1. Introduction
Oral cancer encompasses malignancies arising from various tissues within the oral cavity, including the lips, tongue, gingiva, floor of the mouth, buccal mucosa, and hard palate. Each of these tissues can give rise to different types of oral squamous cell carcinoma (OSCC), which together represent the most common form of oral cancer [
1]. OSCC is a common cancer in the oral cavity, accounting for over 90% of all oral malignancies [
2]. This disease is marked by its tendency to aggressively invade nearby tissues and its high likelihood of spreading to other parts of the body, leading to considerable suffering and death [
3]. Oral cancer is one of the ten most common cancers globally, though its incidence varies significantly across different regions. This variation is largely influenced by factors such as alcohol consumption, tobacco use, and human papillomavirus (HPV) infection [
4]. Despite significant advances in treatment, including surgery, radiation therapy, and chemotherapy, the prognosis for OSCC patients remains poor, particularly in advanced stages [
5]. Therefore, there is an urgent need for novel therapeutic agents that can effectively target OSCC cells with minimal side effects.
Natural compounds have garnered significant attention in cancer research due to their potential efficacy and lower toxicity compared to conventional chemotherapeutics [
6]. Gastrodia elata is a well-known medicinal herb used in traditional medicine across various countries in East Asia, including China, Korea, and Japan. Its therapeutic benefits are well documented, encompassing neuroprotective, anti-inflammatory, and antioxidant effects [
7]. Parishin A (the chemical structure shown in
Figure 1A), a bioactive compound extracted from
Gastrodia elata, has traditionally been used for its anti-inflammatory and neuroprotective properties. Recent studies have shown that Parishin A possesses significant anti-inflammatory effects, aiding conditions such as tendinopathy [
8]. It also provides neuroprotective benefits, particularly for brain disorders and long-term potentiation deficits [
9]. Furthermore, Parishin A can enhance Klotho expression, helping to mitigate vascular endothelial cell aging and delay vascular senescence [
10]. Parishin markedly lowers the levels of aging-related biomarkers such as GDF15, IL-6, and p16 Ink 4 a. It also enhances the balance of the intestinal microbiota and metabolome, while alleviating pathological changes in cardiopulmonary tissues [
11]. Parishin A also exhibits antiviral activity by inhibiting Zika virus entry and offers cardioprotective effects against cardiac aging [
12]. Based on recent studies, Parishin A and its related compounds have shown promising effects on various aspects of cancer treatment. Specifically, Parishin has been found to moderately reduce the activity of the multidrug resistance (MDR) efflux pump in lymphoma cells, which is crucial for overcoming chemotherapy resistance. Additionally, Parishin-related compounds have demonstrated the ability to significantly enhance antibody-dependent cellular cytotoxicity (ADCC), which is vital for improving immune responses against cancer cells. These findings suggest that Parishin compounds, including Parishin A, may contribute to cancer treatment not only through direct cytotoxic effects but also by modulating the immune system to enhance anti-cancer activities [
13]. Furthermore, the anti-cancer potential of Parishin A may extend to its effects on other cancers, such as hepatocellular carcinoma (HCC), where G. elata derivatives have been shown to exhibit immunomodulatory properties that enhance the effectiveness of immune cells in targeting cancer cells [
14]. However, its specific effects on OSCC have not been extensively studied.
This study aimed to explore the anti-cancer properties of Parishin A in OSCC. We utilized the OSCC cell lines YD-10B and Ca9-22 as in vitro models to evaluate how Parishin A affects cell viability and metastatic behavior. Understanding the mechanisms underlying the effects that Parishin A exerts could provide valuable insights, enabling the development of new therapeutic strategies for OSCC.
3. Discussion
OSCC poses a significant health burden due to its high prevalence and aggressive nature, resulting in severe morbidity and mortality [
15]. Current treatments, including surgery, radiation, and chemotherapy, often fail to improve the prognosis for patients with advanced OSCC, highlighting the urgent need for new and effective therapies [
16]. Natural compounds such as Parishin A, derived from
Gastrodia elata, offer a promising alternative due to their potential efficacy and reduced toxicity [
8]. Parishin A has demonstrated various beneficial properties, such as neuroprotection and anti-inflammatory effects, and emerging evidence suggests it may also possess anti-cancer capabilities [
9,
14]. Therefore, this study sought to evaluate the effects of Parishin A on OSCC and investigate its potential therapeutic mechanisms.
