Multifaceted Heparin: Diverse Applications beyond Anticoagulant Therapy
Abstract
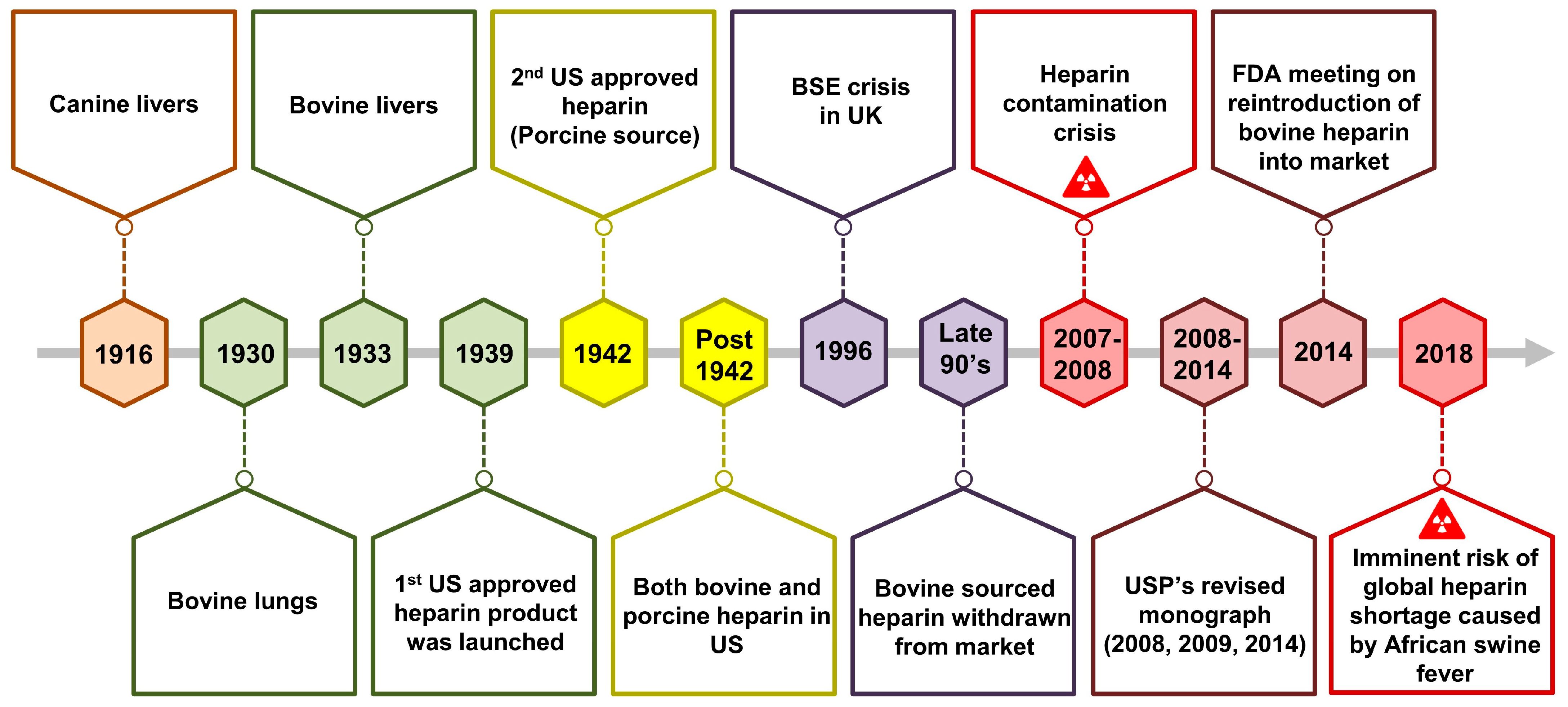
1. Introduction
2. Heparin within Glycosaminoglycans
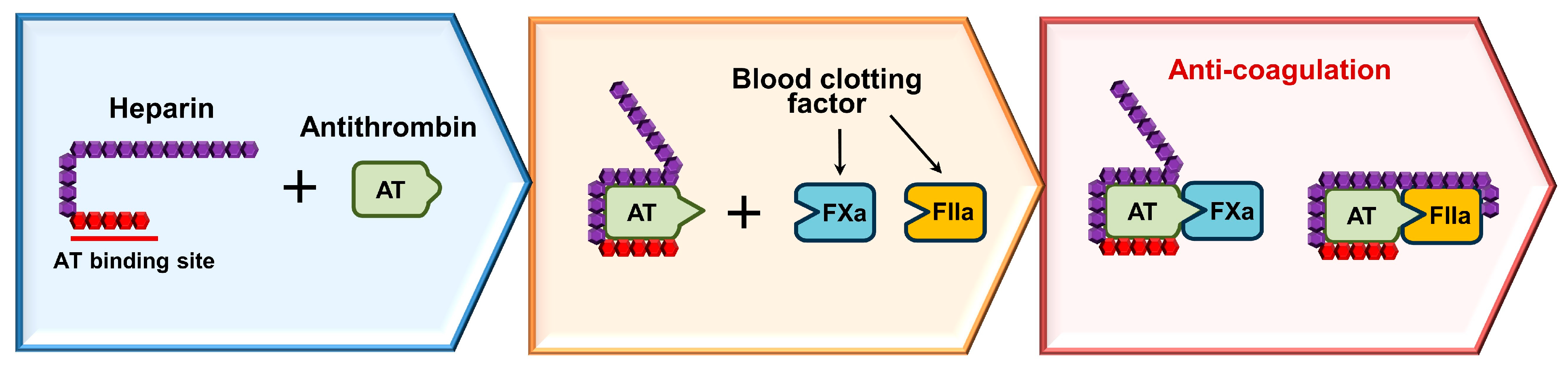
3. How Heparin Works as an Anticoagulant?
4. Synthesis and Modifications
4.1. Chemical Synthesis
4.2. Chemoenzymatic Synthesis
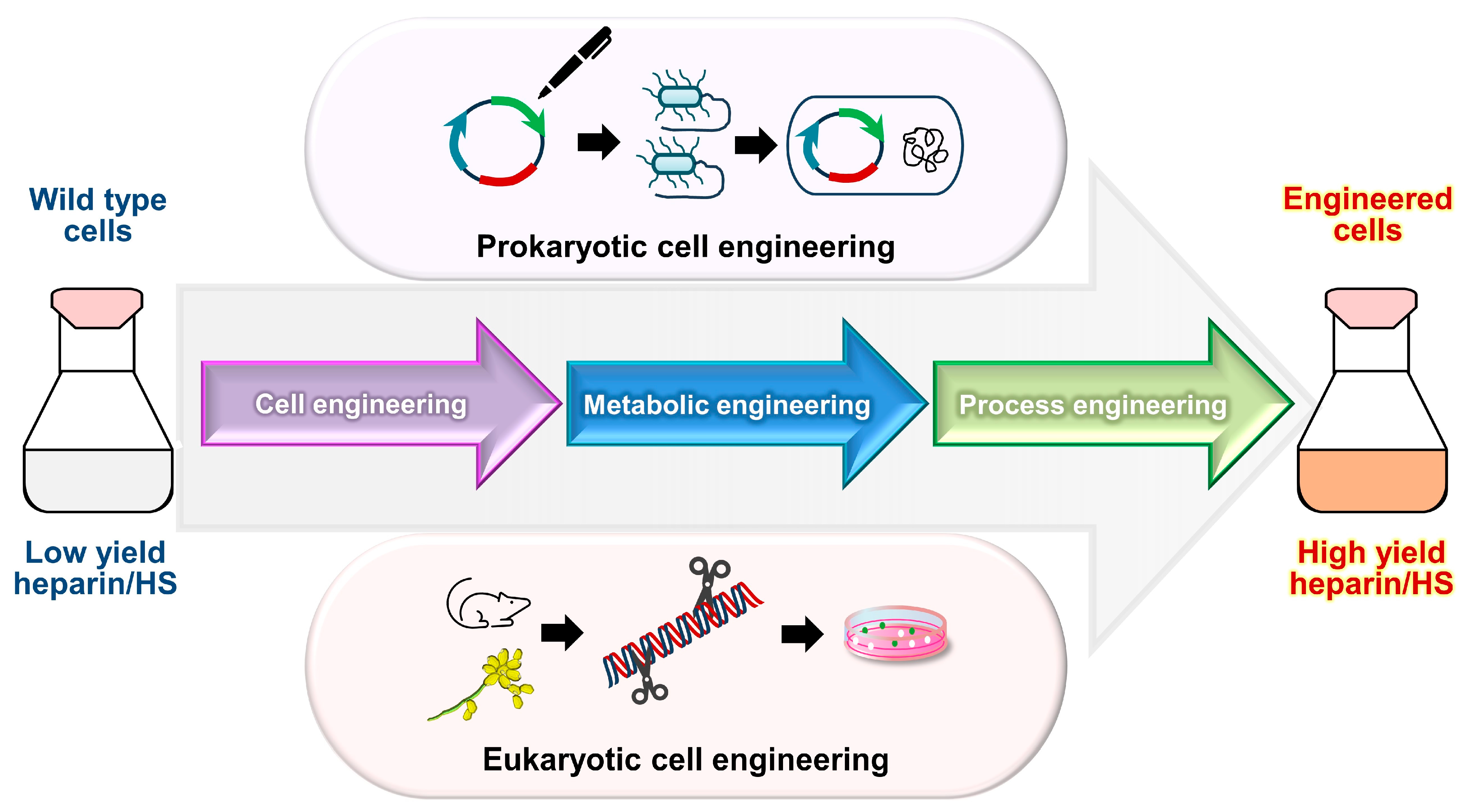
4.3. Advances in Bioengineering
5. Commonly Used Heparin and Its Derivatives
6. Diverse Applications of Heparin
6.1. Heparin in Anti-Inflammatory Therapies
6.2. Heparin in COVID-19 and Other Infectious Diseases
6.3. Heparin in Oncology
6.4. Heparin in Nephropathy
6.5. Heparin in Cardiopathy
6.6. Heparin in Neuroprotection
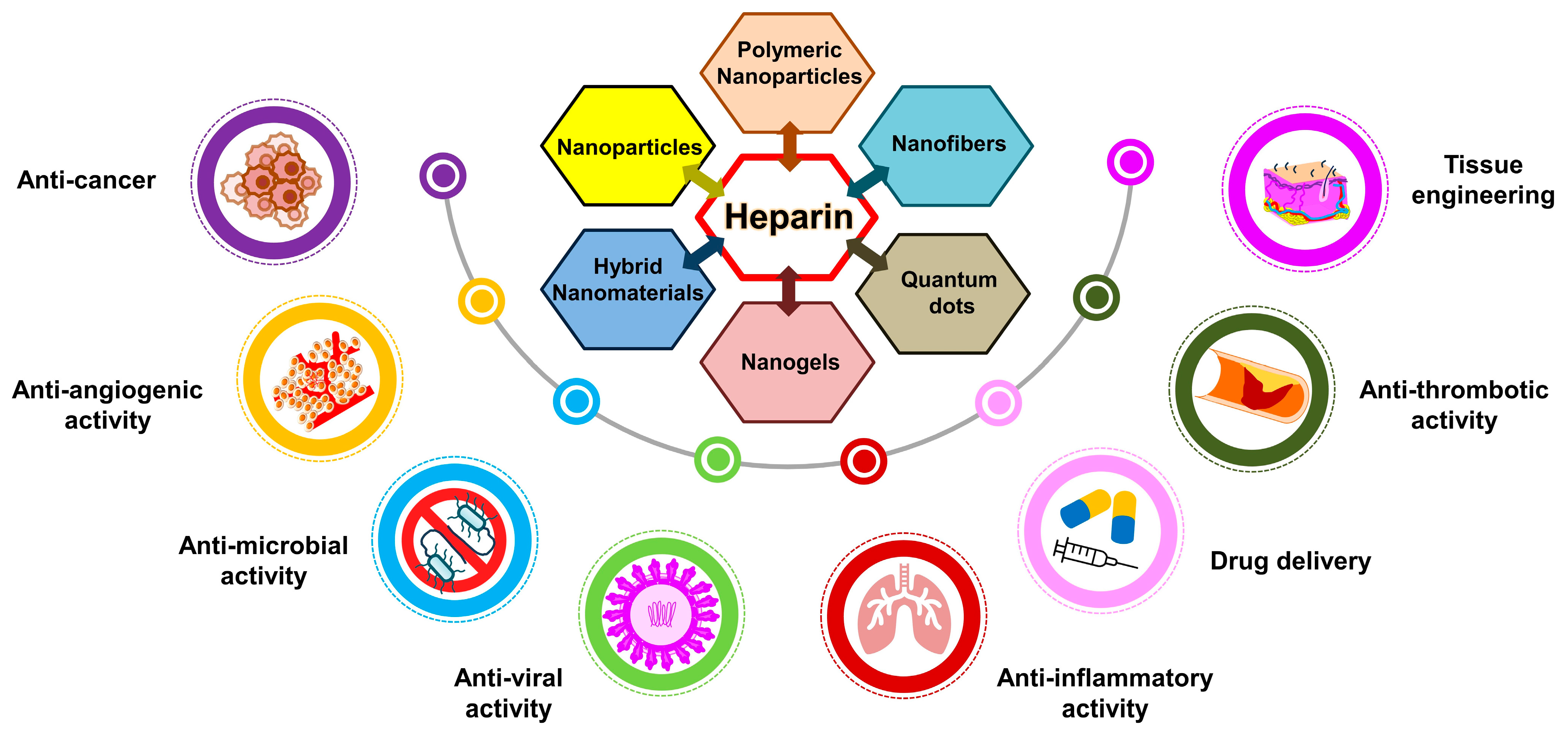
6.7. Heparin in Nanomedical Research and Drug Delivery Systems
6.7.1. Suppressing Cancer Progression with Heparin Nanocomposites
6.7.2. Targeting Angiogenesis with Heparin-Functionalized Nanoparticles
6.7.3. Tailored Heparin Nanocomposites for Enhanced Regeneration
6.7.4. Heparin in Smart Drug Delivery Systems
7. Challenges and Opportunities
8. Advancing Heparin Research
9. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D. Heparin beyond anti-coagulation. Curr. Res. Transl. Med. 2021, 69, 103300. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Huang, S.; Luo, C.; Wu, Z.; Liang, B.; Huang, H.; Ci, Z.; Zhang, D.; Han, L.; Lin, J. Pharmacological and clinical application of heparin progress: An essential drug for modern medicine. Biomed. Pharmacother. 2021, 139, 111561. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Xu, H.; Yu, L.; Zhang, L. Heparin: An essential drug for modern medicine. Prog. Mol. Biol. Transl. Sci. 2019, 163, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Zhu, H.; Wang, K.; Liu, Y.; Yu, F.; Zhao, W. Not Just Anticoagulation—New and Old Applications of Heparin. Molecules 2022, 27, 6968. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chi, L.; Zhang, Z.; Zhao, H.; Zhang, F.; Linhardt, R.J. Heparin: An old drug for new clinical applications. Carbohydr. Polym. 2022, 295, 119818. [Google Scholar] [CrossRef]
- McLean, J. The thromboplastic action of cephalin. Am. J. Physiol.-Leg. Content 1916, 41, 250–257. [Google Scholar] [CrossRef]
- Hemker, H.C. A century of heparin: Past, present and future. J. Thromb. Haemost. 2016, 14, 2329–2338. [Google Scholar] [CrossRef]
- Best, C.H. Preparation of heparin and its use in the first clinical cases. Circulation 1959, 19, 79–86. [Google Scholar] [CrossRef]
- Brinkhous, K.M.; Smith, H.P.; Warner, E.D.; Seegers, W.H. The inhibition of lood clotting: An unidentified substance which acts in conjunction with heparin to prevent the conversion of prothrombin into thrombin. Am. J. Physiol.-Leg. Content 1939, 125, 683–687. [Google Scholar] [CrossRef]
- Waugh, D.F.; Fitzgerald, M.A. Quantitative aspects of antithrombin and heparin in plasma. Am. J. Physiol.-Leg. Content 1956, 184, 627–639. [Google Scholar] [CrossRef]
- Monkhouse, F.C.; France, E.S.; SEEGERS, W.H. Studies on the antithrombin and heparin co-factor activities of a fraction adsorbed from plasma by aluminum hydroxide. Circ. Res. 1955, 3, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, U. Highly purified antithrombin III with heparin cofactor activity prepared by disc electrophoresis. Scand. J. Clin. Lab. Investig. 1968, 21, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Hogwood, J.; Mulloy, B.; Lever, R.; Gray, E.; Page, C.P. Pharmacology of Heparin and Related Drugs: An Update. Pharmacol. Rev. 2023, 75, 328–379. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B.; Hogwood, J.; Gray, E.; Lever, R.; Page, C.P. Pharmacology of heparin and related drugs. Pharmacol. Rev. 2016, 68, 76–141. [Google Scholar] [CrossRef]
- Thacker, B.E.; Thorne, K.J.; Cartwright, C.; Park, J.; Glass, K.; Chea, A.; Kellman, B.P.; Lewis, N.E.; Wang, Z.; Di Nardo, A. Multiplex genome editing of mammalian cells for producing recombinant heparin. Metab. Eng. 2022, 70, 155–165. [Google Scholar] [CrossRef]
- Aláez-Versón, C.R.; Lantero, E.; Fernàndez-Busquets, X. Heparin: New life for an old drug. Nanomedicine 2017, 12, 1727–1744. [Google Scholar] [CrossRef]
- Folkman, J.; Langer, R.; Linhardt, R.J.; Haudenschild, C.; Taylor, S. Angiogenesis Inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science 1983, 221, 719–725. [Google Scholar] [CrossRef]
- Lever, R.; Page, C.P. Novel drug development opportunities for heparin. Nat. Rev. Drug Discov. 2002, 1, 140–148. [Google Scholar] [CrossRef]
- Lindahl, U. ‘Heparin’–from anticoagulant drug into the new biology. Glycoconj. J. 2000, 17, 597–605. [Google Scholar] [CrossRef]
- Shi, D.; Sheng, A.; Chi, L. Glycosaminoglycan-protein interactions and their roles in human disease. Front. Mol. Biosci. 2021, 8, 639666. [Google Scholar] [CrossRef]
- Torri, G.; Naggi, A. Heparin centenary–an ever-young life-saving drug. Int. J. Cardiol. 2016, 212, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Hippensteel, J.A.; LaRiviere, W.B.; Colbert, J.F.; Langouët-Astrié, C.J.; Schmidt, E.P. Heparin as a therapy for COVID-19: Current evidence and future possibilities. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L211–L217. [Google Scholar] [CrossRef] [PubMed]
- Dutch, C.; Thrombosis, C. Early Effects of unfractionated heparin on clinical and radiological signs and D-dimer levels in patients with COVID-19 associated pulmonary embolism: An observational cohort study. Thromb. Res. 2021, 200, 130–132. [Google Scholar] [CrossRef]
- Ennemoser, M.; Rieger, J.; Muttenthaler, E.; Gerlza, T.; Zatloukal, K.; Kungl, A.J. Enoxaparin and pentosan polysulfate bind to the SARS-CoV-2 spike protein and human ACE2 receptor, inhibiting vero cell infection. Biomedicines 2021, 10, 49. [Google Scholar] [CrossRef]
- Kow, C.S.; Ramachandram, D.S.; Hasan, S.S. The effect of higher-intensity dosing of anticoagulation on the clinical outcomes in hospitalized patients with COVID-19: A meta-analysis of randomized controlled trials. J. Infect. Chemother. 2022, 28, 257–265. [Google Scholar] [CrossRef]
- Seffer, M.-T.; Cottam, D.; Forni, L.G.; Kielstein, J.T. Heparin 2.0: A new approach to the infection crisis. Blood Purif. 2021, 50, 28–34. [Google Scholar] [CrossRef]
- Thachil, J. The versatile heparin in COVID-19. J. Thromb. Haemost. 2020, 18, 1020–1022. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, T.; Lu, H.; Li, S.; Lv, K.; Tuffour, A.; Zhang, L.; Ding, K.; Li, J.-P.; Li, H. Heparin mimetics as potential intervention for COVID-19 and their bio-manufacturing. Synth. Syst. Biotechnol. 2023, 8, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, U.; Bäckström, G.; Höök, M.; Thunberg, L.; Fransson, L.-A.; Linker, A. Structure of the Antithrombin-Binding Site in Heparin. Proc. Natl. Acad. Sci. USA 1979, 76, 3198–3202. [Google Scholar] [CrossRef]
- Dementiev, A.; Petitou, M.; Herbert, J.-M.; Gettins, P.G.W. The Ternary Complex of Antithrombin–Anhydrothrombin–Heparin Reveals the Basis of Inhibitor Specificity. Nat. Struct. Mol. Biol. 2004, 11, 863–867. [Google Scholar] [CrossRef]
- Petitou, M.; van Boeckel, C.A.A. A Synthetic Antithrombin III Binding Pentasaccharide Is Now a Drug! What Comes Next? Angew. Chem. Int. Ed. Engl. 2004, 43, 3118–3133. [Google Scholar] [CrossRef] [PubMed]
- van Boeckel, C.A.A.; Petitou, M. The Unique Antithrombin III Binding Domain of Heparin: A Lead to New Synthetic Antithrombotics. Angew. Chem. Int. Ed. Engl. 1993, 32, 1671–1690. [Google Scholar] [CrossRef]
- Rosenberg, R.; Bauer, K. The heparin-antithrombin system: A natural anticoagulant mechanism. In Hemostasis and Thrombosis: Basic Principles and Clinical Practice, 3rd ed.; Colman, R.W., Hirsh, J., Marder, V.J., Salzman, E.W., Eds.; J.B. Lippincott & Co.: Philadelphia, PA, USA, 1994; pp. 837–860. [Google Scholar]
- Rosenberg, R.D.; Lam, L. Correlation between structure and function of heparin. Proc. Natl. Acad. Sci. USA 1979, 76, 1218–1222. [Google Scholar] [CrossRef]
- Kim, H.N.; Whitelock, J.M.; Lord, M.S. Structure-activity relationships of bioengineered heparin/heparan sulfates produced in different bioreactors. Molecules 2017, 22, 806. [Google Scholar] [CrossRef]
- Fu, L.; Suflita, M.; Linhardt, R.J. Bioengineered heparins and heparan sulfates. Adv. Drug Deliv. Rev. 2016, 97, 237–249. [Google Scholar] [CrossRef]
- Capila, I.; Linhardt, R.J. Heparin–protein interactions. Angew. Chem. Int. Ed. Engl. 2002, 41, 390–412. [Google Scholar] [CrossRef]
