Comparison of Cardioprotective Potential of Cannabidiol and β-Adrenergic Stimulation Against Hypoxia/Reoxygenation Injury in Rat Atria and Ventricular Papillary Muscles
Abstract
1. Introduction
2. Results
2.1. General
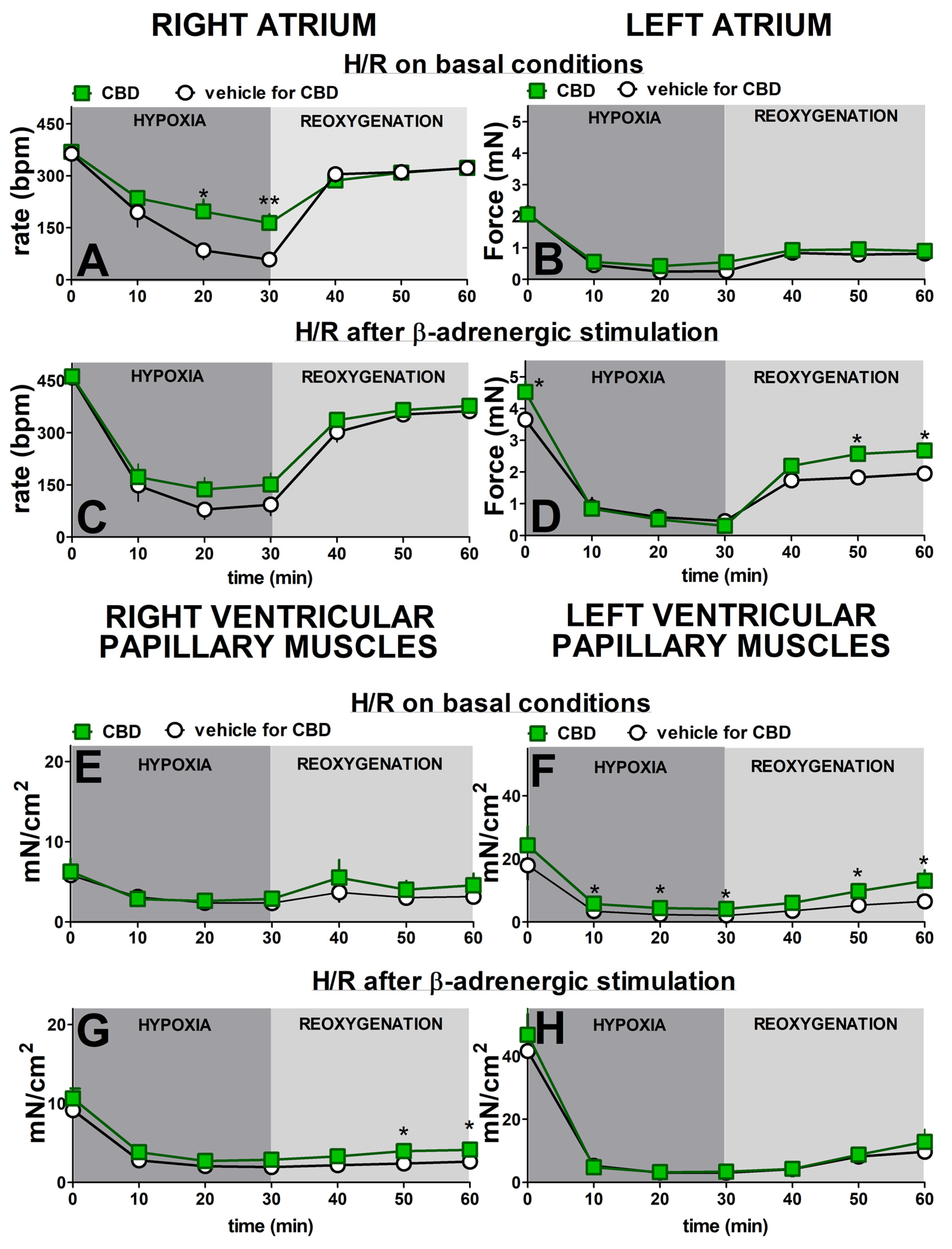
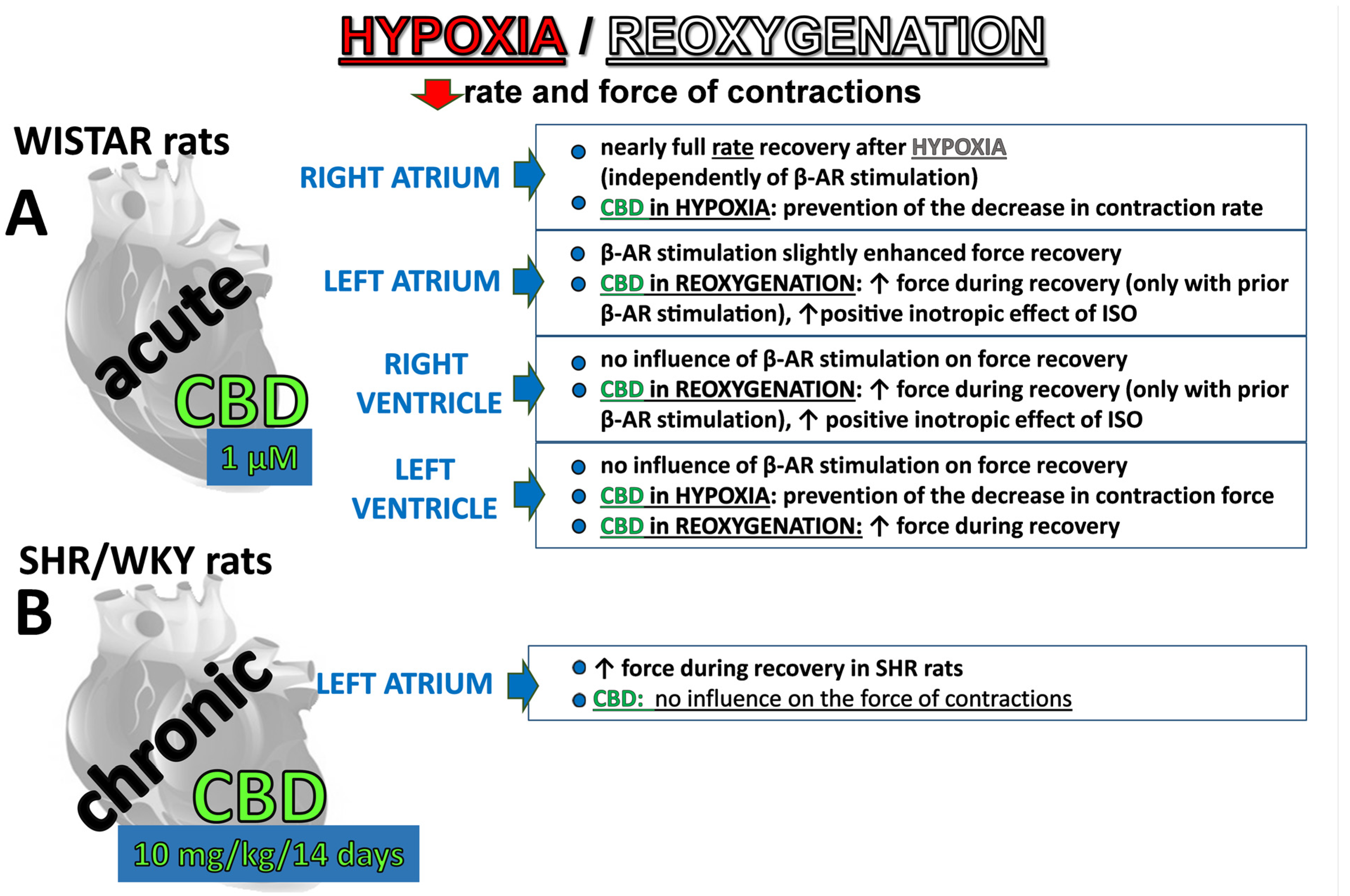
2.2. Influence of β-Adrenergic Stimulation and Cannabidiol on Time-Dependent Hypoxia-Reoxygenation-Induced Changes in Rate and Force of Contractions
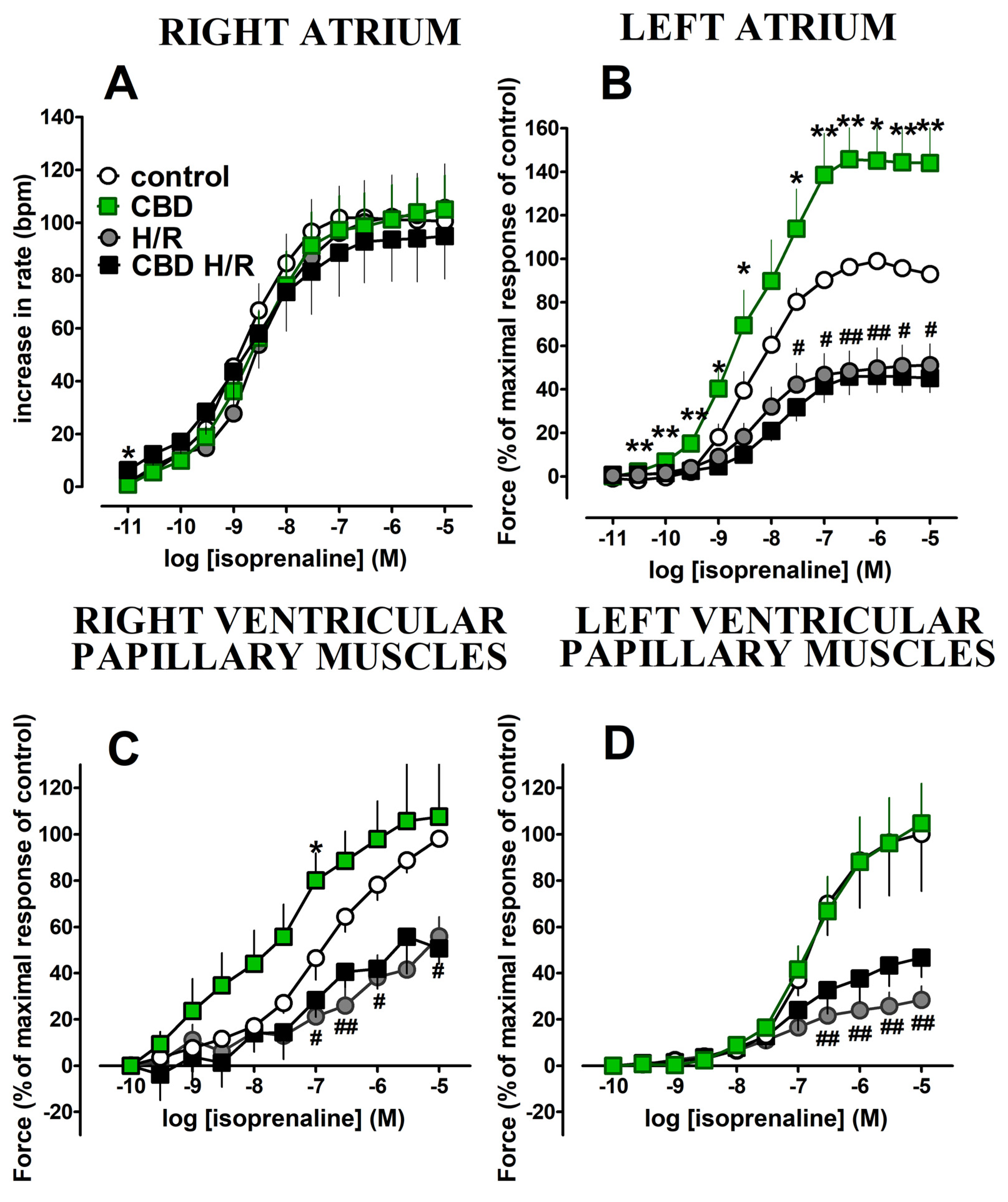
2.3. Influence of Acute Treatment with Cannabidiol and Hypoxia/Reoxygenation on Isoprenaline-Induced Cardiostimulatory Effects
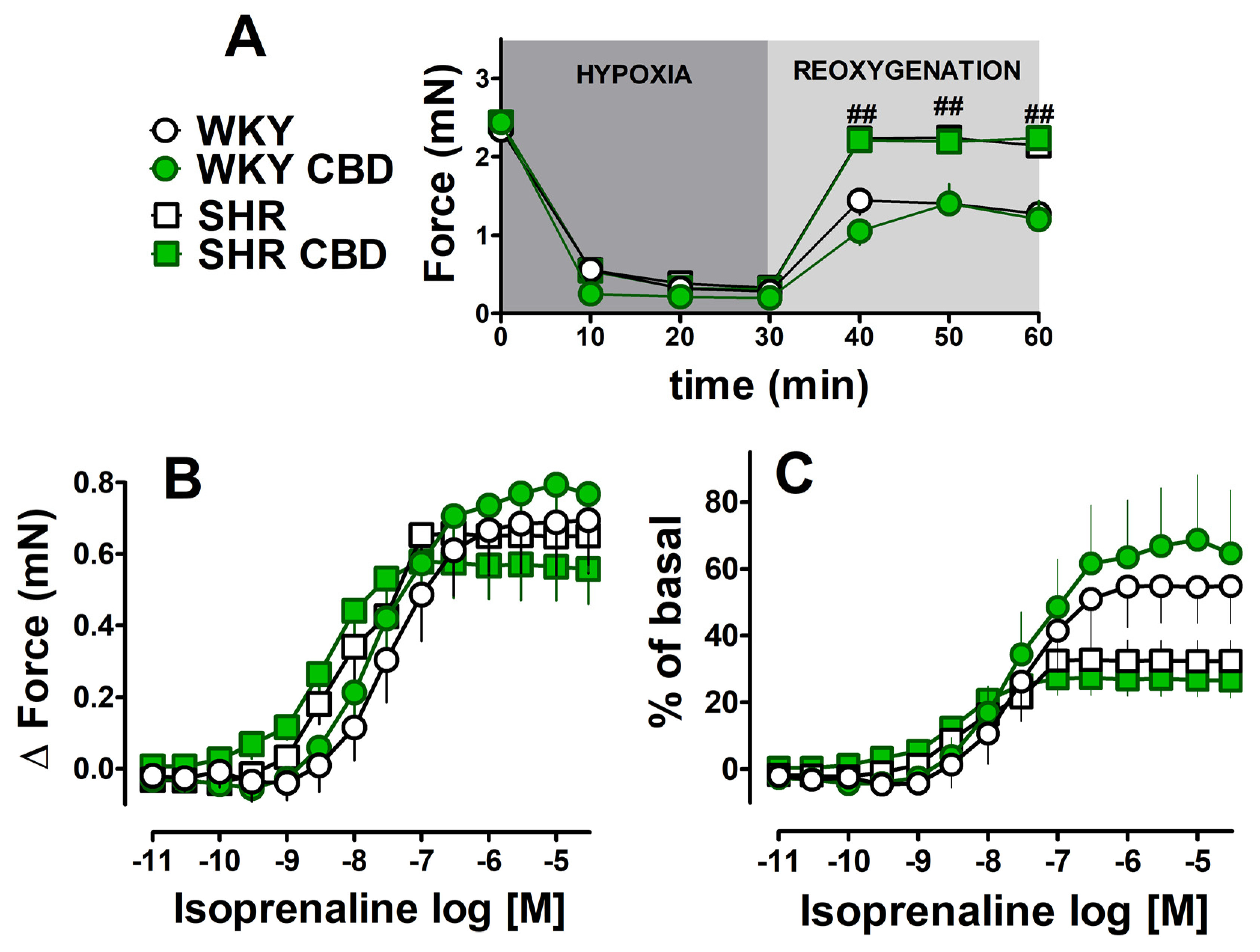
2.4. Influence of Chronic Treatment with CBD on the Hypoxia/Reoxygenation-Induced Changes and Cardiostimulatory Effect of Isoprenaline in Left Atria Isolated from HR and WKY Rats
3. Discussion
3.1. General
3.2. Influence of Hypoxia/Reoxygenation on the Function of Four Cardiac Compartments and the Significance of β-Adrenergic Stimulation
3.3. Potential Beneficial Influence of CBD against Hypoxia/Reoxygenation Injury in Four Cardiac Compartments
3.4. Influence of Hypoxia/Reoxygenation and CBD on Cardiostimulatory Effects of Isoprenaline in Four Cardiac Compartments
3.5. Influence of Chronic Administration of CBD on Changes Induced by Hypoxia/Reoxygenation and Isoprenaline in SHR and WKY Rats
4. Strengths and Limitations
5. Materials and Methods
5.1. Animals
5.2. Chronic Treatment with Cannabidiol
5.3. Isolated Atrial Preparations
5.4. Isolated Papillary Muscle
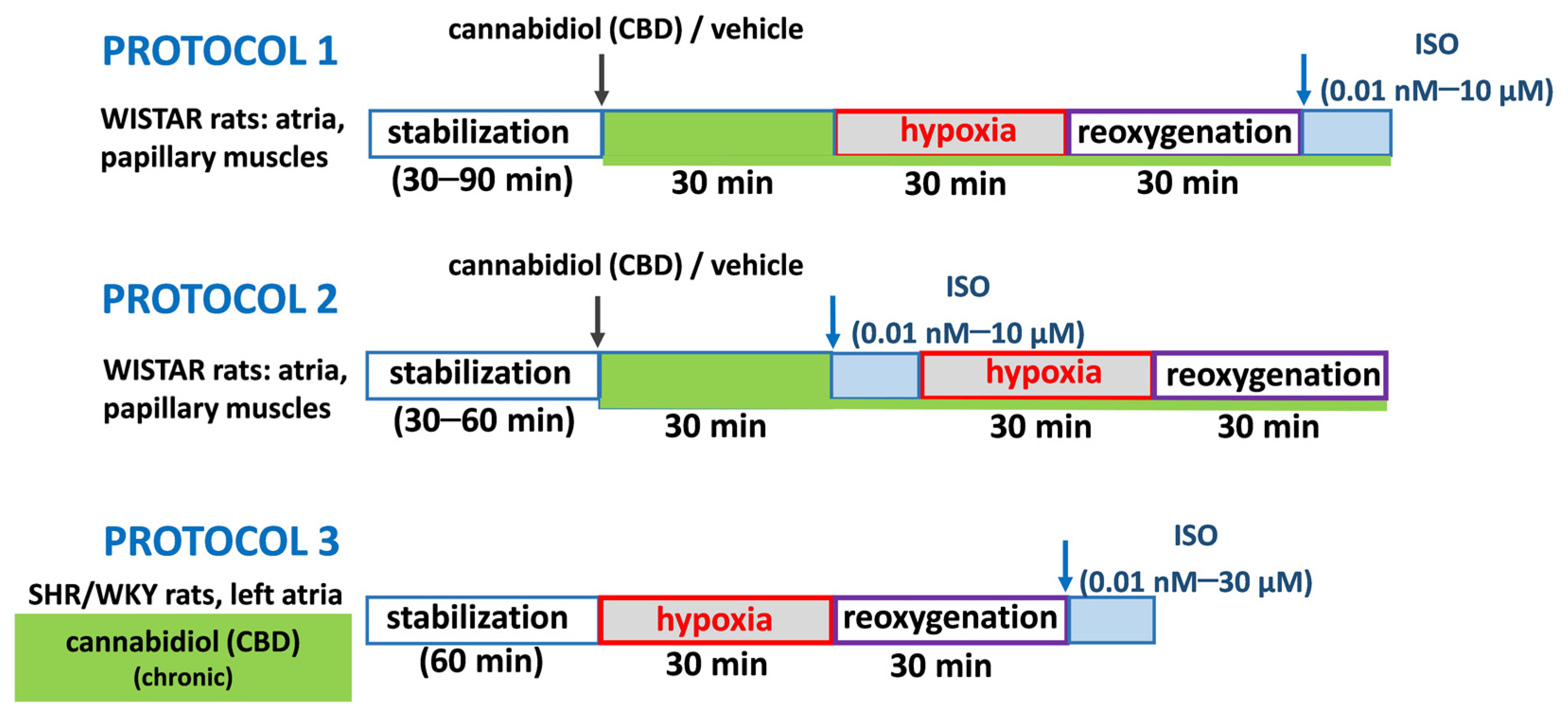
5.5. Hypoxia/Reoxygenation Experimental Protocols
5.6. Drugs
5.7. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blebea, N.M.; Pricopie, A.I.; Vlad, R.A.; Hancu, G. Phytocannabinoids: Exploring Pharmacological Profiles and Their Impact on Therapeutical Use. Int. J. Mol. Sci. 2024, 25, 4204. [Google Scholar] [CrossRef] [PubMed]
- Naya, M.N.; Kelly, J.; Corna, G.; Golino, M.; Polizio, A.H.; Abbate, A.; Toldo, S.; Mezzaroma, E. An Overview of Cannabidiol as a Multifunctional Drug: Pharmacokinetics and Cellular Effects. Molecules 2024, 29, 473. [Google Scholar] [CrossRef] [PubMed]
- de Fátima Dos Santos Sampaio, M.; de Paiva, Y.B.; Sampaio, T.B.; Pereira, M.G.; Coimbra, N.C. Therapeutic applicability of cannabidiol and other phytocannabinoids in epilepsy, multiple sclerosis and Parkinson's disease and in comorbidity with psychiatric disorders. Basic. Clin. Pharmacol. Toxicol. 2024, 134, 574–601. [Google Scholar] [CrossRef]
- Liu, Y. Alzheimer’s disease, aging, and cannabidiol treatment: A promising path to promote brain health and delay aging. Mol. Biol. Rep. 2024, 51, 121. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, R.; Deng, J.; Guo, W. Research progress in the management of vascular disease with cannabidiol: A review. J. Cardiothorac. Surg. 2024, 19, 6. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Ramos-González, M.; Lozano, O.; Jerjes-Sánchez, C.; García-Rivas, G. Therapeutic Applications of Cannabinoids in Cardiomyopathy and Heart Failure. Oxid. Med. Cell Longev. 2020, 4587024. [Google Scholar] [CrossRef]