Cell proliferation and colony formation are fundamental processes in cancer progression [
17]. Uncontrolled cell proliferation allows cancer cells to multiply rapidly, leading to the formation of tumors that can invade surrounding tissues [
18]. In our study, Parishin A inhibited OSCC cell viability in a dose- and time-dependent manner, with significant reductions observed at concentrations of 40 μM and higher (
Figure 1C,D). Previous studies have shown that the natural compound Dioscin can effectively inhibit the proliferation and cloning ability of OSCC, which is similar to our results [
19]. However, our study further tested the toxic effects on normal cells. This selective inhibition, without affecting normal human gingival fibroblasts (
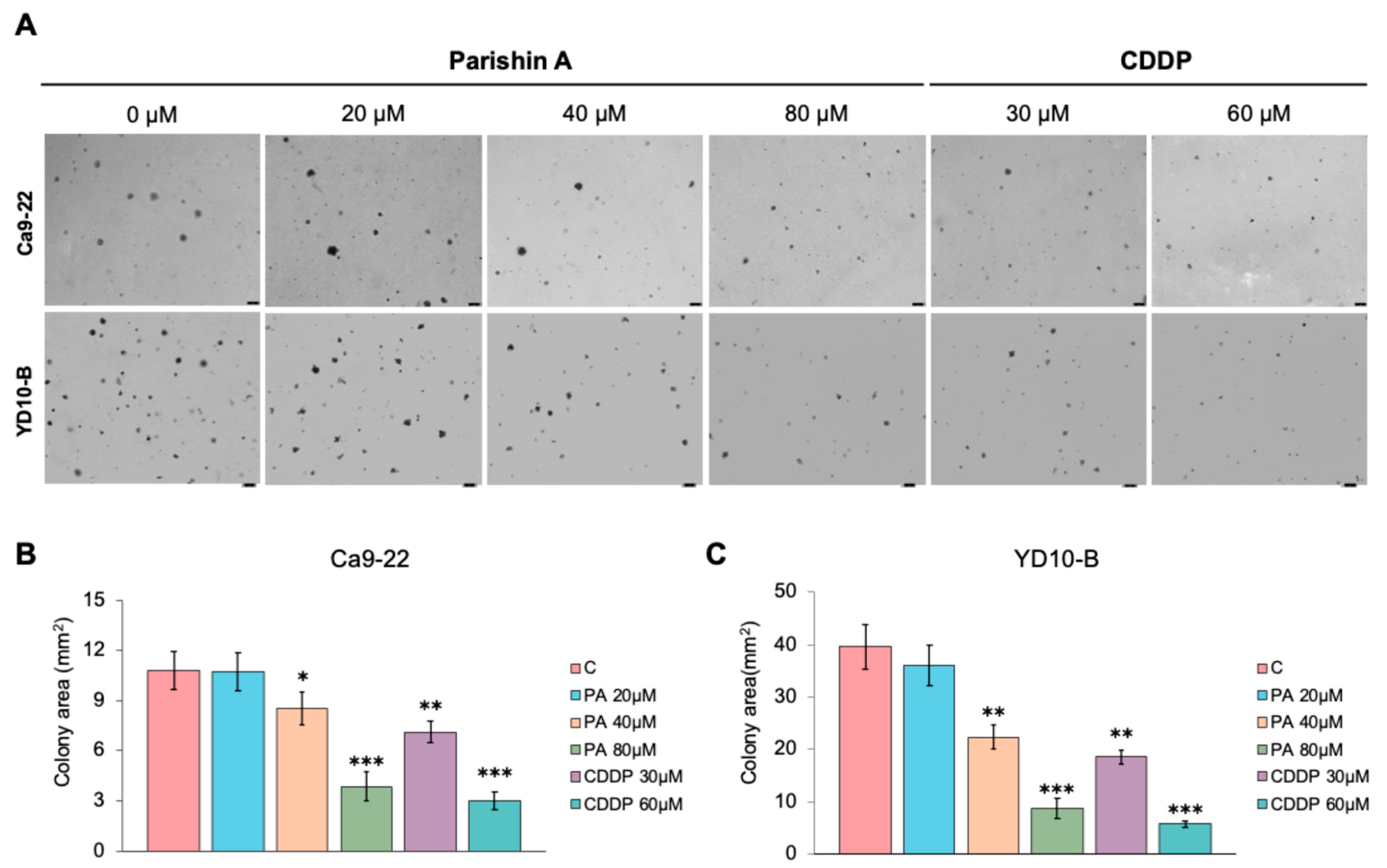
Figure 1B), underscores the potential of Parishin A as a targeted anti-cancer agent, minimizing the side effects typically associated with conventional chemotherapy. Colony formation in soft agar assays is a hallmark of anchorage-independent growth, a characteristic of malignant cells that contributes to their metastatic potential. Parishin A significantly reduced the colony-forming ability of OSCC cells, highlighting its ability to impair the capacity of cells to grow and survive independently (
Figure 2A–C). The comparison between Parishin A and the reference drug, cisplatin, demonstrates that Parishin A, at its highest concentration (80 µM), yields similar inhibitory effects on colony formation to cisplatin at 50 µM. This suggests that while cisplatin is a well-established chemotherapeutic agent, Parishin A can achieve comparable potency, especially at higher doses. The ability of Parishin A to inhibit colony growth at levels similar to cisplatin positions it as a potential alternative therapeutic option, particularly given its lower toxicity profile toward normal cells. Due to its inhibitory effects on both cell proliferation and colony formation, Parishin A could potentially disrupt the early stages of metastasis, which are critical for cancer progression and spread. These promising results provide a solid foundation for designing further experiments with Parishin A, particularly in exploring its potential as a lead compound in anti-metastatic drug development.