- Casu, B.; Naggi, A.; Torri, G. Heparin-derived heparan sulfate mimics to modulate heparan sulfate-protein interaction in inflammation and cancer. Matrix Biol. 2010, 29, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Gulberti, S.; Mao, X.; Bui, C.; Fournel-Gigleux, S. The role of heparan sulfate maturation in cancer: A focus on the 3O-sulfation and the enigmatic 3O-sulfotransferases (HS3STs). Semin. Cancer Biol. 2020, 62, 68–85. [Google Scholar] [CrossRef]
- Baytas, S.N.; Linhardt, R.J. Advances in the preparation and synthesis of heparin and related products. Drug Discov. Today 2020, 25, 2095–2109. [Google Scholar] [CrossRef]
- Bhaskar, U.; Sterner, E.; Hickey, A.M.; Onishi, A.; Zhang, F.; Dordick, J.S.; Linhardt, R.J. Engineering of routes to heparin and related polysaccharides. Appl. Microbiol. Biotechnol. 2012, 93, 1–16. [Google Scholar] [CrossRef]
- Oduah, E.I.; Linhardt, R.J.; Sharfstein, S.T. Heparin: Past, Present, and Future. Pharmaceuticals 2016, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Suflita, M.; Fu, L.; He, W.; Koffas, M.; Linhardt, R.J. Heparin and related polysaccharides: Synthesis using recombinant enzymes and metabolic engineering. Appl. Microbiol. Biotechnol. 2015, 99, 7465–7479. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, P.; Kjellén, L. Heparin biosynthesis. In Heparin—A Century of Progress; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 207, pp. 23–41. [Google Scholar] [CrossRef]
- Lidholt, K.; Riesenfeld, J.; Jacobsson, K.G.; Feingold, D.S.; Lindahl, U. Biosynthesis of heparin. Modulation of polysaccharide chain length in a cell-free System. Biochem. J. 1988, 254, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Rabenstein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef]
- Vaidyanathan, D.; Williams, A.; Dordick, J.S.; Koffas, M.A.G.; Linhardt, R.J. Engineered heparins as new anticoagulant drugs. Bioeng. Transl. Med. 2017, 2, 17–30. [Google Scholar] [CrossRef]
- Glass, C.A. Recombinant heparin—New opportunities. Front. Med. 2018, 5, 341. [Google Scholar] [CrossRef]
- Zare, E.N.; Khorsandi, D.; Zarepour, A.; Yilmaz, H.; Agarwal, T.; Hooshmand, S.; Mohammadinejad, R.; Ozdemir, F.; Sahin, O.; Adiguzel, S. Biomedical applications of engineered heparin-based materials. Bioact. Mater. 2024, 31, 87–118. [Google Scholar] [CrossRef]
- Meher, M.K.; Naidu, G.; Mishra, A.; Poluri, K.M. A review on multifaceted biomedical applications of heparin nanocomposites: Progress and prospects. Int. J. Biol. Macromol. 2024, 260, 129379. [Google Scholar] [CrossRef]
- Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A. Heparin-based nanoparticles: An overview of their applications. J. Nanomater. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Kumar, B.D.; Balaraju, M.; Chandra, J.S.; Ghori, S.S.; Khan, N.M.; Ansari, M.S.; Emmanuel, K.A.; Murthy, K. Heparin-based nanoparticles: A summary of their uses. Chelonian Res. Found. 2023, 18, 2290–2305. [Google Scholar] [CrossRef]
- Saurav, S.; Mohan, A.; Tabassum, Z.; Girdhar, M. Recent trends in polymer-based nanocomposites and its application in bone tissue engineering. AIP Conf. Proc. 2024, 2986, 030138. [Google Scholar] [CrossRef]
- Kemp, M.M.; Kumar, A.; Mousa, S.; Dyskin, E.; Yalcin, M.; Ajayan, P.; Linhardt, R.J.; Mousa, S.A. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology 2009, 20, 455104. [Google Scholar] [CrossRef]
- Sultana, R.; Kamihira, M. Bioengineered heparin: Advances in production technology. Biotechnol. Adv. 2024, 77, 108456. [Google Scholar] [CrossRef]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. Diverse Roles of Heparan Sulfate and Heparin in Wound Repair. BioMed Res. Int. 2015, 2015, 549417. [Google Scholar] [CrossRef]
- Casu, B.; Lindahl, U. Structure and Biological Interactions of Heparin and Heparan Sulfate. Adv. Carbohydr. Chem. Biochem. 2001, 57, 159–206. [Google Scholar] [CrossRef]
- Onishi, A.; St Ange, K.; Dordick, J.S.; Linhardt, R.J. Heparin and Anticoagulation. Front. Biosci. (Landmark Ed.) 2016, 21, 1372–1392. [Google Scholar] [CrossRef]
- Lane, D.A.; Denton, J.; Flynn, A.M.; Thunberg, L.; Lindahl, U. Anticoagulant Activities of Heparin Oligosaccharides and Their Neutralization by Platelet Factor 4. Biochem. J. 1984, 218, 725–732. [Google Scholar] [CrossRef]
- Oosta, G.M.; Gardner, W.T.; Beeler, D.L.; Rosenberg, R.D. Multiple Functional Domains of the Heparin Molecule. Proc. Natl. Acad. Sci. USA 1981, 78, 829–833. [Google Scholar] [CrossRef]
- Rezaie, A.R. Calcium Enhances Heparin Catalysis of the Antithrombin-Factor Xa Reaction by a Template Mechanism: Evidence That Calcium Alleviates Gla Domain Antagonism of Heparin Binding to Factor Xa. J. Biol. Chem. 1998, 273, 16824–16827. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sinha, U.; Betz, A. Antithrombin Binding of Low Molecular Weight Heparins and Inhibition of Factor Xa. Biochim. Biophys. Acta 2001, 1526, 105–113. [Google Scholar] [CrossRef]
- Barrowcliffe, T.W.; Le Shirley, Y. The Effect of Calcium Chloride on Anti-Xa Activity of Heparin and Its Molecular Weight Fractions. Thromb. Haemost. 1989, 62, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Huntington, J.A. Shape-Shifting Serpins–Advantages of a Mobile Mechanism. Trends. Biochem. Sci. 2006, 31, 427–435. [Google Scholar] [CrossRef]
- Johnson, D.J.D.; Langdown, J.; Huntington, J.A. Molecular Basis of Factor IXa Recognition by Heparin-Activated Antithrombin Revealed by a 1.7-Å Structure of the Ternary Complex. Proc. Natl. Acad. Sci. USA 2010, 107, 645–650. [Google Scholar] [CrossRef]
- Wiebe, E.M.; Stafford, A.R.; Fredenburgh, J.C.; Weitz, J.I. Mechanism of Catalysis of Inhibition of Factor IXa by Antithrombin in the Presence of Heparin or Pentasaccharide. J. Biol. Chem. 2003, 278, 35767–35774. [Google Scholar] [CrossRef]
- Gozzo, A.J.; Nunes, V.A.; Cruz-Silva, I.; Carmona, A.K.; Nader, H.B.; Faljoni-Alario, A.; Sampaio, M.U.; Araújo, M.S. Heparin Modulation of Human Plasma Kallikrein on Different Substrates and Inhibitors. Biol. Chem. 2006, 387, 1129–1138. [Google Scholar] [CrossRef]
- Olson, S.T.; Swanson, R.; Raub-Segall, E.; Bedsted, T.; Sadri, M.; Petitou, M.; Hérault, J.-P.; Herbert, J.-M.; Björk, I. Accelerating Ability of Synthetic Oligosaccharides on Antithrombin Inhibition of Proteinases of the Clotting and Fibrinolytic Systems Comparison with Heparin and Low-Molecular-Weight Heparin. Thromb. Haemost. 2004, 92, 929–939. [Google Scholar] [CrossRef]
- Martínez-Martínez, I.; Ordóñez, A.; Pedersen, S.; de la Morena-Barrio, M.E.; Navarro-Fernández, J.; Kristensen, S.R.; Miñano, A.; Padilla, J.; Vicente, V.; Corral, J. Heparin Affinity of Factor VIIa: Implications on the Physiological Inhibition by Antithrombin and Clearance of Recombinant Factor VIIa. Thromb. Res. 2011, 127, 154–160. [Google Scholar] [CrossRef]
- Petitou, M.; Hérault, J.-P.; Bernat, A.; Driguez, P.-A.; Duchaussoy, P.; Lormeau, J.-C.; Herbert, J.-M. Synthesis of thrombin-inhibiting heparin mimetics without side effects. Nature 1999, 398, 417–422. [Google Scholar] [CrossRef]
- Baráth, M.; Hansen, S.U.; Dalton, C.E.; Jayson, G.C.; Miller, G.J.; Gardiner, J.M. Modular synthesis of heparin-related tetra-, hexa-and octasaccharides with differential O-6 protections: Programming for regiodefined 6-O-modifications. Molecules 2015, 20, 6167–6180. [Google Scholar] [CrossRef]
- Hansen, S.U.; Miller, G.J.; Cliff, M.J.; Jayson, G.C.; Gardiner, J.M. Making the longest sugars: A chemical synthesis of heparin-related [4]n oligosaccharides from 16-mer to 40-mer. Chem. Sci. 2015, 6, 6158–6164. [Google Scholar] [CrossRef]
- Roy, S.; El Hadri, A.; Richard, S.; Denis, F.; Holte, K.; Duffner, J.; Yu, F.; Galcheva-Gargova, Z.; Capila, I.; Schultes, B. Synthesis and biological evaluation of a unique heparin mimetic hexasaccharide for structure–activity relationship studies. J. Med. Chem. 2014, 57, 4511–4520. [Google Scholar] [CrossRef]
- Lu, L.-D.; Shie, C.-R.; Kulkarni, S.S.; Pan, G.-R.; Lu, X.-A.; Hung, S.-C. Synthesis of 48 disaccharide building blocks for the assembly of a heparin and heparan sulfate oligosaccharide library. Org. Lett. 2006, 8, 5995–5998. [Google Scholar] [CrossRef]
- Pawar, N.J.; Wang, L.; Higo, T.; Bhattacharya, C.; Kancharla, P.K.; Zhang, F.; Baryal, K.; Huo, C.; Liu, J.; Linhardt, R.J. Expedient synthesis of core disaccharide building blocks from natural polysaccharides for heparan sulfate oligosaccharide assembly. Angew. Chem. Int. Ed. Eng. 2019, 131, 18750–18756. [Google Scholar] [CrossRef]