- Rabino, M.; Mallia, S.; Castiglioni, E.; Rovina, D.; Pompilio, G.; Gowran, A. The Endocannabinoid System and Cannabidiol: Past, Present, and Prospective for Cardiovascular Diseases. Pharmaceuticals 2021, 14, 936. [Google Scholar] [CrossRef]
- McNamara, D.M.; Cooper, L.T.; Arbel, Y.; Bhimaraj, A.; Bocchi, E.; Friedrich, M.G.; Kerneis, M.; Liu, P.; Parker, A.B.; Smith, E.R.; et al. ARCHER Study Group. Impact of cannabidiol on myocardial recovery in patients with acute myocarditis: Rationale & design of the ARCHER trial. ESC Heart Fail. 2024, 11, 3416–3424. [Google Scholar]
- Shaffer, B.L.; Davis, G.M.; Incitti, M.A.; Piper, B.J.; Entler, B.V. Application of medical cannabis in unstable angina and coronary artery disease: A case report. Medicine 2021, 100, e25172. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, W.; Li, C.; Zhao, R.; Lu, H.; Song, S.; Zhou, Y.; Hu, Y.; Shi, B.; Ge, J. Hypoxia-induced signaling in the cardiovascular system: Pathogenesis and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 431. [Google Scholar] [CrossRef]
- Sagris, M.; Apostolos, A.; Theofilis, P.; Ktenopoulos, N.; Katsaros, O.; Tsalamandris, S.; Tsioufis, K.; Toutouzas, K.; Tousoulis, D. Myocardial Ischemia-Reperfusion Injury: Unraveling Pathophysiology, Clinical Manifestations, and Emerging Prevention Strategies. Biomedicines. 2024, 12, 802. [Google Scholar] [CrossRef] [PubMed]
- Durst, R.; Danenberg, H.; Gallily, R.; Mechoulam, R.; Meir, K.; Grad, E.; Beeri, R.; Pugatsch, T.; Tarsish, E.; Lotan, C. Cannabidiol, a nonpsychoactive Cannabis constituent, protects against myocardial ischemic reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3602–H3607. [Google Scholar] [CrossRef] [PubMed]
- Franco-Vadillo, A.; Toledo-Blass, M.; Rivera-Herrera, Z.; Guevara-Balcazar, G.; Orihuela-Rodriguez, O.; Morales-Carmona, J.A.; Kormanovski-Kovzova, A.; Lopez-Sanchez, P.; Rubio-Gayosso, I.; Castillo-Hernandez, M.D.C. Cannabidiol-mediated RISK PI3K/AKT and MAPK/ERK pathways decreasing reperfusion myocardial damage. Pharmacol. Res. Perspect. 2021, 9, e00784. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, Y.; Cai, A.; Yu, Y.; Wang, X.; Lan, L.; Guo, X.; Yan, H.; Gao, X.; Li, H.; et al. Cannabidiol represses miR-143 to promote cardiomyocyte proliferation and heart regeneration after myocardial infarction. Eur. J. Pharmacol. 2024, 963, 176245. [Google Scholar] [CrossRef]
- Walsh, S.K.; Hepburn, C.Y.; Kane, K.A.; Wainwright, C.L. Acute administration of cannabidiol in vivo suppresses ischaemia-induced cardiac arrhythmias and reduces infarct size when given at reperfusion. Br. J. Pharmacol. 2010, 160, 1234–1242. [Google Scholar] [CrossRef]
- Gonca, E.; Darıcı, F. The effect of cannabidiol on ischemia/reperfusion-induced ventricular arrhythmias: The role of adenosine A1 receptors. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 76–83. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, F.; Yin, T.; Xia, Q.; Liu, Y.; Huang, G.; Zhang, J.; Oyen, R.; Ni, Y. Pharmacologic Effects of Cannabidiol on Acute Reperfused Myocardial Infarction in Rabbits: Evaluated With 3.0T Cardiac Magnetic Resonance Imaging and Histopathology. J. Cardiovasc. Pharmacol. 2015, 66, 354–363. [Google Scholar] [CrossRef]
