor receptors | In vitro | Curcumin induces autophagy in both AR-positive and AR-negative prostate cancer cells by reducing the activity and expression of the androgen receptor (AR) and related cofactors AP-1, NF-κB, and CBP. | [230,231] |
| In vitro | The Her2-Akt signaling pathway is suppressed by curcumin (18 μmol/L), resulting in the inhibition of cell proliferation in the human breast cancer cell line. Furthermore, the low-dose (1.5 μmol/L) combination of curcumin and lapatinib resulted in a more potentiated inhibitory effect on the Her2-Akt signaling pathway and the inhibition of cancer cell growth. | [232] | ||
| In vitro | Curcumin inhibits EGFR by repressing the expression of genes and proteins in the EGFR-PI3K-AKT pathway, thereby overcoming Lenvatinib’s resistance in hepatocellular carcinoma cells. | [233] | ||
| In vivo and in vitro | Curcumin enhanced the degradation of EGFR protein, thereby suppressing its activation. This, in turn, resulted in the reversal of gefitinib in non-small cell lung cancer in both in vitro and in vivo conditions. | [234] | ||
| 11. | Modulation of enzymes | In vitro and in vivo | Curcumin treatment produces inhibition of NO production, reduced expression of iNOS and COX-2 by inhibiting ERK 1/2, and p38 activation in RAW 264.7 macrophages. Additionally, curcumin produces reversal of CPA-induced changes in body weight, immunoglobulins, and NK cell activity in mice. | [235] |
| In vitro and in vivo | Addition of curcumin (5–10 microM) to epidermal microsomes produces inhibition of arachidonic acid metabolism into PGE2, PGF2α, and PGD2. Topically applied curcumin results in inhibition of the activity of LOX and COX in epidermal inflammation. | [236] | ||
| In vivo | Curcumin is less ulcerogenic than phenylbutazone and exerts anti-inflammatory activity that is comparable to that of phenylbutazone. It also impeded the increased levels of SGOT and SGPT that were induced by inflammation. | [237] | ||
| In vitro | Curcumin inhibits the AMPK-MAPK (mitogen-activated protein kinase) and PKC pathways by reversing PMA-induced PKC activation and suppressing chronic AMPK activation, resulting in decreased synthesis of EMMPRIN, MMP-9, and MMP-13. | [238] | ||
| 12. | Modulation of adhesion molecules | In vitro | Curcumin demonstrated anti-inflammatory activity by suppressing monocytic THP-1 adhesiveness by inhibiting the TNF-α induced ICAM-1 expression in the human keratinocyte cell line. | [239] |
| In vivo | Curcumin inhibited the formation of glial scars by preventing the production of MIP1α, IL-2, and CCL5 and by reducing NF-κB activation. | [240] | ||
| In vitro | Curcumin modulates the PI3K/Akt/MAPK/NF-B pathway to suppress the expression of VCAM-1 induced by tumour necrosis factor-α (TNFα)/lipopolysaccharide (LPS) in human intestinal microvascular endothelial cell culture. | [241] | ||
| 13. | Modulation of apoptosis-related proteins | In vitro | Increased apoptosis and growth inhibition were observed in HT-29 cells that were treated with curcumin. This was accompanied by corresponding changes in apoptosis-related proteins, including a decrease in the expression of Bcl-2, Bcl-xL, and survivin, as well as an increase in Bax and Bad. | [242] |
| 14. | Modulation of cell cycle proteins | In vitro | The expression levels of PCNA, cyclin D1, and Bcl-xL were reduced by curcumin, which prevented human keratinocyte cell differentiation by arresting them in the G1/S phase. | [243] |
| In vitro | Curcumin nanoparticles triggers cellular arrest at the G1/S and G2/M phases, as well as cell death, by inhibiting several cell cycle proteins, including cyclin E in MDA-MB-231 cells. | [244] | ||
| 15. | Modulation of transcription factors and their signaling pathways | In vivo | Curcumin demonstrated an antidepressant effect by inhibiting the activation of NF-κB and reducing the expression of pro-inflammatory cytokines. | [119] |
| In vitro | Curcumin suppresses inflammation induced by LPS by controlling microglia polarization (M1/M2), balancing TREM2/TLR4, and inhibiting NF-κB activity. | [37] | ||
| In vitro | Curcumin lowers cellular viability in human and rat glioma cell lines, which is associated with the suppression of the AP-1 and NFkappaB signaling pathways by preventing JNK and Akt activation. | [245] | ||
| 16. | Ca2+/CaN/NFAT/IL-2 signaling pathway | In vitro | Curcumin reduced inflammation by inhibiting T cell-mediated Ca2+ mobilization and NFAT-regulated IL-2 and NF-κB production in freshly separated T cells and the Jurkat T leukemia cell line. | [246] |
| 17. | CD95/CD95L apoptotic signaling pathway | In vitro | Curcumin induces apoptosis in colorectal cancer cells via altering the Fas-regulated extrinsic pathway, activating caspase 8 and TRAIL binding to death receptors (DR), and upregulating DR5 proteins. | [247] |
| 18. | c-Jun N-terminal kinases 1/2 (JNK1/2) pathways | In vitro | Curcumin inhibits MEKK1-induced JNK activation via interfering with upstream signaling molecules, resulting in the inhibition of AP-1 and NF-kappaB signaling and significant anti-inflammatory and anticancer effects. | [248] |
| 19. | FABP5/ PPARβ/δ pathway | In vitro | Curcumin suppresses the expression of FABP5 and PPARβ/δ, thereby overcoming the resistance to retinoic acid in MDA-MB-231 and MD-MB-468 cell lines (triple-negative breast cancer). | [249] |
| 20. | Modulation of PPARγ/NF-κB signaling pathway | In vitro and in vivo | Curcumin prevents cigarette smoke extract (CSE)-induced inflammation both in vivo and in vitro, possibly by upregulating PPARγ and inhibiting NF-κB activation. | [250] |
| In vitro and in vivo | In in vitro and in vivo models, curcumin suppresses PPAR activation, inhibits NF-κB p65 translocation, and improves the increased expression of MCP-1 and MUC5AC induced by OVA and IL-4. | [251] |
6. Future Perspectives
7. Conclusions
Funding
Conflicts of Interest
References
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of Turmeric and Its Principal Bioactive Curcumin on Human Health: Pharmaceutical, Medicinal, and Food Applications: A Comprehensive Review. Front. Nutr. 2022, 9, 1040259. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R. A Comprehensive Review on the Therapeutic Potential of Curcuma Longa Linn. in Relation to Its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Miłobȩdzka, J.; Kostanecki, S.V.; Lampe, V. Zur Kenntnis Des Curcumins. Berichte Dtsch. Chem. Ges. 1910, 43, 2163–2170. [Google Scholar] [CrossRef]
- Lampe, V.; Milobedzka, J. Studien Über Curcumin. Berichte Dtsch. Chem. Ges. 1913, 46, 2235–2240. [Google Scholar] [CrossRef]
- Pabon, H.J.J. A Synthesis of Curcumin and Related Compounds. Recl. Trav. Chim. des Pays-Bas 1964, 83, 379–386. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Ślifirski, P. Curcumin and Curcuminoids in Quest for Medicinal Status. Acta Biochim. Pol. 2012, 59, 201–212. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—From Molecule to Biological Function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Ramachandra, M.S.; Subbaraju, G.V. Synthesis and Biological Evaluation of Polyhydroxycurcuminoids. Bioorg. Med. Chem. 2005, 13, 6374–6380. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and Curcumin: Biological Actions and Medicinal Applications. Curr. Sci. 2004, 87, 44–53. [Google Scholar]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N. Biological Activities of Curcumin and Its Analogues (Congeners) Made by Man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Li, X. Curcumin, an Active Constiuent of the Ancient Medicinal Herb Curcuma Longa L.: Some Uses and the Establishment and Biological Basis of Medical Efficacy. CNS Neurol. Disord. Drug Targets 2013, 12, 487–497. [Google Scholar] [CrossRef]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and Pharmacokinetic Study of Oral Curcuma Extract in Patients with Colorectal Cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar]
- Aggarwal, B.B.; Sung, B. Pharmacological Basis for the Role of Curcumin in Chronic Diseases: An Age-Old Spice with Modern Targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef]