- Ramadan, S.; Su, G.; Baryal, K.; Hsieh-Wilson, L.C.; Liu, J.; Huang, X. Automated solid phase assisted synthesis of a heparan sulfate disaccharide library. Org. Chem. Front. 2022, 9, 2910–2920. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, L.; Huang, H.; Linhardt, R.J. Chemoenzymatic synthesis of glycosaminoglycans. Acc. Chem. Res. 2019, 53, 335–346. [Google Scholar] [CrossRef]
- Gottschalk, J.; Elling, L. Current state on the enzymatic synthesis of glycosaminoglycans. Curr. Opin. Chem. Biol. 2021, 61, 71–80. [Google Scholar] [CrossRef]
- Lindahl, U.; Li, J.; Kusche-Gullberg, M.; Salmivirta, M.; Alaranta, S.; Veromaa, T.; Emeis, J.; Roberts, I.; Taylor, C.; Oreste, P. Generation of “Neoheparin” from E. coli K5 capsular polysaccharide. J. Med. Chem. 2005, 48, 349–352. [Google Scholar] [CrossRef]
- Casu, B.; Grazioli, G.; Razi, N.; Guerrini, M.; Naggi, A.; Torri, G.; Oreste, P.; Tursi, F.; Zoppetti, G.; Lindahl, U. Heparin-like compounds prepared by chemical modification of capsular polysaccharide from E. coli K5. Carbohydr. Res. 1994, 263, 271–284. [Google Scholar] [CrossRef]
- Datta, P.; Yan, L.; Awofiranye, A.; Dordick, J.S.; Linhardt, R.J. Heparosan Chain Characterization: Sequential depolymerization of E. coli K5 heparosan by a bacterial eliminase heparin lyase iii and a bacterial hydrolase heparanase bp to prepare defined oligomers. Biotechnol. J. 2021, 16, 2000336. [Google Scholar] [CrossRef]
- Xu, Y.; Masuko, S.; Takieddin, M.; Xu, H.; Liu, R.; Jing, J.; Mousa, S.A.; Linhardt, R.J.; Liu, J. Chemoenzymatic Synthesis of Homogeneous Ultralow Molecular Weight Heparins. Science 2011, 334, 498–501. [Google Scholar] [CrossRef]
- Liu, J.; Linhardt, R.J. Chemoenzymatic synthesis of heparan sulfate and heparin oligosaccharides and nmr analysis: Paving the way to a diverse library for glycobiologists. Nat. Prod. Rep. 2014, 31, 7932–7940. [Google Scholar] [CrossRef]
- Xu, Y.; Pempe, E.H.; Liu, J. Chemoenzymatic synthesis of heparin oligosaccharides with both Anti-factor Xa and Anti-factor IIa activities. J. Biol. Chem. 2012, 287, 29054–29061. [Google Scholar] [CrossRef]
- Zha, Z.; Liu, Y.; Miao, Y.; Liao, S.; Wang, S.-Y.; Tang, H.; Yin, H. Preparation and characterization of 2-deacetyl-3-o-sulfo-heparosan and its antitumor effects via the fibroblast growth factor receptor pathway. Int. J. Biol. Macromol. 2022, 201, 47–58. [Google Scholar] [CrossRef]
- Douaisi, M.; Paskaleva, E.E.; Fu, L.; Grover, N.; McManaman, C.L.; Varghese, S.; Brodfuehrer, P.R.; Gibson, J.M.; de Joode, I.; Xia, K. Synthesis of bioengineered heparin chemically and biologically similar to porcine-derived products and convertible to low MW heparin. Proc. Natl. Acad. Sci. USA 2024, 121, e2315586121. [Google Scholar] [CrossRef]
- Deng, J.-Q.; Li, Y.; Wang, Y.-J.; Cao, Y.-L.; Xin, S.-Y.; Li, X.-Y.; Xi, R.-M.; Wang, F.-S.; Sheng, J.-Z. Biosynthetic production of anticoagulant heparin polysaccharides through metabolic and sulfotransferases engineering strategies. Nat. Commun. 2024, 15, 3755. [Google Scholar] [CrossRef]
- Sun, L.; Chopra, P.; Boons, G. Chemoenzymatic synthesis of heparan sulfate oligosaccharides having a domain structure. Angew. Chem. 2022, 134, e202211112. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, T.; Kan, Y.; Li, H.; Li, J. Overview of the current procedures in synthesis of heparin saccharides. Carbohydr. Polym. 2024, 339, 122220. [Google Scholar] [CrossRef]
- Baik, J.Y.; Gasimli, L.; Yang, B.; Datta, P.; Zhang, F.; Glass, C.A.; Esko, J.D.; Linhardt, R.J.; Sharfstein, S.T. Metabolic engineering of chinese hamster ovary cells: Towards a bioengineered heparin. Metab. Eng. 2012, 14, 81–90. [Google Scholar] [CrossRef]
- Baik, J.Y.; Wang, C.L.; Yang, B.; Linhardt, R.J.; Sharfstein, S.T. Toward a bioengineered heparin: Challenges and strategies for metabolic engineering of mammalian cells. Bioengineered 2012, 3, 227–231. [Google Scholar] [CrossRef]
- Datta, P.; Linhardt, R.J.; Sharfstein, S.T. An’omics approach towards CHO cell engineering. Biotechnol. Bioeng. 2013, 110, 1255–1271. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhou, Z.; Wang, P.; Xi, X.; Hu, S.; Xu, R.; Du, G.; Li, J.; Chen, J. Synthesis of bioengineered heparin by recombinant yeast Pichia pastoris. Green Chem. 2022, 24, 3180–3192. [Google Scholar] [CrossRef]
- Cress, B.F.; Toparlak, O.D.; Guleria, S.; Lebovich, M.; Stieglitz, J.T.; Englaender, J.A.; Jones, J.A.; Linhardt, R.J.; Koffas, M.A.G. CRISPathBrick: Modular combinatorial assembly of type II-A CRISPR arrays for dcas9-mediated multiplex transcriptional repression in E. coli. ACS Synth. Biol. 2015, 4, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Li, G.; Yang, B.; Zhao, X.; Baik, J.Y.; Gemmill, T.R.; Sharfstein, S.T.; Linhardt, R.J. Bioengineered chinese hamster ovary cells with Golgi-targeted 3-O-sulfotransferase-1 biosynthesize heparan sulfate with an antithrombin-binding site. J. Biol. Chem. 2013, 288, 37308–37318. [Google Scholar] [CrossRef]
- Leyh, T.S.; Taylor, J.C.; Markham, G.D. The sulfate activation locus of Escherichia coli K12: Cloning, genetic, and enzymatic characterization. J. Biol. Chem. 1988, 263, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Hosoyama, S.; Ohno, A.; Masuko, S.; Yang, B.; Sterner, E.; Toida, T. Photochemical preparation of a novel low molecular weight heparin. Carbohyd. Polym. 2012, 87, 1737–1743. [Google Scholar] [CrossRef]
- Zhi, Z.J.; Li, J.H.; Chen, J.L.; Li, S.; Cheng, H.; Liu, D.H.; Chen, S.G. Preparation of low molecular weight heparin using an ultrasound-assisted Fentonsystem. Ultrason. Sonochem. 2019, 52, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.M.; Liu, Z.F.; Li, J.H.; Wu, D.M.; Zhu, M.; Yan, L.F.; Chen, S.G. Development of low molecular weight heparin by H2O2/ascorbic acid with ultrasonic power and its anti-metastasis property. Int. J. Biol. Macromol. 2019, 133, 101–109. [Google Scholar] [CrossRef]
- Linhardt, R.J.; Liu, J. Synthetic heparin. Curr. Opin. Pharmacol. 2012, 12, 217–219. [Google Scholar] [CrossRef]
- Ding, Y.; Prasad, C.V.V.; Bai, H.; Wang, B. Efficient and practical synthesis of Fondaparinux. Bioorg. Med. Chem. Lett. 2017, 27, 2424–2427. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Zhang, A.; Shao, X.; Liu, T.; Tang, B.; Fang, G. Strategies for sustained release of heparin: A review. Carbohydr. Polym. 2022, 294, 119793. [Google Scholar] [CrossRef]
- Dey, S.; Lo, H.J.; Wong, C.H. Programmable one-pot synthesis of heparin pentasaccharide fondaparinux. Org. Lett. 2020, 22, 4638–4642. [Google Scholar] [CrossRef]
- Jin, H.; Chen, Q.; Zhang, Y.Y.; Hao, K.F.; Zhang, G.Q.; Zhao, W. Preactivation-based, iterative one-pot synthesis of anticoagulant pentasaccharide fondaparinux sodium. Org. Chem. Front. 2019, 6, 3116–3120. [Google Scholar] [CrossRef]
- Linhardt, R.J.; Toida, T. Role of glycosaminoglycans in cellular communication. Acc. Chem. Res. 2004, 37, 431–438. [Google Scholar] [CrossRef]
- Mousavi, S.; Moradi, M.; Khorshidahmad, T.; Motamedi, M. Anti-Inflammatory effects of heparin and its derivatives: A systematic review. Adv. Pharmacol. Pharm. Sci. 2015, 2015, 507151. [Google Scholar] [CrossRef]
- Poterucha, T.J.; Libby, P.; Goldhaber, S.Z. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb. Haemost. 2017, 117, 437–444. [Google Scholar] [CrossRef]
- Severin, I.C.; Soares, A.; Hantson, J.; Teixeira, M.; Sachs, D.; Valognes, D.; Scheer, A.; Schwarz, M.K.; Wells, T.N.C.; Proudfoot, A.E.I. Glycosaminoglycan analogs as a novel anti-inflammatory strategy. Front. Immunol. 2012, 3, 293. [Google Scholar] [CrossRef]
- Yan, Y.; Ji, Y.; Su, N.; Mei, X.; Wang, Y.; Du, S.; Zhu, W.; Zhang, C.; Lu, Y.; Xing, X.-H. Non-anticoagulant effects of low molecular weight heparins in inflammatory disorders: A review. Carbohydr. Polym. 2017, 160, 71–81. [Google Scholar] [CrossRef]
- Gilotti, A.C.; Nimlamool, W.; Pugh, R.; Slee, J.B.; Barthol, T.C.; Miller, E.A.; Lowe-Krentz, L.J. Heparin responses in vascular smooth muscle cells involve CGMP-dependent protein kinase (PKG). J. Cell. Physiol. 2014, 229, 2142–2152. [Google Scholar] [CrossRef]
- Baumgart, D.C. CB-01-05-MMX, a novel oral controlled-release low molecular weight heparin for the potential treatment of ulcerative colitis. Curr. Opin. Investig. Drugs 2010, 11, 571–576. [Google Scholar] [PubMed]
- Qi, L.; Zhang, X.; Wang, X. Heparin inhibits the inflammation and proliferation of human rheumatoid arthritis fibroblast-like synoviocytes through the NF-κB pathway. Mol. Med. Rep. 2016, 14, 3743–3748. [Google Scholar] [CrossRef]
- Malhotra, S.; Bhasin, D.; Shafiq, N.; Pandhi, P. Drug treatment of ulcerative colitis: Unfractionated heparin, low molecular weight heparins and beyond. Expert Opin. Pharmacother. 2004, 5, 329–334. [Google Scholar] [CrossRef]
