Ponatinib: A Review of the History of Medicinal Chemistry behind Its Development
Abstract
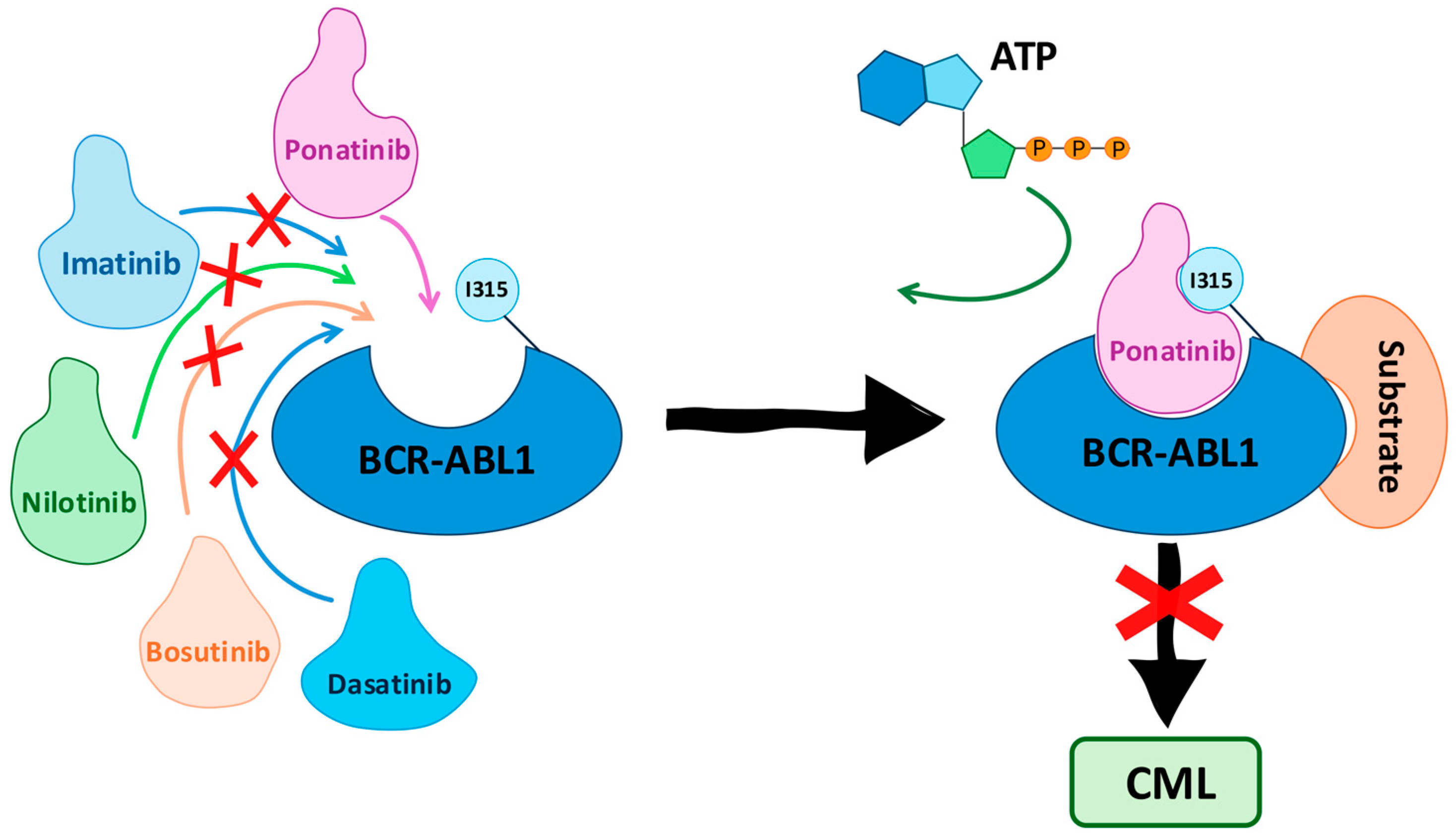
1. Introduction
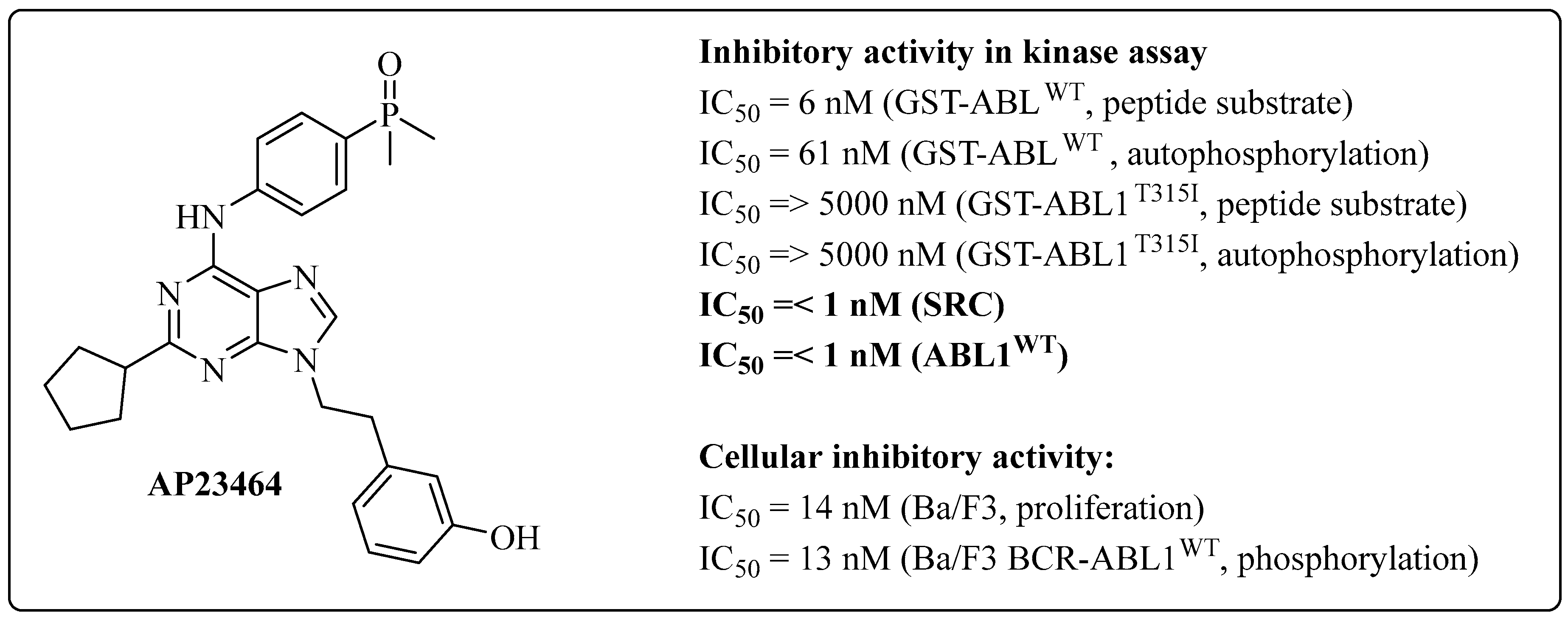
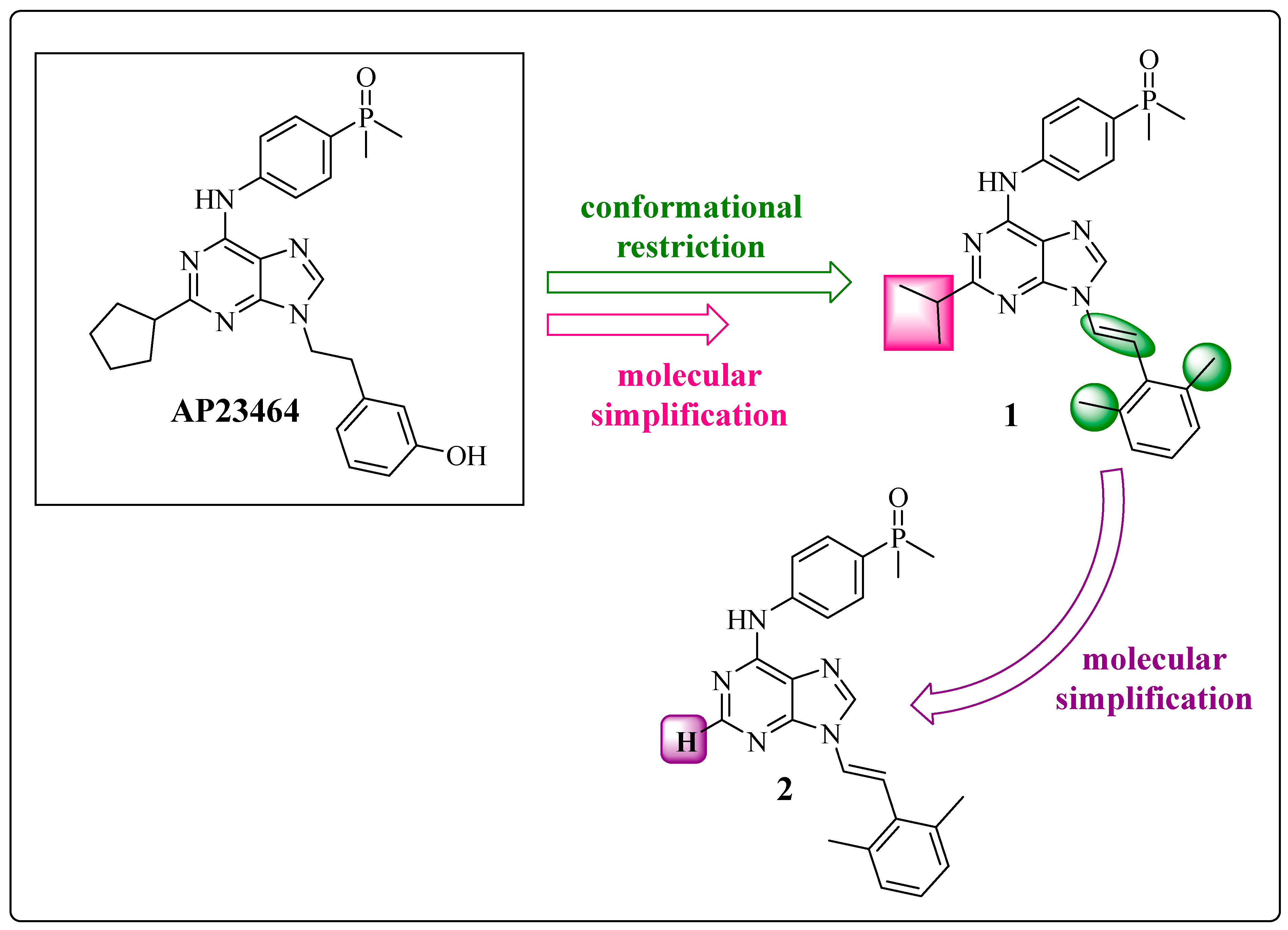
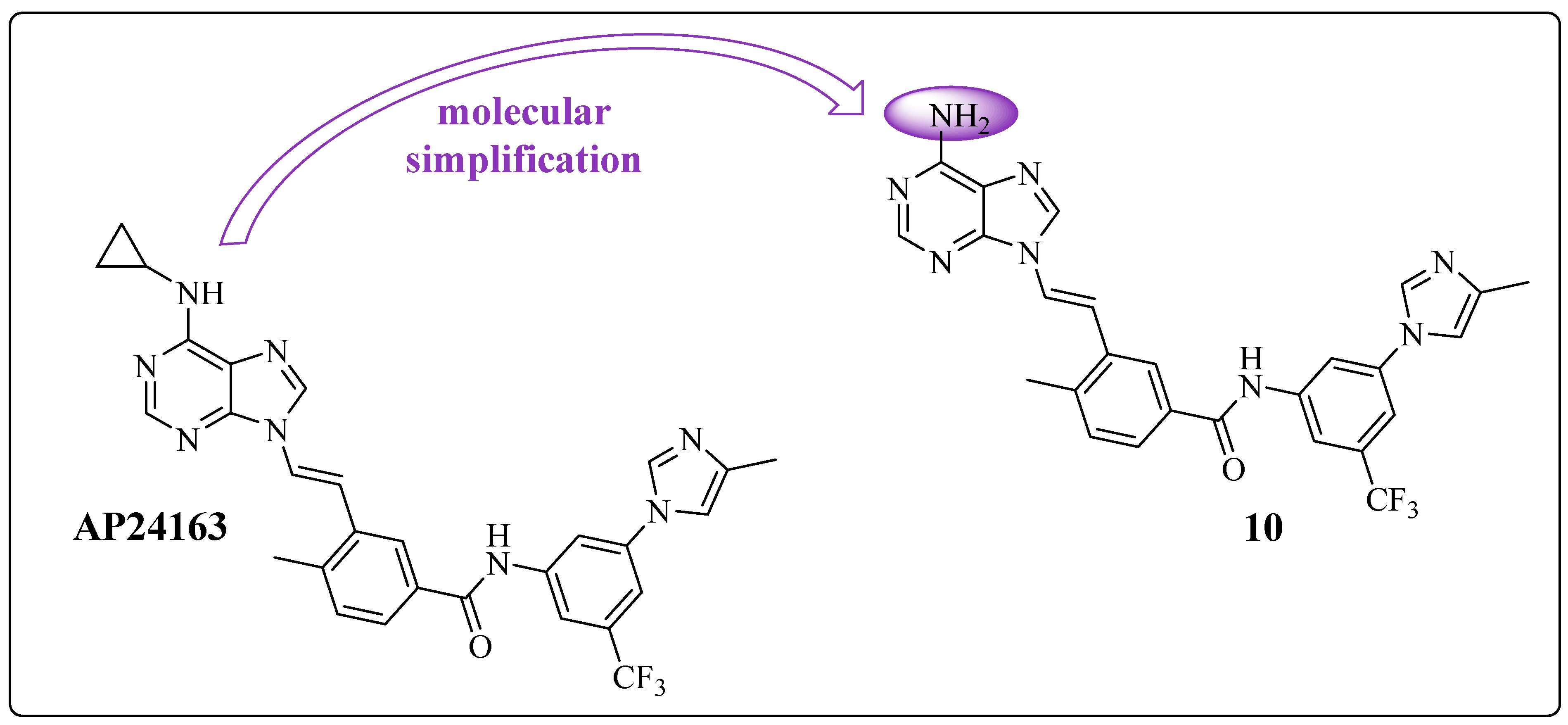
1.1. AP23464: Ponatinib Precursor
1.2. Conformational Restriction of the Spacer as a Medicinal Chemical Tool: Insertion of the Ethylene Group
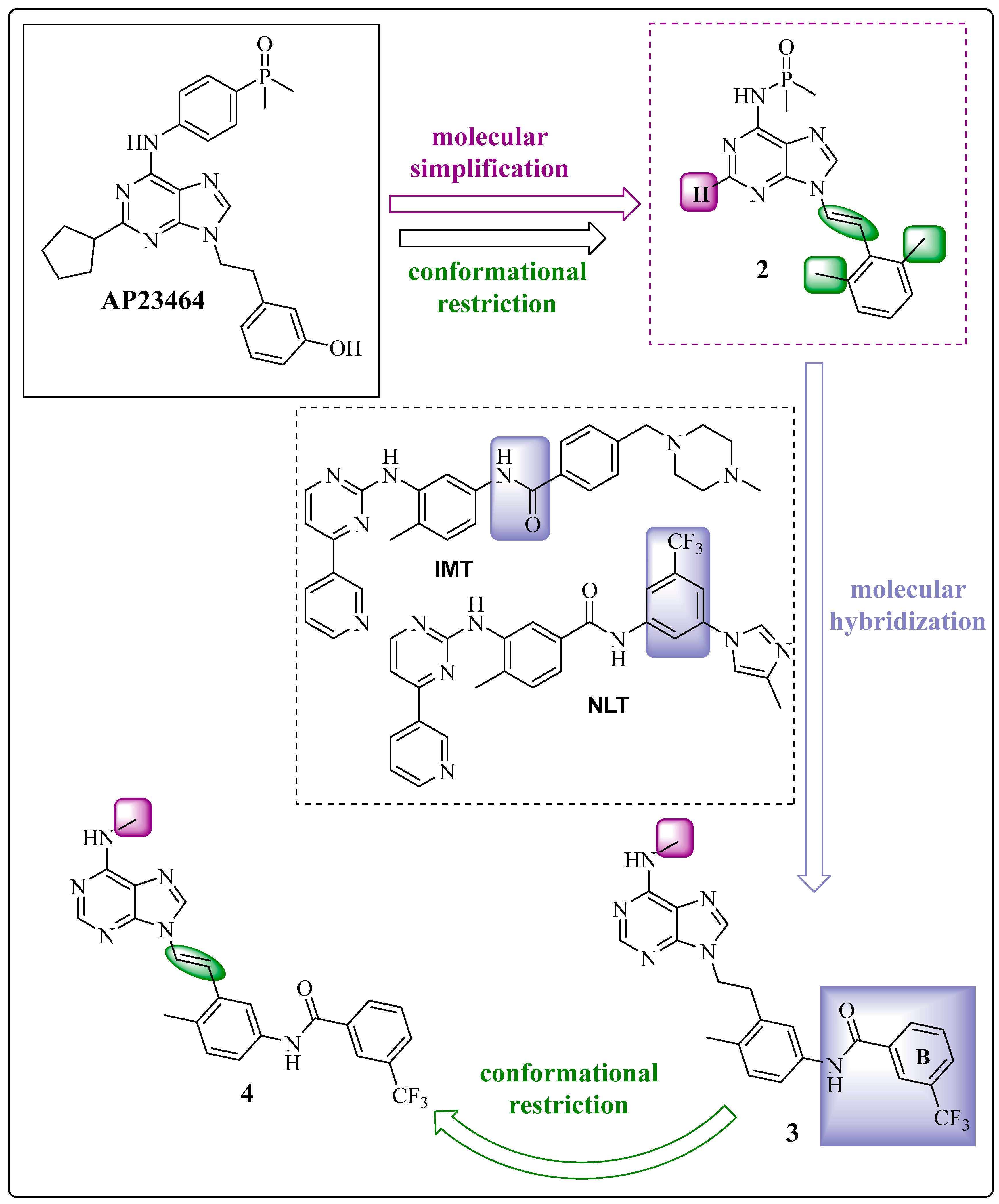
1.3. The Insertion of Arylamines: Targeting the Deep Hydrophobic Pocket of the Enzyme
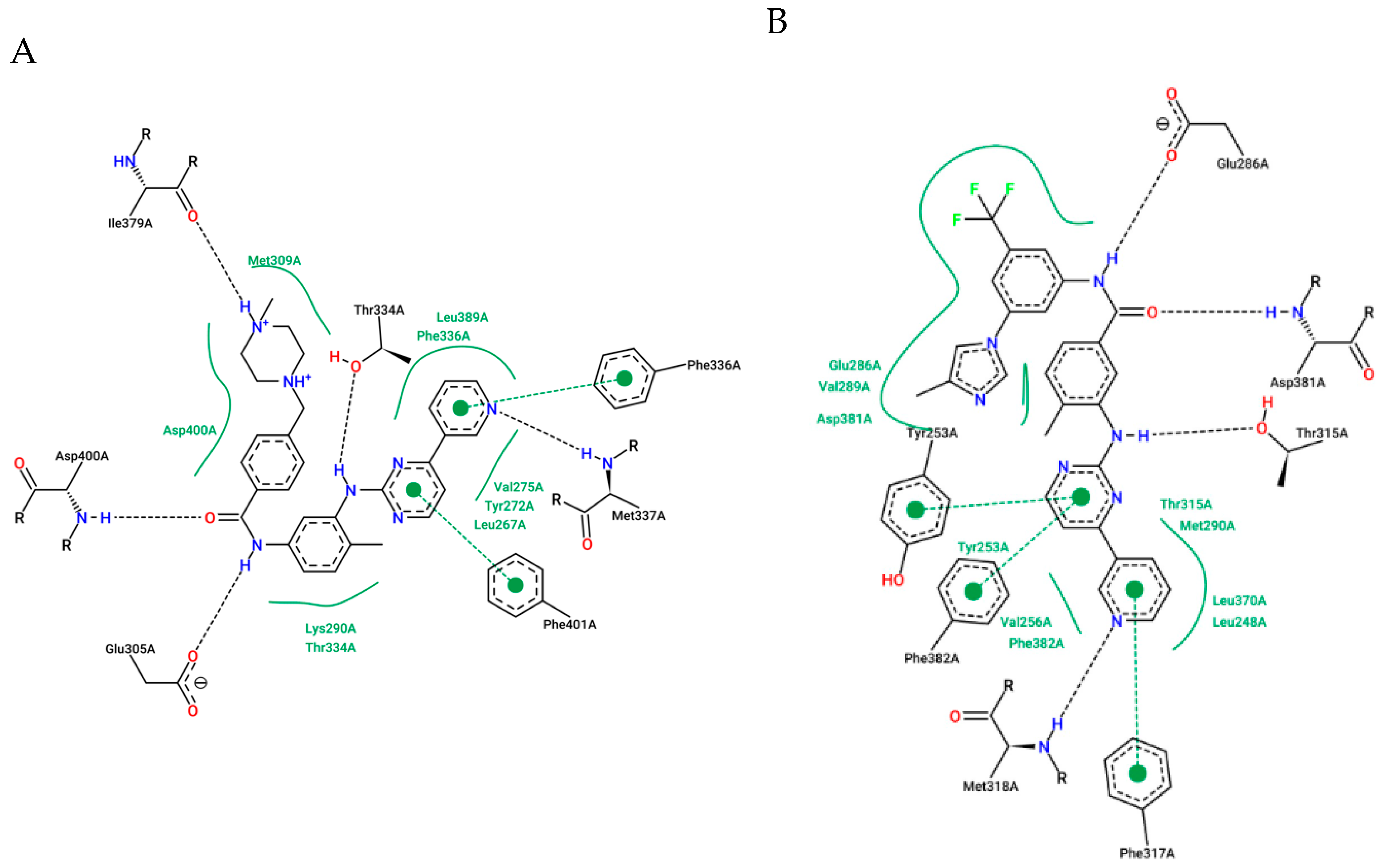
Learning from IMT and NLT: The Importance of the Position of the Amide Group