- Dogan Unlu, M.; Uysal, D.; Karakuyu, N.F.; Asci, S.; Ozmen, O.; Tepebasi, M.Y. Investigation of neuroprotective and therapeutic effects of cannabidiol in an acute coronary syndrome model. Neurosci. Lett. 2024, 825, 137689. [Google Scholar] [CrossRef]
- Ozmen, O.; Asci, H.; Uysal, D.; Ilhan, I.; Taner, R.; Arlıoglu, M.; Milletsever, A.; Tasan, S. The prophylactic and therapeutic effects of cannabidiol on lung injury secondary to cardiac ischemia model in rats via PERK/NRF2/CHOP/BCL2 pathway. Immunopharmacol. Immunotoxicol. 2024, 46, 1–10. [Google Scholar] [CrossRef]
- Stanley, C.P.; Hind, W.H.; Tufarelli, C.; O'Sullivan, S.E. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc. Res. 2015, 107, 568–578. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Sadowska, O.; Kozłowski, M.; Kusaczuk, M.; Kasacka, I.; Malinowska, B. Vasodilatory effects of cannabidiol in human pulmonary and rat small mesenteric arteries: Modification by hypertension and the potential pharmacological opportunities. J. Hypertens. 2020, 38, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Pędzińska-Betiuk, A.; Weresa, J.; Schlicker, E.; Harasim-Symbor, E.; Toczek, M.; Kasacka, I.; Gajo, B.; Malinowska, B. Chronic cannabidiol treatment reduces the carbachol-induced coronary constriction and left ventricular cardiomyocyte width of the isolated hypertensive rat heart. Toxicol. Appl. Pharmacol. 2021, 411, 115368. [Google Scholar] [CrossRef] [PubMed]
- Remiszewski, P.; Jarocka-Karpowicz, I.; Biernacki, M.; Jastrząb, A.; Schlicker, E.; Toczek, M.; Harasim-Symbor, E.; Pędzińska-Betiuk, A.; Malinowska, B. Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 1295. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Drel, V.R.; Obrosova, I.G.; Pacher, P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H610–H619. [Google Scholar] [CrossRef]
- Teichmann, E.; Blessing, E.; Hinz, B. Non-Psychoactive Phytocannabinoids Inhibit Inflammation-Related Changes of Human Coronary Artery Smooth Muscle and Endothelial Cells. Cells 2023, 12, 2389. [Google Scholar] [CrossRef]
- Ali, R.M.; Kury, A.L.T.; Yang, K.H.; Qureshi, A.; Rajesh, M.; Galadari, S.; Shuba, Y.M.; Howarth, F.C.; Oz, M. Effects of cannabidiol on contractions and calcium signaling in rat ventricular myocytes. Cell Calcium. 2015, 57, 290–299. [Google Scholar] [CrossRef]
- Isaev, D.; Shabbir, W.; Dinc, E.Y.; Lorke, D.E.; Petroianu, G.; Oz, M. Cannabidiol Inhibits Multiple Ion Channels in Rabbit Ventricular Cardiomyocytes. Front. Pharmacol. 2022, 13, 821758. [Google Scholar] [CrossRef]
- Kossakowski, R.; Schlicker, E.; Toczek, M.; Weresa, J.; Malinowska, B. Cannabidiol Affects the Bezold-Jarisch Reflex via TRPV1 and 5-HT3 Receptors and Has Peripheral Sympathomimetic Effects in Spontaneously Hypertensive and Normotensive Rats. Front. Pharmacol. 2019, 10, 500. [Google Scholar] [CrossRef]
