Ischemic Optic Neuropathy: A Review of Current and Potential Future Pharmacotherapies
Abstract
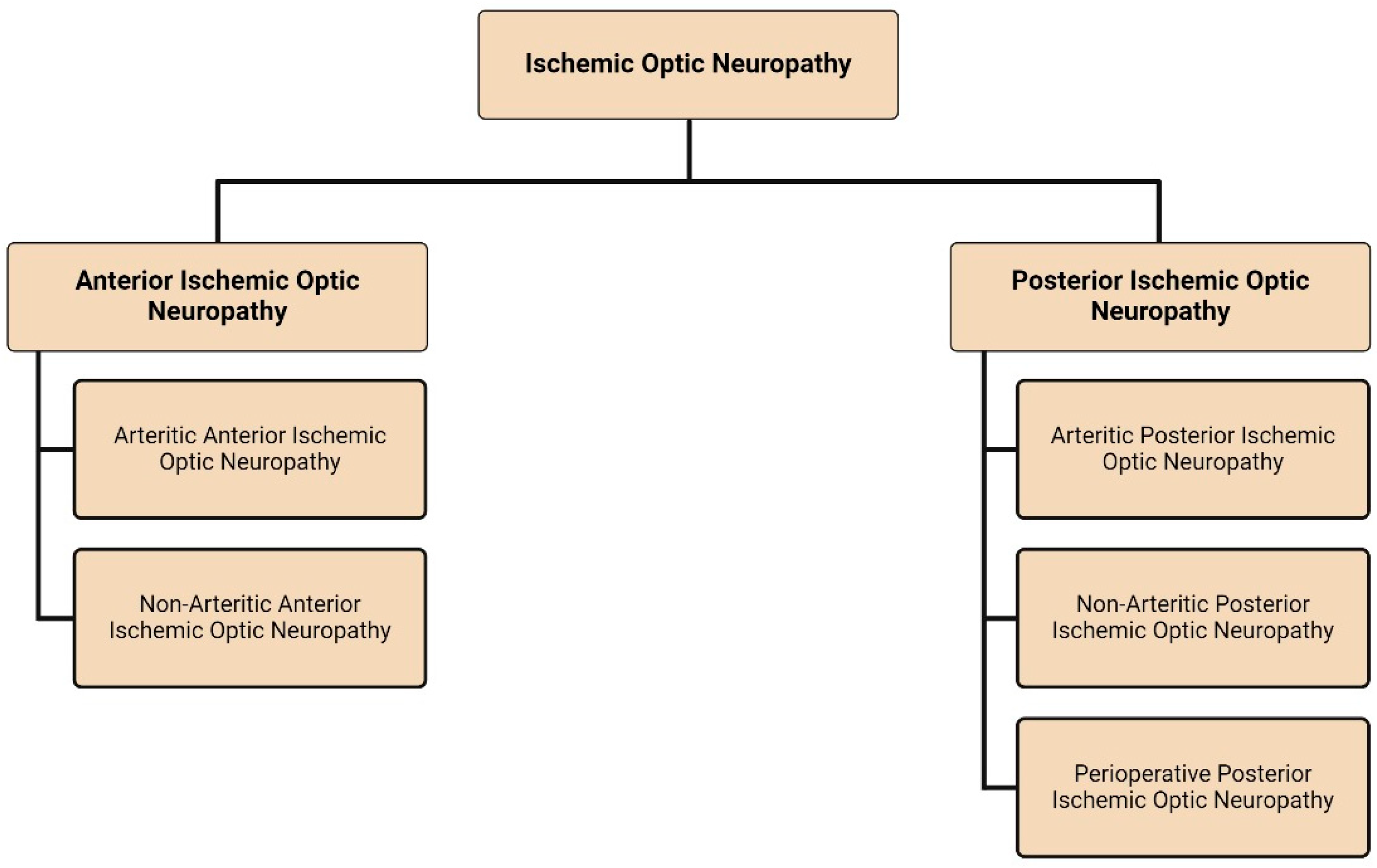
1. Introduction
2. Anterior Ischemic Optic Neuropathy
2.1. Arteritic Anterior Ischemic Optic Neuropathy
2.1.1. Steroid Treatment Strategies
2.1.2. Steroid Sparing Treatment Strategies
- Tocilizumab
- Methotrexate
- Abatacept
- TNF-α inhibitors
- Prostaglandin E1
| Pharmacotherapy | Design of Studies Analyzed | Year of Publication | Conclusions |
|---|---|---|---|
| Corticosteroids | Review [2]. | 2009 | Treatment with a rapidly administered large dose of corticosteroids followed by a long tapering steroid regimen remains the mainstay of AAION treatment. There remains a gap between the length of corticosteroid regimens recommended by ophthalmologists and rheumatologists. |
| Review [8]. | 2011 | ||
| Review [9]. | 2021 | ||
| Guideline Study/Review [10]. | 2020 | ||
| Clinical Trial [11]. | 2000 | ||
| Retrospective Study [12]. | 2001 | ||
| Tocilizumab | Clinical Trial [14]. | 2017 | An FDA-approved for the treatment of GCA, tocilizumab has demonstrated effectiveness as a steroid-sparing therapy. Further studies must be conducted looking at tocilizumab in the context of AAION due to GCA past the currently studied timelines. |
| Randomized, Double-Blind, Placebo-Controlled Trial [15]. | 2016 | ||
| Retrospective Study [16]. | 2016 | ||
| Case Series [17]. | 2011 | ||
| Retrospective Study [18]. | 2015 | ||
| Retrospective Study [19]. | 2012 | ||
| Case Report [20]. | 2014 | ||
| Retrospective Study [22]. | 2021 | ||
| Review [23]. | 2017 | ||
| Methotrexate | Meta-Analysis [25]. | 2007 | Methotrexate was reported to effectively reduce the risk of relapse and corticosteroid exposure in GCA patients. More should be studied about its role in the context of AAION and vision protection. |
| Abatacept | Randomized, Double-Blind Trial [27]. | 2018 | Demonstrated lower remission rates and corticosteroid exposure in the treatment of GCA with a good adverse effect profile. Viable candidate for a future investigation looking at long-term ocular manifestations of GCA. |
| TNF-α inhibitors | Guideline Study/Review [10]. | 2020 | Has not demonstrated significant benefits or efficacy in the treatment of GCA and, therefore, its ocular manifestations. |
| Review [29]. | 2022 | ||
| Prostaglandin E1 | Case Report [30]. | 2010 | In the referenced case report, PGE1 was found to improve and stabilize visual acuity (VA) in two patients on follow-up. Larger studies are necessary to determine its efficacy. |
2.2. Non-Arteritic Anterior Ischemic Optic Neuropathy
2.2.1. Corticosteroids and Associated Pharmacotherapies
- Corticosteroids:
- Triamcinolone:
2.2.2. Neuroprotective Agents
- EPO:
- G-CSF:
- Citicoline:
- Trabodenoson:
- Vincamine:
- CNTF:
- BDNF/LM22A-4:
- Memantine:
- Minocycline:
- Butylidenephthalide:
- Bardoxolone Methyl and Omaveloxolone:
- Prostaglandin J₂ and MAGL/COX inhibitors:
- QPI-1007
- RPh201
- Vitamin B3
- M01, a HECT domain-E3 ubiquitin ligase inhibitor:
- Brimonidine
- Progesterone:
- PLGA-Icariin
- Puerarin:
- miR-124:
2.2.3. Stem Cell Pharmacotherapies
- Mesenchymal Stem Cells:
- Mesenchymal Stem Cell Exosome:
- Mesenchymal Stem Cell-Derived Medium:
2.2.4. Anti-VEGF Pharmacotherapies
- Anti-VEGF:
- Bevacizumab:
- Ranibizumab:
- Aflibercept:
2.2.5. Anti-Parkinson Pharmacotherapies
- Levodopa/Carbidopa:
2.2.6. Blood-Associated Pharmacotherapies
- Aspirin:
- Platelet Rich Plasma:
- Heparin-Induced Extracorporeal LDL/Fibrinogen Precipitation:
- Anticoagulants:
2.2.7. Miscellaneous Pharmacotherapies
- A Multivitamin, Mineral, Carotenoid, and Antioxidant Supplement Regimen:
- 4-PBA:
- 4-phenylbutyric acid (4-PBA) is a chemical chaperone that has been studied in the treatment of cystic fibrosis, liver injury, and animal models of vision loss including glaucoma [93]. The unfolded protein response pathway is used by cells to control the endoplasmic reticulum (ER) and, in the case of cellular insult, to initially act as a defense line activating pro-survival pathways. However, after prolonged endoplasmic reticulum stress, pro-apoptotic pathways are upregulated, and this leads to cell death. Kumar et al. aimed to use intraperitoneally administered 4-BPA to reduce this ER stress and therefore preserve cell survivability in a mouse model of NAAION [93]. 4-PBA-treated NAAION eyes had a significant 22% higher number of RGC, and a significantly higher (5-μm) ganglion cell complex thickness on OCT imaging after induction of NAAION compared to the saline-treated group [93]. These results highlight the unfolded protein response pathway and decreasing ER stress using 4-PBA as valid therapeutic target candidates to be evaluated in human studies and serve in the treatment of NAAION.