- Abdelaty, N.; Abd-Elsalam, M. Efficacy of inhaled heparin is effective in the treatment of acute exacerbation of asthma. In Allergy; Blackwell Publishing: Oxford, UK, 2007; Volume 62, p. 216. [Google Scholar]
- Bendstrup, K.E.; Jensen, J.I. Inhaled heparin is effective in exacerbations of asthma. Respir. Med. 2000, 94, 174–175. [Google Scholar] [CrossRef]
- Ghonim, M.A.; Wang, J.; Ibba, S.V.; Luu, H.H.; Pyakurel, K.; Benslimane, I.; Mousa, S.; Boulares, A.H. Sulfated non-anticoagulant heparin blocks Th2-induced asthma by modulating the IL-4/signal transducer and activator of transcription 6/janus kinase 1 pathway. J. Transl. Med. 2018, 16, 243. [Google Scholar] [CrossRef]
- Shute, J.K.; Puxeddu, E.; Calzetta, L. Therapeutic use of heparin and derivatives beyond anticoagulation in patients with bronchial asthma or COPD. Curr. Opin. Pharmacol. 2018, 40, 39–45. [Google Scholar] [CrossRef]
- Cai, X.; Wang, K.; Wang, J.; Zheng, Y.; Hu, W. Effects of low molecular weight heparin calcium combined with insulin on immune function, inflammatory response, haemorheology and coagulation in patients with high triglyceride acute pancreatitis. Acta Medica Mediterr. 2020, 36, 1557–1561. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Dembinski, A.; Warzecha, Z.; Dembinski, M.; Cieszkowski, J.; Rembisz, K.; Konturek, S.J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Pawlik, W.W. Protective and therapeutic effect of heparin in acute pancreatitis. J. Physiol. Pharmacol. 2008, 59, 103–125. [Google Scholar] [PubMed]
- Granell, S.; Gironella, M.; Bulbena, O.; Panés, J.; Mauri, M.; Sabater, L.; Aparisi, L.; Gelpí, E.; Closa, D. Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit. Care Med. 2003, 31, 525–530. [Google Scholar] [CrossRef]
- Shute, J.K. Heparin, Low molecular weight heparin, and non-anticoagulant derivatives for the treatment of inflammatory lung disease. Pharmaceuticals 2023, 16, 584. [Google Scholar] [CrossRef]
- Fath, M.A.; Wu, X.; Hileman, R.E.; Linhardt, R.J.; Kashem, M.A.; Nelson, R.M.; Wright, C.D.; Abraham, W.M. Interaction of secretory leukocyte protease inhibitor with heparin inhibits proteases involved in asthma. J. Biol. Chem. 1998, 273, 13563–13569. [Google Scholar] [CrossRef]
- Derhaschnig, U.; Pernerstorfer, T.; Knechtelsdorfer, M.; Hollenstein, U.; Panzer, S.; Jilma, B. Evaluation of antiinflammatory and antiadhesive effects of heparins in human endotoxemia. Crit. Care Med. 2003, 31, 1108–1112. [Google Scholar] [CrossRef]
- Li, X.; Ma, X. The role of heparin in sepsis: Much more than just an anticoagulant. Br. J. Haematol. 2017, 179, 389–398. [Google Scholar] [CrossRef]
- Li, L.; Ling, Y.; Huang, M.; Yin, T.; Gou, S.-M.; Zhan, N.-Y.; Xiong, J.-X.; Wu, H.-S.; Yang, Z.-Y.; Wang, C.-Y. Heparin inhibits the inflammatory response induced by LPS and HMGB1 by blocking the binding of HMGB1 to the surface of macrophages. Cytokine 2015, 72, 36–42. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Li, Z.; He, Z.; Yang, X.; Cheng, X.; Peng, Y.; Xue, Q.; Bai, Y.; Zhang, R. Heparin prevents caspase-11-dependent septic lethality independent of anticoagulant properties. Immunity 2021, 54, 454–467. [Google Scholar] [CrossRef]
- Hogwood, J.; Pitchford, S.; Mulloy, B.; Page, C.; Gray, E. Heparin and non-anticoagulant heparin attenuate histone-induced inflammatory responses in whole blood. PLoS ONE 2020, 15, e0233644. [Google Scholar] [CrossRef]
- Wang, T.; Liu, L.; Voglmeir, J. Chemoenzymatic synthesis of ultralow and low-molecular weight heparins. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140301. [Google Scholar] [CrossRef]
- Wildhagen, K.C.A.A.; García de Frutos, P.; Reutelingsperger, C.P.; Schrijver, R.; Aresté, C.; Ortega-Gómez, A.; Deckers, N.M.; Hemker, H.C.; Soehnlein, O.; Nicolaes, G.A.F. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood 2014, 123, 1098–1101. [Google Scholar] [CrossRef]
- Zhang, X.; Han, X.; Xia, K.; Xu, Y.; Yang, Y.; Oshima, K.; Haeger, S.M.; Perez, M.J.; McMurtry, S.A.; Hippensteel, J.A. Circulating heparin oligosaccharides rapidly target the hippocampus in sepsis, potentially impacting cognitive functions. Proc. Natl. Acad. Sci. USA 2019, 116, 9208–9213. [Google Scholar] [CrossRef]
- Yini, S.; Heng, Z.; Xin, A.; Xiaochun, M. Effect of unfractionated heparin on endothelial glycocalyx in a septic shock model. Acta Anaesthesiol. Scand. 2015, 59, 160–169. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Luo, M.; Xi, Y.; Li, C.; Wang, S.; Yang, R. Therapeutic effect of low-molecular-weight heparin on adult sepsis: A meta-analysis. Ann. Palliat. Med. 2021, 10, 3113127–3115127. [Google Scholar] [CrossRef]
- Fu, S.; Yu, S.; Wang, L.; Ma, X.; Li, X. Unfractionated Heparin Improves the Clinical Efficacy in Adult Sepsis Patients: A Systematic Review and Meta-Analysis. BMC Anesthesiol. 2022, 22, 28. [Google Scholar] [CrossRef]
- Kyriakoulis, K.G.; Kollias, A.; Kyriakoulis, I.G.; Kyprianou, I.A.; Papachrysostomou, C.; Makaronis, P.; Kotronias, R.A.; Terentes-Printzios, D.; Toskas, I.; Mikhailidis, D.P. Thromboprophylaxis in patients with COVID-19: Systematic review of national and international clinical guidance reports. Curr. Vasc. Pharmacol. 2022, 20, 96–110. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Poli, D.; Antonucci, E.; Ageno, W.; Prandoni, P.; Palareti, G.; Marcucci, R. Low in-hospital mortality rate in patients with COVID-19 receiving thromboprophylaxis: Data from the multicentre observational START-COVID register. Intern. Emerg. Med. 2022, 17, 1013–1021. [Google Scholar] [CrossRef]
- van Haren, F.M.P.; van Loon, L.M.; Steins, A.; Smoot, T.L.; Sas, C.; Staas, S.; Vilaseca, A.B.; Barbera, R.A.; Vidmar, G.; Beccari, H. Inhaled nebulised unfractionated heparin for the treatment of hospitalised patients with COVID-19: A multicentre case series of 98 patients. Br. J. Clin. Pharmacol. 2022, 88, 2802–2813. [Google Scholar] [CrossRef]
- Zhai, Z.; Kan, Q.; Li, W.; Qin, X.; Qu, J.; Shi, Y.; Xu, R.; Xu, Y.; Zhang, Z.; Wang, C. VTE Risk Profiles and Prophylaxis in medical and surgical inpatients: The identification of chinese hospitalized patients’ risk profile for venous thromboembolism (DissolVE-2)—A cross-sectional study. Chest 2019, 155, 114–122. [Google Scholar] [CrossRef]
- Rentsch, C.T.; Beckman, J.A.; Tomlinson, L.; Gellad, W.F.; Alcorn, C.; Kidwai-Khan, F.; Skanderson, M.; Brittain, E.; King, J.T.; Ho, Y.-L. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: Cohort study. BMJ 2021, 372, n311. [Google Scholar] [CrossRef]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.; Clark, C.; Iba, T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell 2020, 183, 1043–1057. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Su, D.; Pagani, I.; Rudd, T.R.; Elli, S.; Gandhi, N.S.; Guimond, S.E.; Miller, G.J.; Meneghetti, M.C.Z.; Nader, H.B. Heparin inhibits cellular invasion by SARS-CoV-2: Structural dependence of the interaction of the spike S1 receptor-binding domain with heparin. Thromb. Haemost. 2020, 120, 1700–1715. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, C.Z.; Swaroop, M.; Xu, M.; Wang, L.; Lee, J.; Wang, A.Q.; Pradhan, M.; Hagen, N.; Chen, L. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 2020, 6, 80. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Pang, H.; Li, S.J. Heparin interacts with the main protease of SARS-CoV-2 and inhibits its activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120595. [Google Scholar] [CrossRef]
- Cron, R.Q.; Goyal, G.; Chatham, W.W. Cytokine storm syndrome. Annu. Rev. Med. 2023, 74, 321–337. [Google Scholar] [CrossRef]
- Yu, X. Potential of heparin in the treatment of COVID-19–associated myocarditis. Pediatr. Emerg. Care 2022, 38, e504. [Google Scholar] [CrossRef]
- Xue, M.; Zeng, Y.; Qu, H.-Q.; Zhang, T.; Li, N.; Huang, H.; Zheng, P.; Hu, H.; Zhou, L.; Duan, Z. Heparin-binding protein levels correlate with aggravation and multiorgan damage in severe COVID-19. ERJ Open Res. 2021, 7, 00741–02020. [Google Scholar] [CrossRef]
- Copeland, R.; Balasubramaniam, A.; Tiwari, V.; Zhang, F.; Bridges, A.; Linhardt, R.J.; Shukla, D.; Liu, J. Using a 3-O-sulfated heparin octasaccharide to inhibit the entry of Herpes Simplex Virus Type 1. Biochemistry 2008, 47, 5774–5783. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Lei, H.-Y.; Lin, Y.-S.; Yeh, T.-M.; Chen, S.-H.; Liu, H.-S. Heparin inhibits dengue-2 virus infection of five human liver cell lines. Antiviral Res. 2002, 56, 93–96. [Google Scholar] [CrossRef]