EMT is a crucial process in cancer metastasis, during which epithelial cells lose their cell–cell adhesion properties and acquire mesenchymal traits, enhancing the migratory and invasive capabilities [
20]. This process is crucial for the spread of cancer cells from the original tumor to distant organs [
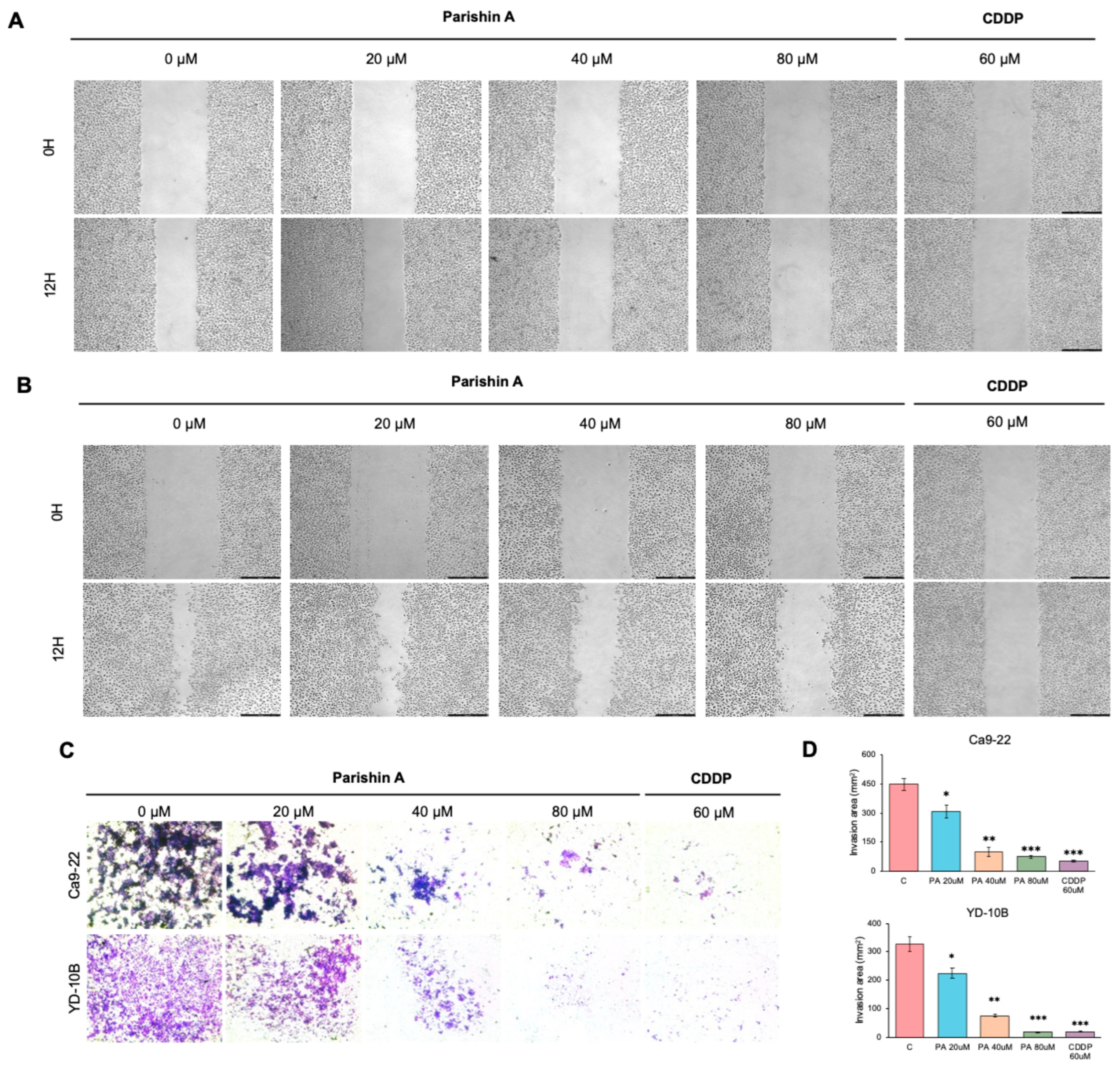
21]. In our study, Parishin A significantly suppressed the migration and invasion of OSCC cells, as evidenced by decreased wound closure and reduced invasion through Matrigel (
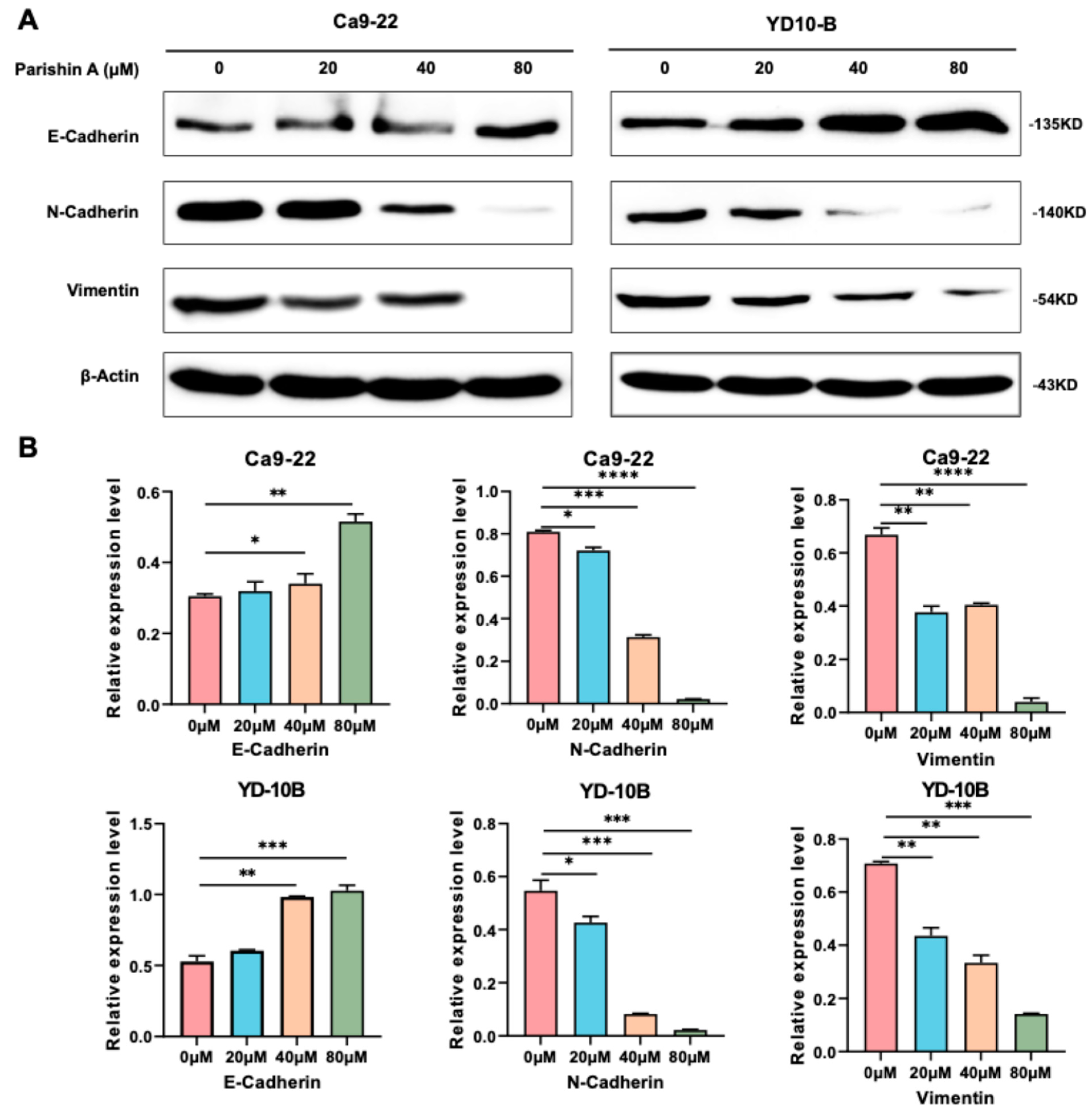
Figure 3A–C). Notably, when compared to the reference drug (likely DMSO-treated control or another therapeutic agent), Parishin A exhibited a similar or even stronger inhibitory effect at higher concentrations (40 μM and 80 μM). The potency of Parishin A in preventing cell migration and invasion is thus comparable, if not superior, to the reference drug, highlighting its potential as a promising therapeutic candidate for OSCC treatment. These findings were supported by alterations in EMT markers, with E-cadherin levels rising and both N-cadherin and vimentin levels falling (
Figure 4A,B). By inhibiting EMT, Parishin A effectively reduces the metastatic potential of OSCC cells, underscoring its role in preventing cancer spread, which is a major cause of cancer-related mortality. Given the critical role of EMT in metastasis, further experiments could focus on investigating the detailed molecular mechanisms through which Parishin A modulates EMT-related pathways, potentially leading to the development of new therapeutic strategies targeting cancer metastasis.
