Synthesis and Evaluation of Glucosyl-, Acyl- and Silyl- Resveratrol Derivatives as Retinoprotective Agents: Piceid Octanoate Notably Delays Photoreceptor Degeneration in a Retinitis Pigmentosa Mouse Model
Abstract
1. Introduction
2. Results and Discussion
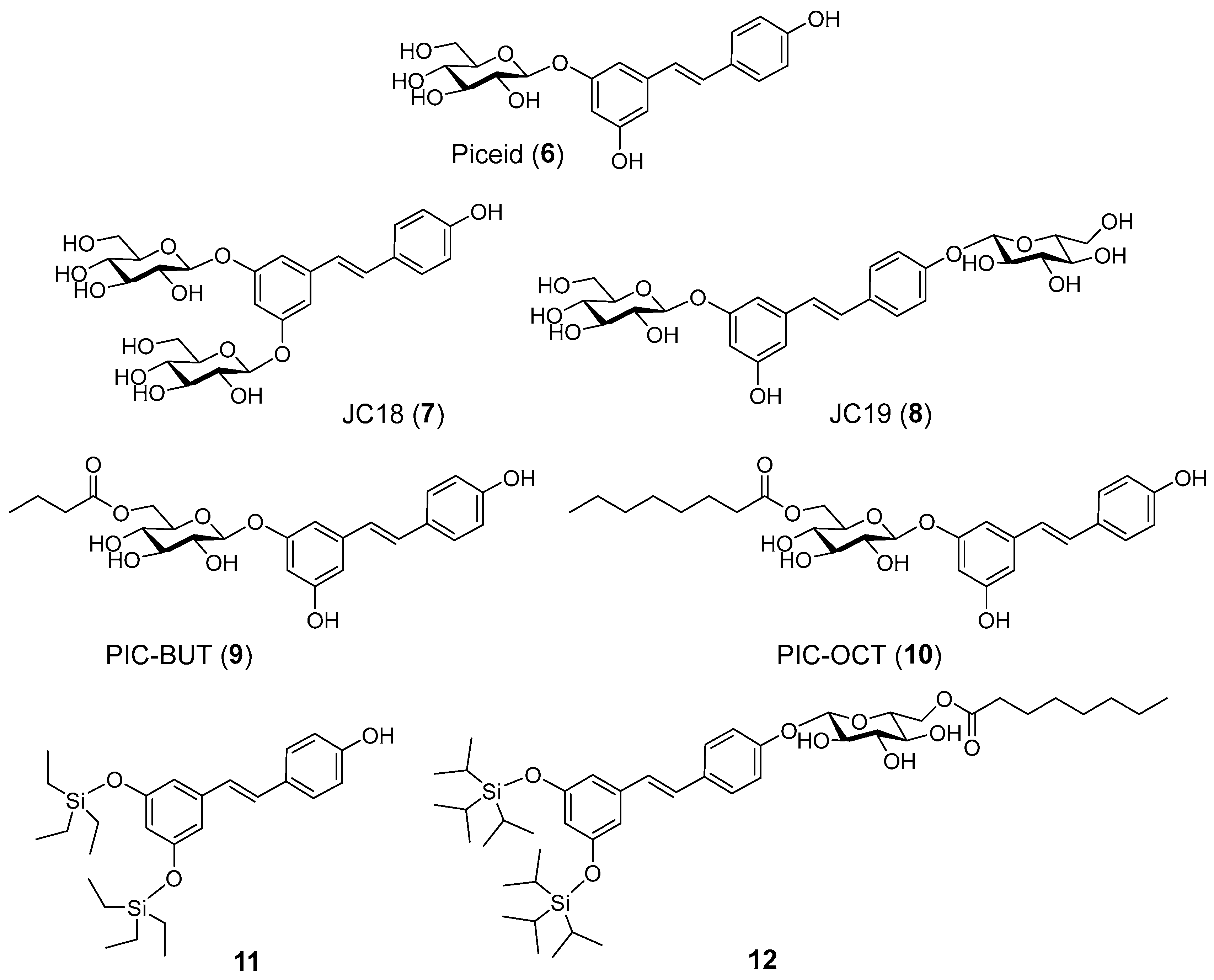
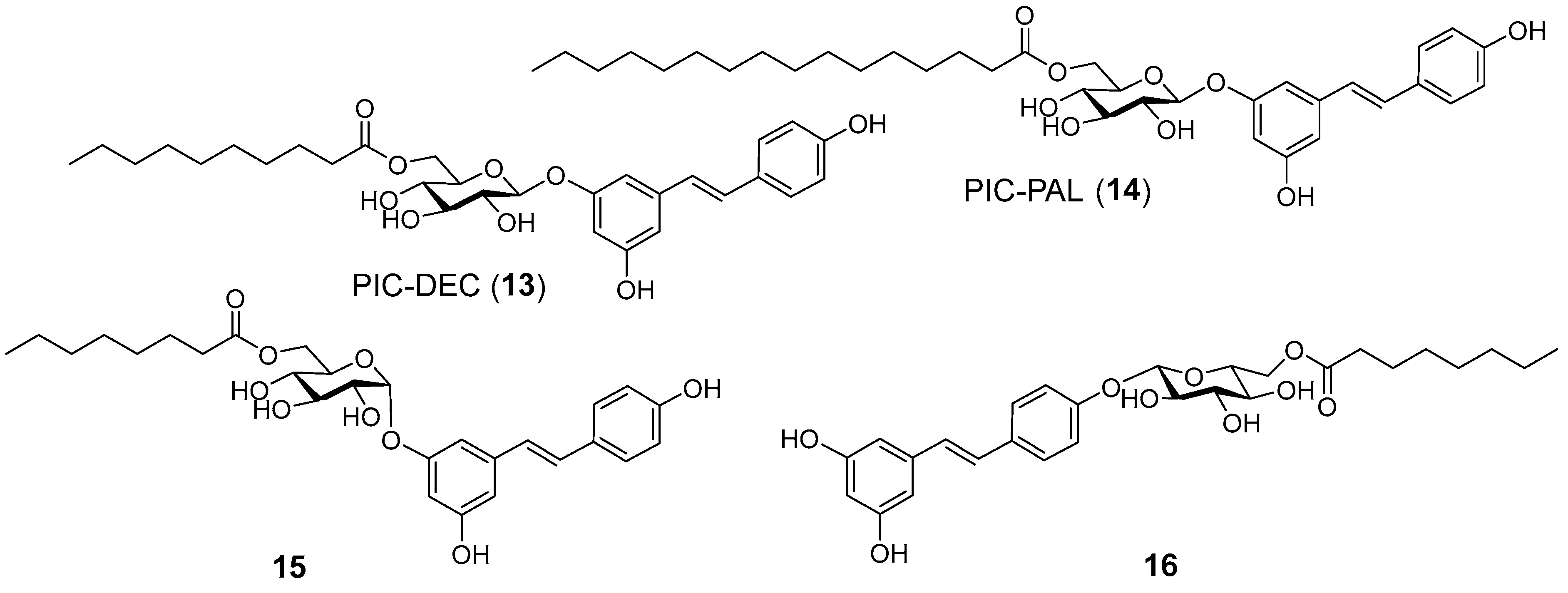
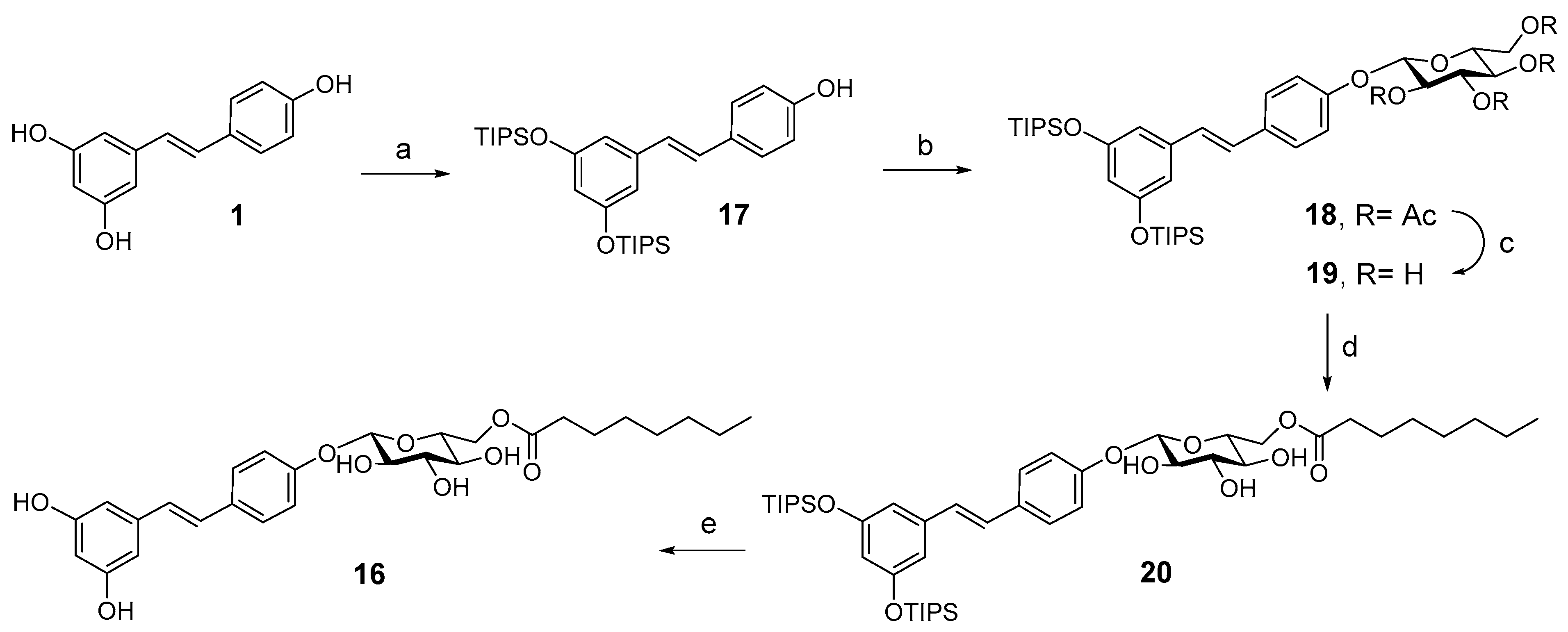
2.1. Chemistry
2.2. Biology
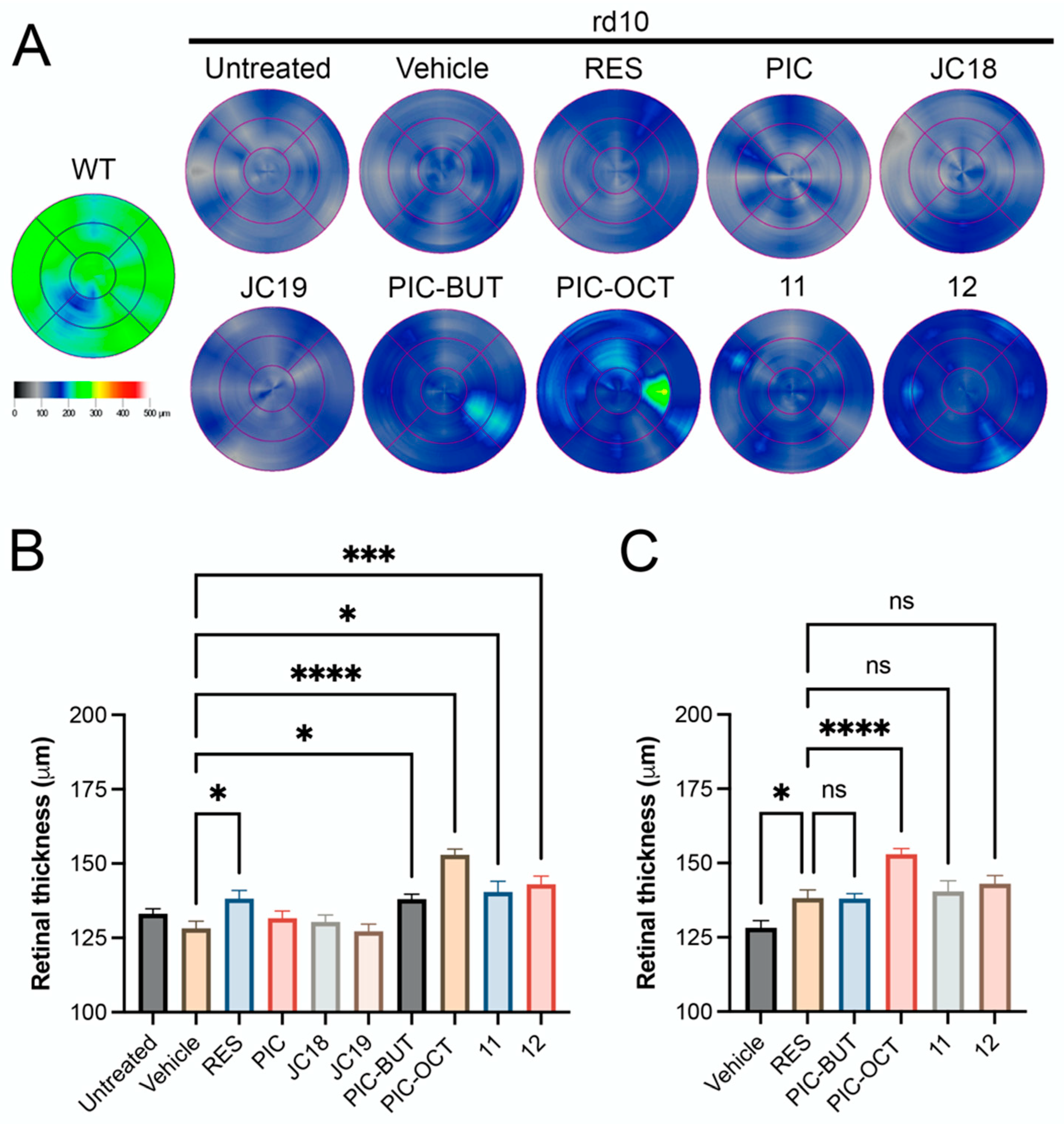
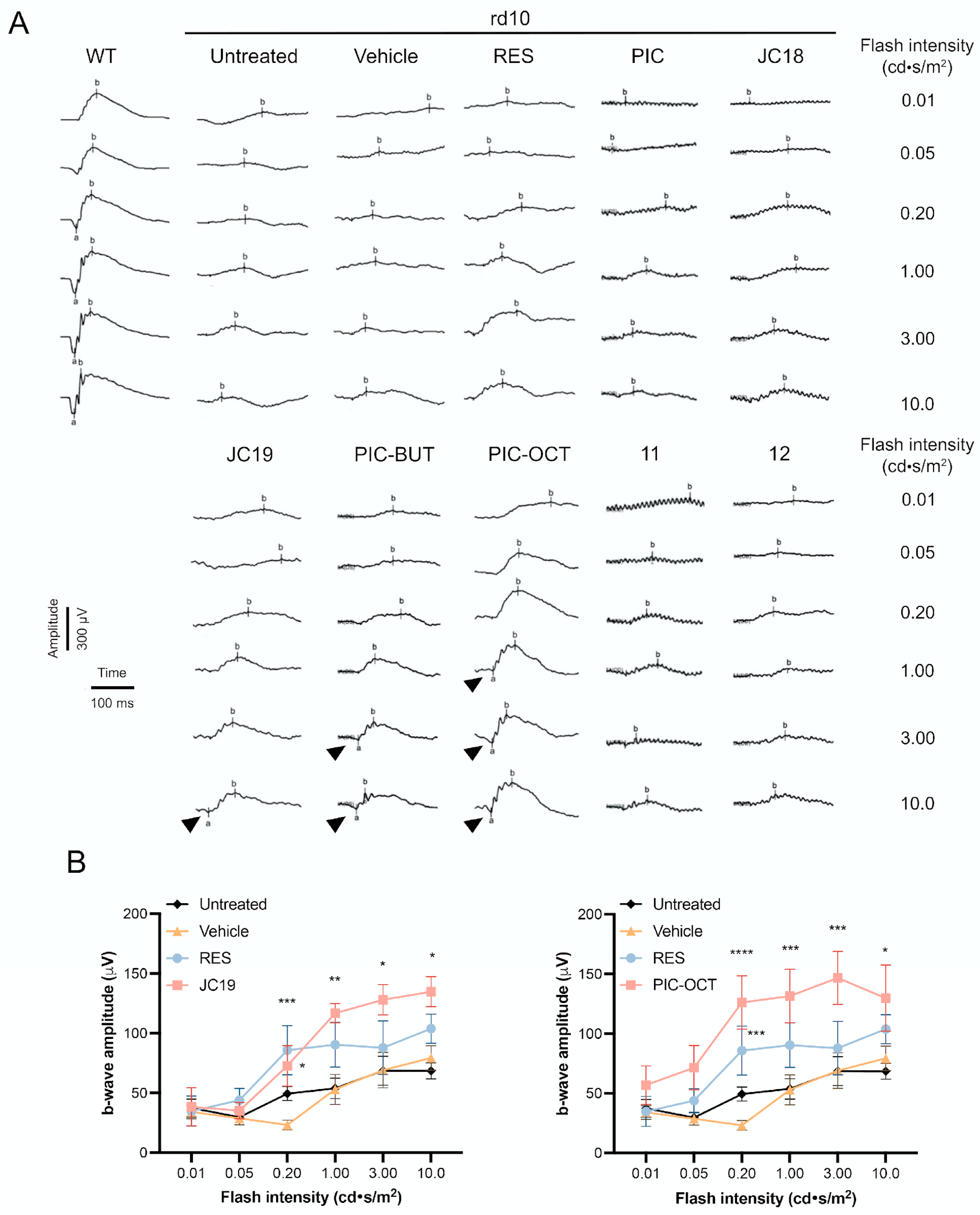
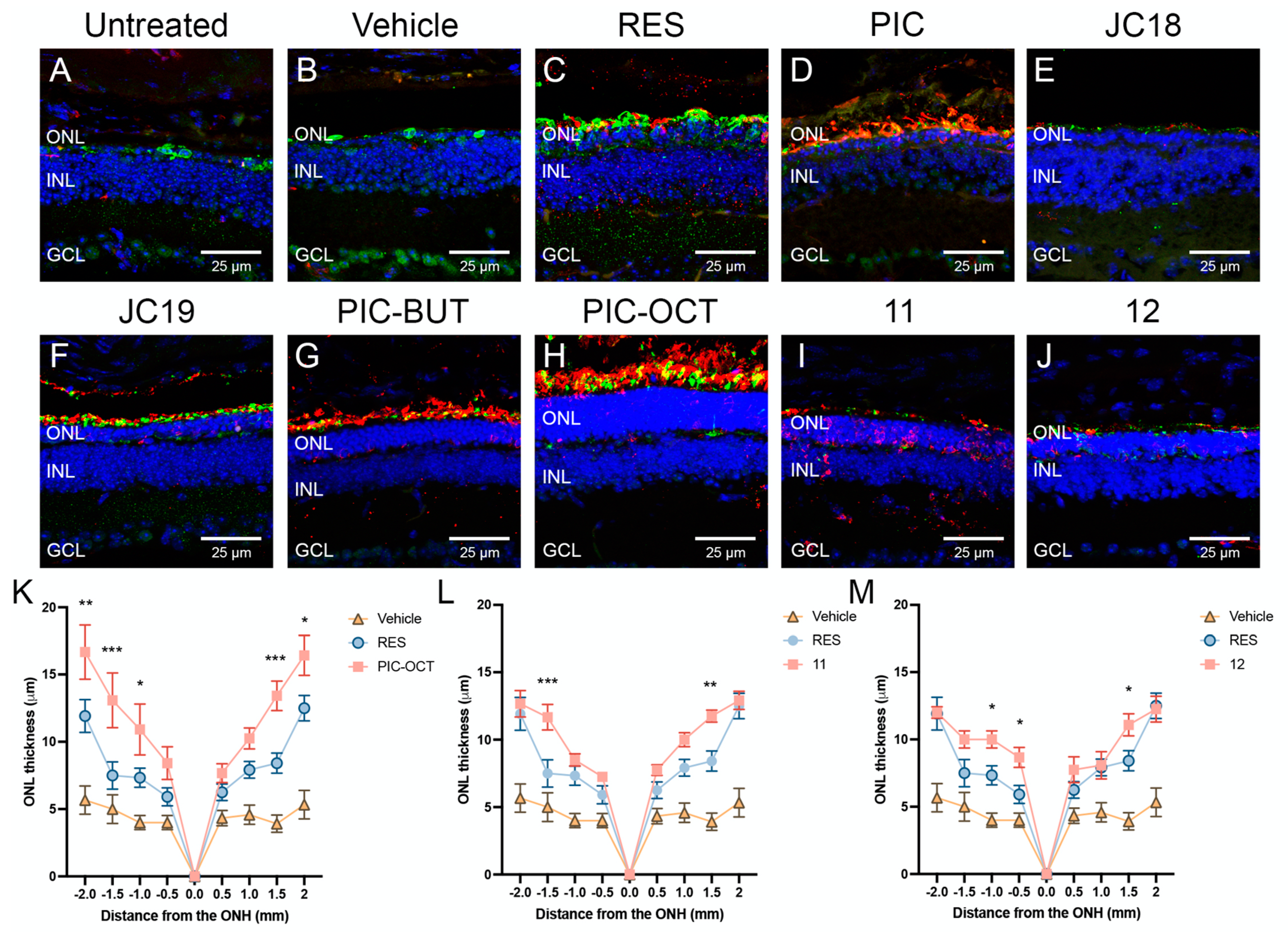
2.2.1. RES Derivative PIC-OCT (10) Preserves Efficiently Photoreceptors in rd10 Mice
2.2.2. RES Derivative PIC-OCT (10) Shows Dose–Response Activity in rd10 Mice
2.2.3. Acyl Chain Length, Carbohydrate Position and Configuration Are Key for Delaying Retinal Degeneration by PIC-OCT (10) in rd10 Mice
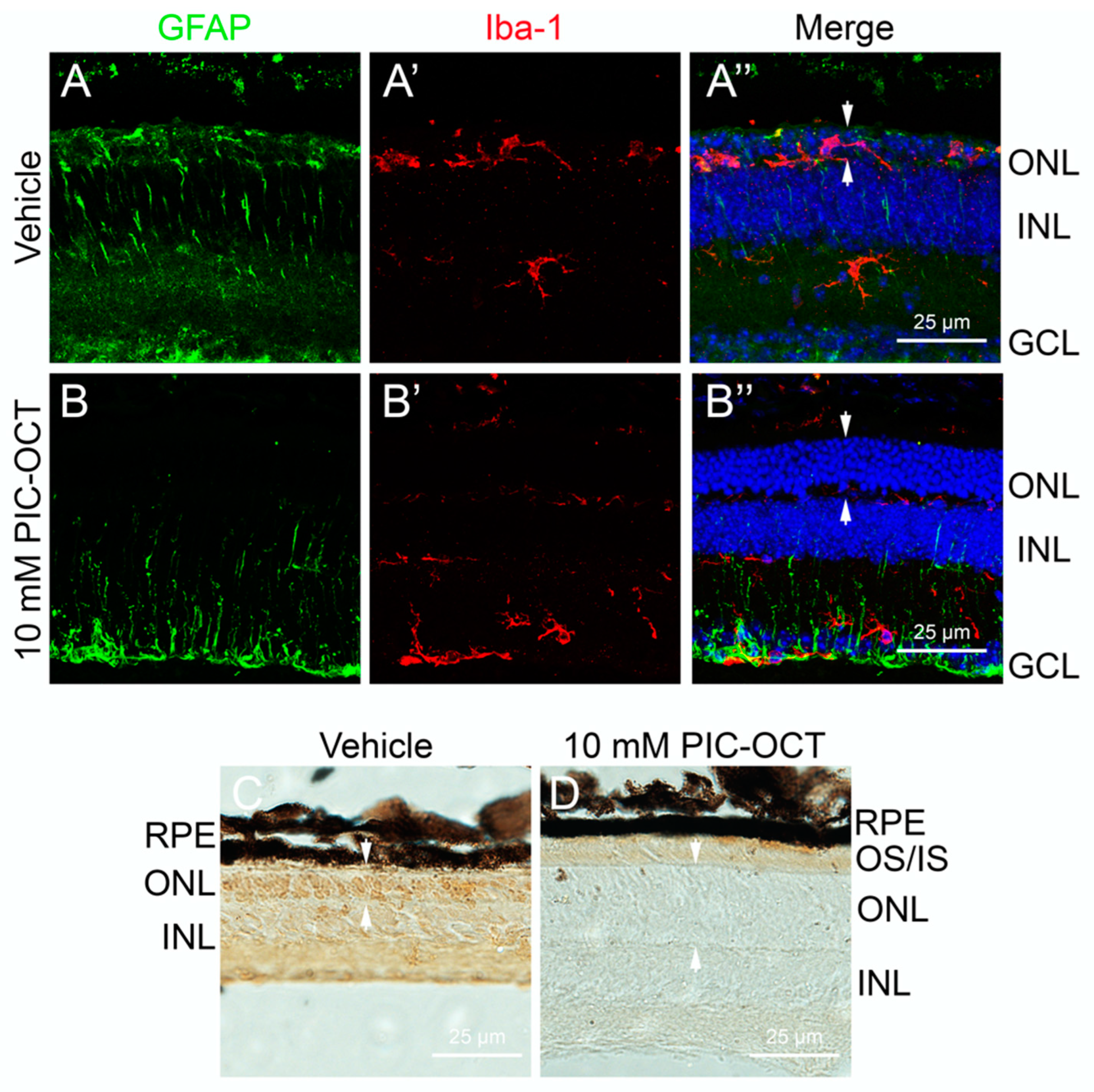
2.2.4. PIC-OCT Reduces Microglial Migration and PARP1 Expression in the rd10 Retina
3. Conclusions
4. Materials and Methods
4.1. Chemistry
4.1.1. Preparation of Compound 13 (PIC-C10)
- 3-O-(6′-O-decanoyl)-β-D-glucopyranosyl resveratrol
4.1.2. Preparation of Compound 15
- 3-O-(6′-O-octanoyl)-α-D-glucopyranosyl resveratrol (15)
4.1.3. Preparation of Compound 16
- 3,5-O,O-di-triisopropylsilyl-4′-O-[(2,3,4,6-O-tetraacetyl)-β-D-glucopyranosyl] resveratrol (18)
- 3,5-O,O-di-triisopropylsilyl-4′-O-β-D-glucopyranosyl resveratrol (19)
- 3,5-O,O-di-triisopropylsilyl-4′-O-(6′-O-octanoyl)-β-D-glucopyranosyl resveratrol (20)
- 4′-O-(6′-O-octanoyl)-β-D-glucopyranosyl resveratrol (16)
4.2. Biology
4.2.1. Animal Handling
4.2.2. Compound Administration
4.2.3. Ocular Coherence Tomography (OCT)
4.2.4. Electroretinogram (ERG)
4.2.5. Histology and Outer Nuclear Layer (ONL) Thickness
4.2.6. Immunofluorescence and Immunocytochemistry Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant Foods and Herbal Sources of Resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Bradamante, S.; Barenghi, L.; Villa, A. Cardiovascular Protective Effects of Resveratrol. Cardiovasc. Drug Rev. 2004, 22, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Zhang, L.; Jiang, S.; Bai, Y. Beneficial Effects of Resveratrol on Atherosclerosis. J. Med. Food 2008, 11, 610–614. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, B.; Ammazzalorso, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R. Anticancer Activity of Stilbene-Based Derivatives. ChemMedChem 2017, 12, 558–570. [Google Scholar] [CrossRef]