- DiSilvestro, R.A.; Joseph, E.; Zhao, S.; Bomser, J. Diverse Effects of a Low Dose Supplement of Lipidated Curcumin in Healthy Middle Aged People. Nutr. J. 2012, 11, 79. [Google Scholar] [CrossRef]
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined Inhibitory Effects of Soy Isoflavones and Curcumin on the Production of Prostate-specific Antigen. Prostate 2010, 70, 1127–1133. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M. Phase I Clinical Trial of Oral Curcumin: Biomarkers of Systemic Activity and Compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y. A Phase I/II Study of Gemcitabine-Based Chemotherapy plus Curcumin for Patients with Gemcitabine-Resistant Pancreatic Cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Rauf, A.; Akash, S.; Trisha, S.I.; Nasim, A.H.; Akter, M.; Dhar, P.S.; Ogaly, H.A.; Hemeg, H.A.; Wilairatana, P. Targeted Therapies of Curcumin Focus on Its Therapeutic Benefits in Cancers and Human Health: Molecular Signaling Pathway-Based Approaches and Future Perspectives. Biomed. Pharmacother. 2024, 170, 116034. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-Y.; Shi, C.-B.; Wen, H.; Li, F.-L.; Wang, B.-L.; Wang, J. Upregulation of P53 Expression in Patients with Colorectal Cancer by Administration of Curcumin. Cancer Invest. 2011, 29, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.H.; Kumar, A.P.; Sethi, G. The Multifaceted Role of Curcumin in Cancer Prevention and Treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef]
- Sivani, B.M.; Azzeh, M.; Patnaik, R.; Pantea Stoian, A.; Rizzo, M.; Banerjee, Y. Reconnoitering the Therapeutic Role of Curcumin in Disease Prevention and Treatment: Lessons Learnt and Future Directions. Metabolites 2022, 12, 639. [Google Scholar] [CrossRef]
- Moon, D.-O. Curcumin in Cancer and Inflammation: An In-Depth Exploration of Molecular Interactions, Therapeutic Potentials, and the Role in Disease Management. Int. J. Mol. Sci. 2024, 25, 2911. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Paciello, F.; Mezzogori, D.; Rolesi, R.; Eramo, S.L.M.; Paludetti, G.; Troiani, D. Molecular Targets for Anticancer Redox Chemotherapy and Cisplatin-Induced Ototoxicity: The Role of Curcumin on PSTAT3 and Nrf-2 Signalling. Br. J. Cancer 2015, 113, 1434–1444. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical Effects of Curcumin in Enhancing Cancer Therapy: A Systematic Review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Screening, P.D.Q.; Board, P.E. Colorectal Cancer Screening (PDQ®). In PDQ Cancer Information Summaries [Internet]; National Cancer Institute (US): Bethesda, MD, USA, 2023. [Google Scholar]
- Sharma, N.; Alam, M.S.; Sharma, A.; Garg, S.; Maity, M.K. Colorectal Cancer in Young Adults: Epidemiology, Risk Factors, Development, Symptoms, Traditional Herbal Therapy And Prevention. J. Pharm. Negat. Results 2022, 14, 1370–1382. [Google Scholar]
- Garcea, G.; Berry, D.P.; Jones, D.J.L.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the Putative Chemopreventive Agent Curcumin by Cancer Patients: Assessment of Curcumin Levels in the Colorectum and Their Pharmacodynamic Consequences. Cancer Epidemiol. Biomark. Prev. 2005, 14, 120–125. [Google Scholar] [CrossRef]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr. Phase IIa Clinical Trial of Curcumin for the Prevention of Colorectal Neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Cruz–Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination Treatment with Curcumin and Quercetin of Adenomas in Familial Adenomatous Polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef]
- Gao, Y.; Zhuang, Z.; Lu, Y.; Tao, T.; Zhou, Y.; Liu, G.; Wang, H.; Zhang, D.; Wu, L.; Dai, H. Curcumin Mitigates Neuro-Inflammation by Modulating Microglia Polarization through Inhibiting TLR4 Axis Signaling Pathway Following Experimental Subarachnoid Hemorrhage. Front. Neurosci. 2019, 13, 496029. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin Inhibits LPS-Induced Neuroinflammation by Promoting Microglial M2 Polarization via TREM2/TLR4/NF-ΚB Pathways in BV2 Cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin Therapy in Inflammatory Bowel Disease: A Pilot Study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef]
- Lahiff, C.; Moss, A.C. Curcumin for Clinical and Endoscopic Remission in Ulcerative Colitis. Inflamm. Bowel Dis. 2011, 17, E66. [Google Scholar] [CrossRef]
- Sadeghi, N.; Mansoori, A.; Shayesteh, A.; Hashemi, S.J. The Effect of Curcumin Supplementation on Clinical Outcomes and Inflammatory Markers in Patients with Ulcerative Colitis. Phyther. Res. 2020, 34, 1123–1133. [Google Scholar] [CrossRef]
- Epstein, J.; Docena, G.; MacDonald, T.T.; Sanderson, I.R. Curcumin Suppresses P38 Mitogen-Activated Protein Kinase Activation, Reduces IL-1β and Matrix Metalloproteinase-3 and Enhances IL-10 in the Mucosa of Children and Adults with Inflammatory Bowel Disease. Br. J. Nutr. 2010, 103, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Young, K.N.; Moniruzzaman, M.; Beyene, A.M.; Do, K.; Kalaiselvi, S.; Min, T. Curcumin and Its Modified Formulations on Inflammatory Bowel Disease (IBD): The Story so Far and Future Outlook. Pharmaceutics 2021, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Kaitha, S.; Bashir, M.; Ali, T. Iron Deficiency Anemia in Inflammatory Bowel Disease. World J. Gastrointest. Pathophysiol. 2015, 6, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wilkinson, J., IV; Di, X.; Wang, W.; Hatcher, H.; Kock, N.D.; D’Agostino, R., Jr.; Knovich, M.A.; Torti, F.M.; Torti, S.V. Curcumin, a Cancer Chemopreventive and Chemotherapeutic Agent, Is a Biologically Active Iron Chelator. Blood J. Am. Soc. Hematol. 2009, 113, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Nicoliche, T.; Maldonado, D.C.; Faber, J.; da Silva, M.C.P. Evaluation of the Articular Cartilage in the Knees of Rats with Induced Arthritis Treated with Curcumin. PLoS ONE 2020, 15, e0230228. [Google Scholar] [CrossRef]
- Yan, D.; He, B.; Guo, J.; Li, S.; Wang, J. Involvement of TLR4 in the Protective Effect of Intra-Articular Administration of Curcumin on Rat Experimental Osteoarthritis. Acta Cirúrgica Bras. 2019, 34, e201900604. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.; Zhang, H.; Li, H.; Liu, J.; Zhang, F.; Jiang, T.; Jiang, S. Curcumin Prevents Osteoarthritis by Inhibiting the Activation of Inflammasome NLRP3. J. Interf. Cytokine Res. 2017, 37, 449–455. [Google Scholar] [CrossRef]
- Guo, C.; Fu, R.; Wang, S.; Huang, Y.; Li, X.; Zhou, M.; Zhao, J.; Yang, N. NLRP3 Inflammasome Activation Contributes to the Pathogenesis of Rheumatoid Arthritis. Clin. Exp. Immunol. 2018, 194, 231–243. [Google Scholar] [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-Associated Uric Acid Crystals Activate the NALP3 Inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Er, B.; Durmus, A.S.; Ozercan, I.H.; Sahin, N.; Padigaru, M.; Morde, A.; Rai, D. Protective Effect of a Novel Highly Bioavailable Formulation of Curcumin in Experimentally Induced Osteoarthritis Rat Model. Curr. Dev. Nutr. 2020, 4, 1765. [Google Scholar] [CrossRef]
- Hamdalla, H.M.; Ahmed, R.R.; Galaly, S.R.; Naguib, I.A.; Alghamdi, B.S.; Ahmed, O.M.; Farghali, A.; Abdul-Hamid, M. Ameliorative Effect of Curcumin Nanoparticles against Monosodium Iodoacetate-Induced Knee Osteoarthritis in Rats. Mediat. Inflamm. 2022, 2022, 8353472. [Google Scholar] [CrossRef] [PubMed]
- Paultre, K.; Cade, W.; Hernandez, D.; Reynolds, J.; Greif, D.; Best, T.M. Therapeutic Effects of Turmeric or Curcumin Extract on Pain and Function for Individuals with Knee Osteoarthritis: A Systematic Review. BMJ Open Sport Exerc. Med. 2021, 7, e000935. [Google Scholar] [CrossRef] [PubMed]
- Chandran, B.; Goel, A. A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phyther. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Efficacy and Safety of Meriva®, a Curcumin-Phosphatidylcholine Complex, during Extended Administration in Osteoarthritis Patients. Altern. Med. Rev. 2010, 15, 337–344. [Google Scholar]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Product-Evaluation Registry of Meriva®, a Curcumin-Phosphatidylcholine Complex, for the Complementary Management of Osteoarthritis. Panminerva Med. 2010, 52, 55–62. [Google Scholar]