- Endothelin Receptor Antagonists: Bosentan:
- Endothelin, a vasoconstrictive peptide released by both endothelial cells and vascular smooth muscle, is strongly implicated in cardiovascular disorders and in obstructive sleep apnea, a condition present in up to 70–85% of patients with NAAION [94]. Studies looking at blocking the effects of endothelin with endothelin receptor antagonist bosentan have found that it increased retinal blood flow at the ONH in both healthy and glaucoma patients [94]. For these reasons, Chiquet et al. considered bosentan a good candidate to target the vasoconstriction caused by endothelin in the acute phase of NAAION and are conducting a multicenter randomized controlled trial looking at change in VF, VA, quality of life, and macular ganglion cell layer thickness. As of 1 July 2024, the results of the clinical trial have not been published, but it remains a promising treatment option for NAAION, which should be followed up.
- Omega-3 Polyunsaturated Fatty Acids:
- Omega-3 polyunsaturated fatty acids (ω-3 PUFAs) are a common food supplement demonstrated to modulate different signaling pathways and inhibit both inflammation and cell apoptosis in multiple models [95,96]. This was studied by Georgiou et al. in rat models of AION. The rats were given ω-3 PUFAs by gavage for 10 days and compared to those given saline. Rats receiving the ω-3 PUFAs were then found to have higher RGC densities, higher amplitudes of FVEP, lower numbers of apoptotic cells in the RGC layer, reduced macrophage recruitment at the ON, and increased M2 macrophage anti-inflammatory markers than in the saline group [97]. This is yet to be evaluated in humans, and further experiments need to be performed to elucidate its uses in NAAION.
- Bioengineered Algae Oil:
- ω-3 PUFAs can contain different ratios of docosahexaenoic acid (DHA) to eicosapentaenoic acid (EPA), and with the background that one study showed that pure DHA or a combination containing more DHA than EPA promoted more expression of neurotrophins and their receptors in neuron cell lines [98], Huang et al. investigated the effects of algae oil from bioengineered marine microalgae Schizochytrium sp., which is a DHA-rich ω-3 PUFA in a model of rAION [99]. Huang et al. found significantly higher FVEP and density of RGCs in the algae oil-treated group [99]. In future human studies, the importance of DHA/EPA ratios in ω-3 PUFAs should be investigated as different ratios could play a role in treatment effectiveness.
- P-Selectin:
- P-selectin plays a role in the recruitment of leukocytes to platelet aggregates and in inflammatory leukocyte extravasations [100]; therefore, targeting this pathway could decrease the number of ischemic injuries in NAAION patients. Kapupara et al. investigated soluble recombinant P-selectin immunoglobulin G chimeric fusion protein in rat AION models and demonstrated an increased RGC survival rate through stabilization of the blood–ON barrier and increased Nrf2 transcription factors levels and activating its signaling pathway [100]. This target needs to be further evaluated in human experiments to assess its efficacy in NAAION patients.
- Anti-Nogo Antibody:
- Nogo-A is an inhibitory protein in the central nervous system that prevents the continued expansion of neurons at the end of development [101]. Johnson et al. investigated the role of anti-NOGO receptor monoclonal antibody 11C7mAb in a rat model of non-arteritic anterior ischemic optic neuropathy and found a higher rate of FVEP preservation and a reduction in microglia, extrinsic macrophages with axon sparing, decreased extracellular debris, and less myelin damage in those receiving the antibody versus the group receiving the vehicle only [101]. Further human studies are required to evaluate 11C7mAb as a treatment for NAAION.
2.2.8. Future Pharmacotherapy Study Targets
- As an effective treatment for NAAION has not been conclusively found, studies looking at potential targets for the treatment of NAAION must be highlighted as only further research can achieve a breakthrough.
- A proteomics study of systemic inflammatory markers in acute and chronic NAAION patients conducted by Mesentier-Luoro et al. identified with immunoprofiling a multitude of markers in both acute and chronic NAAION patients which were significantly unique to each group when compared to the controls, with some overlap between the acute and chronic patients. Since multiple aforementioned studies on corticosteroids aiming to decrease the inflammation component seen in NAAION have not been successful as treatments, a closer, more targeted approach to blunt the inflammatory response could be a possible treatment. Candidate novel specific targets found by Mesentier-Luoro et al. most notably included Eotaxin-3, MCP-2, TPO, and TRAIL in acute NAAION patients and in chronic NAAION, IL-1α, and CXCL10 [102]. These biomarkers reveal more specifics about the systemic inflammation profile of NAAION patients and could be targeted for treatment and help treat the inflammatory component of NAAION. It is important to note the small sample size of the study and the need for a natural history study to have a longitudinal follow-up of patients and try to decrease the effect of inter-patient variability.
- In the previously mentioned study by Kumar et al. (Section 2.2.7, 4-PBA), the unfolded protein response pathway could also serve as a promising area for future research. Kumar et al. identified within those pathways increased expressions of pro-apoptotic transcriptional regulator C/EBP homologous protein (CHOP) and decreased pro-survival chaperon glucose-regulated protein 78 (GRP78) levels in both the ON and RGCs after NAAION induction in mouse models [93]. These elucidated pathways can be further studied or targeted to help further our understanding of treating NAAION.
- When investigating the effects of M01 as a neuroprotector in the aforementioned study, Chien et al. (Section 2.2.2, M01, a HECT domain-E3 ubiquitin ligase inhibitor) found that the protective effect of M01 on RGCs following ON ischemia through upregulating Nr2 was independent of the pathway they hypothesized would be involved, specifically the NEDD4 protein, as it is a known down-regulator of Nrf2. As mentioned by Chien et al., the surprising result calls for more investigation of the E3 ubiquitin ligase inhibitor pathway, or possibly another treatment that could achieve a more potent neuroprotective effect through action on NEDD4.
