Neuroprotective Actions of Hydrogen Sulfide-Releasing Compounds in Isolated Bovine Retinae
Abstract
1. Introduction
2. Results
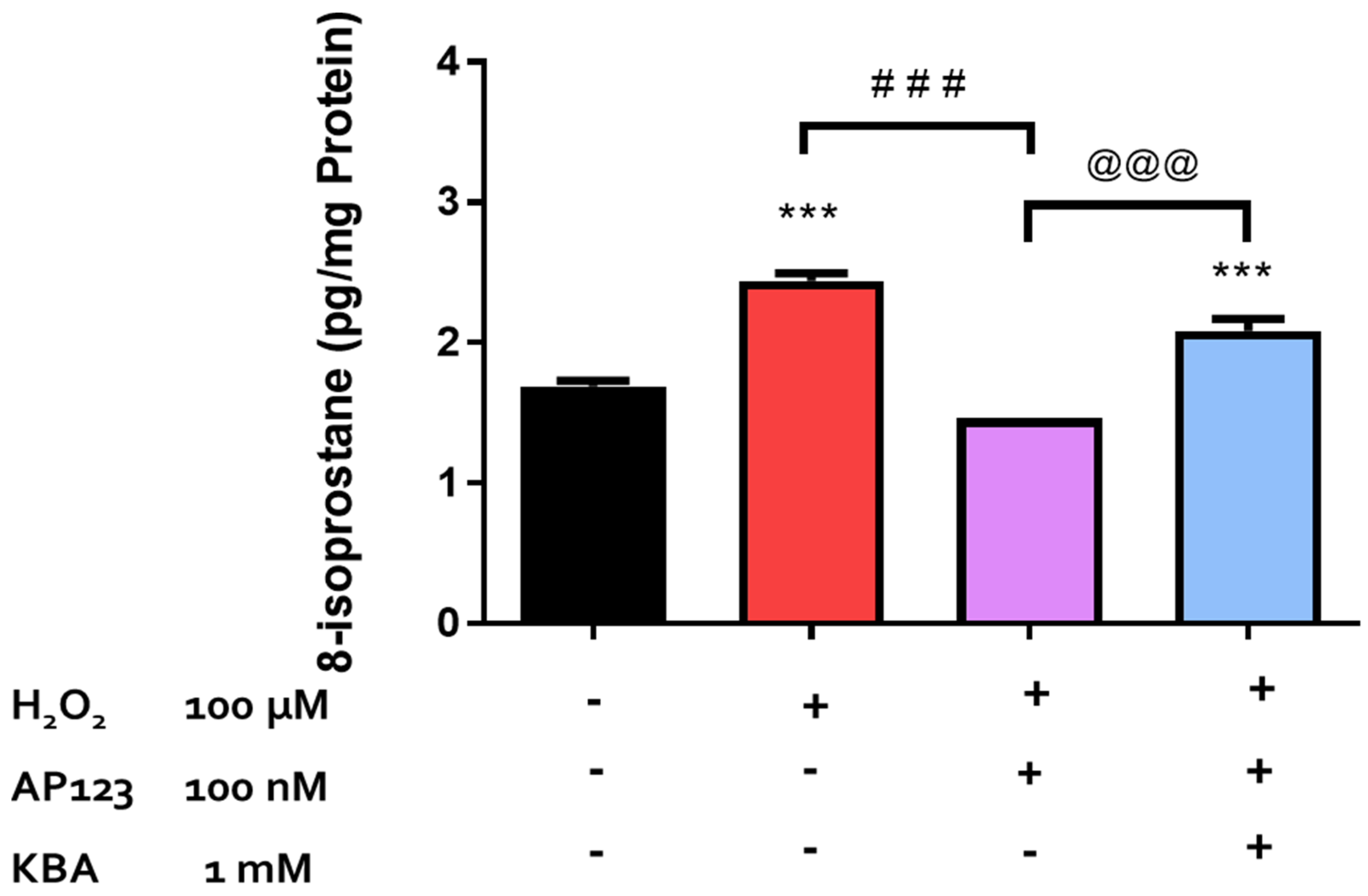
2.1. Effect of H2O2 on 8-Isoprostane Production in the Retina
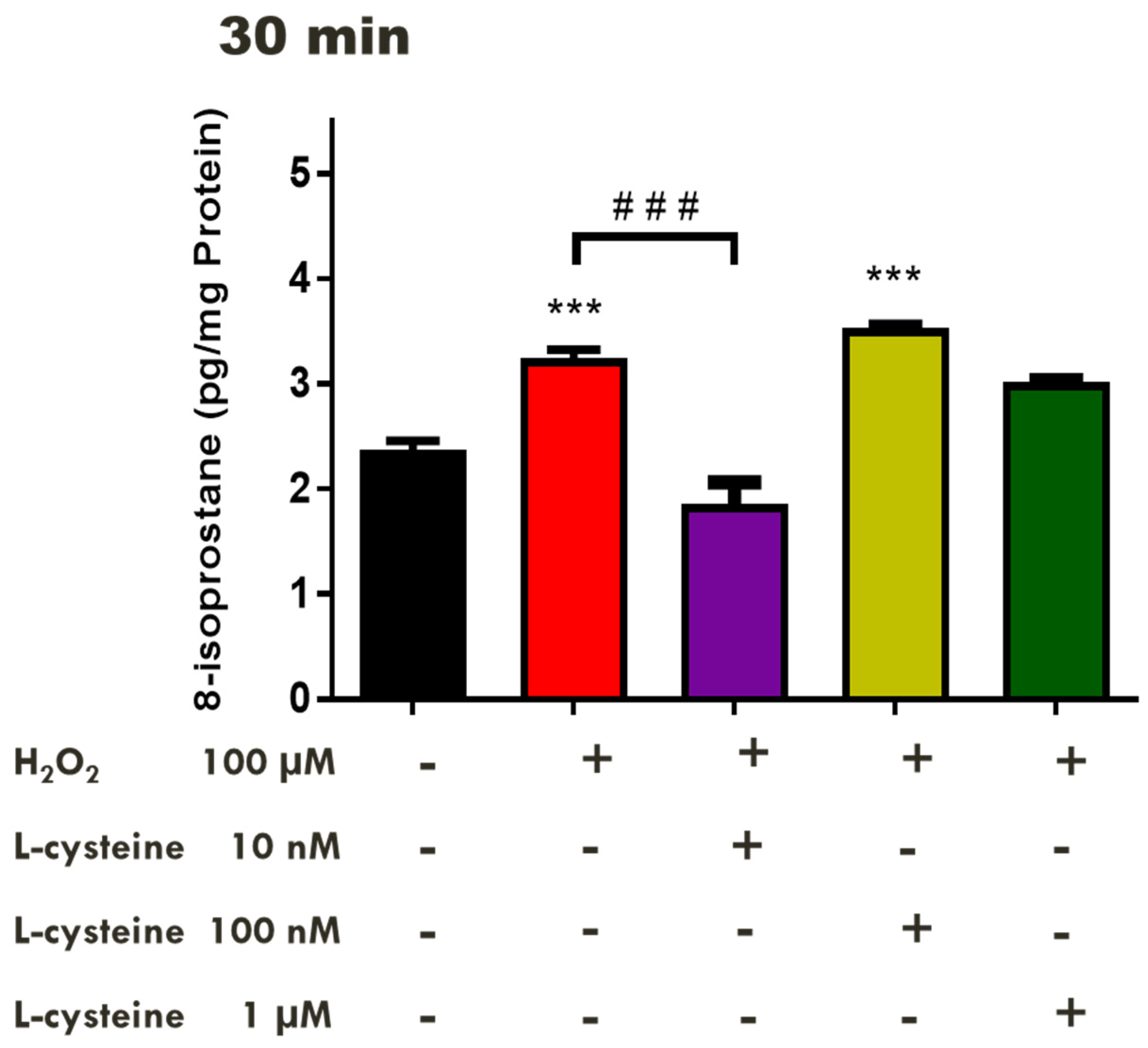
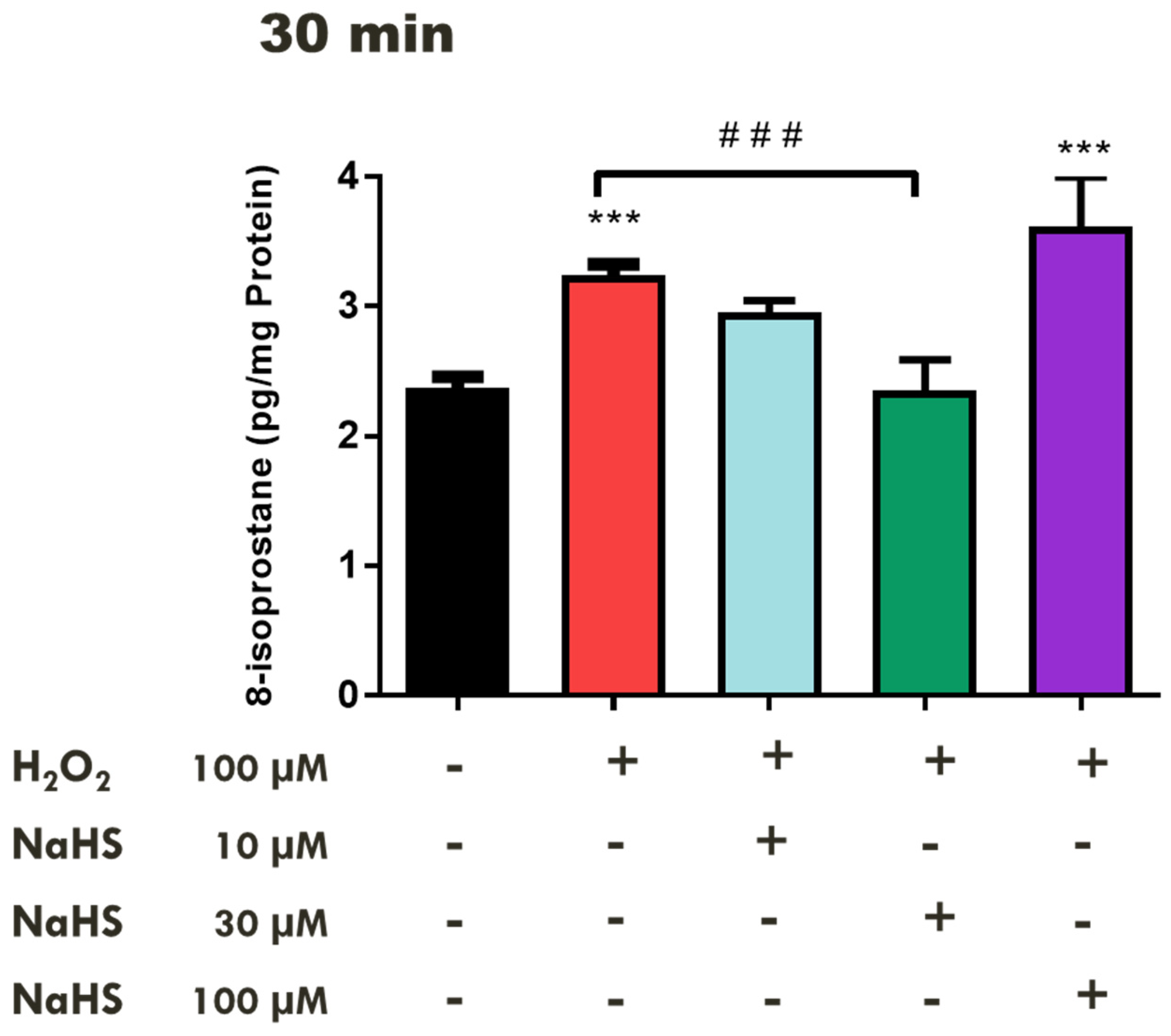
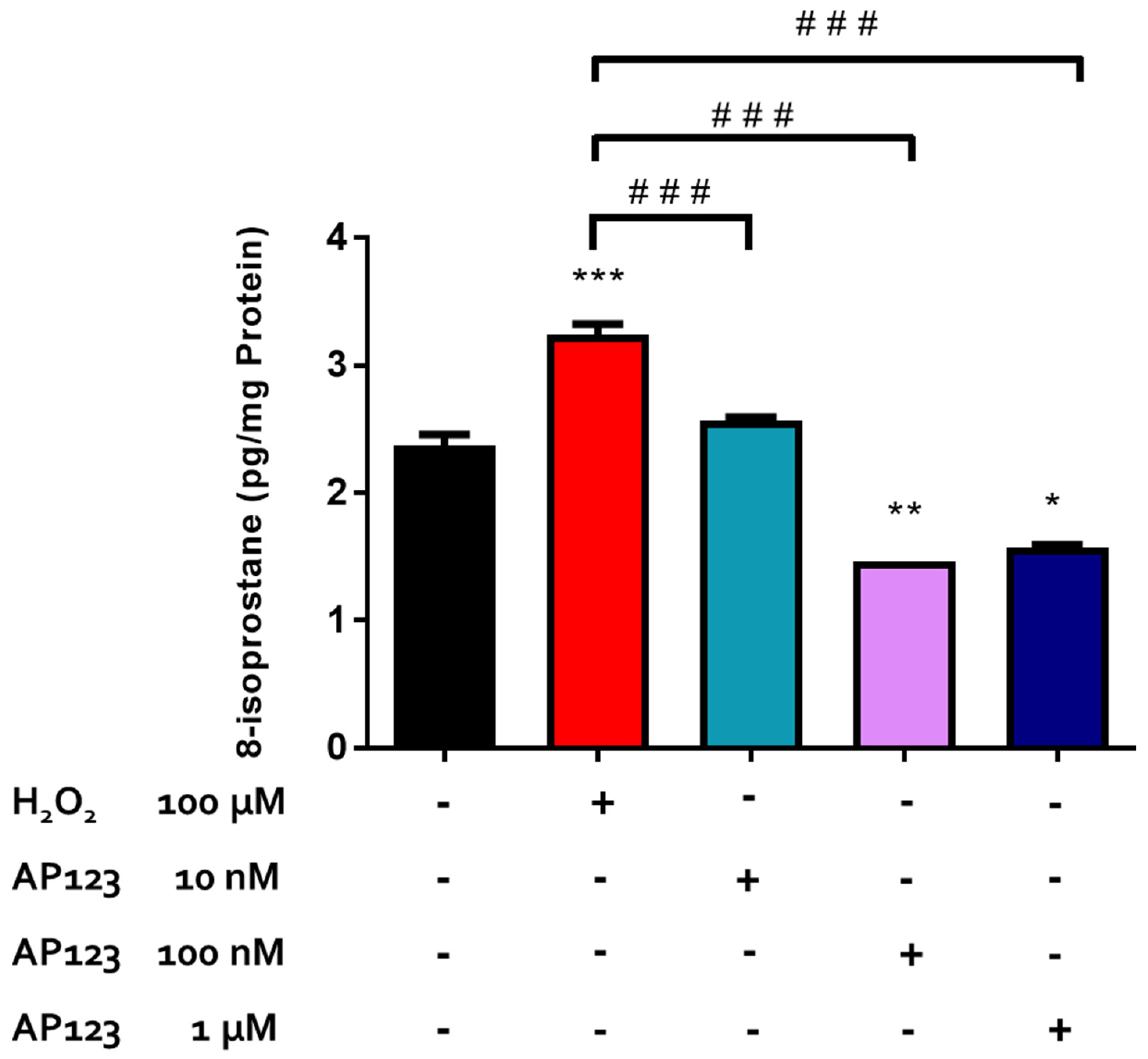
2.2. Effects of H2S-Releasing Compounds on Lipid Peroxidation in the Bovine Retina
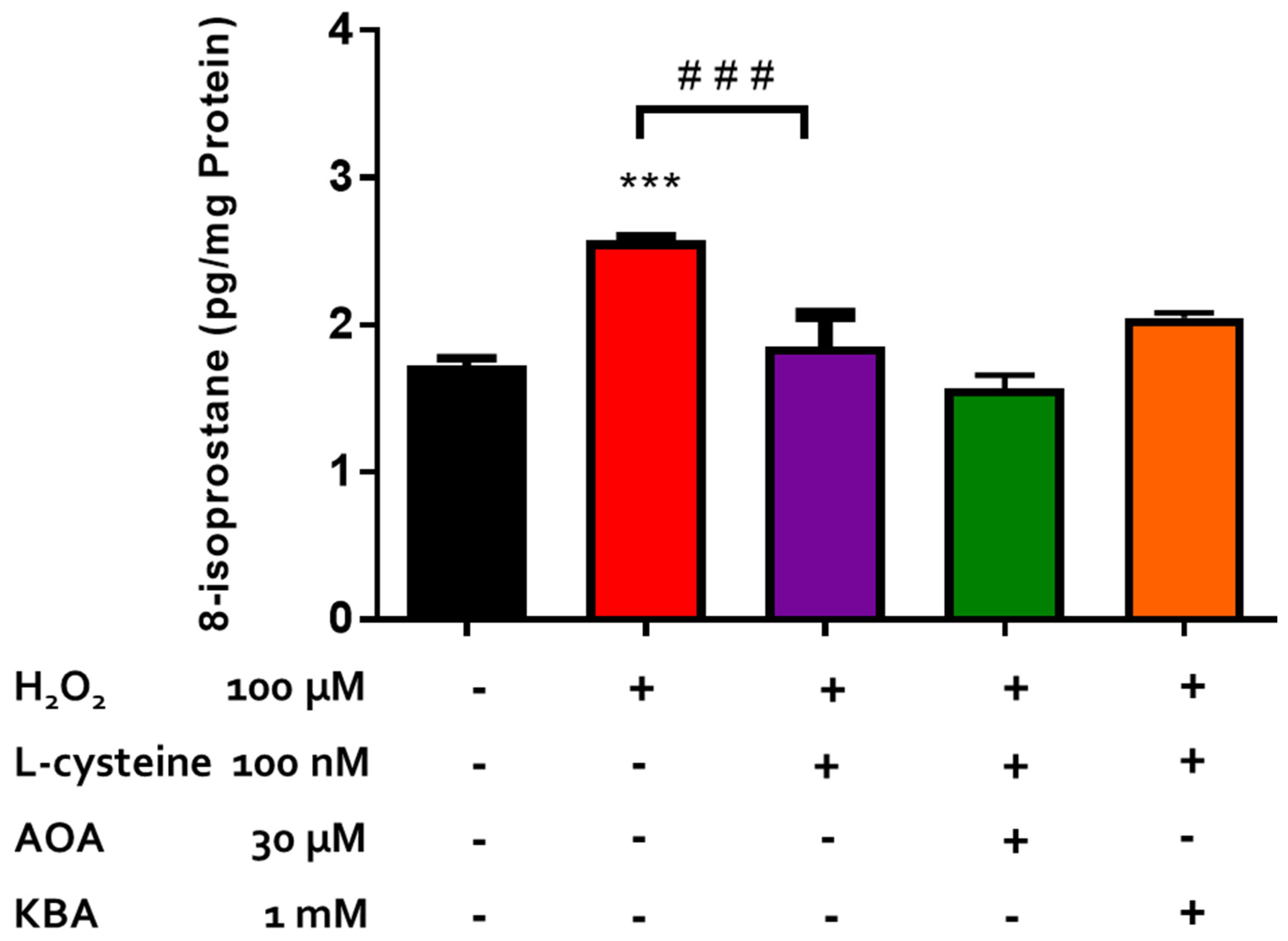
2.3. Effect of Inhibitors of CBS/CSE and 3-MST on the Antioxidant Actions of H2S-Producing Compounds
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Tissue Preparation
4.3. 8-Isoprostane ELISA Assay
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Njie-Mbye, Y.F.; Kulkarni-Chitnis, M.; Opere, C.A.; Barrett, A.; Ohia, S.E. Lipid peroxidation: Pathophysiological and pharmacological implications in the eye. Front. Physiol. 2013, 4, 366. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Smith, S. Prediction of diabetic retinopathy: Role of oxidative stress and relevance of apoptotic biomarkers. EPMA J. 2010, 1, 56–72. [Google Scholar] [CrossRef]
- Berthoud, V.M.; Beyer, E.C. Oxidative stress, lens gap junctions, and cataracts. Antioxid. Redox Signal. 2009, 11, 339–353. [Google Scholar] [CrossRef]
- Sacca, S.; Bolognesi, C.; Battistella, A.; Bagnis, A.; Izzotti, A. Gene–environment interactions in ocular diseases. Mutat. Res. Mol. Mech. Mutagen. 2009, 667, 98–117. [Google Scholar] [CrossRef] [PubMed]
- Hollyfield, J.G. Age-related macular degeneration: The molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: The Proctor lecture. Investig. Opthalmology Vis. Sci. 2010, 51, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- De La Paz, M.A.; Zhang, J.; Fridovich, I. Antioxidant enzymes of the human retina: Effect of age on enzyme activity of macula and periphery. Curr. Eye Res. 1996, 15, 273–278. [Google Scholar] [CrossRef]
- Shih, A.Y.; Erb, H.; Sun, X.; Toda, S.; Kalivas, P.W.; Murphy, T.H. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J. Neurosci. 2006, 26, 10514–10523. [Google Scholar] [CrossRef] [PubMed]
