Locally Injectable Chitosan/β-Glycerophosphate Hydrogel Doped with Triptolide–Human Serum Albumin Nanoparticles for Treating Rheumatoid Arthritis
Abstract
1. Introduction
2. Results and Discussion
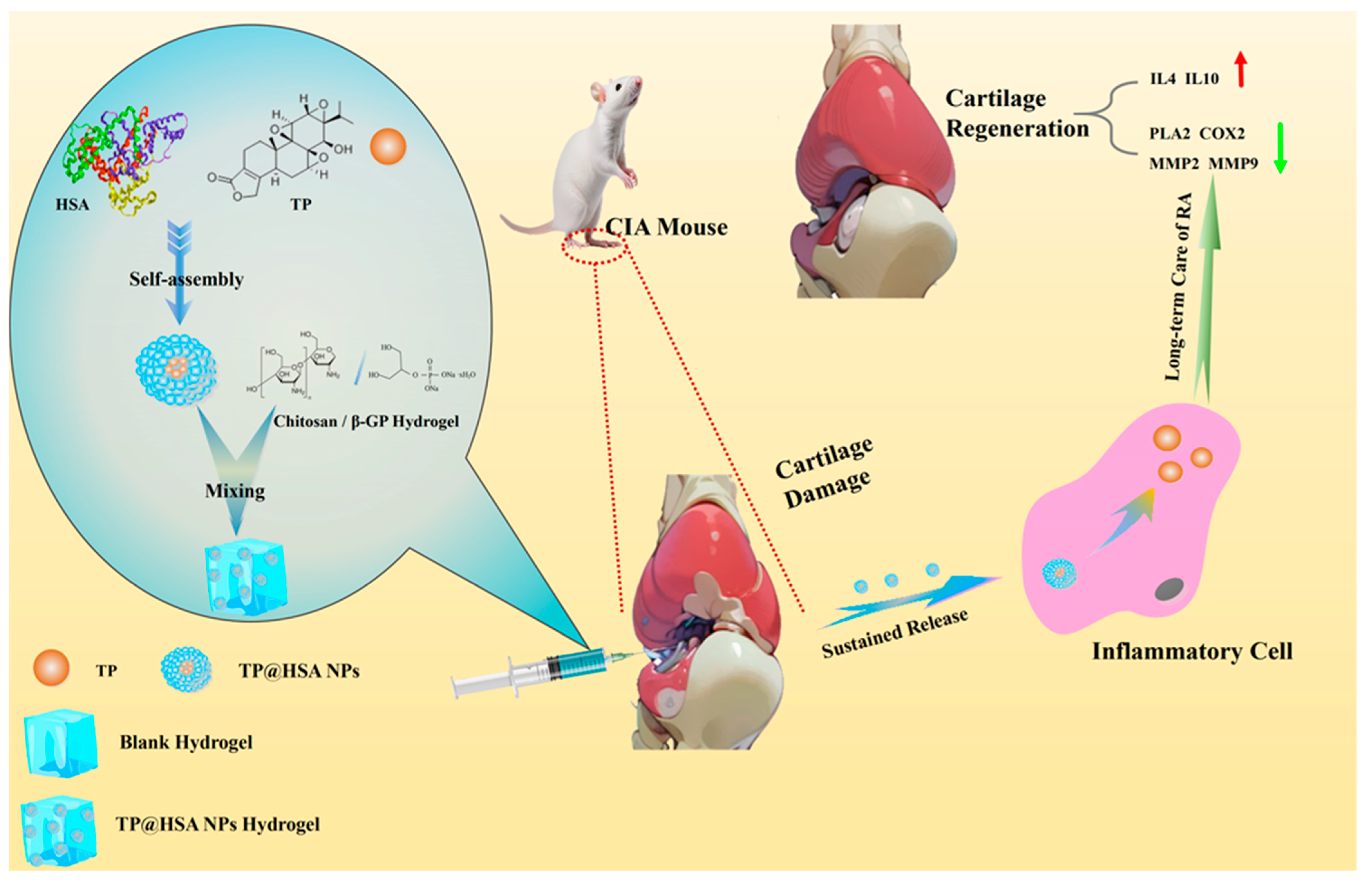
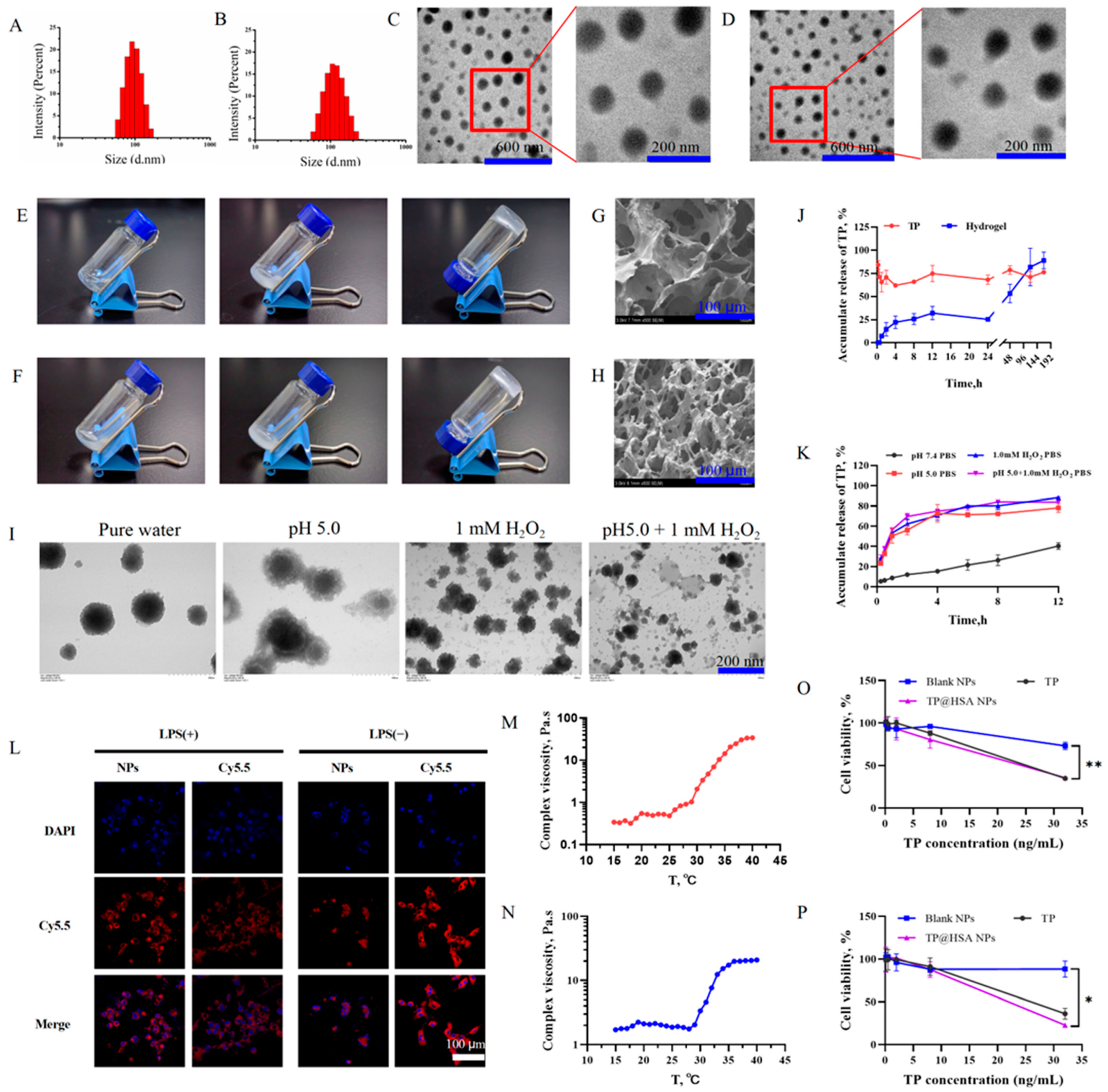
2.1. TP@HSA NP Assembly and TP@HSA NP Hydrogel Fabrication
2.2. TP Release from NPs and Hydrogel
2.3. In Vitro Cytotoxicity Study of TP@HSA NPs
2.4. Intracellular Uptake
2.5. In Vivo Degradation of Hydrogel
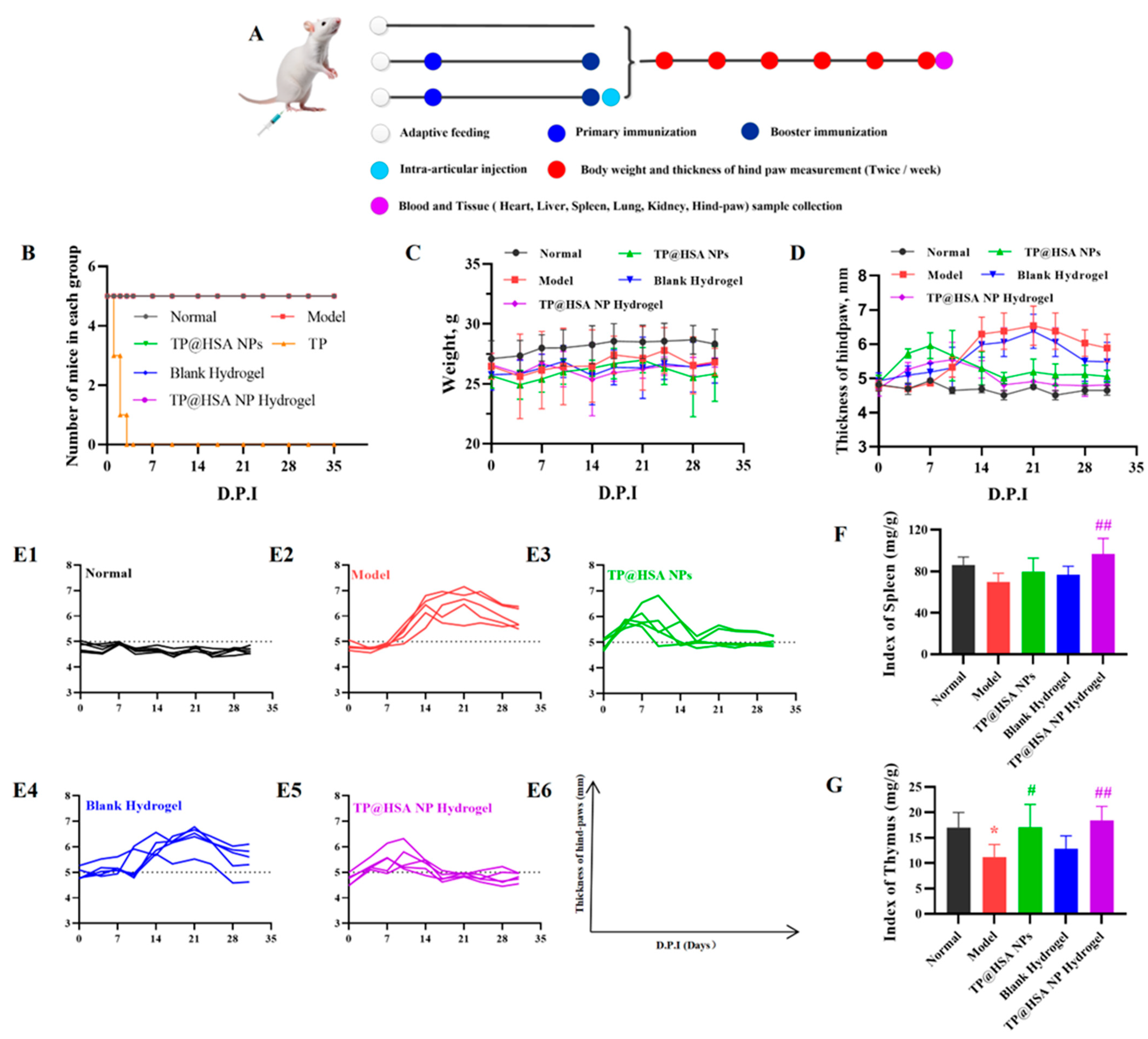
2.6. Biotoxicity
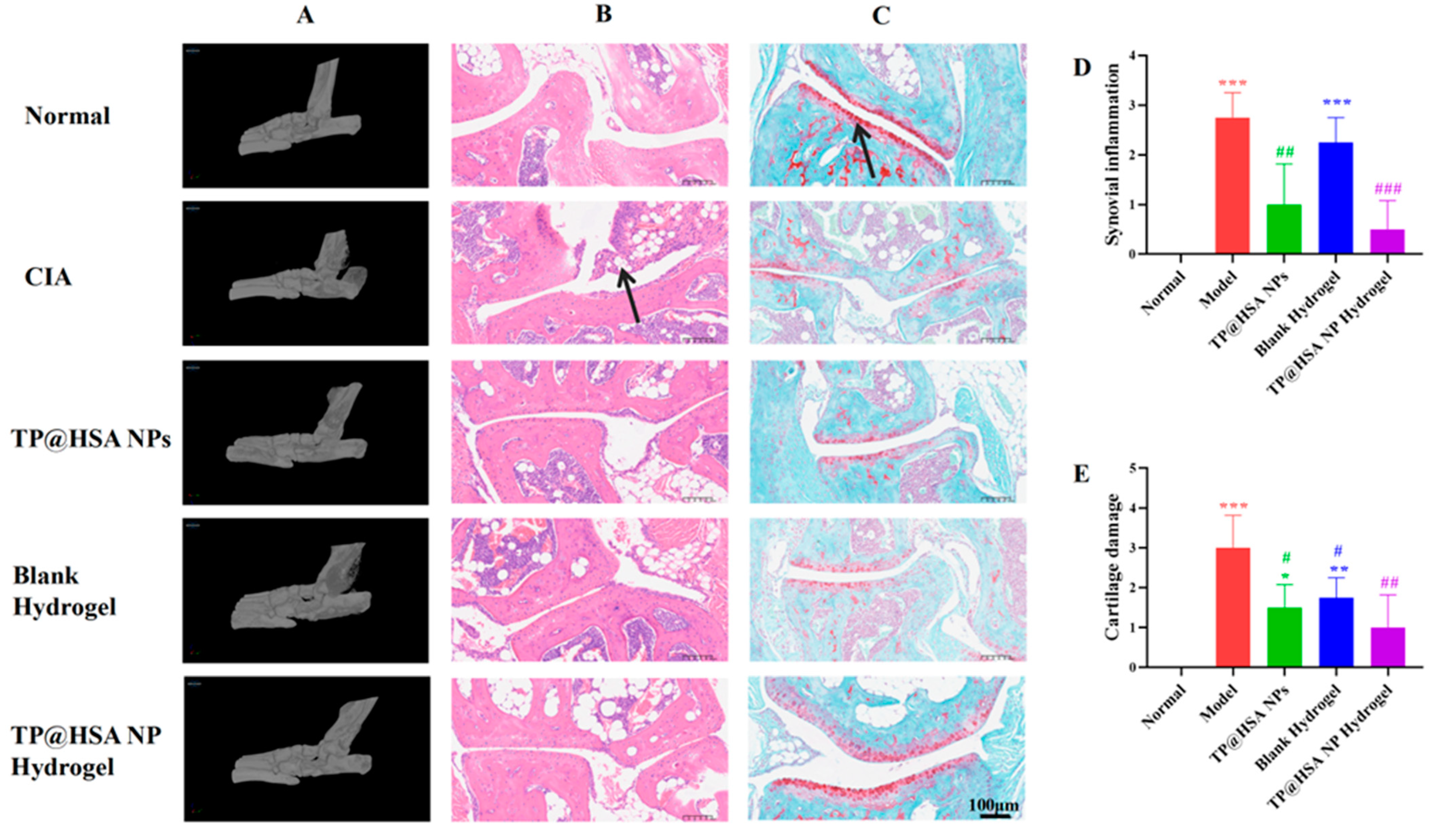
2.7. Anti-Inflammation and Cartilage Degeneration Prevention Effects Following Injection of TP@HSA NP Hydrogel in CIA Mice
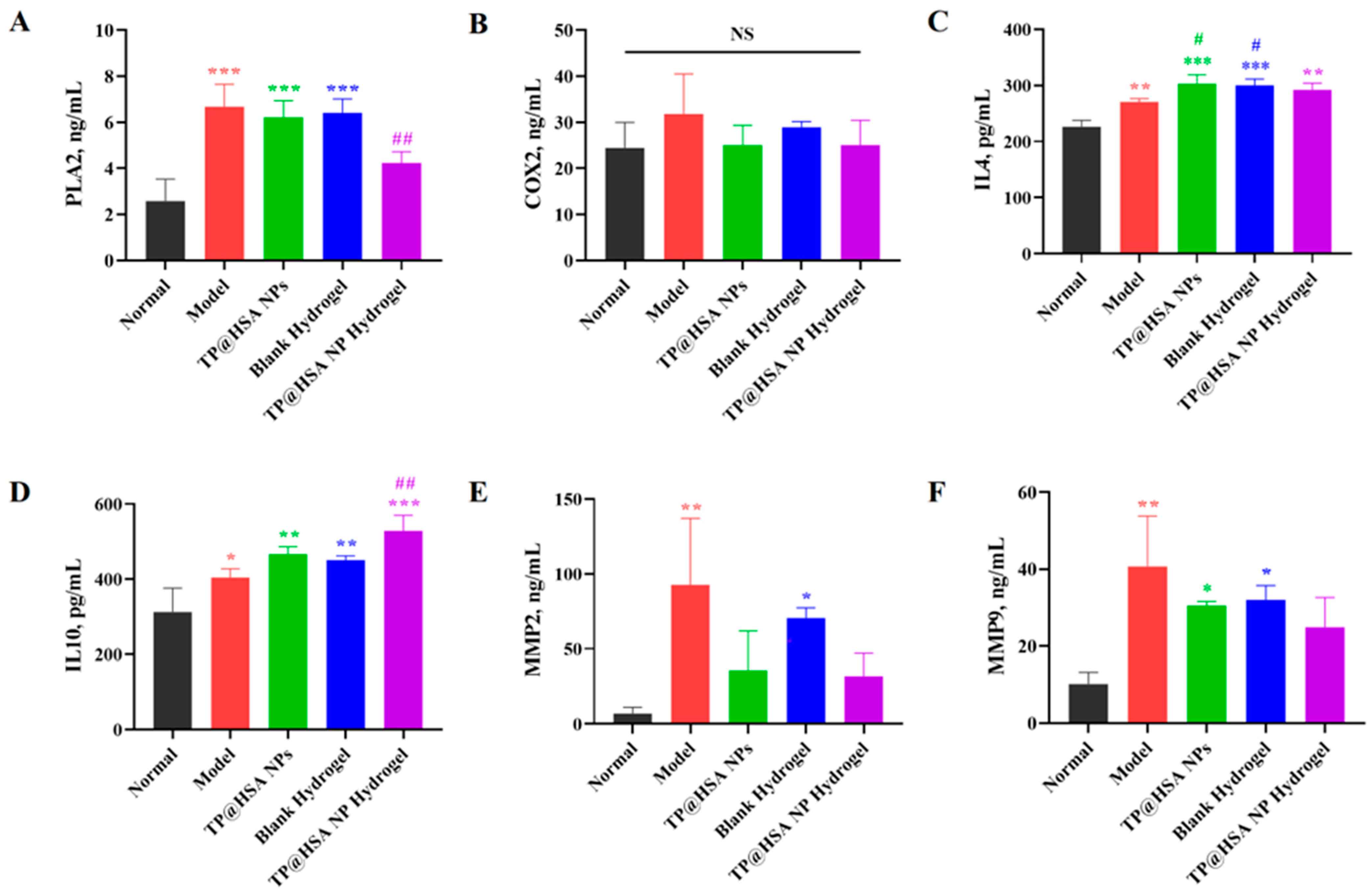
2.8. ELISAs
3. Materials and Methods
3.1. Materials
3.1.1. Reagents and Instruments
3.1.2. Cells
3.1.3. Animals
3.2. TP@HSA NP Assembly
3.3. TP@HSA NP Hydrogel Fabrication
3.4. TP Release from TP@HSA NPs and TP@HSA NP Hydrogel
3.5. Cell Viability
3.6. Intracellular Uptake
3.7. Degradation of Hydrogel
3.8. Long-Term Toxicity
3.9. In Vivo
3.9.1. Pharmacodynamics
3.9.2. Micro-CT
3.9.3. Bone Tissue Staining
3.9.4. Serum ELISAs
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; Firestein, G.S.; McInnes, I.B. The pathogenesis of rheumatoid arthritis. Immunity 2022, 55, 2255–2270. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Alfredsson, L. Prevention vs treatment of rheumatoid arthritis. Immunother. Adv. 2023, 3, ltad016. [Google Scholar] [CrossRef]
