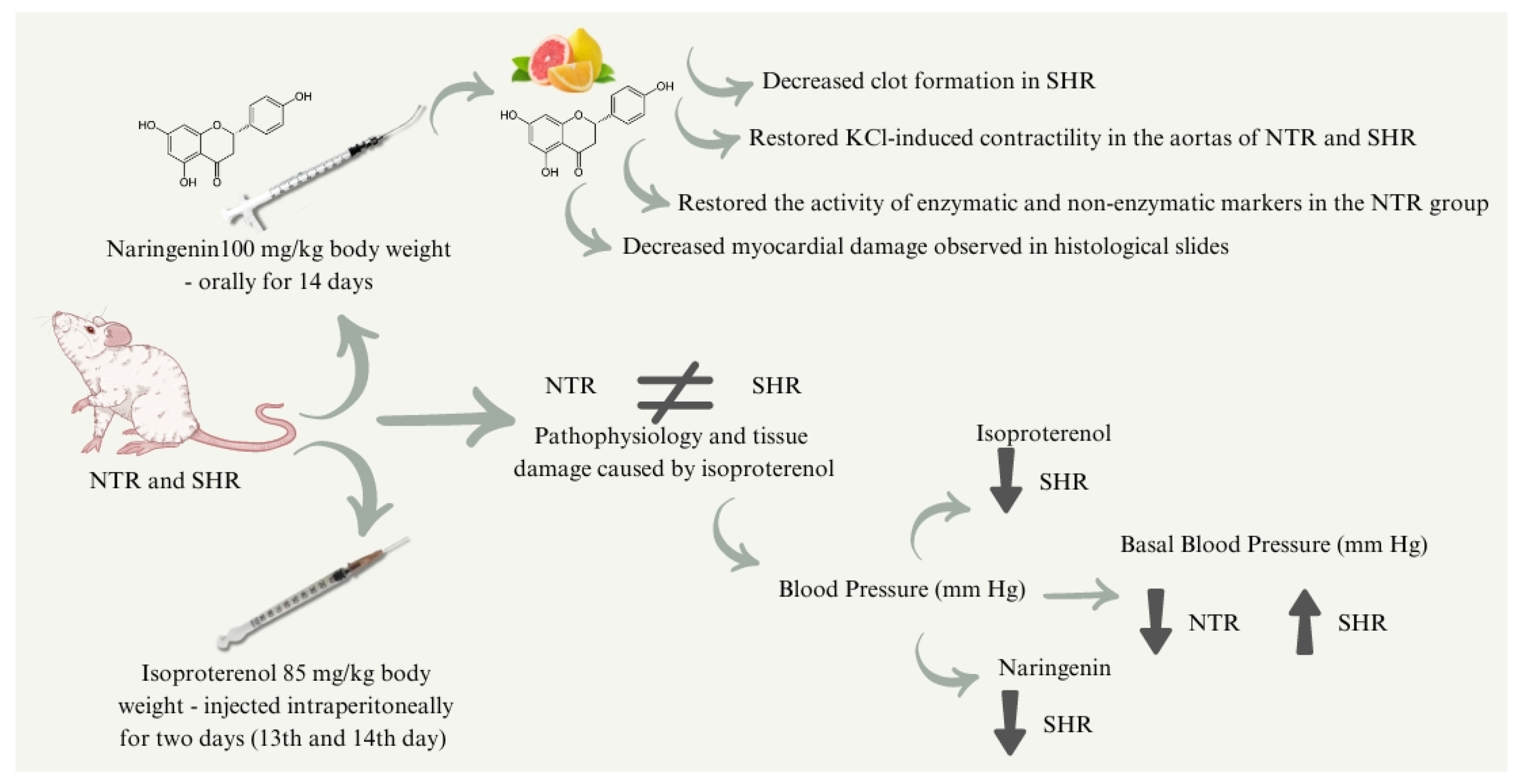
Comparative Analysis of the Protective Effect of Naringenin on Cardiovascular Parameters of Normotensive and Hypertensive Rats Subjected to the Myocardial Infarction Model
Abstract
1. Introduction
2. Results
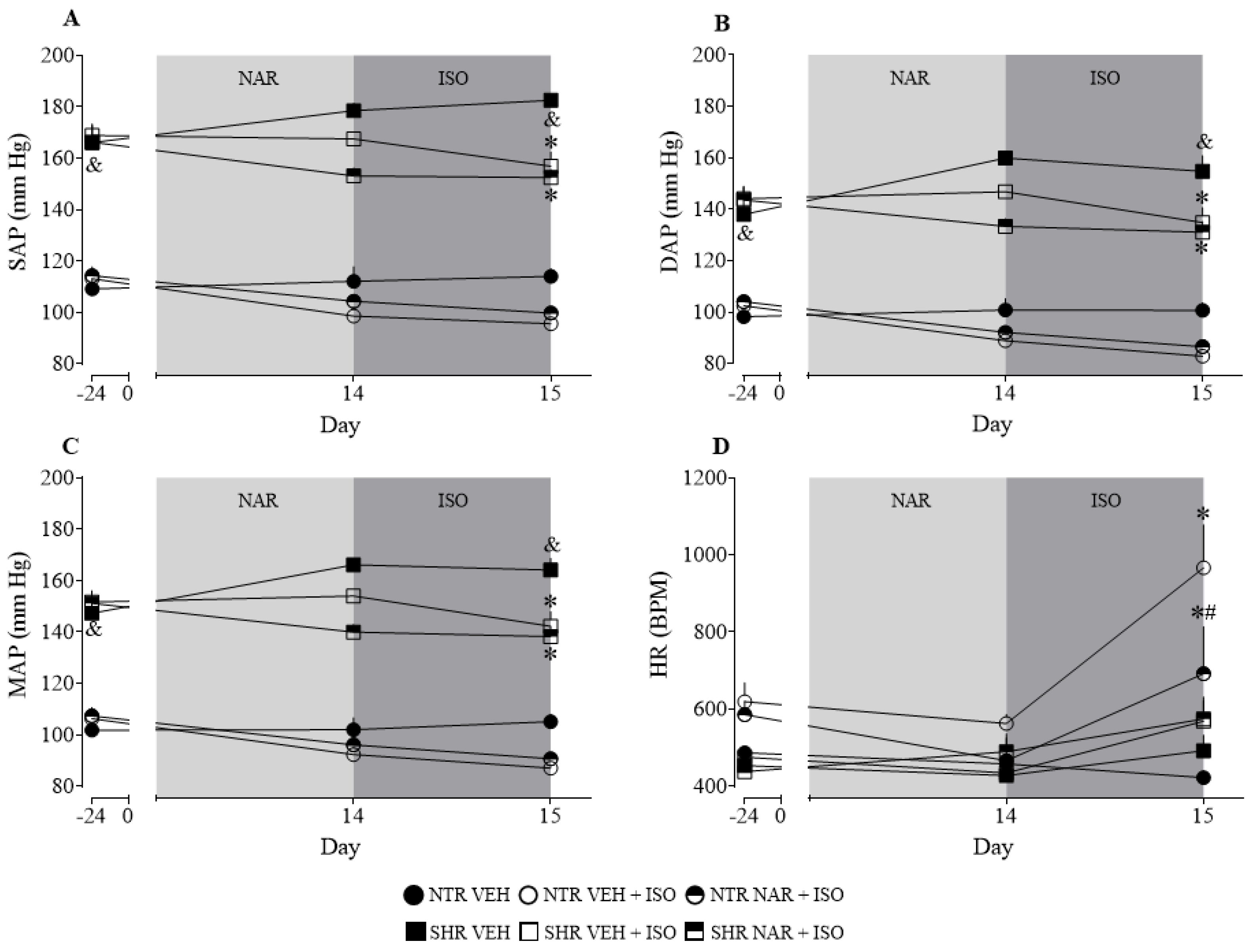
2.1. Analysis of Baseline and after Treatment Arterial Pressure Values in NTR and SHR Groups
2.2. Evaluation of Body Weight, Water Consumption, and Feed Intake in the NTR and SHR Groups
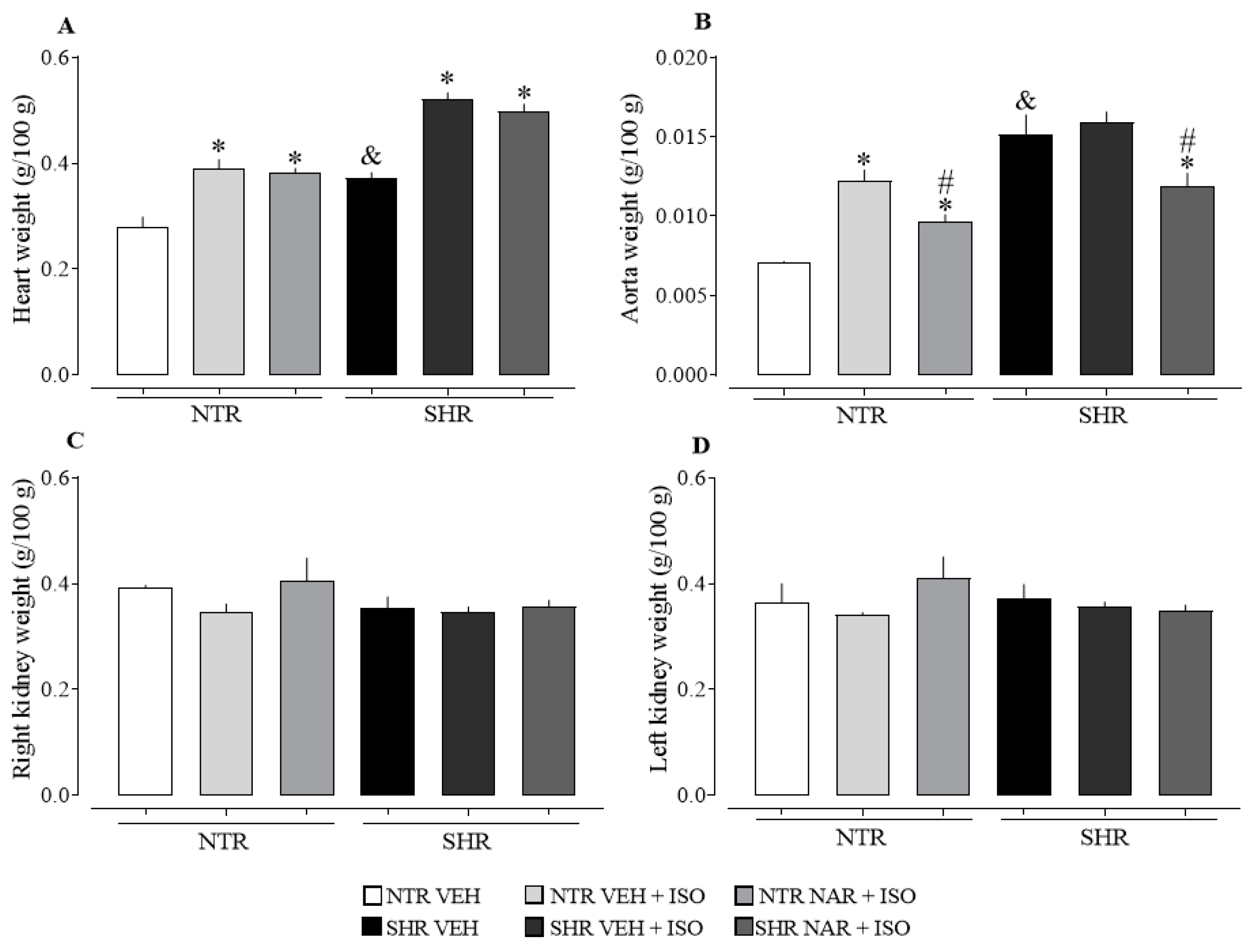
2.3. Evaluation of the Relative Weight of the Heart, Aorta, Kidneys, and Liver
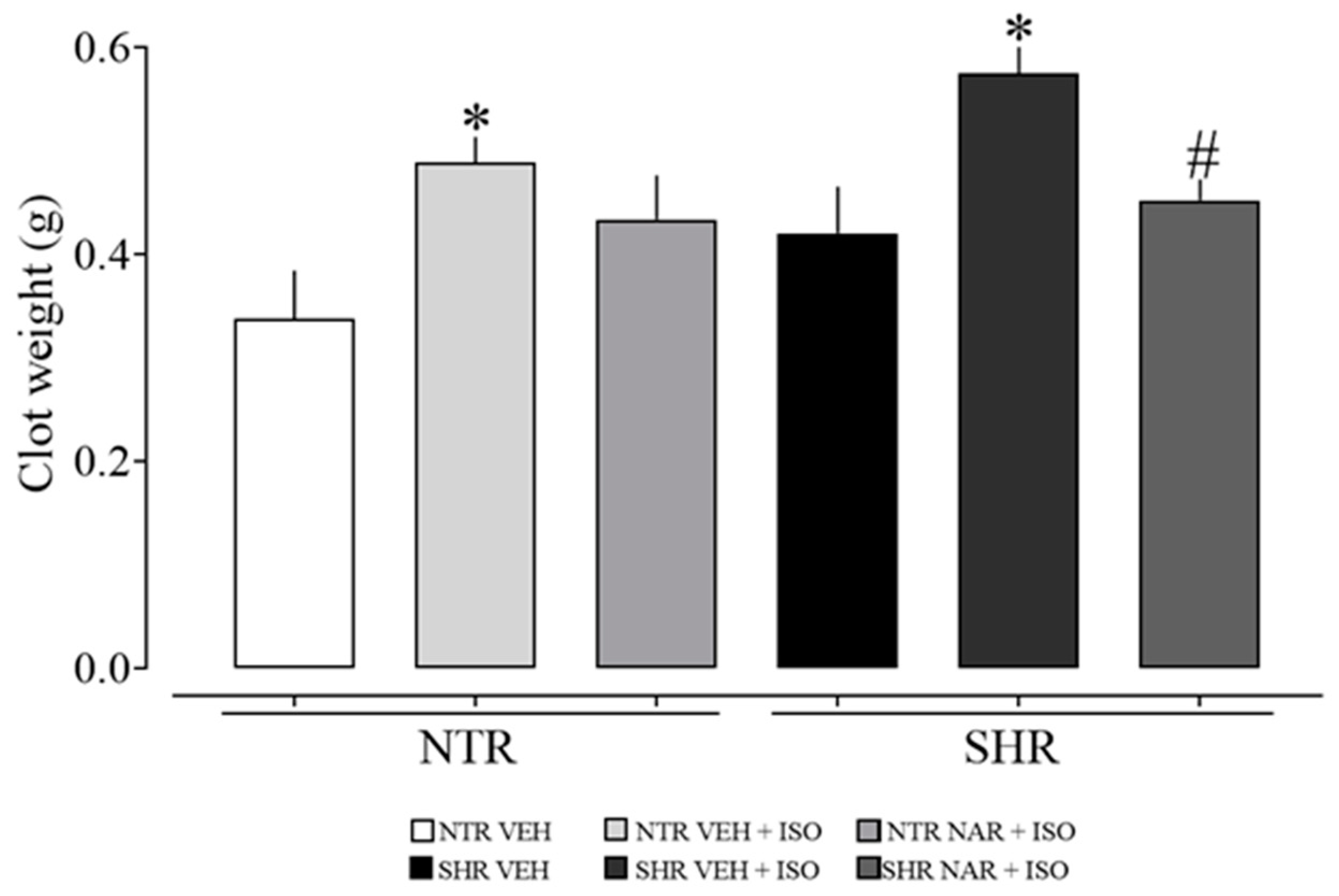
2.4. Analysis of Clot Formation in Blood Samples from NTR and SHR Groups
2.5. Evaluation of Blood Parameters in NTR and SHR Groups
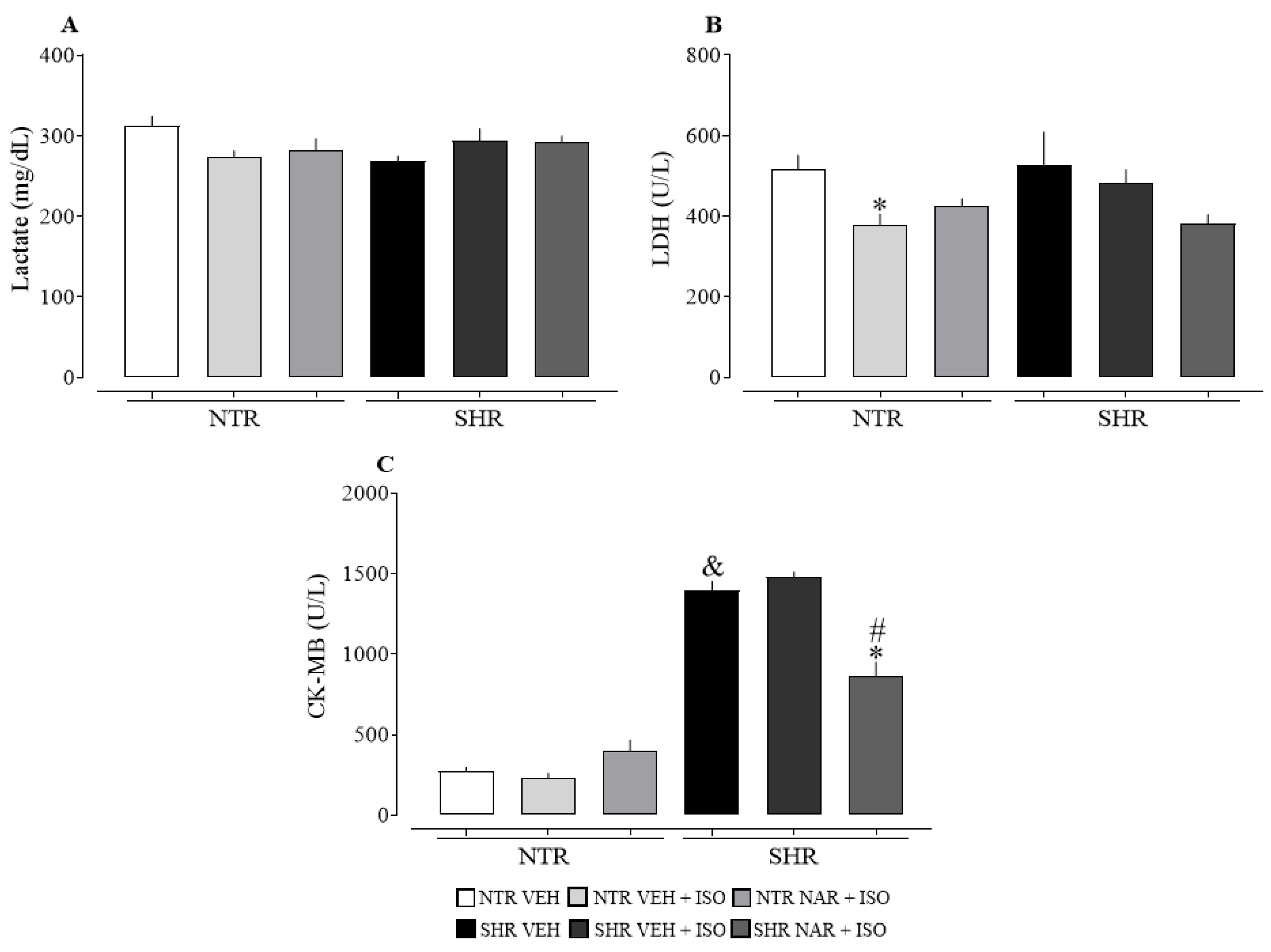
2.6. Analysis of Cardiac Damage Markers NTR and SHR Groups
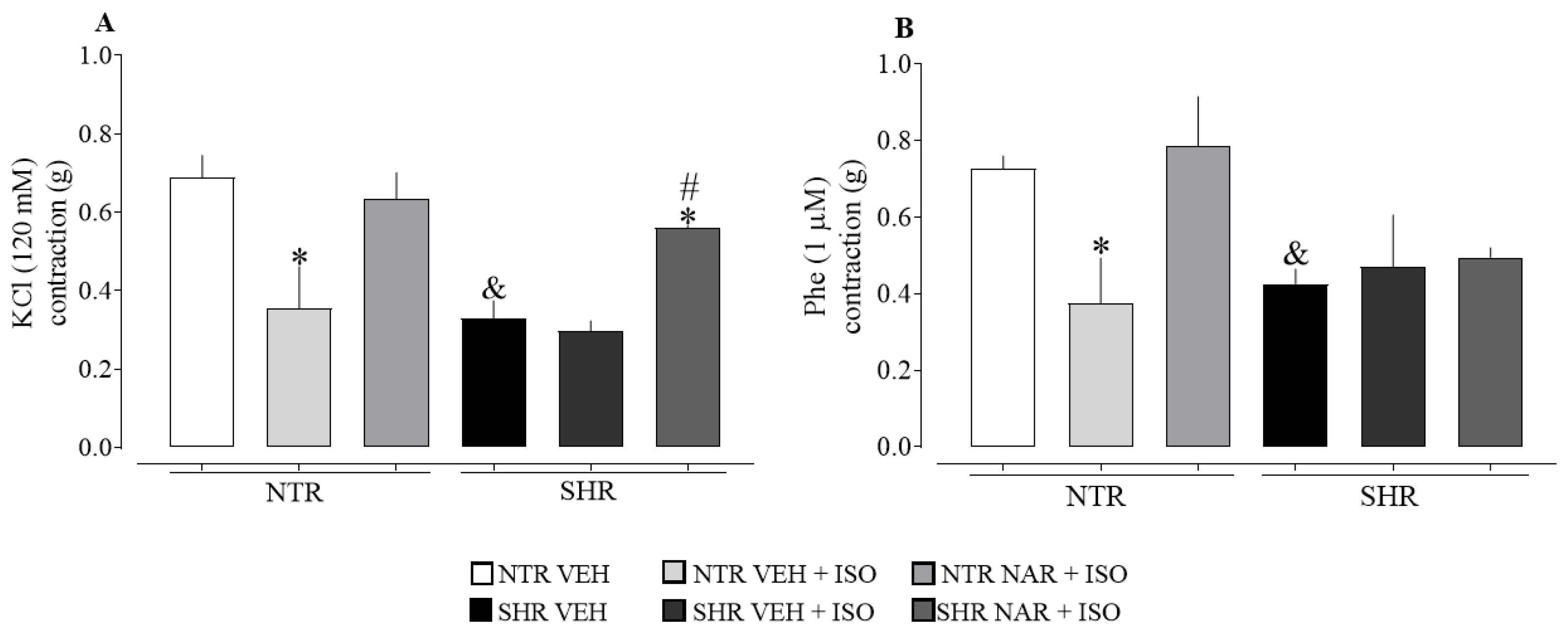
2.7. Evaluation of Aortic Responsiveness to Vasoconstrictors Obtained from NTR and SHR Groups
2.8. Analysis of Enzymatic and Non-Enzymatic Markers of Oxidative Stress
2.9. Evaluation of Inflammatory Markers in the Cardiac Tissue of NTR and SHR Groups
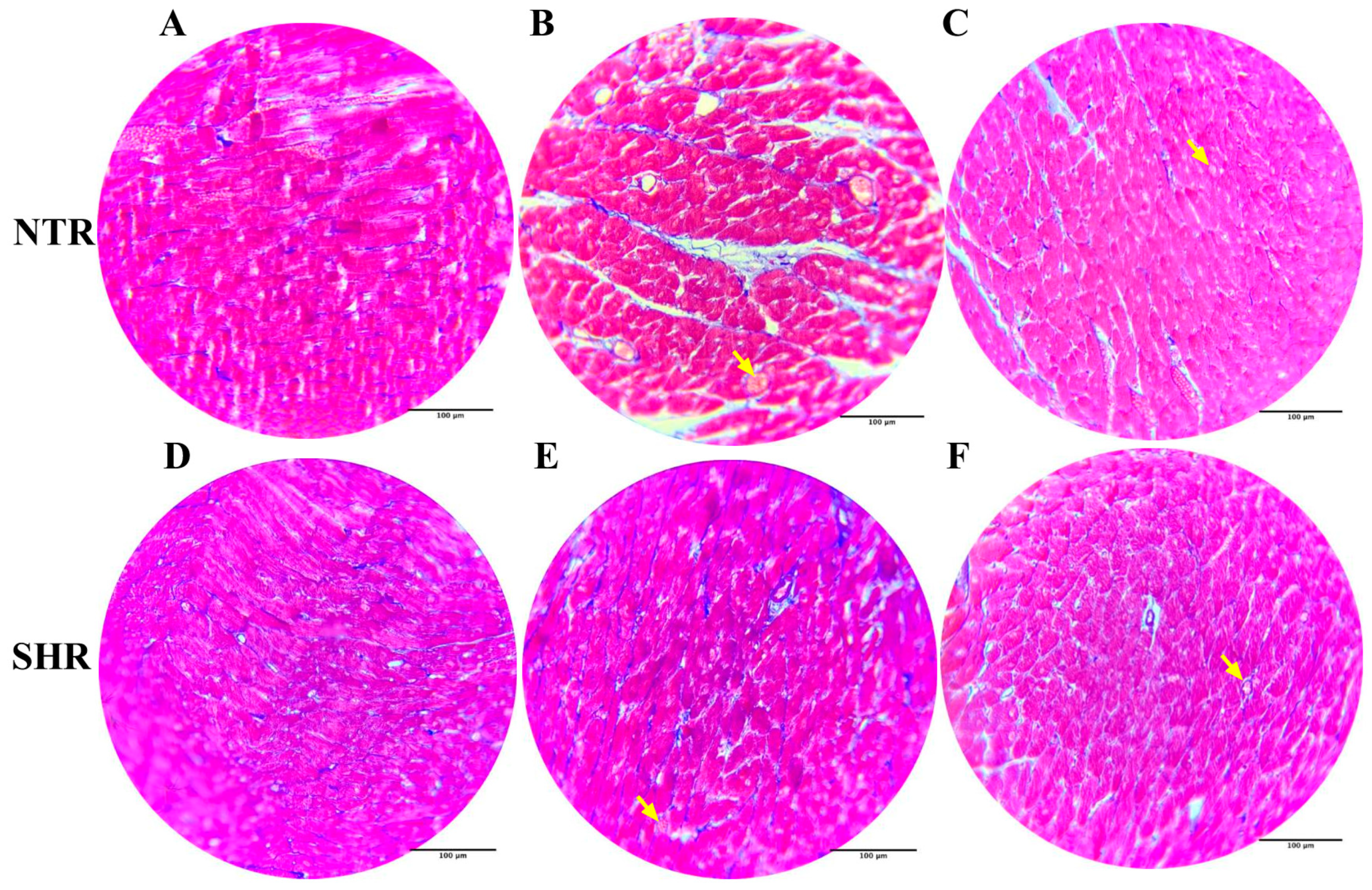
2.10. Histological Analysis with Hematoxylin and Eosin Staining
2.11. Histological Analysis with Masson’s Trichrome Staining
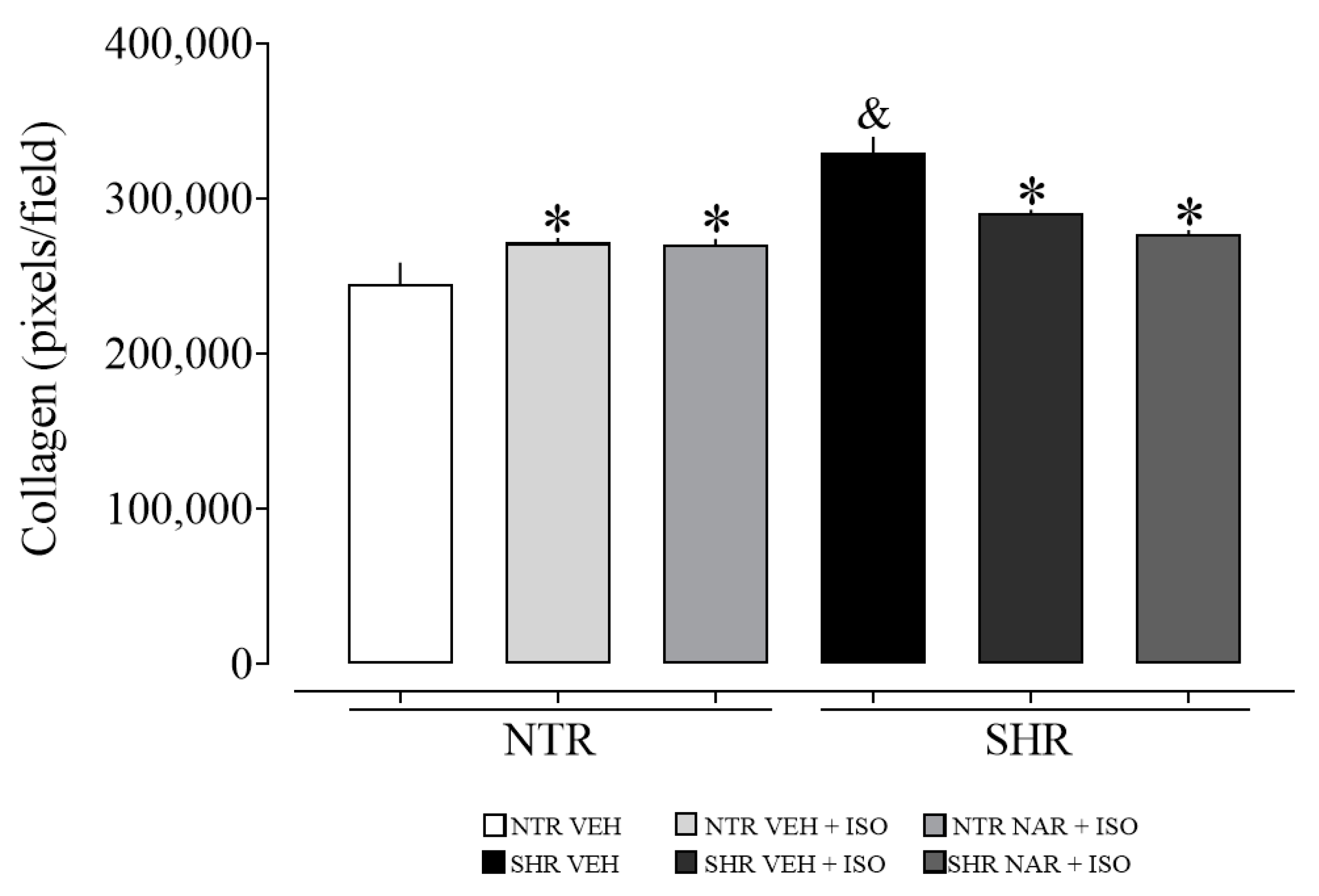
2.12. Evaluation of Collagen Content in Myocardial Samples from NTR and SHR Groups
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Animals
4.3. Blood Pressure Measurements via Plethysmography (Tail-Cuff)
4.4. Induction of Infarction by Isoproterenol
4.5. Evaluation of Clot Formation
4.6. Blood Tests
4.7. Evaluation of the Relative Weight of the Aorta, Kidney, Liver, and Heart
4.8. Evaluation of Weight Gain, Food, and Water Consumption
4.9. Evaluation of Vascular Reactivity in an Isolated Aorta Model
4.10. Determination of Oxidative and Inflammatory Parameters
4.11. Histological Analysis and Collagen Quantification