- Vervandier-Fasseur, D.; Latruffe, N. The Potential Use of Resveratrol for Cancer Prevention. Molecules 2019, 24, 4506. [Google Scholar] [CrossRef]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Influence of Age and Dose on the Effect of Resveratrol for Glycemic Control in Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis. Molecules 2022, 27, 5232. [Google Scholar] [CrossRef]
- Mancuso, R.; del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Núñez, O.; Pallás, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol Improves Motoneuron Function and Extends Survival in SOD1(G93A) ALS Mice. Neurother. J. Am. Soc. Exp. Neurother. 2014, 11, 419–432. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol Regulates Neuro-Inflammation and Induces Adaptive Immunity in Alzheimer’s Disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health Benefits of Resveratrol: Evidence from Clinical Studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Goutham, G.; Manikandan, R.; Beulaja, M.; Thiagarajan, R.; Arulvasu, C.; Arumugam, M.; Setzer, W.N.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. A Focus on Resveratrol and Ocular Problems, Especially Cataract: From Chemistry to Medical Uses and Clinical Relevance. Biomed. Pharmacother. 2017, 86, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.; Daescu, A.; Rugina, D.; Pintea, A. Resveratrol: Its Path from Isolation to Therapeutic Action in Eye Diseases. Antioxidants 2022, 11, 2447. [Google Scholar] [CrossRef] [PubMed]
- Bryl, A.; Falkowski, M.; Zorena, K.; Mrugacz, M. The Role of Resveratrol in Eye Diseases—A Review of the Literature. Nutrients 2022, 14, 2974. [Google Scholar] [CrossRef]
- Losso, J.N.; Truax, R.E.; Richard, G. Trans-Resveratrol Inhibits Hyperglycemia-Induced Inflammation and Connexin Downregulation in Retinal Pigment Epithelial Cells. J. Agric. Food Chem. 2010, 58, 8246–8252. [Google Scholar] [CrossRef] [PubMed]