- Bergstra, S.A.-O.; Sepriano, A.A.-O.; Kerschbaumer, A.A.-O.; van der Heijde, D.A.-O.X.; Caporali, R.; Edwards, C.J.; Verschueren, P.A.-O.; de Souza, S.A.-O.; Pope, J.A.-O.; Takeuchi, T.A.-O.; et al. Efficacy, duration of use and safety of glucocorticoids: A systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 2023, 82, 81–94. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, F.; Xiao, X.; Wu, Z.; Hu, Q.; Jiang, Y.; Zhang, W.; Wei, S.; Ma, X.; Zhang, X. Tripterygium hypoglaucum (Lévl.) Hutch and Its Main Bioactive Components: Recent Advances in Pharmacological Activity, Pharmacokinetics and Potential Toxicity. Front. Pharmacol. 2021, 12, 715359. [Google Scholar] [CrossRef] [PubMed]
- Viegas, J.S.R.; Praca, F.G.; Kravicz, M.; Bentley, M. Therapeutic applications and delivery systems for triptolide. Drug Deliv. Transl. Res. 2020, 10, 1584–1600. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, C.H.; Wang, L.J.; Cui, Y.X.; Qi, R.B.; Yang, C.R.; Zhang, Y.J.; Wei, X.Y.; Lu, D.X.; Wang, Y.F. In vitro anti-viral activity of the total alkaloids from Tripterygium hypoglaucum against herpes simplex virus type 1. Virol. Sin. 2010, 25, 107–114. [Google Scholar] [CrossRef]