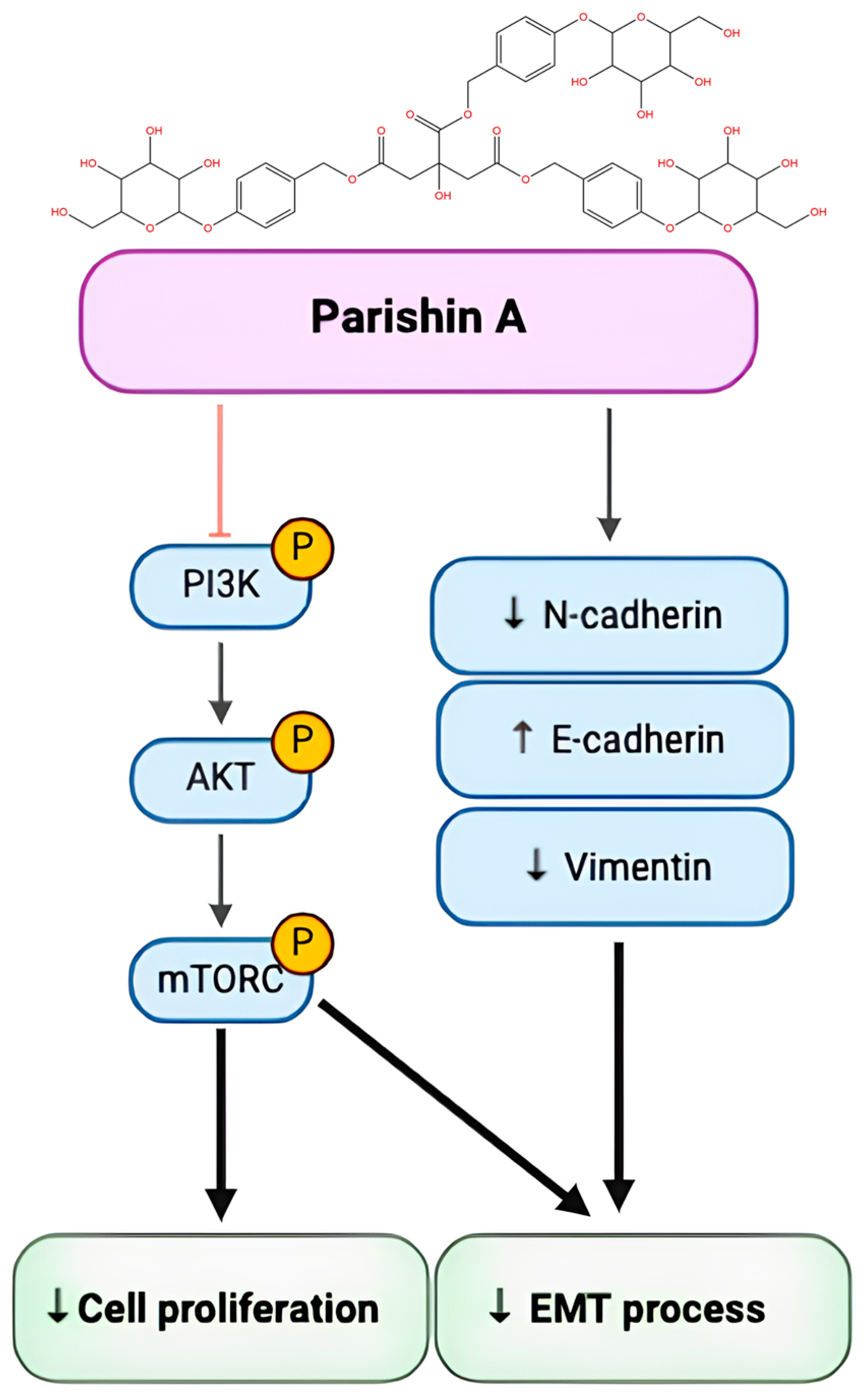
The PI3K/AKT/mTOR signaling pathway plays a vital role in regulating key cellular processes such as growth, proliferation, and survival, and is often dysregulated in various cancers, including OSCC [
22]. This pathway is triggered when growth factors bind to receptor tyrosine kinases, leading to the phosphorylation and activation of PI3K [
23]. Activated PI3K generates PIP3, which attracts AKT to the cell membrane, where it is then phosphorylated by PDK1 and mTORC2. Activated AKT subsequently phosphorylates various downstream targets, including mTOR, promoting cell growth, survival, and proliferation. Dysregulation of this pathway contributes to tumor progression and metastasis, making it a critical target for cancer therapy [
24,
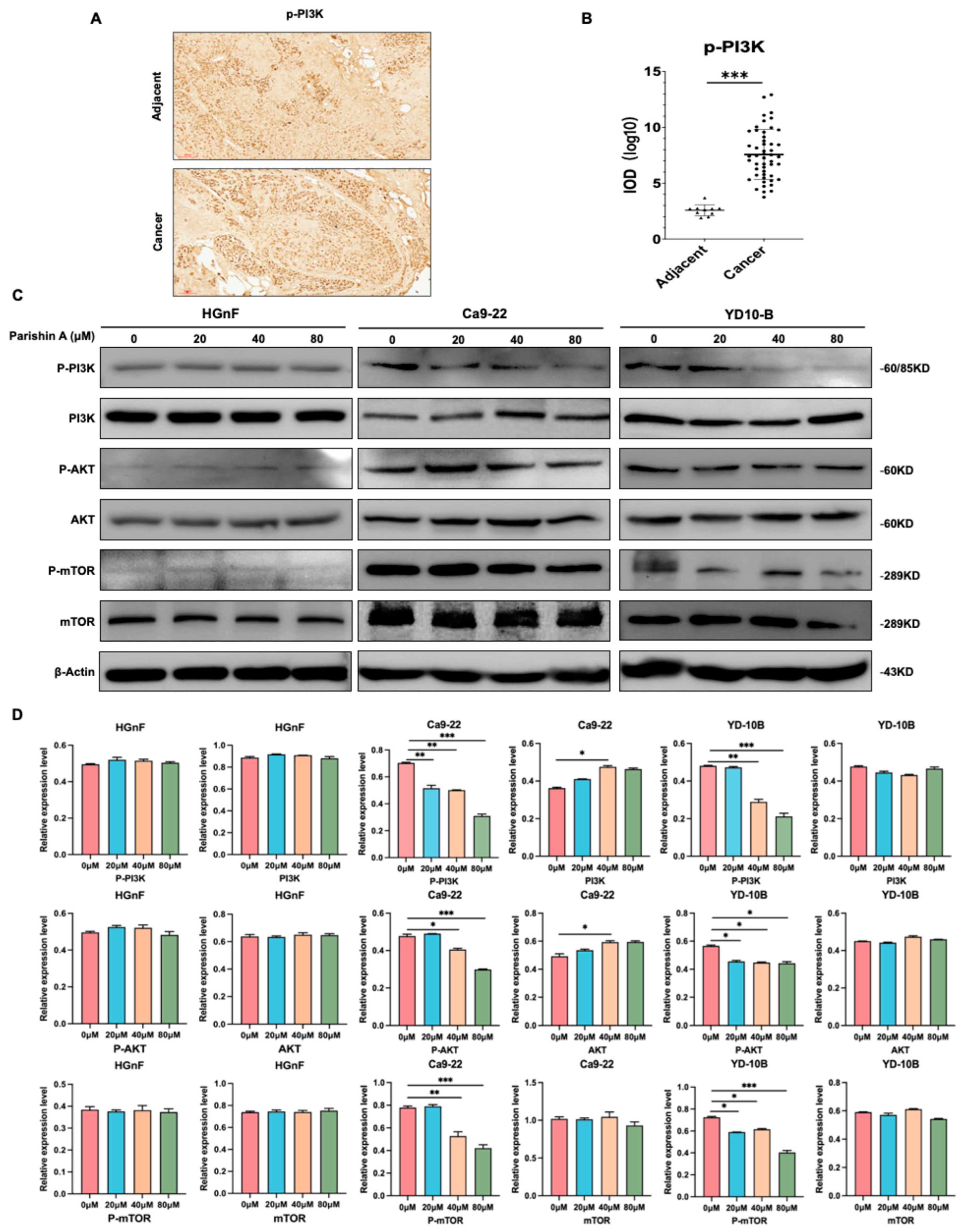
25]. In our study, IHC and Western blot analyses revealed that Parishin A treatment decreases the phosphorylation levels of PI3K, AKT, and mTOR, whereas the total protein levels remain unchanged (
Figure 5C,D). This indicates that Parishin A effectively inhibits the activation of the PI3K/AKT/mTOR pathway, thereby disrupting the signaling mechanisms essential for cancer cell maintenance. The activation level of the PI3K/AKT pathway is relatively low in normal cells, which contrasts sharply with its often-elevated activity in cancer cells [
26]. In normal cells, the PI3K/AKT pathway primarily maintains basic cell survival and metabolic functions, while in cancer cells, it is hyperactivated to promote cell proliferation, inhibit apoptosis, and enhance metabolic activities [
27]. This is also verified in our results (