- Weresa, J.; Pędzińska-Betiuk, A.; Kossakowski, R.; Malinowska, B. Cannabinoid CB1 and CB2 receptors antagonists AM251 and AM630 differentially modulate the chronotropic and inotropic effects of isoprenaline in isolated rat atria. Pharmacol. Rep. 2019, 71, 82–89. [Google Scholar] [CrossRef]
- Mazeh, A.C.; Angus, J.A.; Wright, C.E. Cannabidiol selectively inhibits the contraction of rat small resistance arteries: Possible role for CGRP and voltage-gated calcium channels. Eur. J. Pharmacol. 2021, 891, 173767. [Google Scholar] [CrossRef]
- Menezes-Rodrigues, F.S.; Errante, P.R.; Tavares, J.G.P.; Ferraz, R.R.N.; Gomes, W.J.; Taha, M.O.; Scorza, C.A.; Scorza, F.A.; Caricati-Neto, A. Pharmacological modulation of b-adrenoceptors as a new cardioprotective strategy for therapy of myocardial dysfunction induced by ischemia and reperfusion. Acta Cir. Bras. 2019, 34, e201900505. [Google Scholar] [CrossRef] [PubMed]
- Broadley, K.J.; Penson, P.E. The roles of alpha- and beta-adrenoceptor stimulation in myocardial ischaemia. Auton. Autacoid Pharmacol. 2004, 24, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Maslov, L.N.; Naryzhnaya, N.V.; Voronkov, N.S.; Kurbatov, B.K.; Derkachev, I.A.; Ryabov, V.V.; Vyshlov, E.V.; Kolpakov, V.V.; Tomilova, E.A.; Sapozhenkova, E.V.; et al. The role of β-adrenergic receptors in the regulation of cardiac tolerance to ischemia/reperfusion. Why do β-adrenergic receptor agonists and antagonists protect the heart? Fundam. Clin. Pharmacol. 2024, 38, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Penson, P.E.; Ford, W.R.; Kidd, E.J.; Broadley, K.J. Activation of beta-adrenoceptors mimics preconditioning of rat-isolated atria and ventricles against ischaemic contractile dysfunction. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 378, 589–597. [Google Scholar] [CrossRef]
- Nasa, Y.; Yabe, K.; Takeo, S. Beta-adrenoceptor stimulation-mediated preconditioning-like cardioprotection in perfused rat hearts. J. Cardiovasc. Pharmacol. 1997, 29, 436–443. [Google Scholar] [CrossRef]
- Min, J.Y.; Ding, B.; Wang, J.F.; Sullivan, M.F.; Morgan, J.P. Metoprolol attenuates postischemic depressed myocardial function in papillary muscles isolated from normal and postinfarction rat hearts. Eur. J. Pharmacol. 2001, 422, 115–125. [Google Scholar] [CrossRef]
- Maier, T.; Schreckenberg, R.; Schlüter, K.D. Effect of preischemic beta-adrenoceptor stimulation on postischemic contractile dysfunction. Life Sci. 2009, 84, 437–443. [Google Scholar] [CrossRef]
- Derici, M.K.; Sadi, G.; Cenik, B.; Güray, T.; Demirel-Yilmaz, E. Differential expressions and functions of phosphodiesterase enzymes in different regions of the rat heart. Eur. J. Pharmacol. 2019, 844, 118–129. [Google Scholar] [CrossRef]
- Lookin, O.; Balakin, A.; Protsenko, Y. Differences in Effects of Length-Dependent Regulation of Force and Ca2+ Transient in the Myocardial Trabeculae of the Rat Right Atrium and Ventricle. Int. J. Mol. Sci. 2023, 24, 8960. [Google Scholar] [CrossRef]
- Lisin, R.; Balakin, A.; Mukhlynina, E.; Protsenko, Y. Differences in Mechanical, Electrical and Calcium Transient Performance of the Isolated Right Atrial and Ventricular Myocardium of Guinea Pigs at Different Preloads (Lengths). Int. J. Mol. Sci. 2023, 24, 15524. [Google Scholar] [CrossRef]
