Innovative Use of Nanomaterials in Treating Retinopathy of Prematurity
Abstract
1. Introduction
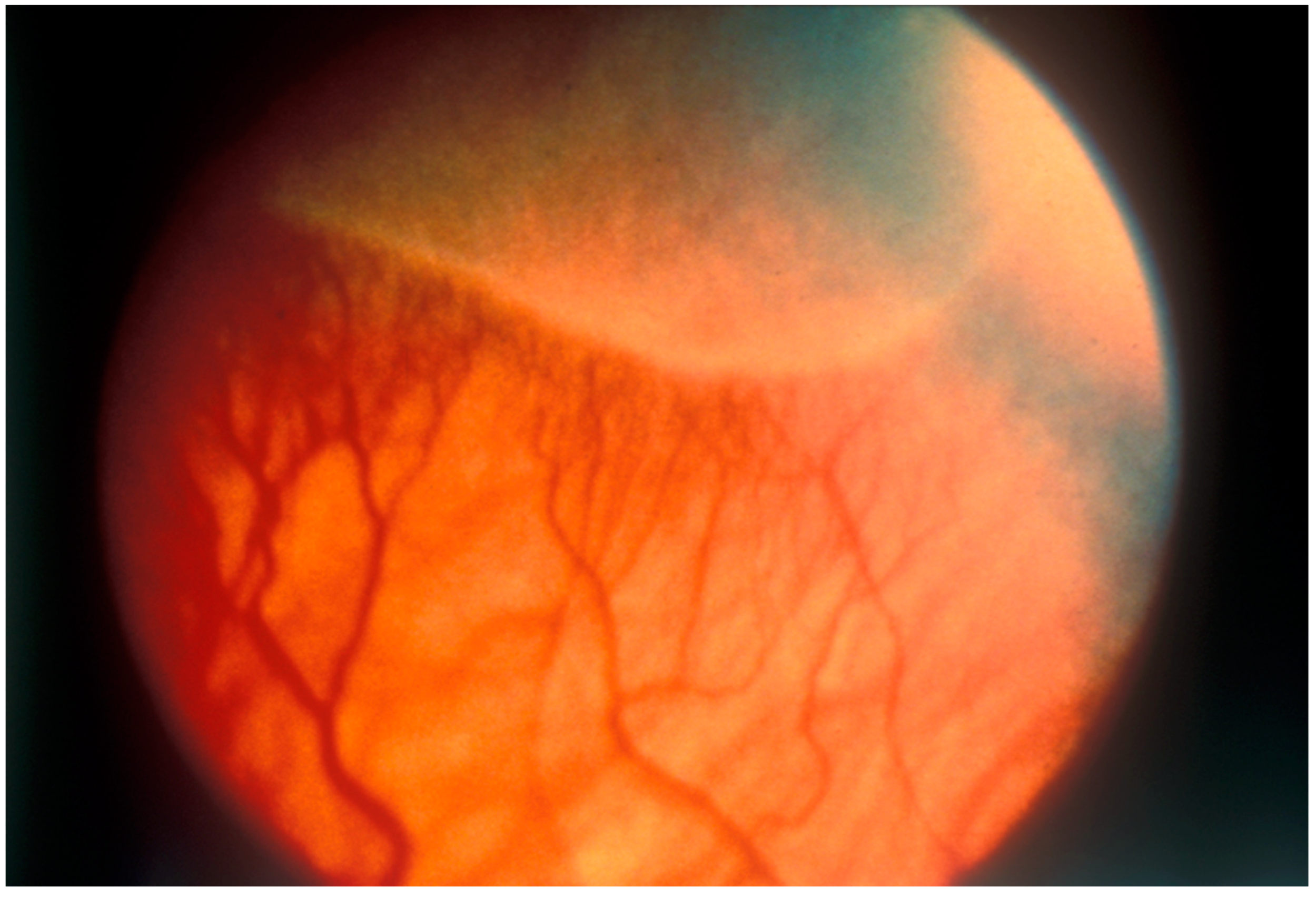
2. Overview of Retinopathy of Prematurity (ROP)
3. Managing Retinopathy of Prematurity
3.1. Cryotherapy
3.2. Laser Photocoagulation
3.3. Anti-Vascular Endothelial Growth Factor Therapy
3.4. Current Challenges in Managing Retinopathy of Prematurity
4. Nano-Based Therapeutic Approaches
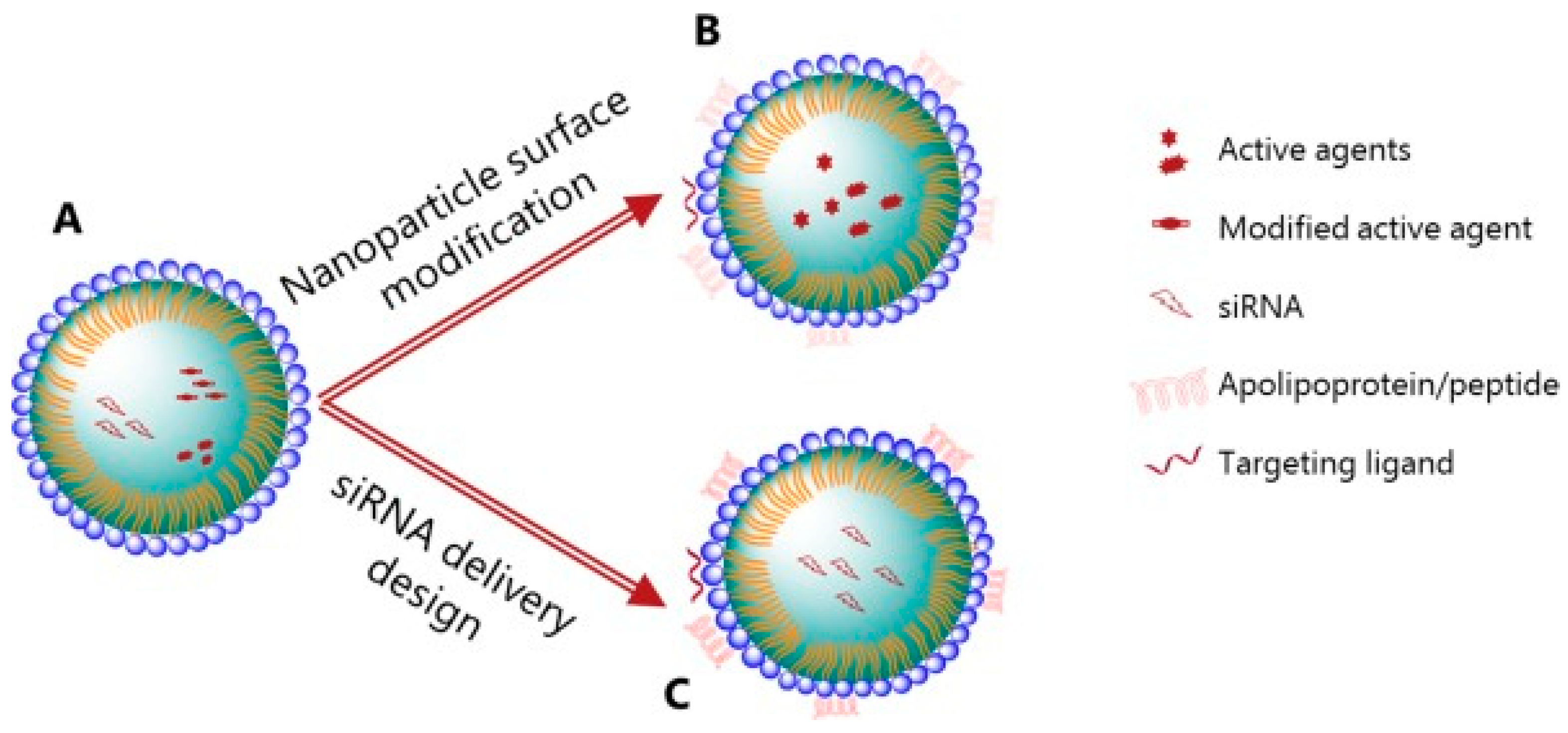
4.1. Various Nano-Based Drug Delivery Systems
4.2. Lipid Nanoparticles
4.3. Gold Nanoparticles
4.4. Polymeric Nanoparticles
5. Clinical Barriers and Future Perspectives
5.1. Commercial Interest
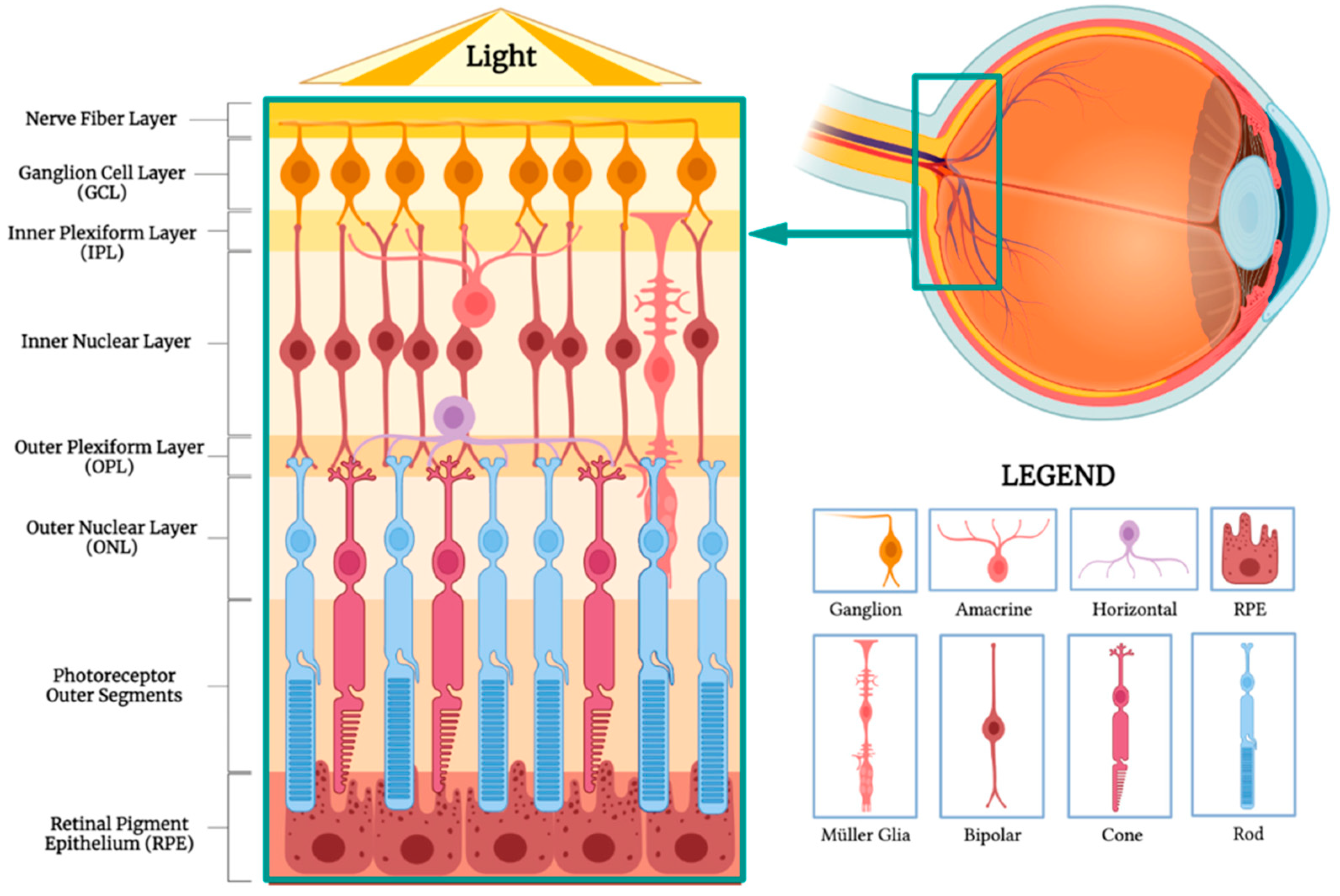
5.2. Retinal Pigment Epithelium Challenges and Potential
6. Emerging Trends
6.1. Gene Therapy
6.2. Exosomes
6.3. Combination Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, L.E.H. Pathogenesis of Retinopathy of Prematurity. Semin. Neonatol. 2003, 8, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Nwanyanwu, K. Retinopathy of Prematurity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: Ophthalmological Outcomes at 10 Years. Arch. Ophthalmol. 2001, 119, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Good, W.V.; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final Results of the Early Treatment for Retinopathy of Prematurity (ETROP) Randomized Trial. Trans. Am. Ophthalmol. Soc. 2004, 102, 233–248; discussion 248–250. [Google Scholar] [PubMed]
- Mintz-Hittner, H.A.; Kennedy, K.A.; Chuang, A.Z.; BEAT-ROP Cooperative Group. Efficacy of Intravitreal Bevacizumab for Stage 3+ Retinopathy of Prematurity. N. Engl. J. Med. 2011, 364, 603–615. [Google Scholar] [CrossRef]
- Wu, K.Y.; Joly-Chevrier, M.; Akbar, D.; Tran, S.D. Overcoming Treatment Challenges in Posterior Segment Diseases with Biodegradable Nano-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1094. [Google Scholar] [CrossRef]
- Kim, S.J.; Port, A.D.; Swan, R.; Campbell, J.P.; Chan, R.V.P.; Chiang, M.F. Retinopathy of Prematurity: A Review of Risk Factors and Their Clinical Significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar] [CrossRef]