- Saber, M.M.; Mahmoud, M.M.; Amin, H.M.; Essam, R.M. Therapeutic Effects of Combining Curcumin and Swimming in Osteoarthritis Using a Rat Model. Biomed. Pharmacother. 2023, 166, 115309. [Google Scholar] [CrossRef]
- Singh, L.; Sharma, S.; Xu, S.; Tewari, D.; Fang, J. Curcumin as a Natural Remedy for Atherosclerosis: A Pharmacological Review. Molecules 2021, 26, 4036. [Google Scholar] [CrossRef]
- Chen, Y.; Chai, Y.; Xie, K.; Yu, F.; Wang, C.; Lin, S.; Yang, Y.; Xu, F. Curcumin Promotes the Expression of IL-35 by Regulating Regulatory T Cell Differentiation and Restrains Uncontrolled Inflammation and Lung Injury in Mice. Inflammation 2020, 43, 1913–1924. [Google Scholar] [CrossRef]
- Cox, F.F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. Protective Effects of Curcumin in Cardiovascular Diseases—Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Hu, G.; Xu, C.; Jiang, H. Curcumin Attenuates Hydrogen Peroxide-Induced Premature Senescence via the Activation of SIRT1 in Human Umbilical Vein Endothelial Cells. Biol. Pharm. Bull. 2015, 38, 1134–1141. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and Radical Scavenging Properties of Curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-Atom Transfer Is a Preferred Antioxidant Mechanism of Curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [PubMed]
- Faten, R.A.; Ibrahim, A.E.; Khaled, A.E. Protective and Modulatory Effects of Curcumin and L-Carnitine against Methotrexate-Induced Oxidative Stress in Albino Rats. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 744–754. [Google Scholar]
- Gao, S.; Zhang, W.; Zhao, Q.; Zhou, J.; Wu, Y.; Liu, Y.; Yuan, Z.; Wang, L. Curcumin Ameliorates Atherosclerosis in Apolipoprotein E Deficient Asthmatic Mice by Regulating the Balance of Th2/Treg Cells. Phytomedicine 2019, 52, 129–135. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Abdollahi, E.; Nikfar, B.; Chaichian, S.; Ekhlasi-Hundrieser, M. Curcumin as a Potential Modulator of M1 and M2 Macrophages: New Insights in Atherosclerosis Therapy. Heart Fail. Rev. 2019, 24, 399–409. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, J.; Li, P.; Zheng, X.; Feng, D. Curcumin Protects against Atherosclerosis in Apolipoprotein E-Knockout Mice by Inhibiting Toll-like Receptor 4 Expression. J. Agric. Food Chem. 2018, 66, 449–456. [Google Scholar] [CrossRef]
- Han, Y.; Sun, H.-J.; Tong, Y.; Chen, Y.-Z.; Ye, C.; Qiu, Y.; Zhang, F.; Chen, A.-D.; Qi, X.-H.; Chen, Q. Curcumin Attenuates Migration of Vascular Smooth Muscle Cells via Inhibiting NFκB-Mediated NLRP3 Expression in Spontaneously Hypertensive Rats. J. Nutr. Biochem. 2019, 72, 108212. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Pirro, M.; Gotto, A.M., Jr.; Banach, M.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Lipid-Modifying Activity of Curcuminoids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1178–1187. [Google Scholar] [CrossRef]
- Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Imaizumi, A.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Komiyama, M.; Wada, H.; et al. Highly Absorptive Curcumin Reduces Serum Atherosclerotic Low-Density Lipoprotein Levels in Patients with Mild COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2029–2034. [Google Scholar] [CrossRef]
- Sahebkar, A. A Systematic Review and Meta-Analysis of Randomized Controlled Trials Investigating the Effects of Curcumin on Blood Lipid Levels. Clin. Nutr. 2014, 33, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Czekaj, R.; Majka, J.; Magierowska, K.; Sliwowski, Z.; Magierowski, M.; Pajdo, R.; Ptak-Belowska, A.; Surmiak, M.; Kwiecien, S.; Brzozowski, T. Mechanisms of Curcumin-Induced Gastroprotection against Ethanol-Induced Gastric Mucosal Lesions. J. Gastroenterol. 2018, 53, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Xu, D.; Xu, S.; Zheng, Y.; Xu, S. Novel Role of Zn (II)–Curcumin in Enhancing Cell Proliferation and Adjusting Proinflammatory Cytokine-Mediated Oxidative Damage of Ethanol-Induced Acute Gastric Ulcers. Chem. Biol. Interact. 2012, 197, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Mahattanadul, S.; Nakamura, T.; Panichayupakaranant, P.; Phdoongsombut, N.; Tungsinmunkong, K.; Bouking, P. Comparative Antiulcer Effect of Bisdemethoxycurcumin and Curcumin in a Gastric Ulcer Model System. Phytomedicine 2009, 16, 342–351. [Google Scholar] [CrossRef]
- Tuorkey, M.; Karolin, K. Anti-Ulcer Activity of Curcumin on Experimental Gastric Ulcer in Rats and Its Effect on Oxidative Stress/Antioxidant, IL-6 and Enzyme Activities. Biomed. Environ. Sci. 2009, 22, 488–495. [Google Scholar] [CrossRef]
- Mahattanadul, S.; Mustafa, M.W.; Kuadkaew, S.; Pattharachayakul, S.; Ungphaiboon, S.; Sawanyawisuth, K. Oral Ulcer Healing and Anti-Candida Efficacy of an Alcohol-Free Chitosan-Curcumin Mouthwash. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7020–7023. [Google Scholar]
- Kuadkaew, S.; Ungphaiboon, S.; Phdoongsombut, N.; Kaewsuwan, S.; Mahattanadul, S. Efficacy of a Chitosan-Curcumin Mixture in Treating Indomethacininduced Acute Gastric Ulcer in Rats. Curr. Pharm. Biotechnol. 2021, 22, 1919–1931. [Google Scholar] [CrossRef]
- Heikal, E.J.; Kaoud, R.M.; Gad, S.; Mokhtar, H.I.; Aldahish, A.A.; Alzlaiq, W.A.; Zaitone, S.A.; Moustafa, Y.M.; Hammady, T.M. Design and Optimization of Omeprazole-Curcumin-Loaded Hydrogel Beads Coated with Chitosan for Treating Peptic Ulcers. Pharmaceuticals 2023, 16, 795. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J.; Fernandez-Botran, R. Effects of Curcumin on Helicobacter Pylori Infection. Ann. Transl. Med. 2016, 4, 479. [Google Scholar] [CrossRef]
- Kositchaiwat, C.; Kositchaiwat, S.; Havanondha, J. Curcuma Longa Linn. in the Treatment of Gastric Ulcer Comparison to Liquid Antacid: A Controlled Clinical Trial. J. Med. Assoc. Thail. 1993, 76, 601–605. [Google Scholar]
- Prucksunand, C.; Indrasukhsri, B.; Leethochawalit, M.; Hungspreugs, K. Phase II Clinical Trial on Effect of the Long Turmeric (Curcuma Longa Linn.) on Healing of Peptic Ulcer. Southeast Asian J. Trop. Med. Public Health 2001, 32, 208–215. [Google Scholar] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; He, H.; Chen, Y.; Huang, W.; Cheng, J.; Ye, J.; Wang, A.; Tao, J.; Wang, C.; Liu, Q. Identification of a Selective and Direct NLRP3 Inhibitor to Treat Inflammatory Disorders. J. Exp. Med. 2017, 214, 3219. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.; Ceballos-Olvera, I.; del Barrio, L.; Re, F. Role of the Inflammasome, IL-1beta, and IL-18 in Bacterial Infections. Sci. World J. 2011, 11, 2037–2050. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Marín-Palma, D.; Tabares-Guevara, J.H.; Zapata-Cardona, M.I.; Flórez-Álvarez, L.; Yepes, L.M.; Rugeles, M.T.; Zapata-Builes, W.; Hernandez, J.C.; Taborda, N.A. Curcumin Inhibits in Vitro SARS-CoV-2 Infection in Vero E6 Cells through Multiple Antiviral Mechanisms. Molecules 2021, 26, 6900. [Google Scholar] [CrossRef]
- Rattis, B.A.C.; Ramos, S.G.; Celes, M. Curcumin as a Potential Treatment for COVID-19. Front. Pharmacol. 2021, 12, 675287. [Google Scholar] [CrossRef]
- Tahmasebi, S.; Saeed, B.Q.; Temirgalieva, E.; Yumashev, A.V.; El-Esawi, M.A.; Navashenaq, J.G.; Valizadeh, H.; Sadeghi, A.; Aslani, S.; Yousefi, M. Nanocurcumin Improves Treg Cell Responses in Patients with Mild and Severe SARS-CoV2. Life Sci. 2021, 276, 119437. [Google Scholar] [CrossRef]
- Golpour-Hamedani, S.; Pourmasoumi, M.; Askari, G.; Bagherniya, M.; Majeed, M.; Guest, P.C.; Sahebkar, A. Antiviral Mechanisms of Curcumin and Its Derivatives in Prevention and Treatment of COVID-19: A Review. Adv. Exp. Med. Biol. 2023, 1412, 397–411. [Google Scholar] [CrossRef]
- Saber-Moghaddam, N.; Salari, S.; Hejazi, S.; Amini, M.; Taherzadeh, Z.; Eslami, S.; Rezayat, S.M.; Jaafari, M.R.; Elyasi, S. Oral Nano-curcumin Formulation Efficacy in Management of Mild to Moderate Hospitalized Coronavirus Disease-19 Patients: An Open Label Nonrandomized Clinical Trial. Phyther. Res. 2021, 35, 2616–2623. [Google Scholar] [CrossRef]