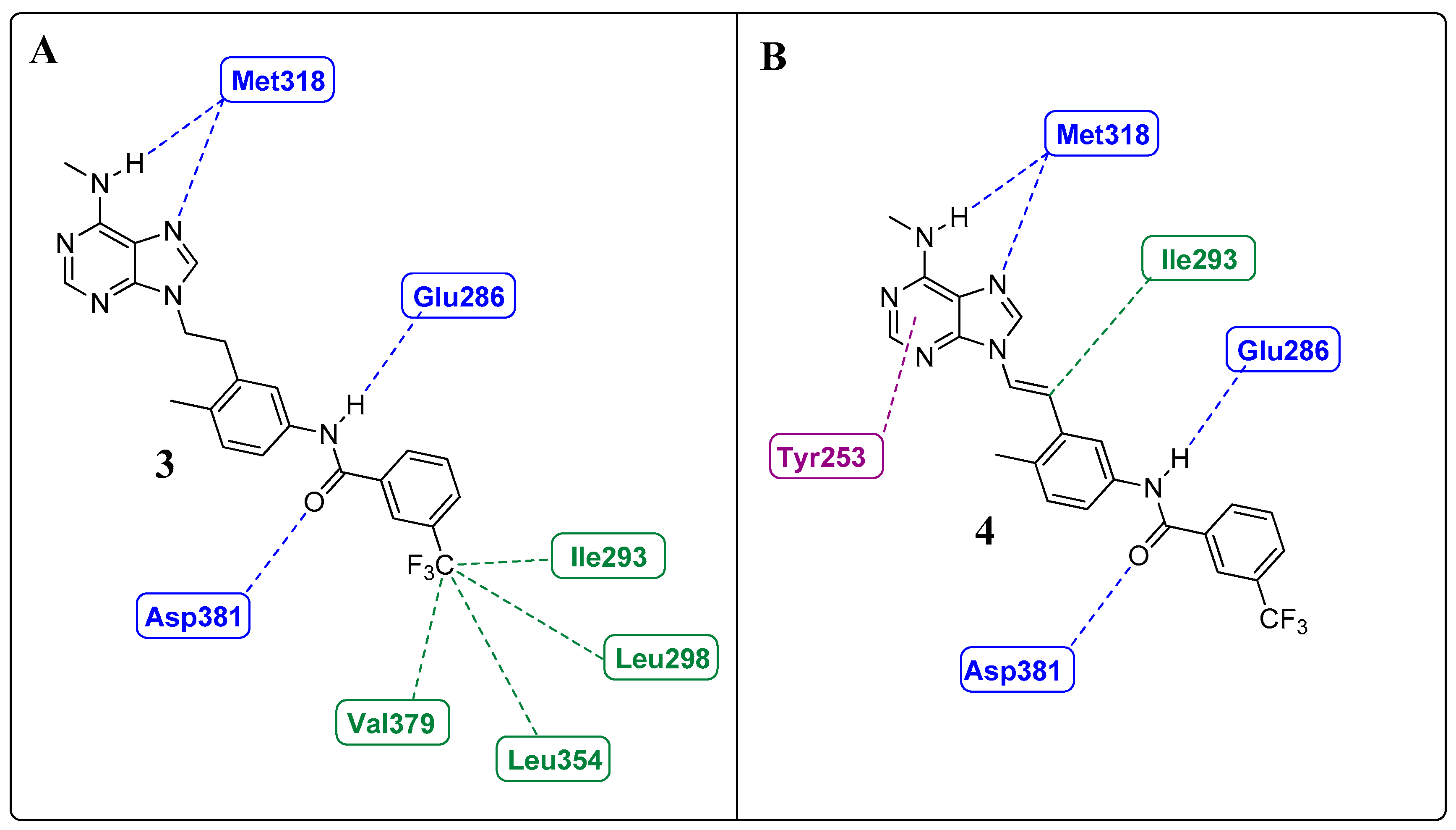
1.4. Focus on the Selective Hydrophobic Pocket in the Deep Region of the Kinase: The Terminal Aromatic Ring of Compounds
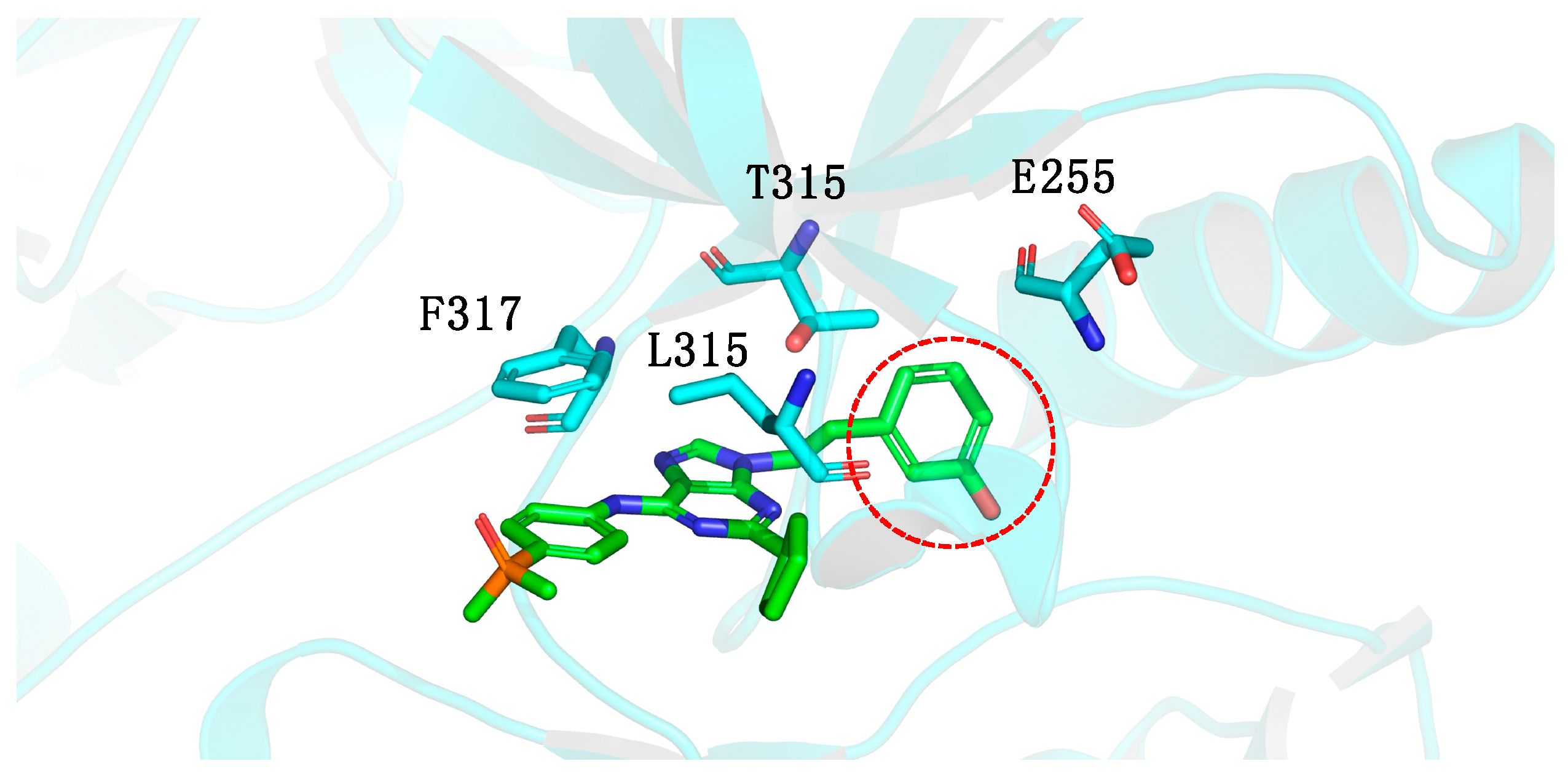
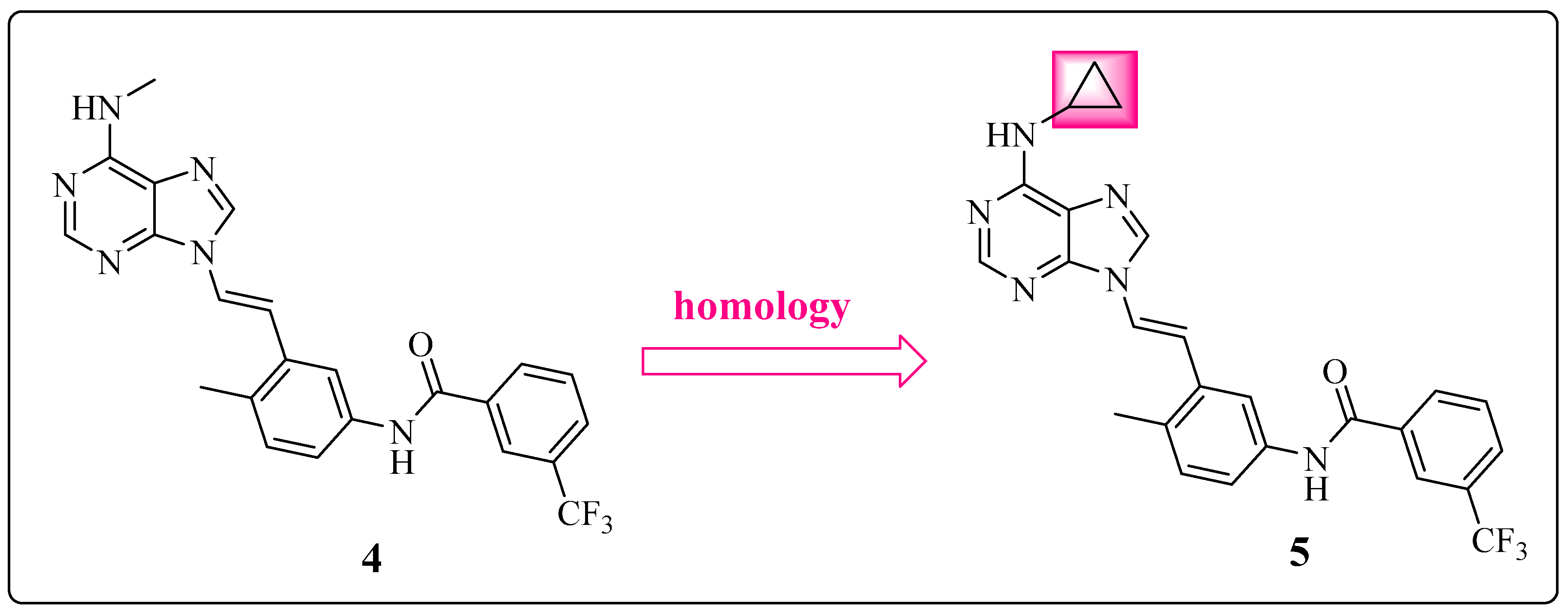
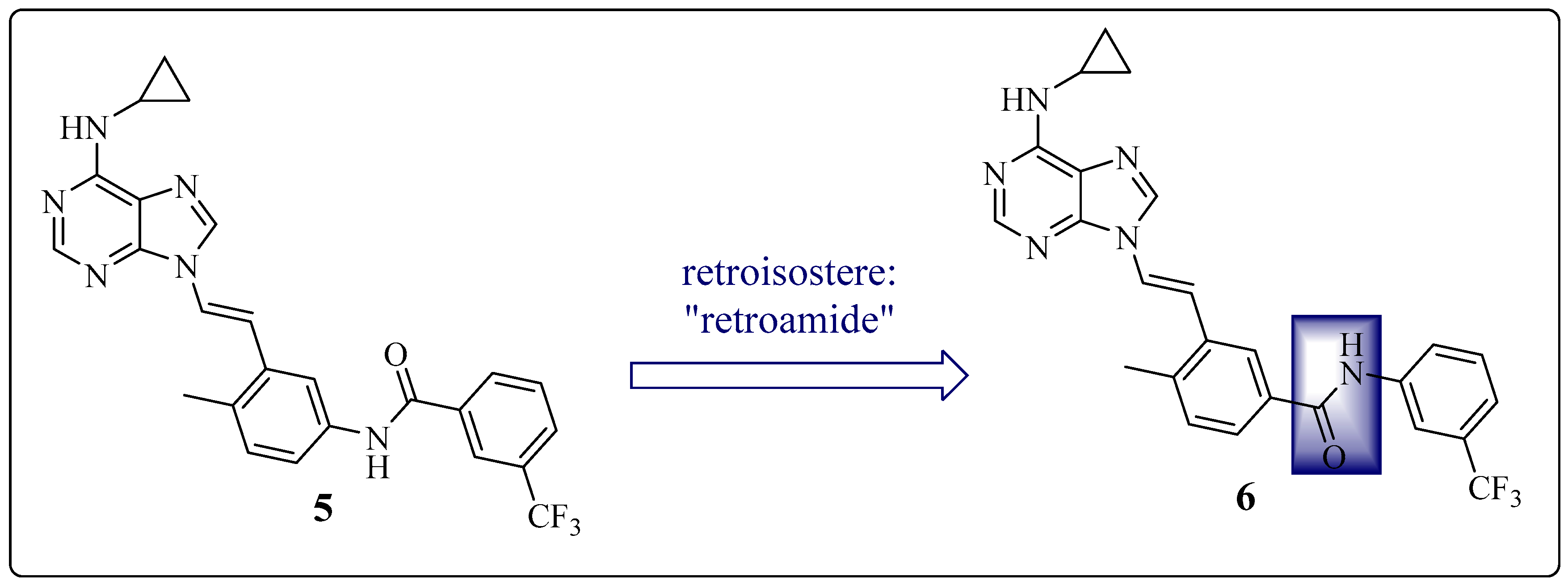
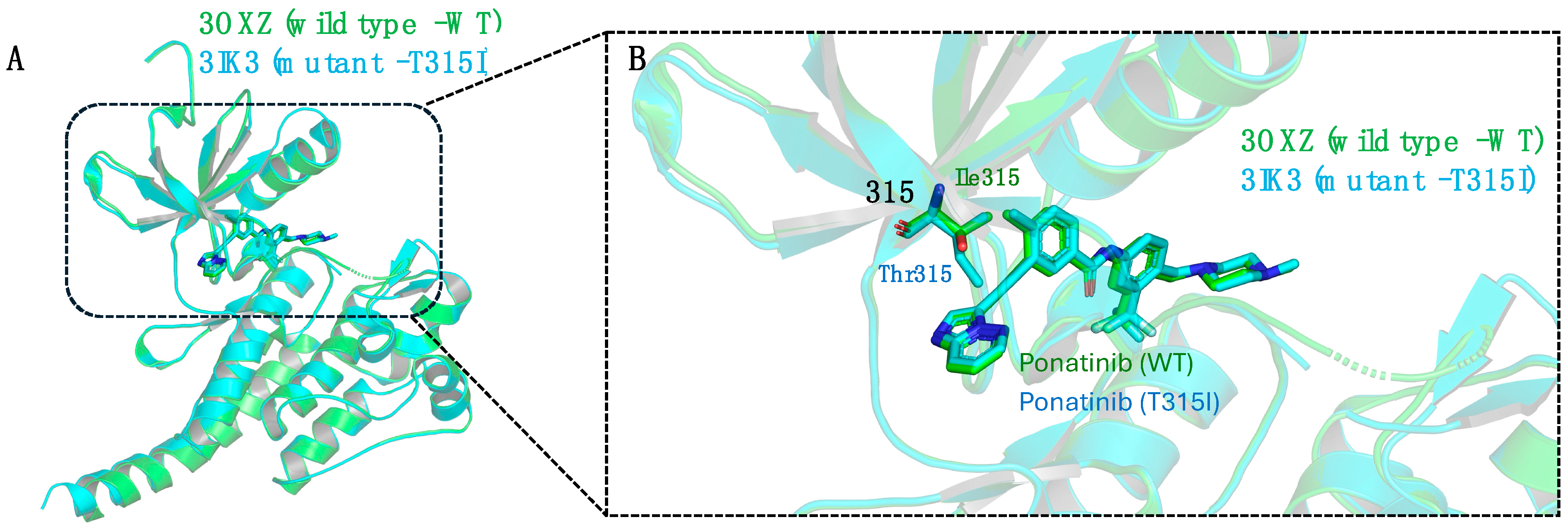
1.5. Large Structural Change in the T315I Mutation: The Introduction of Acetylene as a Spacer
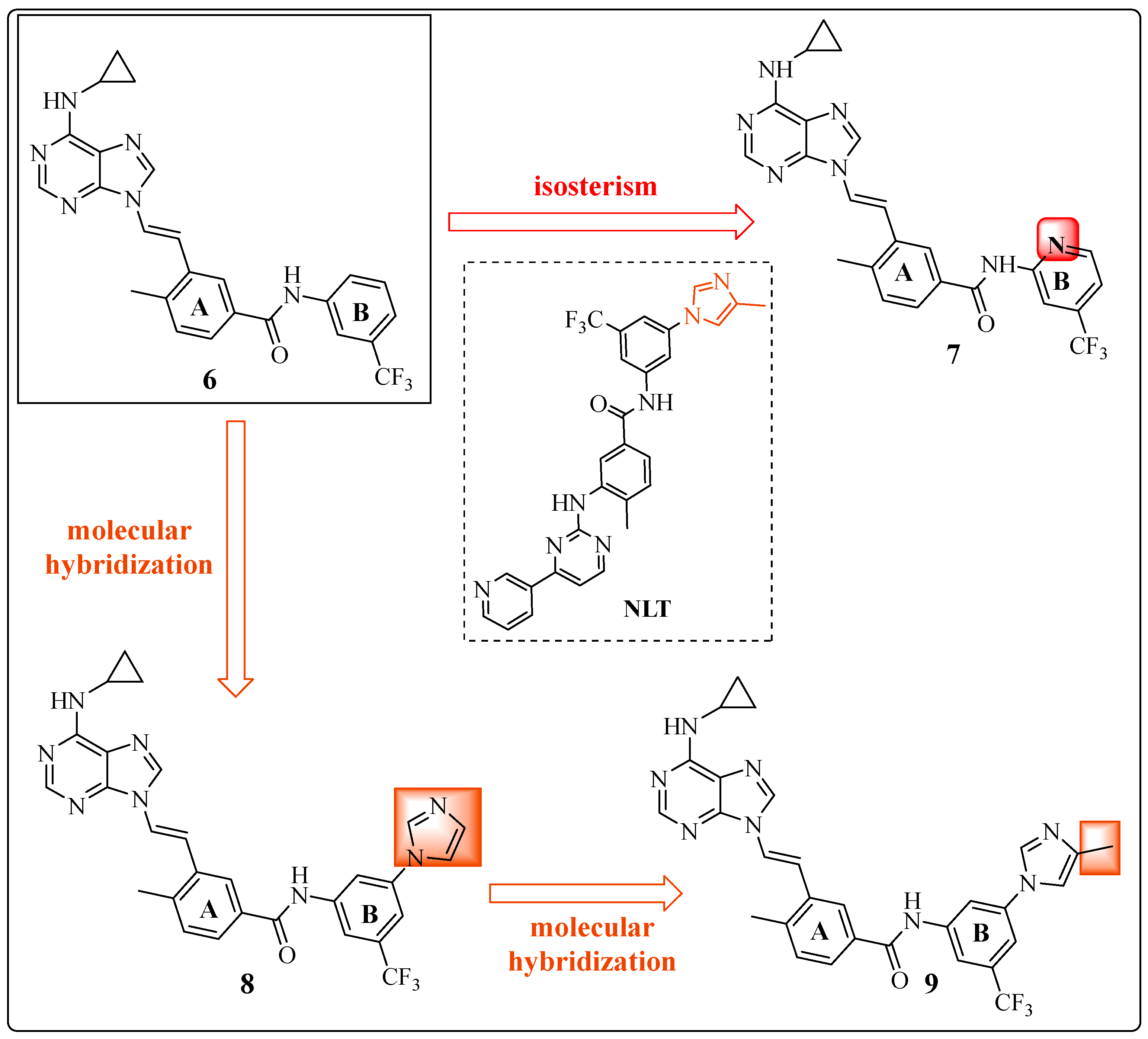
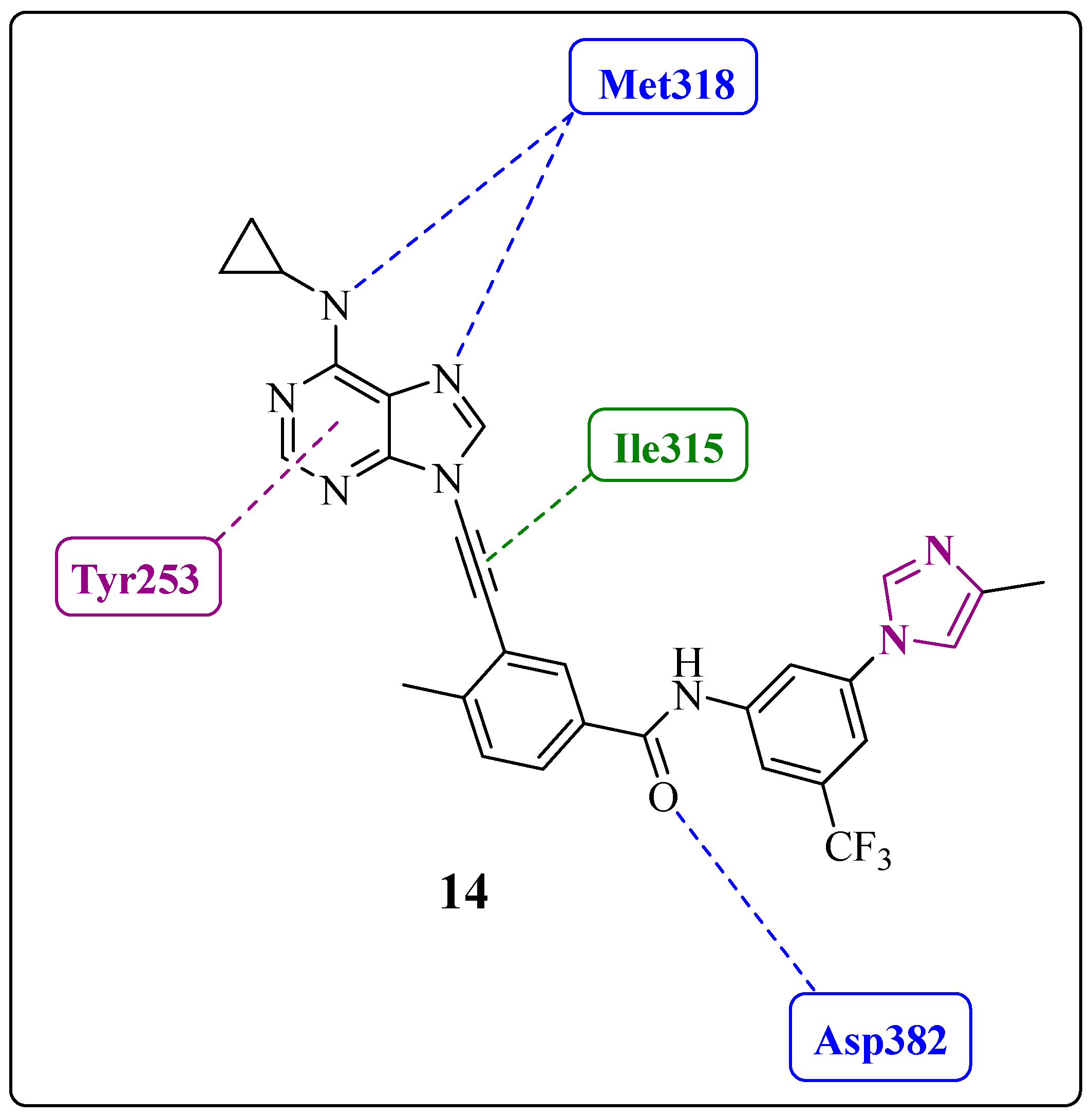
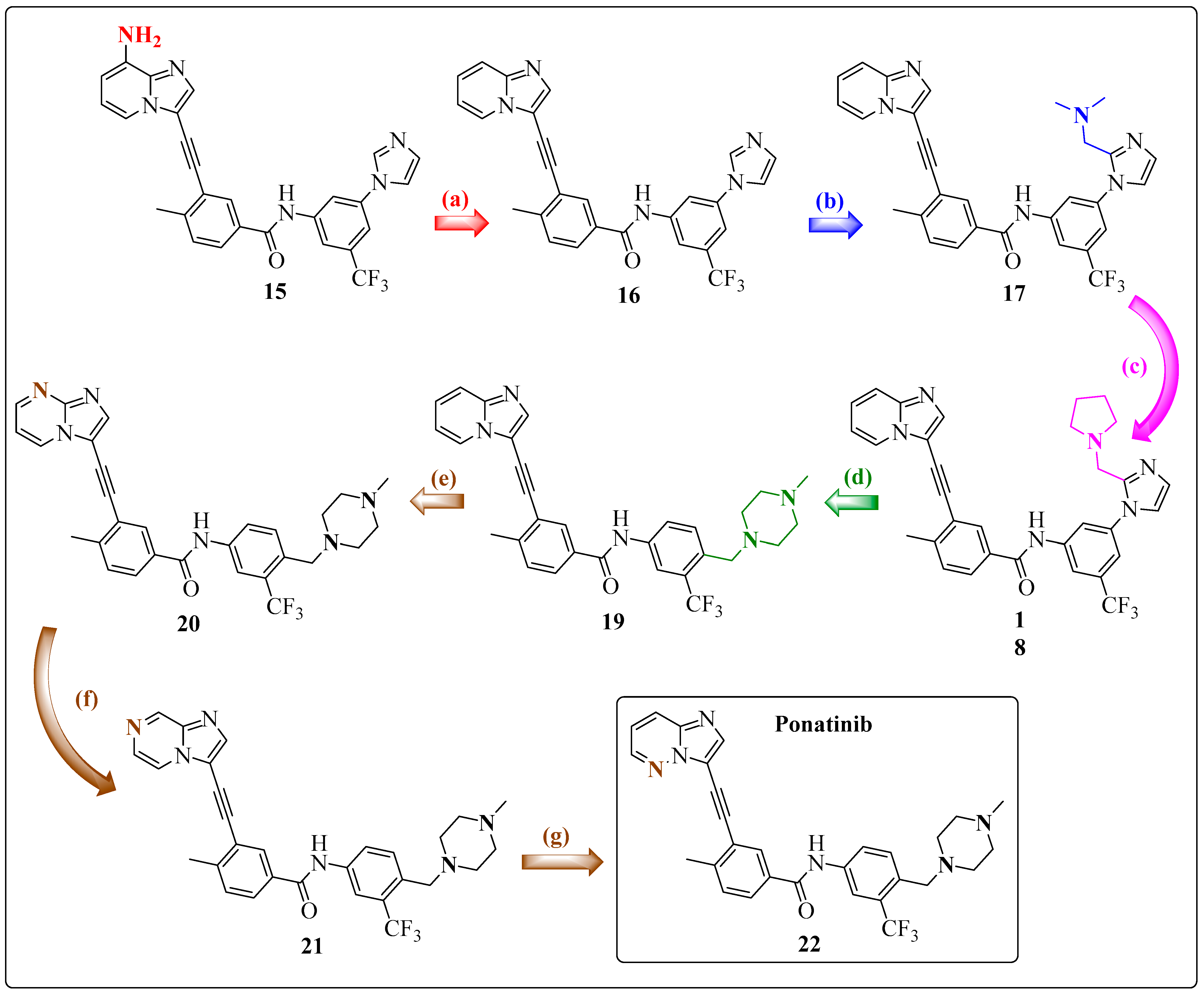
1.6. Final Adjustments: Focus on the Hinge Region and ADMET Properties