- He, L.; Liang, Z.; Zhao, F.; Peng, L.; Chen, Z. Modulation of IL-37 expression by triptolide and triptonide in THP-1 cells. Cell. Mol. Immunol. 2014, 12, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, N.; Chen, T.; Chen, W.; Kong, J.; Zheng, W.; Ruan, J. Triptolide Suppressed the Microglia Activation to Improve Spinal Cord Injury Through miR-96/IKKβ/NF-κB Pathway. Spine 2019, 44, E707–E714. [Google Scholar] [CrossRef]
- Li, X.J.; Jiang, Z.Z.; Zhang, L.Y. Triptolide: Progress on research in pharmacodynamics and toxicology. J. Ethnopharmacol. 2014, 155, 67–79. [Google Scholar] [CrossRef]
- Xi, C.; Peng, S.; Wu, Z.; Zhou, Q.; Zhou, J. Toxicity of triptolide and the molecular mechanisms involved. Biomed. Pharmacother. 2017, 90, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, K.; Zhu, Z.; Shao, F.; Qian, R.; Wang, C.; Dong, H.; Li, Y.; Gao, Z.; Zhao, J. Triptolide with hepatotoxicity and nephrotoxicity used in local delivery treatment of myocardial infarction by thermosensitive hydrogel. J. Nanobiotechnol. 2023, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Guo, Q.; Shen, J.; Zheng, K.; Lu, C.; Zhang, G.; Lu, A.; He, X. The Effect of Triptolide in Rheumatoid Arthritis: From Basic Research towards Clinical Translation. Int. J. Mol. Sci. 2018, 19, 376. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Cao, Q.; Sun, F.; Wang, Y.; Wang, H.; Shen, H.; Yang, F.; Wang, X.; Wu, D. Synergistic control of dual cross-linking strategy toward tailor-made hydrogels. Sci. China Chem. 2020, 63, 1793–1798. [Google Scholar] [CrossRef]