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Précoma, D.B.; de Oliveira, G.M.M.; Simão, A.F.; Dutra, O.P.; Coelho-Filho, O.R.; de O. Izar, M.C.; dos S. Póvoa, R.M.; de C. B. Giuliano, I.; de Alencar Filho, A.C.; Machado, C.A.; et al. Updated Cardiovascular Prevention Guideline of the Brazilian Society of Cardiology—2019. Arq. Bras. Cardiol. 2019, 4, 787–891. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, P.; Kaur, K.; Fatima, S.; Mahak, F.; Noman, M.; Siddenthi, S.M.; Surksha, M.A.; Munir, M.; Fatima, F.; Sultana, S.S.; et al. Advancements in Myocardial Infarction Management: Exploring Novel Approaches and Strategies. Cureus 2023, 15, e45578. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Upadhyaya, V.D.; Wong, C.; Zakir, R.M.; Aghili, N.; Faraz, H.; Kapur, N.K. Management of Myocardial Infarction: Emerging Paradigms for the Future. Methodist Debakey Cardiovasc. J. 2024, 20, 54–63. [Google Scholar] [CrossRef]
- Vasanthi, H.R.; ShriShriMal, N.; Das, D.K. Retraction Notice: Phytochemicals from Plants to Combat Cardiovascular Disease. Curr. Med. Chem. 2012, 19, 2242–2251. [Google Scholar] [CrossRef] [PubMed]
- Heidary Moghaddam, R.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and Naringin in Cardiovascular Disease Prevention: A Preclinical Review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef]
- Peterson, J.J.; Dwyer, J.T.; Beecher, G.R.; Bhagwat, S.A.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in Oranges, Tangerines (Mandarins), Tangors, and Tangelos: A Compilation and Review of the Data from the Analytical Literature. J. Food Compos. Anal. 2006, 19, S66–S73. [Google Scholar] [CrossRef]
- Dickhout, J.G.; Lee, R.M.K.W. Blood Pressure and Heart Rate Development in Young Spontaneously Hypertensive Rats. Am. J. Physiol.-Heart Circulatory Physiol. 1998, 274, H794–H800. [Google Scholar] [CrossRef]
- Wong, Z.W.; Thanikachalam, P.V.; Ramamurthy, S. Molecular Understanding of the Protective Role of Natural Products on Isoproterenol-Induced Myocardial Infarction: A Review. Biomed. Pharmacother. 2017, 94, 1145–1166. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid Intake and Risk of Chronic Diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef]
- Orhan, I.; Nabavi, S.; Daglia, M.; Tenore, G.; Mansouri, K.; Nabavi, S. Naringenin and Atherosclerosis: A Review of Literature. Curr. Pharm. Biotechnol. 2015, 16, 245–251. [Google Scholar] [CrossRef]
- Testai, L.; Calderone, V. Nutraceutical Value of Citrus Flavanones and Their Implications in Cardiovascular Disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Snoke, D.B.; Nishikawa, Y.; Cole, R.M.; Ni, A.; Angelotti, A.; Vodovotz, Y.; Belury, M.A. Dietary Naringenin Preserves Insulin Sensitivity and Grip Strength and Attenuates Inflammation but Accelerates Weight Loss in a Mouse Model of Cancer Cachexia. Mol. Nutr. Food Res. 2021, 65, 2100268. [Google Scholar] [CrossRef]