- Nagineni, C.N.; Raju, R.; Nagineni, K.K.; Kommineni, V.K.; Cherukuri, A.; Kutty, R.K.; Hooks, J.J.; Detrick, B. Resveratrol Suppresses Expression of VEGF by Human Retinal Pigment Epithelial Cells: Potential Nutraceutical for Age-Related Macular Degeneration. Aging Dis. 2014, 5, 88–100. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Song, J.-Y.; Fan, B.; Wang, Y.; Pan, Y.-R.; Che, L.; Sun, Y.-J.; Li, G.-Y. Resveratrol Protects Photoreceptors by Blocking Caspase- and PARP-Dependent Cell Death Pathways. Free Radic. Biol. Med. 2018, 129, 569–581. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, J.; Li, H.; Wei, H.; Bi, F.; Liu, S.; Tang, K.; Guo, H.; Liu, W. Resveratrol Exhibits an Effect on Attenuating Retina Inflammatory Condition and Damage of Diabetic Retinopathy via PON1. Exp. Eye Res. 2019, 181, 356–366. [Google Scholar] [CrossRef]
- Xiao, K.; Ma, X.-H.; Zhong, Z.; Zhao, Y.; Chen, X.-H.; Sun, X.-F. Low-Dose Trans-Resveratrol Ameliorates Diabetes-Induced Retinal Ganglion Cell Degeneration via TyrRS/c-Jun Pathway. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2. [Google Scholar] [CrossRef]
- Hua, J.; Guerin, K.I.; Chen, J.; Michán, S.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Dennison, R.J.; Juan, A.M.; Hatton, C.J.; et al. Resveratrol Inhibits Pathologic Retinal Neovascularization in Vldlr−/− Mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2809–2816. [Google Scholar] [CrossRef]
- Richer, S.; Stiles, W.; Ulanski, L.; Carroll, D.; Podella, C. Observation of Human Retinal Remodeling in Octogenarians with a Resveratrol Based Nutritional Supplement. Nutrients 2013, 5, 1989–2005. [Google Scholar] [CrossRef]
- Richer, S.; Patel, S.; Sockanathan, S.; Ulanski, L.J.; Miller, L.; Podella, C. Resveratrol Based Oral Nutritional Supplement Produces Long-Term Beneficial Effects on Structure and Visual Function in Human Patients. Nutrients 2014, 6, 4404–4420. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Richer, S.B.A. Improved Visual Acuity and Retinal Integrity with Resveratrol Based Supplementation in Patients with Macular Degeneration. Int. J. Ophthalmol. Clin. Res. 2017, 4, 82. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and Bioavailability of Trans-Resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol Bioavailability and Toxicity in Humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.P.S. Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health. Antioxidants 2021, 10, 1893. [Google Scholar] [CrossRef]