- Chang, S.; Wang, S.; Liu, Z.; Wang, X. Advances of Stimulus-Responsive Hydrogels for Bone Defects Repair in Tissue Engineering. Gels 2022, 8, 389. [Google Scholar] [CrossRef]
- Kim, T.; Suh, J.; Kim, W.J. Polymeric Aggregate-Embodied Hybrid Nitric-Oxide-Scavenging and Sequential Drug-Releasing Hydrogel for Combinatorial Treatment of Rheumatoid Arthritis. Adv. Mater. 2021, 33, e2008793. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- Ren, S.; Liu, H.; Wang, X.; Bi, J.; Lu, S.; Zhu, C.; Li, H.; Kong, W.; Chen, R.; Chen, Z. Acupoint nanocomposite hydrogel for simulation of acupuncture and targeted delivery of triptolide against rheumatoid arthritis. J. Nanobiotechnol. 2021, 19, 409. [Google Scholar] [CrossRef]
- Li, C.; Liu, R.; Song, Y.; Chen, Y.; Zhu, D.; Yu, L.; Huang, Q.; Zhang, Z.; Xue, Z.; Hua, Z.; et al. Hyaluronic Acid Hydrogels Hybridized With Au-Triptolide Nanoparticles for Intraarticular Targeted Multi-Therapy of Rheumatoid Arthritis. Front. Pharmacol. 2022, 13, 849101. [Google Scholar] [CrossRef]
- Luo, Y.; Li, J.; Hu, Y.; Gao, F.; Pak-Heng Leung, G.; Geng, F.; Fu, C.; Zhang, J. Injectable thermo-responsive nano-hydrogel loading triptolide for the anti-breast cancer enhancement via localized treatment based on “two strikes” effects. Acta Pharm. Sin. B 2020, 10, 2227–2245. [Google Scholar] [CrossRef]