Figure 5C,D); this difference may explain the selective effects of Parishin A on normal versus cancer cells, further supporting its potential as an anti-cancer agent. The inhibition of this pathway by Parishin A is consistent with the observed reductions in cell viability, colony formation, migration, and invasion. By suppressing the PI3K/AKT/mTOR pathway, Parishin A reduces the signaling required for cancer cell proliferation and survival, leading to decreased tumor growth and potentially limiting metastasis. This suppression of the pathway likely also contributes to the inhibition of the EMT process, as AKT signaling is known to promote EMT through the regulation of various transcription factors and proteins involved in cell adhesion and motility [
28]. The reliability of Parishin A as a candidate for additional experimental research is strengthened by its consistent inhibition of crucial cancer signaling pathways, indicating it is a promising molecule for further investigation in both preclinical and clinical contexts.
In conclusion, Parishin A treatment increases the expression of E-cadherin while decreasing the expression of N-cadherin and vimentin, thereby inhibiting the EMT process. Concurrently, Parishin A suppresses the phosphorylation of PI3K, AKT, and mTOR, thereby decreasing cell proliferation. These findings illustrate the dual impact of Parishin A in inhibiting both cell proliferation and the EMT process (
Figure 6). This provides a strong foundation for further research and development of Parishin A as a novel therapeutic agent for OSCC, with the potential to improve patient prognosis and reduce the burden of this aggressive cancer.
4. Materials and Methods
4.1. Ethical Statement
All protocols described herein were approved by the Institutional Review Board of Kyungpook National University (KNUDH-2022-07-02-00).