- Ferraz da Costa, D.C.; Pereira Rangel, L.; Quarti, J.; Santos, R.A.; Silva, J.L.; Fialho, E. Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy. Molecules 2020, 25, 3531. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, J.; Wang, Z.; Yu, L.; Wang, J. Piceatannol Protects Human Retinal Pigment Epithelial Cells against Hydrogen Peroxide Induced Oxidative Stress and Apoptosis through Modulating PI3K/Akt Signaling Pathway. Nutrients 2019, 11, 1515. [Google Scholar] [CrossRef]
- Koskela, A.; Reinisalo, M.; Hyttinen, J.M.; Kaarniranta, K.; Karjalainen, R.O. Pinosylvin-Mediated Protection against Oxidative Stress in Human Retinal Pigment Epithelial Cells. Mol. Vis. 2014, 20, 760–769. [Google Scholar]
- Millán, I.; Desco, M.D.; Torres-Cuevas, I.; Pérez, S.; Pulido, I.; Mena-Mollá, S.; Mataix, J.; Asensi, M.; Ortega, Á.L. Pterostilbene Prevents Early Diabetic Retinopathy Alterations in a Rabbit Experimental Model. Nutrients 2020, 12, 82. [Google Scholar] [CrossRef]
- Lee, S.J.; Roh, Y.J.; Kim, J.E.; Jin, Y.J.; Song, H.J.; Seol, A.; Park, S.H.; Douangdeuane, B.; Souliya, O.; Choi, S.I.; et al. Protective Effects of Dipterocarpus Tuberculatus in Blue Light-Induced Macular Degeneration in A2E-Laden ARPE19 Cells and Retina of Balb/c Mice. Antioxidants 2023, 12, 329. [Google Scholar] [CrossRef]
- Li, Q.-S.; Li, Y.; Deora, G.S.; Ruan, B.-F. Derivatives and Analogues of Resveratrol: Recent Advances in Structural Modification. Mini Rev. Med. Chem. 2019, 19, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Lizard, G.; Latruffe, N.; Vervandier-Fasseur, D. Aza- and Azo-Stilbenes: Bio-Isosteric Analogs of Resveratrol. Molecules 2020, 25, 605. [Google Scholar] [CrossRef] [PubMed]