- Angeletti, P.C. Seeing HPV in the new light offers a glimpse of heparin. Structure 2017, 25, 213. [Google Scholar] [CrossRef][Green Version]
- Nassar, R.A.; Browne, E.P.; Chen, J.; Klibanov, A.M. Removing Human Immunodeficiency Virus (HIV) from human blood using immobilized heparin. Biotechnol. Lett. 2012, 34, 853–856. [Google Scholar] [CrossRef]
- Plochmann, K.; Horn, A.; Gschmack, E.; Armbruster, N.; Krieg, J.; Wiktorowicz, T.; Weber, C.; Stirnnagel, K.; Lindemann, D.; Rethwilm, A. Heparan sulfate is an attachment factor for foamy virus entry. J. Virol. 2012, 86, 10028–10035. [Google Scholar] [CrossRef]
- Urbinati, C.; Milanesi, M.; Lauro, N.; Bertelli, C.; David, G.; D’Ursi, P.; Rusnati, M.; Chiodelli, P. HIV-1 tat and heparan sulfate proteoglycans orchestrate the setup of in cis and in trans cell-surface interactions functional to lymphocyte trans-endothelial migration. Molecules 2021, 26, 7488. [Google Scholar] [CrossRef]
- Skidmore, M.A.; Kajaste-Rudnitski, A.; Wells, N.M.; Guimond, S.E.; Rudd, T.R.; Yates, E.A.; Vicenzi, E. Inhibition of influenza H5N1 invasion by modified heparin derivatives. Med. Chem. Commun. 2015, 6, 640–646. [Google Scholar] [CrossRef]
- Ghezzi, S.; Cooper, L.; Rubio, A.; Pagani, I.; Capobianchi, M.R.; Ippolito, G.; Pelletier, J.; Meneghetti, M.C.Z.; Lima, M.A.; Skidmore, M.A. Heparin prevents Zika virus induced-cytopathic effects in human neural progenitor cells. Antiviral Res. 2017, 140, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Koetzner, C.A.; Payne, A.F.; Nierode, G.J.; Yu, Y.; Wang, R.; Barr, E.; Dordick, J.S.; Kramer, L.D.; Zhang, F. Glycosaminoglycan compositional analysis of relevant tissues in Zika virus pathogenesis and in vitro evaluation of heparin as an antiviral against Zika Virus infection. Biochemistry 2019, 58, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Zhao, J.; Liu, X.; Fraser, K.; Lin, L.; Zhang, X.; Zhang, F.; Dordick, J.S.; Linhardt, R.J. Interaction of Zika virus envelope protein with glycosaminoglycans. Biochemistry 2017, 56, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.; Essler, L.; Loy, A.; Quinn, F.; Giri, P. Heparin inhibits intracellular Mycobacterium tuberculosis bacterial replication by reducing iron levels in human macrophages. Sci. Rep. 2018, 8, 7296. [Google Scholar] [CrossRef] [PubMed]
- Hills, F.A.; Abrahams, V.M.; González-Timón, B.; Francis, J.; Cloke, B.; Hinkson, L.; Rai, R.; Mor, G.; Regan, L.; Sullivan, M. Heparin prevents programmed cell death in human trophoblast. Mol. Hum. Reprod. 2006, 12, 237–243. [Google Scholar] [CrossRef]
- Leitgeb, A.M.; Blomqvist, K.; Cho-Ngwa, F.; Samje, M.; Nde, P.; Titanji, V.; Wahlgren, M. Low Anticoagulant heparin disrupts Plasmodium falciparum rosettes in fresh clinical isolates. Am. J. Trop. Med. Hyg. 2011, 84, 390. [Google Scholar] [CrossRef]
- Marques, J.; Moles, E.; Urbán, P.; Prohens, R.; Busquets, M.A.; Sevrin, C.; Grandfils, C.; Fernàndez-Busquets, X. Application of heparin as a dual agent with antimalarial and liposome targeting activities toward plasmodium-infected red blood cells. Nanomedicine 2014, 10, 1719–1728. [Google Scholar] [CrossRef]
- Sinnis, P.; Coppi, A.; Toida, T.; Toyoda, H.; Kinoshita-Toyoda, A.; Xie, J.; Kemp, M.M.; Linhardt, R.J. Mosquito heparan sulfate and its potential role in malaria infection and transmission. J. Biol. Chem. 2007, 282, 25376–25384. [Google Scholar] [CrossRef]
- San Anselmo, M.; Lantero, E.; Avalos-Padilla, Y.; Bouzón-Arnáiz, I.; Ramírez, M.; Postigo, A.; Serrano, J.L.; Sierra, T.; Hernández-Ainsa, S.; Fernàndez-Busquets, X. Heparin-coated dendronized hyperbranched polymers for antimalarial targeted delivery. ACS Appl. Polym. Mater. 2022, 5, 381–390. [Google Scholar] [CrossRef]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primers 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-P.; Yu, Y.; Marcinkiewicz, A.L.; Lederman, P.; Hart, T.M.; Zhang, F.; Linhardt, R.J. Non-anticoagulant heparin as a pre-exposure prophylaxis prevents lyme disease infection. ACS Infect. Dis. 2020, 6, 503–514. [Google Scholar] [CrossRef]
- Axelsson, J.; Ferreira, M.; Adolfsson, L.; McCrea, K.; Ward, R.; Larm, O. Cytokines in blood from septic patients interact with surface-immobilized heparin. ASAIO J. 2010, 56, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Buitrago-Pabón, A.L.; Ruiz-Sáenz, S.; Jiménez-Alberto, A.; Aparicio-Ozores, G.; Castelán-Vega, J.A.; Ribas-Aparicio, R.M. An update on Zika virus vaccine development and new research approaches. Microbiol. Res. 2024, 15, 667–692. [Google Scholar] [CrossRef]
- Goubran, H.A.; Burnouf, T.; Radosevic, M.; El-Ekiaby, M. The platelet–cancer loop. Eur. J. Intern. Med. 2013, 24, 393–400. [Google Scholar] [CrossRef]
- Metharom, P.; Falasca, M.; Berndt, M.C. The history of Armand Trousseau and cancer-associated thrombosis. Cancers 2019, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Gervaso, L.; Dave, H.; Khorana, A.A. Venous and arterial thromboembolism in patients with cancer: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. 2021, 3, 173–190. [Google Scholar] [CrossRef]
- Cosmi, B. An update on the efficacy and safety of novel anticoagulants for cancer associated thrombosis. Expert Opin. Pharmacother. 2021, 22, 583–594. [Google Scholar] [CrossRef]
- Smorenburg, S.M.; Van Noorden, C.J.F. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacol. Rev. 2001, 53, 93–106. [Google Scholar]
- Ma, S.-N.; Mao, Z.-X.; Wu, Y.; Liang, M.-X.; Wang, D.-D.; Chen, X.; Chang, P.; Zhang, W.; Tang, J.-H. The anti-cancer properties of heparin and its derivatives: A review and prospect. Cell Adhes. Migr. 2020, 14, 118–128. [Google Scholar] [CrossRef]
- Falanga, A.; Marchetti, M. Cancer-associated thrombosis: Enhanced awareness and pathophysiologic complexity. J. Thromb. Haemost. 2023, 21, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Coombe, D.R.; Gandhi, N.S. Heparanase: A challenging cancer drug target. Front. Oncol. 2019, 9, 1316. [Google Scholar] [CrossRef]
- Kaur, R.; Deb, P.K.; Diwan, V.; Saini, B. Heparanase inhibitors in cancer progression: Recent advances. Curr. Pharm. Des. 2021, 27, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, U.; Li, J.-P. Heparanase—Discovery and targets. Adv. Exp. Med. Biol. 2020, 1221, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Vlodavsky, I.; Abboud-Jarrous, G.; Elkin, M.; Naggi, A.; Casu, B.; Sasisekharan, R.; Ilan, N. The impact of heparanese and heparin on cancer metastasis and angiogenesis. Pathophysiol. Haemost. Thromb. 2006, 35, 116–127. [Google Scholar] [CrossRef]
- Lanzi, C.; Zaffaroni, N.; Cassinelli, G. Targeting heparan sulfate proteoglycans and their modifying enzymes to enhance anticancer chemotherapy efficacy and overcome drug resistance. Curr. Med. Chem. 2017, 24, 2860–2886. [Google Scholar] [CrossRef]
- Kilarski, W.W.; Bikfalvi, A. Recent developments in tumor angiogenesis. Curr. Pharm. Biotechnol. 2007, 8, 3–9. [Google Scholar] [CrossRef]
- Duckworth, C.A.; Guimond, S.E.; Sindrewicz, P.; Hughes, A.J.; French, N.S.; Lian, L.-Y.; Yates, E.A.; Pritchard, D.M.; Rhodes, J.M.; Turnbull, J.E. Chemically modified, non-anticoagulant heparin derivatives are potent galectin-3 binding inhibitors and inhibit circulating galectin-3-promoted metastasis. Oncotarget 2015, 6, 23671. [Google Scholar] [CrossRef]
- Atallah, J.; Khachfe, H.H.; Berro, J.; Assi, H.I. The use of heparin and heparin-like molecules in cancer treatment: A review. Cancer Treat. Res. Commun. 2020, 24, 100192. [Google Scholar] [CrossRef]
- Ek, L.; Gezelius, E.; Bergman, B.; Bendahl, P.O.; Anderson, H.; Sundberg, J.; Wallberg, M.; Falkmer, U.; Verma, S.; Belting, M. Randomized Phase III trial of low-molecular-weight heparin enoxaparin in addition to standard treatment in small-cell lung cancer: The RASTEN trial. Ann. Oncol. 2018, 29, 398–404. [Google Scholar] [CrossRef]
- Gezelius, E.; Bendahl, P.O.; de Oliveira, K.G.; Ek, L.; Bergman, B.; Sundberg, J.; Strandberg, K.; Krämer, R.; Belting, M. Low-molecular-weight heparin adherence and effects on survival within a randomised phase III lung cancer trial (RASTEN). Eur. J. Cancer 2019, 118, 82–90. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.M.; Mahalingam, D.; Roach, J.M.; Miller, P.J.; Rosano, M.E.; Krause, S.; Avery, W.; Bekaii-Saab, T.S.; Shao, S.H.; Richards, D.A.; et al. Necuparanib combined with nab-paclitaxel+ gemcitabine in patients with metastatic pancreatic cancer: Phase 2 results. J. Clin. Oncol. 2017, 35, 370. [Google Scholar] [CrossRef]