- Burke, A.C.; Telford, D.E.; Edwards, J.Y.; Sutherland, B.G.; Sawyez, C.G.; Huff, M.W. Naringenin Supplementation to a Chow Diet Enhances Energy Expenditure and Fatty Acid Oxidation, and Reduces Adiposity in Lean, Pair-Fed Ldlr −/− Mice. Mol. Nutr. Food Res. 2019, 63, 1800833. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Che, J.; Yao, W. Naringenin Protects against Hypertension by Regulating Lipid Disorder and Oxidative Stress in a Rat Model. Kidney Blood Press. Res. 2022, 47, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Nova York, NY, USA, 2007; Volume 1, p. 851. [Google Scholar]
- Li, Y.; He, B.; Zhang, C.; He, Y.; Xia, T.; Zeng, C. Naringenin Attenuates Isoprenaline-Induced Cardiac Hypertrophy by Suppressing Oxidative Stress through the AMPK/NOX2/MAPK Signaling Pathway. Nutrients 2023, 15, 1340. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, C.; Yang, M.; Zhang, L.; Wei, R.; Meng, K.; Bao, Y.; Zhang, L.; Zheng, J. Four Citrus Flavanones Exert Atherosclerosis Alleviation Effects in ApoE−/− Mice via Different Metabolic and Signaling Pathways. J. Agric. Food Chem. 2021, 69, 5226–5237. [Google Scholar] [CrossRef]
- Jin, X.; Jin, L.; Wu, B.; Xu, D. Naringenin protects myocardial ischemia/reperfusion injury by regulating miR-24-3p to inhibit cell death-inducing p53 target 1 expression. Gen. Physiol. Biophys. 2024, 43, 13–23. [Google Scholar] [CrossRef]
- Sabbatini, M.; Antonio Vega, J.; Amenta, F. Peripheral Nerve Vascular Changes in Spontaneously Hypertensive Rats. Neurosci. Lett. 1996, 217, 85–88. [Google Scholar] [CrossRef]
- Wang, Y.; Anesi, J.; Maier, M.C.; Myers, M.A.; Oqueli, E.; Sobey, C.G.; Drummond, G.R.; Denton, K.M. Sympathetic Nervous System and Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 13132. [Google Scholar] [CrossRef]