- The International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity Revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Hamad, A.E.; Moinuddin, O.; Blair, M.P.; Schechet, S.A.; Shapiro, M.J.; Quiram, P.A.; Mammo, D.A.; Berrocal, A.M.; Prakhunhungsit, S.; Cernichiaro-Espinosa, L.A.; et al. Late-Onset Retinal Findings and Complications in Untreated Retinopathy of Prematurity. Ophthalmol. Retin. 2020, 4, 602–612. [Google Scholar] [CrossRef]
- Tufail, A.; Singh, A.J.; Haynes, R.J.; Dodd, C.R.; McLeod, D.; Charteris, D.G. Late Onset Vitreoretinal Complications of Regressed Retinopathy of Prematurity. Br. J. Ophthalmol. 2004, 88, 243–246. [Google Scholar] [CrossRef]
- Fierson, W.M.; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists; Chiang, M.F.; Good, W.; Phelps, D.; Reynolds, J.; Robbins, S.L.; et al. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics 2018, 142, e20183061. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists; Fierson, W.M.; Saunders, R.A.; Good, W.; Palmer, E.A.; Phelps, D.; Reynolds, J.; et al. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics 2013, 131, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Cryotherapy for Retinopathy of Prematurity Cooperative Group and the National Eye Institute Author. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: Preliminary Results. Arch. Ophthalmol. 1988, 106, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.P.; Ng, E.Y.J.; McNamara, J.A.; Regillo, C.D.; Vander, J.F.; Tasman, W. A Comparison of Laser Photocoagulation with Cryotherapy for Threshold Retinopathy of Prematurity at 10 Years: Part 2. Refractive outcome. Ophthalmology 2002, 109, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.Y.J.; Connolly, B.P.; McNamara, J.A.; Regillo, C.D.; Vander, J.F.; Tasman, W. A Comparison of Laser Photocoagulation with Cryotherapy for Threshold Retinopathy of Prematurity at 10 Years: Part 1. Visual function and structural outcome. Ophthalmology 2002, 109, 928–934. [Google Scholar] [CrossRef]
- Clark, D.; Mandal, K. Treatment of Retinopathy of Prematurity. Early Hum. Dev. 2008, 84, 95–99. [Google Scholar] [CrossRef]
- McNamara, J.A.; Tasman, W.; Brown, G.C.; Federman, J.L. Laser Photocoagulation for Stage 3+ Retinopathy of Prematurity. Ophthalmology 1991, 98, 576–580. [Google Scholar] [CrossRef]