- Li, H.; Gou, R.; Liao, J.; Wang, Y.; Qu, R.; Tang, Q.; Gan, J.; Zou, L.; Shi, S. Recent advances in nano-targeting drug delivery systems for rheumatoid arthritis treatment. Acta Mater. Medica 2023, 2, 23–41. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Shi, Y.; Jia, F. Editorial: Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Front. Chem. 2020, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Tian, H.; Liu, L.; Chen, Z.; Liang, R.; Chen, Z.; Wu, Z.; Ma, A.; Zheng, M.; Cai, L. Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose. Theranostics 2018, 8, 3584–3596. [Google Scholar] [CrossRef]
- Prasad, P.; Verma, S.; Surbhi; Ganguly, N.K.; Chaturvedi, V.; Mittal, S.A. Rheumatoid arthritis: Advances in treatment strategies. Mol. Cell. Biochem. 2023, 478, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Schnell, C.N.; Galván, M.V.; Zanuttini, M.A.; Mocchiutti, P. Hydrogels from xylan/chitosan complexes for the controlled release of diclofenac sodium. Cellulose 2019, 27, 1465–1481. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, T.; Peng, L.; Sun, Q.; Wei, Y.; Han, B. Advancements in Hydrogel-Based Drug Sustained Release Systems for Bone Tissue Engineering. Front. Pharmacol. 2020, 11, 622. [Google Scholar] [CrossRef]