- Chhabra, M.; Wilson, J.C.; Wu, L.; Davies, G.J.; Gandhi, N.S.; Ferro, V. Structural insights into Pixatimod (PG545) inhibition of heparanase, a key enzyme in cancer and viral infections. Chemistry 2022, 28, e202104222. [Google Scholar] [CrossRef]
- Dredge, K.; Brennan, T.V.; Hammond, E.; Lickliter, J.D.; Lin, L.; Bampton, D.; Handley, P.; Lankesheer, F.; Morrish, G.; Yang, Y. A Phase I study of the novel immunomodulatory agent PG545 (Pixatimod) in subjects with advanced solid tumours. Br. J. Cancer. 2018, 118, 1035–1041. [Google Scholar] [CrossRef]
- Khorana, A.A.; McCrae, K.R.; Milentijevic, D.; Fortier, J.; Nelson, W.W.; Laliberté, F.; Crivera, C.; Lefebvre, P.; Yannicelli, D.; Schein, J. Current practice patterns and patient persistence with anticoagulant treatments for cancer-associated thrombosis. Res. Pract. Thromb. Haemost. 2017, 1, 14–22. [Google Scholar] [CrossRef]
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C. Comparison of an oral factor xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J. Clin. Oncol. 2018, 36, 2017–2023. [Google Scholar] [CrossRef]
- Schrag, D.; Uno, H.; Rosovsky, R.; Rutherford, C.; Sanfilippo, K.; Villano, J.L.; Drescher, M.; Jayaram, N.; Holmes, C.; Feldman, L. Direct oral anticoagulants vs low-molecular-weight heparin and recurrent vte in patients with cancer: A randomized clinical trial. JAMA 2023, 329, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Planquette, B.; Bertoletti, L.; Charles-Nelson, A.; Laporte, S.; Grange, C.; Mahé, I.; Pernod, G.; Elias, A.; Couturaud, F.; Falvo, N. Rivaroxaban vs Dalteparin in cancer-associated thromboembolism: A randomized trial. Chest 2022, 161, 781–790. [Google Scholar] [CrossRef]
- McBane, R.D.; Wysokinski, W.E.; Le-Rademacher, J.G.; Zemla, T.; Ashrani, A.; Tafur, A.; Perepu, U.; Anderson, D.; Gundabolu, K.; Kuzma, C. Apixaban and Dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE Trial. J. Thromb. Haemost. 2020, 14662, 411–421. [Google Scholar] [CrossRef]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A. Apixaban for the treatment of venous thromboembolism associated with cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef]
- Raskob, G.E.; Van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F. Edoxaban for the treatment of cancer-associated venous thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, T.-T.; Ye, L.; Ma, J.-J.; Zhang, J.-H. Efficacy and safety of direct oral anticoagulants versus low-molecular-weight heparin for thromboprophylaxis after cancer surgery: A systematic review and meta-analysis. World J. Surg. Oncol. 2024, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Frackiewicz, A.; Kalaska, B.; Miklosz, J.; Mogielnicki, A. The methods for removal of direct oral anticoagulants and heparins to improve the monitoring of hemostasis: A narrative literature review. Thromb. J. 2023, 21, 58. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Coombe, D.R. Heparin mimetics: Their therapeutic potential. Pharmaceuticals 2017, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Nakanishi, K.; Sako, M.; Oba, M.S.; Mori, R.; Ota, E.; Ishikura, K.; Hataya, H.; Honda, M.; Ito, S. A multicenter randomized trial indicates initial prednisolone treatment for childhood nephrotic syndrome for two months is not inferior to six-month treatment. Kidney Int. 2015, 87, 225–232. [Google Scholar] [CrossRef]
- Sinha, A.; Saha, A.; Kumar, M.; Sharma, S.; Afzal, K.; Mehta, A.; Kalaivani, M.; Hari, P.; Bagga, A. Extending initial prednisolone treatment in a randomized control trial from 3 to 6 months did not significantly influence the course of illness in children with steroid-sensitive nephrotic syndrome. Kidney Int. 2015, 87, 217–224. [Google Scholar] [CrossRef]
- Muso, E.; Mune, M.; Hirano, T.; Hattori, M.; Kimura, K.; Watanabe, T.; Yokoyama, H.; Sato, H.; Uchida, S.; Wada, T. Immediate Therapeutic efficacy of low-density lipoprotein apheresis for drug-resistant nephrotic syndrome: Evidence from the short-term results from the POLARIS Study. Clin. Exp. Nephrol. 2015, 19, 379–386. [Google Scholar] [CrossRef]
- Medjeral-Thomas, N.; Ziaj, S.; Condon, M.; Galliford, J.; Levy, J.; Cairns, T.; Griffith, M. Retrospective analysis of a novel regimen for the prevention of venous thromboembolism in nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 2014, 9, 478–483. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Zeng, H.; Lu, W.; Fang, Y. Effects of low molecular weight heparin combined with prednisone on coagulation and kidney function of pediatricwith nephrotic syndrome. Int. J. Clin. Exp. Med. 2019, 12, 6032–6037. [Google Scholar]
- Li, R.; Xing, J.; Mu, X.; Wang, H.; Zhang, L.; Zhao, Y.; Zhang, Y. Sulodexide therapy for the treatment of diabetic nephropathy, a meta-analysis and literature review. Drug Des. Devel. Ther. 2015, 9, 6275–6283. [Google Scholar] [CrossRef][Green Version]
- Stopschinski, B.E.; Thomas, T.L.; Nadji, S.; Darvish, E.; Fan, L.; Holmes, B.B.; Modi, A.R.; Finnell, J.G.; Kashmer, O.M.; Estill-Terpack, S. A synthetic heparinoid blocks tau aggregate cell uptake and amplification. J. Biol. Chem. 2020, 295, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Sanjanwala, D.; Londhe, V.; Trivedi, R.; Bonde, S.; Sawarkar, S.; Kale, V.; Patravale, V. Polysaccharide-based hydrogels for medical devices, implants and tissue engineering: A review. Int. J. Biol. Macromol. 2024, 256, 128488. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.; Xu, X. Abnormal glomerular permeability characteristics in diabetic nephropathy: Implications for the therapeutic use of low–molecular weight heparin. Diabetes Care 2008, 31, S202–S207. [Google Scholar] [CrossRef] [PubMed]
- Abbadi, A.; Loftis, J.; Wang, A.; Yu, M.; Wang, Y.; Shakya, S.; Li, X.; Maytin, E.; Hascall, V. Heparin inhibits proinflammatory and promotes anti-inflammatory macrophage polarization under hyperglycemic stress. J. Biol. Chem. 2020, 295, 4849–4857. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ren, J.; Wang, C.P.; Hascall, V.C. Heparin prevents intracellular hyaluronan synthesis and autophagy responses in hyperglycemic dividing mesangial cells and activates synthesis of an extensive extracellular monocyte-adhesive hyaluronan matrix after completing cell division. J. Biol. Chem. 2014, 289, 9418–9429. [Google Scholar] [CrossRef]
- Ceol, M.; Gambaro, G.; Sauer, U.; Baggio, B.; Anglani, F.; Forino, M.; Facchin, S.; Bordin, L.; Weigert, C.; Nerlich, A. Glycosaminoglycan therapy prevents TGF-Β1 overexpression and pathologic changes in renal tissue of long-term diabetic rats. J. Am. Soc. Nephrol. 2000, 11, 2324–2336. [Google Scholar] [CrossRef]
- Myint, K.-M.; Yamamoto, Y.; Doi, T.; Kato, I.; Harashima, A.; Yonekura, H.; Watanabe, T.; Shinohara, H.; Takeuchi, M.; Tsuneyama, K. RAGE Control of diabetic nephropathy in a mouse model: Effects of rage gene disruption and administration of low–molecular weight heparin. Diabetes 2006, 55, 2510–2522. [Google Scholar] [CrossRef]
- Yamamoto, H.; Watanabe, T.; Yamamoto, Y.; Yonekura, H.; Munesue, S.; Harashima, A.; Ooe, K.; Hossain, S.; Saito, H.; Murakami, N. RAGE in diabetic nephropathy. Curr. Mol. Med. 2007, 7, 752–757. [Google Scholar] [CrossRef]
- Tian, Y. Observation of curative effect of fushen decoction combined with low molecular weight heparin on nephritis with anaphylactic purpura of heat-wet stasis syndrome in children. Mod. J. Integr. Tradit. Chin. West. Med. 2016, 25, 3562–3565+3569. [Google Scholar]
- Chen, J.-Y.; Mao, J.-H. Henoch-Schönlein purpura nephritis in children: Incidence, pathogenesis and management. World J. Pediatr. 2015, 11, 29–34. [Google Scholar] [CrossRef]
- Cao Lei, M.F.W.J. Anticoagulant effect of low molecular weight heparin in hemodialysis treatment of acute renal failure. China Pract. Med. 2019, 14, 119–120. [Google Scholar]
- Wong, S.S.-M.; Lau, W.-Y.; Chan, P.-K.; Wan, C.-K.; Cheng, Y.-L. Low-molecular weight heparin infusion as anticoagulation for haemodialysis. Clin. Kidney J. 2016, 9, 630–635. [Google Scholar] [CrossRef]
- Guo, J.W.S.J.; Zhong, G.Y.S.X. Guidelines for rational drug use in coronary heart disease (Second Ed.). Chin. J. Front. Med. Sci. (Electron. Ed.) 2018, 10, 1–130. [Google Scholar]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with st-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with st-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Guidelines for rational use of thrombolytic therapy in patients with acute ST segment elevation myocardial infarction (Second Ed.). Chin. J. Front. Med. Sci. (Electron. Ed.) 2019, 11, 40–65.