- Kaiser, R.S.; Trese, M.T. Iris Atrophy, Cataracts, and Hypotony Following Peripheral Ablation for Threshold Retinopathy of Prematurity. Arch. Ophthalmol. 2001, 119, 615–617. [Google Scholar]
- Gaitan, J.R.; Berrocal, A.M.; Murray, T.G.; Hess, D.; Johnson, R.A.; Mavrofrides, E.C. Anterior Segment Ischemia Following Laser Therapy for Threshold Retinopathy of Prematurity. Retina 2008, 28, S55–S57. [Google Scholar] [CrossRef]
- Lambert, S.R.; Capone, A.; Cingle, K.A.; Drack, A.V. Cataract and Phthisis Bulbi after Laser Photoablation for Threshold Retinopathy of Prematurity. Am. J. Ophthalmol. 2000, 129, 585–591. [Google Scholar] [CrossRef]
- Ajjarapu, A.; Dumitrescu, A. Delayed Anterior Segment Complications after the Treatment of Retinopathy of Prematurity with Laser Photocoagulation. Front. Ophthalmol. 2023, 3, 1270591. [Google Scholar] [CrossRef]
- Ahmad, A.; Nawaz, M.I. Molecular Mechanism of VEGF and Its Role in Pathological Angiogenesis. J. Cell. Biochem. 2022, 123, 1938–1965. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Balakrishnan, D.; Zeynalova, Z.; Padhi, T.R.; Rani, P.K. Serious Adverse Events and Visual Outcomes of Rescue Therapy Using Adjunct Bevacizumab to Laser and Surgery for Retinopathy of Prematurity. The Indian Twin Cities Retinopathy of Prematurity Screening Database Report Number 5. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F327–F333. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, Y.; Wu, W.-C.; Nitulescu, C.E.; Chan, R.V.P.; Thanos, A.; Thomas, B.J.; Todorich, B.; Drenser, K.A.; Trese, M.T.; Capone, A. Progressive Retinal Detachment in Infants with Retinopathy of Prematurity Treated with Intravitreal Bevacizumab or Ranibizumab. Retina 2018, 38, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, M.; Wang, H.; Jiang, Y.; Smith, G.W.; Strange, J.; Hartnett, M.E. Anti-VEGF Antibody Leads to Later Atypical Intravitreous Neovascularization and Activation of Angiogenic Pathways in a Rat Model of Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2020–2026. [Google Scholar] [CrossRef]