- Yao, P.; Wang, X.; Wang, Q.; Dai, Q.; Peng, Y.; Yuan, Q.; Mou, N.; Lv, S.; Weng, B.; Wang, Y.; et al. Cyclic RGD-Functionalized pH/ROS Dual-Responsive Nanoparticle for Targeted Breast Cancer Therapy. Pharmaceutics 2023, 15, 1827. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Wang, X.; Yao, P.; Dai, Q.; Qi, X.; Yang, M.; Zhang, X.; Huang, R.; Yang, J.; et al. Docetaxel-loaded pH/ROS dual-responsive nanoparticles with self-supplied ROS for inhibiting metastasis and enhancing immunotherapy of breast cancer. J. Nanobiotechnol. 2023, 21, 286. [Google Scholar] [CrossRef]
- Qi, X.; Qin, X.; Yang, R.; Qin, J.; Li, W.; Luan, K.; Wu, Z.; Song, L. Intra-articular Administration of Chitosan Thermosensitive In Situ Hydrogels Combined with Diclofenac Sodium–Loaded Alginate Microspheres. J. Pharm. Sci. 2016, 105, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Song, G.; Fu, X.; Song, R.; Li, L.; Pu, W.; Gao, J.; Hu, J.; Liu, Q.; He, F.; et al. Reactive oxygen species-responsive dexamethasone-loaded nanoparticles for targeted treatment of rheumatoid arthritis via suppressing the iRhom2/TNF-alpha/BAFF signaling pathway. Biomaterials 2020, 232, 119730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zeng, L.; Yu, H.; He, Z.; Huang, C.; Li, C.; Nie, Y.; Li, L.; Zhou, F.; Liu, B.; et al. Injectable spontaneous hydrogen-releasing hydrogel for long-lasting alleviation of osteoarthritis. Acta Biomater. 