- Polyamidoamine Dendrimer Nanoparticles:
| Pharmacotherapy | Has This Pharmacotherapy Been Used in Human Patients? | Summary |
|---|---|---|
| Corticosteroids | Yes [3,4,31,33,34,35] | Potential benefit in improving BCVA in the acute phase with MP. Otherwise, no clinically significant benefit improvement in outcome measures. |
| Triamcinolone | Yes [4] | A systematic review and meta-analysis found this drug to improve VA and VF in two studies, which had a relatively small number of cases. Larger, more comprehensive studies are needed to support this data. |
| EPO | Yes [34,35] | EPO administration within five days of NAAION diagnosis led to a functional and structural neuroprotective effect on the ONs at the 6-month follow-up. |
| G-CSF | Yes [43] | In this study, intravitreal injection of G-CSF within 2 weeks of NAAION onset resulted in a BCVA improvement at the 1-month follow-up, but this effect was not seen in the final BCVA measurement indicating the short-term effect of this drug. |
| Citicoline | Yes [45] | A 500 mg/day oral solution of citicoline exerted a neuro-enhancing and neuroprotective effect in a randomized pilot study that enrolled 36 NAAION patients and 20 age-matched controls. These results are promising and need to be verified with larger studies. |
| Trabodenoson | No | Topical trabodenoson has shown promising results in a rodent NAION model but has yet to be tried on human NAAION patients [46]. |
| Vincamine | Yes [48] | Vincamine led to statistically significant improvement in mean deviation of the visual field and RNFL and ganglion cell complex thickness in a study with 27 NAAION patients and 15 age-matched controls. These promising results must be verified with a larger trial. |
| CNTF | No | The positive neuroprotective effects of this drug have been shown in an rAION model but are yet to be verified in human studies [50]. |
| BDNF/LM22A-4 | No | BDNF and LM22A-4 have promising beneficial direct and indirect effects on animal models of NAAION, but no human studies have been performed yet [51,52]. |
| Memantine | Yes [4] | A systematic review and meta-analysis found this drug to only improve VA when analyzed as a continuous variable but not as a categorical variable. Additionally, no improvement in VF was found. |
| Minocycline | No | Compared to previously mentioned neuroprotective drugs, minocycline does not show promise in its ability to preserve RGC in an rNAION model [53]. |
| Butylidenephthalide | No | While the early experimental results in an rAION model are promising, it is essential to follow the experimental evidence with human studies to validate the effect of this drug in NAAION patients [54]. |
| Bardoxolone methyl and omaveloxolone | No | A study of these two treatments has been conducted in an rAION and revealed that out of the two drugs, bardoxolone methyl could be a potential treatment for NAAION, but this needs to be verified with human studies [55]. |
| Prostaglandin J₂ and MAGL/COX inhibitors | No | PGJ2 has been shown to be neuroprotective in rNAION and pNAAION models only [56]. MAGL/COX inhibitors are neuroprotective in rNAION models only when used independently [57]. |
| QPI-1007 | Yes [58,59] | Phase 1 studies showed some promise for QPI-1007 in improving VA [58]; however, phase 2/3 RCTs were terminated and data showed no significant difference [59]. |
| RPh201 | Yes [60] | Phase 1 studies established the safety of RPh201 [61], and Phase 2a demonstrated non-statistically significant improvement of BCVA [60]. Completed Phase 3 study results are unpublished and cannot be assessed. |
| Vitamin B3 | Yes, as part of a multivitamin regimen, not alone [92] | Vitamin B3 showed neuroprotective effects in rat models of NAAION [63]. Evaluated as part of a multivitamin, mineral, and carotenoid regimen in a case series where VFI improved in NAAION patients. However, bigger studies with a comparison group must be held, and vitamin B3 alone has not been assessed [92]. |
| M01, a HECT domain-E3 ubiquitin ligase inhibitor | No | Findings in rNAION models present the modulation of HECT domain-E3 ubiquitin ligase pathways as a new approach toward the treatment of NAAION that needs to be investigated in humans [64]. |
| Brimonidine | Yes [4,49,66,67,68] | Neuroprotective ability in animal studies [65]; however, human trials did not find significant improvement of VA or VF [4,49,66,67,68]. |
| Progesterone | No | Progesterone showed no neuroprotective effects in models of NAAION [69]. |
| PLGA-Icariin | No | Demonstrated a neuroprotective effect in rat NAAION models only [70]. |
| Puerarin | No | Demonstrated a neuroprotective effect in rat NAAION models only [72]. |
| miR-124 | No | Demonstrated a neuroprotective effect in rat NAAION models only [73]. |
| Mesenchymal stem cells | Yes [76] | A prospective, non-randomized phase II study conducted on five NAAION patients ascertained that the treatment was safe, generally well tolerated, and showed positive results, albeit in a limited number of patients. More extensive studies are needed to verify these findings. |
| Mesenchymal stem cell exosome | No | This treatment has yet to be tested on animal NAAION models or human NAAION patients. |
| Mesenchymal stem cell-derived medium | No | The efficacy of MDCM was assessed in the rAION model and was found to preserve visual function and RGC density and reduce inflammation in the ON [74]. These findings are promising but need to be supported by human studies. |
| Bevacizumab | Yes [78,80,81] | The evidence for this drug in human patients is mixed, but the larger studies indicate that bevacizumab is ineffective for NAAION. |
| Ranibizumab | No | Studies in rAION and pNAION models have reported the drug to be ineffective for NAAION [82,83]. |
| Aflibercept | Yes [84,85,86] | Human studies have shown promising results, which need to be supported by larger studies. |
| Levodopa/Carbidopa | Yes [4,90,91,92,93] | Overall, there seems to be contradicting evidence within human studies; therefore, more studies must be carried out to support one conclusion. |
| Aspirin | Yes [49,79,90] | Aspirin was found to be ineffective as a treatment for NAAION. As a preventative measure for the development of NAAION in the second eye, aspirin was found to have mixed results but is still recommended after an episode of NAAION in patients with vasculopathic risk factors. |
| Platelet-rich plasma | Yes. [91] | A prospective nonrandomized controlled trial revealed that this modality is ineffective in NAAION patients when compared to controls. |
| Heparin-induced extracorporeal LDL/fibrinogen precipitation | Yes [4] | This modality did not improve VA as a categorical variable when assessed in a systematic review and meta-analysis. |
| Anticoagulants | Yes [4] | This modality was ineffective in patients with NAAION. |
| A multivitamin, mineral, carotenoid, and antioxidant supplement regimen | Yes [92] | Evaluated in a case series where VFI improved in NAAION patients. However, bigger studies with a comparison group must be held [92]. |
| 4-PBA | No | Demonstrated a neuroprotective effect in rat NAAION models only [93]. |
| Bosentan | In progress [94] | Ongoing multicenter randomized controlled trial on bosentan in NAAION [94]. |