- Khalili, S.; Shifrin, Y.; Pan, J.; Belik, J.; Mireskandari, K. The Effect of a Single Anti-Vascular Endothelial Growth Factor Injection on Neonatal Growth and Organ Development: In-Vivo Study. Exp. Eye Res. 2018, 169, 54–59. [Google Scholar] [CrossRef]
- Okabe, K.; Fukada, H.; Tai-Nagara, I.; Ando, T.; Honda, T.; Nakajima, K.; Takeda, N.; Fong, G.-H.; Ema, M.; Kubota, Y. Neuron-Derived VEGF Contributes to Cortical and Hippocampal Development Independently of VEGFR1/2-Mediated Neurotrophism. Dev. Biol. 2020, 459, 65–71. [Google Scholar] [CrossRef]
- Dao, D.T.; Nandivada, P.; Vuong, J.T.; Anez-Bustillos, L.; Pan, A.; Kishikawa, H.; Mitchell, P.D.; Baker, M.A.; Fell, G.L.; Martin, T.; et al. Vascular Endothelial Growth Factor Accelerates Compensatory Lung Growth by Increasing the Alveolar Units. Pediatr. Res. 2018, 83, 1182–1189. [Google Scholar] [CrossRef]
- Morin, J.; Luu, T.M.; Superstein, R.; Ospina, L.H.; Lefebvre, F.; Simard, M.-N.; Shah, V.; Shah, P.S.; Kelly, E.N.; the Canadian Neonatal Network and the Canadian Neonatal Follow-Up Network Investigators. Neurodevelopmental Outcomes Following Bevacizumab Injections for Retinopathy of Prematurity. Pediatrics 2016, 137, e20153218. [Google Scholar] [CrossRef]
- Natarajan, G.; Shankaran, S.; Nolen, T.L.; Sridhar, A.; Kennedy, K.A.; Hintz, S.R.; Phelps, D.L.; DeMauro, S.B.; Carlo, W.A.; Gantz, M.G.; et al. Neurodevelopmental Outcomes of Preterm Infants with Retinopathy of Prematurity by Treatment. Pediatrics 2019, 144, e20183537. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.H.C.D.; Butera, A.P.; Cabeça, L.F.; Ribeiro-Viana, R.M. Liposome Surface Modification by Phospholipid Chemical Reactions. Chem. Phys. Lipids 2021, 237, 105084. [Google Scholar] [CrossRef] [PubMed]
- Bohley, M.; Dillinger, A.E.; Schweda, F.; Ohlmann, A.; Braunger, B.M.; Tamm, E.R.; Goepferich, A. A Single Intravenous Injection of Cyclosporin A—Loaded Lipid Nanocapsules Prevents Retinopathy of Prematurity. Sci. Adv. 2022, 8, eabo6638. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Sinskey, A.J.; Lodish, H.F. Oxidized Redox State of Glutathione in the Endoplasmic Reticulum. Science 1992, 257, 1496–1502. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, A.; Zhang, H.; Wang, M.; Tang, Q.; Huang, Y.; Wang, L. Inhibition of Retinal Neovascularization by VEGF siRNA Delivered via Bioreducible Lipid-like Nanoparticles. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2407–2418. [Google Scholar] [CrossRef]