- Molina, C.E.; Heijman, J.; Dobrev, D. Differences in Left Versus Right Ventricular Electrophysiological Properties in Cardiac Dysfunction and Arrhythmogenesis. Arrhythm. Electrophysiol. Rev. 2016, 5, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Walklate, J.; Ferrantini, C.; Johnson, C.A.; Tesi, C.; Poggesi, C.; Geeves, M.A. Alpha and beta myosin isoforms and human atrial and ventricular contraction. Cell Mol. Life Sci. 2021, 78, 7309–7337. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.M.; Bell, S.P. Preparing Excitable Cardiac Papillary Muscle and Cardiac Slices for Functional Analyses. Front. Physiol. 2022, 13, 817205. [Google Scholar] [CrossRef]
- Uhl, S.; Freichel, M.; Mathar, I. Contractility Measurements on Isolated Papillary Muscles for the Investigation of Cardiac Inotropy in Mice. J. Vis. Exp. 2015, 103, 53076. [Google Scholar]
- Wheal, A.J.; Jadoon, K.; Randall, M.D.; O’Sullivan, S.E. In vivo cannabidiol treatment improves endothelium-dependent vasorelaxation in mesenteric arteries of Zucker diabetic fatty rats. Front. Pharmacol. 2017, 8, 248. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef]
- Cassol, O.J., Jr.; Comim, C.M.; Silva, B.R.; Hermani, F.V.; Constantino, L.S.; Felisberto, F.; Petronilho, F.; Hallak, J.E.; De Martinis, B.S.; Zuardi, A.W.; et al. Treatment with cannabidiol reverses oxidative stress parameters, cognitive impairment and mortality in rats submitted to sepsis by cecal ligation and puncture. Brain Res. 2010, 1348, 128–138. [Google Scholar] [CrossRef]
- Hao, E.; Mukhopadhyay, P.; Cao, Z.; Erdélyi, K.; Holovac, E.; Liaudet, L.; Lee, W.S.; Haskó, G.; Mechoulam, R.; Pacher, P. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol. Med. 2015, 21, 38–45. [Google Scholar] [CrossRef]
- Lee, W.S.; Erdelyi, K.; Matyas, C.; Mukhopadhyay, P.; Varga, Z.V.; Liaudet, L.; Haskó, G.; Čiháková, D.; Mechoulam, R.; Pacher, P. Cannabidiol limits T cell-mediated chronic autoimmune myocarditis: Implications to autoimmune disorders and organ transplantation. Mol. Med. 2016, 22, 136–146. [Google Scholar] [CrossRef]
- Froldi, G.; Guerra, L.; Pandolfo, L.; Chinellato, A.; Ragazzi, E.; Caparrotta, L.; Borea, P.A.; Fassina, G. Phentolamine and hypoxia: Modulation of contractility and alpha 1-adrenoceptors in isolated rat atria. Naunyn Schmiedebergs Arch. Pharmacol. 1994, 350, 563–568. [Google Scholar] [CrossRef]
- Lin, Y.K.; Lai, M.S.; Chen, Y.C.; Cheng, C.C.; Huang, J.H.; Chen, S.A.; Chen, Y.J.; Lin, C.I. Hypoxia and reoxygenation modulate the arrhythmogenic activity of the pulmonary vein and atrium. Clin. Sci. 2012, 122, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Frances, C.; Nazeyrollas, P.; Prevost, A.; Moreau, F.; Pisani, J.; Davani, S.; Kantelip, J.P.; Millart, H. Role of beta 1- and beta 2-adrenoceptor subtypes in preconditioning against myocardial dysfunction after ischemia and reperfusion. J. Cardiovasc. Pharmacol. 2003, 41, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Asimakis, G.K.; Inners-McBride, K.; Conti, V.R.; Yang, C.J. Transient beta adrenergic stimulation can precondition the rat heart against postischaemic contractile dysfunction. Cardiovasc. Res. 1994, 28, 1726–1734. [Google Scholar] [CrossRef]