- Jordão, M.T.; Ladd, F.V.L.; Coppi, A.A.; Chopard, R.P.; Michelini, L.C. Exercise Training Restores Hypertension-Induced Changes in the Elastic Tissue of the Thoracic Aorta. J. Vasc. Res. 2011, 48, 513–524. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, C.L.B.; Cechinel-Filho, V.; Boeing, T.; Mariano, L.N.B.; da Silva, L.M.; de Andrade, S.F.; de Souza, P. Prolonged Diuretic and Saluretic Effect of Nothofagin Isolated from Leandra Dasytricha (A. Gray) Cogn. Leaves in Normotensive and Hypertensive Rats: Role of Antioxidant System and Renal Protection. Chem. Biol. Interact. 2018, 279, 227–233. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, L.; Chen, G.; You, H. Effect of Fraxetin on Oxidative Damage Caused by Isoproterenol-Induced Myocardial Infarction in Rats. Appl. Biochem. Biotechnol. 2022, 194, 5666–5679. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I.; Ahmmed, I.; Ahmed, R.; Tanvir, E.M.; Afroz, R.; Paul, S.; Gan, S.H.; Alam, N. Amelioration of Isoproterenol-Induced Oxidative Damage in Rat Myocardium by Withania somnifera Leaf Extract. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in the Framingham Heart Study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef]
- Aronow, W.S.; Ahn, C.; Kronzon, I.; Koenigsberg, M. Congestive Heart Failure, Coronary Events and Atherothrombotic Brain Infarction in Elderly Blacks and Whites with Systemic Hypertension and with and without Echocardiographic and Electrocardiographic Evidence of Left Ventricular Hypertrophy. Am. J. Cardiol. 1991, 67, 295–299. [Google Scholar] [CrossRef]
- Koren, M.J.; Devereux, R.B.; Casale, P.N.; Savage, D.D.; Laragh, J.H. Relation of Left Ventricular Mass and Geometry to Morbidity and Mortality in Uncomplicated Essential Hypertension. Ann. Intern. Med. 1991, 114, 345–352. [Google Scholar] [CrossRef]
- González, A.; Schelbert, E.B.; Díez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef]
- Stanchev, S.; Stamenov, N.; Kirkov, V.; Dzhambazova, E.; Nikolov, D.; Paloff, A. Differential Collagen Expression in Kidney and Heart during Hypertension. Bratisl. Med. J. 2020, 121, 73–78. [Google Scholar] [CrossRef]
- Weber, K.T.; Janicki, J.S.; Shroff, S.G.; Pick, R.; Chen, R.M.; Bashey, R.I. Collagen Remodeling of the Pressure-Overloaded, Hypertrophied Nonhuman Primate Myocardium. Circ. Res. 1988, 62, 757–765. [Google Scholar] [CrossRef]