- Yaghmur, A.; Østergaard, J.; Mu, H. Lipid Nanoparticles for Targeted Delivery of Anticancer Therapeutics: Recent Advances in Development of siRNA and Lipoprotein-Mimicking Nanocarriers. Adv. Drug Deliv. Rev. 2023, 203, 115136. [Google Scholar] [CrossRef]
- Mukherjee, P.; Bhattacharya, R.; Wang, P.; Wang, L.; Basu, S.; Nagy, J.A.; Atala, A.; Mukhopadhyay, D.; Soker, S. Antiangiogenic Properties of Gold Nanoparticles. Clin. Cancer Res. 2005, 11, 3530–3534. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Kim, K.-W.; Kim, M.H.; Yu, Y.S. Intravenously Administered Gold Nanoparticles Pass through the Blood–Retinal Barrier Depending on the Particle Size, and Induce No Retinal Toxicity. Nanotechnology 2009, 20, 505101. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.H.; Jo, D.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. The Inhibition of Retinal Neovascularization by Gold Nanoparticles via Suppression of VEGFR-2 Activation. Biomaterials 2011, 32, 1865–1871. [Google Scholar] [CrossRef]
- Song, H.B.; Wi, J.-S.; Jo, D.H.; Kim, J.H.; Lee, S.-W.; Lee, T.G.; Kim, J.H. Intraocular Application of Gold Nanodisks Optically Tuned for Optical Coherence Tomography: Inhibitory Effect on Retinal Neovascularization without Unbearable Toxicity. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1901–1911. [Google Scholar] [CrossRef]
- Söderstjerna, E.; Bauer, P.; Cedervall, T.; Abdshill, H.; Johansson, F.; Johansson, U.E. Silver and Gold Nanoparticles Exposure to In Vitro Cultured Retina—Studies on Nanoparticle Internalization, Apoptosis, Oxidative Stress, Glial- and Microglial Activity. PLoS ONE 2014, 9, e105359. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, S.; Ali, S.; Martirossian, A.N.; Hu, X.; Al-Enazy, S.; Albekairi, N.; Motamedi, M.; Rytting, E. Nanoparticle-Medicated Delivery of Hydrophobic Compounds to Retinal Microvascular Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4160. [Google Scholar]