- Kahkhaie, K.R.; Mirhosseini, A.; Aliabadi, A.; Mohammadi, A.; Mousavi, M.J.; Haftcheshmeh, S.M.; Sathyapalan, T.; Sahebkar, A. Curcumin: A Modulator of Inflammatory Signaling Pathways in the Immune System. Inflammopharmacology 2019, 27, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Nicoliche, T.; Bartolomeo, C.S.; Lemes, R.M.R.; Pereira, G.C.; Nunes, T.A.; Oliveira, R.B.; Nicastro, A.L.M.; Soares, É.N.; da Cunha Lima, B.F.; Rodrigues, B.M. Antiviral, Anti-Inflammatory and Antioxidant Effects of Curcumin and Curcuminoids in SH-SY5Y Cells Infected by SARS-CoV-2. Sci. Rep. 2024, 14, 10696. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Niziński, P.; Hawrył, A.; Gancarz, M.; Hawrył, D.; Oliwa, W.; Pałka, M.; Markowska, J.; Oniszczuk, A. Potential of Curcumin in the Management of Skin Diseases. Int. J. Mol. Sci. 2024, 25, 3617. [Google Scholar] [CrossRef] [PubMed]
- Bahraini, P.; Rajabi, M.; Mansouri, P.; Sarafian, G.; Chalangari, R.; Azizian, Z. Turmeric Tonic as a Treatment in Scalp Psoriasis: A Randomized Placebo-Control Clinical Trial. J. Cosmet. Dermatol. 2018, 17, 461–466. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Liu, L.; Sun, X.; Zhou, Y.; Chen, S.; Lu, Y.; Cai, X.; Hu, M.; Yan, G.; et al. Efficacy and Safety of Curcumin in Psoriasis: Preclinical and Clinical Evidence and Possible Mechanisms. Front. Pharmacol. 2022, 13, 903160. [Google Scholar] [CrossRef]
- Jain, A.; Doppalapudi, S.; Domb, A.J.; Khan, W. Tacrolimus and Curcumin Co-Loaded Liposphere Gel: Synergistic Combination towards Management of Psoriasis. J. Control. Release 2016, 243, 132–145. [Google Scholar] [CrossRef]
- Mao, K.-L.; Fan, Z.-L.; Yuan, J.-D.; Chen, P.-P.; Yang, J.-J.; Xu, J.; ZhuGe, D.-L.; Jin, B.-H.; Zhu, Q.-Y.; Shen, B.-X.; et al. Skin-Penetrating Polymeric Nanoparticles Incorporated in Silk Fibroin Hydrogel for Topical Delivery of Curcumin to Improve Its Therapeutic Effect on Psoriasis Mouse Model. Colloids Surf. B Biointerfaces 2017, 160, 704–714. [Google Scholar] [CrossRef]
- Kim, H.; Choi, M.R.; Jeon, S.H.; Jang, Y.; Yang, Y.D. Pathophysiological Roles of Ion Channels in Epidermal Cells, Immune Cells, and Sensory Neurons in Psoriasis. Int. J. Mol. Sci. 2024, 25, 2756. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Banaeeyeh, S.; Eisvand, F.; Sathyapalan, T.; Hashemzaei, M.; Sahebkar, A. Effects of Curcumin on Ion Channels and Pumps: A Review. IUBMB Life 2019, 71, 812–820. [Google Scholar] [CrossRef]
- Kang, D.; Li, B.; Luo, L.; Jiang, W.; Lu, Q.; Rong, M.; Lai, R. Curcumin Shows Excellent Therapeutic Effect on Psoriasis in Mouse Model. Biochimie 2016, 123, 73–80. [Google Scholar] [CrossRef]
- Reddy, S.; Aggarwal, B.B. Curcumin Is a Non-Competitive and Selective Inhibitor of Phosphorylase Kinase. FEBS Lett. 1994, 341, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, C.; Fu, J.; Wang, Y.; Yang, D.; Peng, B.; Liu, X.; Han, X.; Meng, Y.; Feng, F.; et al. CD44 Targeted Indirubin Nanocrystal-Loaded Hyaluronic Acid Hydrogel for the Treatment of Psoriasis. Int. J. Biol. Macromol. 2023, 243, 125239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, Q.; Li, Y.; He, Z.; Li, Z.; Guo, T.; Wu, Z.; Feng, N. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: A New Strategy for Clustering Drug in Inflammatory Skin. Theranostics 2019, 9, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Z.; Wang, L.; Cun, D.; Tong, H.H.Y.; Yan, R.; Chen, X.; Wang, R.; Zheng, Y. Enhanced Topical Penetration, System Exposure and Anti-Psoriasis Activity of Two Particle-Sized, Curcumin-Loaded PLGA Nanoparticles in Hydrogel. J. Control. Release Off. J. Control. Release Soc. 2017, 254, 44–54. [Google Scholar] [CrossRef]
- Jin, N.; Lin, J.; Yang, C.; Wu, C.; He, J.; Chen, Z.; Yang, Q.; Chen, J.; Zheng, G.; Lv, L.; et al. Enhanced Penetration and Anti-Psoriatic Efficacy of Curcumin by Improved SmartPearls Technology with the Addition of Glycyrrhizic Acid. Int. J. Pharm. 2020, 578, 119101. [Google Scholar] [CrossRef]
- Heng, M.C.Y.; Song, M.K.; Harker, J.; Heng, M.K. Drug-induced Suppression of Phosphorylase Kinase Activity Correlates with Resolution of Psoriasis as Assessed by Clinical, Histological and Immunohistochemical Parameters. Br. J. Dermatol. 2000, 143, 937–949. [Google Scholar] [CrossRef]
- Kurd, S.K.; Smith, N.; VanVoorhees, A.; Troxel, A.B.; Badmaev, V.; Seykora, J.T.; Gelfand, J.M. Oral Curcumin in the Treatment of Moderate to Severe Psoriasis Vulgaris: A Prospective Clinical Trial. J. Am. Acad. Dermatol. 2008, 58, 625–631. [Google Scholar] [CrossRef]
- Asawanonda, P.; Klahan, S.-O. Tetrahydrocurcuminoid Cream plus Targeted Narrowband UVB Phototherapy for Vitiligo: A Preliminary Randomized Controlled Study. Photomed. Laser Surg. 2010, 28, 679–684. [Google Scholar] [CrossRef]
- Vaccaro, M.; Bagnato, G.; Cristani, M.; Borgia, F.; Spatari, G.; Tigano, V.; Saja, A.; Guarneri, F.; Cannavò, S.P.; Gangemi, S. Oxidation Products Are Increased in Patients Affected by Non-Segmental Generalized Vitiligo. Arch. Dermatol. Res. 2017, 309, 485–490. [Google Scholar] [CrossRef]
- Sun, Y.P.; Gu, J.F.; Tan, X.B.; Wang, C.F.; Jia, X.B.; Feng, L.; Liu, J.P. Curcumin Inhibits Advanced Glycation End Product-Induced Oxidative Stress and Inflammatory Responses in Endothelial Cell Damage via Trapping Methylglyoxal. Mol. Med. Rep. 2016, 13, 1475–1486. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [PubMed]
- Jalalmanesh, S.; Mansouri, P.; Rajabi, M.; Monji, F. Therapeutic Effects of Turmeric Topical Cream in Vitiligo: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Cosmet. Dermatol. 2022, 21, 4454–4461. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, A.A.; Bharti, R.K. Spontaneous Reversal of Vitiligo, a Rare Phenomenon Reported in a Case in Saudi Arabia with an Insight into Metabolic Biochemical Derangements. Medicina 2023, 59, 427. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 Signaling Pathway by Polyphenols: A Novel Therapeutic Strategy for Neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Liu, B.; Yu, S.Y. Curcumin Protects against Chronic Stress-Induced Dysregulation of Neuroplasticity and Depression-like Behaviors via Suppressing IL-1β Pathway in Rats. Neuroscience 2018, 392, 92–106. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Han, W.; Yang, M.; Wen, L.; Wang, K.; Jiang, P. Curcumin Relieves Depressive-like Behaviors via Inhibition of the NLRP3 Inflammasome and Kynurenine Pathway in Rats Suffering from Chronic Unpredictable Mild Stress. Int. Immunopharmacol. 2019, 67, 138–144. [Google Scholar] [CrossRef]
- Fattore, L.; Amchova, P.; Fadda, P.; Ruda-Kucerova, J. Olfactory Bulbectomy Model of Depression Lowers Responding for Food in Male and Female Rats: The Modulating Role of Caloric Restriction and Response Requirement. Biomedicines 2023, 11, 2481. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.-S.; Yao, H.-Y.; Lin, Y.-H.; Ma, X.; Zhang, Y.-H.; Li, X.-J. Antidepressant Effects of Curcumin in the Forced Swim Test and Olfactory Bulbectomy Models of Depression in Rats. Pharmacol. Biochem. Behav. 2005, 82, 200–206. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Y.; Wu, H.-L.; Li, Y.-B.; Li, Y.-H.; Guo, J.-B.; Li, X.-J. The Antidepressant Effects of Curcumin in the Forced Swimming Test Involve 5-HT1 and 5-HT2 Receptors. Eur. J. Pharmacol. 2008, 578, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.L.; Akinfiresoye, L.; Nwulia, E.; Kamiya, A.; Kulkarni, A.A.; Tizabi, Y. Antidepressant-like Effects of Curcumin in WKY Rat Model of Depression Is Associated with an Increase in Hippocampal BDNF. Behav. Brain Res. 2013, 239, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.K.; Bishnoi, M.; Kulkarni, S.K. Anti-Depressant like Effect of Curcumin and Its Combination with Piperine in Unpredictable Chronic Stress-Induced Behavioral, Biochemical and Neurochemical Changes. Pharmacol. Biochem. Behav. 2009, 92, 39–43. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Hosny, E.N.; Magdy, M.; Mohammed, H.S. Antidepressant Effects of Curcumin-Coated Iron Oxide Nanoparticles in a Rat Model of Depression. Eur. J. Pharmacol. 2021, 908, 174384. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Aboalasaad, F.A.; Mohamed, A.S.; Elhusseiny, F.A.; Khadrawy, Y.A.; Elmekawy, A. Evaluation of the Therapeutic Effect of Curcumin-Conjugated Zinc Oxide Nanoparticles on Reserpine-Induced Depression in Wistar Rats. Biol. Trace Elem. Res. 2024, 202, 2630–2644. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; Li, N.; Pi, C.; Zhao, W.; Zhong, Y.; Li, W.; Shen, H.; Yang, Y.; Zheng, W. Preliminary Investigation Into the Antidepressant Effects of a Novel Curcumin Analogue (CACN136) In Vitro and In Vivo. Mol. Neurobiol. 2024, 1–24. [Google Scholar] [CrossRef]