- Wei, W.; Smrcka, A.V. Subcellular β-Adrenergic Receptor Signaling in Cardiac Physiology and Disease. J. Cardiovasc. Pharmacol. 2022, 80, 334–341. [Google Scholar] [CrossRef]
- Le Marois, M.; Ballet, V.; Sanson, C.; Maizières, M.A.; Carriot, T.; Chantoiseau, C.; Partiseti, M.; Bohme, G.A. Cannabidiol inhibits multiple cardiac ion channels and shortens ventricular action potential duration in vitro. Eur. J. Pharmacol. 2020, 886, 173542. [Google Scholar] [CrossRef]
- Orvos, P.; Pászti, B.; Topal, L.; Gazdag, P.; Prorok, J.; Polyák, A.; Kiss, T.; Tóth-Molnár, E.; Csupor-Löffler, B.; Bajtel, Á.; et al. The electrophysiological effect of cannabidiol on hERG current and in guinea-pig and rabbit cardiac preparations. Sci. Rep. 2020, 10, 16079. [Google Scholar] [CrossRef]
- Topal, L.; Naveed, M.; Orvos, P.; Pászti, B.; Prorok, J.; Bajtel, Á.; Kiss, T.; Csupor-Löffler, B.; Csupor, D.; Baczkó, I.; et al. The electrophysiological effects of cannabidiol on action potentials and transmembrane potassium currents in rabbit and dog cardiac ventricular preparations. Arch. Toxicol. 2021, 95, 2497–2505. [Google Scholar] [CrossRef]
- Ravens, U.; Wettwer, E. Electrophysiological aspects of changes in heart rate. Basic. Res. Cardiol. 1998, 93, 60–65. [Google Scholar] [CrossRef]
- Haghdoost, M.; Young, S.; Holloway, A.K.; Roberts, M.; Zvorsky, I.; Bonn-Miller, M.O. CBD Versus CBDP: Comparing In Vitro Receptor-Binding Activities. Int. J. Mol. Sci. 2024, 25, 7724. [Google Scholar] [CrossRef]
- Sterin-Borda, L.; Del Zar, C.F.; Borda, E. Differential CB1 and CB2 cannabinoid receptor-inotropic response of rat isolated atria: Endogenous signal transduction pathways. Biochem. Pharmacol. 2005, 69, 1705–1713. [Google Scholar] [CrossRef]
- Hudson, B.D.; Hébert, T.E.; Kelly, M.E. Physical and functional interaction between CB1 cannabinoid receptors and beta2-adrenoceptors. Br. J. Pharmacol. 2010, 160, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekera, P.C.; Wan, T.C.; Gizewski, E.T.; Auchampach, J.A.; Lasley, R.D. Adenosine A1 receptors heterodimerize with β1- and β2-adrenergic receptors creating novel receptor complexes with altered G protein coupling and signaling. Cell Signal. 2013, 25, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J.; Bloom, A.S. Delta 9-Tetrahydrocannabinol-induced changes in beta-adrenergic receptor binding in mouse cerebral cortex. Brain Res. 1982, 235, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Dörner, M.F.; Boknik, P.; Köpp, F.; Buchwalow, I.B.; Neumann, J.; Gergs, U. Mechanisms of Systolic Cardiac Dysfunction in PP2A, PP5 and PP2AxPP5 Double Transgenic Mice. Int. J. Mol. Sci. 2021, 22, 9448. [Google Scholar] [CrossRef]
- Yano, T.; Miki, T.; Tanno, M.; Kuno, A.; Itoh, T.; Takada, A.; Sato, T.; Kouzu, H.; Shimamoto, K.; Miura, T. Hypertensive hypertrophied myocardium is vulnerable to infarction and refractory to erythropoietin-induced protection. Hypertension 2011, 57, 110–115. [Google Scholar] [CrossRef]