- Zhang, X.-P.; Sun, J.-G.; Yao, J.; Shan, K.; Liu, B.-H.; Yao, M.-D.; Ge, H.-M.; Jiang, Q.; Zhao, C.; Yan, B. Effect of Nanoencapsulation Using Poly (Lactide-Co-Glycolide) (PLGA) on Anti-Angiogenic Activity of Bevacizumab for Ocular Angiogenesis Therapy. Biomed. Pharmacother. 2018, 107, 1056–1063. [Google Scholar] [CrossRef]
- Zhao, M.; Shi, X.; Liang, J.; Miao, Y.; Xie, W.; Zhang, Y.; Li, X. Expression of Pro- and Anti-Angiogenic Isoforms of VEGF in the Mouse Model of Oxygen-Induced Retinopathy. Exp. Eye Res. 2011, 93, 921–926. [Google Scholar] [CrossRef]
- Mezu-Ndubuisi, O.J.; Wang, Y.; Schoephoerster, J.; Falero-Perez, J.; Zaitoun, I.S.; Sheibani, N.; Gong, S. Intravitreal Delivery of VEGF-A165-Loaded PLGA Microparticles Reduces Retinal Vaso-Obliteration in an In Vivo Mouse Model of Retinopathy of Prematurity. Curr. Eye Res. 2019, 44, 275–286. [Google Scholar] [CrossRef]
- Vinekar, A.; Dogra, M.; Azad, R.; Gilbert, C.; Gopal, L.; Trese, M. The Changing Scenario of Retinopathy of Prematurity in Middle and Low Income Countries: Unique Solutions for Unique Problems. Indian. J. Ophthalmol. 2019, 67, 717–719. [Google Scholar] [CrossRef]
- Metry, D.W.; Hebert, A.A. Topical therapies and medications in the pediatric patient. Pediatr. Clin. N. Am. 2000, 47, 867–876. [Google Scholar] [CrossRef]
- Joseph, P.D.; Craig, J.C.; Caldwell, P.H.Y. Clinical Trials in Children. Br. J. Clin. Pharmacol. 2015, 79, 357–369. [Google Scholar] [CrossRef]
- Bohley, M.; Dillinger, A.E.; Tamm, E.R.; Goepferich, A. Targeted Drug Delivery to the Retinal Pigment Epithelium: Untapped Therapeutic Potential for Retinal Diseases. Drug Discov. Today 2022, 27, 2497–2509. [Google Scholar] [CrossRef]
- Peynshaert, K.; Devoldere, J.; Minnaert, A.-K.; De Smedt, S.C.; Remaut, K. Morphology and Composition of the Inner Limiting Membrane: Species-Specific Variations and Relevance toward Drug Delivery Research. Curr. Eye Res. 2019, 44, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, C.; Wood, M.J.A. Antisense Oligonucleotides: The next Frontier for Treatment of Neurological Disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef]
- Hagigit, T.; Abdulrazik, M.; Valamanesh, F.; Behar-Cohen, F.; Benita, S. Ocular Antisense Oligonucleotide Delivery by Cationic Nanoemulsion for Improved Treatment of Ocular Neovascularization: An in-Vivo Study in Rats and Mice. J. Control. Release 2012, 160, 225–231. [Google Scholar] [CrossRef]