- Ng, Q.X.; Koh, S.S.H.; Chan, H.W.; Ho, C.Y.X. Clinical Use of Curcumin in Depression: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2017, 18, 503–508. [Google Scholar] [CrossRef]
- Kanchanatawan, B.; Tangwongchai, S.; Sughondhabhirom, A.; Suppapitiporn, S.; Hemrunrojn, S.; Carvalho, A.F.; Maes, M. Add-on Treatment with Curcumin Has Antidepressive Effects in Thai Patients with Major Depression: Results of a Randomized Double-Blind Placebo-Controlled Study. Neurotox. Res. 2018, 33, 621–633. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M.; Attia, M.F.; Hussein, J.; Mekawi, E.M.; Galal, H.M.; Ahmed, E.I.; Allam, A.A.; El-Naggar, M.E. Curcumin Nanoparticles Have Potential Antioxidant Effect and Restore Tetrahydrobiopterin Levels in Experimental Diabetes. Biomed. Pharmacother. 2020, 131, 110688. [Google Scholar] [CrossRef]
- Noorafshan, A.; Ashkani-Esfahani, S. A Review of Therapeutic Effects of Curcumin. Curr. Pharm. Des. 2013, 19, 2032–2046. [Google Scholar] [PubMed]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic Applications of Curcumin Nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Mobarhan, M.G.; Oskuee, R.K. The Effect of Nano-Curcumin on HbA1c, Fasting Blood Glucose, and Lipid Profile in Diabetic Subjects: A Randomized Clinical Trial. Avicenna J. Phytomedicine 2016, 6, 567. [Google Scholar]
- Na, L.; Li, Y.; Pan, H.; Zhou, X.; Sun, D.; Meng, M.; Li, X.; Sun, C. Curcuminoids Exert Glucose-lowering Effect in Type 2 Diabetes by Decreasing Serum Free Fatty Acids: A Double-blind, Placebo-controlled Trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef]
- Funamoto, M.; Shimizu, K.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Wada, H.; Hasegawa, K.; et al. Effects of Highly Absorbable Curcumin in Patients with Impaired Glucose Tolerance and Non-Insulin-Dependent Diabetes Mellitus. J. Diabetes Res. 2019, 2019, 8208237. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcuminoids plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef]
- Agharazi, M.; Gazerani, S.; Huntington, M.K. Topical Turmeric Ointment in the Treatment of Diabetic Foot Ulcers: A Randomized, Placebo-Controlled Study. Int. J. Low. Extrem. Wounds 2022, 15347346221143222. [Google Scholar] [CrossRef]
- Mokhtari, M.; Razzaghi, R.; Momen-Heravi, M. The Effects of Curcumin Intake on Wound Healing and Metabolic Status in Patients with Diabetic Foot Ulcer: A Randomized, Double-blind, Placebo-controlled Trial. Phyther. Res. 2021, 35, 2099–2107. [Google Scholar] [CrossRef]
- Cui, L.; Miao, J.; Cui, L. Cytotoxic Effect of Curcumin on Malaria Parasite Plasmodium Falciparum: Inhibition of Histone Acetylation and Generation of Reactive Oxygen Species. Antimicrob. Agents Chemother. 2007, 51, 488–494. [Google Scholar] [CrossRef]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for Malaria Therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef]
- Nandakumar, D.N.; Nagaraj, V.A.; Vathsala, P.G.; Rangarajan, P.; Padmanaban, G. Curcumin-Artemisinin Combination Therapy for Malaria. Antimicrob. Agents Chemother. 2006, 50, 1859–1860. [Google Scholar] [CrossRef] [PubMed]
- Tjahjani, S.; Tjokropranoto, R. Interaction of Alphamangostin and Curcumin with Dihydroartemisinin as Antimalaria in Vitro. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 125, p. 12017. [Google Scholar]
- Akide Ndunge, O.B.; Kilian, N.; Salman, M.M. Cerebral Malaria and Neuronal Implications of Plasmodium Falciparum Infection: From Mechanisms to Advanced Models. Adv. Sci. 2022, 9, e2202944. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An Orally Bioavailable Blocker of TNF and Other pro-Inflammatory Biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Dende, C.; Meena, J.; Nagarajan, P.; Nagaraj, V.A.; Panda, A.K.; Padmanaban, G. Nanocurcumin Is Superior to Native Curcumin in Preventing Degenerative Changes in Experimental Cerebral Malaria. Sci. Rep. 2017, 7, 10062. [Google Scholar] [CrossRef]
- Ghosh, A.; Banerjee, T.; Bhandary, S.; Surolia, A. Formulation of Nanotized Curcumin and Demonstration of Its Antimalarial Efficacy. Int. J. Nanomed. 2014, 9, 5373–5387. [Google Scholar] [CrossRef]
- Dandekar, P.P.; Jain, R.; Patil, S.; Dhumal, R.; Tiwari, D.; Sharma, S.; Vanage, G.; Patravale, V. Curcumin-Loaded Hydrogel Nanoparticles: Application in Anti-Malarial Therapy and Toxicological Evaluation. J. Pharm. Sci. 2010, 99, 4992–5010. [Google Scholar] [CrossRef]
- Rashidzadeh, H.; Salimi, M.; Sadighian, S.; Rostamizadeh, K.; Ramazani, A. In Vivo Antiplasmodial Activity of Curcumin-Loaded Nanostructured Lipid Carriers. Curr. Drug Deliv. 2019, 16, 923–930. [Google Scholar] [CrossRef]
- Akhtar, F.; Rizvi, M.M.A.; Kar, S.K. Oral Delivery of Curcumin Bound to Chitosan Nanoparticles Cured Plasmodium Yoelii Infected Mice. Biotechnol. Adv. 2012, 30, 310–320. [Google Scholar] [CrossRef]
- Dos Santos, R.B.; Nakama, K.A.; Pacheco, C.O.; de Gomes, M.G.; de Souza, J.F.; de Souza Pinto, A.C.; de Oliveira, F.A.; da Fonseca, A.L.; Varotti, F.; Fajardo, A.R. Curcumin-Loaded Nanocapsules: Influence of Surface Characteristics on Technological Parameters and Potential Antimalarial Activity. Mater. Sci. Eng. C 2021, 118, 111356. [Google Scholar] [CrossRef]
- Memvanga, P.B.; Coco, R.; Préat, V. An Oral Malaria Therapy: Curcumin-Loaded Lipid-Based Drug Delivery Systems Combined with β-Arteether. J. Control. Release 2013, 172, 904–913. [Google Scholar] [CrossRef]
- Khairani, S.; Fauziah, N.; Wiraswati, H.L.; Panigoro, R.; Setyowati, E.Y.; Berbudi, A. The Potential Use of a Curcumin-Piperine Combination as an Antimalarial Agent: A Systematic Review. J. Trop. Med. 2021, 2021, 9135617. [Google Scholar] [CrossRef] [PubMed]
- Schraufstätter, E.; Bernt, H. Antibacterial Action of Curcumin and Related Compounds. Nature 1949, 164, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K. Chemistry and Biomedical Applications of Cumin and Turmeric: A Review, Challenge and Perspective. Chem. Afr. 2022, 5, 1191–1213. [Google Scholar] [CrossRef]
- Rai, D.; Singh, J.K.; Roy, N.; Panda, D. Curcumin Inhibits FtsZ Assembly: An Attractive Mechanism for Its Antibacterial Activity. Biochem. J. 2008, 410, 147–155. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef]
- Sharifian, P.; Yaslianifard, S.; Fallah, P.; Aynesazi, S.; Bakhtiyari, M.; Mohammadzadeh, M. Investigating the Effect of Nano-Curcumin on the Expression of Biofilm Regulatory Genes of Pseudomonas Aeruginosa. Infect. Drug Resist. 2020, 13, 2477–2484. [Google Scholar] [CrossRef]
- Teow, S.-Y.; Liew, K.; Ali, S.A.; Khoo, A.S.-B.; Peh, S.-C. Antibacterial Action of Curcumin against Staphylococcus Aureus: A Brief Review. J. Trop. Med. 2016, 2016, 2853045. [Google Scholar] [CrossRef]
- Zandi, K.; Ramedani, E.; Mohammadi, K.; Tajbakhsh, S.; Deilami, I.; Rastian, Z.; Fouladvand, M.; Yousefi, F.; Farshadpour, F. Evaluation of Antiviral Activities of Curcumin Derivatives against HSV-1 in Vero Cell Line. Nat. Prod. Commun. 2010, 5, 1934578X1000501220. [Google Scholar] [CrossRef]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial Activity of Curcumin against Helicobacter Pylori Isolates from India and during Infections in Mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef]
- Hettiarachchi, S.S.; Perera, Y.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, S.; Rajapakse, R.M.G. Comparison of Antibacterial Activity of Nanocurcumin with Bulk Curcumin. ACS Omega 2022, 7, 46494–46500. [Google Scholar] [CrossRef]
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T. Synergistic Antibacterial Effect of Curcumin against Methicillin-Resistant Staphylococcus Aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Teow, S.-Y.; Ali, S.A. Synergistic Antibacterial Activity of Curcumin with Antibiotics against Staphylococcus Aureus. Pak. J. Pharm. Sci. 2015, 28, 2109–2114. [Google Scholar] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, P.; Fan, Z.; He, X.; Liu, J.; Li, M.; Chen, F. Dandelion Polysaccharide Treatment Protects against Dextran Sodium Sulfate-induced Colitis by Suppressing NF-κB/NLRP3 Inflammasome-mediated Inflammation and Activating Nrf2 in Mouse Colon. Food Sci. Nutr. 2023, 11, 7271–7282. [Google Scholar] [CrossRef]