- Ogas, T.; Kondratyuk, T.P.; Pezzuto, J.M. Resveratrol Analogs: Promising Chemopreventive Agents. Ann. N. Y. Acad. Sci. 2013, 1290, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Micale, N.; Molonia, M.S.; Citarella, A.; Cimino, F.; Saija, A.; Cristani, M.; Speciale, A. Natural Product-Based Hybrids as Potential Candidates for the Treatment of Cancer: Focus on Curcumin and Resveratrol. Molecules 2021, 26, 4665. [Google Scholar] [CrossRef]
- Biasutto, L.; Mattarei, A.; Azzolini, M.; La Spina, M.; Sassi, N.; Romio, M.; Paradisi, C.; Zoratti, M. Resveratrol Derivatives as a Pharmacological Tool. Ann. N. Y. Acad. Sci. 2017, 1403, 27–37. [Google Scholar] [CrossRef]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic Versatility of Resveratrol Derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef]
- Moine, E.; Brabet, P.; Guillou, L.; Durand, T.; Vercauteren, J.; Crauste, C. New Lipophenol Antioxidants Reduce Oxidative Damage in Retina Pigment Epithelial Cells. Antioxidants 2018, 7, 197. [Google Scholar] [CrossRef]
- Moine, E.; Boukhallat, M.; Cia, D.; Jacquemot, N.; Guillou, L.; Durand, T.; Vercauteren, J.; Brabet, P.; Crauste, C. New Lipophenols Prevent Carbonyl and Oxidative Stresses Involved in Macular Degeneration. Free Radic. Biol. Med. 2021, 162, 367–382. [Google Scholar] [CrossRef]
- Larrosa, M.; Tome-Carneiro, J.; Yanez-Gascon, M.J.; Alcantara, D.; Selma, M.V.; Beltran, D.; Garcia-Conesa, M.T.; Urban, C.; Lucas, R.; Tomas-Barberan, F.; et al. Preventive Oral Treatment with Resveratrol Pro-Prodrugs Drastically Reduce Colon Inflammation in Rodents. J. Med. Chem. 2010, 53, 7365–7376. [Google Scholar] [CrossRef]
- Falomir, E.; Lucas, R.; Penalver, P.; Marti-Centelles, R.; Dupont, A.; Zafra-Gomez, A.; Carda, M.; Morales, J.C. Cytotoxic, Antiangiogenic and Antitelomerase Activity of Glucosyl- and Acyl- Resveratrol Prodrugs and Resveratrol Sulfate Metabolites. Chembiochem 2016, 17, 1343–1348. [Google Scholar] [CrossRef]
- Peñalver, P.; Belmonte-Reche, E.; Adán, N.; Caro, M.; Mateos-Martín, M.L.; Delgado, M.; González-Rey, E.; Morales, J.C. Alkylated Resveratrol Prodrugs and Metabolites as Potential Therapeutics for Neurodegenerative Diseases. Eur. J. Med. Chem. 2018, 146, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Belmonte-Reche, E.; Peñalver, P.; Caro-Moreno, M.; Mateos-Martín, M.L.; Adán, N.; Delgado, M.; González-Rey, E.; Morales, J.C. Silyl Resveratrol Derivatives as Potential Therapeutic Agents for Neurodegenerative and Neurological Diseases. Eur. J. Med. Chem. 2021, 223, 113655. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Sánchez, L.; García-Delgado, A.B.; Montero-Sánchez, A.; de la Cerda, B.; Lucas, R.; Peñalver, P.; Morales, J.C.; Bhattacharya, S.S.; Díaz-Corrales, F.J. The Resveratrol Prodrug JC19 Delays Retinal Degeneration in Rd10 Mice BT—Retinal Degenerative Diseases. Adv. Exp. Med. Biol. 2019, 1185, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-Syndromic Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Dirks-Hofmeister, M.E.; Verhaeghe, T.; De Winter, K.; Desmet, T. Creating Space for Large Acceptors: Rational Biocatalyst Design for Resveratrol Glycosylation in an Aqueous System. Angew. Chemie Int. Ed. 2015, 54, 9289–9292. [Google Scholar] [CrossRef]
- Gonzalez-Alfonso, J.L.; Ubiparip, Z.; Jimenez-Ortega, E.; Poveda, A.; Alonso, C.; Coderch, L.; Jimenez-Barbero, J.; Sanz-Aparicio, J.; Ballesteros, A.O.; Desmet, T.; et al. Enzymatic Synthesis of Phloretin α-Glucosides Using a Sucrose Phosphorylase Mutant and Its Effect on Solubility, Antioxidant Properties and Skin Absorption. Adv. Synth. Catal. 2021, 363, 3079–3089. [Google Scholar] [CrossRef]
- Crauste, C.; Vigor, C.; Brabet, P.; Picq, M.; Lagarde, M.; Hamel, C.; Durand, T.; Vercauteren, J. Synthesis and Evaluation of Polyunsaturated Fatty Acid–Phenol Conjugates as Anti-Carbonyl-Stress Lipophenols. European J. Org. Chem. 2014, 2014, 4548–4561. [Google Scholar] [CrossRef]
- Wang, T.; Reingruber, J.; Woodruff, M.L.; Majumder, A.; Camarena, A.; Artemyev, N.O.; Fain, G.L.; Chen, J. The PDE6 Mutation in the Rd10 Retinal Degeneration Mouse Model Causes Protein Mislocalization and Instability and Promotes Cell Death through Increased Ion Influx. J. Biol. Chem. 2018, 293, 15332–15346. [Google Scholar] [CrossRef]
- Pennesi, M.E.; Michaels, K.V.; Magee, S.S.; Maricle, A.; Davin, S.P.; Garg, A.K.; Gale, M.J.; Tu, D.C.; Wen, Y.; Erker, L.R.; et al. Long-Term Characterization of Retinal Degeneration in Rd1 and Rd10 Mice Using Spectral Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4644–4656. [Google Scholar] [CrossRef]