- Patel, D.K.; Desai, S.N.; Gandhi, H.P.; Devkar, R.V.; Ramachandran, A.V. Cardio Protective Effect of Coriandrum Sativum L. on Isoproterenol Induced Myocardial Necrosis in Rats. Food Chem. Toxicol. 2012, 50, 3120–3125. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Mateen, S.; Naeem, S.S.; Akhtar, K.; Rizvi, W.; Moin, S. Syringic Acid Protects from Isoproterenol Induced Cardiotoxicity in Rats. Eur. J. Pharmacol. 2019, 849, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular Smooth Muscle Contraction in Hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef] [PubMed]
- Gendron, G.; Gobeil, F.; Morin, J.; D’Orléans-Juste, P.; Regoli, D. Contractile Responses of Aortae from WKY and SHR to Vasoconstrictors. Clin. Exp. Hypertens. 2004, 26, 511–523. [Google Scholar] [CrossRef]
- Begonha, R.; Moura, D.; Guimarães, S. Vascular β-Adrenoceptor-Mediated Relaxation and the Tone of the Tissue in Canine Arteries. J. Pharm. Pharmacol. 2011, 47, 510–513. [Google Scholar] [CrossRef]
- Averett, R.D.; Menn, B.; Lee, E.H.; Helms, C.C.; Barker, T.; Guthold, M. A Modular Fibrinogen Model That Captures the Stress-Strain Behavior of Fibrin Fibers. Biophys. J. 2012, 103, 1537–1544. [Google Scholar] [CrossRef]
- Ait Aissa, K.; Lagrange, J.; Mohamadi, A.; Louis, H.; Houppert, B.; Challande, P.; Wahl, D.; Lacolley, P.; Regnault, V. Vascular Smooth Muscle Cells Are Responsible for a Prothrombotic Phenotype of Spontaneously Hypertensive Rat Arteries. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 930–937. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Lindahl, B. Acute coronary syndrome—The present and future role of biomarkers. Clin. Chem. Lab. Med. 2013, 51, 1699–1706. [Google Scholar] [CrossRef]
- Khurana, S.; Piche, M.; Hollingsworth, A.; Venkataraman, K.; Tai, T.C. Oxidative Stress and Cardiovascular Health: Therapeutic Potential of Polyphenols. Can. J. Physiol. Pharmacol. 2013, 91, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Srivastava, S.; Singhal, S.S. Lipid Peroxidation Products in Human Health and Disease 2016. Oxid. Med. Cell Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Angelone, T.; Rocca, C.; Lionetti, V.; Penna, C.; Pagliaro, P. Expanding the Frontiers of Guardian Antioxidant Selenoproteins in Cardiovascular Pathophysiology. Antioxid. Redox Signal. 2024, 40, 369–432. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.V.; Kitts, D.D.; Godin, D.V. Heart and Red Blood Cell Antioxidant Status and Plasma Lipid Levels in the Spontaneously Hypertensive and Normotensive Wistar-Kyoto Rat. Can. J. Physiol. Pharmacol. 1996, 74, 290–297. [Google Scholar] [CrossRef]