| Omega-3 polyunsaturated fatty acid | No | Demonstrated a neuroprotective effect in rat NAAION models only [97]. |
| Bioengineered algae oil | No | Demonstrated a neuroprotective effect in rat NAAION models only [99]. |
| P-Selectin | No | Demonstrated a neuroprotective effect in rat NAAION models only [100]. |
| Anti-Nogo antibody | No | Demonstrated a neuroprotective effect in rat NAAION models only [101]. |
3. Posterior Ischemic Optic Neuropathy
3.1. Arteritic Posterior Ischemic Optic Neuropathy
3.2. Non-Arteritic Posterior Ischemic Optic Neuropathy
3.3. Perioperative Posterior Ischemic Optic Neuropathy
| Drug | Mechanism of Action | Effectiveness | Arteritic PION | Non-Arteritic PION | Perioperative PION | References |
|---|---|---|---|---|---|---|
| Systemic steroids (e.g., prednisone) | Reduces inflammation | Prevents further visual deterioration | ✓ | [8,104] | ||
| High-dose steroids | Reduces inflammation | Significant improvement in visual acuity and fields, though not always effective | ✓ | [8,104,112] | ||
| PGE1 and high-dose steroids | Vasodilation and neuroprotection | Marked vision improvement within one day when started early | ✓ | [112] | ||
| EPO with prednisone | Enhances oxygen delivery to damaged tissue | Improvement in vision when administered within five days | ✓ | [110] | ||
| IV methylprednisolone (MP) | Reduces inflammation | Mild visual improvement, though inconsistent | ✓ | [120,121] | ||
| Hyperbaric oxygen therapy with steroids | Enhances oxygenation | Successfully restored vision postoperatively | ✓ | [122] |
3.4. Potential Future Treatments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Salvetat, M.L.; Pellegrini, F.; Spadea, L.; Salati, C.; Zeppieri, M. Non-Arteritic Anterior Ischemic Optic Neuropathy (NA-AION): A Comprehensive Overview. Vision 2023, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Ischemic optic neuropathy. Prog. Retin. Eye Res. 2009, 28, 34–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.F.; Chen, Y.; Li, T.P.; Wang, Y.; Lin, H.J.; Yang, J.F.; Chen, L.; Tan, S.Y.; Liang, J.J.; Cen, L.P. Visual outcome of various dose of glucocorticoids treatment in nonarteritic anterior ischemic optic neuropathy—A retrospective analysis. BMC Ophthalmol. 2024, 24, 100. [Google Scholar] [CrossRef]
- Lantos, K.; Dömötör, Z.R.; Farkas, N.; Kiss, S.; Szakács, Z.; Garami, A.; Varga, G.; Lujber, L.; Kanaan, R.; Hegyi, P.; et al. Efficacy of Treatments in Nonarteritic Ischemic Optic Neuropathy: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 2718. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Wen, Y.T.; Chang, C.H.; Chang, S.W.; Lin, K.H.; Tsai, R.K. Early Methylprednisolone Treatment Can Stabilize the Blood-Optic Nerve Barrier in a Rat Model of Anterior Ischemic Optic Neuropathy (rAION). Investig. Ophthalmol. Vis. Sci. 2017, 58, 1628–1636. [Google Scholar] [CrossRef]
- Teja, S.; Patel, V.R. Ischemic Optic Neuropathies: Diagnosis and Management. Int. Ophthalmol. Clin. 2019, 59, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Chourpiliadis, C.; Aeddula, N.R. Physiology, Glucocorticoids. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hayreh, S. Management of ischemic optic neuropathies. Indian J. Ophthalmol. 2011, 59, 123. [Google Scholar] [CrossRef]
- Bajpai, V.; Madan, S.; Beri, S. Arteritic anterior ischaemic optic neuropathy: An update. Eur. J. Ophthalmol. 2021, 31, 2818–2827. [Google Scholar] [CrossRef]
- Mackie, S.L.; Dejaco, C.; Appenzeller, S.; Camellino, D.; Duftner, C.; Gonzalez-Chiappe, S.; Mahr, A.; Mukhtyar, C.; Reynolds, G.; de Souza, A.W.S.; et al. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis: Executive summary. Rheumatology 2020, 59, 487–494. [Google Scholar] [CrossRef]
- Chevalet, P.A.; Barrier, J.H.; Pottier, P.I.; Magadur-Joly, G.E.; Pottier, M.A.; Hamidou, M.O.; Planchon, B.E.; El Kouri, D.O.; Connan, L.O.; Dupond, J.L.; et al. A randomized, multicenter, controlled trial using intravenous pulses of methylprednisolone in the initial treatment of simple forms of giant cell arteritis: A one year followup study of 164 patients. J. Rheumatol. 2000, 27, 1484–1491. [Google Scholar]
- Chan, C.C.K. Steroid management in giant cell arteritis. Br. J. Ophthalmol. 2001, 85, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Goyal, A.; Sonthalia, S. Corticosteroid Adverse Effects. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Stone, J.H.; Tuckwell, K.; Dimonaco, S.; Klearman, M.; Aringer, M.; Blockmans, D.; Brouwer, E.; Cid, M.C.; Dasgupta, B.; Rech, J.; et al. Trial of Tocilizumab in Giant-Cell Arteritis. N. Engl. J. Med. 2017, 377, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Villiger, P.M.; Adler, S.; Kuchen, S.; Wermelinger, F.; Dan, D.; Fiege, V.; Bütikofer, L.; Seitz, M.; Reichenbach, S. Tocilizumab for induction and maintenance of remission in giant cell arteritis: A phase 2, andomized, double-blind, placebo-controlled trial. Lancet 2016, 387, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Régent, A.; Redeker, S.; Deroux, A.; Kieffer, P.; Ly, K.H.; Dougados, M.; Liozon, E.; Larroche, C.; Guillevin, L.; Bouillet, L.; et al. Tocilizumab in Giant Cell Arteritis: A Multicenter Retrospective Study of 34 Patients. J. Rheumatol. 2016, 43, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Seitz, M.; Reichenbach, S.; Bonel, H.; Adler, S.; Wermelinger, F.; Villiger, P. Rapid induction of remission in large vessel vasculitis by IL-6 blockade. Swiss Med. Wkly. 2011, 141, w13156. [Google Scholar]
- Loricera, J.; Blanco, R.; Hernández, J.L.; Castañeda, S.; Mera, A.; Pérez-Pampín, E.; Peiró, E.; Humbría, A.; Calvo-Alén, J.; Aurrecoechea, E.; et al. Tocilizumab in giant cell arteritis: Multicenter open-label study of 22 patients. Semin. Arthritis Rheum. 2015, 44, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Unizony, S.; Arias-Urdaneta, L.; Miloslavsky, E.; Arvikar, S.; Khosroshahi, A.; Keroack, B.; Stone, J.R.; Stone, J.H. Tocilizumab for the treatment of large-vessel vasculitis (giant cell arteritis, Takayasu arteritis) and polymyalgia rheumatica. Arthritis Care Res. 2012, 64, 1720–1729. [Google Scholar] [CrossRef]
- Oliveira, F.; Butendieck, R.R.; Ginsburg, W.W.; Parikh, K.; Abril, A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin. Exp. Rheumatol. 2014, 32 (Suppl. 82), 76–78. [Google Scholar]
- Preuss, C.V.; Anjum, F. Tocilizumab. StatPearls [Internet]. 12 February 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570644/ (accessed on 1 August 2024).
- Unizony, S.; McCulley, T.J.; Spiera, R.; Pei, J.; Sidiropoulos, P.N.; Best, J.H.; Birchwood, C.; Pavlov, A.; Stone, J.H. Clinical outcomes of patients with giant cell arteritis treated with tocilizumab in real-world clinical practice: Decreased incidence of new visual manifestations. Arthritis Res. Ther. 2021, 23, 8. [Google Scholar] [CrossRef]
- Vionnet, J.; Buss, G.; Mayer, C.; Sokolov, A.A.; Borruat, F.X.; Spertini, F. Tocilizumab for giant cell arteritis with corticosteroid-resistant progressive anterior ischemic optic neuropathy. Jt. Bone Spine 2017, 84, 615–619. [Google Scholar] [CrossRef]
- Hanoodi, M.; Mittal, M. Methotrexate. Neuroimaging Pharmacopoeia, Second Edition [Internet]. 16 August 2023; pp. 149–161. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556114/ (accessed on 1 August 2024).