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Yu, S.Y. Neuroprotective Effects of Curcumin on IL-1β-Induced Neuronal Apoptosis and Depression-like Behaviors Caused by Chronic Stress in Rats. Front. Cell. Neurosci. 2019, 12, 516. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Wang, X.; Yu, S.; Li, L.; Wu, X.; Chen, Y.; Zhao, J.; Zhao, Y. Neuroprotection by Curcumin in Ischemic Brain Injury Involves the Akt/Nrf2 Pathway. PLoS ONE 2013, 8, e59843. [Google Scholar] [CrossRef]
- Qi, X.-J.; Liu, X.-Y.; Tang, L.-M.-Y.; Li, P.-F.; Qiu, F.; Yang, A.-H. Anti-Depressant Effect of Curcumin-Loaded Guanidine-Chitosan Thermo-Sensitive Hydrogel by Nasal Delivery. Pharm. Dev. Technol. 2020, 25, 316–325. [Google Scholar] [CrossRef]
- Zbarsky, V.; Datla, K.P.; Parkar, S.; Rai, D.K.; Aruoma, O.I.; Dexter, D.T. Neuroprotective Properties of the Natural Phenolic Antioxidants Curcumin and Naringenin but Not Quercetin and Fisetin in a 6-OHDA Model of Parkinson’s Disease. Free Radic. Res. 2005, 39, 1119–1125. [Google Scholar] [CrossRef]
- Agrawal, S.S.; Gullaiya, S.; Dubey, V.; Singh, V.; Kumar, A.; Nagar, A.; Tiwari, P. Neurodegenerative Shielding by Curcumin and Its Derivatives on Brain Lesions Induced by 6-OHDA Model of Parkinson’s Disease in Albino Wistar Rats. Cardiovasc. Psychiatry Neurol. 2012, 2012, 942981. [Google Scholar] [CrossRef]
- Song, S.; Nie, Q.; Li, Z.; Du, G. Curcumin Improves Neurofunctions of 6-OHDA-Induced Parkinsonian Rats. Pathol. Res. Pract. 2016, 212, 247–251. [Google Scholar] [CrossRef]
- Yang, J.; Song, S.; Li, J.; Liang, T. Neuroprotective Effect of Curcumin on Hippocampal Injury in 6-OHDA-Induced Parkinson’s Disease Rat. Pathol. Res. Pract. 2014, 210, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Li, C.; Chen, Z.; Gao, Q.; Han, M.; Zhao, F.; Chen, D.; Chen, Q.; Hu, M.; et al. The Dosage of Curcumin to Alleviate Movement Symptoms in a 6-Hydroxydopamine-Induced Parkinson’s Disease Rat Model. Heliyon 2023, 9, e16921. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Risk Factors for Alzheimer’s Disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin Inhibits Formation of Amyloid Beta Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in Vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, Y. Curcumin Protects against Intracellular Amyloid Toxicity in Rat Primary Neurons. Int. J. Clin. Exp. Med. 2012, 5, 44–49. [Google Scholar]
- Shi, H.; Yin, Z.; Koronyo, Y.; Fuchs, D.-T.; Sheyn, J.; Davis, M.R.; Wilson, J.W.; Margeta, M.A.; Pitts, K.M.; Herron, S.; et al. Regulating Microglial MiR-155 Transcriptional Phenotype Alleviates Alzheimer’s-Induced Retinal Vasculopathy by Limiting Clec7a/Galectin-3(+) Neurodegenerative Microglia. Acta Neuropathol. Commun. 2022, 10, 136. [Google Scholar] [CrossRef]
- Teter, B.; Morihara, T.; Lim, G.P.; Chu, T.; Jones, M.R.; Zuo, X.; Paul, R.M.; Frautschy, S.A.; Cole, G.M. Curcumin Restores Innate Immune Alzheimer’s Disease Risk Gene Expression to Ameliorate Alzheimer Pathogenesis. Neurobiol. Dis. 2019, 127, 432–448. [Google Scholar] [CrossRef]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.-P.; Liu, J.; Merrill, D.A. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef]
- Das, S.S.; Gopal, P.M.; Thomas, J.V.; Mohan, M.C.; Thomas, S.C.; Maliakel, B.P.; Krishnakumar, I.M.; Pulikkaparambil Sasidharan, B.C. Influence of CurQfen®-Curcumin on Cognitive Impairment: A Randomized, Double-Blinded, Placebo-Controlled, 3-Arm, 3-Sequence Comparative Study. Front. Dement. 2023, 2, 1222708. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and Cognition: A Randomised, Placebo-Controlled, Double-Blind Study of Community-Dwelling Older Adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and Pharmacodynamics of Curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar] [PubMed]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of Curcumin in Buffer Solutions and Characterization of Its Degradation Products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a Golden Spice with a Low Bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal-Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef]
- Baek, J.-S.; Cho, C.-W. Surface Modification of Solid Lipid Nanoparticles for Oral Delivery of Curcumin: Improvement of Bioavailability through Enhanced Cellular Uptake, and Lymphatic Uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Li, Z.; Peng, S.; Chen, X.; Zhu, Y.; Zou, L.; Liu, W.; Liu, C. Pluronics Modified Liposomes for Curcumin Encapsulation: Sustained Release, Stability and Bioaccessibility. Food Res. Int. 2018, 108, 246–253. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Efficacy and Safety of Curcumin and Curcuma Longa Extract in the Treatment of Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Immunol. 2022, 13, 891822. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Zielińska, D.; Setzer, W.N. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 550909. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Are Curcuminoids Effective C-Reactive Protein-Lowering Agents in Clinical Practice? Evidence from a Meta-Analysis. Phytother. Res. 2014, 28, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Pelletier, J. Examen Chimique de La Racine de Curcuma. J. Pharm 1815, 1, 289–300. [Google Scholar]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of Curcumin, a Component of Golden Spice, and Its Miraculous Biological Activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. Acute and Subchronic Toxicity as Well as Mutagenic Evaluation of Essential Oil from Turmeric (Curcuma Longa L). Food Chem. Toxicol. 2013, 53, 52–61. [Google Scholar] [CrossRef]
- Kim, D.-C.; Ku, S.-K.; Bae, J.-S. Anticoagulant Activities of Curcumin and Its Derivative. BMB Rep. 2012, 45, 221–226. [Google Scholar] [CrossRef]
- Shah, B.H.; Nawaz, Z.; Pertani, S.A.; Roomi, A.; Mahmood, H.; Saeed, S.A.; Gilani, A.H. Inhibitory Effect of Curcumin, a Food Spice from Turmeric, on Platelet-Activating Factor-and Arachidonic Acid-Mediated Platelet Aggregation through Inhibition of Thromboxane Formation and Ca2+ Signaling. Biochem. Pharmacol. 1999, 58, 1167–1172. [Google Scholar] [CrossRef]
- Chaudhari, S.P.; Tam, A.Y.; Barr, J.A. Curcumin: A Contact Allergen. J. Clin. Aesthet. Dermatol. 2015, 8, 43–48. [Google Scholar]
- Sathyabhama, M.; Priya Dharshini, L.C.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 2022, 12, 1405. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Vinqvist, M.R.; Mukai, K.; Goto, H.; Hashimoto, Y.; Tokunaga, A.; Uno, H. On the Antioxidant Mechanism of Curcumin: Classical Methods Are Needed to Determine Antioxidant Mechanism and Activity. Org. Lett. 2000, 2, 2841–2843. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, F.; Abas, F.; Lajis, N.H.; Othman, I.; Naidu, R. Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer. Biomolecules 2019, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Beevers, C.S.; Huang, S. The Targets of Curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef]
- Zhang, F.; Altorki, N.K.; Mestre, J.R.; Subbaramaiah, K.; Dannenberg, A.J. Curcumin Inhibits Cyclooxygenase-2 Transcription in Bile Acid-and Phorbol Ester-Treated Human Gastrointestinal Epithelial Cells. Carcinogenesis 1999, 20, 445–451. [Google Scholar] [CrossRef]
- Setyono, J.; Harini, I.M.; Sarmoko, S.; Rujito, L. Supplementation of Curcuma Domestica Extract Reduces Cox-2 and Inos Expression on Raw 264.7 Cells. J. Phys. Conf. Ser. 2019, 1246, 12059. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Cho, S.-S.; Li, Y.; Bae, C.-S.; Park, K.M.; Park, D.-H. Anti-Inflammatory Effect of Curcuma Longa and Allium Hookeri Co-Treatment via NF-ΚB and COX-2 Pathways. Sci. Rep. 2020, 10, 5718. [Google Scholar] [CrossRef]
- Sreejayan, X.X.; Rao, M.N.A. Nitric Oxide Scavenging by Curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar] [CrossRef]
- Sreejayan, N.; Rao, M.N. Free Radical Scavenging Activity of Curcuminoids. Arzneimittelforschung 1996, 46, 169–171. [Google Scholar]