- Matsuhisa, S.; Otani, H.; Okazaki, T.; Yamashita, K.; Akita, Y.; Sato, D.; Moriguchi, A.; Imamura, H.; Iwasaka, T. Angiotensin II type 1 receptor blocker preserves tolerance to ischemia-reperfusion injury in Dahl salt-sensitive rat heart. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2473–H2479. [Google Scholar] [CrossRef]
- Wagner, C.; Ebner, B.; Tillack, D.; Strasser, R.H.; Weinbrenner, C. Cardioprotection by ischemic postconditioning is abrogated in hypertrophied myocardium of spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 2013, 61, 35–41. [Google Scholar] [CrossRef]
- Mozaffari, M.S.; Schaffer, S.W. Effect of hypertension and hypertension-glucose intolerance on myocardial ischemic injury. Hypertension. 2003, 42, 1042–1049. [Google Scholar] [CrossRef][Green Version]
- Neckář, J.; Kopkan, L.; Husková, Z.; Kolář, F.; Papoušek, F.; Kramer, H.J.; Hwang, S.H.; Hammock, B.D.; Imig, J.D.; Malý, J.; et al. Inhibition of soluble epoxide hydrolase by cis-4-[4-(3-adamantan-1-ylureido) cyclohexyl-oxy] benzoic acid exhibits antihypertensive and cardioprotective actions in transgenic rats with angiotensin II-dependent hypertension. Clin. Sci. 2012, 122, 513–525. [Google Scholar] [CrossRef]
- Alánová, P.; Husková, Z.; Kopkan, L.; Sporková, A.; Jíchová, Š.; Neckář, J.; Imig, J.D.; Klevstig, M.; Kolář, F.; Rami Reddy, N.; et al. Orally active epoxyeicosatrienoic acid analog does not exhibit antihypertensive and reno- or cardioprotective actions in two-kidney, one-clip Goldblatt hypertensive rats. Vasc. Pharmacol. 2015, 73, 45–56. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Kusaczuk, M.; Harasim-Symbor, E.; Biernacki, M.; Kasacka, I.; Malinowska, B. Vasoprotective Endothelial Effects of Chronic Cannabidiol Treatment and Its Influence on the Endocannabinoid System in Rats with Primary and Secondary Hypertension. Pharmaceuticals 2021, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Kockskämper, J.; Pluteanu, F. Left Atrial Myocardium in Arterial Hypertension. Cells 2022, 11, 3157. [Google Scholar] [CrossRef] [PubMed]
- Mellors, L.J.; Barclay, C.J. The energetics of rat papillary muscles undergoing realistic strain patterns. J. Exp. Biol. 2001, 204, 3765–3777. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Bödicker, K.; Buchwalow, I.B.; Schmidbaur, C.; Ramos, G.; Frantz, S.; Hofmann, U.; Gergs, U. Effects of acute ischemia and hypoxia in young and adult calsequestrin (CSQ2) knock-out and wild-type mice. Mol. Cell Biochem. 2022, 477, 1789–1801. [Google Scholar] [CrossRef]
- Bollmann, P.; Werner, F.; Jaron, M.; Bruns, T.A.; Wache, H.; Runte, J.; Boknik, P.; Kirchhefer, U.; Müller, F.U.; Buchwalow, I.B.; et al. Initial Characterization of Stressed Transgenic Mice with Cardiomyocyte-Specific Overexpression of Protein Phosphatase 2C. Front. Pharmacol. 2021, 11, 591773. [Google Scholar] [CrossRef]
| Control | CBD | H/R | CBD H/R | Control | CBD | H/R | CBD H/R | ||
|---|---|---|---|---|---|---|---|---|---|
| Right Atrium | Left Atrium | ||||||||