- Mahr, A.D.; Jover, J.A.; Spiera, R.F.; Hernández-García, C.; Fernández-Gutiérrez, B.; LaValley, M.P.; Merkel, P.A. Adjunctive methotrexate for treatment of giant cell arteritis: An individual patient data meta-analysis. Arthritis Rheum. 2007, 56, 2789–2797. [Google Scholar] [CrossRef]
- Leon, L.; Rodriguez-Rodriguez, L.; Morado, I.; Rosales, Z.; Vadillo, C.; Freites, D.; Macarron, P.; Fernandez-Gutierrez, B.; Blanco, M.; Jover, J.A.; et al. Treatment with methotrexate and risk of relapses in patients with giant cell arteritis in clinical practice. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 111), 121–128. [Google Scholar] [PubMed]
- Langford, C.A.; Cuthbertson, D.; Ytterberg, S.R.; Khalidi, N.; Monach, P.A.; Carette, S.; Seo, P.; Moreland, L.W.; Weisman, M.; Koening, C.L.; et al. A Randomized, Double-Blind Trial of Abatacept (CTLA-4Ig) for the Treatment of Giant Cell Arteritis. Arthritis Rheumatol. 2017, 69, 837–845. [Google Scholar] [CrossRef]
- Gerriets, V.; Goyal, A.; Khaddour, K. Tumor Necrosis Factor Inhibitors. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Vilares-Morgado, R.; Nunes, H.M.M.; dos Reis, R.S.; Barbosa-Breda, J. Management of ocular arterial ischemic diseases: A review. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 261, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Steigerwalt, R.D.; Cesarone, M.R.; Belcaro, G.; Pascarella, A.; De Angelis, M.; Gattegna, R.; Nebbioso, M. Arteritic anterior ischemic optic neuropathy treated with intravenous prostaglandin E 1 and steroids. Int. J. Angiol. 2010, 19, e113–e115. [Google Scholar] [CrossRef][Green Version]
- Saxena, R.; Singh, D.; Sharma, M.; James, M.; Sharma, P.; Menon, V. Steroids versus No Steroids in Nonarteritic Anterior Ischemic Optic Neuropathy. Ophthalmology 2018, 125, 1623–1627. [Google Scholar] [CrossRef]
- Huang, T.L.; Huang, S.P.; Chang, C.H.; Lin, K.H.; Chang, S.W.; Tsai, R.K. Protective effects of systemic treatment with methylprednisolone in a rodent model of non-arteritic anterior ischemic optic neuropathy (rAION). Exp. Eye Res. 2015, 131, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, M.; Sanjari, N.; Esfandiari, H.; Pakravan, P.; Yaseri, M. The effect of high-dose steroids, and normobaric oxygen therapy, on recent onset non-arteritic anterior ischemic optic neuropathy: A randomized clinical trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 2043–2048. [Google Scholar] [CrossRef]
- Pakravan, M.; Esfandiari, H.; Hassanpour, K.; Razavi, S.; Pakravan, P. The Effect of Combined Systemic Erythropoietin and Steroid on Non-arteritic Anterior Ischemic Optic Neuropathy: A Prospective Study. Curr. Eye Res. 2017, 42, 1079–1084. [Google Scholar] [CrossRef]
- Nikkhah, H.; Golalipour, M.; Doozandeh, A.; Pakravan, M.; Yaseri, M.; Esfandiari, H. The effect of systemic erythropoietin and oral prednisolone on recent-onset non-arteritic anterior ischemic optic neuropathy: A randomized clinical trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2291–2297. [Google Scholar] [CrossRef]
- Radoi, C.; Garcia, T.; Brugniart, C.; Ducasse, A.; Arndt, C. Intravitreal triamcinolone injections in non-arteritic anterior ischemic optic neuropathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 339–345. [Google Scholar] [CrossRef]
- Pereira, L.S.; Ávila, M.P.; Salustiano, L.X.; Paula, A.C.; Arnhold, E.; McCulley, T.J. Intravitreal Triamcinolone Acetonide Injection in a Rodent Model of Anterior Ischemic Optic Neuropathy. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2018, 38, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Wen, Y.T.; Chang, C.H.; Chang, S.W.; Lin, K.H.; Tsai, R.K. Efficacy of Intravitreal Injections of Triamcinolone Acetonide in a Rodent Model of Nonarteritic Anterior Ischemic Optic Neuropathy. Investig. Opthalmol. Vis. Sci. 2016, 57, 1878. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsai, R.K.; Lin, K.L.; Huang, C.T.; Wen, Y.T. Transcriptomic Analysis Reveals That Granulocyte Colony-Stimulating Factor Trigger a Novel Signaling Pathway (TAF9-P53-TRIAP1-CASP3) to Protect Retinal Ganglion Cells after Ischemic Optic Neuropathy. Int. J. Mol. Sci. 2022, 23, 8359. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Huang, T.L.; Huang, S.P.; Tsai, R.K. Neuroprotective effects of recombinant human granulocyte colony-stimulating factor (G-CSF) in a rat model of anterior ischemic optic neuropathy (rAION). Exp. Eye Res. 2014, 118, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.T.; Huang, T.L.; Huang, S.P.; Chang, C.H.; Tsai, R.K. Early applications of granulocyte colony-stimulating factor (G-CSF) can stabilize the blood-optic nerve barrier and further ameliorate optic nerve inflammation in a rat model of anterior ischemic optic neuropathy (rAION). Dis. Models Mech. 2016, 9, 1193–1202. [Google Scholar] [CrossRef]
- Liu, P.K.; Wen, Y.T.; Lin, W.; Kapupara, K.; Tai, M.; Tsai, R.K. Neuroprotective effects of low-dose G-CSF plus meloxicam in a rat model of anterior ischemic optic neuropathy. Sci. Rep. 2020, 10, 10351. [Google Scholar] [CrossRef]
- Abri Aghdam, K.; Aghajani, A.; Ashraf Khorasani, M.; Soltan Sanjari, M.; Chaibakhsh, S.; Habibi, A.; Falavarjani, K.G. Intravitreal Injection Of The Granulocyte-Colony Stimulating Factor For The Treatment Of Non-Arteritic Anterior Ischemic Optic Neuropathy: A Pilot Study. Semin. Ophthalmol. 2021, 36, 649–657. [Google Scholar] [CrossRef]
- Oddone, F.; Rossetti, L.; Parravano, M.; Sbardella, D.; Coletta, M.; Ziccardi, L.; Roberti, G.; Carnevale, C.; Romano, D.; Manni, G.; et al. Citicoline in Ophthalmological Neurodegenerative Disease: A Comprehensive Review. Pharmaceuticals 2021, 14, 281. [Google Scholar] [CrossRef]
- Parisi, V.; Barbano, L.; di Renzo, A.; Coppola, G.; Ziccardi, L. Neuroenhancement and neuroprotection by oral solution citicoline in non-arteritic ischemic optic neuropathy as a model of neurodegeneration: A randomized pilot study. PLoS ONE 2019, 14, e0220435. [Google Scholar] [CrossRef]
- Guo, Y.; Mehrabian, Z.; Johnson, M.A.; Albers, D.S.; Rich, C.C.; Baumgartner, R.A.; Bernstein, S.L. Topical Trabodenoson Is Neuroprotective in a Rodent Model of Anterior Ischemic Optic Neuropathy (rNAION). Transl. Vis. Sci. Technol. 2019, 8, 47. [Google Scholar] [CrossRef]