1.7. Ponatinib (PNT)
- ✓
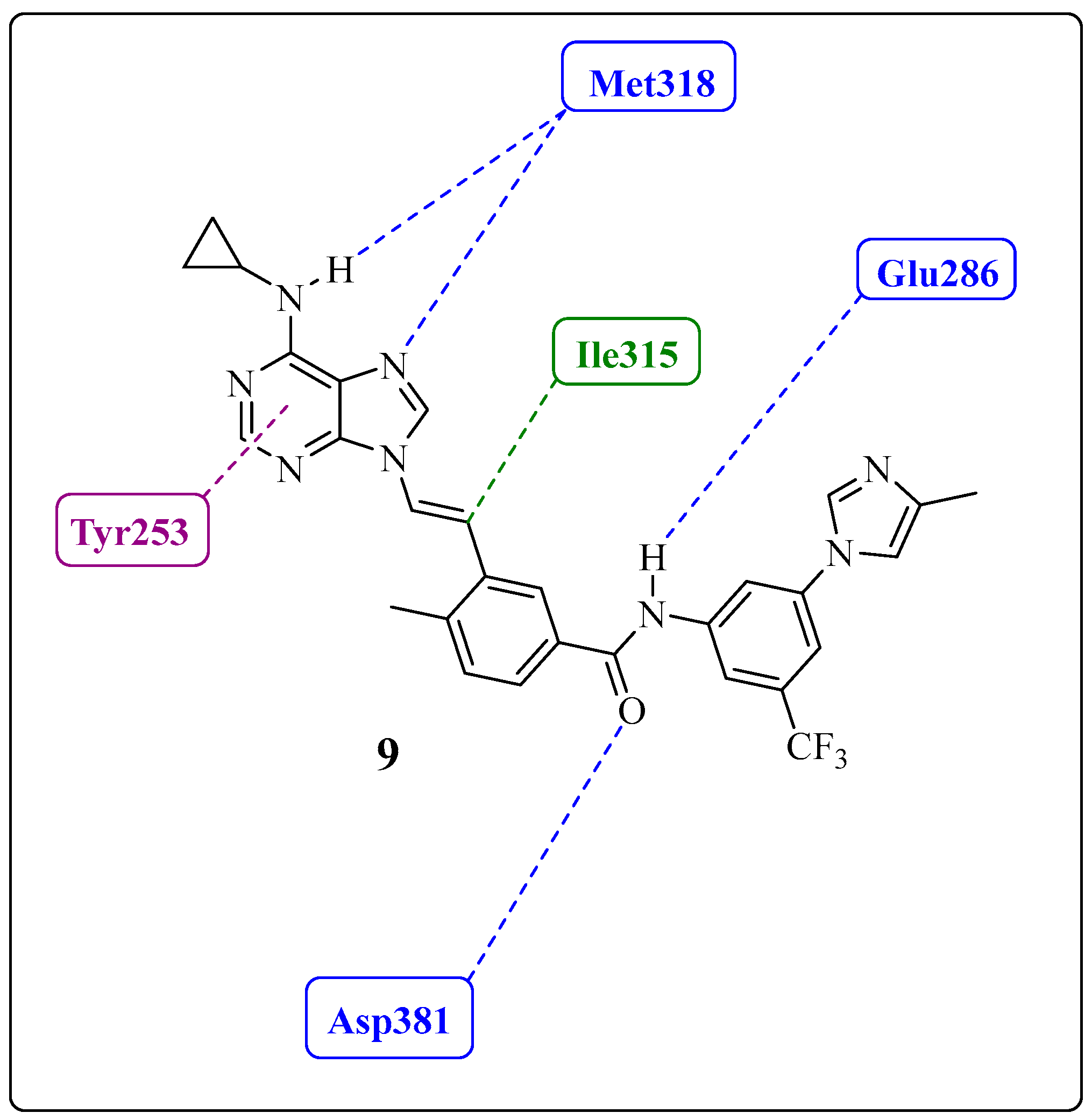
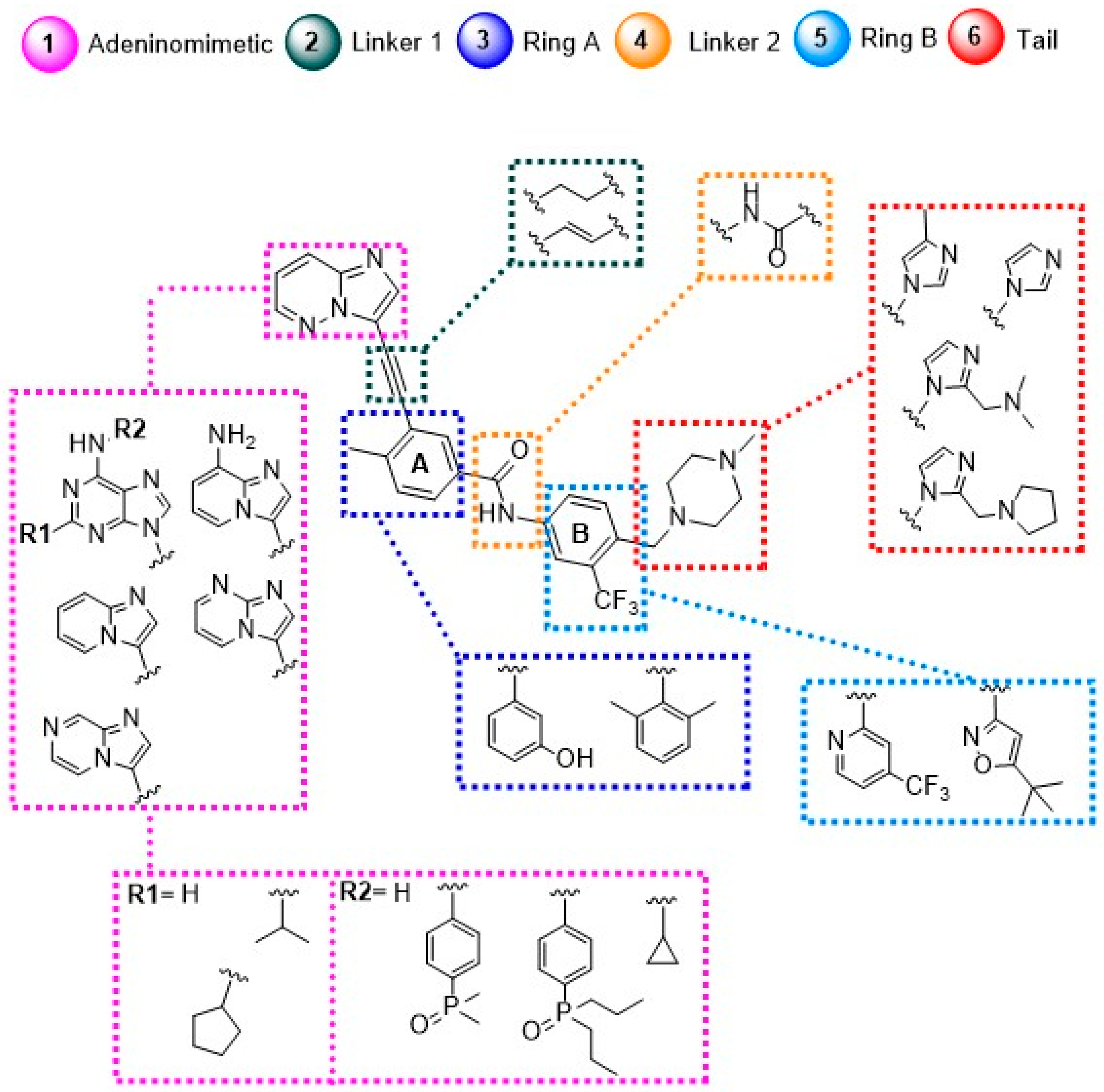
- Adeninomimetic-hinge core (imidazolo[1,2-b]pyridazine): To mimic the adenine nucleus of ATP, fused heteroaromatic (nitrogenated) rings, culminating in imidazolo[1,2-b]pyridazine, were explored. The imidazole fits into the hinge region through a hydrogen bond-like interaction with Met318, establishing van der Waals interactions with residues Met318 and Phe317. The pyridazine ring establishes hydrophobic interactions with specific amino acids, such as Phe382 in the DFG triad and Tyr253 in the P-loop region (Figure 19) [81].