- Wielgus, A.R.; Sarna, T. Ascorbate enhances photogeneration of hydrogen peroxide mediated by the iris melanin. Photochem. Photobiol. 2008, 84, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Hara, H.; Kondo, M.; Hong, S.; Matsugi, T. Oxidative Stress in Retinal Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 4076518. [Google Scholar] [CrossRef]
- Greene, E.L.; Paller, M.S. Xanthine oxidase produces O2−. in posthypoxic injury of renal epithelial cells. Am. J. Physiol. Physiol. 1992, 263 Pt 2, F251–F255. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef] [PubMed]
- Shichi, H. Cataract formation and prevention. Expert Opin. Investig. Drugs 2004, 13, 691–701. [Google Scholar] [CrossRef] [PubMed]
- van Reyk, D.M.; Gillies, M.C.; Davies, M.J. The retina: Oxidative stress and diabetes. Redox Rep. 2003, 8, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ohia, S.E.; Opere, C.A.; LeDay, A.M. Pharmacological consequences of oxidative stress in ocular tissues. Mutat. Res. Mol. Mech. Mutagen. 2005, 579, 22–36. [Google Scholar] [CrossRef]
- Łowicka, E.; Bełtowski, J. Hydrogen sulfide (H2S)—The third gas of interest for pharmacologists. Pharmacol. Rep. 2007, 59, 4–24. [Google Scholar]
- Goodwin, L.R.; Francom, D.; Dieken, F.P.; Taylor, J.D.; Warenycia, M.W.; Reiffenstein, R.J.; Dowling, G. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: Postmortem studies and two case reports. J. Anal. Toxicol. 1989, 13, 105–109. [Google Scholar] [CrossRef]
- Lefer, D.J. A new gaseous signaling molecule emerges: Cardioprotective role of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2007, 104, 17907–17908. [Google Scholar] [CrossRef]
- Savage, J.; Gould, D. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J. Chromatogr. 1990, 526, 540–545. [Google Scholar] [CrossRef]
- Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Tan, B.H.; Wong, P.T.-H.; Bian, J.-S. Hydrogen sulfide: A novel signaling molecule in the central nervous system. Neurochem. Int. 2010, 56, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Yamada, M.; Kimura, H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J. Biol. Chem. 2011, 286, 39379–39386. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Kimura, H. Production of hydrogen sulfide from d-cysteine and its therapeutic potential. Front. Endocrinol. 2013, 4, 57041. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef]