- Li, L.; Su, Y.; Liu, J.; Chen, C. Efficacy of Vincamine treatment in a rat model of anterior ischemic optic neuropathy. Eur. J. Ophthalmol. 2021, 31, 3442–3449. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Q.; Chen, C.Z.; Su, Y.; Yi, Z.H. Therapeutic effect and safety of vincamine in anterior non-arteritic ischemic optic neuropathy. Guoji Yanke Zazhi (Int. Eye Sci.) 2017, 17, 1845–1848. [Google Scholar]
- Peeler, C.; Cestari, D.M. Non-Arteritic Anterior Ischemic Optic Neuropathy (NAION): A Review and Update on Animal Models. Semin. Ophthalmol. 2016, 31, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mathews, M.K.; Guo, Y.; Langenberg, P.; Bernstein, S.L. Ciliary neurotrophic factor (CNTF)-mediated ganglion cell survival in a rodent model of non-arteritic anterior ischaemic optic neuropathy (NAION). Br. J. Ophthalmol. 2015, 99, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Ali Shariati, M.; Kumar, V.; Yang, T.; Chakraborty, C.; Barres, B.A.; Longo, F.M.; Liao, Y.J. A Small Molecule TrkB Neurotrophin Receptor Partial Agonist as Possible Treatment for Experimental Nonarteritic Anterior Ischemic Optic Neuropathy. Curr. Eye Res. 2018, 43, 1489–1499. [Google Scholar] [CrossRef]
- Goldenberg-Cohen, N.; Avraham-Lubin, B.C.R.; Sadikov, T.; Askenasy, N. Effect of Coadministration of Neuronal Growth Factors on Neuroglial Differentiation of Bone Marrow–Derived Stem Cells in the Ischemic Retina. Investig. Opthalmol. Vis. Sci. 2014, 55, 502. [Google Scholar] [CrossRef][Green Version]
- Mehrabian, Z.; Guo, Y.; Weinreich, D.; Bernstein, S.L. Oligodendrocyte death, neuroinflammation, and the effects of minocycline in a rodent model of nonarteritic anterior ischemic optic neuropathy (rNAION). Mol. Vis. 2017, 23, 963–976. [Google Scholar]
- Chou, Y.Y.; Chien, J.Y.; Ciou, J.W.; Huang, S.P. The Protective Effects of n-Butylidenephthalide on Retinal Ganglion Cells during Ischemic Injury. Int. J. Mol. Sci. 2022, 23, 2095. [Google Scholar] [CrossRef]
- Chien, J.Y.; Chou, Y.Y.; Ciou, J.W.; Liu, F.Y.; Huang, S.P. The Effects of Two Nrf2 Activators, Bardoxolone Methyl and Omaveloxolone, on Retinal Ganglion Cell Survival during Ischemic Optic Neuropathy. Antioxidants 2021, 10, 1466. [Google Scholar] [CrossRef]
- Miller, N.R.; Johnson, M.A.; Nolan, T.; Guo, Y.; Bernstein, A.M.; Bernstein, S.L. Sustained Neuroprotection From a Single Intravitreal Injection of PGJ 2 in a Nonhuman Primate Model of Nonarteritic Anterior Ischemic Optic Neuropathy. Investig. Opthalmol. Vis. Sci. 2014, 55, 7047–7056. [Google Scholar] [CrossRef][Green Version]
- Mehrabian, Z.; Guo, Y.; Miller, N.R.; Henderson, A.D.; Roth, S.; Bernstein, S.L. Approaches to Potentiated Neuroprotective Treatment in the Rodent Model of Ischemic Optic Neuropathy. Cells 2021, 10, 1440. [Google Scholar] [CrossRef] [PubMed]
- Antoszyk, A.; Katz, B.; Singh, R.; Gurses-Ozden, R.; Erlich, S.; Rothenstein, D.; Sharon, N.; Hodge, J.; Levin, L.; Miller, N. A Phase I Open Label, Dose Escalation Trial Of QPI-1007 Delivered By A Single Intravitreal (IVT) Injection To Subjects with Low Visual Acuity And Acute Non-Arteritic Anterior Ischemic Optic Neuropathy (NAION). Investig. Ophthalmol. Vis. Sci. 2013, 54, 4575. [Google Scholar]
- EU Clinical Trials Register [Internet]. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2015-003079-31/DE (accessed on 1 August 2024).
- Rath, E.Z.; Hazan, Z.; Adamsky, K.; Solomon, A.; Segal, Z.I.; Levin, L.A. Randomized Controlled Phase 2a Study of RPh201 in Previous Nonarteritic Anterior Ischemic Optic Neuropathy. J. Neuro-Ophthalmol. 2019, 39, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Hazan, Z.; Adamsky, K.; Lucassen, A.; Levin, L.A. A First-in-Human Phase 1 Randomized Single and Multiple Ascending Dose Study of RPh201 in Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2020, 9, 366–374. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Zimmerman, M.B. Nonarteritic Anterior Ischemic Optic Neuropathy. Ophthalmology 2008, 115, 298–305.e2. [Google Scholar] [CrossRef]
- Chen, T.W.; Wu, P.Y.; Wen, Y.T.; Desai, T.D.; Huang, C.T.; Liu, P.K.; Tsai, R.K. Vitamin B3 Provides Neuroprotection via Antioxidative Stress in a Rat Model of Anterior Ischemic Optic Neuropathy. Antioxidants 2022, 11, 2422. [Google Scholar] [CrossRef]
- Chien, J.Y.; Ciou, J.W.; Yen, Y.; Huang, S.P. Protective effects of compound M01 on retinal ganglion cells in experimental anterior ischemic optic neuropathy by inhibiting TXNIP/NLRP3 inflammasome pathway. Biomed. Pharmacother. 2023, 169, 115861. [Google Scholar] [CrossRef]
- Maciulaitiene, R.; Kalesnykas, G.; Pauza, D.H.; Januleviciene, I. A combination of topical and systemic administration of brimonidine is neuroprotective in the murine optic nerve crush model. PLoS ONE 2024, 19, e0308671. [Google Scholar] [CrossRef]
- Berry, S.; Lin, W.; Sadaka, A.; Lee, A. Nonarteritic anterior ischemic optic neuropathy: Cause, effect, and management. Eye Brain 2017, 9, 23–28. [Google Scholar] [CrossRef]
- Hayreh, S.S. Controversies on neuroprotection therapy in non-arteritic anterior ischaemic optic neuropathy. Br. J. Ophthalmol. 2020, 104, 153–156. [Google Scholar] [CrossRef]
- Wilhelm, B.; Lüdtke, H.; Wilhelm, H. Efficacy and tolerability of 0.2% brimonidine tartrate for the treatment of acute non-arteritic anterior ischemic optic neuropathy (NAION): A 3-month, double-masked, andomized, placebo-controlled trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 551–558. [Google Scholar]
- Allen, R.S.; Olsen, T.W.; Sayeed, I.; Cale, H.A.; Morrison, K.C.; Oumarbaeva, Y.; Lucaciu, I.; Boatright, J.H.; Pardue, M.T.; Stein, D.G. Progesterone Treatment in Two Rat Models of Ocular Ischemia. Investig. Opthalmol. Vis. Sci. 2015, 56, 2880–2891. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.D.; Wen, Y.T.; Daddam, J.R.; Cheng, F.; Chen, C.C.; Pan, C.L.; Lin, K.L.; Tsai, R.K. Long term therapeutic effects of icariin-loaded PLGA microspheres in an experimental model of optic nerve ischemia via modulation of CEBP-β/G-CSF /noncanonical NF κB axis. Bioeng. Transl. Med. 2022, 7, e10289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Peng, C. Puerarin: A Review of Pharmacological Effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef]
- Nguyen Ngo Le, M.A.; Wen, Y.T.; Ho, Y.C.; Kapupara, K.; Tsai, R.K. Therapeutic Effects of Puerarin Against Anterior Ischemic Optic Neuropathy Through Antiapoptotic and Anti-Inflammatory Actions. Investig. Opthalmol. Vis. Sci. 2019, 60, 3481. [Google Scholar] [CrossRef]