4.2. Reagents and Antibodies
Parishin A, with a purity greater than 98% (Harvey Biotech Co., Beijing, China), was dissolved in dimethyl sulfoxide (DMSO) at various working concentrations. Cisplatin (CDDP) was generously provided by Dr. Yong-Gun Kim from the Department of Periodontology, Kyungpook National University School of Dentistry, Daegu, Korea. The primary antibodies used in this study, obtained from Santa Cruz Biotechnology Inc. (Dallas, TX, USA), included E-cadherin (sc-21791), N-cadherin (sc-59987), vimentin (sc-6260), and β-actin (sc-47778). Additionally, antibodies for phosphorylated AKT (p-AKT; #9271), AKT (#9272), phosphorylated PI3K (p-PI3K; #17366), PI3K (#4249), phosphorylated mTOR (p-mTOR; #5536), and mTOR (#2983) were procured from Cell Signaling Technology (Danvers, USA).
4.3. Cell Culture
Using a previously described tissue explant technique [
29], primary human gingival fibroblasts (HGnFs) were isolated from three healthy individuals under 35 years old with no systemic or periodontal conditions. These fibroblasts were derived from 1 mm tissue biopsies and cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco™, Waltham, USA), supplemented with 10% fetal bovine serum (FBS; GenDEPOT, Katy, USA) and 1% penicillin/streptomycin (PS; Gibco™, Waltham, USA). The cells, which did not exceed seven passages, were deprived of nutrients by being cultured in medium with 0.3% FBS before experimental treatment. Human oral squamous cell carcinoma (OSCC) lines, YD-10B and Ca9-22, were provided by the Department of Oral Biology at Yonsei University, Seoul, Republic of Korea. These OSCC cell lines were maintained in DMEM with 10% FBS and 1% PS and incubated in a humidified chamber with 5% CO
2 at 37 °C.