- Gerő, D.; Torregrossa, R.; Perry, A.; Waters, A.; Le-Trionnaire, S.; Whatmore, J.L.; Wood, M.; Whiteman, M. The novel mitochondria-targeted hydrogen sulfide (H2S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol. Res. 2016, 113 Pt A, 186–198. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Zhao, Y.; Biggs, T.D.; Xian, M. Hydrogen sulfide (H2S) releasing agents: Chemistry and biological applications. Chem. Commun. 2014, 50, 11788–11805. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Cheng, Y.; Ndisang, J.F.; Tang, G.; Cao, K.; Wang, R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2316–H2323. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, R.; Matsuki, N.; Kimura, H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide as a neuromodulator. Mol. Neurobiol. 2002, 26, 013–020. [Google Scholar] [CrossRef]
- Qu, K.; Lee, S.W.; Bian, J.S.; Low, C.-M.; Wong, P.T.-H. Hydrogen sulfide: Neurochemistry and neurobiology. Neurochem. Int. 2008, 52, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Mikami, Y.; Osumi, K.; Tsugane, M.; Oka, J.; Kimura, H. Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J. 2013, 27, 2451–2457. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Yang, G.; Wu, L.; Wang, R. Interaction of H2S with Calcium Permeable Channels and Transporters. Oxid. Med. Cell Longev. 2015, 2015, 323269. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Njie-Mbye, Y.F.; Okpobiri, I.; Zhao, M.; Opere, C.A.; Ohia, S.E. Endogenous production of hydrogen sulfide in isolated bovine eye. Neurochem. Res. 2011, 36, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Bankhele, P.; Salvi, A.; Jamil, J.; Njie-Mbye, F.; Ohia, S.; Opere, C.A. Comparative Effects of Hydrogen Sulfide-Releasing Compounds on [3H]D-Aspartate Release from Bovine Isolated Retinae. Neurochem. Res. 2018, 43, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Njie-Mbye, Y.F.; Bongmba, O.Y.N.; Onyema, C.C.; Chitnis, A.; Kulkarni, M.; Opere, C.A.; LeDay, A.M.; Ohia, S.E. Effect of hydrogen sulfide on cyclic AMP production in isolated bovine and porcine neural retinae. Neurochem. Res. 2010, 35, 487–494. [Google Scholar] [CrossRef]
- Njie-Mbye, Y.F.; Kulkarni, M.; Opere, C.A.; Ohia, S.E. Mechanism of action of hydrogen sulfide on cyclic AMP formation in rat retinal pigment epithelial cells. Exp. Eye Res. 2012, 98, 16–22. [Google Scholar] [CrossRef]