- Chen, Y.H.; Huang, Y.C.; Chen, C.H.; Wen, Y.T.; Tsai, R.K.; Chen, C. Investigation of the Protective Effect of Extracellular Vesicle miR-124 on Retinal Ganglion Cells Using a Photolabile Paper-Based Chip. Investig. Opthalmol. Vis. Sci. 2023, 64, 17. [Google Scholar] [CrossRef]
- Wen, Y.T.; Ho, Y.C.; Lee, Y.C.; Ding, D.C.; Liu, P.K.; Tsai, R.K. The Benefits and Hazards of Intravitreal Mesenchymal Stem Cell (MSC) Based-Therapies in the Experimental Ischemic Optic Neuropathy. Int. J. Mol. Sci. 2021, 22, 2117. [Google Scholar] [CrossRef]
- Li, D.; Gong, Y. A Promising Strategy for Non-Arteritic Anterior Ischemic Optic Neuropathy: Intravitreal Mesenchymal Stem Cell Exosome. Curr. Stem Cell Res. Ther. 2021, 16, 109–114. [Google Scholar] [CrossRef]
- Pastor, J.C.; Pastor-Idoate, S.; López-Paniagua, M.; Para, M.; Blazquez, F.; Murgui, E.; García, V.; Coco-Martín, R.M. Intravitreal allogeneic mesenchymal stem cells: A non-randomized phase II clinical trial for acute non-arteritic optic neuropathy. Stem Cell Res. Ther. 2023, 14, 261. [Google Scholar] [CrossRef]
- Gaier, E.D.; Torun, N. The enigma of nonarteritic anterior ischemic optic neuropathy. Curr. Opin. Ophthalmol. 2016, 27, 498–504. [Google Scholar] [CrossRef]
- Rootman, D.B.; Gill, H.S.; Margolin, E.A. Intravitreal bevacizumab for the treatment of nonarteritic anterior ischemic optic neuropathy: A prospective trial. Eye 2013, 27, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.R.; Arnold, A.C. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye 2015, 29, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.L.; Thomas, S.; Olson, J.L.; Mandava, N. Treatment of Nonarteritic Anterior Ischemic Optic Neuropathy with Intravitreal Bevacizumab. J. Neuro-Ophthalmol. 2007, 27, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.; Pappuru, R. An unusual presentation of nonarteritic ischemic optic neuropathy with subretinal fluid treated with intravitreal bevacizumab. Indian J. Ophthalmol. 2016, 64, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Chang, C.H.; Chang, S.W.; Lin, K.H.; Tsai, R.K. Efficacy of Intravitreal Injections of Antivascular Endothelial Growth Factor Agents in a Rat Model of Anterior Ischemic Optic Neuropathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2290–2296. [Google Scholar] [CrossRef][Green Version]
- Miller, N.R.; Johnson, M.A.; Nolan, T.; Guo, Y.; Bernstein, S.L. A Single Intravitreal Injection of Ranibizumab Provides No Neuroprotection in a Nonhuman Primate Model of Moderate-to-Severe Nonarteritic Anterior Ischemic Optic Neuropathy. Investig. Opthalmol. Vis. Sci. 2015, 56, 7679–7686. [Google Scholar] [CrossRef]
- Ayhan, Z.; Kocaoğlu, G.; Yaman, A.; Bajin, M.S.; Saatci, A.O. Single intravitreal aflibercept injection for unilateral acute nonarteritic ischemic optic neuropathy. Case Rep. Ophthalmol. Med. 2015, 2015, 783241. [Google Scholar] [CrossRef]
- Cheng, K.C.; Chiu, C.C.; Chen, K.J.; Chang, Y.C. Intravitreal Aflibercept for Patients with Acute Nonarteritic Anterior Ischemic Optic Neuropathy: A Retrospective Trial. J. Clin. Med. 2023, 12, 4868. [Google Scholar] [CrossRef]
- Lyttle, D.P.; Johnson, L.N.; Margolin, E.A.; Madsen, R.W. Levodopa as a possible treatment of visual loss in nonarteritic anterior ischemic optic neuropathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 757–764. [Google Scholar] [CrossRef]
- Johnson, L.N.; Guy, M.E.; Krohel, G.B.; Madsen, R.W. Levodopa may improve vision loss in recent-onset, nonarteritic anterior ischemic optic neuropathy. Ophthalmology 2000, 107, 521–526. [Google Scholar] [CrossRef]
- Johnson, L.N.; Gould, T.J.; Krohel, G.B. Effect of Levodopa and Carbidopa on Recovery of Visual Function in Patients with Nonarteritic Anterior Ischemic Optic Neuropathy of Longer Than Six Months’ Duration. Am. J. Ophthalmol. 1996, 121, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Simsek, T.; Eryilmaz, T.; Acaroglu, G. Efficacy of levodopa and carbidopa on visual function in patients with non-arteritic anterior ischaemic optic neuropathy. Int. J. Clin. Pract. 2005, 59, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Bialer, O.Y.; Stiebel-Kalish, H. Evaluation and management of nonarteritic anterior ischemic optic neuropathy: A national survey. Graefe’s Arch. Clin. Exp. Ophthalmol. 2024, Online ahead of print. [Google Scholar] [CrossRef]
- Jin, X.; Fu, J.; Lv, R.; Hao, X.; Wang, S.; Sun, M.; Xu, G.; Zhang, Q.; Zhang, L.; Li, Y.; et al. Efficacy and safety of platelet-rich plasma for acute nonarteritic anterior ischemic optic neuropathy: A prospective cohort study. Front. Med. 2024, 11, 1344107. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Vega, B.; Nicieza, J.; Álvarez-Barrios, A.; Álvarez, L.; García, M.; Fernández-Vega, C.; Vega, J.A.; González-Iglesias, H. The Use of Vitamins and Coenzyme Q10 for the Treatment of Vascular Occlusion Diseases Affecting the Retina. Nutrients 2020, 12, 723. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mesentier-Louro, L.A.; Oh, A.J.; Heng, K.; Shariati, M.A.; Huang, H.; Hu, Y.; Liao, Y.J. Increased ER Stress after Experimental Ischemic Optic Neuropathy and Improved RGC and Oligodendrocyte Survival after Treatment with Chemical Chaperon. Investig. Opthalmol. Vis. Sci. 2019, 60, 1953–1966. [Google Scholar] [CrossRef]
- Chiquet, C.; Vignal, C.; Gohier, P.; Heron, E.; Thuret, G.; Rougier, M.B.; Lehmann, A.; Flet, L.; Quesada, J.L.; Roustit, M.; et al. Treatment of nonarteritic anterior ischemic optic neuropathy with an endothelin antagonist: ENDOTHELION (ENDOTHELin antagonist receptor in Ischemic Optic Neuropathy)—A multicentre andomized controlled trial protocol. Trials 2022, 23, 916. [Google Scholar] [CrossRef]
- Ghasemi Fard, S.; Wang, F.; Sinclair, A.J.; Elliott, G.; Turchini, G.M. How does high DHA fish oil affect health? A systematic review of evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 1684–1727. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Fan, S.; Wu, S.; Yang, F.; Fang, Z.; Fu, H.; Li, Y. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2018, 15, 116. [Google Scholar] [CrossRef]