4.4. Cell Viability Assay
Cell viability was assessed using the Cell Counting Kit-8 (CCK-8) assay. YD-10B and Ca9-22 cells were seeded in 96-well plates at a density of 5 × 103 cells per well and allowed to adhere overnight. The cells were then treated with PA at various concentrations (0, 10, 20, 40, 60, and 80 µM) for different time intervals (0, 24, 48, 72, and 96 h). After treatment, 10 µL of CCK-8 reagent was added to each well, and the plates were incubated for an additional 2 h at 37 °C. Absorbance was measured at 450 nm using a microplate reader.
4.5. Colony Formation Assay
The effect of PA on the colony-forming ability of OSCC cells was evaluated using agarose gel. YD-10B and Ca9-22 cells were plated in 6-well plates at a density of 500 cells per well and treated with various concentrations of PA (0, 10, 20, 40, 60, and 80 µM). A base layer of 0.6% agarose in DMEM with 10% FBS was first prepared. The cells were suspended in a top layer of 0.3% agarose in DMEM with 10% FBS and then overlaid onto the base layer. The cells were then incubated for 14 days to allow colony formation. Colonies were stained with 0.3% crystal violet and counted under a microscope.
4.6. Wound Healing Assay
The wound healing assay was used to evaluate the migratory ability of OSCC cells in response to PA treatment. YD-10B and Ca9-22 cells were seeded in 6-well plates at a density of 1 × 105 cells per well and allowed to grow to near-confluence. A sterile 200 µL pipette tip was used to create a uniform scratch across the cell monolayer. The cells were then washed with phosphate-buffered saline (PBS) to remove detached cells and debris. Cells were treated with various concentrations of PA (0, 20, 40, and 80 µM) in serum-free DMEM. The initial wound width was recorded, and images of the wound area were captured at 0 and 12 h using an inverted microscope. The migratory distance of the cells was measured using the ImageJ software 1.54.
4.7. Cell Invasion Assay
Cell invasion was examined using a Matrigel invasion assay. Transwell inserts (8 µm pore size) were first coated with Matrigel (Corning Costar, Lowell, MA, USA). YD-10B and Ca9-22 cells were seeded in the upper chamber of the transwell inserts at a density of 1 × 105 cells per well in serum-free DMEM. The lower chamber was filled with DMEM containing 10% FBS as a chemoattractant. After 48 h of incubation, non-invading cells were removed from the upper surface of the membrane, and the invading cells on the lower surface were fixed, stained with crystal violet, and counted under a microscope.
4.8. Western Blot Analysis
Cells were lysed in ethylenediaminetetraacetic acid (EDTA)-free RIPA II cell lysis buffer (1×) supplemented with Triton a protease inhibitor cocktail (100×). Protein concentrations were determined using the BCA protein assay kit (Thermo Fisher Scientific, Waltham, USA). Equal amounts of protein (30 µg) were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were blocked with 5% non-fat milk in TBST and probed with primary antibodies. After incubation with HRP-conjugated secondary antibodies, signals were detected using an ECL detection system (Bio-Rad, Hercules, USA) and imaged with the ImageQuant LAS 500 System.
4.9. Tissue Array
The Fifth-Generation Tissue Array (T-BO-1-TARP) was acquired from the National Cancer Institute Array program. This array encompasses oral squamous cell carcinoma tissue samples along with marginal or paracancerous tissues. Specifically, it contains 50 cases of squamous cell carcinoma and 10 cases of adjacent normal or cancer-adjacent tissues, with a single core per case. Detailed parameters of the array are provided in
Supplementary Table S1.
4.10. Immunohistochemistry Analysis
Immunohistochemistry (IHC) analyses were conducted using 4 μm thick paraffin-embedded tissue sections. The sections were blocked with 1% BSA and incubated overnight at 4 °C with the primary antibody p-PI3K (AF3242, Affinity Biosciences, Jiangsu, China). The tissue sections were then deparaffinized, rehydrated, and permeabilized with 0.5% Triton X-100 in PBS for 10 min. After three PBS washes, the sections were incubated with an appropriate secondary antibody. Protein targets were visualized using 3,3′-Diaminobenzidine (DAB) staining according to the manufacturer’s instructions. The sections were counterstained with hematoxylin for 2 min and imaged under a microscope, after which the results were analyzed and quantified using the Image-Pro Plus software (v.6.1) from Media Cybernetics.
4.11. Statistical Analysis
All experiments were carried out in triplicate, and the results are presented as the mean ± standard deviation (SD). Statistical analysis was conducted using SPSS software (version 23.0; IBM Inc., Chicago, IL, USA). Group differences were assessed using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test for multiple comparisons. A p-value of less than 0.05 was deemed statistically significant.