- ✓
- Gatekeeper-linker 1 (ethynyl group): The SAR analysis of PNT revealed that the conformational restriction of the linker plays a crucial role in its ability to inhibit ABL1T315I. This may be due to steric relief to the gatekeeper residue (Ile315), allowing access to both the hinge region and the deeper region of the enzyme in the selective hydrophobic pocket provided by the inactive DFG-out conformation (as shown in Figure 17) [81].
- ✓
- Hydrophobic pocket-ring A (arene moiety): This ring occupies the hydrophobic cavity of the BCR-ABL1 enzyme located behind the gatekeeper residue (Figure 19). The presence of the methyl group is essential because it allows access to the deeper region of the enzyme through a conformational restriction in the IMT, NLT, and PNT inhibitors. Changing positions or removing them can reduce their biological activity [81].
- ✓
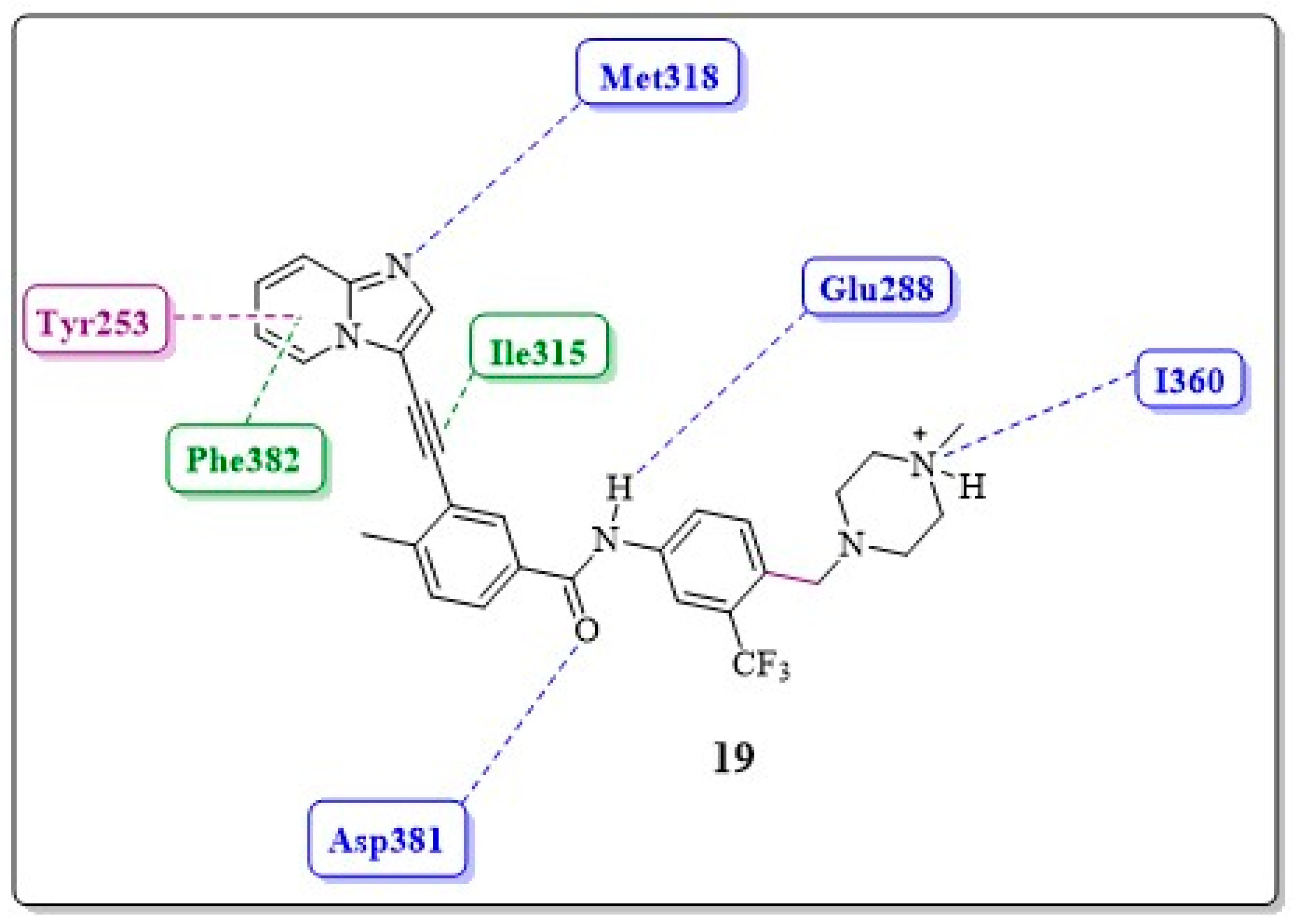
- DFG-linker 2 interaction region (amide group): This peptide bond between aromatic rings A and B results in crucial hydrogen bond-type interactions in the enzyme between the carbonyl oxygen of the amide and the Asp381 residue and the NH group with the Glu286 residue. These interactions anchor the inhibitor in the active site (Figure 19) [81].