- Napoli, D.; Biagioni, M.; Billeri, F.; Di Marco, B.; Orsini, N.; Novelli, E.; Strettoi, E. Retinal Pigment Epithelium Remodeling in Mouse Models of Retinitis Pigmentosa. Int. J. Mol. Sci. 2021, 22, 5381. [Google Scholar] [CrossRef]
- Arango-Gonzalez, B.; Trifunović, D.; Sahaboglu, A.; Kranz, K.; Michalakis, S.; Farinelli, P.; Koch, S.; Koch, F.; Cottet, S.; Janssen-Bienhold, U.; et al. Identification of a Common Non-Apoptotic Cell Death Mechanism in Hereditary Retinal Degeneration. PLoS ONE 2014, 9, e112142. [Google Scholar] [CrossRef] [PubMed]
- Rösch, S.; Johnen, S.; Müller, F.; Pfarrer, C.; Walter, P. Correlations between ERG, OCT, and Anatomical Findings in the Rd10 Mouse. J. Ophthalmol. 2014, 2014, 874751. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.G.; Frishman, L.J.; Grigg, J.; Hamilton, R.; Jeffrey, B.G.; Kondo, M.; Li, S.; McCulloch, D.L. ISCEV Standard for full-field clinical electroretinography (2022 update). Doc. Ophthalmol. 2022, 144, 165–177. [Google Scholar] [CrossRef]
- Bhatt, Y.; Hunt, D.M.; Carvalho, L.S. The origins of the full-field flash electroretinogram b-wave. Front. Mol. Neurosci. 2023, 16, 1153934. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- Moshtaghion, S.M.; Caballano-Infantes, E.; Plaza Reyes, Á.; Valdés-Sánchez, L.; Fernández, P.G.; de la Cerda, B.; Riga, M.S.; Álvarez-Dolado, M.; Peñalver, P.; Morales, J.C.; et al. Piceid Octanoate Protects Retinal Cells against Oxidative Damage by Regulating the Sirtuin 1/Poly-ADP-Ribose Polymerase 1 Axis In Vitro and in Rd10 Mice. Antioxidants 2024, 13, 201. [Google Scholar] [CrossRef]
- Pensado, A.; Diaz-Corrales, F.J.; De la Cerda, B.; Valdés-Sánchez, L.; Del Boz, A.A.; Rodriguez-Martinez, D.; García-Delgado, A.B.; Seijo, B.; Bhattacharya, S.S.; Sanchez, A. Span poly-L-arginine nanoparticles are efficient non-viral vectors for PRPF31 gene delivery: An approach of gene therapy to treat retinitis pigmentosa. Nanomedicine 2016, 12, 2251–2260. [Google Scholar] [CrossRef]


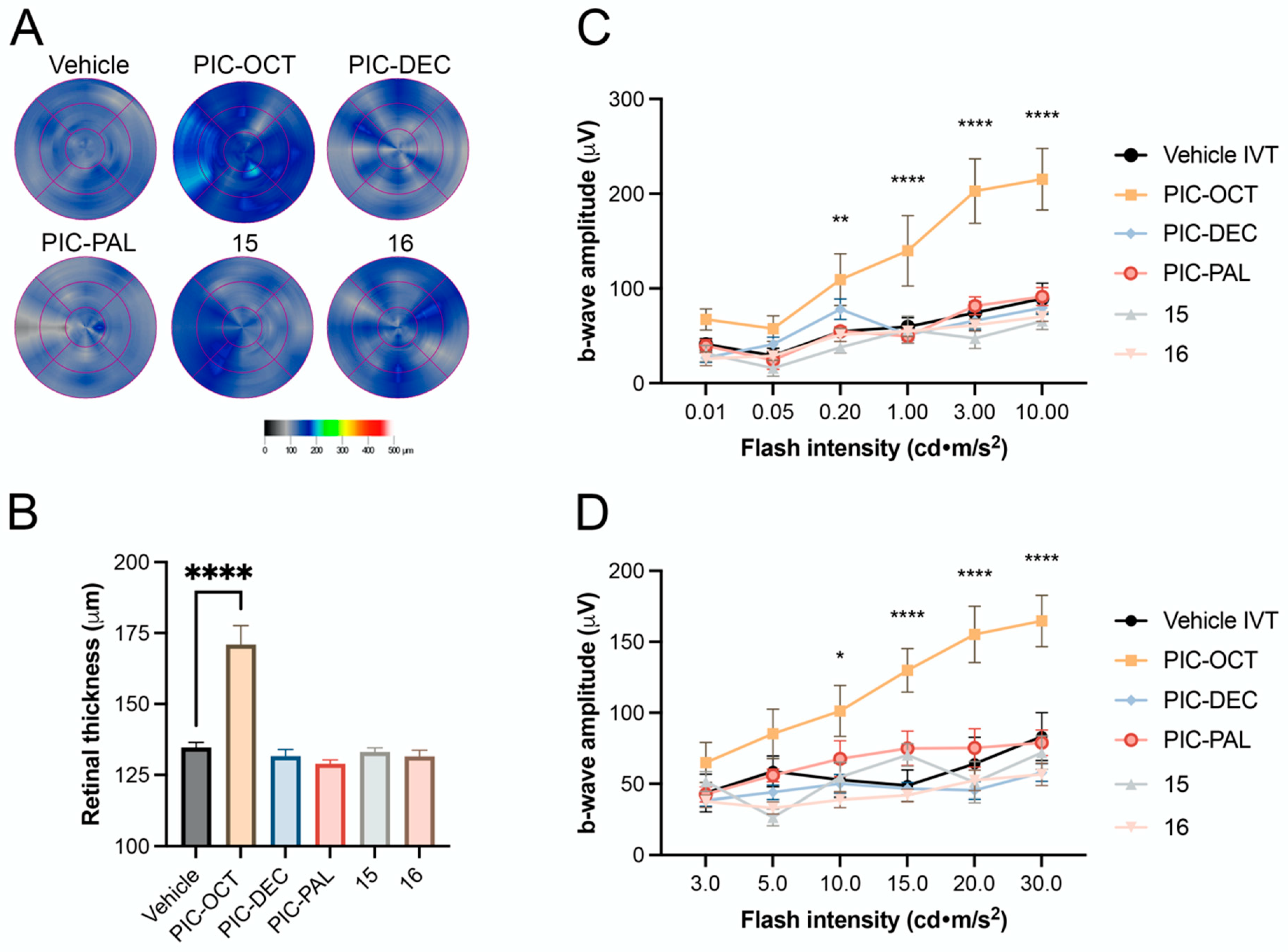
| Test | WT (Mean) | Vehicle IVT (Mean) | 10 mM PIC-OCT (Mean) | WT vs. Vehicle Loss (%) | WT vs. PIC-OCT Loss (%) | PIC-OCT vs. Vehicle Improvement (%) |