- Huang, K.; Lin, Z.; Ge, Y.; Chen, X.; Pan, Y.; Lv, Z.; Sun, X.; Yu, H.; Chen, J.; Yao, Q. Immunomodulation of MiRNA-223-Based Nanoplatform for Targeted Therapy in Retinopathy of Prematurity. J. Control. Release 2022, 350, 789–802. [Google Scholar] [CrossRef]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, R.; Lee, K.; Tyagi, P.; Ding, L.; Kompella, U.B.; Chen, J.; Xu, X.; Ma, J. Nanoparticle-Mediated Expression of a Wnt Pathway Inhibitor Ameliorates Ocular Neovascularization. ATVB 2015, 35, 855–864. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Y.; Hu, Z.; Sun, L.; Dou, G.; Zhang, Z.; Wang, H.; Guo, C.; Wang, Y. Exosomes from Microglia Attenuate Photoreceptor Injury and Neovascularization in an Animal Model of Retinopathy of Prematurity. Mol. Ther. Nucleic Acids 2019, 16, 778–790. [Google Scholar] [CrossRef]
- Li, J.; Fan, W.; Hao, L.; Li, Y.; Yu, G.; Sun, W.; Luo, X.; Zhong, J. Inhibition of VEGF-A Expression in Hypoxia-Exposed Fetal Retinal Microvascular Endothelial Cells by Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells. Biocell 2023, 47, 2485–2494. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, T.; Ma, H.; Pan, Y.; Wang, S.; Liu, X.; Dai, X.; Zheng, Y.; Lee, L.P.; Liu, F. Discovering the Secret of Diseases by Incorporated Tear Exosomes Analysis via Rapid-Isolation System: iTEARS. ACS Nano 2022, 16, 11720–11732. [Google Scholar] [CrossRef]
- Autrata, R.; Krejčířová, I.; Šenková, K.; Holoušová, M.; Doležel, Z.; Borek, I. Intravitreal Pegaptanib Combined with Diode Laser Therapy for Stage 3+ Retinopathy of Prematurity in Zone I and Posterior Zone II. Eur. J. Ophthalmol. 2012, 22, 687–694. [Google Scholar] [CrossRef]
- Modrzejewska, M.; Nazwalska, M. The Long-Term Observation of the Beneficial Effects of Treatment: 0.12 Mg Anti-VEGF Monotherapy or Anti-VEGF Combined Therapy and Diode-Laser in Various Stages of Retinopathy of Prematurity—Series of Cases. JCM 2023, 12, 5644. [Google Scholar] [CrossRef]

| NP | Characteristics | Observations | Stage | Year | Ref. |
|---|---|---|---|---|---|
| ROP | |||||
| Lipid nanocapsules (LNCs) | Surface cRGD loaded with Cyclosporin A |