- ✓
- Selective hydrophobic pocket-ring B (arene moiety): This pocket-ring makes hydrophobic contact with the biomacromolecule. The presence of the trifluoromethyl group allows the exploration of new hydrophobic contacts in the deep region of the enzyme [81].
- ✓
- Terminal residue or Tail (N-methylpiperazine moiety): Although the authors do not consider it one of the main fragments of PNT, crucial hydrogen bond-like interactions are established between the methylpiperazine group and His361 and Ile360 residues of the ABL1T315I kinase structure (Figure 19) [81].
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nowell, P.C.; Hungerford, D.A. A Minute Chromosome in Human Chronic Granulocytic Leukemia. Science 1960, 132, 1497–1501. [Google Scholar] [CrossRef]
- Groffen, J.; Stephenson, J.; Heisterkamp, N.; Deklein, A.; Bartram, C.; Grosveld, G. Philadelphia Chromosomal Breakpoints Are Clustered within a Limited Region, Bcr, on Chromosome 22. Cell 1984, 36, 93–99. [Google Scholar] [CrossRef]
- Lugo, T.G.; Pendergast, A.-M.; Muller, A.J.; Witte, O.N. Tyrosine Kinase Activity and Transformation Potency of Bcr-Abl Oncogene Products. Science 1990, 247, 1079–1082. [Google Scholar] [CrossRef]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y.S. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 2018, 17, 36. [Google Scholar] [CrossRef]
- Schindler, T.; Bornmann, W.; Pellicena, P.; Miller, W.T.; Clarkson, B.; Kuriyan, J. Structural Mechanism for STI-571 Inhibition of Abelson Tyrosine Kinase. Science 2000, 289, 1938–1942. [Google Scholar] [CrossRef]
- Cortes, J.; Lang, F. Third-Line Therapy for Chronic Myeloid Leukemia: Current Status and Future Directions. J. Hematol. Oncol. 2021, 14, 44. [Google Scholar] [CrossRef]
- Talati, C.; Pinilla-Ibarz, J. Resistance in Chronic Myeloid Leukemia: Definitions and Novel Therapeutic Agents. Curr. Opin. Hematol. 2018, 25, 154–161. [Google Scholar] [CrossRef]
- Donato, N.J.; Wu, J.Y.; Stapley, J.; Gallick, G.; Lin, H.; Arlinghaus, R.; Talpaz, M. BCR-ABL Independence and LYN Kinase Overexpression in Chronic Myelogenous Leukemia Cells Selected for Resistance to STI571. Blood 2003, 101, 690–698. [Google Scholar] [CrossRef]
- Talpaz, M. Imatinib Induces Durable Hematologic and Cytogenetic Responses in Patients with Accelerated Phase Chronic Myeloid Leukemia: Results of a Phase 2 Study. Blood 2002, 99, 1928–1937. [Google Scholar] [CrossRef]
- Gorre, M.E.; Mohammed, M.; Ellwood, K.; Hsu, N.; Paquette, R.; Rao, P.N.; Sawyers, C.L. Clinical Resistance to STI-571 Cancer Therapy Caused by BCR-ABL Gene Mutation or Amplification. Science 2001, 293, 876–880. [Google Scholar] [CrossRef]
- Balabanov, S.; Braig, M.; Brümmendorf, T.H. Current Aspects in Resistance against Tyrosine Kinase Inhibitors in Chronic Myelogenous Leukemia. Drug Discov. Today Technol. 2014, 11, 89–99. [Google Scholar] [CrossRef]
- Miller, G.D.; Bruno, B.J.; Lim, C.S. Resistant Mutations in CML and Ph+ALL-Role of Ponatinib. Biol. Targets Ther. 2014, 8, 243. [Google Scholar] [CrossRef]
- Pauli, F.P.; Barreiro, E.J.; Barbosa, M.L.C. Structural Characteristics of Protein Kinases and Their Inhibitors in Clinical Use. Rev. Virtual Quim. 2018, 10, 1280–1303. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, P.; Gupta, S.K.; Ali, V.; Verma, M. Transport and Metabolism of Tyrosine Kinase Inhibitors Associated with Chronic Myeloid Leukemia Therapy: A Review. Mol. Cell Biochem. 2022, 477, 1261–1279. [Google Scholar] [CrossRef]
- Cheng, S.; Jin, P.; Li, H.; Pei, D.; Shu, X. Evaluation of CML TKI Induced Cardiovascular Toxicity and Development of Potential Rescue Strategies in a Zebrafish Model. Front. Pharmacol. 2021, 12, 740529. [Google Scholar] [CrossRef]
- Hughes, T.; Saglio, G.; Branford, S.; Soverini, S.; Kim, D.-W.; Müller, M.C.; Martinelli, G.; Cortes, J.; Beppu, L.; Gottardi, E.; et al. Impact of Baseline BCR-ABL Mutations on Response to Nilotinib in Patients with Chronic Myeloid Leukemia in Chronic Phase. J. Clin. Oncol. 2009, 27, 4204–4210. [Google Scholar] [CrossRef]
- Huang, W.-S.; Metcalf, C.A.; Sundaramoorthi, R.; Wang, Y.; Zou, D.; Thomas, R.M.; Zhu, X.; Cai, L.; Wen, D.; Liu, S.; et al. Discovery of 3-[2-(Imidazo [1,2-b]Pyridazin-3-Yl)Ethynyl]-4-Methyl-N-{4-[(4-Methylpiperazin-1-Yl)Methyl]-3-(Trifluoromethyl)Phenyl}benzamide (AP24534), a Potent, Orally Active Pan-Inhibitor of Breakpoint Cluster Region-Abelson (BCR-ABL) Kinase Including the T315I Gatekeeper Mutant. J. Med. Chem. 2010, 53, 4701–4719. [Google Scholar] [CrossRef]
- Shacham-Abulafia, A.; Raanani, P.; Lavie, D.; Volchek, Y.; Ram, R.; Helman, I.; Shargian, L.; Gourevitch, A.; Chubar, E.; Ratzon, R.; et al. Real-Life Experience with Ponatinib in Chronic Myeloid Leukemia: A Multicenter Observational Study. Cl. Lymph. Myelom. Leuk. 2018, 18, e295–e301. [Google Scholar] [CrossRef]
- Qian, H.; Gang, D.; He, X.; Jiang, S. A Review of the Therapeutic Role of the New Third-Generation TKI Olverembatinib in Chronic Myeloid Leukemia. Front. Oncol. 2022, 12, 1036437. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Chifotides, H.T.; Haddad, F.G.; Short, N.J.; Loghavi, S.; Jabbour, E. Ponatinib-review of historical development, current status, and future research. Am. J. Hematol. 2024, 99, 1576–1585. [Google Scholar] [CrossRef]
- Lussana, F.; Intermesoli, T.; Stefanoni, P.; Rambaldi, A. Mechanisms of resistance to targeted therapies in chronic myeloid leukemia. Mech. Drug Resist. Cancer Ther. 2018, 249, 231–250. [Google Scholar] [CrossRef]
- Tan, F.H.; Putoczki, T.L.; Stylli, S.S.; Luwor, R.B. Ponatinib: A Novel Multi-Tyrosine Kinase Inhibitor against Human Malignancies. Oncotargets Ther. 2019, 12, 635–645. [Google Scholar] [CrossRef]
- Huang, W.-S.; Zhu, X.; Wang, Y.; Azam, M.; Wen, D.; Sundaramoorthi, R.; Thomas, R.M.; Liu, S.; Banda, G.; Lentini, S.P.; et al. 9-(Arenethenyl)Purines as Dual Src/Abl Kinase Inhibitors Targeting the Inactive Conformation: Design, Synthesis, and Biological Evaluation. J. Med. Chem. 2009, 52, 4743–4756. [Google Scholar] [CrossRef]
- Mahon, F.-X.; Hayette, S.; Lagarde, V.; Belloc, F.; Turcq, B.; Nicolini, F.; Belanger, C.; Manley, P.W.; Leroy, C.; Etienne, G.; et al. Evidence That Resistance to Nilotinib May Be Due to BCR-ABL, Pgp, or Src Kinase Overexpression. Cancer Res. 2008, 68, 9809–9816. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Giles, F.; Quintás-Cardama, A.; Cortes, J. Important Therapeutic Targets in Chronic Myelogenous Leukemia. Clin. Cancer Res. 2007, 13, 1089–1097. [Google Scholar] [CrossRef]
- Schenone, S.; Manetti, F.; Botta, M. Last Findings on Dual Inhibitors of Abl and Src Tyrosine-Kinases. Mini-Rev. Med. Chem. 2007, 7, 191–201. [Google Scholar] [CrossRef]
- Martinelli, G.; Soverini, S.; Rosti, G.; Baccarani, M. Dual Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia. Leukemia 2005, 19, 1872–1879. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Pelletier, S.; Buchdunger, E.; Warmuth, M.; Fabbro, D.; Hallek, M.; Van Etten, R.A.; Li, S. Requirement of Src Kinases Lyn, Hck and Fgr for BCR-ABL1-Induced B-Lymphoblastic Leukemia but Not Chronic Myeloid Leukemia. Nat. Genet. 2004, 36, 453–461. [Google Scholar] [CrossRef]
- Lombardo, L.J.; Lee, F.Y.; Chen, P.; Norris, D.; Barrish, J.C.; Behnia, K.; Castaneda, S.; Cornelius, L.A.M.; Das, J.; Doweyko, A.M.; et al. Discovery of N-(2-Chloro-6-Methyl-Phenyl)-2-(6-(4-(2-Hydroxyethyl)-Piperazin-1-Yl)-2-Methylpyrimidin-4-Ylamino)Thiazole-5-Carboxamide (BMS-354825), a Dual Src/Abl Kinase Inhibitor with Potent Antitumor Activity in Preclinical Assays. J. Med. Chem. 2004, 47, 6658–6661. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kim, D.-W.; Pinilla-Ibarz, J.; Le Coutre, P.D.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. Ponatinib Efficacy and Safety in Philadelphia Chromosome–Positive Leukemia: Final 5-Year Results of the Phase 2 PACE Trial. Blood 2018, 132, 393–404. [Google Scholar] [CrossRef]
- Hnatiuk, A.P.; Bruyneel, A.A.N.; Tailor, D.; Pandrala, M.; Dheeraj, A.; Li, W.; Serrano, R.; Feyen, D.A.M.; Vu, M.M.; Amatya, P.; et al. Reengineering Ponatinib to Minimize Cardiovascular Toxicity. Cancer Res. 2022, 82, 2777–2791. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia: 2022 Update on Diagnosis, Therapy, and Monitoring. Am. J. Hematol. 2022, 97, 1236–1256. [Google Scholar] [CrossRef]
- Singh, A.P.; Umbarkar, P.; Tousif, S.; Lal, H. Cardiotoxicity of the BCR-ABL1 Tyrosine Kinase Inhibitors: Emphasis on Ponatinib. Int. J. Cardiol. 2020, 316, 214–221. [Google Scholar] [CrossRef]
- Kort, A.; Van Hoppe, S.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Brain Accumulation of Ponatinib and Its Active Metabolite, N-Desmethyl Ponatinib, Is Limited by P-Glycoprotein (P-GP/ABCB1) and Breast Cancer Resistance Protein (BCRP/ABCG2). Mol. Pharmaceut. 2017, 14, 3258–3268. [Google Scholar] [CrossRef]
- Douxfils, J.; Haguet, H.; Mullier, F.; Chatelain, C.; Graux, C.; Dogné, J.-M. Association Between BCR-ABL Tyrosine Kinase Inhibitors for Chronic Myeloid Leukemia and Cardiovascular Events, Major Molecular Response, and Overall Survival: A Systematic Review and Meta-Analysis. JAMA Oncol. 2016, 2, 625. [Google Scholar] [CrossRef]
- Iclusig (Ponatinib) [Prescribing Information]. Available online: https://www.iclusig.com/sites/default/files/2023-02/iclusig-prescribing-information.pdf (accessed on 28 June 2024).