- Carvalho, D.d.M.; Takeuchi, K.P.; Geraldine, R.M.; de Moura, C.J.; Torres, M.C.L. Production, Solubility and Antioxidant Activity of Curcumin Nanosuspension. Food Sci. Technol. 2015, 35, 115–119. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of Phenolic O-H and Methylene Hydrogen on the Free Radical Reactions and Antioxidant Activity of Curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant Activities of Curcumin, Demethoxycurcumin and Bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin Attenuates Oxidative Stress in RAW264. 7 Cells by Increasing the Activity of Antioxidant Enzymes and Activating the Nrf2-Keap1 Pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar]
- Ren, L.; Zhan, P.; Wang, Q.; Wang, C.; Liu, Y.; Yu, Z.; Zhang, S. Curcumin Upregulates the Nrf2 System by Repressing Inflammatory Signaling-Mediated Keap1 Expression in Insulin-Resistant Conditions. Biochem. Biophys. Res. Commun. 2019, 514, 691–698. [Google Scholar] [CrossRef]
- Liu, F.; Gao, S.; Yang, Y.; Zhao, X.; Fan, Y.; Ma, W.; Yang, D.; Yang, A.; Yu, Y. Antitumor Activity of Curcumin by Modulation of Apoptosis and Autophagy in Human Lung Cancer A549 Cells through Inhibiting PI3K/Akt/MTOR Pathway. Oncol. Rep. 2018, 39, 1523–1531. [Google Scholar] [CrossRef]
- Kuttikrishnan, S.; Siveen, K.S.; Prabhu, K.S.; Khan, A.Q.; Ahmed, E.I.; Akhtar, S.; Ali, T.A.; Merhi, M.; Dermime, S.; Steinhoff, M. Curcumin Induces Apoptotic Cell Death via Inhibition of PI3-Kinase/AKT Pathway in B-Precursor Acute Lymphoblastic Leukemia. Front. Oncol. 2019, 9, 484. [Google Scholar] [CrossRef]
- Panahi, Y.; Rahimnia, A.; Sharafi, M.; Alishiri, G.; Saburi, A.; Sahebkar, A. Curcuminoid Treatment for Knee Osteoarthritis: A Randomized Double-blind Placebo-controlled Trial. Phyther. Res. 2014, 28, 1625–1631. [Google Scholar] [CrossRef]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Sahebkar, A. Adjuvant Therapy with Bioavailability-Boosted Curcuminoids Suppresses Systemic Inflammation and Improves Quality of Life in Patients with Solid Tumors: A Randomized Double-Blind Placebo-Controlled Trial. Phytother. Res. 2014, 28, 1461–1467. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcumin on Serum Cytokine Concentrations in Subjects with Metabolic Syndrome: A Post-Hoc Analysis of a Randomized Controlled Trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef]
- Abe, Y.; Hashimoto, S.H.U.; Horie, T. Curcumin Inhibition of Inflammatory Cytokine Production by Human Peripheral Blood Monocytes and Alveolar Macrophages. Pharmacol. Res. 1999, 39, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Salvatore, R.; Panaro, M.A. PI3k/Akt Signalling Pathway Plays a Crucial Role in the Anti-Inflammatory Effects of Curcumin in LPS-Activated Microglia. Int. Immunopharmacol. 2016, 36, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.S.; Mishra, K.P.; Singh, D.P.; Mehrotra, S.; Singh, V.K. Immunomodulatory Effects of Curcumin. Immunopharmacol. Immunotoxicol. 2005, 27, 485–497. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Xiao, X.; Han, L.; Wu, Z.; Ma, Q.; Cao, L. Curcumin Attenuates Hyperglycemia-Driven EGF-Induced Invasive and Migratory Abilities of Pancreatic Cancer via Suppression of the ERK and AKT Pathways. Oncol. Rep. 2019, 41, 650–658. [Google Scholar] [CrossRef]
- Mohan, R.; Sivak, J.; Ashton, P.; Russo, L.A.; Pham, B.Q.; Kasahara, N.; Raizman, M.B.; Fini, M.E. Curcuminoids Inhibit the Angiogenic Response Stimulated by Fibroblast Growth Factor-2, Including Expression of Matrix Metalloproteinase Gelatinase B. J. Biol. Chem. 2000, 275, 10405–10412. [Google Scholar] [CrossRef]
- El-Azab, M.; Hishe, H.; Moustafa, Y.; El-Awady, E.-S. Anti-Angiogenic Effect of Resveratrol or Curcumin in Ehrlich Ascites Carcinoma-Bearing Mice. Eur. J. Pharmacol. 2011, 652, 7–14. [Google Scholar] [CrossRef]
- Bae, M.-K.; Kim, S.-H.; Jeong, J.-W.; Lee, Y.M.; Kim, H.-S.; Kim, S.-R.; Yun, I.; Bae, S.-K.; Kim, K.-W. Curcumin Inhibits Hypoxia-Induced Angiogenesis via down-Regulation of HIF-1. Oncol. Rep. 2006, 15, 1557–1562. [Google Scholar] [CrossRef]
- Nakamura, K.; Yasunaga, Y.; Segawa, T.; Ko, D.; Moul, J.W.; Srivastava, S.; Rhim, J.S. Curcumin Down-Regulates AR Gene Expression and Activation in Prostate Cancer Cell Lines. Int. J. Oncol. 2002, 21, 825–830. [Google Scholar] [CrossRef]
- Dorai, T.; Gehani, N.; Katz, A. Therapeutic Potential of Curcumin in Human Prostate Cancer—I. Curcumin Induces Apoptosis in Both Androgen-Dependent and Androgen-Independent Prostate Cancer Cells. Prostate Cancer Prostatic Dis. 2000, 3, 84–93. [Google Scholar] [CrossRef]
- Saxena, A.R.; Ilic, Z.; Sripada, V.; Crawford, D.R. Lower Concentrations of Curcumin Inhibit Her2-Akt Pathway Components in Human Breast Cancer Cells, and Other Dietary Botanicals Potentiate This and Lapatinib Inhibition. Nutr. Res. 2020, 78, 93–104. [Google Scholar] [CrossRef]
- Miyazaki, K.; Morine, Y.; Xu, C.; Nakasu, C.; Wada, Y.; Teraoku, H.; Yamada, S.; Saito, Y.; Ikemoto, T.; Shimada, M.; et al. Curcumin-Mediated Resistance to Lenvatinib via EGFR Signaling Pathway in Hepatocellular Carcinoma. Cells 2023, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Lee, Y.-M.; Chang, G.-C.; Yu, S.-L.; Hsieh, W.-Y.; Chen, J.J.W.; Chen, H.-W.; Yang, P.-C. Curcumin Induces EGFR Degradation in Lung Adenocarcinoma and Modulates P38 Activation in Intestine: The Versatile Adjuvant for Gefitinib Therapy. PLoS ONE 2011, 6, e23756. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-G.; Cho, J.-H.; Choi, J.; Ju, E.-M.; Adam, G.O.; Hwang, D.-I.; Lee, J.-H.; An, S.-Y.; Choi, H.-K.; Park, C.-B. Immunomodulatory Effects of Curcuma Longa L. and Carthamus Tinctorius L. on RAW 264.7 Macrophages and Cyclophosphamide-Induced Immunosuppression C57BL/6 Mouse Models. J. Funct. Foods 2022, 91, 105000. [Google Scholar] [CrossRef]
- Huang, M.-T.; Lysz, T.; Ferraro, T.; Abidi, T.F.; Laskin, J.D.; Conney, A.H. Inhibitory Effects of Curcumin on in Vitro Lipoxygenase and Cyclooxygenase Activities in Mouse Epidermis. Cancer Res. 1991, 51, 813–819. [Google Scholar]
- Srimal, R.C.; Dhawan, B.N. Pharmacology of Diferuloyl Methane (Curcumin), a Non-steroidal Anti-inflammatory Agent. J. Pharm. Pharmacol. 1973, 25, 447–452. [Google Scholar] [CrossRef]
- Cao, J.; Han, Z.; Tian, L.; Chen, K.; Fan, Y.; Ye, B.; Huang, W.; Wang, C.; Huang, Z. Curcumin Inhibits EMMPRIN and MMP-9 Expression through AMPK-MAPK and PKC Signaling in PMA Induced Macrophages. J. Transl. Med. 2014, 12, 266. [Google Scholar] [CrossRef]
- Youn, G.S.; Kwon, D.-J.; Ju, S.M.; Choi, S.Y.; Park, J. Curcumin Ameliorates TNF-α-Induced ICAM-1 Expression and Subsequent THP-1 Adhesiveness via the Induction of Heme Oxygenase-1 in the HaCaT Cells. BMB Rep. 2013, 46, 410–415. [Google Scholar] [CrossRef]
- Machova Urdzikova, L.; Karova, K.; Ruzicka, J.; Kloudova, A.; Shannon, C.; Dubisova, J.; Murali, R.; Kubinova, S.; Sykova, E.; Jhanwar-Uniyal, M.; et al. The Anti-Inflammatory Compound Curcumin Enhances Locomotor and Sensory Recovery after Spinal Cord Injury in Rats by Immunomodulation. Int. J. Mol. Sci. 2015, 17, 49. [Google Scholar] [CrossRef]
- Binion, D.G.; Heidemann, J.; Li, M.S.; Nelson, V.M.; Otterson, M.F.; Rafiee, P. Vascular Cell Adhesion Molecule-1 Expression in Human Intestinal Microvascular Endothelial Cells Is Regulated by PI 3-Kinase/Akt/MAPK/NF-KappaB: Inhibitory Role of Curcumin. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G259–G268. [Google Scholar] [CrossRef]
- Wang, J.; Qi, L.; Zheng, S.; Wu, T. Curcumin Induces Apoptosis through the Mitochondria-Mediated Apoptotic Pathway in HT-29 Cells. J. Zhejiang Univ. Sci. B 2009, 10, 93–102. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Peng, Z.; Song, J.; Ren, J. Curcumin Down-Regulates PCNA, Cyclin D1 and Bcl-XL Expression in Human Keratinocyte Cell Lines. J. Med. Coll. PLA 2010, 25, 321–330. [Google Scholar]