2023, 158, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Xing, W.; Shi, X.; Zhang, T.; Lou, H.; Fan, P. Antitumor and toxicity study of mitochondria-targeted triptolide derivatives using triphenylphosphine (TPP(+)) as a carrier. Bioorganic Med. Chem. 2021, 50, 116466. [Google Scholar] [CrossRef] [PubMed]
- Solakivi, T.; Kunnas, T.; Karkkainen, S.; Jaakkola, O.; Nikkari, S.T. Arachidonic acid increases matrix metalloproteinase 9 secretion and expression in human monocytic MonoMac 6 cells. Lipids Health Dis. 2009, 8, 11. [Google Scholar] [CrossRef]
- Seo, B.B.; Kwon, Y.; Kim, J.; Hong, K.H.; Kim, S.E.; Song, H.R.; Kim, Y.M.; Song, S.C. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact. Mater. 2022, 7, 14–25. [Google Scholar] [CrossRef]
- Lin, N.; Liu, C.; Xiao, C.; Jia, H.; Imada, K.; Wu, H.; Ito, A. Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem. Pharmacol. 2007, 73, 136–146. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, X.; Wang, W.; Peng, Y.; Bi, K.; Dai, R. Targeted profiling of arachidonic acid and eicosanoids in rat tissue by UFLC–MS/MS: Application to identify potential markers for rheumatoid arthritis. Talanta 2017, 162, 479–487. [Google Scholar] [CrossRef]