- Cornwell, A.; Badiei, A. The role of hydrogen sulfide in the retina. Exp. Eye Res. 2023, 234, 109568. [Google Scholar] [CrossRef] [PubMed]
- Tribble, J.R.; Hui, F.; Quintero, H.; El Hajji, S.; Bell, K.; Di Polo, A.; Williams, P.A. Neuroprotection in glaucoma: Mechanisms beyond intraocular pressure lowering. Mol. Asp. Med. 2023, 92, 101193. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.J.; Chakraborty, S.; Miller, E.; Pieper, A.A.; Paul, B.D. Hydrogen sulfide signalling in neurodegenerative diseases. Br. J. Pharmacol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Perumal, N.; Manicam, C.; Mercieca, K.; Prokosch, V. Proteomics reveals the potential protective mechanism of hydrogen sulfide on retinal ganglion cells in an ischemia/reperfusion injury animal model. Pharmaceuticals 2020, 13, 213. [Google Scholar] [CrossRef]
- Sakamoto, K.; Suzuki, Y.; Kurauchi, Y.; Mori, A.; Nakahara, T.; Ishii, K. Hydrogen sulfide attenuates NMDA-induced neuronal injury via its anti-oxidative activity in the rat retina. Exp. Eye Res. 2014, 120, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Delanty, N.; Reilly, M.P.; Pratico, D.; Lawson, J.A.; McCarthy, J.F.; Wood, A.E.; Ohnishi, S.T.; Fitzgerald, D.J.; FitzGerald, G.A. 8-epi PGF2 alpha generation during coronary reperfusion. A potential quantitative marker of oxidant stress in vivo. Circulation 1997, 95, 2492–2499. [Google Scholar] [CrossRef]
- Gopaul, N.K.; Anggard, E.E.; Mallet, A.I.; Betteridge, D.J.; Wolff, S.P.; Nourooz-Zadeh, J. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. 1995, 368, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004, 18, 1165–1167. [Google Scholar] [CrossRef]
- Baskin, S.I.; Porter, D.W.; Rockwood, G.A.; Romano, J.J.A.; Patel, H.C.; Kiser, R.C.; Cook, C.M.; Ternay, J.A.L. In vitro andin vivo comparison of sulfur donors as antidotes to acute cyanide intoxication. J. Appl. Toxicol. 1999, 19, 173–183. [Google Scholar] [CrossRef]
- Milatovic, D.; Montine, T.J.; Aschner, M. Measurement of isoprostanes as markers of oxidative stress. Methods Mol Biol. 2011, 758, 195–204. [Google Scholar]