- Eikelboom, J.W.; Anand, S.S.; Malmberg, K.; Weitz, J.I.; Ginsberg, J.S.; Yusuf, S. Unfractionated heparin and low-molecular-weight heparin in acute coronary syndrome without st elevation: A Meta-Analysis. Lancet 2000, 355, 1936–1942. [Google Scholar] [CrossRef]
- Cohen, M.; Mahaffey, K.W.; Pieper, K.; Pollack, C.V.; Antman, E.M.; Hoekstra, J.; Goodman, S.G.; Langer, A.; Col, J.J.; White, H.D. A subgroup analysis of the impact of prerandomization antithrombin therapy on outcomes in the sYNERGY Trial: Enoxaparin versus unfractionated heparin in Non–ST-Segment elevation acute coronary syndromes. J. Am. Coll. Cardiol. 2006, 48, 1346–1354. [Google Scholar] [CrossRef]
- Bikdeli, B.; Erlinge, D.; Valgimigli, M.; Kastrati, A.; Han, Y.; Steg, P.G.; Stables, R.H.; Mehran, R.; James, S.K.; Frigoli, E. Bivalirudin versus heparin during PCI in NSTEMI: Individual patient data meta-analysis of large randomized trials. Circulation 2023, 148, 1207–1219. [Google Scholar] [CrossRef]
- Erlinge, D.; Omerovic, E.; Fröbert, O.; Linder, R.; Danielewicz, M.; Hamid, M.; Swahn, E.; Henareh, L.; Wagner, H.; Hårdhammar, P. Bivalirudin versus Heparin monotherapy in myocardial infarction. N. Engl. J. Med. 2017, 377, 1132–1142. [Google Scholar] [CrossRef]
- Patel, H.; Garris, R.; Bhutani, S.; Shah, P.; Rampal, U.; Vasudev, R.; Melki, G.; Ghalyoun, B.A.; Virk, H.; Bikkina, M. Bivalirudin versus heparin during percutaneous coronary intervention in patients with acute myocardial infarction. Cardiol. Res. 2019, 10, 278. [Google Scholar] [CrossRef]
- Centurión, O.A. Heparin versus bivalirudin in acute myocardial infarction: Unfractionated heparin monotherapy elevated to primary treatment in contemporary percutaneous coronary intervention. Open Cardiovasc. Med. J. 2016, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Deharo, P.; Johnson, T.W.; Rahbi, H.; Kandan, R.; Bowles, R.; Mozid, A.; Dorman, S.; Strange, J.W.; Baumbach, A. Bivalirudin versus heparin in primary pci: Clinical outcomes and cost analysis. Open Heart 2018, 5, e000767. [Google Scholar] [CrossRef]
- Rashid, M.K.; Singh, K.; Bernick, J.; Wells, G.A.; Hibbert, B.; Russo, J.; So, D.Y.; Le May, M.R. Periprocedural bivalirudin versus unfractionated heparin during percutaneous coronary intervention following fibrinolysis for ST-Segment elevation myocardial infarction. J. Invasive Cardiol. 2019, 31, E387–E391. [Google Scholar] [PubMed]
- Al-Abdouh, A.; Mhanna, M.; Jabri, A.; Madanat, L.; Alhuneafat, L.; Mostafa, M.R.; Kundu, A.; Gupta, V. Bivalirudin versus unfractionated heparin in patients with myocardial infarction undergoing percutaneous coronary intervention: A systematic review and meta-analysis of randomized controlled trials. Cardiovasc. Revasc. Med. 2024, 61, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Gargiulo, G.; Capranzano, P.; Mehran, R.; Tamburino, C.; Stone, G.W. Bivalirudin versus heparin with or without glycoprotein IIb/IIIa inhibitors in patients with STEMI undergoing primary PCI: An updated meta-analysis of 10,350 patients from five randomized clinical trials. Eur. Heart J. Acute Cardiovasc. Care. 2016, 5, 253–262. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H. The ‘Ten Commandments’ for the 2020 ESC Guidelines for the Management of Acute coronary syndromes in patients presenting without persistent ST-segment elevation 2020. Eur. Heart J. 2020, 41, 3495–3497. [Google Scholar] [CrossRef]
- Bergamaschini, L.; Rossi, E.; Vergani, C.; De Simoni, M.G. Alzheimer’s disease: Another target for heparin therapy. Sci. World J. 2009, 9, 891–908. [Google Scholar] [CrossRef]
- Ma, Q.; Cornelli, U.; Hanin, I.; Jeske, W.P.; Linhardt, R.J.; Walenga, J.M.; Fareed, J.; Lee, J.M. Heparin oligosaccharides as potential therapeutic agents in senile dementia. Curr. Pharm. Des. 2007, 13, 1607–1616. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Snow, A.D.; Cummings, J.A.; Lake, T. The unifying hypothesis of Alzheimer’s Disease: Heparan sulfate proteoglycans/glycosaminoglycans are key as first hypothesized over 30 years ago. Front. Aging Neurosci. 2021, 13, 710683. [Google Scholar] [CrossRef]
- Timmer, N.M.; van Dijk, L.; van der Zee, C.E.E.M.; Kiliaan, A.; de Waal, R.M.W.; Verbeek, M.M. Enoxaparin treatment administered at both early and late stages of amyloid β deposition improves cognition of APPswe/PS1dE9 mice with differential effects on brain Aβ Levels. Neurobiol. Dis. 2010, 40, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Leveugle, B.; Ding, W.; Laurence, F.; Dehouck, M.; Scanameo, A.; Cecchelli, R.; Fillit, H. Heparin oligosaccharides that pass the blood-brain barrier inhibit β-amyloid precursor protein secretion and heparin binding to Β-Amyloid peptide. J. Neurochem. 1998, 70, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, Y.; Song, X.; Xiao, Y.; Su, G.; Liu, X.; Wang, Z.; Xu, Y.; Liu, J.; Eliezer, D. 3-O-Sulfation of heparan sulfate enhances tau interaction and cellular uptake. Angew. Chem. Int. Ed. 2020, 59, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Mycroft-West, C.J.; Devlin, A.J.; Cooper, L.C.; Guimond, S.E.; Procter, P.; Guerrini, M.; Miller, G.J.; Fernig, D.G.; Yates, E.A.; Lima, M.A. Glycosaminoglycans from litopenaeus vannamei inhibit the Alzheimer’s disease β Secretase, BACE1. Mar. Drugs 2021, 19, 203. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Cooper, L.C.; Devlin, A.J.; Procter, P.; Guimond, S.E.; Guerrini, M.; Fernig, D.G.; Lima, M.A.; Yates, E.A.; Skidmore, M.A. A Glycosaminoglycan extract from portunus pelagicus inhibits BACE1, the β Secretase Implicated in Alzheimer’s Disease. Mar. Drugs 2019, 17, 293. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Devlin, A.J.; Cooper, L.C.; Procter, P.; Miller, G.J.; Fernig, D.G.; Guerrini, M.; Guimond, S.E.; Lima, M.A.; Yates, E.A. Inhibition of BACE1, the β-Secretase implicated in Alzheimer’s Disease, by a chondroitin sulfate extract from Sardina pilchardus. Neural Regen. Res. 2020, 15, 1546–1553. [Google Scholar] [CrossRef]
- Wang, Z.; Patel, V.N.; Song, X.; Xu, Y.; Kaminski, A.M.; Doan, V.U.; Su, G.; Liao, Y.; Mah, D.; Zhang, F. Increased 3-O-Sulfated Heparan Sulfate in Alzheimer’s Disease Brain Is Associated with Genetic Risk Gene HS3ST1. Sci. Adv. 2023, 9, eadf6232. [Google Scholar] [CrossRef]
- Shin, H.-W.; Hong, S.-W.; Youn, Y.C. Clinical aspects of the differential diagnosis of Parkinson’s Disease and parkinsonism. J. Clin. Neurol. 2022, 18, 259. [Google Scholar] [CrossRef]
- Wang, Q.; Bu, C.; Wang, H.; Zhang, B.; Chen, Q.; Shi, D.; Chi, L. Distinct mechanisms underlying the therapeutic effects of low-molecular-weight heparin and chondroitin sulfate on Parkinson’s Disease. Int. J. Biol. Macromol. 2024, 262, 129846. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Marchetti, M.; Vignoli, A. Coagulation and cancer: Biological and clinical aspects. J. Thromb. Haemost. 2013, 11, 223–233. [Google Scholar] [CrossRef]
- Ejaz, U.; Akhtar, F.; Xue, J.; Wan, X.; Zhang, T.; He, S. Inhibitory potential of low molecular weight heparin in cell adhesion; emphasis on tumor metastasis. Eur. J. Pharmacol. 2021, 892, 173778. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Luo, Y.; Xu, D.; Ke, X.; Ci, T. Low molecular weight heparin modified bone targeting liposomes for orthotopic osteosarcoma and breast cancer bone metastatic tumors. Int. J. Biol. Macromol. 2020, 164, 2583–2597. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Q.; Zou, Y.; Guo, Q.; Huang, J.; Tao, L.; Shen, X.; Peng, J. Co-delivery of doxorubicin and epacadostat via heparin coated ph-sensitive liposomes to suppress the lung metastasis of melanoma. Int. J. Pharm. 2020, 584, 119446. [Google Scholar] [CrossRef]
- Cao, D.; Li, H.; Luo, Y.; Feng, N.; Ci, T. Heparin modified photosensitizer-loaded liposomes for tumor treatment and alleviating metastasis in phototherapy. Int. J. Biol. Macromol. 2021, 168, 526–536. [Google Scholar] [CrossRef]
- Mei, L.; Liu, Y.; Zhang, H.; Zhang, Z.; Gao, H.; He, Q. Antitumor and antimetastasis activities of heparin-based micelle served as both carrier and drug. ACS Appl. Mater. Interfaces 2016, 8, 9577–9589. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, Y.; Zhang, Y.; Gao, S.; Yang, X.; Ye, L.; Zhai, G. Cancer cell membrane camouflaged biomimetic nanosheets for enhanced chemo-photothermal-starvation therapy and tumor microenvironment remodeling. Appl. Mater. Today 2022, 29, 101677. [Google Scholar] [CrossRef]
- Erpenbeck, L.; Schön, M.P. Deadly Allies: The fatal interplay between platelets and metastasizing cancer cells. Blood 2010, 115, 3427–3436. [Google Scholar] [CrossRef]
- Norrby, K. Low-molecular-weight heparins and angiogenesis. Apmis 2006, 114, 79–102. [Google Scholar] [CrossRef]
- Akl, M.R.; Nagpal, P.; Ayoub, N.M.; Tai, B.; Prabhu, S.A.; Capac, C.M.; Gliksman, M.; Goy, A.; Suh, K.S. Molecular and clinical significance of fibroblast growth factor 2 (FGF2/BFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget 2016, 7, 44735. [Google Scholar] [CrossRef]
- Knaack, S.; Lode, A.; Hoyer, B.; Rösen-Wolff, A.; Gabrielyan, A.; Roeder, I.; Gelinsky, M. Heparin Modification of a biomimetic bone matrix for controlled release of VEGF. J. Biomed. Mater. Res. Part A 2014, 102, 3500–3511. [Google Scholar] [CrossRef]