- Gao, Y.; Ding, Y.; Tai, X.-R.; Zhang, C.; Wang, D. Ponatinib: An update on its drug targets, therapeutic potential and safety. BBA–Rev. Cancer 2023, 1878, 188949. [Google Scholar] [CrossRef]
- Mercy, M.S. Targeted Therapies: A Life Saving Approach for Cancer. World J. Pharm. Pharm. Sci. 2016, 5, 612–626. [Google Scholar]
- Li, J.; Gong, C.; Zhou, H.; Liu, J.; Xia, X.; Ha, W.; Jiang, Y.; Liu, Q.; Xiong, H. Kinase Inhibitors and Kinase-Targeted Cancer Therapies: Recent Advances and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 5489. [Google Scholar] [CrossRef]
- Shah, N.H.; Amacher, J.F.; Nocka, L.M.; Kuriyan, J. The Src Module: An Ancient Scaffold in the Evolution of Cytoplasmic Tyrosine Kinases. Crit. Rev. Biochem. Mol. 2018, 53, 535–563. [Google Scholar] [CrossRef]
- Konig, H.; Copland, M.; Chu, S.; Jove, R.; Holyoake, T.L.; Bhatia, R. Effects of Dasatinib on Src Kinase Activity and Downstream Intracellular Signaling in Primitive Chronic Myelogenous Leukemia Hematopoietic Cells. Cancer Res. 2008, 68, 9624–9633. [Google Scholar] [CrossRef]
- Azam, M.; Nardi, V.; Shakespeare, W.C.; Metcalf, C.A.; Bohacek, R.S.; Wang, Y.; Sundaramoorthi, R.; Sliz, P.; Veach, D.R.; Bornmann, W.G.; et al. Activity of Dual SRC-ABL Inhibitors Highlights the Role of BCR/ABL Kinase Dynamics in Drug Resistance. Proc. Natl. Acad. Sci. USA 2006, 103, 9244–9249. [Google Scholar] [CrossRef]
- Schenone, S.; Brullo, C.; Musumeci, F.; Botta, M. Novel Dual Src/Abl Inhibitors for Hematologic and Solid Malignancies. Expert Opin. Inv. Drug 2010, 19, 931–945. [Google Scholar] [CrossRef]
- Noble, M.E.M.; Endicott, J.A.; Johnson, L.N. Protein Kinase Inhibitors: Insights into Drug Design from Structure. Science 2004, 303, 1800–1805. [Google Scholar] [CrossRef]
- Rickles, R.J.; Botfield, M.C.; Weng, Z.; Taylor, J.A.; Green, O.M.; Brugge, J.S.; Zoller, M.J. Identification of Src, Fyn, Lyn, PI3K and Abl SH3 Domain Ligands Using Phage Display Libraries. EMBO J. 1994, 13, 5598–5604. [Google Scholar] [CrossRef]
- Weng, Z.; Rickles, R.J.; Feng, S.; Richard, S.; Shaw, A.S.; Schreiber, S.L.; Brugge, J.S. Structure-Function Analysis of SH3 Domains: SH3 Binding Specificity Altered by Single Amino Acid Substitutions. Mol. Cel. Biol. 1995, 15, 5627–5634. [Google Scholar] [CrossRef]
- O’Hare, T.; Pollock, R.; Stoffregen, E.P.; Keats, J.A.; Abdullah, O.M.; Moseson, E.M.; Rivera, V.M.; Tang, H.; Metcalf, C.A.; Bohacek, R.S.; et al. Inhibition of Wild-Type and Mutant Bcr-Abl by AP23464, a Potent ATP-Based Oncogenic Protein Kinase Inhibitor: Implications for CML. Blood 2004, 104, 2532–2539. [Google Scholar] [CrossRef]
- Corbin, A.S.; Demehri, S.; Griswold, I.J.; Wang, Y.; Metcalf, C.A.; Sundaramoorthi, R.; Shakespeare, W.C.; Snodgrass, J.; Wardwell, S.; Dalgarno, D.; et al. In Vitro and in Vivo Activity of ATP-Based Kinase Inhibitors AP23464 and AP23848 against Activation-Loop Mutants of Kit. Blood 2005, 106, 227–234. [Google Scholar] [CrossRef]
- Wang, Y.; Shakespeare, W.C.; Huang, W.-S.; Sundaramoorthi, R.; Lentini, S.; Das, S.; Liu, S.; Banda, G.; Wen, D.; Zhu, X.; et al. Novel N9-Arenethenyl Purines as Potent Dual Src/Abl Tyrosine Kinase Inhibitors. Bioorg. Med. Chem. Let. 2008, 18, 4907–4912. [Google Scholar] [CrossRef]
- Zhou, T.; Commodore, L.; Huang, W.; Wang, Y.; Sawyer, T.K.; Shakespeare, W.C.; Clackson, T.; Zhu, X.; Dalgarno, D.C. Structural Analysis of DFG-in and DFG-out Dual Src-Abl Inhibitors Sharing a Common Vinyl Purine Template. Chem. Biol. Drug Des. 2010, 75, 18–28. [Google Scholar] [CrossRef]
- Braun, T.P.; Eide, C.A.; Druker, B.J. Response and Resistance to BCR-ABL1-Targeted Therapies. Cancer Cell 2020, 37, 530–542. [Google Scholar] [CrossRef]
- Corbin, A.S.; Buchdunger, E.; Pascal, F.; Druker, B.J. Analysis of the Structural Basis of Specificity of Inhibition of the Abl Kinase by STI571. J. Biol. Chem. 2002, 277, 32214–32219. [Google Scholar] [CrossRef]
- Dalgarno, D.; Stehle, T.; Narula, S.; Schelling, P.; Rose Van Schravendijk, M.; Adams, S.; Andrade, L.; Keats, J.; Ram, M.; Jin, L.; et al. Structural Basis of Src Tyrosine Kinase Inhibition with a New Class of Potent and Selective Trisubstituted Purine-based Compounds. Chem. Biol. Drug Des. 2006, 67, 46–57. [Google Scholar] [CrossRef]
- Lima, L.M.; Da Silva, T.F.; Da Silva Monteiro, C.E.; Aparecida-Silva, C.; Bispo Júnior, W.; De Queiroz, A.C.; Alexandre-Moreira, M.S.; Zapata-Sudo, G.; Barreiro, E.J. Design and Synthesis in Silico Drug-like Prediction and Pharmacological Evaluation of Cyclopolymethylenic Homologous of LASSBio-1514. Molecules 2021, 26, 4828. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. Available online: http://www.swissadme.ch/index.php (accessed on 12 February 2024). [CrossRef]
- Noronha, G.; Cao, J.; Zeng, B.; Mak, C.; McPherson, A.; Renick, J.; Pathak, V.P.; Chow, C.; Palanki, M.; Soll, R.M.; et al. Thiazole Inhibitors Targeting Resistant Kinase Mutations. U.S. Patent 11/591,252, 12 July 2007. [Google Scholar]
- Getlik, M.; Grütter, C.; Simard, J.R.; Klüter, S.; Rabiller, M.; Rode, H.B.; Robubi, A.; Rauh, D. Hybrid Compound Design to Overcome the Gatekeeper T338M Mutation in cSrc. J. Med. Chem. 2009, 52, 3915–3926. [Google Scholar] [CrossRef]
- Tokarski, J.S.; Newitt, J.A.; Chang, C.Y.J.; Cheng, J.D.; Wittekind, M.; Kiefer, S.E.; Kish, K.; Lee, F.Y.F.; Borzillerri, R.; Lombardo, L.J.; et al. The Structure of Dasatinib (BMS-354825) Bound to Activated ABL Kinase Domain Elucidates Its Inhibitory Activity against Imatinib-Resistant ABL Mutants. Cancer Res. 2006, 66, 5790–5797. [Google Scholar] [CrossRef]
- Barreiro, E.J.; Fraga, C.A.M. Química Medicinal: As Bases Moleculares da Ação dos Fármacos, 3rd ed.; Artmed: Porto Alegre, Brazil, 2014; ISBN 978-85-8271-117-0. [Google Scholar]
- Hari, S.B.; Perera, B.G.K.; Ranjitkar, P.; Seeliger, M.A.; Maly, D.J. Conformation-Selective Inhibitors Reveal Differences in the Activation and Phosphate-Binding Loops of the Tyrosine Kinases Abl and Src. ACS Chem. Biol. 2013, 8, 2734–2743. [Google Scholar] [CrossRef]
- Wermuth, C.G.; Aldous, D.J.; Raboisson, P.; Rognan, D. The Practice of Medicinal Chemistry, 4th ed.; Elsevier Academic Press: London, UK, 2015; ISBN 978-0-12-417205-0. [Google Scholar]
- Rossari, F.; Minutolo, F.; Orciuolo, E. Past, Present, and Future of Bcr-Abl Inhibitors: From Chemical Development to Clinical Efficacy. J. Hematol. Oncol. 2018, 11, 84. [Google Scholar] [CrossRef]
- Liu, Y.; Gray, N.S. Rational Design of Inhibitors That Bind to Inactive Kinase Conformations. Nat. Chem. Biol. 2006, 2, 358–364. [Google Scholar] [CrossRef]
- Okram, B.; Nagle, A.; Adrián, F.J.; Lee, C.; Ren, P.; Wang, X.; Sim, T.; Xie, Y.; Wang, X.; Xia, G.; et al. A General Strategy for Creating “Inactive-Conformation” Abl Inhibitors. Chem. Biol. 2006, 13, 779–786. [Google Scholar] [CrossRef]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel Procedure for Modeling Ligand/Receptor Induced Fit Effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef]
- Weisberg, E.; Barrett, R.; Liu, Q.; Stone, R.; Gray, N.; Griffin, J.D. FLT3 Inhibition and Mechanisms of Drug Resistance in Mutant FLT3-Positive AML. Drug Resist. Updates 2009, 12, 81–89. [Google Scholar] [CrossRef]
- Alkorta, I.; Rozas, I.; Elguero, J. Non-Conventional Hydrogen Bonds. Chem. Soc. Rev. 1998, 27, 163. [Google Scholar] [CrossRef]
- Bonn, B.; Masimirembwa, C.M.; Aristei, Y.; Zamora, I. The Molecular Basis of CYP2D6-Mediated N-Dealkylation: Balance between Metabolic Clearance Routes and Enzyme Inhibition. Drug Metab. Dispos. 2008, 36, 2199–2210. [Google Scholar] [CrossRef]