- Switala, J.; Loewen, P.C. Diversity of Properties among Catalases. Arch. Biochem. Biophys. 2002, 401, 145–154. [Google Scholar] [CrossRef]
- Álvarez, M.C.; Caldiz, C.; Fantinelli, J.C.; Garciarena, C.D.; Console, G.M.; Chiappe de Cingolani, G.E.; Mosca, S.M. Is Cardiac Hypertrophy in Spontaneously Hypertensive Rats the Cause or the Consequence of Oxidative Stress? Hypertens. Res. 2008, 31, 1465–1476. [Google Scholar] [CrossRef]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of Cutaneous Inflammation: Estimation of Neutrophil Content with an Enzyme Marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef]
- Schultz, J.; Kaminker, K. Myeloperoxidase of the Leucocyte of Normal Human Blood. I. Content and Localization. Arch. Biochem. Biophys. 1962, 96, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Magierowski, M.; Magierowska, K.; Kwiecien, S.; Brzozowski, T. Gaseous Mediators Nitric Oxide and Hydrogen Sulfide in the Mechanism of Gastrointestinal Integrity, Protection and Ulcer Healing. Molecules 2015, 20, 9099–9123. [Google Scholar] [CrossRef]
- Tejero, J.; Stuehr, D. Tetrahydrobiopterin in Nitric Oxide Synthase. IUBMB Life 2013, 65, 358–365. [Google Scholar] [CrossRef]
- DeMartino, A.W.; Kim-Shapiro, D.B.; Patel, R.P.; Gladwin, M.T. Nitrite and Nitrate Chemical Biology and Signalling. Br. J. Pharmacol. 2019, 176, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.; Trujillo, M.; Telleri, R.; Radi, R. Kinetics of Cytochrome C2+ Oxidation by Peroxynitrite: Implications for Superoxide Measurements in Nitric Oxide-Producing Biological-Systems. Arch. Biochem. Biophys. 1995, 319, 491–497. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Altura, B.; Altura, B. Peroxynitrite-Induced Relaxation in Isolated Rat Aortic Rings and Mechanisms of Action. Toxicol. Appl. Pharmacol. 2005, 209, 269–276. [Google Scholar] [CrossRef]
- Khan, V.; Sharma, S.; Bhandari, U.; Sharma, N.; Rishi, V.; Haque, S.E. Suppression of Isoproterenol-Induced Cardiotoxicity in Rats by Raspberry Ketone via Activation of Peroxisome Proliferator Activated Receptor-α. Eur. J. Pharmacol. 2019, 842, 157–166. [Google Scholar] [CrossRef]