- Kadiiska, M.B.; Gladen, B.C.; Baird, D.; Germolec, D.; Graham, L.B.; Parker, C.E.; Nyska, A.; Wachsman, J.T.; Ames, B.N.; Basu, S.; et al. Biomarkers of oxidative stress study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005, 38, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, C.F.; Broek, N.J.F.v.D.; Postma, P.; Berger, R.; Brenkman, A.B. A protocol for quantifying lipid peroxidation in cellular systems by F2-isoprostane analysis. PLoS ONE 2013, 8, e80935. [Google Scholar] [CrossRef] [PubMed]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Czerska, M.; Mikołajewska, K.; Zieliński, M.; Gromadzińska, J.; Wąsowicz, W. Today’s oxidative stress markers. Med. Pr. 2015, 66, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Nourooz-Zadeh, J.; Pereira, P. F2 isoprostanes, potential specific markers of oxidative damage in human retina. Ophthalmic Res. 2000, 32, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, F.; Cervellati, C.; Romani, A.; Cremonini, E.; Sticozzi, C.; Belmonte, G.; Pessina, F.; Valacchi, G. Hypoxia induces cell damage via oxidative stress in retinal epithelial cells. Free Radic. Res. 2014, 48, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.L.; Pye, Q.N.; Chen, L.; Seal, S.; McGinnis, J.F. Defining the catalytic activity of nanoceria in the P23H-1 rat, a photoreceptor degeneration model. PLoS ONE 2015, 10, e0121977. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, N.; Xu, W.; Xu, G. Mesenchymal stem cells attenuate hydrogen peroxide-induced oxidative stress and enhance neuroprotective effects in retinal ganglion cells. Vitr. Cell. Dev. Biol.-Anim. 2017, 53, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Guo, J.; Zhou, L.; Zhu, S.; Wang, C.; Liu, J.; Hu, S.; Yang, M.; Lin, C. Hydrogen sulfide protects retinal pigment epithelial cells from oxidative stress-induced apoptosis and affects autophagy. Oxid. Med. Cell. Longev. 2020, 2020, 8868564. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhang, X.; Feng, Y.; Yuan, Y. Neuroprotective effects of idebenone on hydrogen peroxide-induced oxidative damage in retinal ganglion cells-5. Int. Ophthalmol. 2023, 43, 3831–3839. [Google Scholar] [CrossRef]