| basal (bpm) | 341 ± 16 | 346 ± 17 | 347 ± 8 | 343 ± 18 | basal (mN) | 2.4 ± 0.1 | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.1 |
| 30 min after CBD/vehicle (bpm) | 359 ± 15 | 373 ± 12 | 323 ± 18 | 323 ± 14 | 30 min after CBD/vehicle (mN) | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 |
| Emax 1 | 111 ± 16 | 90 ± 10 | 106 ± 17 | 95 ± 16 | Emax 2 | 99 ± 1 | 146 ± 21 * | 51 ± 10 # | 46 ± 8 |
| pEC50 | 8.8 ± 0.2 | 8.5 ± 0.1 | 8.5 ± 0.2 | 8.7 ± 0.2 | pEC50 | 8.3 ± 0.1 | 8.4 ± 0.2 | 8.2 ± 0.2 | 7.9 ± 0.2 |
| n | 10 | 10 | 10 | 10 | n | 10 | 10 | 10 | 10 |
| Right Ventricular Papillary Muscles | Left Ventricular Papillary Muscles | ||||||||
| Basal (mN/cm2) | 7.9 ± 0.2 | 7.9 ± 0.9 | 7.8 ± 0.9 | 8.7 ± 1.0 | basal (mN/cm2) | 23.0 ± 6.5 | 23.4 ± 4.4 | 20.0 ± 4.2 | 23.8 ± 5.4 |
| 30 min after CBD/vehicle (mN/cm2) | 7.5 ± 0.5 | 8.0 ± 0.7 | 6.1 ± 0.6 | 6.3 ± 1.6 | 30 min after CBD/vehicle(mN/cm2) | 25.0 ± 6.7 | 26.1 ± 4.0 | 18.0 ± 4.5 | 24.3 ± 6.1 |
| Emax 2 | 98 ± 4 | 108 ± 29 | 56 ± 8 # | 56 ± 16 | Emax 2 | 100 ± 25 | 105 ± 17 | 28 ± 6 ## | 47 ± 8 |
| pEC50 | 7.0 ± 0.1 | 7.6 ± 0.3 | 6.5 ± 0.2 | 7.1 ± 0.3 | pEC50 | 6.8 ± 0.2 | 6.8 ± 0.2 | 7.3 ± 0.2 | 7.0 ± 0.3 |
| n | 6 | 6 | 7 | 9 | n | 7 | 6 | 10 | 9 |
| WKY | WKY CBD | SHR | SHR CBD | |
|---|---|---|---|---|
| basal (mN) | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.1 |
| Emax 1 (Δ, mN) | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.7 ± 0.1 | 0.6 ± 0.1 |
| pEC50 1 | 7.4 ± 0.2 | 7.6 ± 0.2 | 8.1 ± 0.2 # | 8.5 ± 0.2 |
| Emax 2 (% of basal) | 54.9 ± 11.1 | 68.7 ± 19.4 | 32.5 ± 6.0 | 27.1 ± 5.2 |
| pEC50 2 | 7.5 ± 0.2 | 7.6 ± 0.2 | 8.0 ± 0.2 | 8.5 ± 0.2 |
| n | 5 | 7 | 7 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pędzińska-Betiuk, A.; Gergs, U.; Weresa, J.; Remiszewski, P.; Harasim-Symbor, E.; Malinowska, B. Comparison of Cardioprotective Potential of Cannabidiol and β-Adrenergic Stimulation Against Hypoxia/Reoxygenation Injury in Rat Atria and Ventricular Papillary Muscles. Pharmaceuticals 2024, 17, 1379. https://doi.org/10.3390/ph17101379
Pędzińska-Betiuk A, Gergs U, Weresa J, Remiszewski P, Harasim-Symbor E, Malinowska B. Comparison of Cardioprotective Potential of Cannabidiol and β-Adrenergic Stimulation Against Hypoxia/Reoxygenation Injury in Rat Atria and Ventricular Papillary Muscles. Pharmaceuticals. 2024; 17(10):1379. https://doi.org/10.3390/ph17101379
Chicago/Turabian StylePędzińska-Betiuk, Anna, Ulrich Gergs, Jolanta Weresa, Patryk Remiszewski, Ewa Harasim-Symbor, and Barbara Malinowska. 2024. "Comparison of Cardioprotective Potential of Cannabidiol and β-Adrenergic Stimulation Against Hypoxia/Reoxygenation Injury in Rat Atria and Ventricular Papillary Muscles" Pharmaceuticals 17, no. 10: 1379. https://doi.org/10.3390/ph17101379
APA StylePędzińska-Betiuk, A., Gergs, U., Weresa, J., Remiszewski, P., Harasim-Symbor, E., & Malinowska, B. (2024). Comparison of Cardioprotective Potential of Cannabidiol and β-Adrenergic Stimulation Against Hypoxia/Reoxygenation Injury in Rat Atria and Ventricular Papillary Muscles. Pharmaceuticals, 17(10), 1379. https://doi.org/10.3390/ph17101379