|---|---|---|---|---|---|---|
| OCT (µm) | 220.7 | 134.7 | 171.0 | 39.0 | 22.5 | 16.5 |
| 10 DA ERG (µV) | 470.9 | 109.9 | 215.4 | 76.7 | 54.3 | 22.4 |
| 30 LA ERG (µV) | 194.2 | 83.3 | 164.7 | 57.1 | 15.2 | 41.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés-Sánchez, L.; Moshtaghion, S.M.; Caballano-Infantes, E.; Peñalver, P.; Rodríguez-Ruiz, R.; González-Alfonso, J.L.; Plou, F.J.; Desmet, T.; Morales, J.C.; Díaz-Corrales, F.J. Synthesis and Evaluation of Glucosyl-, Acyl- and Silyl- Resveratrol Derivatives as Retinoprotective Agents: Piceid Octanoate Notably Delays Photoreceptor Degeneration in a Retinitis Pigmentosa Mouse Model. Pharmaceuticals 2024, 17, 1482. https://doi.org/10.3390/ph17111482
Valdés-Sánchez L, Moshtaghion SM, Caballano-Infantes E, Peñalver P, Rodríguez-Ruiz R, González-Alfonso JL, Plou FJ, Desmet T, Morales JC, Díaz-Corrales FJ. Synthesis and Evaluation of Glucosyl-, Acyl- and Silyl- Resveratrol Derivatives as Retinoprotective Agents: Piceid Octanoate Notably Delays Photoreceptor Degeneration in a Retinitis Pigmentosa Mouse Model. Pharmaceuticals. 2024; 17(11):1482. https://doi.org/10.3390/ph17111482
Chicago/Turabian StyleValdés-Sánchez, Lourdes, Seyed Mohamadmehdi Moshtaghion, Estefanía Caballano-Infantes, Pablo Peñalver, Rosario Rodríguez-Ruiz, José Luis González-Alfonso, Francisco José Plou, Tom Desmet, Juan C. Morales, and Francisco J. Díaz-Corrales. 2024. "Synthesis and Evaluation of Glucosyl-, Acyl- and Silyl- Resveratrol Derivatives as Retinoprotective Agents: Piceid Octanoate Notably Delays Photoreceptor Degeneration in a Retinitis Pigmentosa Mouse Model" Pharmaceuticals 17, no. 11: 1482. https://doi.org/10.3390/ph17111482
APA StyleValdés-Sánchez, L., Moshtaghion, S. M., Caballano-Infantes, E., Peñalver, P., Rodríguez-Ruiz, R., González-Alfonso, J. L., Plou, F. J., Desmet, T., Morales, J. C., & Díaz-Corrales, F. J. (2024). Synthesis and Evaluation of Glucosyl-, Acyl- and Silyl- Resveratrol Derivatives as Retinoprotective Agents: Piceid Octanoate Notably Delays Photoreceptor Degeneration in a Retinitis Pigmentosa Mouse Model. Pharmaceuticals, 17(11), 1482. https://doi.org/10.3390/ph17111482