- Wallace, J.L.; Wang, R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, Q.; Zhu, Y. Therapeutic application of hydrogen sulfide donors: The potential and challenges. Front. Med. 2015, 10, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, H.; van den Born, J.C.; Hillebrands, J.-L.; Joles, J.A. Hydrogen sulfide in hypertension. Curr. Opin. Nephrol. Hypertens. 2016, 25, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Sattar, M.; Ahmad, A.; Rathore, H.; Khan, S.; Lazhari, M.; Afzal, S.; Hashmi, F.; Abdullah, N.; Johns, E. A critical review of pharmacological significance of Hydrogen Sulfide in hypertension. Indian J. Pharmacol. 2015, 47, 243–247. [Google Scholar] [CrossRef]
- Kida, K.; Ichinose, F. Hydrogen Sulfide and Neuroinflammation. Handb. Exp. Pharmacol. 2015, 230, 181–189. [Google Scholar] [PubMed]
- Biermann, J.; Lagrèze, W.A.; Schallner, N.; Schwer, C.I.; Goebel, U. Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Mol. Vis. 2011, 17, 1275–1286. [Google Scholar]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Ohia, S.E.; Opere, C.A.; Awe, S.O.; Adams, L.; Sharif, N.A. Human, bovine, and rabbit retinal glutamate-induced [3H]D-aspartate release: Role in excitotoxicity. Neurochem. Res. 2000, 25, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Opere, C.A.; Heruye, S.; Njie-Mbye, Y.-F.; Ohia, S.E.; Sharif, N.A. Regulation of excitatory amino acid transmission in the retina: Studies on neuroprotection. J. Ocul. Pharmacol. Ther. 2018, 34, 107–118. [Google Scholar] [CrossRef]
- Heruye, S.H.; Mbye, Y.F.; Ohia, S.E.; Opere, C.A. Protective action of hydrogen sulfide-releasing compounds against oxidative stress-induced cataract formation in cultured bovine lenses. Curr. Eye Res. 2022, 47, 239–245. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Hu, Q.; Liu, S.; Bai, X.; Xie, Y.; Zhang, T.; Bo, S.; Gao, X.; Wu, S.; et al. Neuroprotective Roles of l-Cysteine in Attenuating Early Brain Injury and Improving Synaptic Density via the CBS/H2S Pathway Following Subarachnoid Hemorrhage in Rats. Front. Neurol. 2017, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, D.-X.; Wang, F.-W.; Zhang, Q.; Du, Z.-X.; Zhan, J.-M.; Yuan, Q.-H.; Ling, E.-A.; Hao, A.-J. L-Cysteine promotes the proliferation and differentiation of neural stem cells via the CBS/H(2)S pathway. Neuroscience 2013, 237, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Qanungo, S.; Wang, M.; Nieminen, A.L. N-Acetyl-L-cysteine enhances apoptosis through inhibition of nuclear factor-kappaB in hypoxic murine embryonic fibroblasts. J. Biol. Chem. 2004, 279, 50455–50464. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Li, L.; Rose, P.; Tan, C.-H.; Parkinson, D.B.; Moore, P.K. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 2010, 12, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Goto, Y.-I.; Kimura, H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Okoro, E.; Ezuedu, C.; Bush, L.; Opere, C.A.; Ohia, S.E.; Njie-Mbye, Y.F. Effects of Hydrogen Sulfide-Releasing Compounds on Aqueous Humor Outflow Facility in Porcine Ocular Anterior Segments, Ex Vivo. J. Ocul. Pharmacol. Ther. 2017, 33, 91–97. [Google Scholar] [CrossRef]
- Ohia, S.E.; Opere, C.A.; Monjok, E.M.; Kouamou, G.; Leday, A.M.; Njie-Mbye, Y.F. Role of hydrogen sulfide production in inhibitory action of L-cysteine on isolated porcine irides. Curr. Eye Res. 2010, 35, 402–407. [Google Scholar] [CrossRef]
- Suzuki, K.; Olah, G.; Modis, K.; Coletta, C.; Kulp, G.; Gerö, D.; Szoleczky, P.; Chang, T.; Zhou, Z.; Wu, L.; et al. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc. Natl. Acad. Sci. USA 2011, 108, 13829–13834. [Google Scholar] [CrossRef]
- Franklin, C.C.; Backos, D.S.; Mohar, I.; White, C.C.; Forman, H.J.; Kavanagh, T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Asp. Med. 2008, 30, 86–98. [Google Scholar] [CrossRef]
- Salvi, A.; Bankhele, P.; Jamil, J.; Chitnis, M.K.; Njie-Mbye, Y.F.; Ohia, S.E.; Opere, C.A. Effect of hydrogen sulfide donors on intraocular pressure in rabbits. J. Ocul. Pharmacol. Ther. 2016, 32, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Ohia, S.E.; Robinson, J.; Mitchell, L.; Ngele, K.K.; Heruye, S.; Opere, C.A.; Njie-Mbye, Y.F. Regulation of aqueous humor dynamics by hydrogen sulfide: Potential role in glaucoma pharmacotherapy. J. Ocul. Pharmacol. Ther. 2018, 34, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Ramlogan-Steel, C.A.; Steel, J.C.; Layton, C.J. Characterisation and validation of the 8-fold quadrant dissected human retinal explant culture model for pre-clinical toxicology investigation. Toxicol. Vitr. 2020, 63, 104716. [Google Scholar] [CrossRef]
- Kulkarni, P.; Payne, S. Eicosanoids in bovine retinal microcirculation. J. Ocul. Pharmacol. Ther. 1997, 13, 139–149. [Google Scholar] [CrossRef] [PubMed]