| OIR mouse in vivo, intravenous | 2022 | [34] |
| Gold NPs | Empty gold NPs |
| HRMECs in vitro and intravitreal OIR mouse in vivo | 2011 | [40] |
| PLGA | VEGF-A165 loaded |
| Intravitreal OIR mouse | 2019 | [47] |
| Folic acid–chitosan-modified mesoporous silica nanoparticles | MiRNA-223 loaded |
| Microglia/macrophages cells (BV2 and Raw 264.7), HRMECs and HUVECs in vitro, and intravitreal OIR mouse in vivo | 2022 | [55] |
| Exosomes | Derived from microglial |
| 661w cells in vitro and intravitreal OIR mouse in vivo | 2019 | [58] |
| Exosomes | hucMSC-Exos |
| HfRMECs | 2023 | [59] |
| Retinal neo-vascularization | |||||
| Lipid-like nanoparticles | Loaded with VEGF siRNA smart release |
| HUVECs in vitro and intravitreal OIR mouse in vivo | 2020 | [36] |
| Gold nanodiscs | Optimized anatomy of nanodiscs |
| Intravitreal OIR mouse in vivo | 2017 | [41] |
| PLGA | Bevacizumab loaded |
| HUVECs in vitro and intravitreal OIR mouse in vivo | 2018 | [45] |
| Ischemic retinopathy | |||||
| PLGA, PLA | Pazopanib or coumarin-6 loaded |
| RMECs | 2015 | [44] |
| Ocular neo-vascularization | |||||
| DOTAP nanoemulsions | Anti-VEGFR loaded oligonucleotides |
| Intravitreal ROP mouse models in vivo | 2012 | [54] |
| PLGA | Loaded with plasmid expressing VLN |
| HRMEC in vitro and intravitreal OIR VLDR-/-mouse in vivo | 2015 | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Wang, X.C.; Anderson, M.; Tran, S.D. Innovative Use of Nanomaterials in Treating Retinopathy of Prematurity. Pharmaceuticals 2024, 17, 1377. https://doi.org/10.3390/ph17101377
Wu KY, Wang XC, Anderson M, Tran SD. Innovative Use of Nanomaterials in Treating Retinopathy of Prematurity. Pharmaceuticals. 2024; 17(10):1377. https://doi.org/10.3390/ph17101377
Chicago/Turabian StyleWu, Kevin Y., Xingao C. Wang, Maude Anderson, and Simon D. Tran. 2024. "Innovative Use of Nanomaterials in Treating Retinopathy of Prematurity" Pharmaceuticals 17, no. 10: 1377. https://doi.org/10.3390/ph17101377
APA StyleWu, K. Y., Wang, X. C., Anderson, M., & Tran, S. D. (2024). Innovative Use of Nanomaterials in Treating Retinopathy of Prematurity. Pharmaceuticals, 17(10), 1377. https://doi.org/10.3390/ph17101377