- Yang, H.Y.; Jang, M.S.; Li, Y.; Lee, J.H.; Lee, D.S. Multifunctional and Redox-Responsive Self-Assembled Magnetic Nanovectors for Protein Delivery and Dual-Modal Imaging. ACS Appl. Mater. Interfaces 2017, 9, 19184–19192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, P.; Tan, Z.; Weng, B.; Wang, X.; Wang, H.; Yang, G.; Sun, F.; Zhao, Y. Locally Injectable Chitosan/β-Glycerophosphate Hydrogel Doped with Triptolide–Human Serum Albumin Nanoparticles for Treating Rheumatoid Arthritis. Pharmaceuticals 2024, 17, 1312. https://doi.org/10.3390/ph17101312
Yao P, Tan Z, Weng B, Wang X, Wang H, Yang G, Sun F, Zhao Y. Locally Injectable Chitosan/β-Glycerophosphate Hydrogel Doped with Triptolide–Human Serum Albumin Nanoparticles for Treating Rheumatoid Arthritis. Pharmaceuticals. 2024; 17(10):1312. https://doi.org/10.3390/ph17101312
Chicago/Turabian StyleYao, Pu, Zirui Tan, Bangbi Weng, Xiaowen Wang, Hongping Wang, Ge Yang, Fengjun Sun, and Ying Zhao. 2024. "Locally Injectable Chitosan/β-Glycerophosphate Hydrogel Doped with Triptolide–Human Serum Albumin Nanoparticles for Treating Rheumatoid Arthritis" Pharmaceuticals 17, no. 10: 1312. https://doi.org/10.3390/ph17101312
APA StyleYao, P., Tan, Z., Weng, B., Wang, X., Wang, H., Yang, G., Sun, F., & Zhao, Y. (2024). Locally Injectable Chitosan/β-Glycerophosphate Hydrogel Doped with Triptolide–Human Serum Albumin Nanoparticles for Treating Rheumatoid Arthritis. Pharmaceuticals, 17(10), 1312. https://doi.org/10.3390/ph17101312