- LeDay, A.M.; Kulkarni, K.H.; Opere, C.A.; Ohia, S.E. Arachidonic acid metabolites and peroxide-induced inhibition of [3H]D-aspartate release from bovine isolated retinae. Curr. Eye Res. 2004, 28, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Ohnishi, K.; Misaka, E.; Yamazaki, M. Decrease of urinary prostaglandin E2 and prostaglandin F2 alpha excretion by nonsteroidal anti-inflammatory drugs in rats. Relationship to anti-inflammatory activity. Biochem. Pharmacol. 1983, 32, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.-L.; Ohia, S.; Camras, C.; Ohia, E.; Wang, Y. Superior cervical ganglionectomy-induced lowering of intraocular pressure in rabbits: Role of prostaglandins and neuropeptide Y. Gen. Pharmacol. Vasc. Syst. 1999, 32, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Nucci, C.; Gasperi, V.; Tartaglione, R.; Cerulli, A.; Terrinoni, A.; Bari, M.; De Simone, C.; Agro, A.F.; Morrone, L.A.; Corasaniti, M.T.; et al. Involvement of the endocannabinoid system in retinal damage after high intraocular pressure-induced ischemia in rats. Investig. Opthalmology Vis. Sci. 2007, 48, 2997–3004. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bush, L.; Robinson, J.; Okolie, A.; Muili, F.; Opere, C.A.; Whiteman, M.; Ohia, S.E.; Njie Mbye, Y.F. Neuroprotective Actions of Hydrogen Sulfide-Releasing Compounds in Isolated Bovine Retinae. Pharmaceuticals 2024, 17, 1311. https://doi.org/10.3390/ph17101311
Bush L, Robinson J, Okolie A, Muili F, Opere CA, Whiteman M, Ohia SE, Njie Mbye YF. Neuroprotective Actions of Hydrogen Sulfide-Releasing Compounds in Isolated Bovine Retinae. Pharmaceuticals. 2024; 17(10):1311. https://doi.org/10.3390/ph17101311
Chicago/Turabian StyleBush, Leah, Jenaye Robinson, Anthonia Okolie, Fatima Muili, Catherine A. Opere, Matthew Whiteman, Sunny E. Ohia, and Ya Fatou Njie Mbye. 2024. "Neuroprotective Actions of Hydrogen Sulfide-Releasing Compounds in Isolated Bovine Retinae" Pharmaceuticals 17, no. 10: 1311. https://doi.org/10.3390/ph17101311
APA StyleBush, L., Robinson, J., Okolie, A., Muili, F., Opere, C. A., Whiteman, M., Ohia, S. E., & Njie Mbye, Y. F. (2024). Neuroprotective Actions of Hydrogen Sulfide-Releasing Compounds in Isolated Bovine Retinae. Pharmaceuticals, 17(10), 1311. https://doi.org/10.3390/ph17101311