- Meena, R.; Kumar, S.; Gaharwar, U.S.; Rajamani, P. PLGA-CTAB Curcumin Nanoparticles: Fabrication, Characterization and Molecular Basis of Anticancer Activity in Triple Negative Breast Cancer Cell Lines (MDA-MB-231 Cells). Biomed. Pharmacother. 2017, 94, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, K.M.; Mahesh, V.B.; Brann, D.W. Curcumin Suppresses Growth and Chemoresistance of Human Glioblastoma Cells via AP-1 and NFkappaB Transcription Factors. J. Neurochem. 2007, 102, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Kliem, C.; Merling, A.; Giaisi, M.; Köhler, R.; Krammer, P.H.; Li-Weber, M. Curcumin Suppresses T Cell Activation by Blocking Ca2+ Mobilization and Nuclear Factor of Activated T Cells (NFAT) Activation. J. Biol. Chem. 2012, 287, 10200–10209. [Google Scholar] [CrossRef]
- Jung, E.M.; Lim, J.H.; Lee, T.J.; Park, J.-W.; Choi, K.S.; Kwon, T.K. Curcumin Sensitizes Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)-Induced Apoptosis through Reactive Oxygen Species-Mediated Upregulation of Death Receptor 5 (DR5). Carcinogenesis 2005, 26, 1905–1913. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Tan, T.-H. Inhibition of the C-Jun N-Terminal Kinase (JNK) Signaling Pathway by Curcumin. Oncogene 1998, 17, 173–178. [Google Scholar] [CrossRef]
- Thulasiraman, P.; McAndrews, D.J.; Mohiudddin, I.Q. Curcumin Restores Sensitivity to Retinoic Acid in Triple Negative Breast Cancer Cells. BMC Cancer 2014, 14, 724. [Google Scholar] [CrossRef]
- Li, Q.; Sun, J.; Mohammadtursun, N.; Wu, J.; Dong, J.; Li, L. Curcumin Inhibits Cigarette Smoke-Induced Inflammation via Modulating the PPARγ-NF-ΚB Signaling Pathway. Food Funct. 2019, 10, 7983–7994. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Z.; Chen, G.; Wang, D.; Tang, S.; Deng, H.; Wang, J.; Li, S.; Lan, J.; Tong, J.; et al. Curcumin Attenuates Asthmatic Airway Inflammation and Mucus Hypersecretion Involving a PPARγ-Dependent NF-ΚB Signaling Pathway in Vivo and in Vitro. Mediat. Inflamm. 2019, 2019, 4927430. [Google Scholar] [CrossRef]
- Bradley, J. TNF-mediated Inflammatory Disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Anwar, M.J.; Alenezi, S.K.; Alhowail, A.H. Molecular Insights into the Pathogenic Impact of Vitamin D Deficiency in Neurological Disorders. Biomed. Pharmacother. 2023, 162, 114718. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.J.; Hernanz, A.; Medina, S.; Bayón, C.; Iglesias, P. Serum Concentrations of Tumour Necrosis Factor-alpha (TNF-α) and Soluble TNF-α Receptor P55 in Patients with Hypothyroidism and Hyperthyroidism before and after Normalization of Thyroid Function. Clin. Endocrinol. 2002, 57, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-α and Obesity. TNF Pathophysiol. 2010, 11, 145–156. [Google Scholar]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I. Inhibition of the NF-ΚB Signaling Pathway by the Curcumin Analog, 3, 5-Bis (2-Pyridinylmethylidene)-4-Piperidone (EF31): Anti-Inflammatory and Anti-Cancer Properties. Int. Immunopharmacol. 2012, 12, 368–377. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, Y.; Wen, Y.; Chen, Y.F.; Na, L.X.; Li, S.T.; Sun, C.H. Curcumin, a Potential Inhibitor of up-Regulation of TNF-Alpha and IL-6 Induced by Palmitate in 3T3-L1 Adipocytes through NF-KappaB and JNK Pathway. Biomed. Environ. Sci. 2009, 22, 32–39. [Google Scholar] [CrossRef]
- Panaro, M.A.; Corrado, A.; Benameur, T.; Paolo, C.F.; Cici, D.; Porro, C. The Emerging Role of Curcumin in the Modulation of TLR-4 Signaling Pathway: Focus on Neuroprotective and Anti-Rheumatic Properties. Int. J. Mol. Sci. 2020, 21, 2299. [Google Scholar] [CrossRef]
- Ben, P.; Liu, J.; Lu, C.; Xu, Y.; Xin, Y.; Fu, J.; Huang, H.; Zhang, Z.; Gao, Y.; Luo, L. Curcumin Promotes Degradation of Inducible Nitric Oxide Synthase and Suppresses Its Enzyme Activity in RAW 264.7 Cells. Int. Immunopharmacol. 2011, 11, 179–186. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Bahroudi, Z.; Hussen, B.M.; Talebi, S.F.; Taheri, M.; Ayatollahi, S.A. Nrf2-Related Therapeutic Effects of Curcumin in Different Disorders. Biomolecules 2022, 12, 82. [Google Scholar] [CrossRef]
- Atiaa, A.; Abdullah, A. NQO1 Enzyme and Its Role in Cellular Protection; an Insight. Iberoam. J. Med. 2020, 2, 306–313. [Google Scholar] [CrossRef]
- Battino, M.; Giampieri, F.; Pistollato, F.; Sureda, A.; de Oliveira, M.R.; Pittalà, V.; Fallarino, F.; Nabavi, S.F.; Atanasov, A.G.; Nabavi, S.M. Nrf2 as Regulator of Innate Immunity: A Molecular Swiss Army Knife! Biotechnol. Adv. 2018, 36, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.S.; Nam, H.; Morrison, R.F. Curcumin Inhibits 3T3-L1 Preadipocyte Proliferation by Mechanisms Involving Post-Transcriptional P27 Regulation. Biochem. Biophys. Rep. 2016, 5, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yang, B.; Najafi, M. Targeting of Cancer Cell Death Mechanisms by Curcumin: Implications to Cancer Therapy. Basic Clin. Pharmacol. Toxicol. 2021, 129, 397–415. [Google Scholar] [CrossRef]
- Shen, Q.; Hang, M.; Shi, Y. Curcumin Boosts Natural Killer Cell-Based Immunotherapy in Impeding Progression of Hepatocellular Carcinoma Through Androgen Receptor/UL16 Binding Protein-2 Signal. Sci. Adv. Mater. 2022, 14, 188–195. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Md Sakib Hossain, D.; Mohanty, S.; Sankar Sen, G.; Chattopadhyay, S.; Banerjee, S.; Chakraborty, J.; Das, K.; Sarkar, D.; Das, T.; et al. Curcumin Reverses T Cell-Mediated Adaptive Immune Dysfunctions in Tumor-Bearing Hosts. Cell. Mol. Immunol. 2010, 7, 306–315. [Google Scholar] [CrossRef]
- Alinejad, S.; Khademvatan, S.; Amani, S.; Asadi, N.; Tappeh, K.H.; Yousefi, E.; Miandoabi, T. The Effect of Curcumin on the Expression of INFγ, TNF-α, and INOS Genes in PBMCs Infected with Leishmania Major [MRHO/IR/75/ER]. Infect. Disord. Drug Targets 2022, 22, e040422203031. [Google Scholar] [CrossRef]
- Draghiciu, O.; Lubbers, J.; Nijman, H.W.; Daemen, T. Myeloid Derived Suppressor Cells-An Overview of Combat Strategies to Increase Immunotherapy Efficacy. Oncoimmunology 2015, 4, e954829. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Jiang, H.; Ni, M.; Zou, Y.; Chen, Y.; Wu, T.; Ding, D.; Xu, H.; Li, X. Targeted Regulation of Tumor Microenvironment through the Inhibition of MDSCs by Curcumin Loaded Self-Assembled Nano-Filaments. Mater. Today Bio 2022, 15, 100304. [Google Scholar] [CrossRef]
- Ahmad, I.; Hoque, M.; Alam, S.S.M.; Zughaibi, T.A.; Tabrez, S. Curcumin and Plumbagin Synergistically Target the PI3K/Akt/MTOR Pathway: A Prospective Role in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 6651. [Google Scholar] [CrossRef]
- Song, J.; Choi, B.; Jin, E.-J.; Yoon, Y.; Choi, K.-H. Curcumin Suppresses Streptococcus Mutans Adherence to Human Tooth Surfaces and Extracellular Matrix Proteins. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, T.; Bais, H.P. Curcumin, a Known Phenolic from Curcuma Longa, Attenuates the Virulence of Pseudomonas Aeruginosa PAO1 in Whole Plant and Animal Pathogenicity Models. J. Agric. Food Chem. 2008, 56, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, V.M.; Peram, M.R.; Kugaji, M.S.; Shah, T.; Patil, S.P.; Muddapur, U.M.; Bhat, K.G. Effect of Curcumin on Growth, Biofilm Formation and Virulence Factor Gene Expression of Porphyromonas Gingivalis. Odontology 2021, 109, 18–28. [Google Scholar] [CrossRef]
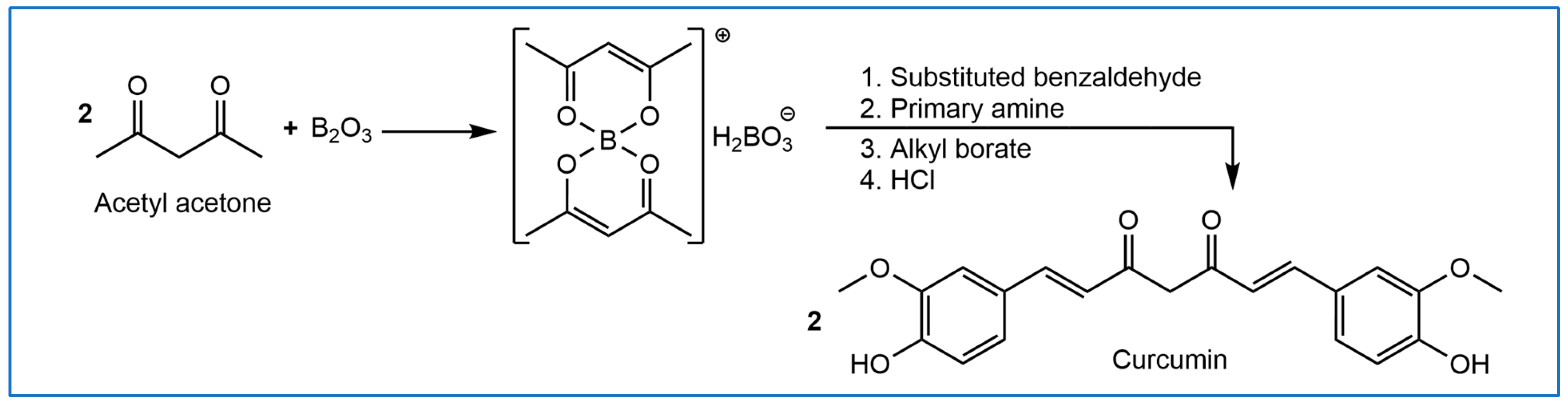
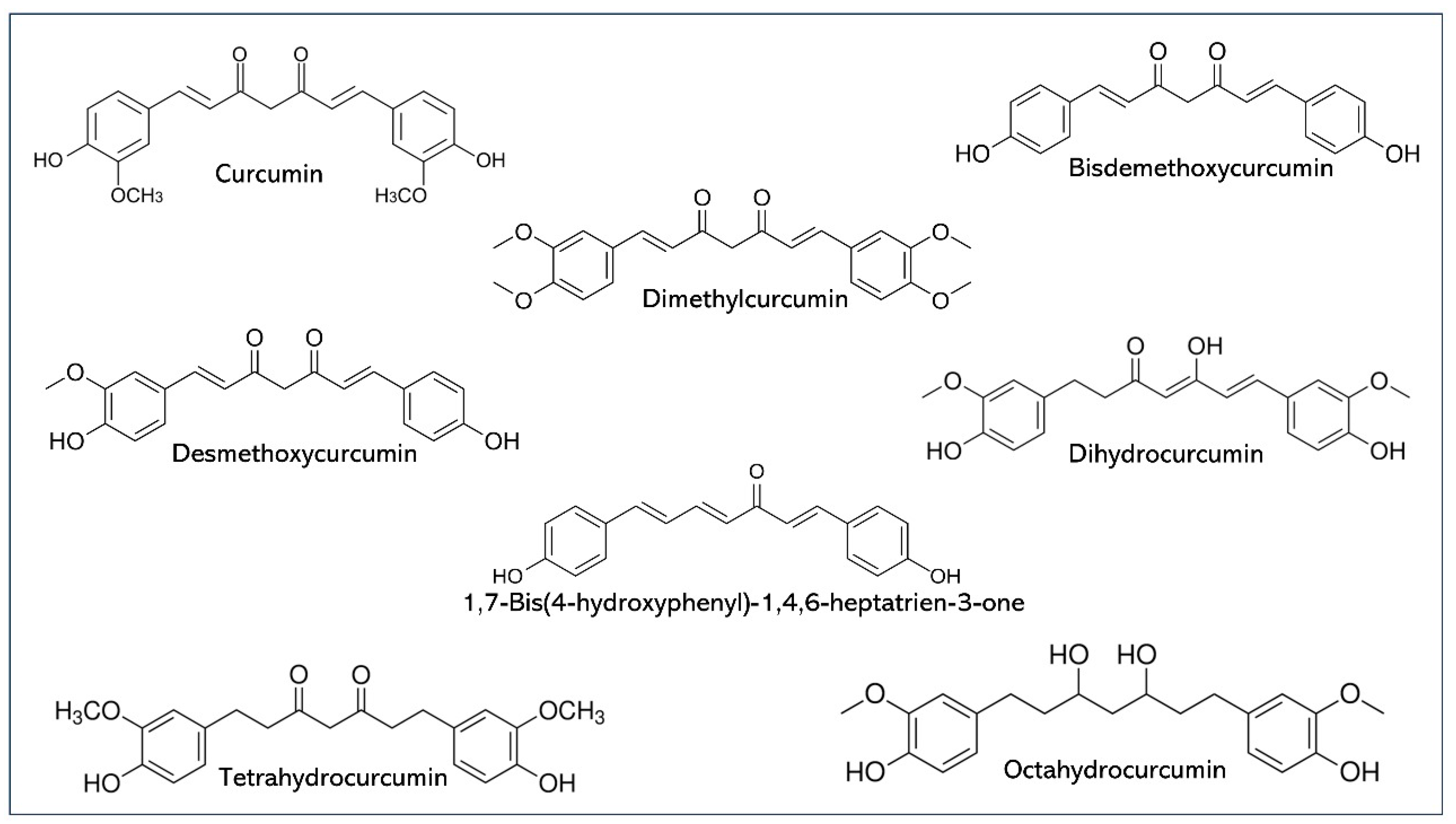



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.S.; Anwar, M.J.; Maity, M.K.; Azam, F.; Jaremko, M.; Emwas, A.-H. The Dynamic Role of Curcumin in Mitigating Human Illnesses: Recent Advances in Therapeutic Applications. Pharmaceuticals 2024, 17, 1674. https://doi.org/10.3390/ph17121674
Alam MS, Anwar MJ, Maity MK, Azam F, Jaremko M, Emwas A-H. The Dynamic Role of Curcumin in Mitigating Human Illnesses: Recent Advances in Therapeutic Applications. Pharmaceuticals. 2024; 17(12):1674. https://doi.org/10.3390/ph17121674
Chicago/Turabian StyleAlam, Md Shamshir, Md Jamir Anwar, Manish Kumar Maity, Faizul Azam, Mariusz Jaremko, and Abdul-Hamid Emwas. 2024. "The Dynamic Role of Curcumin in Mitigating Human Illnesses: Recent Advances in Therapeutic Applications" Pharmaceuticals 17, no. 12: 1674. https://doi.org/10.3390/ph17121674
APA StyleAlam, M. S., Anwar, M. J., Maity, M. K., Azam, F., Jaremko, M., & Emwas, A.-H. (2024). The Dynamic Role of Curcumin in Mitigating Human Illnesses: Recent Advances in Therapeutic Applications. Pharmaceuticals, 17(12), 1674. https://doi.org/10.3390/ph17121674