- Georgiou, T.; Wen, Y.T.; Chang, C.H.; Kolovos, P.; Kalogerou, M.; Prokopiou, E.; Neokleous, A.; Huang, C.T.; Tsai, R.K. Neuroprotective Effects of Omega-3 Polyunsaturated Fatty Acids in a Rat Model of Anterior Ischemic Optic Neuropathy. Investig. Opthalmol. Vis. Sci. 2017, 58, 1603. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Brown, R.E.; Zhang, P.C.; Zhao, Y.T.; Ju, X.H.; Song, C. DHA, EPA and their combination at various ratios differently modulated Aβ25-35-induced neurotoxicity in SH-SY5Y cells. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Wen, Y.T.; Ho, Y.C.; Wang, J.K.; Lin, K.H.; Tsai, R.K. Algae Oil Treatment Protects Retinal Ganglion Cells (RGCs) via ERK Signaling Pathway in Experimental Optic Nerve Ischemia. Mar. Drugs 2020, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Kapupara, K.; Wen, Y.T.; Tsai, R.K.; Huang, S.P. Soluble P-selectin promotes retinal ganglion cell survival through activation of Nrf2 signaling after ischemia injury. Cell Death Dis. 2017, 8, e3172. [Google Scholar] [CrossRef]
- Johnson, M.A.; Mehrabian, Z.; Guo, Y.; Ghosh, J.; Brigell, M.G.; Bernstein, S.L. Anti-NOGO Antibody Neuroprotection in a Rat Model of NAION. Transl. Vis. Sci. Technol. 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Mesentier-Louro, L.A.; Stell, L.; Yan, Y.; Montague, A.A.; de Jesus Perez, V.; Liao, Y.J. Immunoprofiling of Nonarteritic Anterior Ischemic Optic Neuropathy. Transl. Vis. Sci. Technol. 2021, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Johnson, M.A.; Mehrabian, Z.; Mishra, M.K.; Kannan, R.; Miller, N.R.; Bernstein, S.L. Dendrimers Target the Ischemic Lesion in Rodent and Primate Models of Nonarteritic Anterior Ischemic Optic Neuropathy. PLoS ONE 2016, 11, e0154437. [Google Scholar] [CrossRef]
- Hayreh, S.S. Posterior ischaemic optic neuropathy: Clinical features, pathogenesis, and management. Eye 2004, 18, 1188–1206. [Google Scholar] [CrossRef]
- Brewer, R.; Sadun, A.A. Posterior ischemic optic neuropathy: Perioperative risk factors. Taiwan J. Ophthalmol. 2020, 10, 167–173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Isayama, Y.; Takahashi, T.; Inoue, M.; Jimura, T. Posterior Ischemic Optic Neuropathy. Ophthalmologica 1983, 187, 141–147. [Google Scholar] [CrossRef]
- Inoue, M. Vascular Optic Neuropathy in Diabetes Mellitus. Jpn. J. Ophthalmol. 1997, 41, 328–331. [Google Scholar] [CrossRef]
- Yonezawa, Y.; Koga, K.; Higashi, Y.; Hasebe, M.; Fukushima, C.; Omiya, C.; Nishioka, K.; Yahata, K. A Successfully Treated Case of Posterior Ischemic Optic Neuropathy That Developed during Antihypertensive Therapy for Hypertensive Emergency. Intern. Med. 2024, 63, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Buono, L.M.; Foroozan, R.; Savino, P.J.; Danesh-Meyer, H.v.; Stanescu, D. Posterior ischemic optic neuropathy after hemodialysis. Ophthalmology 2003, 110, 1216–1218. [Google Scholar] [CrossRef] [PubMed]
- Paez-Escamilla, M.; Abo-Zed, A.; Abramovitz, B.; Stefko, S.T.; Waxman, E. Recovery of vision after treatment of hemodialysis related bilateral optic nerve ischemia. Am. J. Ophthalmol. Case Rep. 2022, 25, 101373. [Google Scholar] [CrossRef] [PubMed]
- Gerber, C.J.; Neil-Dwyer, G.; Kennedy, P. Posterior ischaemic optic neuropathy after a spontaneous extradural haematoma. J. Neurol. Neurosurg. Psychiatry 1992, 55, 630. [Google Scholar] [CrossRef]
- Steigerwalt, R.D.; Cesarone, M.R.; Belcaro, G.; de Angelis, M.; Pascarella, A.; Nebbioso, M. Non-arteritic Posterior Ischaemic Optic Neuropathy Treated with Intravenous Prostaglandin E1 and Oral Corticosteroids. Neuro-Ophthalmol. 2011, 35, 81–84. [Google Scholar] [CrossRef]
- Sperber, J.; Owolo, E.; Zachem, T.J.; Bishop, B.; Johnson, E.; Lad, E.M.; Goodwin, C.R. Perioperative Blindness in Spine Surgery: A Scoping Literature Review. J. Clin. Med. 2024, 13, 1051. [Google Scholar] [CrossRef]
- Hayreh, S.S. Ischemic optic neuropathies—Where are we now? Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 1873–1884. [Google Scholar] [CrossRef]
- Srinivasan, S.; Moorthy, S.; Sreekumar, K.; Kulkarni, C. Diffusion-weighted MRI in acute posterior ischemic optic neuropathy. Indian J. Radiol. Imaging 2012, 22, 106–107. [Google Scholar] [CrossRef]
- Yang, T.H.; Lin, M.C. Using diffuse weighted image and apparent diffusion coefficient in MRI for diagnosis of posterior ischemic optic neuropathy in a young male: A case report and literature review. BMC Ophthalmol. 2022, 22, 168. [Google Scholar] [CrossRef]
- Newman, N.J. Perioperative Visual Loss After Nonocular Surgeries. Am. J. Ophthalmol. 2008, 145, 604–610.e1. [Google Scholar] [CrossRef]
- Postoperative Visual Loss Study Group. Risk Factors Associated with Ischemic Optic Neuropathy after Spinal Fusion Surgery. Anesthesiology 2012, 116, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.F.; Sweis, R.T.; Nockels, R.P. Reversible postoperative blindness caused by bilateral status epilepticus amauroticus following thoracolumbar deformity correction: Case report. J. Neurosurg. Spine 2017, 27, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Shifa, J.; Abebe, W.; Bekele, N.; Habte, D. A case of bilateral visual loss after spinal cord surgery. Pan Afr. Med. J. 2016, 23, 119. [Google Scholar] [CrossRef] [PubMed]
- der Kelen, L.; Mommaerts, M. Visual loss after cosmetic blepharoplasty using local anaesthesia containing epinephrine—A case series. Ann. Maxillofac. Surg. 2021, 11, 340. [Google Scholar] [CrossRef]
- Allashem, H.M.; Sward, D.G.; Sethuraman, K.; Matthews, M.K. Hyperbaric oxygen therapy for perioperative posterior ischemic optic neuropathy: A case report. Undersea Hyperb. Med. 2019, 46, 701–707. [Google Scholar] [CrossRef]
- Wang, Y.; Brown, D.P.; Watson, B.D.; Goldberg, J.L. Rat Model of Photochemically-Induced Posterior Ischemic Optic Neuropathy. J. Vis. Exp. 2015, 105, 52402. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badla, O.; Badla, B.A.; Almobayed, A.; Mendoza, C.; Kishor, K.; Bhattacharya, S.K. Ischemic Optic Neuropathy: A Review of Current and Potential Future Pharmacotherapies. Pharmaceuticals 2024, 17, 1281. https://doi.org/10.3390/ph17101281
Badla O, Badla BA, Almobayed A, Mendoza C, Kishor K, Bhattacharya SK. Ischemic Optic Neuropathy: A Review of Current and Potential Future Pharmacotherapies. Pharmaceuticals. 2024; 17(10):1281. https://doi.org/10.3390/ph17101281
Chicago/Turabian StyleBadla, Omar, Beshr Abdulaziz Badla, Amr Almobayed, Carlos Mendoza, Krishna Kishor, and Sanjoy K. Bhattacharya. 2024. "Ischemic Optic Neuropathy: A Review of Current and Potential Future Pharmacotherapies" Pharmaceuticals 17, no. 10: 1281. https://doi.org/10.3390/ph17101281
APA StyleBadla, O., Badla, B. A., Almobayed, A., Mendoza, C., Kishor, K., & Bhattacharya, S. K. (2024). Ischemic Optic Neuropathy: A Review of Current and Potential Future Pharmacotherapies. Pharmaceuticals, 17(10), 1281. https://doi.org/10.3390/ph17101281