- Jiang, Z.; Woollard, A.C.S.; Wolff, S.P. Lipid Hydroperoxide Measurement by Oxidation of Fe2+ in the Presence of Xylenol Orange. Comparison with the TBA Assay and an Iodometric Method. Lipids 1991, 26, 853–856. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of Total, Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Young, L.M.; Kheifets, J.B.; Ballaron, S.J.; Young, J.M. Edema and Cell Infiltration in the Phorbol Ester-Treated Mouse Ear Are Temporally Separate and Can Be Differentially Modulated by Pharmacologic Agents. Agents Actions 1989, 26, 335–341. [Google Scholar] [CrossRef]
- Bailey, P.J. Sponge Implants as Models. Methods Enzymol. 1988, 162, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Analysis of Nitrite and Nitrate in Biological Fluids by Assays Based on the Griess Reaction: Appraisal of the Griess Reaction in the l-Arginine/Nitric Oxide Area of Research. J. Chromatogr. B 2007, 851, 51–70. [Google Scholar] [CrossRef] [PubMed]

| Groups | Weight (g) Day 0 | Weight (g) Day 15 |
|---|---|---|
| NTR VEH | 337 ± 21 | 340 ± 25 |
| NTR VEH + ISO | 340 ± 25 | 298 ± 19 |
| NTR NAR + ISO | 336 ± 14 | 287 ± 18 (p = 0.0547) |
| SHR VEH | 291 ± 7 | 319 ± 15 |
| SHR VEH + ISO | 293 ± 7 | 291 ± 5 |
| SHR NAR + ISO | 291 ± 7 | 280 ± 6 |
| Groups | Urea (mg/dL) | Creatinine (mg/dL) | Na+ (mmol/L) | K+ (mmol/L) | Cl− (mmol/L) | Ca2+ (mg/dL) |
|---|---|---|---|---|---|---|
| NTR VEH | 47.1 ± 1.3 | 0.5 ± 0.1 | 164.0 ± 11.5 | 21.2 ± 2.1 | 230.6 ± 9.2 | 9.6 ± 1.4 |
| NTR VEH + ISO | 85.8 ± 6.8 * | 0.1 ± 0.01 * | 138.0 ± 3.8 | 15.6 ± 2.5 | 209.2 ± 2.2 | 5.5 ± 0.1 * |
| NTR NAR + ISO | 59.8 ± 7.0 # | 0.2 ± 0.06 | 133.3 ± 5.1 | 15.4 ± 2.0 | 231.1 ± 7.2 | 6.0 ± 0.3 * |
| SHR VEH | 51.8 ± 3.9 | 0.3 ± 0.03 | 163.2 ± 6.0 | 17 ± 1.7 | 207.1 ± 1.9 | 5.8 ± 0.4 & |
| SHR VEH + ISO | 86.4 ± 3.4 * | 0.2 ± 0.03 | 173.0 ± 6.3 | 15.6 ± 1.6 | 205.2 ± 2.1 | 5.5 ± 0.2 |
| SHR NAR + ISO | 81.0 ± 2.3 * | 0.3 ± 0.06 | 192.0 ± 5.3 * | 24.1 ± 2.8 # | 211.1 ± 4.2 | 5.7 ± 0.1 |
| Groups | LOOH (μmol/g of Tissue) | GSH (μg/g of Tissue) | SOD (U/ mg Protein) | CAT (μmol/mg/min) | GST (μmol/mg Protein/min) |
|---|---|---|---|---|---|
| NTR VEH | 1.27 ± 0.10 | 868.5 ± 79.9 | 0.16 ± 0.02 | 0.09 ± 0.003 | 0.005 ± 0.003 |
| NTR VEH + ISO | 1.59 ± 0.14 | 794.1 ± 49.8 | 0.11 ± 0.006 | 1.03 ± 0.31 * | 0.018 ± 0.003 * |
| NTR NAR + ISO | 0.99 ± 0.01 # | 824.7 ± 24.3 | 0.13 ± 0.01 | 0.16 ± 0.03 # | 0.008 ± 0.001 # |
| SHR VEH | 1.53 ± 0.16 | 1416 ± 109.1 & | 0.17 ± 0.01 | 0.36 ± 0.04 & | 0.015 ± 0.003 |