- Nawaz, A.; Zaman Safi, S.; Sikandar, S.; Zeeshan, R.; Zulfiqar, S.; Mehmood, N.; Alobaid, H.M.; Rehman, F.; Imran, M.; Tariq, M. Heparin-loaded alginate hydrogels: Characterization and molecular mechanisms of their angiogenic and anti-microbial potential. Materials 2022, 15, 6683. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Y.; Zhang, Y.; Huang, M.; Zhou, Z.; Luo, W.; Tang, J.; Wang, J.; Xiao, Q.; Chen, H. Dual-targeting heparin-based nanoparticles that re-assemble in blood for glioma therapy through both anti-proliferation and anti-angiogenesis. Adv. Funct. Mater. 2016, 26, 7873–7885. [Google Scholar] [CrossRef]
- Nourreddine, F.Z.; Oussedik-Oumehdi, H.; Laraba-Djebari, F. Myotoxicity induced by cerastes cerastes venom: Beneficial effect of heparin in skeletal muscle tissue regeneration. Acta Trop. 2020, 202, 105274. [Google Scholar] [CrossRef]
- Saliba, M.J., Jr. Heparin in the treatment of burns: A review. Burns 2001, 27, 349–358. [Google Scholar] [CrossRef]
- Ye, Y.; Zhou, Y.; Jing, Z.; Xu, Y.; Yin, D. Electrospun heparin-loaded nano-fiber sutures for the amelioration of achilles tendon rupture regeneration: In vivo evaluation. J. Mater. Chem. B 2021, 9, 4154–4168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, G. An effective wound healing material based on gold incorporation into a heparin-polyvinyl alcohol nanocomposite: Enhanced in vitro and in vivo care of perioperative period. J. Clust. Sci. 2022, 33, 1655–1665. [Google Scholar] [CrossRef]
- Gulati, K.; Meher, M.K.; Poluri, K.M. Glycosaminoglycan-based resorbable polymer composites in tissue refurbishment. Regen. Med. 2017, 12, 431–457. [Google Scholar] [CrossRef]
- Liang, Y.; Kiick, K.L. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014, 10, 1588–1600. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M. Customized Ca–P/PHBV Nanocomposite scaffolds for bone tissue engineering: Design, fabrication, surface modification and sustained release of growth factor. J. R. Soc. Interface 2010, 7, S615–S629. [Google Scholar] [CrossRef]
- Conzelmann, C.; Müller, J.A.; Perkhofer, L.; Sparrer, K.M.J.; Zelikin, A.N.; Münch, J.; Kleger, A. Inhaled and systemic heparin as a repurposed direct antiviral drug for prevention and treatment of COVID-19. Clin. Med. 2020, 20, e218. [Google Scholar] [CrossRef]
- Ludwig, R.J. Therapeutic use of heparin beyond anticoagulation. Curr. Drug Discov. Technol. 2009, 6, 281–289. [Google Scholar] [CrossRef]
- Zaferani, A.; Talsma, D.; Richter, M.K.S.; Daha, M.R.; Navis, G.J.; Seelen, M.A.; van den Born, J. Heparin/heparan sulphate interactions with complement—A possible target for reduction of renal function loss? Nephrol. Dial. Transplant. 2014, 29, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, L.; Shi, Y.; Yu, S.; Ma, X. Different signaling pathways involved in the anti-inflammatory effects of unfractionated heparin on lipopolysaccharide-stimulated human endothelial cells. J. Inflamm. 2020, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Ferrara, F. Low molecular weight heparin, anti-inflammatory/immunoregulatory and antiviral effects, a short update. Cardiovasc. Drugs Ther. 2023, 37, 277–281. [Google Scholar] [CrossRef]
- Rider, C.C. The potential for heparin and its derivatives in the therapy and prevention of HIV-1 Infection. Glycoconj. J. 1997, 14, 639–642. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, T.; Zhang, W.; Sun, Q.; Li, H.; Li, J. Elucidating the interactions between heparin/heparan sulfate and SARS-CoV-2-related proteins—An important strategy for developing novel therapeutics for the COVID-19 pandemic. Front. Mol. Biosci. 2021, 7, 628551. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jin, W.; Sood, A.; Montgomery, D.W.; Grant, O.C.; Fuster, M.M.; Fu, L.; Dordick, J.S.; Woods, R.J.; Zhang, F. Characterization of heparin and severe acute respiratory syndrome-related Coronavirus 2 (SARS-CoV-2) Spike glycoprotein binding interactions. Antiviral Res. 2020, 181, 104873. [Google Scholar] [CrossRef] [PubMed]
- Wasik, D.; Mulchandani, A.; Yates, M. V A Heparin-Functionalized Carbon Nanotube-Based Affinity Biosensor for Dengue Virus. Biosens. Bioelectron. 2017, 91, 811–816. [Google Scholar] [CrossRef]
- Hendricks, G.L.; Velazquez, L.; Pham, S.; Qaisar, N.; Delaney, J.C.; Viswanathan, K.; Albers, L.; Comolli, J.C.; Shriver, Z.; Knipe, D.M. Heparin octasaccharide decoy liposomes inhibit replication of multiple viruses. Antiviral Res. 2015, 116, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Wu, W.; Xu, X.; Xu, H.; Zhang, T. Recent progress of paclitaxel delivery systems: Covalent and noncovalent approaches. Adv. Ther. 2023, 6, 2200281. [Google Scholar] [CrossRef]
- Ye, L.; Gao, Z.; Zhou, Y.; Yin, X.; Zhang, X.; Zhang, A.; Feng, Z. A PH-sensitive binary drug delivery system based on poly (Caprolactone)–heparin conjugates. J. Biomed. Mater. Res. Part A 2014, 102, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gan, L.; Tao, H.; Wang, Q.; Ye, L.; Zhang, A.; Feng, Z. The synthesis and application of heparin-based smart drug carrier. Carbohydr. Polym. 2016, 140, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, C.-K.; Law, W.-C.; Mok, J.; Zou, J.; Prasad, P.N.; Cheng, C. Well-defined degradable brush polymer–drug conjugates for sustained delivery of Paclitaxel. Mol. Pharm. 2013, 10, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Thacker, B.; Glass, C.; Sharfstein, S. Advancing to recombinant heparin. Am. Pharm. Rev. 2021, 24, 10–14. [Google Scholar]
- Eidi, H.; Joubert, O.; Attik, G.; Duval, R.E.; Bottin, M.C.; Hamouia, A.; Maincent, P.; Rihn, B.H. Cytotoxicity assessment of heparin nanoparticles in NR8383 macrophages. Int. J. Pharm. 2010, 396, 156–165. [Google Scholar] [CrossRef]
- Paluck, S.J.; Nguyen, T.H.; Maynard, H.D. Heparin-mimicking polymers: Synthesis and biological applications. Biomacromolecules. 2016, 17, 3417–3440. [Google Scholar] [CrossRef]
- Yu, H.; Frederiksen, J.; Sullenger, B.A. Applications and future of aptamers that achieve rapid-onset anticoagulation. RNA 2023, 29, 455–462. [Google Scholar] [CrossRef]

| LMWHs | Brand Name | Manufacturing | MW (Da) | Anti-Xa:IIa Ratio | Half-Life (hours) | Primary Indications | Major Adverse Effects | Dosing Route | Bioavailability |
|---|---|---|---|---|---|---|---|---|---|
| Tinzaparin | Innohep | Heparinase-induced beta-eliminative cleavage | 5500–7500 | 2.8:1 | 3–4 | VTE and PE | Bleeding and thrombocytopenia | SC | 90% |
| Dalteparin | Fragmin | Deamination-induced cleavage with nitrous acid | 5000 (14–26% are >8000) | 2.7:1 | 3–5 | DVT, PE, and UA/NSTEMI | Bleeding and thrombocytopenia | SC | 87% |
| Certoparin | Sandoparin | Cleavage through deamination using isoamyl nitrite | 5400 | 2.0–2.2:1 | 5–6 | DVT | Bleeding and thrombocytopenia | SC | >90% |
| Parnaparin | Fluxum | Copper-catalyzed oxidative depolymerization using hydrogen peroxide | 4500 | 2.3:1 | 4 | DVT, PE, and MI | Bleeding and thrombocytopenia | SC | ~100% |
| Enoxaparin | Lovenox, Clexane | The alkaline-induced cleavage of the benzyl ester of heparin via beta-elimination | 2000–8000 (average 4500) | 2.7–4:1 | 4–7 | VTE, PE, and ACS | Bleeding and thrombocytopenia | SC | ~100% |
| Reviparin | Clivarin | Deamination-induced cleavage with nitrous acid | 4400 | 4.2:1 | 3 | DVT, PE, and VTE prophylaxis | Bleeding and thrombocytopenia | SC | 95% |
| Nadroparin | Fraxiparin | Deamination-induced cleavage with nitrous acid | 5000 | 3.3:1 | 3.5 | DVT, PE, and VTE prophylaxis | Bleeding and thrombocytopenia | SC | 89% |
| Bemiparin | Beparine | Heparin depolymerized through alkaline degradation | 3600 | 8:1 | 5–6 | DVT, VTE prophylaxis, and ACS | Bleeding and thrombocytopenia | SC | 96% |
| Ardeparin | Normiflo | The peroxide degradation of heparin | 5500–6500 | 1.7–2.4:1 | 3.3 | DVT and PE | Bleeding and thrombocytopenia | SC | 92% |
| ULMWHs | Brand Name | Manufacturing | MW (Da) | Anti-Xa:IIa Ratio | Half-Life (hours) | Primary Indications | Major Adverse Effects | Dosing Route | Bioavailability |
| Fondaparinux | Arixtra | Diverse synthetic routes | 1508 | 2–4:1 | 17–21 | DVT, PE, VTE, NSTEMI, STEMI, and UA | Bleeding, thrombocytopenia, and HIT (rare) | SC | ~100% |
| Semuloparin | AVE5026 | Selective depolymerization with a phosphazene base induces beta-eliminative cleavage | 2000–3000 (average 2400) | 80:1 | 16–20 | VTE and PE | Bleeding and thrombocytopenia | SC | 90% |
| RO-14 | - | Selective chemical depolymerization induces beta-eliminative cleavage in a non-aqueous medium | 1800–3000 (average 2200) | >20:1 | 8.05 | VTE prophylaxis | - | SC | ~80–100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, R.; Kamihira, M. Multifaceted Heparin: Diverse Applications beyond Anticoagulant Therapy. Pharmaceuticals 2024, 17, 1362. https://doi.org/10.3390/ph17101362
Sultana R, Kamihira M. Multifaceted Heparin: Diverse Applications beyond Anticoagulant Therapy. Pharmaceuticals. 2024; 17(10):1362. https://doi.org/10.3390/ph17101362
Chicago/Turabian StyleSultana, Razia, and Masamichi Kamihira. 2024. "Multifaceted Heparin: Diverse Applications beyond Anticoagulant Therapy" Pharmaceuticals 17, no. 10: 1362. https://doi.org/10.3390/ph17101362
APA StyleSultana, R., & Kamihira, M. (2024). Multifaceted Heparin: Diverse Applications beyond Anticoagulant Therapy. Pharmaceuticals, 17(10), 1362. https://doi.org/10.3390/ph17101362