- Rydberg, P.; Olsen, L. Ligand-Based Site of Metabolism Prediction for Cytochrome P450 2D6. ACS Med. Chem. Lett. 2012, 3, 69–73. [Google Scholar] [CrossRef]
- Yang, J.; Campobasso, N.; Biju, M.P.; Fisher, K.; Pan, X.-Q.; Cottom, J.; Galbraith, S.; Ho, T.; Zhang, H.; Hong, X.; et al. Discovery and Characterization of a Cell-Permeable, Small-Molecule c-Abl Kinase Activator That Binds to the Myristoyl Binding Site. Chem. Biol. 2011, 18, 177–186. [Google Scholar] [CrossRef][Green Version]
- Tadesse, F.; Asres, G.; Abubeker, A.; Gebremedhin, A.; Radich, J. Spectrum of BCR-ABL Mutations and Treatment Outcomes in Ethiopian Imatinib-Resistant Patients with Chronic Myeloid Leukemia. JCO Glob. Oncol. 2021, 7, 1187–1193. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Hughes, T.P.; Larson, R.A.; Kim, D.-W.; Issaragrisil, S.; Le Coutre, P.; Etienne, G.; Boquimpani, C.; Pasquini, R.; Clark, R.E.; et al. Long-Term Outcomes with Frontline Nilotinib versus Imatinib in Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase: ENESTnd 10-Year Analysis. Leukemia 2021, 35, 440–453. [Google Scholar] [CrossRef]
- Manley, P.W.; Cowan-Jacob, S.W.; Fendrich, G.; Mestan, J. Molecular Interactions between the Highly Selective Pan-Bcr-Abl Inhibitor, AMN107, and the Tyrosine Kinase Domain of Abl. Blood 2005, 106, 3365. [Google Scholar] [CrossRef]
- Weisberg, E.; Manley, P.W.; Breitenstein, W.; Brüggen, J.; Cowan-Jacob, S.W.; Ray, A.; Huntly, B.; Fabbro, D.; Fendrich, G.; Hall-Meyers, E.; et al. Characterization of AMN107, a Selective Inhibitor of Native and Mutant Bcr-Abl. Cancer Cell 2005, 7, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, C.G. Similarity in Drugs: Reflections on Analogue Design. Drug Discov. Today 2006, 11, 348–354. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, T.; Corbin, A.S.; Druker, B.J. Targeted CML Therapy: Controlling Drug Resistance, Seeking Cure. Curr. Opin. Genet. Dev. 2006, 16, 92–99. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, T.; Shakespeare, W.C.; Zhu, X.; Eide, C.A.; Rivera, V.M.; Wang, F.; Adrian, L.T.; Zhou, T.; Huang, W.-S.; Xu, Q.; et al. AP24534, a Pan-BCR-ABL Inhibitor for Chronic Myeloid Leukemia, Potently Inhibits the T315I Mutant and Overcomes Mutation-Based Resistance. Cancer Cell 2009, 16, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Massaro, F.; Molica, M.; Breccia, M. Ponatinib: A Review of Efficacy and Safety. Curr. Cancer Drug Tar. 2018, 18, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Quintás-Cardama, A.; Cortes, J. Therapeutic Options Against BCR-ABL1 T315I-Positive Chronic Myelogenous Leukemia. Clin. Cancer Res. 2008, 14, 4392–4399. [Google Scholar] [CrossRef]
- Sanford, D.; Kantarjian, H.; Skinner, J.; Jabbour, E.; Cortes, J. Phase II Trial of Ponatinib in Patients with Chronic Myeloid Leukemia Resistant to One Previous Tyrosine Kinase Inhibitor. Haematologica 2015, 100, e494–e495. [Google Scholar] [CrossRef]
- Zhou, T.; Commodore, L.; Huang, W.; Wang, Y.; Thomas, M.; Keats, J.; Xu, Q.; Rivera, V.M.; Shakespeare, W.C.; Clackson, T.; et al. Structural Mechanism of the Pan-BCR-ABL Inhibitor Ponatinib (AP24534): Lessons for Overcoming Kinase Inhibitor Resistance. Chem. Biol. Drug. Des. 2011, 77, 1–11. [Google Scholar] [CrossRef]
- Bishop, A.C.; Ubersax, J.A.; Petsch, D.T.; Matheos, D.P.; Gray, N.S.; Blethrow, J.; Shimizu, E.; Tsien, J.Z.; Schultz, P.G.; Rose, M.D.; et al. A Chemical Switch for Inhibitor-Sensitive Alleles of Any Protein Kinase. Nature 2000, 407, 395–401. [Google Scholar] [CrossRef]
- Lee, B.J.; Shah, N.P. Identification and Characterization of Activating ABL1 1b Kinase Mutations: Impact on Sensitivity to ATP-Competitive and Allosteric ABL1 Inhibitors. Leukemia 2017, 31, 1096–1107. [Google Scholar] [CrossRef]
- Xie, T.; Saleh, T.; Rossi, P.; Kalodimos, C.G. Conformational States Dynamically Populated by a Kinase Determine Its Function. Science 2020, 370, eabc2754. [Google Scholar] [CrossRef]
- Ayaz, P.; Lyczek, A.; Paung, Y.; Mingione, V.R.; Iacob, R.E.; De Waal, P.W.; Engen, J.R.; Seeliger, M.A.; Shan, Y.; Shaw, D.E. Structural Mechanism of a Drug-Binding Process Involving a Large Conformational Change of the Protein Target. Nat. Commun. 2023, 14, 1885. [Google Scholar] [CrossRef] [PubMed]

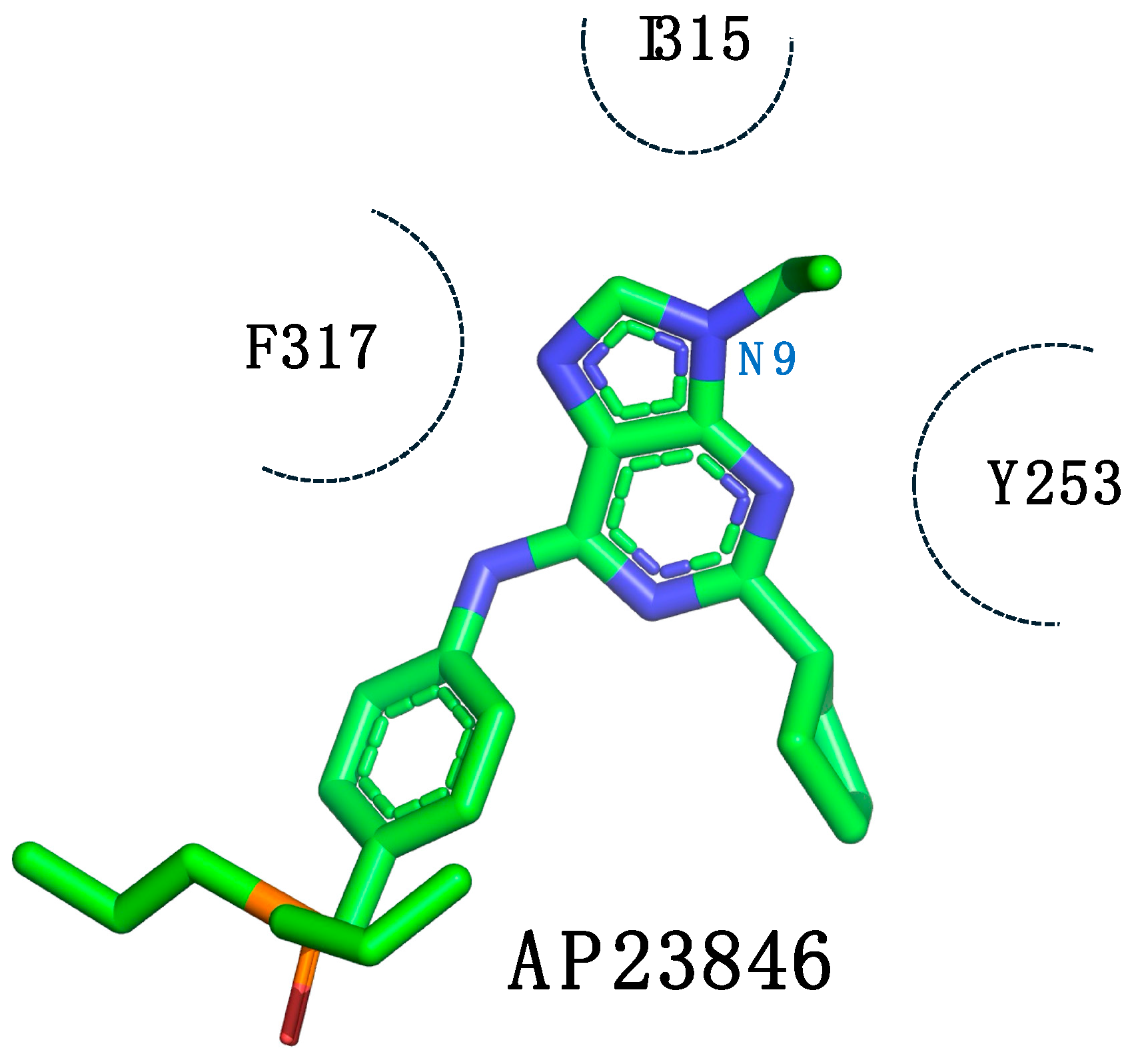


| Compound | IC50 (ABL1T315I) (nM) | cLogP a |
|---|---|---|
| AP23464 | >5000 | 3.99 |
| AP23848 | 5.1 | 5.36 |
| AP23846 | 6.4 | 4.20 |
| AP23980 | 297 | 4.62 |
| Compound | IC50 (ABL1WT) a (nM) | cLogP b |
|---|---|---|
| AP23464 | 61 | 3.99 |
| 1 | 15.8 | 4.67 |
| 2 | 3.58 | 3.68 |
| Compound | IC50 (ABL1WT) a (nM) | IC50 (SRC) (nM) | cLogP b |
|---|---|---|---|
| AP23464 | 61 | ≤1 | 3.99 |
| 2 | 3.58 | - | 4.67 |
| 3 | 96 | 300 | 4.10 |
| 4 | 25 | 52 | 4.13 |
| Compound | IC50 (K562) a | IC50 (Ba/F3) (BCR-ABL1WT) a | IC50 (Ba/F3) (Parenteral) a | IC50 (ABL1WT) a | IC50 (SRC) a | cLogP b |
|---|---|---|---|---|---|---|
| 4 | 67 | 47 | >1000 | 25 | 52 | 4.13 |
| 5 | 95 | 57 | 7857 | 74 | 91 | 4.57 |
| Compound | IC50 (ABL1WT) (nM) | IC50 (SRC) (nM) |
|---|---|---|
| 5 | 74 | 91 |
| 6 | 23 | 74 |
| Compound | IC50 (Ba/F3) (BCR-ABL1T315I) a | IC50 (Ba/F3) (Parenteral) a | IC50 (ABL1WT) a | IC50 (ABL1T315I) a | IC50 (SRC) a | cLogP b |
|---|---|---|---|---|---|---|
| 6 | - | 4353 | 23 | - | 11 | 3.92 |
| 7 | - | 4894 | 20 | - | 7 | 3.12 |
| 8 | 298 | 7120 | 13 | 542 | 8.0 | 4.36 |
| 9 | 422 | 6455 | 25 | 478 | 7.6 | 4.71 |
| Compound | IC50 (ABL1T315I) a | IC50 T315I (Ba/F3) a | IC50 (ABL1WT) a | cLogP b |
|---|---|---|---|---|
| AP24163 | 478 | 422 | 25 | 3.99 |
| 10 | 386 | 295 | 1.6 | 3.41 |
| Compound | IC50 (ABL1WT) a | IC50 (ABL1T315I) a | cLogP b |
|---|---|---|---|
| 7 | 20 | 14142 | 3.12 |
| 11 | 23 | 15513 | 3.74 |
| 12 | 30 | 524 | 3.32 |
| 13 | 28 | 359 | 3.78 |
| Compound | IC50 (ABL1WT) a | IC50 (ABL1T315I) a | F% |
|---|---|---|---|
| 15 | 2.3 | 1216 | 5.5 |
| 16 | 26 | 102 | 42.4 |
| 17 | 45 | 168 | ND |
| 18 | 26 | 185 | ND |
| 19 | 9 | 56 | 29.0 |
| 20 | 69 | 639 | ND |
| 21 | 2.3 | 9 | 16.7 |
| 22 | 8.6 | 40 | 18.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, M.; Moura, S.; Parra, L.; Vasconcellos, V.; Costa, G.; Leite, D.; Dias, M.; Fernandes, T.V.A.; Hoelz, L.; Pimentel, L.; et al. Ponatinib: A Review of the History of Medicinal Chemistry behind Its Development. Pharmaceuticals 2024, 17, 1361. https://doi.org/10.3390/ph17101361
Nascimento M, Moura S, Parra L, Vasconcellos V, Costa G, Leite D, Dias M, Fernandes TVA, Hoelz L, Pimentel L, et al. Ponatinib: A Review of the History of Medicinal Chemistry behind Its Development. Pharmaceuticals. 2024; 17(10):1361. https://doi.org/10.3390/ph17101361
Chicago/Turabian StyleNascimento, Mayara, Stefany Moura, Lidia Parra, Valeska Vasconcellos, Gabriela Costa, Debora Leite, Maria Dias, Tácio Vinício Amorim Fernandes, Lucas Hoelz, Luiz Pimentel, and et al. 2024. "Ponatinib: A Review of the History of Medicinal Chemistry behind Its Development" Pharmaceuticals 17, no. 10: 1361. https://doi.org/10.3390/ph17101361
APA StyleNascimento, M., Moura, S., Parra, L., Vasconcellos, V., Costa, G., Leite, D., Dias, M., Fernandes, T. V. A., Hoelz, L., Pimentel, L., Bastos, M., & Boechat, N. (2024). Ponatinib: A Review of the History of Medicinal Chemistry behind Its Development. Pharmaceuticals, 17(10), 1361. https://doi.org/10.3390/ph17101361