| SHR VEH + ISO | 2.16 ± 0.18 * | 1299 ± 26.3 | 0.17 ± 0.008 | 0.20 ± 0.04 * | 0.005 ± 0.001 * |
| SHR NAR + ISO | 1.60 ± 0.08 # | 1312 ± 80.2 | 0.17 ± 0.009 | 0.21 ± 0.02 * | 0.004 ± 0.0008 * |
| Groups | MPO (mD.O/μg Protein) | NAG (mD.O/μg Protein) | Nitrite (μM) |
|---|---|---|---|
| NTR VEH | 3 ± 0.2 | 15.58 ± 1.38 | 0.48 ± 0.18 |
| NTR VEH + ISO | 3 ± 0.2 | 25.09 ± 1.65 * | 5.01 ± 1.54 * |
| NTR NAR + ISO | 4 ± 0.1 | 21.09 ± 0.82 * | 3.21 ± 0.87 |
| SHR VEH | 4 ± 0.2 | 36.59 ± 2.34 & | 5.47 ± 1.36 & |
| SHR VEH + ISO | 4 ± 0.2 | 41.68 ± 2.24 | 10.94 ± 2.04 * |
| SHR NAR + ISO | 4 ± 0.1 | 37.94 ± 3.17 | 6.32 ± 0.61 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dada, A.; da Silva, R.d.C.V.; Zanovello, M.; Moser, J.C.; Orengo, S.L.D.; Cavichiolo, M.O.; Bidinha, E.R.; Boeing, T.; Cechinel-Filho, V.; de Souza, P. Comparative Analysis of the Protective Effect of Naringenin on Cardiovascular Parameters of Normotensive and Hypertensive Rats Subjected to the Myocardial Infarction Model. Pharmaceuticals 2024, 17, 1324. https://doi.org/10.3390/ph17101324
Dada A, da Silva RdCV, Zanovello M, Moser JC, Orengo SLD, Cavichiolo MO, Bidinha ER, Boeing T, Cechinel-Filho V, de Souza P. Comparative Analysis of the Protective Effect of Naringenin on Cardiovascular Parameters of Normotensive and Hypertensive Rats Subjected to the Myocardial Infarction Model. Pharmaceuticals. 2024; 17(10):1324. https://doi.org/10.3390/ph17101324
Chicago/Turabian StyleDada, Anelize, Rita de Cássia Vilhena da Silva, Mariana Zanovello, Jeniffer C. Moser, Sabrina L. D. Orengo, Martina O. Cavichiolo, Eleine R. Bidinha, Thaise Boeing, Valdir Cechinel-Filho, and Priscila de Souza. 2024. "Comparative Analysis of the Protective Effect of Naringenin on Cardiovascular Parameters of Normotensive and Hypertensive Rats Subjected to the Myocardial Infarction Model" Pharmaceuticals 17, no. 10: 1324. https://doi.org/10.3390/ph17101324
APA StyleDada, A., da Silva, R. d. C. V., Zanovello, M., Moser, J. C., Orengo, S. L. D., Cavichiolo, M. O., Bidinha, E. R., Boeing, T., Cechinel-Filho, V., & de Souza, P. (2024). Comparative Analysis of the Protective Effect of Naringenin on Cardiovascular Parameters of Normotensive and Hypertensive Rats Subjected to the Myocardial Infarction Model. Pharmaceuticals, 17(10), 1324. https://doi.org/10.3390/ph17101324







